Abstract
The liver expresses high levels of two proteins with high affinity for long-chain fatty acids (LCFAs): liver fatty acid binding protein (L-FABP) and sterol carrier protein-2 (SCP-2). Real-time confocal microscopy of cultured primary hepatocytes from gene-ablated (L-FABP, SCP-2/SCP-x, and L-FABP/SCP-2/SCP-x null) mice showed that the loss of L-FABP reduced cellular uptake of 12-N-methyl-(7-nitrobenz-2-oxa-1,3-diazo)-aminostearic acid (a fluorescent-saturated LCFA analog) by ∼50%. Importantly, nuclear targeting of the LCFA was enhanced when L-FABP was upregulated (SCP-2/SCP-x null) but was significantly reduced when L-FABP was ablated (L-FABP null), thus impacting LCFA nuclear targeting. These effects were not associated with a net decrease in expression of key membrane proteins involved in LCFA or glucose transport. Since hepatic LCFA uptake and metabolism are closely linked to glucose uptake, the effect of glucose on L-FABP-mediated LCFA uptake and nuclear targeting was examined. Increasing concentrations of glucose decreased cellular LCFA uptake and even more extensively decreased LCFA nuclear targeting. Loss of L-FABP exacerbated the decrease in LCFA nuclear targeting, while loss of SCP-2 reduced the glucose effect, resulting in enhanced LCFA nuclear targeting compared with control. Simply, ablation of L-FABP decreases LCFA uptake and even more extensively decreases its nuclear targeting.
Keywords: liver fatty acid binding protein, sterol carrier protein-2, glucose
hepatic long-chain fatty acids (LCFAs) and glucose are metabolically linked through the intracellular LCFA pool arising from exogenous (dietary) uptake and de novo synthesis from glucose. High blood levels of LCFAs, as well as glucose, are significant risk factors in the pathogenesis of diabetes and associated cardiovascular disease (CVD) (87). The liver plays a major role in maintaining blood LCFA, as well as glucose, homeostasis (64). Exogenous LCFAs are translocated into the hepatocyte via bifunctional membrane-bound fatty acid transport proteins (FATPs) with acyl-coenzyme A (CoA) synthase activity (FATP2, FATP4, and FATP5) (reviewed in Ref. 78). The liver contains high levels of two soluble proteins, liver fatty acid binding protein (L-FABP) and sterol carrier protein-2 (SCP-2), with high affinity for LCFA, as well as LCFA-CoA (reviewed in Refs. 19, 20, 52, 59, 82).
The impact of L-FABP and SCP-2 on LCFA uptake has been examined in transformed cell lines and in L-FABP gene-targeted mice. Overexpression of L-FABP in L-cell fibroblasts, cells with a very low level of endogenous FABP or SCP-2, enhanced cellular LCFA uptake (7, 49). While L-FABP antisense treatment of HepG2 hepatoma cells decreased cellular LCFA uptake, such hepatoma cells normally express 3- to 10-fold less L-FABP and SCP-2 than the liver (34, 83). This issue was resolved with L-FABP null mice, which exhibit decreased hepatic LCFA uptake in vivo (40, 51). SCP-2 overexpression in L-cell fibroblasts also enhanced LCFA uptake (8, 48). Conversely, nothing is known about the effect of SCP-2 gene ablation on hepatic LCFA uptake (40, 51). Furthermore, interpretation of hepatic LCFA uptake in mouse models is complicated by the high prevalence of SCP-2 and L-FABP, as well as concomitant upregulation of L-FABP expression, in SCP-2 null liver (21, 68, 72). Thus the relative contributions of L-FABP and SCP-2 to LCFA uptake in liver hepatocytes remain to be resolved.
Although L-FABP facilitates intracellular targeting of LCFA to nuclei of transformed cell lines, its role in LCFA targeting into hepatocyte nuclei is not completely resolved, and nothing is known regarding the role of SCP-2 in LCFA nuclear targeting. In vitro studies showed that L-FABP stimulated cotransport of LCFA into purified nuclei (35). Real-time confocal and multiphoton imaging demonstrated that L-FABP overexpression in L-cell fibroblasts preferentially increased the targeting of fluorescent analogs of saturated and polyunsaturated LCFAs into nuclei (30, 31, 45). While L-FABP gene ablation reduced the distribution of LCFA into nuclei of primary hepatocytes in culture, the effect of SCP-2 gene ablation has not been reported (46). Again the potential contribution of SCP-2 in L-FABP null liver and the concomitant upregulation of L-FABP in SCP-2 null liver preclude clear interpretation of the respective protein's roles (21, 68). Consequently, the individual contributions of L-FABP and SCP-2 to nuclear targeting of LCFAs in the liver remain to be further resolved.
Additionally, studies with primary hepatocytes may be complicated by glucose concentrations that are much higher (11–28 mM) than physiological (6 mM) in the culture medium (32, 46, 53). Glucose concentration is much higher in liver and liver-derived cells than in most other tissues, and intracellular glucose levels rapidly rise in response to high (20–30 mM) extracellular glucose (17, 25, 27, 81). High glucose alters L-FABP interaction with peroxisome proliferator-activated receptor-α (PPARα), a key nuclear receptor in transcription of LCFA β-oxidative enzymes (28, 29, 71). However, the impact of high glucose on L-FABP-mediated LCFA uptake and distribution to the nucleus of cultured primary hepatocytes is not known.
The work presented here begins to address these questions by real-time confocal imaging of 12-N-methyl-(7-nitrobenz-2-oxa-1,3-diazo)-aminostearic acid (NBD-stearic acid) uptake in cultured primary hepatocytes. Hepatocytes were obtained from livers of wild-type (WT), L-FABP null, SCP-2/SCP-x null, and L-FABP/SCP-2/SCP-x null mice. Studies were performed not only with radiolabeled stearic acid, but also with NBD-stearic acid, a poorly metabolized fluorescent LCFA that reflects direct uptake, rather than secondary uptake driven by intracellular LCFA metabolism (30, 31).
MATERIALS AND METHODS
Materials.
Antisera against FATP2, FATP4, glucokinase (GCK), and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antisera against FATP5, insulin receptor, glucose transport protein (GLUT)-1, GLUT2, and cytochrome c oxidase subunit IV were obtained from Abcam (Cambridge, MA). Rabbit anti-glutamic oxaloacetic transaminase (GOT) was produced as described elsewhere (6). Rabbit polyclonal antibodies against rat L-FABP and human SCP-2 were produced as previously described (40, 41). NBD-stearic acid was obtained from Avanti Polar Lipids (Alabaster, AL). Alkaline phosphatase-conjugated rabbit anti-goat IgG and goat anti-rabbit IgG were purchased from Sigma-Aldrich (St. Louis, MO). Donkey anti-rabbit IgG conjugated to 15-nm gold and donkey anti-goat IgG conjugated to 6-nm gold, as well as LR White resin, were obtained from Electron Microscopy Sciences (Hatfield, PA). d(+)-glucose and d(+)-maltose were obtained from Sigma (St. Louis, MO). Rat liver recombinant L-FABP was isolated as described elsewhere (37). d-[1-3H]glucose (8.00 Ci/mmol) was purchased from Amersham/GE Healthcare (Piscataway, NJ). [9,10-3H]stearic acid (1 nmol/63.5 μCi) was obtained from Moravek Biochemicals (Brea, CA). All reagents and solvents were of the highest grade available and were cell culture tested as necessary.
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee at Texas A & M University. Male WT C57BL/6NCr mice (8–10 wk old, 20–25 g body wt) were purchased from the National Cancer Institute (Frederick Cancer Research and Developmental Center, Frederick, MD). L-FABP null mice were produced as described earlier by our laboratory and backcrossed to C57BL/6NCr mice to ≥10 generations (40). SCP-2/SCP-x null mice were generated by this laboratory by targeted disruption of the SCP-2 gene through homologous recombination and backcrossed to C57BL/6NCr mice to ≥10 generations (3). L-FABP/SCP-2/SCP-x null mice were generated and backcrossed to the C57BL/6NCr background to ≥10 generations by this laboratory, as described elsewhere (72). Mice were kept under a 12:12-h light-dark cycle in a temperature-controlled facility (25°C) with access to food (standard rodent chow mix, 4% fat calories) and water ad libitum. All mice in the facility were free of all known rodent pathogens, as monitored quarterly.
Hepatocyte isolation and culture.
Cultured primary hepatocytes were isolated from the livers of 10- to 12-wk-old male WT or null mice, as described elsewhere (72). Briefly, mice were euthanized by CO2 asphyxiation, and the liver was removed and perfused with buffer A [10 mM HEPES, pH 7.4, in calcium/magnesium-free HBSS, gentamicin sulfate (1 mg/ml medium), and 0.5 mM EGTA]. Hepatocytes were released by perfusion in buffer B [buffer A without EGTA, supplemented with 5 mM CaCl2 and 0.2 mg/ml collagenase] with gentle palpation of the liver capsule during perfusion. Hepatocytes were washed twice in cold DMEM-F12 medium with 5% FBS and plated on collagen-coated dishes in the DMEM-F12 medium with 5% FBS. DMEM-F12 medium contained 17.5 mM d-glucose, while the only insulin available in the culture medium was in the FBS.
NBD-stearic acid uptake by cultured primary hepatocytes: laser scanning microscopy.
Hepatocytes isolated from male WT and L-FABP null, SCP-2/SCP-x null, and SCP-2/SCP-x/L-FABP null mice were plated at a density of 2 × 105 cells/well on collagen-coated two-chamber cover glasses (Nalge Nunc Lab-Tek). After the cells were cultured overnight in DMEM-F12 medium with 5% FBS, individual wells were processed for a time-course analysis of NBD-stearic acid uptake. Cells were washed twice with warm (37°C) PBS, incubated for 15 min in PBS at 37°C under 5% CO2, and transferred to a 37°C heated microscope stage on a laser scanning confocal microscopy system (MRC-1024MP, Bio-Rad Laboratories, Hercules, CA) equipped with an inverted microscope (Zeiss Axiovert, Carl Zeiss MicroImaging, Thornwood, NY), a band-pass filter (no. HQ530/40, Chroma Technology, Bellow Falls, VT), and a ×40 oil objective (Zeiss Apochromat). The 488-nm excitation line of the argon-krypton ion laser source was set to 1% power, and LaserSharp 3.0 software (Bio-Rad) was used to capture images of the cells at 1-min intervals after addition of 0.01–10.4 μM NBD-stearic acid.
For examination of the effects of high or low glucose, hepatocytes were washed as described above and then incubated with 0, 6, or 20 mM glucose or maltose before (15 min) and during uptake of NBD-stearic acid. Hepatocytes were only used for 2 days in culture after isolation (Fig. 1).
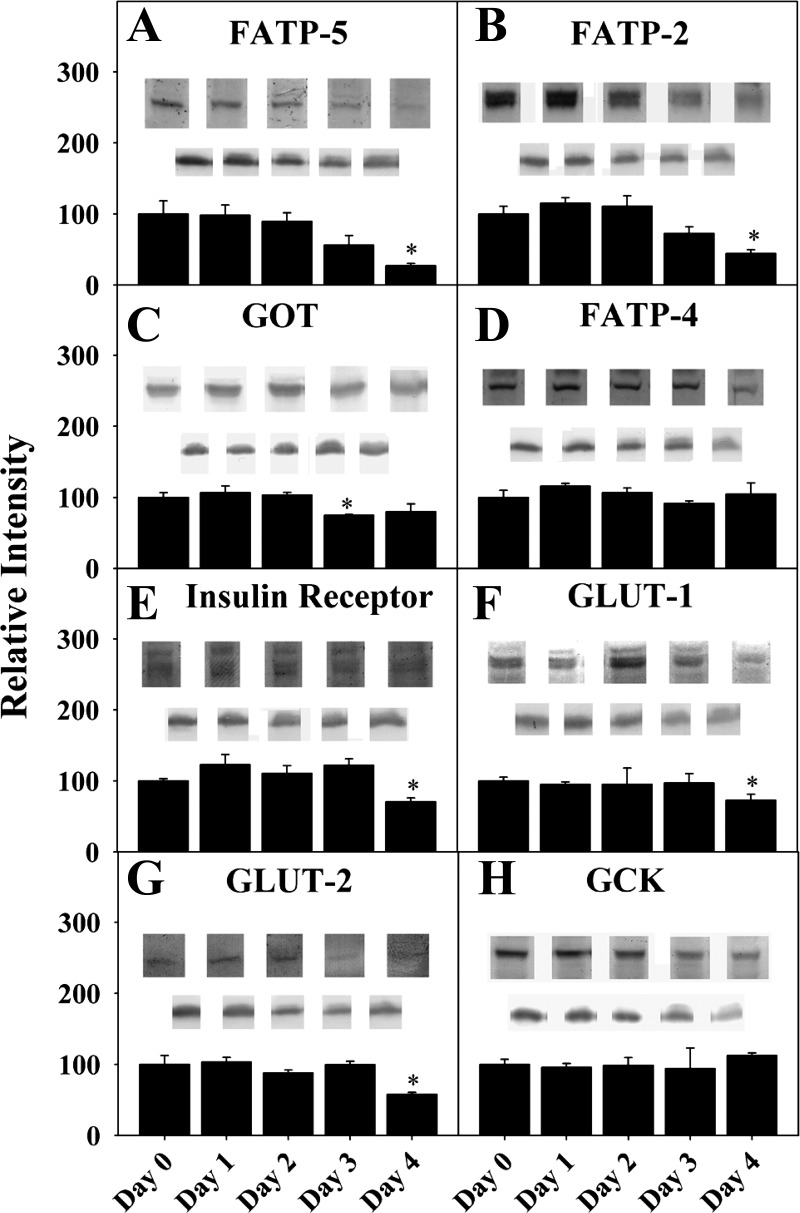
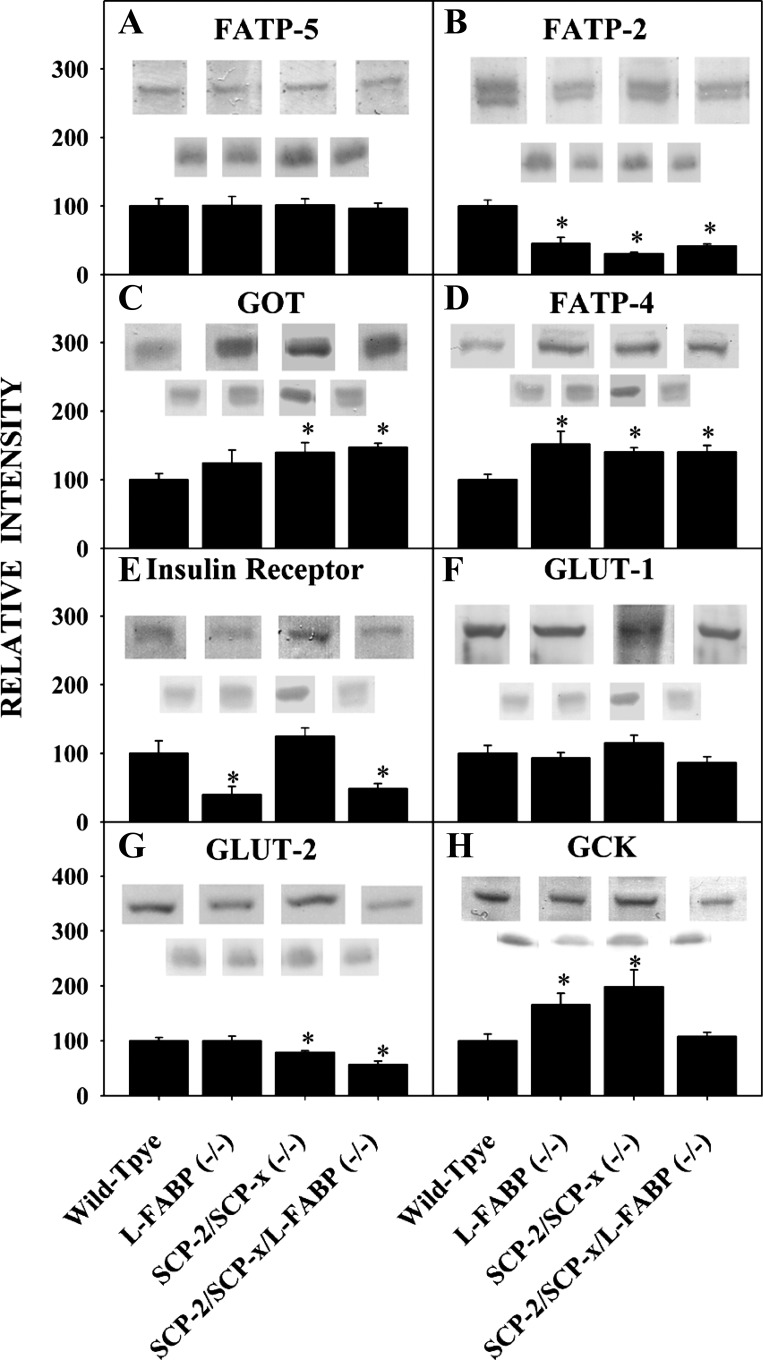
Fig. 1.
Expression of proteins involved in transport and metabolism of fatty acids and glucose across membranes in cultured primary hepatocytes from days 0–4 postisolation. Western blotting of wild-type (WT) hepatocyte lysates taken at days 0–4 after cell isolation was performed using GAPDH or other housekeeper internal control. A: fatty acid transport protein (FATP)-5. B: FATP2. C: glutamic oxaloacetic transaminase (GOT). D: FATP4. E: insulin receptor. F: glucose transporter (GLUT)-1. G: GLUT2. H: glucokinase (GCK). Values are means ± SE; n = 6–8. *P < 0.05 vs. day 0.
Quantitation of NBD-stearic acid uptake by cultured primary hepatocytes: laser scanning microscopy.
Metamorph 4.0 (Molecular Devices, Sunnyvale, CA) was used to quantify the fluorescence intensities of individual cells and nuclei throughout each time course. Excel (Microsoft, Redmond, WA) and SigmaPlot (Systat Software, San Jose, CA) were used for data analysis. The average relative intensity of NBD fluorescence at each time point was calculated from multiple replicates of the same cell type and NBD-stearic acid concentration. Data points were best fit to the Wiebull equation: y = A[1 − e−(t/b)ĉ], where y is NBD-stearic acid fluorescence intensity in hepatocytes over time, 1/b is the apparent rate constant, t is time (in minutes), and c is the shape parameter. Rates were calculated using the first derivative of the Wiebull equation: rate = (Ac/b)(t/b)c − 1e−(t/b)ĉ. Photoshop 7.0 (Adobe Systems, San Jose, CA) and Canvas 7 (ACD Systems International, Victoria, BC, Canada) were used to convert select time-course images to tiff format and to compile final multipanel images, respectively.
Fluorescent fatty acid binding to L-FABP in the presence of glucose.
NBD-stearic acid was titrated into 25 nM L-FABP in 10 mM phosphate buffer (pH 7.4) containing 0, 6, or 20 mM glucose at 24°C, as described elsewhere (20, 50). The concentrations were kept low, so that inner filter effects were negligible. Excitation and emission spectra were collected using a fluorescence spectrophotometer (Cary Eclipse, Agilent Technologies, Santa Clara, CA). NBD-stearic acid was excited at 490 nm with 5-nm bandwidth slits. NBD-stearic acid emission spectra from 515 to 600 nm were obtained using 10-nm bandwidth slits at 0–250 nM NBD-stearic acid. Data were corrected for buffer and scatter effects, and peak intensities were used to determine the final curve in SigmaPlot.
Western blot analysis of hepatocyte membrane proteins involved in LCFA (FATP2, FATP4, FATP5, and GOT) and glucose (GCK, GLUT1, GLUT2, and insulin receptor) uptake, as well as soluble proteins in LCFA uptake (L-FABP and SCP-2).
Relative and absolute protein levels were determined by Western blotting, as described elsewhere (4, 40, 41). Briefly, 2–10 μg of hepatocyte homogenates (day 0 after cell isolation, unless otherwise noted) were loaded onto tricine gels (12%) and run on a Mini-Protean II cell system (Bio-Rad) at 100-V constant voltage for ∼2 h (30 mA per gel). Proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad) by application of 100-V constant voltage for 2 h. After transfer, the blots were blocked in 3% gelatin in TBST (10 mM Tris·HCl, pH 8, 100 mM NaCl, 0.05% Tween 20) for 1 h at room temperature, washed twice with TBST, and incubated overnight at room temperature with the respective primary antibodies in 1% gelatin in TBST. The membranes were washed three times with TBST and incubated for 2 h at room temperature with the appropriate alkaline phosphatase-conjugated secondary antibodies diluted in 1% gelatin in TBST. After a final wash with TBST, the blots were incubated in AP buffer (100 mM Tris-base, pH 9, 100 mM NaCl, and 10 mM MgCl2) for 10 min and then in 5-bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium according to the manufacturer's protocol (Sigma Aldrich) until bands developed. Images of blots were acquired using a dual-lens scanning system at 600 dpi (Perfection V700 PHOTO, Epson America, Long Beach, CA). Relative expression levels of proteins were quantified by densitometric analysis of the images performed in eight-bit gray-scale density using Scion Image beta release 4.0.2 (Scion, Frederick, MD). Adobe Photoshop 7.0 and Canvas 7 were used to compile Western blot scans and produce the final multipanel images.
Immunogold electron microscopy.
Electron microscopy was performed after double-immunogold labeling of FATP5 and L-FABP, as described elsewhere (72, 73).
Quantitation of NBD-stearic acid uptake and metabolism by cultured primary hepatocytes.
The freshly isolated mouse hepatocytes were plated in six-well plates (1 × 106 cells/well) and incubated with DMEM-F12 medium (with 5% FBS) overnight. Medium was removed, and the cells were gently washed twice with PBS. Hepatocytes were incubated with 0.5 nmol of NBD-stearic acid per well in PBS for 1 or 2 h (control cells were incubated with PBS without NBD-stearic acid). The plates were floated in liquid nitrogen to freeze the cells, and the lipids were extracted with hexane-isopropanol (3:2, vol/vol), as described elsewhere (31). For analysis of neutral lipids, samples were first run on TLC Silica Gel G plates with a petroleum ether-diethyl ether-methanol-glacial acetic acid (180:14:4:1, vol/vol) solvent system. The plates were further developed in petroleum ether-diethyl ether-glacial acetic acid (70:30:1, vol/vol) for 2 h. For analysis of phospholipids, lipids were separated on Silica Gel 60 plates with a chloroform-methanol-concentrated hydrochloric acid (100:50:0.375, vol/vol) solvent system. A solid-phase extraction method was used to isolate the acyl-CoAs (14, 31), and samples were run on Silica Gel 60 plates with an n-butanol-acetic acid-water (80:25:40) solvent system.
Fluorescent images of the TLC plates were acquired and analyzed with the ChemiImager System and FluorChem version 2.0 software (Alpha Innotech). Standard curves for quantitation were created using a known amount of NBD-stearic acid standard that was run on the same TLC plate. Protein assays for each sample show the amount of nonmetabolized NBD-stearic acid and metabolites as picomoles per milligram of protein, as well as percentage of total.
Quantitation of [3H]stearic acid and metabolism by cultured primary hepatocytes.
Isolated WT hepatocytes were plated on collagen-coated six-well dishes at 1 × 106 cells/well and incubated overnight in complete DMEM-F12 medium. After removal of the medium, the cells were washed twice with PBS and incubated for 2 h in PBS supplemented with 0.5 μM cold stearic acid and [9,10-3H]stearic acid (0.01 nmol/well) with 6 or 20 mM glucose. Medium was removed and saved, the cells were washed twice with PBS, and the washes were pooled with the appropriated medium samples. The labeled cells were scraped in a hexane-isopropanol (3:2, vol/vol) mixture and centrifuged at 1,500 rpm for 15 min, and the lipid extracts were transferred to acid-washed glass tubes. The remaining protein samples were dried overnight at room temperature and dissolved overnight in 0.2 M KOH (1 ml/sample) for Bradford protein assay. Cellular lipids were dried under nitrogen and resolved by TLC using Silica Gel G plates (Analtech, Newark, DE) and a petroleum ether-diethyl ether-methanol-acetic acid (180:14:4:1, vol/vol/vol/vol) solvent system. Individual lipid bands were identified with lipid standards (TLC 18.5A, Nu-Chek Prep, Elysian, MN), which were then removed from the plate for disintegration-per-minute (DPM) quantification by scintillation counting. The DPM of the medium/wash samples was quantified, and a chloroform-methanol (2:1, vol/vol) solution in combination with a 30-min 1,500-rpm centrifugation was used to remove the cold + labeled stearic acid from the medium/wash samples to quantify DPM of aqueous cellular oxidation products. Samples extracted from cell-free wells and unlabeled feedings were used to control for loss/precipitation of the labeled probe and for label specificity/background, respectively.
Quantitation of [3H]glucose uptake and fatty acid metabolism by cultured primary hepatocytes.
Isolated hepatocytes were plated on collagen-coated six-well culture plates at a density of 1 × 106 cells/well, cultured overnight in DMEM-F12 medium (57, 58, 72), rinsed twice with PBS, and cultured in glucose-free DMEM (1 ml/well) supplemented with 6 or 20 mM glucose for 0, 1, 2, or 6 h (n = 3 wells per medium type per time point). For radiolabeled experiments, 1 μl of [3H]glucose was added to 13 ml of the 6 mM medium (5,171 cpm/μmol glucose), and 1 μl of [3H]glucose was added to 13 ml of the 20 mM medium (1,551 cpm/μmol glucose). At each time point, the medium was removed, and the cells were rinsed twice with 500 μl of PBS; the 1-ml medium and the two 500-μl PBS washes were pooled to give the 2-ml medium for each sample. The cells were frozen/floated in liquid nitrogen, and each well was scraped twice in 500 μl of PBS (1-ml cell sample for each time point).
Hepatocyte cell homogenates were prepared as described previously (2). Cell homogenate and medium total protein and glucose were quantified by the Bradford protein assay (Bio-Rad, Richmond, CA) and the Wako Autokit Glucose (Wako Chemicals, Richmond, VA), respectively, utilizing a microplate reader (Synergy 2, Biotek Instruments, Winooski, VT). Concentration was determined using hepatocyte volume (42). Cell homogenate and medium lipid masses were determined as described previously (2). For nonradioactive samples, the lipid classes were identified by comparison with known standards utilizing TLC according to the method of Marzo et al. (43). Radioactive samples were prepared as described above. The lipids were separated by TLC and visualized by iodine, and each lipid spot was scraped into a scintillation vial. After addition of ScintiSafe gel (Fisher Scientific, Pittsburgh, PA) (5 ml), the amount of radioactivity was quantified utilizing a liquid scintillation counter (model 1600 TR, Packard, Meriden, CT). No detectable [3H]lipid signal above background was measured in any of the medium samples (data not shown).
Statistical analysis.
Values are means ± SE based on the replication number for each experiment. Statistical variance between mean values within groups of three or more was determined using one-way ANOVA and Dunnett's post tests; analysis of groups of two was performed using unpaired t-tests. P < 0.05 indicates statistical significance. All statistical tests were performed within GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Cultured primary mouse hepatocytes maintain expression of key proteins involved in LCFA and glucose uptake and metabolism.
Primary hepatocytes may lose expression of proteins with increasing time in culture (33, 47). Thus it was important to first determine whether mouse hepatocytes in culture stably express the transporters involved in LCFA and glucose uptake. Western blotting was performed on primary mouse hepatocytes immediately after isolation (day 0) or at 1, 2, 3, and 4 days in culture.
Several integral membrane proteins involved in hepatic LCFA uptake are localized in the plasma membrane and peroxisomal membranes. Hepatocytes expressed both key plasma membrane LCFA transporters, FATP5 (Fig. 1A) and GOT (Fig. 1C) at levels similar to those in the liver (day 0) for up to 2 days in culture. Expression of the peroxisomal membrane LCFA transporters FATP2 (Fig. 1B) and FATP4 (Fig. 1D) was similar to that in the liver (day 0) at 2 and 4 days in culture, respectively.
Quantitative Western blotting demonstrated that WT cultured primary mouse hepatocytes expressed nearly fourfold more L-FABP than SCP-2, while L-FABP gene ablation did not alter SCP-2 expression (72). Conversely, ablating SCP-2 elicited 1.7-fold upregulation of L-FABP, as seen previously (72). In contrast, hepatic expression of L-FABP (2) and SCP-2 (not shown) was maintained at the same level as in the liver and remained constant for 3 and 4 days in culture, respectively.
Hepatocytes also express several key proteins involved in glucose uptake, plasma membrane integral proteins and soluble enzymes. Expression of the insulin receptor was similar to that in the liver (day 0) and remained constant or slightly increased for at least 4 days in culture (Fig. 1E). Expression of the plasma membrane glucose transporter found in all hepatocytes, GLUT2, was constant for 4 days in culture (Fig. 1G). Similarly, expression of plasma membrane GLUT1 (Fig. 1F), present in only a small subset (1%) of hepatocytes localized near the central vein (9, 74), was also constant for 4 days in culture. The level of the rate-limiting enzyme in glucose metabolism, GCK, was also similar to that in the liver (day 0) and was maintained for 4 days in culture (Fig. 1H).
Thus expression of all hepatocyte soluble and membrane LCFA transporters was similar to that in the liver and was maintained at constant levels for 3–4 days and 2 days, respectively, in culture. Concentrations of key proteins involved in glucose uptake and metabolism were maintained at levels similar to those in the liver and were stable for at least 4 days. On the basis of these findings, mouse primary hepatocytes were cultured for ≤2 days for all subsequent studies.
Real-time confocal imaging of fluorescent LCFA uptake in cultured primary mouse hepatocytes.
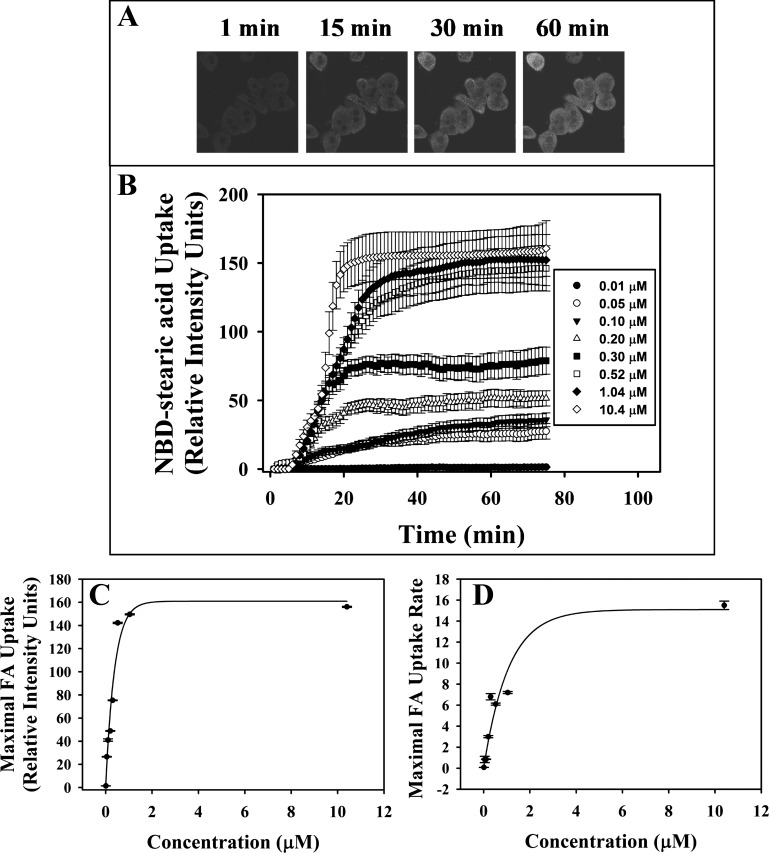
To examine the impact of LCFA binding protein gene ablation on LCFA uptake in living hepatocytes, NBD-stearic acid concentration and incubation time were optimized. Representative confocal images showed that, with increasing time, the primary hepatocytes took up NBD-stearic acid into the cytoplasm and less intensely into nuclei (Fig. 2A). Uptake exhibited saturation by 20–60 min at all concentrations (Fig. 2B). The maximum fatty acid uptake or cellular capacity saturated quickly at >0.5 μM NBD-stearic acid (Fig. 2C). The maximal rate of uptake (Fig. 2D) saturated at around four- to fivefold higher concentration. Therefore, all subsequent studies were undertaken with a nonsaturating NBD-stearic acid concentration of 0.1 μM, a level in the linear range of both curves.
Fig. 2.
A and B: confocal images and time course of 12-N-methyl-(7-nitrobenz-2-oxa-1,3-diazo)-aminostearic acid (NBD-stearic acid) uptake from WT primary cultured hepatocytes. A: representative images of cellular fluorescence 0, 15, 30, and 60 min after addition of 0.1 μM NBD-stearic acid to hepatocytes isolated from WT mice. B: representative time course uptake of fluorescent fatty acid at 1-min intervals after addition of 0.01–10.4 μM NBD-stearic acid. Values are means ± SE; n = 10–15 cells. C and D: uptake curves from each fatty acid (FA) concentration fit using the Wiebull equation. C: maximal integrated fluorescence intensity. D: maximal uptake rate. Values are means ± SE; n = 10–15 cells.
NBD-stearic acid was poorly metabolized in cultured primary mouse hepatocytes.
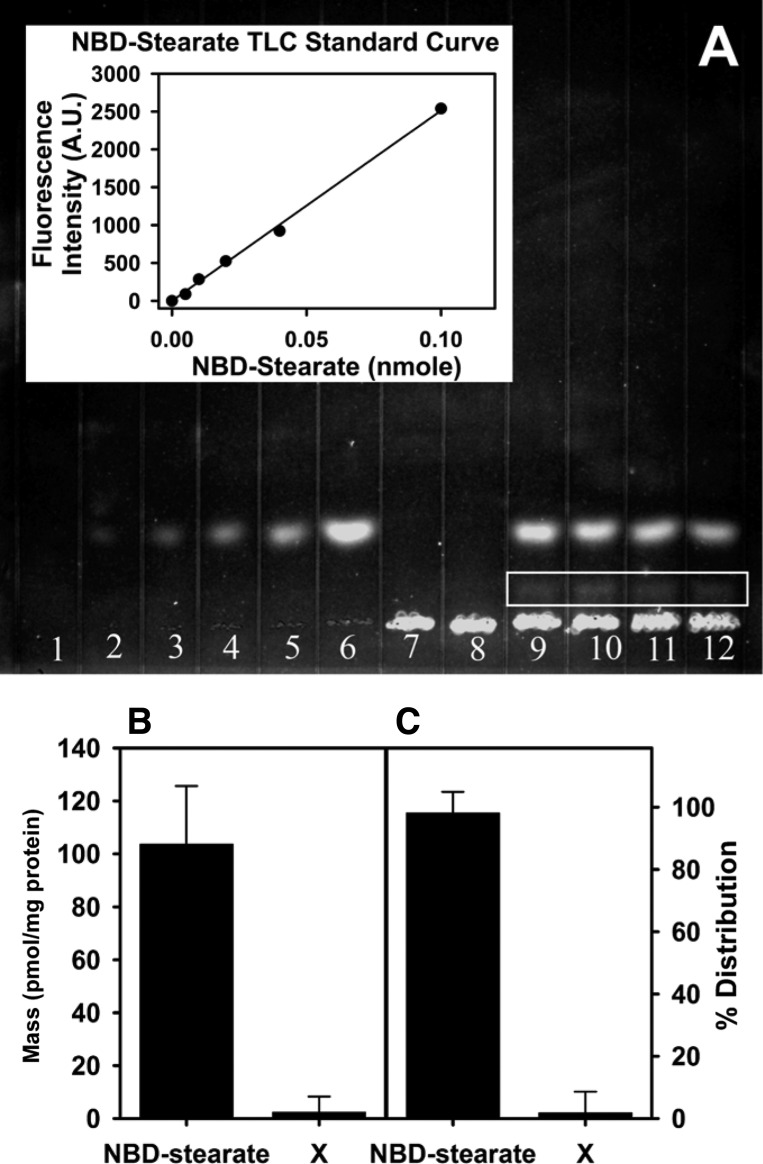
To determine if the NBD-stearic acid uptake and distribution as seen by confocal laser scanning microscopy represented free LCFA and not a metabolite, cultured primary mouse hepatocytes isolated from the liver of WT mice were incubated with NBD-stearic acid in PBS for 1 or 2 h. Lipids were extracted, separated by TLC, and quantitated as described in materials and methods. First, when Silica Gel G plates were developed with a petroleum ether-diethyl ether-methanol-glacial acetic acid (180:14:4:1, vol/vol) solvent system, NBD-stearic acid remained at the origin, with no other fluorescent bands observed above the origin (data not shown). When the same TLC plate was developed with petroleum ether-diethyl ether-glacial acetic acid (70:30:1, vol/vol) for 2 h, the majority of the fluorescence above the origin was NBD-stearic acid (Fig. 3A), as shown by the NBD-stearic acid loaded on the plate for standard curve quantitation. A faint fluorescent band between the origin and the brighter NBD-stearic acid band (Fig. 3A) accounted for ∼2% of the fluorescence as well as the mass (Fig. 3, B and C). No NBD-stearic acid phospholipids or NBD-stearic acid-CoA was detected after 1 or 2 h of incubation (data not shown).
Fig. 3.
NBD-stearic acid uptake and metabolism in WT primary cultured hepatocytes as measured by TLC. A: representative image of TLC plate. Lanes 1–6, NBD-stearic acid standard (0, 0.005, 0.01, 0.02, 0.04, and 0.1 nmol, respectively), with standard curve shown in inset (AU, arbitrary units); lanes 7 and 8, blank (hepatocyte in PBS without addition of NBD-stearic acid); lanes 9–12, primary cultured hepatocytes incubated with NBD-stearic acid in PBS for 2 h. Lipids were extracted and run on a Silica Gel G plate for 2 h using petroleum ether-diethyl ether-glacial acetic acid (70:30:1, vol/vol). Faint band in lanes 9–12 (white outline below NBD-stearic acid) was denoted compound X. B and C: amount of NBD-stearic acid and metabolite (compound X) after 2 h of incubation shown as pmol/mg protein and as percentage of total mass (%distribution).
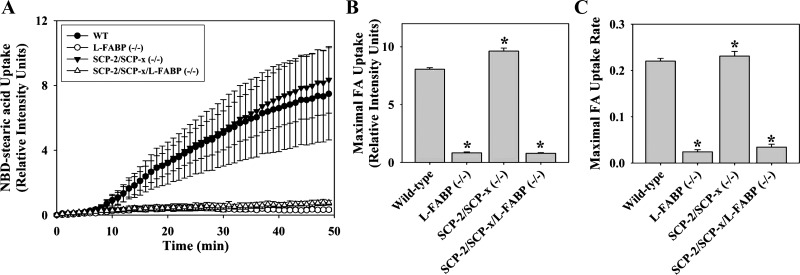
LCFA binding protein gene ablation reduced cellular LCFA uptake.
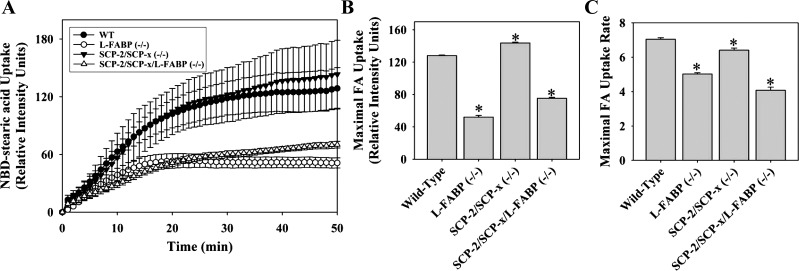
L-FABP gene ablation decreased the cellular uptake of NBD-stearic acid at all time points (Fig. 4A), primarily due to a 60% decrease in maximal uptake (Fig. 4B), rather than a maximal rate of uptake (Fig. 4C). In contrast, SCP-2 gene ablation did not reduce LCFA uptake (Fig. 4A) but, instead, significantly increased maximal uptake (Fig. 4B), likely due to concomitant upregulation of L-FABP (72, 73). Ablation of L-FABP and SCP-2 did not further reduce cellular LCFA uptake into hepatocytes, as did L-FABP ablation alone (Fig. 4, A–C).
Fig. 4.
Alterations in uptake of cellular NBD-stearic acid uptake due to loss of SCP-2 and/or L-FABP. A: time-course analysis of relative cellular fluorescence in WT, L-FABP null, SCP-2/SCP-x null, and SCP-2/SCP-x/L-FABP null hepatocytes after addition of 0.01 μM NBD-stearic acid. Uptake curves were fit using the Wiebull equation. B and C: maximal cellular fluorescence intensity and maximal uptake rate. Values are means ± SE; n = 10–15 cells. *P < 0.05 vs. WT.
Thus ablation of L-FABP, but not SCP-2, reduced cellular LCFA uptake in cultured primary hepatocytes. The residual ability of hepatocytes to take up LCFA in low quantity in L-FABP null and L-FABP/SCP-2/SCP-x null hepatocytes indicated potential contributions of membrane binding, compartmentalization, or as yet unresolved additional cellular factors (2).
LCFA binding protein gene ablation differentially reduced LCFA uptake into nuclei.
L-FABP gene ablation (L-FABP null and L-FABP/SCP-2/SCP-x null) nearly abolished NBD-stearic acid uptake into the nucleus of the cultured hepatocytes at all time points (Fig. 5, A–C). Consequently, the ratio of nuclear to cytoplasmic LCFA uptake was reduced >90% relative to that in WT hepatocytes. SCP-2 gene ablation did not impact LCFA uptake into the nucleus (Fig. 5, A–C). This was likely due to the concomitant upregulation of L-FABP in SCP-2-ablated hepatocytes (72, 73). Conversely, SCP-2 was not upregulated in L-FABP null hepatocytes (72).
Fig. 5.
Alterations in NBD-stearic acid nuclear transport due to loss of SCP-2 and/or L-FABP. A: time-course analysis of relative nuclear fluorescence in WT, L-FABP null, SCP-2/SCP-x null, and SCP-2/SCP-x/L-FABP null hepatocytes after addition of 0.01 μM NBD-stearic acid. Nuclear uptake curves from each genotype were fit using the Wiebull equation. B and C: maximal cellular fluorescence intensity and maximal uptake rate. Values are means ± SE; n = 10–15 cells. *P < 0.05 vs. WT.
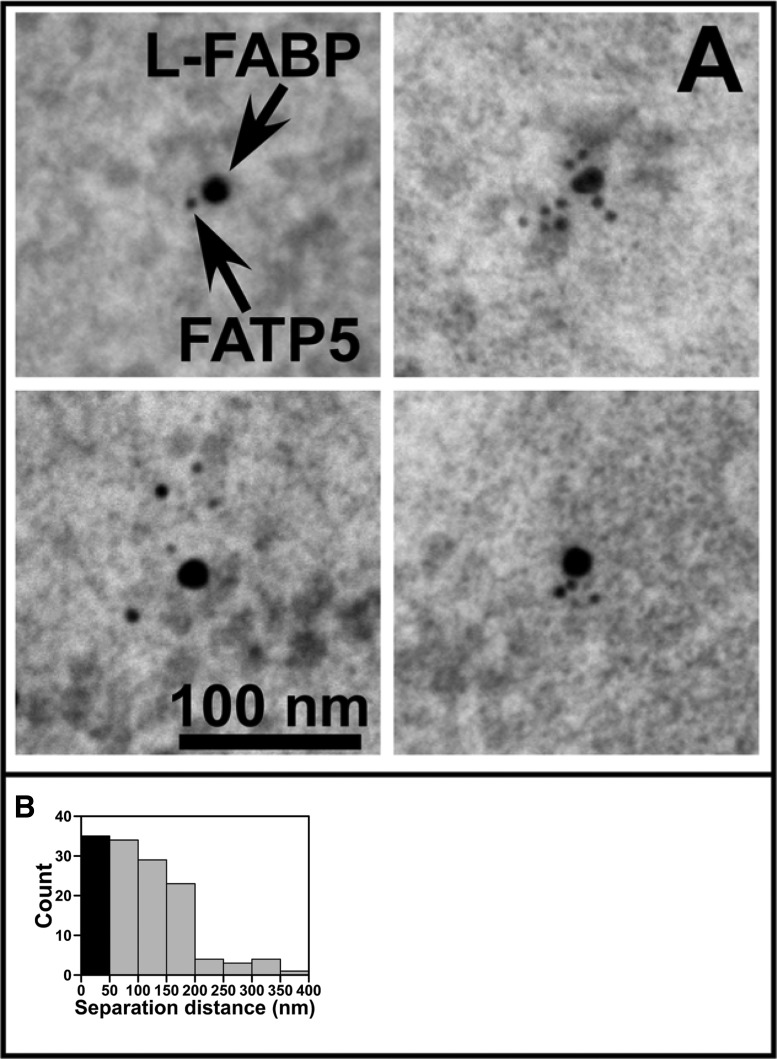
Double-immunogold labeling of FATP5 and L-FABP at the plasma membrane of primary cultured hepatocytes from WT control mice.
It has been suggested that FATP5, which localizes to the basolateral plasma membrane of liver hepatocytes, might interact with L-FABP (15, 16, 70). This possibility was explored by double-immunogold-labeling electron microscopy focusing on the plasma membrane regions of fixed hepatocytes isolated from the liver of WT mice. Representative images of FATP5 and L-FABP in close proximity as determined by immunogold (6 and 15 nm, respectively) are shown in Fig. 6A. A histogram of the edge-to-edge separation distance of FATP5 and L-FABP pairs was prepared from multiple images obtained at or near the plasma membranes of mouse hepatocytes (Fig. 6B). The diameter of each IgG is ∼28 nm (60). On the basis of an interaction distance of 10 nm for the two proteins, FATP5 and L-FABP, the gold particles would distribute at edge-to-edge distances of up to ∼60 nm. The first bin of the histogram (Fig. 6B) was colored black to denote the distribution of immunogold-labeled FATP5 and L-FABP close enough to form protein-protein interactions.
Fig. 6.
Double-immunogold labeling of FATP5 and L-FABP at the plasma membrane of primary cultured hepatocytes from a WT control mouse. A: representative electron micrographs of fixed WT hepatocytes immunolabeled with FATP5 (6 nm gold) and L-FABP (15 nm gold). Arrows denote L-FABP and FATP5 gold particles. B: edge-to-edge separation distance of FATP5 and L-FABP pairs determined from images obtained at or near the plasma membrane. Solid bar (at 0 nm) represents distribution of immunogold-labeled FATP5 and L-FABP that could be involved in protein-protein interactions.
LCFA binding protein gene ablation did not reduce cellular LCFA uptake by downregulating membrane LCFA transport proteins.
The possibility that the reduced LCFA uptake was due at least in part to net reduction of the membrane LCFA transporters was examined. Ablation of L-FABP, SCP-2, or both did not decrease expression of the two major integral plasma membrane proteins involved in LCFA uptake into the hepatocyte. Expression of FATP5 was unaltered (Fig. 7A), while expression of GOT was increased (Fig. 7C). With regard to peroxisomal membrane LCFA transport proteins, ablation of L-FABP, SCP-2, or both decreased expression of FATP2 (Fig. 7B) and increased expression of FATP4 (Fig. 7D). Taken together, these findings indicate that ablation of L-FABP, SCP-2, or both did not result in net downregulation of integral membrane proteins involved in LCFA uptake localized in the plasma membrane or peroxisomal membranes.
Fig. 7.
Effect of ablation of L-FABP, SCP-2/SCP-x, or both on expression of proteins involved in membrane fatty acid and glucose uptake and metabolism. A–H: representative Western blots and analysis of multiple Western blots of hepatocytes isolated from WT, L-FABP null, SCP-2/SCP-x null, and SCP-2/SCP-x/L-FABP null mice for FATP5, FATP2, GOT, FATP4, insulin receptor, GLUT1, GLUT2, and GCK. Values are mean ± SE; n = 6–8. *P < 0.05 vs. WT.
LCFA binding protein gene ablation did not reduce cellular LCFA uptake by downregulating key hepatocyte proteins involved in glucose uptake and metabolism.
The possibility that LCFA binding protein gene ablation adversely impacted key proteins in glucose uptake and metabolism was examined. Loss of L-FABP reduced the expression of insulin receptors in hepatocytes from L-FABP null and L-FABP/SCP-2/SCP-x null mice (Fig. 7E). Expression of the major glucose transporter GLUT2, which is present in all hepatocytes (Fig. 7G), as well as expression of the minor glucose transporter GLUT1, which is present in a small fraction of hepatocytes (Fig. 7F), was not altered by L-FABP gene ablation, but GLUT2 was reduced in hepatocytes from SCP-2/SCP-x null and L-FABP/SCP-2/SCP-x null mice (Fig. 7G). The rate-limiting enzyme in glucose metabolism, GCK, was upregulated in hepatocytes from L-FABP null and SCP-2/SCP-x null mice, but not from L-FABP/SCP-2/SCP-x null mice (Fig. 7H). Taken together, these findings suggest that reduced cellular LCFA uptake in these null hepatocytes did not correlate with the patterns of expression of key plasma membrane proteins involved in glucose transport or intracellular metabolism.
High glucose reduces cellular LCFA uptake and LCFA transport into the nuclei.
Most prior studies of LCFA metabolism in primary hepatocytes have been performed with culture media containing glucose levels much higher than physiological (32, 46, 53). Therefore, the impact of different extracellular glucose concentrations on NBD-stearic acid uptake and nuclear targeting was examined.
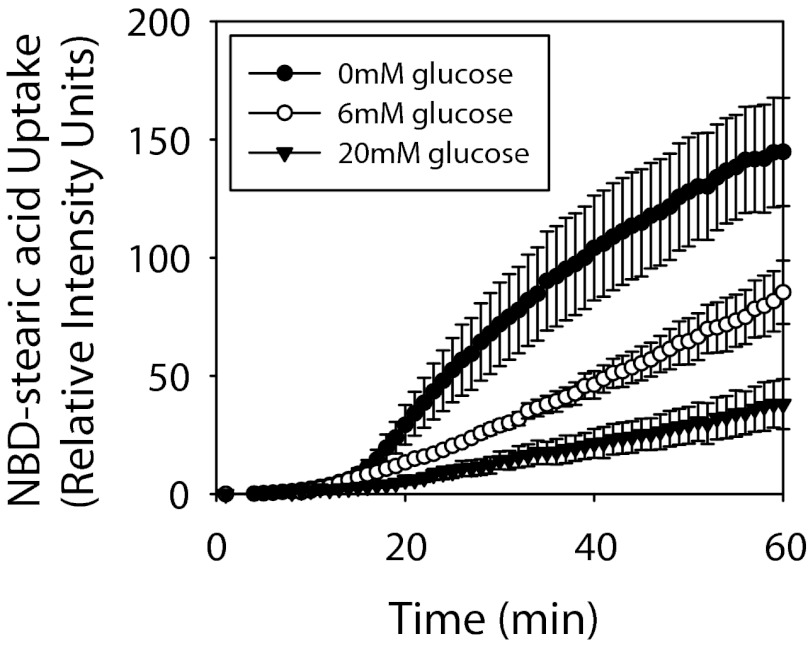
Increasing glucose resulted in decreased cellular LCFA uptake in cultured primary hepatocytes from WT mice. Increasing glucose from 0 to 6 mM (normal physiological level) decreased LCFA uptake at all time points (Fig. 8). Further increasing glucose from 6 to 20 mM reduced cellular (Fig. 8) and maximal (Fig. 9A) LCFA uptake even more extensively. The inhibitory effects of high glucose on cellular LCFA uptake in hepatocytes did not correlate directly with L-FABP or SCP-2 expression, since cellular LCFA uptake was decreased 65–70% in all cases (Fig. 9, A–D). An osmotic control, maltose, did not significantly impact LCFA uptake into hepatocytes (not shown).
Fig. 8.
Alterations to time course of cellular NBD-stearic acid uptake induced by glucose. Relative cellular fluorescence was determined in WT hepatocytes exposed to 0, 6, or 20 mM glucose after addition of 0.01 μM NBD-stearic acid. Values are means ± SE; n = 10–15 cells.
Fig. 9.
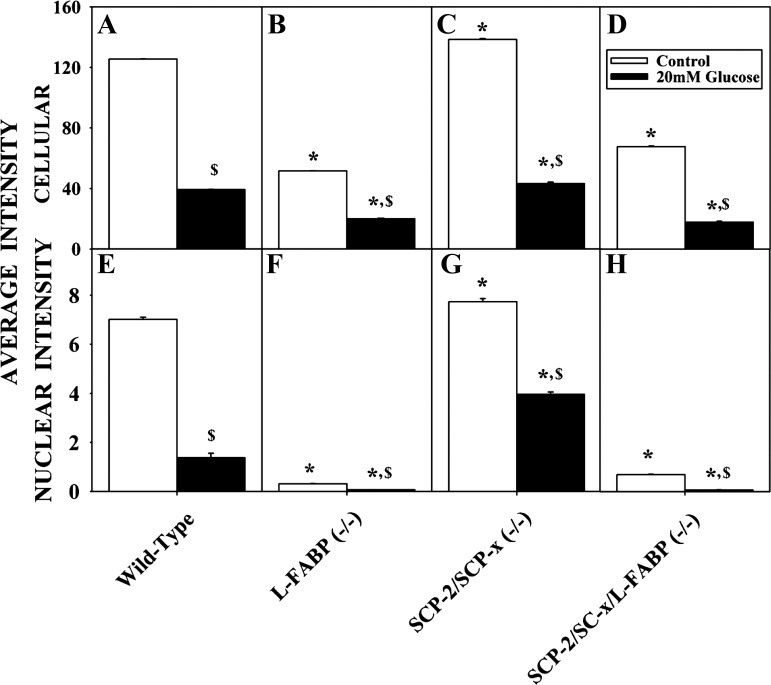
Effect of glucose on maximal NBD-stearic acid uptake into hepatocytes and hepatocyte nuclei. Primary mouse hepatocytes were isolated from WT, L-FABP null, SCP-2/SCP-x null, and SCP-2/SCP-x/L-FABP null mice and incubated with 0 (control) or 20 mM glucose and 0.01 μM NBD-stearic acid. Maximal NBD-stearic acid uptake into hepatocytes (A–D) and nuclei (E–H) was determined as maximal fluorescence intensity from a 0- to 60-min time curve. Values are means ± SE; n = 10–15 cells. *P < 0.05 vs. WT with the same treatment. $P < 0.05 vs. control with the same genotype.
However, the impact of high glucose on LCFA uptake into nuclei in general correlated with L-FABP expression. High glucose decreased LCFA distribution to the nuclei of L-FABP null (Fig. 9F) and L-FABP/SCP-2/SCP-x null (Fig. 9H) hepatocytes to near the limit of detection. In contrast, glucose decreased nuclear LCFA uptake less in WT (Fig. 9E) and SCP-2/SCP-x null hepatocytes (upregulate L-FABP) (Fig. 9H) by 80% and 49%, respectively. An osmotic control, maltose, did not significantly impact LCFA nuclear targeting in hepatocytes (not shown).
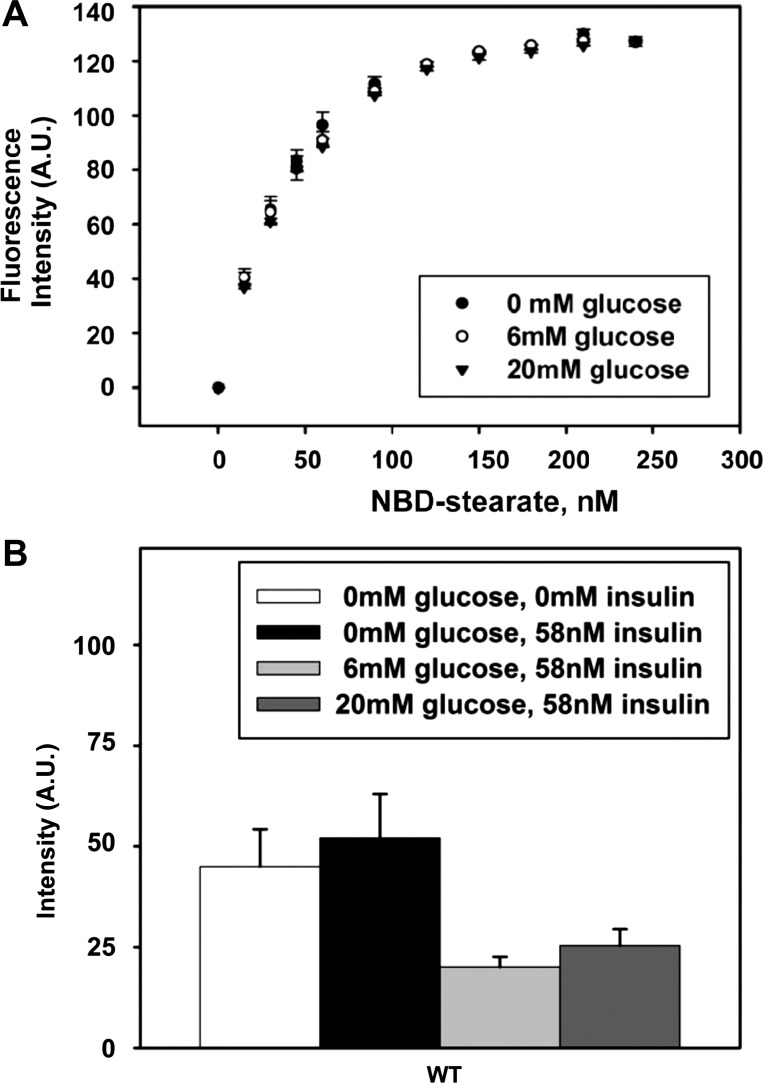
To determine if the decrease in LCFA cellular uptake and/or nuclear targeting was related to glucose directly affecting the binding affinity of NBD-stearic acid to L-FABP or potentially affecting its environment in either ligand binding pocket and, thus, its quantum yield, ligand binding experiments were performed in the presence of 0, 6, and 20 mM glucose. The resulting curves (Fig. 10) overlapped, with no differences in binding capacity, and yielded the same Kd for NBD-stearic acid binding. Therefore, glucose did not enhance or inhibit the binding of NBD-stearic acid to recombinant L-FABP. Thus high glucose-induced decrease in NBD-stearic acid uptake was not due to altered L-FABP binding affinity for NBD-stearic acid.
Fig. 10.
Ligand binding of NBD-stearic acid to recombinant L-FABP and effect of insulin on NBD-stearic acid uptake in the presence of glucose. A: NBD-stearic acid was titrated into phosphate buffer (pH 7.4) containing 25 nM recombinant rat L-FABP in the presence of 0, 6, or 20 mM glucose. Binding curves show lack of change in ligand binding affinity of NBD-stearic acid to L-FABP resulting from the presence of glucose. B: hepatocytes isolated from WT mice were fed 0.1 μM NBD-stearic acid with 0 mM glucose without insulin, 0 mM glucose with 58 nM insulin, 6 mM glucose with 58 nM insulin, or 20 mM glucose with 58 nM insulin and assayed for NBD fluorescence over time. Values (means ± SE; n = 3–16) represent average cellular fluorescence at 55–65 min after NBD-stearic acid addition.
Since insulin receptor expression significantly decreased as a result of the L-FABP gene ablation (Fig. 7E), the glucose-dependent effects on NBD-stearic acid uptake were examined in the presence of insulin. Insulin (58 nM) did not have an effect on NBD-stearic acid uptake in the absence or presence of 6 mM glucose (Fig. 10B). Insulin did not further decrease the maximal uptake of NBD-stearic acid (Fig. 10B), as observed in the presence of 20 mM glucose (Fig. 8).
[3H]glucose uptake and subsequent incorporation of the label into lipids in primary cultured hepatocytes.
The possibility that de novo synthesis of LCFA from high glucose might result in inhibition of LCFA uptake was examined. d-[1-3H]glucose was used as a tracer for intracellular measurements of the incorporation of the glucose carbons and radiolabel into fatty acid biosynthesis. Glucose was taken up rapidly into WT hepatocytes incubated with 6 and 20 mM extracellular glucose (Table 1). For 60 min, total intracellular lipid content was relatively unchanged. Even with the addition of 20 mM glucose, there were no dramatic increases in the incorporation of the glucose radiolabel into the intracellular lipid. These results indicate that there is no significant rise in the intracellular glucose-derived LCFA content of the hepatocytes as a result of increases in extracellular glucose concentrations.
Table 1.
[3H]glucose uptake and lipid incorporation in cultured wild-type primary hepatocytes
| Time, min | 6 mM Glucose | 20 mM Glucose |
|---|---|---|
| Intracellular [3H]glucose, mmol/l | ||
| 2 | 0.94 ± 0.02 | 3.75 ± 0.09 |
| 60 | 2.28 ± 0.01 | 10.47 ± 0.03 |
| Intracellular total [3H]lipid, nmol/mg protein | ||
| 2 | 6.0 ± 0.4 | 9.4 ± 0.3 |
| 60 | 7.0 ± 0.3 | 6.6 ± 0.8 |
| Intracellular total lipid, nmol/mg protein | ||
| 2 | 390 ± 20 | 390 ± 20 |
| 60 | 450 ± 30 | 370 ± 30 |
Values are means ± SE (n = 3).
Effects of extracellular glucose concentration on [3H]stearic acid uptake and metabolism in cultured primary mouse hepatocytes.
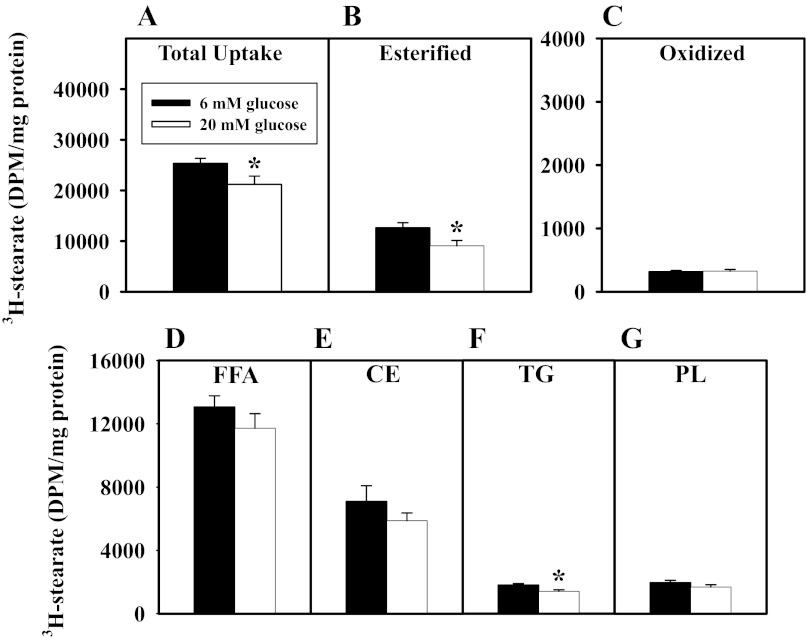
WT primary hepatocytes were plated in medium with 6 or 20 mM glucose supplemented with 0.5 μM stearic acid/[3H]stearic acid (Fig. 11), and total uptake of the [3H]stearic acid (esterified + oxidized), as well as incorporation into cellular free fatty acids, esterified lipids, and oxidation products, was determined. High glucose decreased the total uptake of [3H]stearic acid (Fig. 11A), primarily through incorporation into esterified lipids (Fig. 11B), such as triglycerides (Fig. 11C).
Fig. 11.
Effects of extracellular glucose concentration on [3H]stearic acid uptake and metabolism in cultured primary mouse hepatocytes. WT hepatocytes were plated with physiological (6 mM) or hyperglycemic (20 mM) glucose and 0.5 μM stearic acid/[3H]stearic acid. A: total uptake of labeled stearic acid (esterified + oxidized). B–G: presence of label in cellular esterified lipids, free fatty acids (FFA, D), cholesteryl esters (CE, E), triacylglycerides (TG, F), and phospholipids (PL, G), as well as extracellular aqueous metabolism products (oxidized) (C). Values (means ± SE n = 3–6) are shown as disintegrations per minute (DPM)/mg total cellular protein. *P ≤ 0.05 vs. 6 mM glucose control.
DISCUSSION
Hepatic LCFAs and glucose are metabolically linked. Once taken up by the liver, exogenous LCFAs are stored/secreted (triglycerides/VLDL), partially oxidized to two-carbon units for gluconeogenesis (for storage as glycogen or maintenance of blood glucose levels), or fully oxidized to produce energy. L-FABP impacts hepatic LCFA and glucose metabolism in the short term by enhancing LCFA uptake and in the longer term by enhancing LCFA targeting to nuclei for induction of PPARα transcription of LCFA β-oxidative enzymes (reviewed in Ref. 1). These actions of L-FABP are attributed in part to L-FABP's high affinity for LCFAs and their CoA thioesters (reviewed in Refs. 19, 20, 52, 59, 82). Although SCP-2 also binds LCFAs and LCFA-CoAs with high affinity and enhances LCFA uptake, the impact of SCP-2 on LCFA nuclear targeting is not known (19, 23). Use of L-FABP null and SCP-2/SCP-x null mice alone to resolve the physiological roles of SCP-2 and L-FABP has been complicated by the continued presence of SCP-2 or upregulation of L-FABP in these models, respectively (21, 40, 41, 68). The data presented here, especially with L-FABP/SCP-2/SCP-x null hepatocytes, further our understanding of the respective roles of L-FABP and SCP-2 in cellular LCFA uptake and nuclear targeting.
Ablation of L-FABP or SCP-2/SCP-x differentially regulated expression of the respective nontargeted gene. While concomitant upregulation of SCP-2 was observed in hepatocytes from early-backcross-generation L-FABP null mice (2), this was not the case in cultured primary hepatocytes from a ≥10-backcross generation. Similarly, upregulation of SCP-2 was observed in livers of early-backcross generations of L-FABP null mice, but not later-backcross generations (39, 40). These findings suggest that any effects of L-FABP gene ablation on LCFA uptake and nuclear targeting in hepatocytes are not likely to be complicated by concomitant upregulation of SCP-2. In contrast, L-FABP was upregulated two- to fourfold in ≥10-backcross-generation hepatocytes from SCP-2 null hepatocytes, as well as in the liver (21, 68, 72). Thus upregulation of L-FABP may confound interpretation of SCP-2's effects on LCFA uptake and nuclear targeting in hepatocytes.
Ablation of L-FABP decreased cellular LCFA uptake, as demonstrated using the fluorescent probe NBD-stearic acid not significantly metabolized by hepatocytes and [3H]stearic acid. Roles for L-FABP and SCP-2 in hepatocyte LCFA uptake were also supported by the finding that L-FABP overexpression in L-cell fibroblasts (control L-cell fibroblasts do not express L-FABP and express very low levels of SCP-2) enhanced LCFA uptake (7, 49). Similarly, SCP-2 overexpression in these fibroblasts also increased LCFA uptake (8, 48). The fact that the effects of L-FABP and SCP-2 ablation on LCFA uptake in cultured primary hepatocytes are not additive suggests that the two proteins may have overlapping functions in LCFA uptake in hepatocytes. An analogous potential overlap in function relating to HDL-mediated cholesterol uptake was observed in relation to the distribution of proteins in cholesterol-poor and -rich microdomains (44). Hepatocyte plasma membrane-associated SCP-2 and L-FABP are enriched in cholesterol-rich and -poor microdomains, respectively, in WT control mice (44). Loss of L-FABP resulted in an increase of SCP-2 in the cholesterol-poor microdomains (44). This remodeling of the SCP-2 distribution in hepatocyte plasma membranes in L-FABP gene-ablated mice may give rise to the differences in the maximal LCFA uptake rates compared with those in hepatocytes from SCP-2/SCP-x/L-FABP null mice. Additionally, even when both L-FABP and SCP-2 were ablated, the hepatocytes maintained a residual LCFA uptake into the hepatocyte. The latter may be attributed to a diffusional component and/or integral membrane fatty acid transport proteins known to enhance LCFA uptake through their localization at the plasma membrane (FATP5 and GOT) or at the peroxisomal membrane (FATP2 and FATP4) (78). Since ablation of L-FABP, SCP-2, or both did not change the overall level of these integral membrane fatty acid transport proteins, the basal level of LCFA uptake was maintained at ∼50% of maximal in the hepatocytes.
Ablation of L-FABP inhibited LCFA nuclear targeting compared with control or SCP-2 gene ablation. This finding is consistent with L-FABP upregulation in SCP-2/SCP-x null hepatocytes, compensating for the loss of SCP-2 and with the intracellular distribution of L-FABP, which is found not only in the cytoplasm, but also in the nucleus, of cultured primary hepatocytes, as well as in transfected cells overexpressing L-FABP (28–31, 65). L-FABP cotransports bound LCFAs into purified nuclei in vitro, and overexpression in transformed cells enhances LCFA distribution into the nucleus (30, 31, 35). In contrast, SCP-2 is distributed mainly in the cytosol and peroxisomes, with very little localized in nuclei (reviewed in Ref. 66). There are no reports on the effect of SCP-2 on LCFA nuclear targeting. However, differential uptake of bound ligand (i.e., retinoic acid) has been observed with other FABP family members (i.e., cellular retinoic acid binding proteins I and II) (12, 75). The latter finding suggests that entry of the binding protein into nuclei is essential for ligand-mediated activation of nuclear receptor target (12, 75). Thus L-FABP's role in LCFA nuclear targeting shares more functional similarity to these other LCFA binding proteins than with SCP-2.
High glucose reduced cellular LCFA uptake in all mouse models, but the impact of high glucose on LCFA uptake into nuclei in general correlated with L-FABP expression. High glucose especially reduced nuclear uptake of LCFA in the SCP-2/SCP-x null hepatocytes. This was due not only to concomitant upregulation of L-FABP in SCP-2/SCP-x null hepatocytes but also to glucose interacting with L-FABP and increasing L-FABP's affinity for PPARα (28, 71). Glucose also enhanced LCFA transport into nuclei of L-FABP-overexpressing L cells (28). Taken together, these findings, including the observations from the nonmetabolizable NBD-stearic acid, as well as from [3H]stearic acid and [3H]glucose, suggest that L-FABP and glucose together significantly impact cellular LCFA uptake and nuclear targeting, but not by utilization of glucose through fatty acid biosynthesis or major changes in fatty acid metabolism.
Dietary improvements, exercise, and statins significantly decrease incidence and severity of CVD. However, CVD risk remains higher among individuals with diabetes mellitus, even with optimum statin therapy. Dyslipidemia, particularly hypertriglyceridemia, is the major basis for this high residual CVD risk in statin-treated subjects, especially hypertriglyceridemic diabetic patients. Therapeutic interest in L-FABP has increasingly focused on L-FABP's protection against hepatocellular oxidative stress, as in diabetes (56, 76, 77, 85), and on developing fibrate analogs better bound and targeted by L-FABP for activating PPARα (13, 62, 82). Part of this interest is based on the discovery of the L-FABP T94A variant, which is the most frequently occurring polymorphism in the entire FABP protein family, with a 26–38% minor allele frequency (8.3 + 1.9% frequency of homozygous variant) in multiple populations tested worldwide [mutation annotation format for 1,000 genomes in National Center for Biotechnology Information single-nucleotide polymorphism database; ALFRED database] (11, 18, 38, 54, 61, 80, 84). The human L-FABP T94A variant is associated with high plasma triglyceride (10, 18) and high LDL cholesterol (18, 54), features associated with increased risk of CVD and type 2 diabetes (10, 18). It has been suggested that the L-FABP null mouse has a phenotype similar to that of humans with the L-FABP T94A polymorphism (10, 18). Consistent with this possibility, overexpression of mouse L-FABP or human L-FABP increases LCFA uptake in transfected L-cell fibroblasts, human HepG2 cells, and human Chang liver cells (24, 49, 55, 83). Conversely, L-FABP gene ablation or expression of the human L-FABP T94A variant decreases LCFA uptake (24, 49, 55, 83). Furthermore, the elevated serum triglycerides in L-FABP null mice share a phenotype similar to that of humans with the L-FABP T94A polymorphism (10, 18). Finally, clofibrate- and/or phytol-treated L-FABP null mice with elevated serum triglycerides share a phenotype similar to that of fenofibrate-treated humans expressing the L-FABP T94A variant (10). Fibrates were first developed as less toxic analogs of dietary phytol and its metabolite PPARα agonists. Low levels of clofibrate and/or dietary phytol decreased serum and hepatic triglycerides in WT, but not L-FABP null, mice (4, 5).
In summary, resolving fatty acid and glucose signaling pathways is of great importance to our understanding of human metabolic disorders such as diabetes mellitus and its complications, which is the sixth-leading cause of death in the United States (22, 63, 79, 86). Serum glucose and LCFA are elevated in diabetes (69, 87). The uptake and metabolism of LCFA and glucose respond to increased levels of both in primary rat hepatocytes and podocytes (26, 36). Our findings suggest that the elevated serum LCFA in diabetes may be due not only to increased hepatic de novo synthesis of LCFAs from glucose, but also at least in part to glucose inhibiting LCFA uptake. Our results from cultured primary hepatocytes from L-FABP/SCP-2/SCP-x null mice help clarify the interwoven roles of L-FABP and SCP-2 in hepatic fatty acid uptake. L-FABP and SCP-2 are highly expressed in the liver, and L-FABP is concomitantly upregulated in SCP-2/SCP-x null hepatocytes and the liver (21, 68). In a glucose concentration-dependent manner, our findings demonstrate that L-FABP, much more than SCP-2, impacted hepatocyte cellular LCFA uptake and LCFA targeting to nuclei. Ablation of SCP-2, resulting in the increased expression of L-FABP, enhanced LCFA distribution to the nucleus, consistent with the entry of L-FABP, but not SCP-2, into the nuclei (23, 67). Within the nuclei, L-FABP interaction with and activation of PPARα transcription of LCFA β-oxidative enzymes may be greatly affected by high glucose concentrations (28, 29, 46, 67).
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41402 (F. Schroeder and A. B. Kier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.S., H.H., G.G.M., K.K.L., D.L., and H.R.P. performed the experiments; S.M.S., A.L.M., H.H., G.G.M., K.K.L., D.L., H.R.P., and F.S. analyzed the data; S.M.S., A.L.M., H.H., G.G.M., K.K.L., D.L., A.B.K., and F.S. interpreted the results of the experiments; S.M.S., A.L.M., H.H., K.K.L., and D.L. prepared the figures; S.M.S. and F.S. drafted the manuscript; S.M.S., A.L.M., A.B.K., and F.S. edited and revised the manuscript; S.M.S., A.L.M., H.H., G.G.M., K.K.L., D.L., H.R.P., A.B.K., and F.S. approved the final version of the manuscript; A.L.M., A.B.K., and F.S. are responsible for conception and design of the research.
ACKNOWLEDGMENTS
The facilities of the Microscopy and Imaging Center at Texas A & M University were used for one of the steps of specimen preparation for electron microscopy.
REFERENCES
- 1. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and dietary obesity. J Nutr Biochem 21: 1015– 1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem 279: 30954– 30965, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock K, Maeda N, Kier AB, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res 48: 2193– 2211, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol Cell Physiol 288: C543– C558, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res 45: 812– 830, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Atshaves BP, Petrescu A, Starodub O, Roths J, Kier AB, Schroeder F. Expression and intracellular processing of the 58 kDa sterol carrier protein 2/3-oxoacyl-CoA thiolase in transfected mouse L-cell fibroblasts. J Lipid Res 40: 610– 622, 1999 [PubMed] [Google Scholar]
- 7. Atshaves BP, Storey S, Huang H, Schroeder F. Liver fatty acid binding protein expression enhances branched-chain fatty acid metabolism. Mol Cell Biochem 259: 115– 129, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Atshaves BP, Storey SM, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x expression differentially alters fatty acid metabolism in L-cell fibroblasts. J Lipid Res 44: 1751– 1762, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Bilir BM, Gong TWL, Kwasiborski V, Shen CS, Fillmore CS, Berkowitz CM, Gumucio JJ. Novel control of the position dependent expression of genes in hepatocytes: the GLUT-1 transporter. J Biol Chem 268: 19776– 19784, 1993 [PubMed] [Google Scholar]
- 10. Brouillette C, Bose Y, Perusse L, Gaudet D, Vohl MC. Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate. J Hum Genet 49: 424– 432, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Prac 92: 82– 91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid induced growth arrest. Mol Cell Biol 22: 2632– 2641, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chuang S, Velkov T, Horne J, Wielens J, Chalmers DK, Porter CJH, Scanlon MJ. Probing fibrate binding specificity of rat liver fatty acid binding protein. J Med Chem 52: 5344– 5355, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem 220: 321– 323, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid metabolism. Gastroenterology 130: 1245– 1258, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Doege H, Stahl A. Protein mediated fatty acid uptake: novel insights from in vivo models. Physiology 21: 259– 268, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Fehr M, Takanaga H, Ehrhardt DW, Frommer WB. Evidence for high-capacity bidirectional glucose transport across the endoplasmic reticulum membrane by genetically encoded fluorescence resonance energy transfer nanosensors. Mol Cell Biol 25: 11102– 11112, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher E, Weikert C, Klapper M, Lindner I, Mohlig M, Spranger J, Boeing H, Schrezenmeir J, Doring F. L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol Genet Metab 91: 278– 284, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem 271: 31878– 31884, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry 36: 6545– 6555, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J Biol Chem 276: 48058– 48065, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Gaby AR. Adverse effects of dietary fructose. Altern Med Rev 10: 294– 306, 2005 [PubMed] [Google Scholar]
- 23. Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh A, Martin G, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res 40: 498– 563, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Gao N, Qu X, Yan J, Huang Q, Yuan HY, Ouyang DS. L-FABP T94A decreased fatty acid uptake and altered hepatic triglyceride and cholesterol accumulation in Chang liver cells stably transfected with L-FABP. Mol Cell Biochem 345: 207– 214, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Garrett RH, Grisham CM. Glycolysis. In: Biochemistry, edited by Garrett RH, Grisham CM. Boston, MA: Brooks/Cole, Cengage Learning, 2010, p. 535–562 [Google Scholar]
- 26. Hansson PK, Asztely AK, Clapham JC, Schreyer SA. Glucose and fatty acid metabolism in McA-RH7777 hepatoma cells vs. rat primary hepatocytes: responsiveness to nutrient availability. Biochim Biophys Acta 1684: 54– 62, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Harris RA. Carbohydrate metabolism. I. Major metabolic pathways and their control. In: Textbook of Biochemistry With Clinical Correlations, edited by Devlin TM. Hoboken, NJ: Wiley, 2006, p. 581–635 [Google Scholar]
- 28. Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. Glucose regulates fatty acid binding protein interaction with lipids and PPARα. J Lipid Res 51: 3103– 3116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) interacts with peroxisome proliferator activated receptor-α in cultured primary hepatocytes. J Lipid Res 50: 1663– 1675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang H, Starodub O, McIntosh A, Atshaves BP, Woldegiorgis G, Kier AB, Schroeder F. Liver fatty acid binding protein colocalizes with peroxisome proliferator receptor-α and enhances ligand distribution to nuclei of living cells. Biochemistry 43: 2484– 2500, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid binding protein targets fatty acids to the nucleus: real-time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem 277: 29139– 29151, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Karam WG, Ghanayem BI. Induction of replicative DNA synthesis and PPARα-dependent gene transcription by Wy-14 643 in primary rat hepatocyte and non-parenchymal cell co-cultures. Carcinogenesis 18: 2077– 2083, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Kim JW, Ahn YH. CCAAT/enhancer binding protein regulates the promoter activity of the rat GLUT2 transporter gene in liver cells. Biochem J 336: 83– 90, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kriska T, Levchenko VV, Korytowski W, Atshaves BP, Schroeder F, Girotti AW. Hypersensitivity of sterol carrier protein-2 overexpressing hepatoma cells to lethal peroxidative damage induced by an exogenous cholesterol hydroperoxide. J Biol Chem 281: 23643– 23651, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Lawrence JW, Kroll DJ, Eacho PI. Ligand dependent interaction of hepatic fatty acid binding protein with the nucleus. J Lipid Res 41: 1390– 1401, 2000 [PubMed] [Google Scholar]
- 36. Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ, Tavare JM, Mathieson PW, Saleem MA, Welsh GI. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transpl 24: 3288– 3296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lowe JB, Sacchettini JC, Laposta M, McQuillan JJ, Gordon JI. Expression of rat intestinal fatty acid binding protein in E. coli. Purification and comparison of ligand binding characteristics with that of E. coli derived rat liver fatty acid binding protein. J Biol Chem 262: 5931– 5937, 1987 [PubMed] [Google Scholar]
- 38. Mansego ML, Martinez F, Martinez-Larrad MT, Zabena C, Rojo G, Morcillo S, Soriguer F, Martin-Escudero JC, Serrano-Rios M, Redon J, Chaves FJ. Common variants of the liver fatty acid binding protein gene influence the risk of type 2 diabetes and insulin resistance in Spanish population. PLos One 7: e31853, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem 324: 101– 115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem 278: 21429– 21438, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Martin GG, Huang H, Atshaves BP, Binas B, Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochemistry 42: 11520– 11532, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Martin NC, McCullough CT, Bush PG, Sharp L, Hall AC, Harrison DJ. Functional analysis of mouse hepatocytes differing in DNA content: volume, receptor expression, and effect of IFNγ. J Cell Physiol 191: 138– 144, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Marzo A, Ghirardi P, Sardini D, Meroni G. Simplified measurement of monoglycerides, diglycerides, triglycerides, and free fatty acids in biological samples. Clin Chem 17: 145– 147, 1971 [PubMed] [Google Scholar]
- 44. McIntosh AL, Atshaves BP, Martin GG, Landrock KK, Landrock D, Kier AB, Schroeder F. Loss of liver fatty acid binding protein impacts mouse hepatocyte plasma membrane microdomains. J Lipid Res 53: 467– 480, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McIntosh AL, Huang H, Atshaves BP, Wellburg E, Kuklev DV, Smith WL, Kier AB, Schroeder F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J Biol Chem 285: 18693– 18708, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McIntosh AL, Atshaves BP, Hostetler HA, Huang H, Davis J, Lyuksyutova OI, Landrock D, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-α activity in cultured primary hepatocytes. Arch Biochem Biophys 485: 160– 173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mischoulon D, Rana B, Kotliar N, Pilch PF, Bucher NLR, Farmer SR. Differential regulation of glucose transporter 1 and 2 mRNA expression by epidermal growth factor and transforming growth factor-β in rat hepatocytes. J Cell Physiol 153: 288– 296, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Murphy EJ. Sterol carrier protein-2 expression increases fatty acid uptake and cytoplasmic diffusion in L-cell fibroblasts. Am J Physiol Gastrointest Liver Physiol 275: G237– G243, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta 1301: 191– 198, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Myers-Payne SC, Hubbell T, Pu L, Schnutgen F, Borchers T, Wood WG, Spener F, Schroeder F. Isolation and characterization of two fatty acid binding proteins from mouse brain. J Neurochem 66: 1648– 1656, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem 278: 51664– 51672, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Norris AW, Spector AA. Very long chain n-3 and n-6 polyunsaturated fatty acids bind strongly to liver fatty acid binding protein. J Lipid Res 43: 646– 653, 2002 [PubMed] [Google Scholar]
- 53. Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator activated receptor-α activity in primary rat hepatocytes. J Biol Chem 278: 35931– 35939, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Peng XE, Wu YL, Lu QQ, Ju ZJ, Lin X. Two genetic variants in FABP1 and susceptibility to non-alcoholic fatty liver disease in a Chinese population. Gene 500: 54– 58, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-cells. Lipids 30: 907– 910, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Rajaraman G, Wang GQ, Yan J, Jiang P, Gong Y, Burczynski FJ. Role of cytosolic liver fatty acid binding protein in hepatocellular oxidative stress: effect of dexamethasone and clofibrate treatment. Mol Cell Biochem 295: 27– 34, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Ren X, Hogaboam C, Carpenter A, Colletti L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J Clin Invest 112: 1407– 1418, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Resseguie M, Song J, Niculescu MD, de Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine-N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J 21: 1622– 2632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem 269: 23918– 23930, 1994 [PubMed] [Google Scholar]
- 60. Roberts CJ, Williams PM, Davies J, Dawkes AC, Sefton J, Edwards KC, Haymes AG, Bestwick C, Davies MC, Tendler SBJ. Real-space differentiation of IgG and IgM antibodies deposited on microtiter wells by scanning force microscopy. Langmuir 11: 1822– 1826, 1995 [Google Scholar]
- 61. Robitaille J, Brouillette C, Lemieux S, Perusse L, Gaudet D, Vohl MC. Plasma concentrations of apolipoprotein B are modulated by a gene-diet interaction effect between the L-FABP T94A polymorphism and dietary fat intake in French-Canadian men. Mol Genet Metab 82: 296– 303, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Rolf B, Oudenampsen-Kruger E, Borchers T, Faergeman NJ, Knudsen J, Lezius A, Spener F. Analysis of the ligand binding properties of recombinant bovine liver-type fatty acid binding protein. Biochim Biophys Acta 1259: 245– 253, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65: S13– S23, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Sanderson LM, Degenhart T, Desvergne B, Muller M, Kersten S. The roles of PPARα and PPARβ in liver: dietary vs. endogenous fat sensor. Chem Phys Lipids 154 Suppl: S17, 2008 [Google Scholar]
- 65. Schroeder F, Atshaves BP, Starodub O, Boedeker AL, Smith R, Roths JB, Foxworth WB, Kier AB. Expression of liver fatty acid binding protein alters growth and differentiation of embryonic stem cells. Mol Cell Biochem 219: 127– 138, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Schroeder F, Frolov A, Starodub O, Russell W, Atshaves BP, Petrescu AD, Huang H, Gallegos A, McIntosh A, Tahotna D, Russell D, Billheimer JT, Baum CL, Kier AB. Pro-sterol carrier protein-2: role of the N-terminal presequence in structure, function, and peroxisomal targeting. J Biol Chem 275: 25547– 25555, 2000 [DOI] [PubMed] [Google Scholar]
- 67. Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 43: 1– 17, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev 12: 1189– 1201, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shimabukuro M, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95: 2498– 2502, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stahl A. A current review of fatty acid transport proteins (SLC27). Pflügers Arch 447: 722– 735, 2003 [DOI] [PubMed] [Google Scholar]
- 71. Stewart JM, Dewling VF, Wright TG. Fatty acid binding to rat liver fatty acid-binding protein is modulated by early glycolytic intermediates. Biochim Biophys Acta 1391: 1– 6, 1998 [DOI] [PubMed] [Google Scholar]
- 72. Storey SM, Atshaves BP, McIntosh AL, Landrock KK, Martin GG, Huang H, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from primary cultured mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 299: G244– G254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Storey SM, McIntosh AL, Huang H, Landrock KK, Martin GG, Payne HR, Atshaves BP, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 302: G824– G839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tal M, Schneider DL, Thorens B, Lodish HF. Restricted expression of the erythroid/brain glucose transporter isoform to perivenous hepatocytes in rats. Modulation by glucose. J Clin Invest 86: 986– 992, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W, Noy N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator activated receptors in regulating transcription. Mol Cell Biol 22: 5114– 5127, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang G, Gong Y, Anderson J, Sun D, Minuk G, Robertes MS, Burczynski FJ. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology 42: 871– 879, 2005 [DOI] [PubMed] [Google Scholar]
- 77. Wang G, Shen H, Rajaraman G, Roberts MS, Gong Y, Jiang P, Burczynski FJ. Expression and antioxidant function of liver fatty acid binding protein in normal and bile-duct ligated rats. Eur J Pharmacol 560: 61– 68, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Watkins PA. Very long chain acyl-CoA synthetases. J Biol Chem 283: 1773– 1777, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem 18: 1– 9, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Weikert MO, Loeffelholz CV, Roden M, Chandramouli V, Brehm A, Nowotny P, Osterhoff MA, Isken F, Spranger J, Landau BR, Pfeiffer A, Mohlig M. A Thr94Ala mutation in human liver fatty acid binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucose levels in lipid-exposed subjects. Am J Physiol Endocrinol Metab 293: E1078– E1084, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Williamson DH, Brosnan JT. Concentrations of metabolites in animal tissues. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Academic, 1974, p. 2266–2302 [Google Scholar]
- 82. Wolfrum C, Borchers T, Sacchettini JC, Spener F. Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver. Biochemistry 39: 1469– 1474, 2000 [DOI] [PubMed] [Google Scholar]
- 83. Wolfrum C, Buhlman C, Rolf B, Borchers T, Spener F. Variation of liver fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim Biophys Acta 1437: 194– 201, 1999 [DOI] [PubMed] [Google Scholar]
- 84. Yamada Y, Kato K, Oguri M, Yoshida T, Yokoi K, Watanabe S, Metoki N, Yoshida H, Satoh K, Ichihara S, Aoyagi Y, Yasunaga A, Park H, Tanaka M, Nozawa Y. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med 21: 801– 808, 2008 [PubMed] [Google Scholar]
- 85. Yan J, Gong Y, She YM, Wang G, Roberts MS, Burczynski FJ. Molecular mechanism of recombinant liver fatty acid binding protein's antioxidant activity. J Lipid Res 50: 2445– 2454, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA 103: 1006– 1011, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784– 1789, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]