Abstract
Inflammation of the salivary gland is a well-documented aspect of salivary gland dysfunction that occurs in Sjogren's syndrome (SS), an autoimmune disease, and in γ-radiation-induced injury during treatment of head and neck cancers. Extracellular nucleotides have gained recognition as key modulators of inflammation through activation of cell surface ionotropic and metabotropic receptors, although the contribution of extracellular nucleotides to salivary gland inflammation is not well understood. In vitro studies using submandibular gland (SMG) cell aggregates isolated from wild-type C57BL/6 mice indicate that treatment with ATP or the high affinity P2X7R agonist 3′-O-(4-benzoyl)benzoyl-ATP (BzATP) induces membrane blebbing and enhances caspase activity, responses that were absent in SMG cell aggregates isolated from mice lacking the P2X7R (P2X7R−/−). Additional studies with SMG cell aggregates indicate that activation of the P2X7R with ATP or BzATP stimulates the cleavage and release of α-fodrin, a cytoskeletal protein thought to act as an autoantigen in the development of SS. In vivo administration of BzATP to ligated SMG excretory ducts enhances immune cell infiltration into the gland and initiates apoptosis of salivary epithelial cells in wild-type, but not P2X7R−/−, mice. These findings indicate that activation of the P2X7R contributes to salivary gland inflammation in vivo, suggesting that the P2X7R may represent a novel target for the treatment of salivary gland dysfunction.
Keywords: salivary gland degeneration, apoptosis, proinflammatory response, Sjogren's syndrome
salivary gland dysfunction affects millions of Americans whose quality of life is severely impacted by dry mouth, oral bacterial infections, poor nutrition, and other disorders that are associated with decreased saliva production (4). Loss of saliva production is most common in Sjögren's syndrome (SS), an autoimmune exocrinopathy of unknown etiology in which decreased saliva production is followed by lymphocytic infiltration of the salivary glands and ultimately tissue degeneration (28, 29). In addition, salivary gland inflammation and hypo-salivation are unintended consequences of γ-radiation therapy administered to ∼60,000 head and neck cancer patients in the U.S. annually (4, 27). Chronic inflammation resulting from lymphocytic infiltration of salivary glands is a major characteristic of salivary gland dysfunction (4, 28, 29), although the initiating mechanisms have not been clearly defined. Understanding these inflammatory mechanisms will help elucidate currently unavailable therapeutic options to prevent salivary gland dysfunction.
Accumulating evidence indicates that extracellular ATP (eATP) can act as a host-derived danger signal or damage-associated molecular pattern (19, 74, 92) that is capable of initiating inflammatory responses in a variety of cell and tissue types (8, 25, 46, 74, 83, 84, 107). Under physiological conditions, the concentration of eATP is tightly regulated, but under pathological conditions (i.e., stress, trauma, or inflammation) high levels of ATP are released into the extracellular milieu. Several subtypes of receptors for adenine and/or uridine nucleotides (i.e., ionotropic P2X receptors and metabotropic P2Y receptors) have been associated with inflammatory mechanisms (2, 6, 48, 100, 101), and the P2X7 receptor subtype (P2X7R) is strongly implicated in inflammatory effects initiated by eATP, in part due to its activation requirement of high concentrations of eATP (>100 μM) associated with inflammation (46) or cell apoptosis (21, 26, 71).
Brief activation of the P2X7R with ATP or the high affinity agonist 3′-O-(4-benzoyl)benzoyl-ATP (BzATP) results in the opening of nonselective cation channels the sustained activation of which induces mitochondrial and plasma membrane depolarization, the formation of plasma membrane pores that promote extracellular release of nucleotides, plasma membrane blebbing, production of reactive oxygen species, and ultimately cell death (1, 8, 23, 32, 46, 57, 76, 79, 103, 104, 106). In addition, P2X7R activation leads to the release of proinflammatory cytokines, predominantly interleukin-1β (IL-1β), via increased caspase-1 activity, a key component of the inflammatory cascade regulated by the assembly of the intracellular complex known as the “inflammasome” (19, 46, 94). Several studies (11, 42, 47, 51) using in vivo mouse models of inflammation have shown that mice deficient in the P2X7R (P2X7R−/−) exhibit a decreased inflammatory response. In models of lung inflammation, P2X7R−/− mice displayed dramatically reduced inflammation and inflammation-mediated pulmonary fibrosis (47). In mouse models of chronic inflammation and neuropathic pain, hypersensitivity to pain stimuli was completely absent in P2X7R−/− mice (11). These studies suggest that the P2X7R is a potential therapeutic target in inflammatory diseases as evidenced by phase I and II clinical trials using selective P2X7R antagonists to treat rheumatoid arthritis and inflammatory bowel disease (3, 30). These recent findings have spurred interest in elucidating the role of the P2X7R in other inflammatory diseases.
The P2X7R is expressed in a variety of tissues, including the central nervous system, hematopoietic cells of the bone marrow, and various epithelial tissues among others (14, 39, 59, 84). In salivary gland epithelium, the P2X7R is thought to play a physiological role in controlling saliva secretion. Several studies (58, 60, 68, 80) using freshly dispersed cells from rodent salivary glands or salivary gland cell lines have demonstrated that ATP or BzATP causes a characteristic P2X7R-mediated Ca2+ influx resulting in a sustained increase in the intracellular calcium concentration ([Ca2+]i) a response that promotes saliva secretion (53). Also, a recent study by Nakamoto et al. (58) used ex vivo perfused submandibular gland (SMG) preparations to show that ATP can initiate saliva flow in wild-type, but not P2X7R−/−, mice. Other studies (58, 60) indicate that P2X7R activation in salivary glands can inhibit cholinergic receptor-mediated saliva secretion, suggesting that increased activation or expression of the P2X7R could contribute to hyposalivation. The proinflammatory effects of P2X7R activation in the salivary gland have not been well investigated, although it has been shown that ATP or BzATP treatment of rat parotid Par C5 cells induces membrane blebbing (35), a hallmark of apoptotic cell death, and degradation of the cytoskeletal protein α-fodrin (36), a putative autoantigen associated with SS (54). At the time of this writing, there have been no reports investigating the role of the P2X7R in an in vivo model of salivary gland inflammation.
In this study, we characterize the proinflammatory role of P2X7R activation in freshly dispersed mouse SMG cell aggregates and in mice in which the SMG excretory duct has been ligated, an in vivo model of acute salivary gland inflammation. Our data suggest that P2X7R activation in mouse SMG induces proinflammatory responses and cell apoptosis, suggesting that the P2X7R could play a role in the pathogenesis of salivary gland inflammation associated with SS or γ-radiation-induced damaged of salivary glands.
MATERIALS AND METHODS
Reagents.
Culture media, penicillin-streptomycin 100× solution, Texas Red goat anti-rat IgG antibody, AlexaFluor 488 goat anti-rabbit antibody, Hoescht 33258 nuclear stain, and TRIzol reagent were obtained from Life Technologies (Grand Island, NY). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless stated otherwise.
Mice.
C57BL/6 (stock no. 005304) and P2X7R−/− (stock no. 005576) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the Christopher S. Bond Life Sciences Center Animal Facility of the University of Missouri (Columbia, MO). Animals were housed in vented cages with 12-h light-dark cycles and received food and water ad libitum. Age-matched 6- to 8-wk-old male mice were utilized for all experiments. All mice experimental procedures were reviewed and conducted under the strict guidelines and approval of the University of Missouri Institutional Animal Care and Use Committee.
Preparation of dispersed cell aggregates from mouse SMG.
Dispersed cell aggregates from wild-type C57BL/6 or P2X7R−/− mouse SMG were prepared, as previously described (78). Briefly, eight mice were anesthetized with isoflurane in a chamber and euthanized by cervical dislocation. SMGs were excised and minced in dispersion media [1:1 DMEM:Ham's F-12 media containing 50 U/ml collagenase (Worthington Biochemical, Lakewood, NJ), 400 U/ml hyaluronidase, 1% (wt/vol) BSA, and 0.2 mM CaCl2]. Then, minced SMGs were incubated in dispersion media in a shaking water bath at 37°C under 95% air-5% CO2 for 40 min with further dispersion by pipetting at 20, 30, and 40 min. Dispersed SMG aggregates were washed three times in assay buffer [120 mM NaCl, 4 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1 mM CaCl2, 10 mM glucose, 15 mM HEPES, and 1% (wt/vol) BSA pH 7.4], filtered through a 100-μm cell strainer (Fisher Scientific, St. Louis, MO), and resuspended in serum-free 1:1 DMEM:Ham's F-12 media containing 100 U/ml penicillin and 100 μg/ml streptomycin. Dispersed cell aggregates were incubated for 2 h at 37°C under 95% air-5% CO2 before further procedures were performed.
SDS-PAGE and Western blot analysis.
For measurement of P2X7R levels, whole SMGs were excised and homogenized in T-PER tissue protein extraction reagent (Thermo Scientific, Rockford, IL). Samples were centrifuged at 10,000 g for 5 min to pellet cellular debris, and supernatants were collected. Samples were normalized for protein concentration using a NanoDrop 1000 spectrophotometer, combined 1:1 with 2× Laemmli buffer [20 mM sodium phosphate, pH 7.0, 20% (vol/vol) glycerol, 4% (wt/vol) SDS, 0.01% (wt/vol) bromophenol blue, and 100 mM dithiothreitol] and analyzed by Western blot analysis, as previously described (73). Briefly, samples were subjected to 7.5% (wt/vol) SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 5% (wt/vol) nonfat dry milk in TBS containing 0.1% (vol/vol) Tween-20 (TBST) and incubated overnight at 4°C with rabbit anti-P2X7R antibody (1:1,000 dilution in TBST; Alomone, Jerusalem, Israel) or rabbit anti-ERK1/2 antibody (1:5,000 dilution in TBST; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed three times in TBST and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution in TBST; Santa Cruz Biotechnology) at room temperature for 1 h. Then, blots were washed three times in TBST and incubated in enhanced chemiluminescent reagent (Thermo Scientific) for 1 min. Protein bands were detected on X-ray film and quantified using a computer-driven scanner and Quantity One software (Bio-Rad, Hercules, CA).
Immunofluorescence microscopy.
Whole SMGs were excised and immediately placed in 2-methylbutane and frozen in liquid nitrogen. SMGs were then equilibrated to −20°C before 5-μm sections were cut using a Leica CM1900 cryostat. Sections were adhered to microscope slides and allowed to fully air dry before being analyzed by immunofluorescence. All steps were carried out at room temperature unless otherwise noted. Samples were fixed with 4% (vol/vol) paraformaldehyde in PBS, pH 7.4, for 20 min and then washed three times in PBS. Samples were then treated for 5 min with 0.1% (vol/vol) Triton X-100 in PBS followed by three washes in PBS. To block nonspecific antibody binding, sections were incubated in blocking buffer containing 5% (vol/vol) goat serum, 10 μM digitonin, and 0.3 M glycine for 2 h. Then, sections were incubated for 16 h at 4°C in blocking buffer containing rat anti-P2X7R antibody (1:50 dilution in blocking buffer; Enzo Life Sciences, Farmingdale, NY) and/or rabbit anti-aquaporin 5 antibody (1:1,000 dilution in blocking buffer; EMD Biosciences, San Diego, CA). Following three washes in PBS, sections were incubated for 1 h in blocking buffer containing Texas Red goat anti-rat IgG antibody and/or AlexaFluor 488 goat anti-rabbit IgG antibody, both diluted 1:1,000 in blocking buffer. Following three washes in PBS, sections were incubated for 5 min in Hoechst 33258 nuclear stain diluted 1:5,000 in PBS. Slides were washed three times in PBS and mounted. Fluorescence was visualized using a Nikon TI-E inverted microscope equipped with appropriate filters.
Real-time brightfield microscopy.
Dispersed SMG aggregates from wild-type or P2X7R−/− mice were adhered to chambered coverslips using Cell-Tak cell adhesive (BD Biosciences, Bedford, MA) per the manufacturer's protocol and kept in serum-free 1:1 DMEM:Ham's F-12 media containing 100 U/ml penicillin and 100 μg/ml streptomycin. ATP was prepared in the same media, and pH was neutralized before addition to cells at a final concentration of 3 mM. Following ATP addition, cells were imaged in real time on a Nikon TI-E inverted microscope equipped with a humidified incubation chamber maintained at 37°C with 95% air and 5% CO2.
Single cell intracellular free Ca2+ concentration measurements.
Intracellular free Ca2+ concentration ([Ca2+]i) in individual cells was quantified, as previously described (78). Briefly, dispersed SMG cell aggregates from wild-type or P2X7R−/− mice were adhered to chambered coverslips using Cell-Tak cell adhesive and loaded with the Ca2+-sensitive fluorescent dye fura 2-AM (EMD Biosciences) diluted to 2 μM in assay buffer containing 0.1% (wt/vol) BSA for 30 min at 37°C followed by a 30-min incubation in the absence of fura 2-AM. Then, cells were incubated with 3 mM ATP, pH 7, and changes in the 340/380 nm excitation fluorescence ratio (505 nm emission) were detected using an InCyt dual-wavelength fluorescence imaging system (Intracellular Imaging, Cincinnati, OH). Resulting fluorescence ratios were converted to [Ca2+]i (nM) using a standard curve created with solutions containing known concentrations of Ca2+.
Measurement of extracellular cleaved α-fodrin.
Equal numbers of dispersed SMG cell aggregates were aliquoted into wells of a 12-well plate and incubated with pH neutralized 3 mM ATP, 0.3 mM BzATP, or vehicle for 3 h at 37°C. Cell supernatants (1 ml) were collected and concentrated to 50 μl by centrifugation in a 10-kDa centrifugal filter (Millipore, Billerica, MA) for 5 min at 14,000 g. Samples were combined 4:1 with 5× Laemmli buffer and subjected to immunoblot analysis, as described above, using rabbit anti-α-fodrin antibody (1:1,000 dilution in TBST; Abcam, Cambridge, MA) and horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution in TBST).
Measurement of caspase activity.
Equal numbers of dispersed SMG cell aggregates were aliquoted into wells of a 12-well plate and incubated with pH neutralized 3 mM ATP, 0.3 mM BzATP, or vehicle for 3 h at 37°C. Caspase activity was measured using the caspase-1/ICE or caspase-3/CPP32 fluorometric assay kit (BioVision, Milpitas, CA), as per the manufacturer's instructions. Briefly, cell aggregates were pelleted by centrifugation for 1 min at 200 g, resuspended in chilled cell lysis buffer provided in the kit, and incubated on ice for 10 min. Provided reaction buffer containing 10 mM dithiothreitol and 50 μM caspase-1 substrate [YVAD-(7-amino-4-trifluoromethyl coumarin)] or caspase-3 substrate [DEVD-(7-amino-4-trifluoromethyl coumarin)] was added to the cell lysate, and samples were incubated at 37°C for 1 h. Samples were transferred to wells of a 96-well plate, and caspase activity was assessed by measuring changes in kit-provided caspase substrate fluorescence (400 nm excitation; 505 nm emission) using an EnSpire 2300 multi-label plate reader. Additionally, SMG cell aggregates were aliquoted into wells of a 12-well plate and incubated at pH 7 with 3 mM ATP, 0.3 mM BzATP, 1 μM staurosporine, or vehicle for 24 h at 37°C. Cells were collected in T-PER tissue protein extraction reagent and subjected to immunoblot analysis, as described above, using rabbit anti-caspase-1 antibody (1:1,000 dilution in TBST; Abcam) or rabbit anti-cleaved caspase-3 antibody (1:1,000 dilution in TBST; Cell Signaling, Danvers, MA) and horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution in TBST).
Retrograde perfusion and ligation of the SMG main excretory duct.
A procedure for unilateral ligation of the SMG main excretory duct (2) was modified to include retrograde perfusion of BzATP. Wild-type or P2X7R−/− mice were anesthetized by intraperitoneal injection with Avertin (0.75 mg/g mouse weight), and the main excretory duct on one side of the neck was dissected and separated from surrounding connective tissue under a surgical stereoscope. PE10 polyethylene tubing was stretched over a flame to create a catheter that was attached to a 3/10 cc insulin syringe with a 29-gauge needle. The catheter was inserted into the main excretory duct at a point ∼7-mm distal to the gland hilum, and 20 μl of 3 mM BzATP were slowly perfused over a 2-min period into the gland. Following perfusion, the main excretory duct was ligated proximal to catheter insertion using surgical sutures with particular care taken to avoid ligation of surrounding blood vessels and nerves. The incision was closed using surgical clamps, and the mice were allowed to recover. After 24 h, mice were anesthetized with isoflurane in a chamber and euthanized by cervical dislocation. Perfused ligated glands and contralateral control glands were excised and processed for the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, immunohistochemistry, or real-time PCR (RT-PCR) as described below.
Detection of apoptotic cells in SMG.
Apoptotic cells in BzATP-perfused/ligated and contralateral control SMGs were detected using the TUNEL-based in situ cell death detection kit (Roche Applied Science, Indianapolis, IN) with slight modifications to the manufacturer's instructions. Frozen SMG sections were prepared, as described above for immunofluorescence microscopy. Sections were fixed in 4% (vol/vol) paraformaldehyde at room temperature for 20 min followed by three washes in PBS. Sections were placed in permeabilization solution [0.2% (vol/vol) Triton X-100 and 0.1% (wt/vol) sodium citrate] on ice for 10 min followed by three washes in PBS. TUNEL reaction mixture (50 μl) was placed on each section, and slides were incubated in a humidified chamber at 37°C for 1 h. Samples were washed three times in PBS, mounted, and analyzed by fluorescence microscopy (488-nm excitation, 525-nm emission). TUNEL-positive apoptotic cells were detected by fluorescein-conjugated dUTP incorporation into DNA strand breaks. The number of apoptotic cells in each SMG section was quantified by counting TUNEL-positive cells in five randomly selected high-magnification fields.
Analysis of immune cell infiltration.
Immune cell infiltration of BzATP-perfused/ligated and contralateral control glands from wild-type or P2X7R−/− mice was analyzed by immunohistochemistry and RT-PCR. For immunohistochemistry, glands were excised and placed in 4% (vol/vol) paraformaldehyde in PBS for 24 h at 4°C followed by 70% (vol/vol) ethanol for 24 h at 4°C. Glands were sent to IDEXX RADIL (Columbia, MO) where SMGs were embedded in paraffin, cut into 5-μm sections, and stained with the neutrophil-specific antibody NIMP-R14 (1:200 dilution; Santa Cruz Biotechnology) or the monocyte/macrophage-specific mouse anti-CD68 antibody (1:200 dilution; Abcam). Images were captured on an Olympus Vanox brightfield microscope. For quantitative RT-PCR, glands were excised, homogenized in TRIzol reagent, and incubated at room temperature for 5 min. Chloroform (0.2 ml/ml TRIzol) was added, and the samples were incubated for 5 min at room temperature. The samples were spun at 12,000 g for 15 min at 4°C, and the resulting aqueous phase containing RNA was collected and DNA-free RNA was isolated using the RNeasy Plus Mini kit (Qiagen, Valencia, CA). cDNA was synthesized from 1 μg of purified RNA using the Advantage RT for PCR kit (Clontech Laboratories, Mountain View, CA). TaqMan probes for CD45 (also known as protein tyrosine phosphatase receptor type C, a pan-immune cell marker), Fc gamma RIII (Fcgr3, a neutrophil marker), and IbaI (ionized calcium-binding adaptor molecule 1, a macrophage marker) were obtained from Applied Biosystems (Foster City, CA) and used for RT-PCR on an Applied Biosystems 7500 real-time PCR machine. Data were analyzed using Applied Biosystems software.
Statistical analysis.
Quantitative results are presented as means ± SE of data from three or more experiments. Statistical significance was defined as P < 0.05, as calculated by a two-tailed t-test using GraphPad Prism software.
RESULTS
P2X7R is expressed in acinar and ductal SMG cells of wild-type mice.
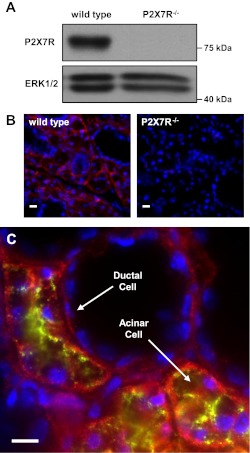
Salivary glands express several subtypes of purinergic P2 receptors, including metabotropic G-protein-coupled P2Y1 and P2Y2 receptors and ionotropic P2X4 and P2X7Rs (17, 97). Previous studies (58, 60, 68) have reported the expression of the P2X7R in mouse parotid and submandibular glands where the P2X7R has been suggested to play a physiological role in the regulation of saliva secretion. Western blot analysis using a P2X7R-specific antibody confirmed the expression of the P2X7R (∼75 kDa) in whole SMG cell lysates prepared from wild-type mice, whereas the ∼75 kDa band was absent in P2X7R−/− mouse SMG (Fig. 1A). Immunofluorescence analysis of frozen SMG sections confirmed the presence of the P2X7R in acinar and ductal cells of wild-type, but not P2X7R−/−, mice (Fig. 1B). To further investigate P2X7R localization in wild-type mouse SMG, we performed dual immunofluorescent staining using specific antibodies to the P2X7R and aquaporin 5, a water channel at the apical membrane of acinar cells in rodent SMGs (50). Results indicate little colocalization of P2X7R- and aquaporin 5-specific staining (Fig. 1C), suggesting that the P2X7R is primarily localized to the basolateral membrane in acinar cells. Furthermore, the absence of P2X7R-specific staining near the ductal lumen (Fig. 1C) suggests that like acinar cells, the P2X7R is primarily expressed on the basolateral membrane of ductal cells. These data indicate that under conditions where eATP is elevated, activation of basolateral P2X7Rs in both acinar and ductal cells should occur.
Fig. 1.
P2X7 receptor (P2X7R) expression in mouse submandibular gland (SMG). Whole SMGs from wild-type or P2X7R−/− mice were excised, homogenized in lysis buffer, and subjected to immunoblot analysis (A) using an anti-P2X7R antibody and an anti-ERK1/2 antibody as a loading control. Alternatively, whole SMGs from wild-type or P2X7R−/− mice were snap-frozen, cut into 5-μm sections, and subjected to immunohistochemical analysis using an anti-P2X7R antibody (B; red) and Hoescht nuclear stain (blue) or anti-P2X7R antibody (C; red), anti-aquaporin 5 antibody (green) and Hoescht nuclear stain (blue). Images represent results from 3 independent experiments. Scale bar = 10 μm.
ATP induces membrane blebbing and sustained increases in [Ca2+]i in SMG cells from wild-type, but not P2X7R−/−, mice.
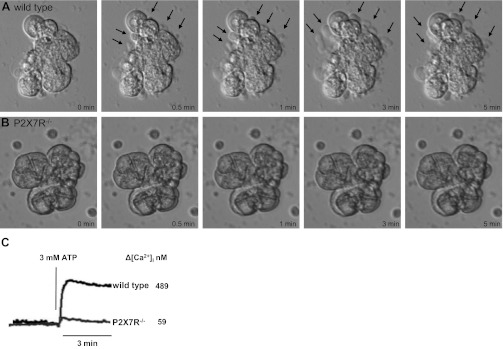
Membrane blebbing involves the extrusion and retraction of portions of the plasma membrane (69) and is an early indicator of cell apoptosis (22). Previous studies (35, 57, 63, 66, 69, 76, 108) have shown that prolonged activation of P2X7Rs can result in extensive membrane blebbing and eventual cell death. To evaluate whether P2X7R activation causes membrane blebbing in mouse SMG, freshly prepared SMG cell aggregates from wild-type or P2X7R−/− mice were stimulated with 3 mM ATP (Figs. 2, A and B) or 0.3 mM BzATP (not shown) and membrane blebbing was monitored by real-time brightfield microscopy, as described in materials and methods. Within 30 s of ATP treatment, membrane blebs (arrows) began to form in wild-type SMG cells (Fig. 2A) but not in SMG cells from P2X7R−/− mice (Fig. 2B), and extensive membrane blebbing was evident in ATP-treated wild-type SMG cells within 3–5 min (Fig. 2A). Since sustained increases in [Ca2+]i have been linked to cell death (93), we tested whether treatment of wild-type SMG cells with 3 mM ATP (Fig. 2C) or 0.3 mM BzATP (not shown) could induce a rapid and sustained increase in [Ca2+]i. Robust increases in [Ca2+]i (489 nM with ATP) were observed in wild-type cells, whereas this response was virtually absent in P2X7R−/− cells. The small increase in [Ca2+]i seen in ATP-treated cells from P2X7R−/− mice may be due to the presence of other P2 receptor subtypes known to be expressed in mouse SMG cells, e.g., P2X4R (58, 97). Collectively, these data suggest that prolonged P2X7R-mediated increases in [Ca2+]i in salivary epithelial cells lead to membrane blebbing associated with cell apoptosis.
Fig. 2.
ATP induces membrane blebbing and increases in intracellular calcium concentration ([Ca2+]i) in SMG cells from wild-type, but not P2X7R−/−, mice. Freshly dispersed SMG cell aggregates from wild-type (A) or P2X7R−/− (B) mice were treated at pH 7 with 3 mM ATP, and cells were monitored by real-time brightfield microscopy for 5 min at 37°C. Select still-frames of cell micrographs are shown at the indicated time points. Membrane blebs (arrows) can be clearly seen within 30 s of ATP treatment in wild-type SMG cells and increase over 5 min. C: ATP-induced changes in [Ca2+]i in single SMG cells were quantified using the Ca2+-sensitive fluorescent dye fura-2 and an InCyt dual-wavelength fluorescence imaging system, as described in materials and methods, and the peak increase in [Ca2+]i for n = 9 cells is indicated. Images and [Ca2+]i traces are representative of results from three independent experiments.
ATP or BzATP induces the release of cleaved α-fodrin in SMG cell aggregates from wild-type, but not P2X7R−/−, mice.
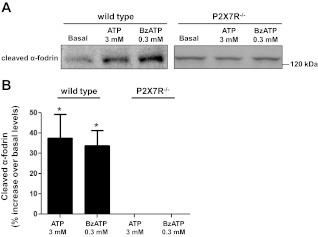
P2X7R-mediated membrane blebbing has been shown to induce the release of microvesicles or microparticles containing a variety of components, including cytokines, soluble proteins, and plasma membrane-derived fragments (56, 67, 69, 70, 72, 91). One of these plasma membrane-derived fragments is α-fodrin (also known as SPTAN1), a member of the spectrin family of cytoskeletal proteins that stabilize the plasma membrane (9). The cleavage of α-fodrin by proteases is known to occur in response to apoptotic stimuli (37), and the resulting NH2-terminal 120-kDa fragment (cleaved α-fodrin) has been suggested to be an autoantigen related to the development of SS (54). To test the hypothesis that P2X7R activation induces the cleavage and release of α-fodrin from SMG cells, we collected cell supernatants from freshly isolated wild-type or P2X7R−/− SMG cell aggregates treated with 3 mM ATP, 0.3 mM BzATP, or vehicle (basal), as described in materials and methods. Western blot analysis revealed that treatment of wild-type SMG cell aggregates with ATP or BzATP significantly increased the concentration of cleaved α-fodrin in the cell supernatant relative to untreated cells, whereas this response was absent in P2X7R−/− cells (Fig. 3). These results are consistent with previous studies in the parotid cell line Par C5 (36) and highlight a possible mechanism by which chronic P2X7R activation might stimulate autoantigen formation and release from salivary gland cells that is phenotypic of SS patients (54).
Fig. 3.
ATP or 3′-O-(4-benzoyl)benzoyl-ATP (BzATP) induces release of cleaved α-fodrin from SMG cell aggregates of wild-type, but not P2X7R−/−, mice. A: equal aliquots of dispersed SMG cell aggregates from wild-type or P2X7R−/− mice were treated with 3 mM ATP, 0.3 mM BzATP, or vehicle (basal) for 3 h. Cell supernatants were concentrated and subjected to immunoblot analysis using anti-cleaved α-fodrin antibody, as described in materials and methods. Blots represent 4 independent experiments with similar results. B: quantification of protein bands was performed using Bio-Rad Quantity One software. Data are presented as the %increase in the concentration of extracellular cleaved α-fodrin compared with basal. Data with ATP/BzATP-treated wild-type SMG cells represent means ± SE (n = 4). *P < 0.05, significant increase over the basal level. Data with ATP/BzATP-treated P2X7R−/− SMG cells (n = 4) were not significantly different from basal levels.
ATP or BzATP increases caspase-1 and caspase-3 activities in SMG cell aggregates from wild-type, but not P2X7R−/−, mice.
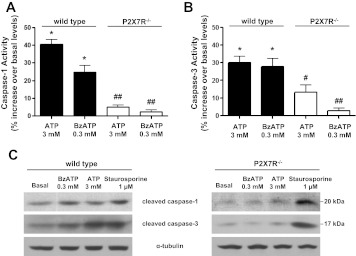
The caspase family of cysteine proteases has long been recognized for its role in the induction of apoptosis, and many studies (19, 24, 35, 41, 70, 99, 103, 104) have shown that caspase-1 and caspase-3 are crucial components of proinflammatory and apoptotic pathways. Previous studies (19, 70) indicate that P2X7R activation increases the activities of both caspases. Caspase-1 has been shown to promote the processing of inactive pro-IL-1β into active 17 kDa IL-1β in immune cells (25, 70, 88, 103). Caspase-3 has been shown to catalyze the cleavage of protein kinase Cδ, the DNA repair enzyme polyADP-ribose polymerase and α-fodrin (12, 24). Both caspase-1 and caspase-3 reside in the cytoplasm as inactive proenzymes and are processed into their active forms in response to extrinsic (i.e., binding of Fas ligand, TNFα, or ATP to their cognate receptors) or intrinsic (i.e., DNA damage, γ-radiation, or mitochondrial stress) apoptotic stimuli (12, 24). Since our observations suggest that P2X7R activation initiates apoptotic responses (i.e., membrane blebbing and α-fodrin cleavage) in freshly isolated SMG cell aggregates from wild-type mice, we investigated the role of the P2X7R in the activation of caspase-1 and caspase-3 in SMG cell aggregates. Treatment of wild-type mouse SMG cell aggregates with 3 mM ATP or 0.3 mM BzATP significantly increased the activities of both caspase-1 and caspase-3, as measured by fluorometric analysis (Fig. 4, A and B), and the levels of cleaved caspase-1 and caspase-3, as measured by immunoblot analysis (Fig. 4C), compared with untreated control cells. Furthermore, the activation or cleavage (i.e., increases in cleaved p20 caspase-1 and p17/19 caspase-3) of both caspases by ATP or BzATP treatment was significantly attenuated in SMG cell aggregates from P2X7R−/− mice, whereas staurosporine-induced caspase-1 and caspase-3 cleavage was unaffected. Interestingly, P2X7R−/− SMG cell aggregates have higher ATP-induced caspase-3 activity than BzATP-treated P2X7R−/− SMG cells. Since BzATP is a relatively selective agonist for the P2X7R and ATP activates multiple P2 receptor subtypes, we speculate that P2 receptors besides P2X7R can regulate caspase-3 activity in the SMG. Taken together, these data suggest that prolonged activation of the P2X7R in salivary epithelium can initiate proinflammatory responses and cell apoptosis through the activation of caspase-1 and caspase-3, respectively.
Fig. 4.
ATP or BzATP stimulates caspase-1 and caspase-3 activity in SMG cells from wild-type, but not P2X7R−/−, mice. Dispersed SMG cell aggregates from wild-type and P2X7R−/− mice were treated at pH 7 with 3 mM ATP, 0.3 mM BzATP, or vehicle for 3 h at 37°C. Cells were collected by centrifugation and caspase-1 (A) or caspase-3 (B) activity was quantified using fluorometric analysis, as described in materials and methods. Data represent means ± SE (n = 4), where *P < 0.05 indicates a significant difference from basal caspase activity, whereas #P < 0.05 and ##P < 0.01 indicate significant differences from ATP/BzATP-induced caspase activity in wild-type SMG. C: alternatively, SMG cell aggregates were treated at pH 7 with 0.3 mM BzATP, 3 mM ATP, 1 μM staurosporine, or vehicle for 24 h at 37°C. Cells were collected, and samples were subjected to immunoblot analysis using anti-caspase-1 or anti-caspase-3 antibody, as described in materials and methods. Blots represent 3 independent experiments with similar results.
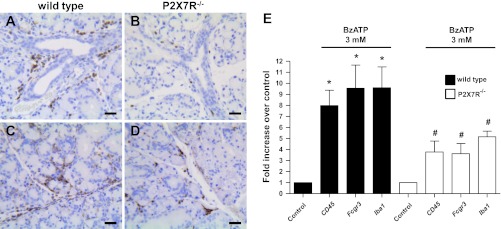
Attenuated immune cell infiltration in BzATP-perfused and duct-ligated SMG of P2X7R−/− mice, compared with wild-type mice.
Previous studies (11, 42, 47, 51) using mouse models of lung inflammation and neuropathic pain have demonstrated improved outcomes upon knockout of the P2X7R. Moreover, the use of P2X7R antagonists in clinical trials for rheumatoid arthritis and inflammatory bowel disease underscores the importance of this receptor in chronic inflammatory diseases (3). To investigate the role of P2X7R activation in salivary gland inflammation in vivo, we utilized the mouse model of unilateral SMG excretory duct ligation, a commonly used model of acute salivary gland inflammation (2, 10, 15, 31, 49, 81, 89, 98). To activate the P2X7R in vivo, 20 μl of 3 mM BzATP were perfused into the main SMG excretory duct of wild-type and P2X7R−/− mice followed by duct ligation and after 24 h the glands were histologically analyzed, as described in materials and methods. Significant neutrophil infiltration was observed in the SMG of wild-type, but not P2X7R−/−, mice, as judged by staining with the neutrophil-specific antibody NIMP-R14 (Figs. 5, A and B). Furthermore, staining for the monocyte/macrophage-specific protein CD68 revealed significant monocyte and macrophage infiltration in the SMG of wild-type mice relative to P2X7R−/− mice (Fig. 5, C and D). Decreased T-cell infiltration was observed in P2X7R−/− SMG relative to wild-type SMG, although T cells represented only a small proportion of the infiltrating immune cells, as judged by staining for the T-cell-specific protein CD3 (data not shown). For a more quantitative analysis of immune cell infiltration in the BzATP-perfused ligated glands, we performed RT-PCR on whole SMG cell lysates using specific primers for the neutrophil marker Fc gamma RIII (Fcgr3), the macrophage marker Iba1, and the pan-immune cell marker CD45 (also known as protein tyrosine phosphatase receptor type C). The results indicate a significant increase in CD45, Fcgr3 and Iba1 expression in the BzATP-perfused ligated wild-type SMG, compared with the contralateral control (Fig. 5E). These increases were significantly attenuated in the BzATP-perfused ligated SMG of P2X7R−/− mice (Fig. 5E). In agreement with previous studies (11, 42, 47, 51) using other in vivo models of inflammation, these results suggest that P2X7R activation in SMG cells in vivo can initiate cellular mechanisms that enhance immune cell infiltration into salivary glands.
Fig. 5.
Attenuated immune cell infiltration in BzATP-perfused and duct-ligated SMG of P2X7R−/− mice. Wild-type or P2X7R−/− mice were subjected to retrograde perfusion of the SMG main excretory duct with 3 mM BzATP followed by duct ligation, as described in materials and methods. After 24 h, SMGs were excised and subjected to immunohistochemical analysis using the neutrophil-specific antibody NIMP-R14 (A and B; brown) or the monocyte/macrophage specific anti-CD68 antibody (C and D; brown). A and C: wild-type SMGs show extensive infiltration of neutrophils and macrophages following 3 mM BzATP perfusion. B and D: in contrast, P2X7R−/− SMGs display a significant reduction in the number of immune cells following 3 mM BzATP perfusion. Images represent 3 independent experiments with similar results. E: alternatively, cDNA was prepared from BzATP-perfused and duct-ligated SMGs and analyzed by RT-PCR using specific primers for the pan-immune cell marker CD45, the neutrophil marker Fcgr3, or the macrophage marker Iba1. RT-PCR data represent means ± SE (n = 7). *P < 0.05, significant increase in marker expression, compared with contralateral control gland. #P < 0.05, significant difference in marker expression in P2X7R−/− SMG, compared with wild-type SMG. Scale bar = 25 μm.
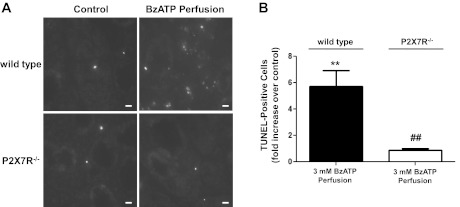
P2X7R activation by BzATP perfusion induces apoptosis in wild-type SMG.
Numerous studies (5, 33, 34, 44, 45) have indicated that apoptosis of salivary gland epithelial cells contributes to salivary gland dysfunction. The presence of apoptotic salivary gland epithelial cells in minor salivary gland biopsies has been reported in a number of patients with SS (40, 82, 87). In a mouse model of γ-radiation-induced salivary gland damage, studies (5, 44) suggest that apoptosis of acinar cells is a major contributor to salivary gland dysfunction, since inhibition of apoptosis was shown to restore function. P2X7R activation has been linked to apoptosis of a variety of cell types (79, 86, 90, 96, 104, 107, 108), and our in vitro data indicate that P2X7R activation initiates apoptotic events in freshly isolated SMG cell aggregates from wild-type mice (Figs. 2–4). DNA damage is a hallmark of late stage apoptosis, and the TUNEL assay has been shown to be a sensitive indicator of DNA damage in apoptotic cells (7). To investigate whether P2X7R activation induces apoptosis in salivary gland epithelium in vivo, BzATP-perfused ligated SMGs from wild-type and P2X7R−/− mice were subjected to the TUNEL assay, as described in materials and methods. The results indicate that BzATP perfusion significantly increases the number of TUNEL-positive cells in duct-ligated SMG of wild-type, but not P2X7R−/−, mice, compared with their respective contralateral control glands (Fig. 6, A and B). This suggests that prolonged P2X7R activation in salivary gland epithelium in vivo can cause late stage apoptosis.
Fig. 6.
BzATP perfusion induces apoptosis in duct-ligated SMG of wild-type, but not P2X7R−/−, mice. Wild-type or P2X7R−/− mice were subjected to retrograde perfusion of the SMG main excretory duct with 3 mM BzATP followed by duct ligation, as described in materials and methods. After 24 h, the SMGs were removed, snap-frozen, and sectioned. A: presence of apoptotic cells was assessed using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-based in situ cell death detection kit, as described in materials and methods. Apoptotic cells are recognized by the incorporation of fluorescein-conjugated dUTP into DNA strand breaks (white nuclei). Images represent 4 independent experiments with similar results. B: number of apoptotic cells in each SMG section was quantified by counting the number of TUNEL-positive cells in five randomly selected high-magnification fields. Data represent means ± SE (n = 4). **P < 0.01, significant increase in apoptotic cells, compared with contralateral control gland. ##P < 0.01, significant decrease in apoptotic cells in BzATP-perfused and duct-ligated P2X7R−/− SMG, compared with wild-type SMG. Scale bar = 10 μm.
DISCUSSION
In recent years, the P2X7R and its endogenous ligand ATP have gained recognition as initiators of inflammation associated with several chronic diseases, although little is known about the role of this receptor in salivary gland inflammation. In this study, we demonstrate that activation of the P2X7R in primary SMG epithelial cells from wild-type mice induces apoptotic responses including sustained increases in the [Ca2+]i, enhanced α-fodrin release, increased caspase-1 and caspase-3 activities, and membrane blebbing, responses that are absent or significantly diminished in SMG cells from P2X7R−/− mice (Figs. 2–4). Furthermore, this study indicates that in vivo activation of the P2X7R by perfusion of SMG with the high affinity P2X7R agonist BzATP promotes neutrophil and macrophage recruitment subsequent to SMG duct ligation, an accepted mouse model of acute inflammation, since neutrophil and macrophage infiltration into BzATP-perfused and duct-ligated SMGs is significantly reduced in P2X7R−/− mice (Fig. 5). These results suggest that activation of the P2X7R in the salivary gland may be an initiator of inflammatory responses associated with SS and γ-radiation-induced salivary gland damage resulting from the treatment of head and neck cancers.
eATP is now recognized as an important signaling molecule that initiates an array of physiological responses, including neurotransmission, immune cell recruitment, regulation of vascular and muscular tone, and perception of pain through the activation of cell surface P2 receptors (8, 18, 74, 85, 92, 95). Under normal conditions, the concentration of eATP, the endogenous P2X7R agonist, is tightly regulated by ecto-ATPases that rapidly hydrolyze eATP (16, 52, 55). However, in the event of tissue damage, disease, or stress, the concentration of eATP rises significantly resulting in cell death by either necrosis or apoptosis, depending on the magnitude and location of ATP release (18). In the microenvironment surrounding a cell, eATP levels likely increase to concentrations sufficient to activate the P2X7R (8, 65). Many studies have shown that the P2X7R is responsible for initiating inflammatory responses and promoting cell apoptosis in a variety of cell and tissue types (8, 25, 46, 74, 83, 84, 107) and proinflammatory cytokines (i.e., TNFα and IFNγ) associated with inflammatory salivary gland disease (i.e., SS) can increase the cellular release of ATP (43, 102), which likely activates P2X7Rs expressed on acinar and ductal cells of the SMG (Fig. 1). Studies in other laboratories indicate that ATP is also released from cells in response to γ-radiation (61), suggesting a possible role for eATP and P2X7R-mediated cell apoptosis in γ-radiation-induced damage to the salivary gland associated with some cancer therapies (27). Recently, it has been demonstrated that activation of the P2X7R can mediate ATP release through interaction with a pannexin hemi-channel (64), thus elaborating an autocrine/paracrine mechanism whereby eATP-induced activation of the P2X7R can prolong inflammation, which likely contributes to tissue degeneration in inflammatory diseases (11, 42, 47, 51). Since P2X7R activation causes sustained increases in [Ca2+]i (Fig. 2C) and has been shown to inhibit cholinergic receptor-mediated saliva secretion (58, 60), we speculate that prolonged eATP-mediated P2X7R activation in vivo can lead to salivary gland dysfunction both by inhibiting saliva secretion and by enhancing inflammatory responses and cell apoptosis in salivary glands. A better understanding of the pathophysiological consequences of prolonged P2X7R activation in damaged and diseased salivary glands could lead to novel approaches for the treatment of salivary gland hypofunction.
Studies in our laboratory and other laboratories (23, 32, 77, 105) have shown that brief stimulation of P2X7Rs by ATP4− can induce depolarizing currents that lead to reversible formation of plasma membrane pores to normally impermeant molecules, whereas prolonged activation of P2X7Rs initiates apoptosis, a highly regulated process of programmed cell death characterized by membrane blebbing, pore formation, and DNA fragmentation (35, 57, 63, 66, 69, 76, 108). Membrane blebbing occurs when sections of the plasma membrane reversibly protrude and retract at the cell surface (69). Our data show that ATP causes rapid (< 5 min) membrane blebbing in SMG cells of wild-type, but not P2X7R−/−, mice (Fig. 2, A and B), consistent with studies using P2X7R-transfected HEK293 cells and RAW 264.7 macrophages that demonstrate the dependence of P2X7R-mediated membrane blebbing and pore formation on sustained increases in [Ca2+]i and activation of Rho, Rho kinase (i.e., ROCK1), and caspase-1 (13, 35, 57, 63, 66, 69, 103). In addition to contributing to cell apoptosis, P2X7R-mediated membrane blebbing induces the release of microvesicles and microparticles that can act as chemoattractants for the recruitment of immune cells (67, 69, 70, 72). These microparticles contain bioactive cytokines, soluble proteins, and plasma membrane-derived fragments (56, 91), one of which is α-fodrin, a membrane-associated cytoskeletal protein suggested to be an autoantigen that contributes to the development of SS (54). Our data show that ATP or BzATP treatment of wild-type mouse SMG cell aggregates significantly increases the release of a 120-kDa fragment of α-fodrin relative to P2X7R−/− mice (Fig. 3). These observations are in agreement with previous studies using Par C5 cells derived from rat parotid gland that demonstrate the induction of membrane blebbing (35) and the degradation of α-fodrin (36) in response to ATP or BzATP. Our results with freshly isolated mouse SMG cell aggregates give strong support to the hypothesis that P2X7Rs can contribute to salivary gland dysfunction by enhancing membrane blebbing and autoantigen production.
In addition to inducing plasma membrane blebbing, P2X7R activation by ATP or BzATP initiates apoptosis by stimulating the activities of caspase-1 and caspase-3 (Fig. 4), members of a family of cysteine proteases known to initiate cell apoptosis (24, 99). P2X7R-mediated caspase-1 activation in immune cells has been shown to regulate the maturation and release of the proinflammatory cytokine IL-1β (19, 25, 70, 88, 103). IL-1β is an important modulator of inflammatory processes (20) and has been shown to be upregulated in the labial salivary glands of SS patients, compared with salivary glands of control subjects (62, 75). The mechanism by which the P2X7R mediates caspase-1 activation is through assembly of the NLRP3 inflammasome (8, 19, 46, 70), a multiprotein complex that regulates inflammatory responses (38). Caspase-3, the principal caspase involved in apoptosis, is stimulated by P2X7R activation in a variety of cell types (35, 41, 103, 104), consistent with our results using SMG cell aggregates (Fig. 4). Interestingly, several studies have shown that α-fodrin is a major substrate cleaved by caspase-3 during apoptosis (12, 24), suggesting that P2X7R-mediated caspase-3 activation (Fig. 4) is directly related to enhanced α-fodrin production in mouse SMG cell aggregates treated with ATP or BzATP (Fig. 3).
The induction of acute salivary gland inflammation by ligation of the primary SMG excretory duct has been used by our group and others (2, 10, 15, 31, 49, 81, 89, 98) for many years as an in vivo model of salivary gland dysfunction. Studies (10, 15, 31, 49, 81, 89) of duct-ligated salivary glands have demonstrated a progressive atrophy of acinar cells, loss of saliva flow, and infiltration of immune cells within 24 h of ligation. Results from the current study demonstrate that BzATP perfusion of the main SMG excretory duct before a 24-h duct ligation induces significant immune cell infiltration, a response mediated by the P2X7R since it is absent in SMG of P2X7R−/− mice (Fig. 5). We speculate that P2X7R activation contributes to immune cell infiltration through caspase-1-mediated IL-1β production and/or caspase-3-mediated autoantigen production. Since P2X7R-mediated cell apoptosis was detected in BzATP-perfused and duct-ligated SMGs of wild-type, but not P2X7R−/−, mice (Fig. 6), it seems likely that caspase-1 and caspase-3 activation and membrane blebbing are early apoptotic responses to P2X7R activation in SMG which, along with immune cell infiltration, likely contribute to salivary gland degeneration under a variety of pathological conditions. These findings are consistent with studies (11, 42, 47, 51) using in vivo mouse models of lung inflammation, pulmonary fibrosis, and neuropathic pain, which demonstrate that deletion of the P2X7R can decrease inflammatory responses. Further studies using other in vivo mouse models of salivary gland inflammation (i.e., γ-radiation-induced salivary gland damage or mouse models of autoimmune exocrinopathy) are needed to assess the contribution of P2X7R-mediated responses to salivary gland dysfunction.
In conclusion, this study demonstrates that the proinflammatory and apoptotic responses arising from acute salivary gland inflammation are mediated by P2X7R activation, highlighting the possible roles of ATP release and P2X7R activation in degenerative diseases of the salivary gland, such as SS, and identifying the P2X7R as a potential therapeutic target in the treatment of salivary gland hypofunction.
GRANTS
The project described was supported by National Institute of Dental and Craniofacial Research Grants R01-DE-017591 and R01-DE-007389.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.T.W., J.M.C., and G.A.W. conception and design of research; L.T.W., J.M.C., and J.M.B. performed experiments; L.T.W., J.M.C., and J.M.B. analyzed data; L.T.W., J.M.C., M.J.P., L.E., and G.A.W. interpreted results of experiments; L.T.W. and J.M.C. prepared figures; L.T.W. and J.M.C. drafted manuscript; L.T.W., J.M.C., M.J.P., L.E., and G.A.W. edited and revised manuscript; L.T.W., J.M.C., J.M.B., M.J.P., L.E., and G.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
REFERENCES
- 1. Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D. P2X7 receptor: death or life? Purinergic Signal 1: 219–227, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn JS, Camden JM, Schrader AM, Redman RS, Turner JT. Reversible regulation of P2Y2 nucleotide receptor expression in the duct-ligated rat submandibular gland. Am J Physiol Cell Physiol 279: C286–C294, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs 20: 897–915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am 49: 309–326, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Avila JL, Grundmann O, Burd R, Limesand KH. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys 73: 523–529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 [corrected] expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol 45: 65–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bortner CD, Oldenburg NB, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol 5: 21–26, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci 3: 1443–1456, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Broderick MJ, Winder SJ. Towards a complete atomic structure of spectrin family proteins. J Struct Biol 137: 184–193, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Carpenter GH, Osailan SM, Correia P, Paterson KP, Proctor GB. Rat salivary gland ligation causes reversible secretory hypofunction. Acta Physiol (Oxf) 189: 241–249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114: 386–396, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Cohen GM. Caspases: the executioners of apoptosis. Biochem J 326: 1–16, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3: 339–345, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology 36: 1277–1283, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Correia PN, Carpenter GH, Osailan SM, Paterson KL, Proctor GB. Acute salivary gland hypofunction in the duct ligation model in the absence of inflammation. Oral Dis 14: 520–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol 61: 301–332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dehaye JP, Moran A, Marino A. Purines, a new class of agonists in salivary glands? Arch Oral Biol 44, Suppl 1: S39–43, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Di Virgilio F. ATP as a death factor. Biofactors 8: 301–303, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Di Virgilio F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci 28: 465–472, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol 41: 1203–1217, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erb L, Lustig KD, Ahmed AH, Gonzalez FA, Weisman GA. Covalent incorporation of 3′-O-(4-benzoyl)benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem 265: 7424–7431, 1990 [PubMed] [Google Scholar]
- 24. Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 37: 719–727, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc 118: 199–208, 2007 [PMC free article] [PubMed] [Google Scholar]
- 27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann NY Acad Sci 842: 132–137, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Fox RI, Kang HI. Pathogenesis of Sjogren's syndrome. Rheum Dis Clin North Am 18: 517–538, 1992 [PubMed] [Google Scholar]
- 29. Fox RI, Maruyama T. Pathogenesis and treatment of Sjogren's syndrome. Curr Opin Rheumatol 9: 393–399, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Friedle SA, Curet MA, Watters JJ. Recent patents on novel P2X7 receptor antagonists and their potential for reducing central nervous system inflammation. Recent Pat CNS Drug Discov 5: 35–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garrett JR, Parsons PA. Changes in the main submandibular salivary duct of rabbits resulting from ductal ligation. Z Mikrosk Anat Forsch 93: 593–608, 1979 [PubMed] [Google Scholar]
- 32. Gonzalez FA, Ahmed AH, Lustig KD, Erb L, Weisman GA. Permeabilization of transformed mouse fibroblasts by 3′-O-(4-benzoyl)benzoyl adenosine 5′-triphosphate and the desensitization of the process. J Cell Physiol 139: 109–115, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer 10: 417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res 88: 894–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hwang SM, Koo NY, Choi SY, Chun GS, Kim JS, Park K. P2X7 receptor-mediated membrane blebbing in salivary epithelial cells. Korean J Physiol Pharmacol 13: 175–179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hwang SM, Li J, Koo NY, Choi SY, Lee SJ, Oh SB, Castro R, Kim JS, Park K. Role of purinergic receptor in α-fodrin degradation in Par C5 cells. J Dent Res 88: 927–932, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem 273: 15540–15545, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 30: 628–631, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442: 527–532, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Kong L, Ogawa N, McGuff HS, Nakabayashi T, Sakata KM, Masago R, Vela-Roch N, Talal N, Dang H. Bcl-2 family expression in salivary glands from patients with primary Sjogren's syndrome: involvement of Bax in salivary gland destruction. Clin Immunol Immunopathol 88: 133–141, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Sun GY, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2X7 nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal 1: 337–347, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168: 6436–6445, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes 10: 251–257, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Limesand KH, Said S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IGF-1. PLos One 4: e4663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol 26: 8840–8856, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm 4: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lucattelli M, Cicko S, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, Zissel G, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol 44: 423–429, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Luttikhuizen DT, Harmsen MC, de Leij LF, van Luyn MJ. Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res 317: 289–298, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Martinez JR, Bylund DB, Cassity N. Progressive secretory dysfunction in the rat submandibular gland after excretory duct ligation. Arch Oral Biol 27: 443–450, 1982 [DOI] [PubMed] [Google Scholar]
- 50. Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res 295: 513–521, 1999 [DOI] [PubMed] [Google Scholar]
- 51. McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience 146: 1817–1828, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Melani A, Corti F, Stephan H, Muller CE, Donati C, Bruni P, Vannucchi MG, Pedata F. Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp Neurol 233: 193–204, 2012 [DOI] [PubMed] [Google Scholar]
- 53. Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Miyazaki K, Takeda N, Ishimaru N, Omotehara F, Arakaki R, Hayashi Y. Analysis of in vivo role of α-fodrin autoantigen in primary Sjogren's syndrome. Am J Pathol 167: 1051–1059, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montalbetti N, Leal Denis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ. Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem 286: 38397–38407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol 33: 469–486, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, Pinton P, Rizzuto R, Olson MF, Di Virgilio F. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell 14: 2655–2664, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamoto T, Brown DA, Catalan MA, Gonzalez-Begne M, Romanenko VG, Melvin JE. Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem 284: 4815–4822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal 4: 237–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Novak I, Jans IM, Wohlfahrt L. Effect of P2X7 receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J Physiol 588: 3615–3627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ohshima Y, Tsukimoto M, Takenouchi T, Harada H, Suzuki A, Sato M, Kitani H, Kojima S. γ-Irradiation induces P2X7 receptor-dependent ATP release from B16 melanoma cells. Biochim Biophys Acta 1800: 40–46, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Oxholm P, Daniels TE, Bendtzen K. Cytokine expression in labial salivary glands from patients with primary Sjogren's syndrome. Autoimmunity 12: 185–191, 1992 [DOI] [PubMed] [Google Scholar]
- 63. Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem 282: 3403–3412, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLos One 3: e2599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol 75: 1173–1182, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood 109: 3856–3864, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Pochet S, Garcia-Marcos M, Seil M, Otto A, Marino A, Dehaye JP. Contribution of two ionotropic purinergic receptors to ATP responses in submandibular gland ductal cells. Cell Signal 19: 2155–2164, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Qu Y, Dubyak GR. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal 5: 163–173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179: 1913–1925, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol 186: 6553–6561, 2011 [DOI] [PubMed] [Google Scholar]
- 72. Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun 78: 5116–5125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ratchford AM, Baker OJ, Camden JM, Rikka S, Petris MJ, Seye CI, Erb L, Weisman GA. P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J Biol Chem 285: 7545–7555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome. Oral Dis 15: 519–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Roger S, Pelegrin P, Surprenant A. Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28: 6393–6401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saribas AS, Lustig KD, Zhang X, Weisman GA. Extracellular ATP reversibly increases the plasma membrane permeability of transformed mouse fibroblasts to large macromolecules. Anal Biochem 209: 45–52, 1993 [DOI] [PubMed] [Google Scholar]
- 78. Schrader AM, Camden JM, Weisman GA. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD. B10 mouse model of Sjogren's syndrome. Arch Oral Biol 50: 533–540, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol Renal Physiol 275: F962–F971, 1998 [DOI] [PubMed] [Google Scholar]
- 80. Seil M, Fontanils U, Etxebarria IG, Pochet S, Garcia-Marcos M, Marino A, Dehaye JP. Pharmacological evidence for the stimulation of NADPH oxidase by P2X7 receptors in mouse submandibular glands. Purinergic Signal 4: 347–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shiba R, Hamada T, Kawakatsu K. Histochemical and electron microscopical studies on the effect of duct ligation of rat salivary glands. Arch Oral Biol 17: 299–309, 1972 [DOI] [PubMed] [Google Scholar]
- 82. Shibata Y, Hishikawa Y, Izumi S, Fujita S, Yamaguchi A, Koji T. Involvement of Fas/Fas ligand in the induction of apoptosis in chronic sialadenitis of minor salivary glands including Sjogren's syndrome. Hum Cell 15: 52–60, 2002 [DOI] [PubMed] [Google Scholar]
- 83. Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24: 337–345, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol 78: 327–346, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29: 5346–5358, 2010 [DOI] [PubMed] [Google Scholar]
- 86. Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci 51: 3236–3243, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Sumida T, Matsumoto I, Murata H, Namekawa T, Matsumura R, Tomioka H, Iwamoto I, Saito Y, Mizushima Y, Hasunuma T, Maeda T, Nishioka K. TCR in Fas-sensitive T cells from labial salivary glands of patients with Sjogren's syndrome. J Immunol 158: 1020–1025, 1997 [PubMed] [Google Scholar]
- 88. Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1β in microglial cells. Crit Rev Immunol 29: 335–345, 2009 [DOI] [PubMed] [Google Scholar]
- 89. Tamarin A. The leukocytic response in ligated rat submandibular glands. J Oral Pathol Med 8: 293–304, 1979 [DOI] [PubMed] [Google Scholar]
- 90. Taylor SR, Gonzalez-Begne M, Dewhurst S, Chimini G, Higgins CF, Melvin JE, Elliott JI. Sequential shrinkage and swelling underlie P2X7-stimulated lymphocyte phosphatidylserine exposure and death. J Immunol 180: 300–308, 2008 [DOI] [PubMed] [Google Scholar]
- 91. Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3: 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal 2: pe6, 2009 [DOI] [PubMed] [Google Scholar]
- 93. Tripathi A, Chaube SK. High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 17: 439–448, 2012 [DOI] [PubMed] [Google Scholar]
- 94. Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010 [DOI] [PubMed] [Google Scholar]
- 95. Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev 63: 222–232, 2009 [DOI] [PubMed] [Google Scholar]
- 96. Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22: 386–395, 2007 [DOI] [PubMed] [Google Scholar]
- 97. Turner JT, Landon LA, Gibbons SJ, Talamo BR. Salivary gland P2 nucleotide receptors. Crit Rev Oral Biol Med 10: 210–224, 1999 [DOI] [PubMed] [Google Scholar]
- 98. Turner JT, Weisman GA, Camden JM. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am J Physiol Cell Physiol 273: C1100–C1107, 1997 [DOI] [PubMed] [Google Scholar]
- 99. Ulukaya E, Acilan C, Yilmaz Y. Apoptosis: why and how does it occur in biology? Cell Biochem Funct 29: 468–480, 2011 [DOI] [PubMed] [Google Scholar]
- 100. Uratsuji H, Tada Y, Kawashima T, Kamata M, Hau CS, Asano Y, Sugaya M, Kadono T, Asahina A, Sato S, Tamaki K. P2Y6 receptor signaling pathway mediates inflammatory responses induced by monosodium urate crystals. J Immunol 188: 436–444, 2012 [DOI] [PubMed] [Google Scholar]
- 101. Varani K, De Mattei M, Vincenzi F, Tosi A, Targa M, Masieri FF, Pellati A, Massari L, Borea PA. P2X1 and P2X3 purinergic receptors differentially modulate the inflammatory response in human osteoarthritic synovial fibroblasts. Cell Physiol Biochem 25: 325–336, 2010 [DOI] [PubMed] [Google Scholar]
- 102. Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-γ. J Immunol 166: 6383–6391, 2001 [DOI] [PubMed] [Google Scholar]
- 103. Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1β release. J Immunol 170: 5728–5738, 2003 [DOI] [PubMed] [Google Scholar]
- 104. Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol 287: C1349–C1358, 2004 [DOI] [PubMed] [Google Scholar]
- 105. Weisman GA, De BK, Pritchard RS. Ionic dependence of the extracellular ATP-induced permeabilization of transformed mouse fibroblasts: role of plasma membrane activities that regulate cell volume. J Cell Physiol 138: 375–383, 1989 [DOI] [PubMed] [Google Scholar]
- 106. Weisman GA, Dunn SD, De BK, Kitagawa T, Friedberg I. On the role of protein phosphorylation in the ATP-dependent permeabilization of transformed cells. J Cell Physiol 118: 124–132, 1984 [DOI] [PubMed] [Google Scholar]
- 107. Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78: 321–332, 2011 [DOI] [PubMed] [Google Scholar]
- 108. Wilson HL, Wilson SA, Surprenant A, North RA. Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J Biol Chem 277: 34017–34023, 2002 [DOI] [PubMed] [Google Scholar]