Abstract
The acid-sensing ion channels (ASICs) are a family of proton-sensing channels expressed throughout the nervous system. Their activity is linked to a variety of complex behaviors including fear, anxiety, pain, depression, learning, and memory. ASICs have also been implicated in neuronal degeneration accompanying ischemia and multiple sclerosis. As a whole, ASICs represent novel therapeutic targets for several clinically important disorders. An understanding of the correlation between ASIC structure and function will help to elucidate their mechanism of action and identify potential therapeutics that specifically target these ion channels. Despite the seemingly simple nature of proton binding, multiple studies have shown that proton-dependent gating of ASICs is quite complex, leading to activation and desensitization through distinct structural components. This review will focus on the structural aspects of ASIC gating in response to both protons and the newly discovered activators GMQ and MitTx. ASIC modulatory compounds and their action on proton-dependent gating will also be discussed. This review is dedicated to the memory of Dale Benos, who made a substantial contribution to our understanding of ASIC activity.
Keywords: acidosis, ischemia, ASICs, acidic pocket, pore domain, proton sensing, neuropeptide, pH, agmatine, spermine, psalmotoxin, channel gating
in the central nervous system (CNS), fluctuations in pH occur as a result of high energy consumption and metabolic demand, elevated neuronal activity, inflammation, hypercapnia, and hypoxia (31, 76, 116, 117). Accordingly, cells utilize specific molecules to measure and respond to these pH changes to maintain proper physiology and react to pathologic insults (31, 116, 117). The acid-sensing ion channels (ASICs) are thought to represent molecular acid-sensors (78, 127). These cation channels are activated by acidic pH changes and are expressed in neurons throughout the body (127). Through work with rodent models, ASICs are known to play critical roles in various processes associated with changes in pH. For example, ASIC1 is required for fear-related behaviors in response to excess CO2 inhalation, a condition which results in acidic pH in the brain (144). ASIC1 also facilitates seizure termination by responding to extracellular acidic pH induced by abnormally high neuronal activity (145). ASICs are important for other processes such as anxiety, depression, pain, sensory transduction, retinal function, as well as learning and memory within the hippocampus, cerebellum, and amygdala (20, 25, 40, 41, 49, 54–56, 94, 118, 130, 131). These findings suggest that acidic pH transients and ASICs play an important signaling role throughout the nervous system. Further, ASIC currents have been observed in glia, smooth muscle cells, and osteoclasts, indicating that ASICs likely play a role in the physiology of nonneuronal cells as well (36, 66, 70, 91).
ASICs also contribute to neuronal death during pathological insults. In particular, inhibiting ASIC1a attenuates neurological damage in mouse models of stroke and multiple sclerosis, two conditions which cause long-lasting extracellular acidosis within the central nervous system (57, 101, 124, 136). These results indicate that ASICs represent novel targets for potential therapeutics (48, 51, 65, 119, 132, 135). Significant progress has been made in our understanding of ASICs. This review highlights recent advances in the structure/function of ASICs with a special emphasis on the mechanisms of ASIC activation and modulation.
Overview of ASIC Gating and Structure
There are four ASIC genes in mammals (ASIC1-4) which encode at least six distinct ASIC subunits termed ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 (4, 11, 16, 24, 46, 58, 62, 68, 92, 103, 128).1 Alternate isoforms, “a” and “b”, of ASIC1 and ASIC2 differ in the NH2-terminal third of the protein, and the location of the alternate splice site is very similar between ASIC1 and ASIC2 (16, 92). Three individual ASIC subunits associate to form active channels which can be homomeric (composed of uniform subunits) or heteromeric (composed of varied subunits) (71). The current kinetics and gating characteristics of ASICs vary dramatically with subunit composition (8, 10, 15, 18, 21, 35, 53, 58, 63, 115). These channel characteristics can be used, along with pharmacological approaches, to determine the subunit composition of ASICs. ASICs are permeable to monovalent cations (Na+ > K+) and protons (27, 127). Some ASICs (ASIC1a homomeric channels, ASIC1a/2b heteromeric channels, and human ASIC1b channels) are also permeable to divalent cations such as calcium, suggesting that they could play a particularly important role in intracellular signaling as well as membrane excitability (64, 115, 127).
ASICs are activated by a rapid increase in the concentration of extracellular protons (acidic pH) (127). ASICs typically desensitize/inactivate rapidly after proton-induced activation and produce characteristic transient currents, although specific channels can show some level of a sustained acid-dependent component to their current (78, 126, 127). Exposure to mildly acidic pH (not low enough to induce substantial activation) or slow acidification results in steady-state desensitization (9). When steady-state desensitization is induced, ASICs fail to respond to additional decreases in extracellular pH. Multiple studies suggest that induction of steady-state desensitization prevents acidosis-mediated death of neurons and results in behavioral changes in rodents (41, 113, 114, 144). Modulators of ASIC activity also affect behavior and neuronal death in rodents (34, 48, 51, 60, 119, 135). In addition to being activated by acidic pH changes, recent data suggest that some ASICs can open in the absence of pH changes by the addition of compounds such as agmatine or MitTx, a heteromeric snake venom toxin (20, 141). These results suggest that other gating mechanisms and activators of ASICs may be employed at neutral pH in vivo.
Like all degenerin (DEG)/epithelial sodium (ENaC) channels, individual ASIC subunits have characteristic topology with intracellular NH2 and COOH termini and two transmembrane domains separated by a large extracellular cysteine-rich region. The publication of two chicken ASIC1 (cASIC1) crystal structures (Protein Data Bank 2QTS and 3HGC) has lent invaluable insight into our understanding of ASIC structure and function, and it has spurred a flurry of investigations into the domains responsible for ASIC gating (59, 71). Both structures are from channels crystallized at low pH and are, presumably, of the desensitized channel. Only the second structure published (3HGC) was solved using a cASIC1 protein which could produce functional acid-gated channels (59). Overall, the structures are similar, but there are significant differences, particularly within the transmembrane domain (59).
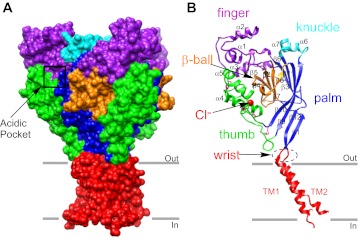
ASICs display a chalice-like shape with the large extracellular domain projecting substantially above the membrane (Fig. 1A) (59, 71). Individual subunits associate in a threefold axis arrangement within the trimeric channel. Within the extracellular domain of the trimeric channel, there are numerous intersubunit contact sites and crevices. The most notable of these represents the acidic pocket, a region of intrasubunit contacts which play a role in pH-dependent gating and binding of some modulators (Fig. 1A) (59, 71). Four main solvent vestibules are arranged vertically within the central core of the protein (upper, central, extracellular, intracellular) (59). The extracellular domain of each subunit resembles a clenched hand, and subregions in the extracellular domain include the wrist, palm, finger, knuckle, thumb, and β-ball domain (Fig. 1B) (59, 71). The palm domain is the central element of each subunit, being composed of four large β-strands that span most of the large extracellular domain (Fig. 1B). The “β-ball” region exists between the palm, thumb, and tip of the finger (59, 71). A chloride ion is bound to each subunit within the extracellular domain at the interface of the thumb, finger, and palm (Fig. 1B) (59, 71). The “wrist” domain connects the “hand” area (palm, finger, knuckle, thumb, and β-ball) to the transmembrane domain through two well-ordered loops. The transmembrane region is composed of six individual continuous α-helices with each subunit contributing two helices (corresponding to transmembrane domain 1 and 2) (59, 71). ASICs also have intracellular NH2 and COOH termini which are not represented in the crystal structures. These domains play an important role in channel gating, ion permeation, intracellular protein-protein contacts, and modulation by intracellular molecules (34).
Fig. 1.
Overview of the acid-sensing ion channel 1 (ASIC1) crystal structure. A: surface view of trimeric chicken ASIC1 (cASIC1a) created with the UCSF Chimera package from Protein Data Bank (PDB) ID: 3HGC (98). The coloration indicates domains identified by Jasti et al. (59, 71): transmembrane domains 1 and 2 (TM1 and TM2, red); wrist (red); palm (blue); knuckle (cyan); finger (purple); thumb (green); and β-ball (orange). Notice that ASICs display a chalice shape with a large extracellular domain. The acidic pocket is located at the interface between 2 subunits and is indicated by the black box (71). B: one subunit of cASIC1 in ribbon format. Coloration indicates the specific domains identified by Jasti et al. (71): The extracellular domain also has 12 β-sheets (β1–12), 7 α-helices (α1–7), and 7 disulfide bonds (illustrated in pink). Note that one of the disulfide bonds is obscured from view by the upper region of the palm domain. The extracellular domain also contains a Cl− (red sphere) binding site in the thumb domain. Images for all figures were rendered using the crystal structure of the functional cASIC1 (PDB ID: 3HGC) and the UCSF Chimera package (98). Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, funded by grants from the National Institutes of Health, National Center for Research Resources (2P41RR001081) and National Institute of General Medical Sciences (9P41GM103311). [Adapted by permission from Macmillan Publishers Ltd: Nature (Ref. 59, copyright 2009, and Ref. 71, copyright 2007).]
The Pore Region
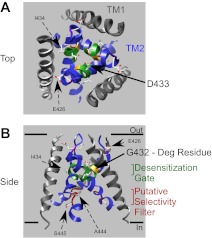
Transmembrane domain 1 (TM1) and transmembrane domain 2 (TM2) form the pore of ASICs (Fig. 2). The α-helices are arranged at an approximately 50° angle to the membrane plane in the crystal structure (59). Transmembrane domain 2 predominantly lines the lumen of the pore. Transmembrane domain 1 contacts the lipid bilayer and makes numerous intrasubunit contacts with TM2 as well as intersubunit contacts with TM1 and TM2 of the adjacent subunit. Only the COOH-terminal portion of TM1 appears to line the pore (86). Within the crystal structure, the extracellular vestibule (approximately 12 Å high by 8 Å wide) is separated from an intracellular vestibule (approximately 10 Å high and 15 Å wide at its base) by the desensitization gate (59). Approximately half of the extracellular vestibule is within the plane of the lipid bilayer. The other half is encased in the extracellular domain of the channel. Ions likely flow into the extracellular vestibule through three oval-shaped fenestrations (approximately 4 × 10 Å) located between subunits at the boundary of the extracellular region and lipid bilayer (59). These three ion pathways are located in the wrist domain which also plays a critical role in channel gating (see below). Although there is no continuous opening through the central axis of the desensitized channel's extracellular domain, conformational changes could create a central pathway which also allows ion passage.
Fig. 2.
Structure of the pore region of ASIC1. The pore of cASIC1 is shown from the top (A) and side (B) view (PDB ID: 3HGC). Transmembrane domain 2 (TM2) is indicated in blue and the desensitization gate (D433-I434-G435-G436) is shown in green. Residue D433 is thought to represent the gate residue and is the most extracellular residue of the desensitization gate located in the narrowest region of the pore when the channel is desensitized (59, 86). The degenerin (Deg) residue (G432) is highlighted in gold. Mutations of this residue have long been known to alter acid-dependent gating of ASICs. A residue critical for Ca2+ block, E426 is highlighted in purple (96). The putative ion selectivity filter (G443-A444-S445) is shown in red (86). Note that only the ribbon of glycine 443 is shown in red. Images were formulated using the UCSF Chimera package (98). [Adapted by permission from Macmillan Publishers Ltd: Nature (Ref. 59, copyright 2009) and Nature Communications (Ref. 86, copyright 2011).]
The desensitization gate.
The second transmembrane domain of each subunit crosses about one third of the way through the bilayer region to form the desensitization gate (59). Within the crystal structure, the desensitization gate constriction is formed from residues D433-G436 with the side chain of D433 forming the extracellular boundary of the desensitization gate (Fig. 2, A and B) (59). Transmembrane domain 2 is highly conserved between ASICs and among DEG/ENaC channels in general (77). It has been known for many years that in other DEG/ENaC channels the residue immediately adjacent to the desensitization gate (G432 in cASIC1) plays a critical role in channel gating (Fig. 2, A and B). This amino acid position is often referred to as the degenerin or “Deg” residue, and mutation of residues in this location can produce channels whose gating is dramatically perturbed. This was first discovered when mutation of this residue in the Caenorhabditis elegans channel DEG-1 produced constitutively open channels which caused neurodegeneration (hence the “Deg” designation) (22). For some ASIC subunits, mutations in the corresponding residue produce channels with whole cell currents showing constitutive activity, delayed desensitization, or alterations in the pH dependence of channel activation (1, 23, 128). Interestingly, such mutations at the Deg residue often do not dramatically affect ion selectivity in ASICs, which is surprising given their location near the tight constriction within the transmembrane pore (23, 59, 83). Mutations of the residue which forms the boundary of the desensitization gate, D433 in cASIC1, also affect channel gating and the side chain at position 433 may be important for stabilizing (or destabilizing) the gate (83). Mutation of D433 also does not profoundly alter ion selectivity, suggesting that the selectivity filter and desensitization gate are separate in ASICs (83, 86).
Ion selectivity.
The TM2 domain predominantly lines the pore and, thus, is assumed to mediate ion permeation and form the selectivity filter. In an elegant series of experiments using lamprey ASIC1, the role of TM2 was examined using cysteine scanning studies and mutational analysis (86). These studies suggest that the location of the narrowest part of the pore is different in the closed versus the open state of the channel. In the closed state, data were consistent with a constriction around D433, similar to that observed in the desensitized crystal structure (59, 86). In the open state, however, the smallest constriction was located mid-way through the pore around G443-A444-S445 in cASIC1 (Fig. 2, A and B) (86). Mutation of G443 profoundly affected ion permeability, supporting the theory that this region is important for ion selectivity (86). Mutation of A445 in human ASIC1a (hASIC1a), which corresponds to A444 in cASIC1a, as well as L441 in hASIC1a also affects ion permeability, further supporting this hypothesis (138). This is in stark contrast to mutations of G432 or D433, which do not profoundly affect ion permeability and are located at the narrowest region in the desensitized channel (83). These results suggest that the selectivity filter in ASICs exists at the 443–445 location within TM2.
The TM2 domains of the different ASIC subunits are highly conserved with few amino acid substitutions. Yet, individual subunits confer distinct ion selectivity to ASICs. Studies capitalizing on the difference between subunits have indicated that the ion selectivity of ASICs is affected by residues in the region intracellular to transmembrane domain 1 (pre-TM1) (16, 42). Specifically, substitutions within this region can alter selectivity for monovalent ions (Na/K) as well as the divalent ion, calcium (16, 42). Since these residues do not appear to line the pore region, it is thought that they affect ion selectivity indirectly. The NH2-terminal pre-TM1 region is also home to the “HG” motif (His-Gly), which plays an important, but not completely understood, role in the activity of DEG/ENaCs (17, 61). Mutation of residues in the pre-TM1 region has been shown to affect channel gating, suggesting it plays a defined and important role in ASIC gating as well as selectivity (14, 16, 42, 99, 107). This region is not illustrated in the current crystal structures and future studies are necessary to determine how the pre-TM1 region contributes to ion selectivity and channel gating.
Pore block.
Multiple compounds are thought to inhibit ASICs via blocking the transmembrane pore. Tetraethylammonium (TEA), a nonspecific inhibitor of potassium channels, inhibits ASIC1a/2a and ASIC1a/2b heteromeric channels at concentrations commonly used to inhibit potassium channels (115). Based on voltage dependence and TEA's action on other channels, it is thought that TEA inhibits heteromeric ASICs via pore blocking. The diuretic amiloride and related compounds also inhibit ASICs (127, 128). It is thought that amiloride and related compounds may inhibit ASICs through a pore-blocking mechanism, and modeling studies have suggested that amiloride interacts with residues near the desensitization gate (105, 110). However, amiloride binding to additional sites within the extracellular domain can also have consequences on channel gating (3, 105, 110).
In addition to pharmacological pore blockers, ASICs are sensitive to calcium-mediated pore block. Calcium has a variety of effects on ASICs (19, 122, 127). Early studies suggested that removal of calcium was sufficient for ASIC3 opening, and a model was proposed in which calcium displacement by proton binding was solely responsible for a transition from the closed state of the channel to the open state (67). However, experiments with ASIC1a suggest that calcium modulation of ASICs is more complex, and that calcium allosterically affects channel opening (96, 143). Yet, ASICs clearly display low-affinity calcium-dependent block that, based on limited voltage-dependence, is likely due to calcium ions binding to an area outside the deepest part of the membrane spanning region (96). Interestingly, mutation of a residue that lines one of the fenestrations thought to be critical for ion permeation (corresponding to E426 in cASIC1) in conjunction with mutation of the “gate” residue at the base of the extracellular vestibule (D433 in cASIC1) has been shown to eliminate calcium block in rat ASIC1a (rASIC1a) (96). Studies of residues around E426 indicate that this region may also undergo conformational changes during gating, suggesting that this region is involved in the translation of signals from the extracellular domain to changes in the conductive state of the pore (95). Whether E426 or D433 is involved directly in coordinating calcium or whether they allosterically affect calcium-mediated block of ASIC1 has yet to be determined.
The Extracellular Region
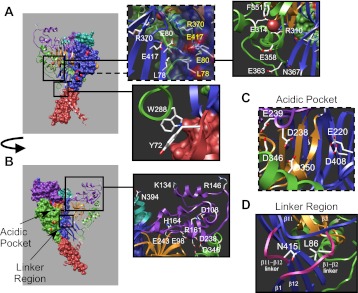
The extracellular region of ASICs is large and encompasses more than two thirds of the protein. It is characterized by specific protein motifs and 14 disulfide bonds which are conserved among DEG/ENaC channels (17). The extracellular domain is responsible for acid-dependent gating, induction of desensitization/inactivation, and the response to extracellular modulators (60). It plays a critical role in channel activation and is a target for therapeutics to inhibit ASIC1 activity (48, 119). There are seven α-helices (α1–7) and 12 β-sheets (β1–12) distributed throughout the knuckle, finger, β-ball, palm, and thumb regions of the extracellular domain (Fig. 1B) (71). Two conserved glycosylation sites are located at N367 within β10 of the palm domain and N394 between the α6 and α7 of the knuckle domain (Fig. 3, A and B) (71, 75, 109). Glycosylation is required for proper trafficking to the surface and affects apparent proton sensitivity (73, 75, 109). Other regions required for proper surface expression are also contained within the extracellular domain. In ASIC3, a salt bridge between D107 located within the α1 of the finger domain (D108 in cASIC1) and R153 within the α3 of the finger domain (R161 in cASIC1) is required for proper surface localization (Fig. 3B) (139). Similarly, interaction between W287 (W288 in cASIC1) within the thumb domain and Y71 (Y72 in cASIC1) within the wrist of TM1 is also important for proper surface expression of ASIC1 and plays an integral role in gating as well (see below) (74). Chloride is coordinated by residues R310, E314, and K212 from the neighboring subunit (Fig. 3A) (71). Substitution of chloride or mutation of chloride coordinating residues results in channels that are expressed on the surface and are activated by acidic pH, but desensitize more rapidly following activation and undergo less tachyphylaxis (79). These results indicate that chloride ions play a prominent role in channel desensitization by unknown mechanisms.
Fig. 3.
The location of amino acids in the extracellular domain known to be involved in ASIC gating. On the left, subunits within the multimeric channel have domains color coded as in Fig. 1. Note that one subunit within the trimeric channel has been removed to better highlight important regions. Boxes on the right indicate higher magnification of the highlighted regions showing the locations of specific amino acid residues. A: the Cl− binding site and residues of interest around the thumb domain are shown in the far right box. Note that one of the residues involved in coordinating Cl− (K212) is located on the adjacent subunit which is not shown. The lower box shows Y72 and W288, critical residues in the wrist domain. The loop shown in this inset is also involved in propagating the signal from extracellular modulators [i.e., RFamides (peptide terminating in the sequence arginine-phenylalanine-amide)] to channel gating (114). Residues corresponding to those involved in GMQ/agmatine binding of ASIC3 are shown in the dashed box. These residues are located within the palm regions of the trimeric channel. Note that residue E80 from one subunit (white label) interacts with E417 from the adjacent subunit (yellow label). B: residues of interest located in the finger and knuckle domains. Note that the arrow indicates that the image in A has been rotated to the right to highlight the important region. In the crystal structure (3HGC), side chains for residues K134 and R146 were unable to be visualized. The side chains shown here were inserted using the swapaa command in Chimera software. The acidic pocket is illustrated with a dashed box. The linker region is outlined by the solid box. C: higher-magnification image of the acidic pocket which is composed of the thumb and finger of one subunit and the upper region of the palm domain from the adjacent subunit. The crystal structure in this image was rotated in relationship to the trimer shown in B to show the two carboxyl-carboxylate and one carbonyl-carboxylate pairs (D238-D350, E239-D346, and E220-D408). D: higher magnification of the linker region of one cASIC1 subunit. The crystal structure in this image was rotated in relation to the ribbon subunit shown in B to highlight the β1-β2 and β11-β12 linkers (pink) and residues of interest. Images were formulated using the UCSF Chimera package and PDB ID: 3HGC (59, 71, 98). [Adapted by permission from Macmillan Publishers Ltd: Nature (Ref. 59, copyright 2009, and Ref. 71, copyright 2007).]
Proton sensing.
Although protons represent the simplest ligand, the structural mechanisms underpinning proton-induced activation of ASICs has not been a simple question to answer. It is known that protons bind the extracellular domain to trigger channel activation and steady-state desensitization (127). The crystal structure of desensitized ASIC shows a solvent pocket containing many acidic residues located at a junction between the finger and thumb of one subunit and the palm domain of another (71). This location has been termed the acidic pocket (Fig. 3, B and C). Within the acidic pocket there are two pairs of carboxyl-carboxylate side chains (Asp238-Asp350 and Glu239-Asp346 in cASIC1a) and one pair of carbonyl-carboxylate side chains within the palm of an adjacent subunit (Glu220-Asp408) which are coordinated via hydrogen bonds (Fig. 3C) (71). These side chains represent excellent candidates for proton-sensing residues because of their highly negative electrostatic potential. Further, amino acids within the acidic pocket are perfectly poised to support conformation changes in the thumb domain which, in turn, could affect conformation change in the wrist and transmembrane domains (71). In support of this theory, mutations of amino acids within the pocket result in ASICs with changes in the concentration of protons required for gating or a change in the Hill coefficient for proton-induced activation (71, 84, 97, 111, 112, 138, 141). However, elimination of all three interacting pairs results in channels with reduced apparent proton affinity, but which ultimately retain the ability to open in response to acid (84). Therefore, other residues clearly play a role in proton-induced opening of ASICs. Amino acids outside the acidic pocket have also been implicated as potential proton sensors or residues critical for apparent proton affinity (13, 39, 69, 90, 97, 112, 120). These residues are distributed throughout the extracellular domain. Yet, defining “proton-sensing” residues is complicated by the fact that many residues can indirectly affect the apparent proton affinity without necessarily affecting proton binding. Alterations that inhibit the conformation changes required for gating, destabilize the open state, or stabilize the closed state can shift the apparent proton affinity. For example, mutation of M85L (Xenopus ASIC1, L86 in cASIC1) within the β1-β2 linker shifts the apparent proton sensitivity of ASIC1 by a mechanism that likely involves stabilizing the closed state (Fig. 3B and D) (85). More rigorous analysis is necessary to determine the connection between amino acid mutation and the proton sensitivity of the channel. Even with these important caveats, the general consensus is that there is no one proton-sensing residue for ASICs and that protonation of multiple residues, possibly at multiple regions throughout extracellular domain, initiates acid-dependent activation.
The role of calcium in proton-dependent activation.
The gating behavior of many ASICs is intricately related to the concentration of extracellular calcium (19, 45, 127). As discussed above, calcium can block the pore of ASICs (67, 96). In addition, the apparent proton sensitivity of ASIC1 and ASIC3 is inversely related to extracellular Ca2+ concentration (67, 112, 127, 143). Multiple studies suggest that Ca2+ and protons compete for binding to the channel, and evidence supports a model where calcium binding favors the closed state whereas proton binding favors the open or desensitized state (67, 127, 143). Work with ASIC3 suggested that a calcium ion blocks the pore of ASIC3 and removal of the calcium ion alone (induced by proton binding to the calcium-coordinating amino acids) could open the channel (67). This model implied that no conformation change was necessary for ASIC3 to open, only calcium removal. These observations were supported by the fact that at very low calcium concentrations, ASIC3 displays a small noninactivating current at basal pH (7.4) (67). Yet, protons dramatically enhanced channel opening even in the absence of calcium, suggesting the presence of protons enhanced gating in a manner independent of calcium block (67). In further support of a more complex gating mechanism, studies with ASIC1 show that there are significant allosteric changes upon opening of the channel (143). Several recent studies on ASIC1a support a twofold effect. Calcium blocks the ion permeation pore of ASIC1a (96). In addition, Ca2+ decreases the apparent proton sensitivity of ASIC1a by an undetermined mechanism (96, 143). This is supported by ASIC1a mutants where calcium pore block is abolished, but the calcium-dependent shifts in apparent proton sensitivity remain intact (96). These studies suggest that there are two calcium-binding sites in ASIC1a: one that predominantly determines pore block and one that predominantly mediates calcium regulation of proton-induced gating. Further, calcium-mediated inhibition of apparent proton sensitivity can be separated from the apparent proton sensitivity of activation, suggesting that multiple regions of the protein are important for this process (112). Whether protons and calcium compete directly for binding at one site or whether the calcium binding affects proton-induced activation allosterically remains to be determined.
Acid-evoked steady-state desensitization.
ASICs undergo steady-state desensitization (also termed steady-state inactivation) in which the channel enters the desensitized state without obviously transitioning through the open state (9). This process occurs with exposure to a mild increase in proton concentration over time on the order of seconds to minutes (9, 67, 122). Once steady-state desensitization has occurred, little to no current is observed with a further increase in proton concentration. In fact, the ASIC inhibitor psalmotoxin 1 (PcTx1) elicits neuroprotective effects by promoting steady-state desensitization of the channel (29, 113, 134). Steady-state desensitization, like activation, is mediated by proton binding to the extracellular domain of the channel (9). Multiple studies suggest that proton-dependent activation and steady-state desensitization are coupled (12, 90, 114). For example, the protein domains involved in steady-state desensitization overlap with those involved in proton-dependent activation (12, 90, 114). Residues in the acidic pocket play a role in the apparent proton sensitivity of steady-state desensitization and activation (141). In addition, the concentration of calcium inversely affects the apparent proton affinity of both steady-state desensitization and activation (9, 122). Finally, the venom peptide PcTx1 affects the proton dependence of both steady-state desensitization and activation of ASIC1a (29). These results suggest that there are similar mechanisms responsible for proton-dependent activation and steady-state desensitization. However, there are important differences between proton-induced steady-state desensitization and activation. The apparent proton affinity for steady-state desensitization is much higher than activation and occurs at more neutral pHs (9). The proton-dose response curve of steady-state desensitization has a greater Hill coefficient and displays significantly more cooperativity (9). These results suggest that steady-state desensitization may utilize “high-affinity” proton-binding sites which foster desensitization over time rather than activation. In support of this idea, alterations of certain titratable residues (specifically E235 and E254 in hASIC1a) can specifically affect the apparent proton sensitivity of steady-state desensitization without profoundly affecting the pH50 for activation (90). Channel modulators can also affect steady-state desensitization specifically, or differentially affect the apparent proton affinity of steady-state desensitization and activation (50, 113, 129). Further, some amino acids play a prominent role in defining the apparent proton sensitivity of activation or steady-state desensitization, suggesting that residues can play distinct roles in proton-dependent gating (43, 85, 90). Importantly, mutation of N415 (cASIC1) within the β11-β12 linker preferentially affects the apparent proton sensitivity of steady-state desensitization (Fig. 3B and D) (82). Another consideration is that the channel conformation evoked by steady-state desensitization is similar to the desensitized state that occurs after proton-mediated activation (67), and no studies have shown that they are distinct conformational states. Thus, protein regions involved in desensitization following activation might also be expected to affect steady-state desensitization. The fact that induction of steady-state desensitization likely requires a large conformational change suggests that mutations in many residues could affect apparent proton sensitivity. Further understanding of the differences between proton-induced steady-state desensitization and activation may lend specific insight into the mechanisms of these two potentially therapeutically important gating states.
Mechanisms of proton-dependent gating.
There is much to be determined on how proton binding to the extracellular domain triggers channel activation and desensitization. The acidic pocket is situated between the thumb and finger, and interactions between these domains have been proposed to be an important aspect of gating (71). Mutations that are calculated to destabilize this interaction reduce the apparent proton sensitivity of channel activation, and mutations that are predicted to stabilize this interaction enhance apparent proton sensitivity (138). The region at the base of the thumb plays a critical role in translating signals in the extracellular domain to changes in the transmembrane regions. Specifically, the loop region at the base of the thumb that links the β9 of the palm to the α4 of the thumb is critical to acid-dependent gating (84, 138). Within this loop, proline residues create a sharp turn which allows tryptophan 288 to interact with tyrosine 72 within the wrist domain near transmembrane domain 1 (Fig. 3) (84). Specific mutations of W288, P287, or Y72 can eliminate acid-dependent gating of ASICs (84, 95, 138). Additional conformational changes in the wrist region, both extracellular to TM1 and TM2, occur with channel gating and are important for normal channel activity (84, 95, 123, 138). It has been proposed that conformational changes in the wrist result in twisting or bending movement of the transmembrane domains to initiate channel gating (59, 86, 123, 137, 138). Multiple lines of evidence indicate that both TM1 and TM2 undergo a conformation change with gating (2, 86, 99). However, a defined twisting movement of TM2 (the transmembrane helix that predominantly lines the pore) has not been supported by accessibility studies of lamprey ASIC1 (86). Instead, data support a model in which activation involves a reduction in the tilt of the transmembrane helices which widens the external mouth and forms a narrowing deeper in the pore (possibly the selectivity filter) (86). A similar mechanism is utilized by P2X receptors which share multiple structural features and gating characteristics with ASICs (59, 80, 81).
The transition from the closed to open to desensitized state involves other conformation changes within the extracellular domain. Mutation of residues within the β1-β2 linker region in the palm, the β1 strand in the palm, the β11-β12 linker as well as the wrist domain affects the kinetics of opening and closing the pore (38, 43, 85, 87, 121). These events are manifested macroscopically as alterations in the rate of desensitization, proton dependence of activation, proton dependence of steady-state desensitization, or sustained channel opening (43, 85, 87, 121). Within the β1 region of the palm of ASIC3, E79 (E80 in cASIC1) becomes less accessible when the channel is in the desensitized state compared with the closed state (43). This supports the idea that this region undergoes a conformation change during gating. Similarly, amino acid 110 in shark ASIC1a (corresponding to A82 in cASIC1) in the β1-β2 linker is also more accessible when the channel is closed compared with desensitized (121). In addition, there appears to be tight apposition between this residue within the β1-β2 linker and a complement residue (corresponding to V413 in cASIC1) in the β11-β12 linker region when the channel is desensitized (121). Other residues within the β11-β12 linker region have also been shown to play a role in channel gating, particularly desensitization, supporting the idea that movement of these regions is critical to normal gating (82). Together these data suggest that gating, and desensitization in particular, involves movement of the palm domains. Mutations of residues within the contact region between the β-ball, finger, and upper palm domain also affect channel gating and surface expression of the channel. Specifically, residues within the β-ball to finger loop (residues 94–97, 101 in hASIC1a), the finger (163–164 in hASIC1a), and the palm to finger loop (224, 226, 228–229 in hASIC1a) are critical for normal activity (12). Further, evidence suggests that this region also undergoes a conformation change with gating (12). Thus, multiple regions of the extracellular domain move during channel gating. This idea is in agreement with other, more generalized, studies suggesting that there is a large conformational change in the extracellular domain in the transition from the closed to the desensitized state (6, 140).
Other modulators and inhibitors.
Many modulators and inhibitors affect ASIC activity through interaction with the extracellular domain (34, 48, 51). For the purpose of this review, we focus on compounds in which there is some understanding into the structural domains of the channel important for modulator action. These modulators include zinc, reducing agents, proteases, peptide toxins, spermine, and neuropeptides (5, 7, 13, 28, 30, 33, 35, 37, 93, 102, 106, 108, 113, 114, 125).
The finger domain plays an important role in zinc and reducing agent-dependent modulation of ASICs. Low concentrations of zinc inhibit ASIC1a by decreasing apparent proton sensitivity of activation through a mechanism that requires K133 (K134 in cASIC1), a residue located at the beginning of α2 within the finger domain (Fig. 3B) (35). K133 is also involved in potentiation of hASIC1a activity by reducing agents (32, 33). Interestingly, residues within the finger domain are required for low-affinity zinc-mediated inhibition of ASIC1b (C149) and zinc-mediated potentiation of ASIC2a (H162) (13, 72). H162 (H164 in cASIC1) corresponds to a conserved histidine which is also one of the residues involved in the contact region between the β-ball, finger, and upper palm domain and is required for proper gating (Fig. 3B) (12). Although exactly how these residues contribute to modulation of ASIC activity is unknown, such results further indicate that the finger domain plays a critical role in ASIC gating and represents a potential target for modulator binding.
The finger domain also plays a prominent role in sensitivity to extracellular proteases. Trypsin and other proteases limit ASIC activity by dampening the proton response, changing ion permeation, and limiting RFamide (peptide terminating with the sequence arginine-phenylalanine-amide)-related neuropeptide modulation (see below) (37, 102, 125). Trypsin cleavage at R145 (R146 just following α2 in the finger domain in cASIC1) is responsible for attenuated rASIC1a activity (125). Further, matripase cleaves rASIC1a at three sites (R145, K185, and K384) with cleavage at R145 producing the majority of the inhibitory activity (37). Thus, proteolytic cleavage within the finger domain can result in dramatic changes in ASIC gating further supporting the idea that the finger domain plays a critical role in ASIC activity.
The acidic pocket also plays a prominent role in ASIC modulation. One of the best studied ASIC inhibitors is the venom peptide psalmotoxin 1 (PcTx1) isolated from the West Indies tarantula Psalmopoeus cambridgei (53). PcTx1 is a 40 amino acid peptide with three disulfide bonds arranged in an inhibitor cysteine knot (ICK) pattern similar to spider and cone snail venoms (52, 53). Low nanomolar concentrations of PcTx1 can inhibit ASIC1a homomeric channels and ASIC1a/2b heteromeric channels by promoting steady-state desensitization (26, 28, 29). PcTx1 is not a classic “blocker” and does not inhibit ASIC activity by blocking the pore of the channel (28, 29). Instead, PcTx1 acts as a gating modifier toxin and inhibits ASIC1a and ASIC1a/2b channels by shifting the pH dependence of steady-state desensitization to more alkaline pHs such that the channels desensitize at basal pH 7.4 (28, 29, 115). PcTx1 also shifts the pH dependence of channel activation to more neutral pHs (28, 29). Thus, if PcTx1 is present at more basic basal pH and steady-state desensitization is not induced, PcTx1 can enhance ASIC1a activation: an effect in opposition to its normally inhibitory nature (29).
PcTx1 folds tightly into the “knottin” fold pattern similar to other toxins that inhibit voltage-gated ion channels (52). However, PcTx1 is unique in this family in targeting ASIC1 channels and displays a novel collection of basic amino acids “K25-R26-R27-R28” that form a strong positive patch at the surface of the toxin (52, 53). Since the discovery of PcTx1, it was hypothesized that these residues mediate ASIC1a-specific interaction (52, 53, 100, 104, 106, 108). Recent functional data confirm that R26 and R27 play a critical role in PcTx1 action (106). Excitingly, the use of chimeric ASIC channels composed of different regions of PcTx1-sensitive and -insensitive ASIC subunits, computer docking of PcTx1 to the crystal structure of cASIC1a, site-directed mutagenesis of ASIC1a, and a recent crystal structure of ASIC1a bound to PcTx1 indicate that PcTx1 binds within the acidic pocket of the channel, the same region that is thought to play a key role in pH-dependent gating (44, 100, 104, 106, 108, 112). Functionally, mutations of residues D349 (D350 in cASIC1 thumb and one of the residues involved in the carboxyl-carboxylate pairs) and E97 (E98 in cASIC1 in a loop connecting the β-ball and finger) dramatically reduce PcTx1 binding (Fig. 3, B and C) (108). Other residues such as E358 in the thumb loop, E363 in the loop connecting β10 of the palm to the thumb, and F351 in the thumb also play a role in the response to PcTx1 (Fig. 3A) (108, 112). The recent crystal structure of PcTx1 bound to ASIC1a indicates that PcTx1 binding involves both a hydrophobic patch of the venom peptide which interacts with the thumb of ASIC1a and the basic cluster of residues of the venom peptide (R27 and R28 in particular) which extend into the acidic pocket of ASIC1a to form strong H-bonds (44). Of the residues in ASIC1a previously implicated in PcTx1 action, the new crystal structure supports a direct role for D350 and F351 in binding (44). The involvement of the acidic pocket in PcTx1 action further supports the role of the acidic pocket in pH-dependent gating of the channel, as PcTx1 shifts the pH dependence of both activation and desensitization to more basic pH values. In addition, amino acids from two adjacent subunits within the multimeric channel contribute to PcTx1 binding. This may explain why PcTx1 affects specific ASIC1-containing heteromeric channels such as ASIC1a and ASIC1a/2b, but not ASIC1a/2a (53).
Several other modulators are believed to also interact with the acidic pocket of ASIC1. The cation spermine enhances ASIC1a activity by slowing inactivation, shifting steady-state desensitization to more acidic pHs, and accelerating recovery from desensitization (9, 50). Spermine competes with the venom peptide PcTx1 for action and likely interacts within the acidic pocket of ASIC to alter channel gating (50). Modulation by spermine is disrupted by mutations of E219 (E220 in cASIC1 located within the palm domain in the acidic pocket and one residue involved in the carbonyl-carboxylate interaction) and E242 (E243 in cASIC1 within the acidic pocket) (Fig. 3, B and C) (50, 71). These residues are also important for steady-state desensitization, suggesting that these residues play a critical role in spermine modulation of steady-state desensitization (50).
Another set of modulators that may also bind to the acidic pocket are the endogenous opioid peptides big dynorphin and dynorphin A. Dynorphins reduce the apparent proton sensitivity of steady-state desensitization of ASIC1 through a mechanism that likely involves binding of the peptide to the extracellular domain of the channel (113). PcTx1 and big dynorphin compete for action on ASIC1a, suggesting that PcTx1 and dynorphin may share an interaction site or that the binding of one compound prevents the action of the other (113). Another set of neuropeptides, the RFamides, also shift the apparent sensitivity of ASIC steady-state desensitization to more acidic values. RFamides interact with the extracellular domain of ASICs to slow desensitization and reduce the apparent proton sensitivity of steady-state desensitization (7, 21, 30, 47, 93, 114). For some ASICs, RFamides can induce a robust sustained acid-dependent current (7, 30, 47, 133). Both mammalian RFamides and invertebrate RFamides can exert these effects, although smaller RFamides are generally more effective (7, 21, 30, 47, 93, 114). Calcium antagonizes and acidic pH enhances RFamide modulation (7, 30, 93). RFamide action is affected by specific amino acids within the loop region at the base of the thumb that links the β9 of the palm to the α4 of the thumb which is critical for translating signals in the extracellular environment to changes in the transmembrane domains (Fig. 3A) (84, 115, 138). Interestingly, alteration of these residues (A274, P285, K291, D298, L299) in hASIC1a does not seem to affect RFamide binding, but it compromises the ability of RFamide binding to alter desensitization (114). Although RFamides and dynorphins result in similar changes in channel gating characteristics, it is not yet clear whether they share a common binding site (113).
Alternate activation mechanisms.
Recently, novel mechanisms of ASIC activation have been identified. ASIC3 can be activated at physiological pH (7.4) by the synthetic compound 2-guanidine-4-methylquinazoline (GMQ), the endogenous compound agmatine, and related chemicals (141). ASIC3 becomes activated by GMQ, but little to no desensitization is induced, resulting in constitutive GMQ-dependent currents (141). GMQ interacts with E79 (E80 in β1 of the palm domain of cASIC1), E423 (E417 in β12 of the palm domain cASIC1a), L77 (L78 in β1 of the palm domain of cASIC1), and R376 (R370 in the β10 of the palm domain), which are located within a shallow depression near the base of the extracellular domain formed by residues of the palm domain (Fig. 3A) (71, 141, 142). Interestingly, this cavity is at the edges of β1 and β12, whose linkers have been shown to play a critical role in ASIC desensitization and production of sustained acid-dependent current with altered ion selectivity (82, 85, 121). Further, E79 of ASIC3 has already been linked to alterations in desensitization (see above) (43). DTNB (5,5′-dithiobis-2-nitrobenzoic acid) modification of E79C mutants of ASIC3 also results in channel activation (141). Interestingly, the classical nonspecific inhibitor of ASICs, amiloride, can activate ASIC3 channels through interaction with this GMQ-binding region (3, 88). Thus, this “non-proton” ligand-sensing domain can induce channel activation at neutral pH in response to multiple compounds (88). The currents induced by GMQ have a different ion selectivity compared with those evoked with protons alone, suggesting that binding of GMQ or activation by GMQ results in changes to the transmembrane domains which are different from those encountered with protons alone (141). This form of activation is further distinguished from proton-induced activation in that mutations that disrupt critical carboxyl-carboxylate proton-binding residues within the acidic pocket (and reduce apparent proton sensitivity) spare GMQ-mediated activation (141). GMQ-mediated activation is not, however, entirely independent of protons or calcium. Low pH potentiates GMQ-mediated activation and, conversely, GMQ potentiates proton response (141). Similarly, low calcium facilitates and high calcium limits GMQ-mediated activation much like the effect of calcium upon proton-dependent activation (141). Together these results suggest that ASIC3 can act as a multifactorial sensor (protons, calcium, and GMQ-related compounds). In support of this idea, agmatine (an endogenous GMQ-like compound) can synergize with other ASIC modulators such as arachidonic acid and hyperosmolarity to enhance ASIC3 current (89). These results suggest that this region is a critical site for binding of new activators and other modulators that affect channel activation, and represents a potentially new therapeutic target for the development of ASIC inhibitors. To date, ASIC1 and ASIC2 channels have not been activated by GMQ and related compounds (141), but, the existence of such a mechanism of ASIC activation is exciting and suggests that other, non-proton ligands for other ASICs may exist in vivo.
A second alternative activation mechanism for ASICs was recently discovered. A heteromeric coral snake toxin called MitTx can activate ASIC1a channels at neutral pH (20). MitTx is a complex of two toxins: a Kunitz-type toxin MitTx-α and a phospholipase-A2-like toxin MitTx-β (20). MitTx induces large sustained amiloride-sensitive currents from ASIC1a and ASIC1b as well as ASIC3 (at approximately 100-fold higher concentration). The currents evoked from ASIC1a lack desensitization and are slow to reverse after removal of the toxin (20). MitTx-evoked currents from ASIC3 display a slower onset and faster washout compared with ASIC1 channels. ASIC2a channels are weakly affected by MitTx at neutral pH (20). MitTx, however, does substantially potentiate proton-induced activation of ASIC2a. MitTx and PcTx1 compete, suggesting that there is functional occlusion of toxin action or the toxins share a binding site (20). The interplay between protons, calcium, and MitTx is not known. Yet, the fact that MitTx can induce currents from ASICs at neutral pH provides another line of evidence to suggest that other endogenous ligands for ASICs may exist and await discovery.
GRANTS
This manuscript was supported, in part, by National Institutes of Health Grant RO1 NS062967. E. N. Frey was supported by a training grant awarded to the Integrated Biomedical Graduate Program from National Institutes of Health (T32GM068412) as well as a fellowship awarded by the Wexner Medical Center. T. W. Sherwood was supported in part through a P30 core grant (NS045758).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.W.S. and C.C.A. interpreted the results of the experiments; T.W.S. and C.C.A. drafted the manuscript; T.W.S., E.N.F., and C.C.A. edited and revised the manuscript; T.W.S., E.N.F., and C.C.A. approved the final version of the manuscript; E.N.F. prepared the figures.
ACKNOWLEDGMENTS
This review is dedicated to the memory of Dale Benos, who made substantial contributions to the field of ASIC structure and function. His work stimulated research in these areas across the world and has had a wide-reaching impact on the field of ASIC physiology. We will miss his groundbreaking ideas and enthusiastic support of all things “ASIC”. We also thank Soluman Culver for helpful discussions and thoughtful editorial comments on this manuscript.
Footnotes
ASIC nomenclature over the years has been exceedingly complex due to 1) multiple names being given to ASIC subunits initially and 2) disparities between the ENTREZ database and the nomenclature adopted within the literature. In this review we adopt the nomenclature commonly utilized in the literature and based on the rodent subunits.
REFERENCES
- 1. Adams CM, Price MP, Snyder PM, Welsh MJ. Tetraethylammonium block of the BNC1 channel. Biophys J 76: 1377–1383, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams CM, Snyder PM, Price MP, Welsh MJ. Protons activate brain Na+ channel 1 by inducing a conformational change that exposes a residue associated with neurodegeneration. J Biol Chem 273: 30204–30207, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Adams CM, Snyder PM, Welsh MJ. Paradoxical stimulation of a DEG/ENaC channel by amiloride. J Biol Chem 274: 15500–15504, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport 11: 2217–2222, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Andrey F, Tsintsadze T, Volkova T, Lozovaya N, Krishtal O. Acid sensing ionic channels: modulation by redox reagents. Biochim Biophys Acta 1745: 1–6, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 26: 133–141, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem 279: 18296–18305, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem 277: 41597–41603, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+. J Biol Chem 275: 28519–28525, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem 72: 51–57, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Bargeton B, Kellenberger S. The contact region between three domains of the extracellular loop of ASIC1a is critical for channel function. J Biol Chem 285: 13816–13826, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem 276: 35361–35367, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Bashari E, Qadri YJ, Zhou ZH, Kapoor N, Anderson SJ, Meltzer RH, Fuller CM, Benos DJ. Two PKC consensus sites on human acid-sensing ion channel 1b differentially regulate its function. Am J Physiol Cell Physiol 296: C372–C384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem 272: 28819–28822, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Grunder S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem 276: 33782–33787, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 520: 631–644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berdiev BK, Mapstone TB, Markert JM, Gillespie GY, Lockhart J, Fuller CM, Benos DJ. pH alterations “reset” Ca2+ sensitivity of brain Na+ channel 2, a degenerin/epithelial Na+ ion channel, in planar lipid bilayers. J Biol Chem 276: 38755–38761, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D, Sanchez EE, Burlingame AL, Basbaum AI, Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 479: 410–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Catarsi S, Babinski K, Seguela P. Selective modulation of heteromeric ASIC proton-gated channels by neuropeptide FF. Neuropharmacology 41: 592–600, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Chalfie M, Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345: 410–416, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Champigny G, Voilley N, Waldmann R, Lazdunski M. Mutations causing neurodegeneration in Caenorhabditis elegans drastically alter the pH sensitivity and inactivation of the mammalian H+-gated Na+ channel MDEG1. J Biol Chem 273: 15418–15422, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen CH, Hsu YT, Chen CC, Huang RC. Acid-sensing ion channels in neurones of the rat suprachiasmatic nucleus. J Physiol 587: 1727–1737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen X, Grunder S. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J Physiol 579: 657–670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen X, Kalbacher H, Grunder S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol 127: 267–276, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen X, Kalbacher H, Grunder S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol 126: 71–79, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen X, Paukert M, Kadurin I, Pusch M, Grunder S. Strong modulation by RFamide neuropeptides of the ASIC1b/3 heteromer in competition with extracellular calcium. Neuropharmacology 50: 964–974, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci 15: 396–402, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Cho JH, Askwith C. Potentiation of acid-sensing ion channels by sulfhydryl compounds. Am J Physiol Cell Physiol 292: C2161–C2174, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Chu XP, Close N, Saugstad JA, Xiong ZG. ASIC1a-specific modulation of acid-sensing ion channels in mouse cortical neurons by redox reagents. J Neurosci 26: 5329–5339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chu XP, Papasian CJ, Wang JQ, Xiong ZG. Modulation of acid-sensing ion channels: molecular mechanisms and therapeutic potential. Int J Physiol Pathophysiol Pharmacol 3: 288–309, 2011 [PMC free article] [PubMed] [Google Scholar]
- 35. Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci 24: 8678–8689, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung WS, Farley JM, Swenson A, Barnard JM, Hamilton G, Chiposi R, Drummond HA. Extracellular acidosis activates ASIC-like channels in freshly isolated cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 298: C1198–C1208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clark EB, Jovov B, Rooj AK, Fuller CM, Benos DJ. Proteolytic cleavage of human acid-sensing ion channel 1 by the serine protease matriptase. J Biol Chem 285: 27130–27143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coric T, Zhang P, Todorovic N, Canessa CM. The extracellular domain determines the kinetics of desensitization in acid-sensitive ion channel 1. J Biol Chem 278: 45240–45247, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Coric T, Zheng D, Gerstein M, Canessa CM. Proton sensitivity of ASIC1 appeared with the rise of fishes by changes of residues in the region that follows TM1 in the ectodomain of the channel. J Physiol 568: 725–735, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci 29: 5381–5388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry 62: 1140–1148, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Coscoy S, de Weille JR, Lingueglia E, Lazdunski M. The pre-transmembrane 1 domain of acid-sensing ion channels participates in the ion pore. J Biol Chem 274: 10129–10132, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Cushman KA, Marsh-Haffner J, Adelman JP, McCleskey EW. A conformation change in the extracellular domain that accompanies desensitization of acid-sensing ion channel (ASIC) 3. J Gen Physiol 129: 345–350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dawson RJ, Benz J, Stohler P, Tetaz T, Joseph C, Huber S, Schmid G, Hugin D, Pflimlin P, Trube G, Rudolph MG, Hennig M, Ruf A. Structure of the acid-sensing ion channel 1 in complex with the gating modifier psalmotoxin 1. Nat Commun 3: 936, 2012 [DOI] [PubMed] [Google Scholar]
- 45. De Weille J, Bassilana F. Dependence of the acid-sensitive ion channel, ASIC1a, on extracellular Ca2+ ions. Brain Res 900: 277–281, 2001 [DOI] [PubMed] [Google Scholar]
- 46. De Weille JR, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett 433: 257–260, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology 44: 662–671, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 128: 549–558, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci 31: 6059–6066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, Cao H, Hansen J, Simon RP, Zhu MX, Xiong ZG, Xu TL. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci 31: 2101–2112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dube GR, Elagoz A, Mangat H. Acid sensing ion channels and acid nociception. Curr Pharm Des 15: 1750–1766, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci 12: 1332–1343, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275: 25116–25121, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J Neurosci 26: 5800–5809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci 50: 2417–2426, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci 24: 1005–1012, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med 13: 1483–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA 94: 1459–1464, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol 2: 73–94, 2010 [PMC free article] [PubMed] [Google Scholar]
- 61. Grunder S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J 16: 899–907, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport 11: 1607–1611, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Hoagland EN, Sherwood TW, Lee KG, Walker CJ, Askwith CC. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J Biol Chem 285: 41852–41862, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol 194: 283–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang C, Hu ZL, Wu WN, Yu DF, Xiong QJ, Song JR, Shu Q, Fu H, Wang F, Chen JG. Existence and distinction of acid-evoked currents in rat astrocytes. Glia 58: 1415–1424, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Ishibashi K, Marumo F. Molecular cloning of a DEG/ENaC sodium channel cDNA from human testis. Biochem Biophys Res Commun 245: 589–593, 1998 [DOI] [PubMed] [Google Scholar]
- 69. Ishikita H. Proton-binding sites of acid-sensing ion channel 1. PLos One 6: e16920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun 337: 349–354, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Jiang Q, Inoue K, Wu X, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zinc-mediated inhibition. Neuroscience 193: 89–99, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci 32: 4080–4091, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jing L, Jiang YQ, Jiang Q, Wang B, Chu XP, Zha XM. The interaction between the first transmembrane domain and the thumb of ASIC1a is critical for its N-glycosylation and trafficking. PLos One 6: e26909, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kadurin I, Golubovic A, Leisle L, Schindelin H, Grunder S. Differential effects of N-glycans on surface expression suggest structural differences between the acid-sensing ion channel (ASIC) 1a and ASIC1b. Biochem J 412: 469–475, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Katsura K, Kristian T, Smith ML, Siesjo BK. Acidosis induced by hypercapnia exaggerates ischemic brain damage. J Cereb Blood Flow Metab 14: 243–250, 1994 [DOI] [PubMed] [Google Scholar]
- 77. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience 5: 2325–2327, 1980 [DOI] [PubMed] [Google Scholar]
- 79. Kusama N, Harding AM, Benson CJ. Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a). J Biol Chem 285: 17425–17431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li M, Chang TH, Silberberg SD, Swartz KJ. Gating the pore of P2X receptor channels. Nat Neurosci 11: 883–887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li M, Kawate T, Silberberg SD, Swartz KJ. Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun 1: 44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li T, Yang Y, Canessa CM. Asn415 in the beta11-beta12 linker decreases proton-dependent desensitization of ASIC1. J Biol Chem 285: 31285–31291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li T, Yang Y, Canessa CM. Asp433 in the closing gate of ASIC1 determines stability of the open state without changing properties of the selectivity filter or Ca2+ block. J Gen Physiol 137: 289–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li T, Yang Y, Canessa CM. Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J Biol Chem 284: 4689–4694, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li T, Yang Y, Canessa CM. Leu85 in the beta1-beta2 linker of ASIC1 slows activation and decreases the apparent proton affinity by stabilizing a closed conformation. J Biol Chem 285: 22706–22712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li T, Yang Y, Canessa CM. Outlines of the pore in open and closed conformations describe the gating mechanism of ASIC1. Nat Commun 2: 399, 2011 [DOI] [PubMed] [Google Scholar]
- 87. Li T, Yang Y, Canessa CM. Two residues in the extracellular domain convert a nonfunctional ASIC1 into a proton-activated channel. Am J Physiol Cell Physiol 299: C66–C73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li WG, Yu Y, Huang C, Cao H, Xu TL. Nonproton ligand sensing domain is required for paradoxical stimulation of acid-sensing ion channel 3 (ASIC3) channels by amiloride. J Biol Chem 286: 42635–42646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li WG, Yu Y, Zhang ZD, Cao H, Xu TL. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain 6: 88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liechti LA, Berneche S, Bargeton B, Iwaszkiewicz J, Roy S, Michielin O, Kellenberger S. A combined computational and functional approach identifies new residues involved in pH-dependent gating of ASIC1a. J Biol Chem 285: 16315–16329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin YC, Liu YC, Huang YY, Lien CC. High-density expression of Ca2+-permeable ASIC1a channels in NG2 glia of rat hippocampus. PLos One 5: e12665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem 272: 29778–29783, 1997 [DOI] [PubMed] [Google Scholar]
- 93. Ostrovskaya O, Moroz L, Krishtal O. Modulatory action of RFamide-related peptides on acid-sensing ionic channels is pH dependent: the role of arginine. J Neurochem 91: 252–255, 2004 [DOI] [PubMed] [Google Scholar]
- 94. Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54: 1408–1415, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Passero CJ, Okumura S, Carattino MD. Conformational changes associated with proton-dependent gating of ASIC1a. J Biol Chem 284: 36473–36481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Paukert M, Babini E, Pusch M, Grunder S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol 124: 383–394, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Paukert M, Chen X, Polleichtner G, Schindelin H, Grunder S. Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem 283: 572–581, 2008 [DOI] [PubMed] [Google Scholar]
- 98. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612, 2004 [DOI] [PubMed] [Google Scholar]
- 99. Pfister Y, Gautschi I, Takeda AN, van Bemmelen M, Kellenberger S, Schild L. A gating mutation in the internal pore of ASIC 1a. J Biol Chem 281: 11787–11791, 2006 [DOI] [PubMed] [Google Scholar]
- 100. Pietra F. On the putative binding site of RFamide-family neuropeptides from the western Atlantic clam Sunray Venus and cephalopods on acid-sensing ion channels. An automated docking and molecular-dynamics study with hASIC1a homology model. Chem Biodivers 8: 816–826, 2011 [DOI] [PubMed] [Google Scholar]
- 101. Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 130: 151–158, 2007 [DOI] [PubMed] [Google Scholar]
- 102. Poirot O, Vukicevic M, Boesch A, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem 279: 38448–38457, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem 271: 7879–7882, 1996 [DOI] [PubMed] [Google Scholar]
- 104. Qadri YJ, Berdiev BK, Song Y, Lippton HL, Fuller CM, Benos DJ. Psalmotoxin-1 docking to human acid-sensing ion channel-1. J Biol Chem 284: 17625–17633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qadri YJ, Song Y, Fuller CM, Benos DJ. Amiloride docking to acid-sensing ion channel-1. J Biol Chem 285: 9627–9635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saez NJ, Mobli M, Bieri M, Chassagnon IR, Malde AK, Gamsjaeger R, Mark AE, Gooley PR, Rash LD, King GF. A dynamic pharmacophore drives the interaction between Psalmotoxin-1 and the putative drug target acid-sensing ion channel 1a. Mol Pharmacol 80: 796–808, 2011 [DOI] [PubMed] [Google Scholar]
- 107. Salinas M, Lazdunski M, Lingueglia E. Structural elements for the generation of sustained currents by the acid pain sensor ASIC3. J Biol Chem 284: 31851–31859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, Lazdunski M. The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J Physiol 570: 339–354, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Saugstad JA, Roberts JA, Dong J, Zeitouni S, Evans RJ. Analysis of the membrane topology of the acid-sensing ion channel 2a. J Biol Chem 279: 55514–55519, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 109: 15–26, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shaikh SA, Tajkhorshid E. Potential cation and H+ binding sites in acid sensing ion channel-1. Biophys J 95: 5153–5164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sherwood T, Franke R, Conneely S, Joyner J, Arumugan P, Askwith C. Identification of protein domains that control proton and calcium sensitivity of ASIC1a. J Biol Chem 284: 27899–27907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci 29: 14371–14380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sherwood TW, Askwith CC. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J Biol Chem 283: 1818–1830, 2008 [DOI] [PubMed] [Google Scholar]
- 115. Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci 31: 9723–9734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Siesjo BK. Acidosis and ischemic brain damage. Neurochem Pathol 9: 31–88, 1988 [DOI] [PubMed] [Google Scholar]
- 117. Siesjo BK, Katsura KI, Kristian T, Li PA, Siesjo P. Molecular mechanisms of acidosis-mediated damage. Acta Neurochir Suppl (Wien) 66: 8–14, 1996 [DOI] [PubMed] [Google Scholar]
- 118. Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 119. Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Devel 12: 693–704, 2009 [PMC free article] [PubMed] [Google Scholar]
- 120. Smith ES, Zhang X, Cadiou H, McNaughton PA. Proton binding sites involved in the activation of acid-sensing ion channel ASIC2a. Neurosci Lett 426: 12–17, 2007 [DOI] [PubMed] [Google Scholar]
- 121. Springauf A, Bresenitz P, Grunder S. The interaction between two extracellular linker regions controls sustained opening of acid-sensing ion channel 1. J Biol Chem 286: 24374–24384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Todorovic N, Coric T, Zhang P, Canessa C. Effects of extracellular calcium on fASIC1 currents. Ann NY Acad Sci 1048: 331–336, 2005 [DOI] [PubMed] [Google Scholar]
- 123. Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1a. J Biol Chem 286: 16297–16307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain 134: 571–584, 2011 [DOI] [PubMed] [Google Scholar]
- 125. Vukicevic M, Weder G, Boillat A, Boesch A, Kellenberger S. Trypsin cleaves acid-sensing ion channel 1a in a domain that is critical for channel gating. J Biol Chem 281: 714–722, 2006 [DOI] [PubMed] [Google Scholar]
- 126. Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997 [DOI] [PubMed] [Google Scholar]
- 127. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 128. Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem 271: 10433–10436, 1996 [DOI] [PubMed] [Google Scholar]
- 129. Wang WZ, Chu XP, Li MH, Seeds J, Simon RP, Xiong ZG. Modulation of asic currents, acid-induced increase of intracellular Ca2+ and acidosis mediated neuronal injury by intracellular pH. J Biol Chem 281: 29369–29378, 2006 [DOI] [PubMed] [Google Scholar]
- 130. Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci 23: 5496–5502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci USA 101: 3621–3626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 133. Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol 89: 2459–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 134. Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol 209: 59–68, 2006 [DOI] [PubMed] [Google Scholar]
- 135. Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol 8: 25–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698, 2004 [DOI] [PubMed] [Google Scholar]
- 137. Yang H, Yu Y, Li WG, Xu TL, Jiang H. Conformational sampling on acid-sensing ion channel 1 (ASIC1): implication for a symmetric conformation. Cell Res 19: 1035–1037, 2009 [DOI] [PubMed] [Google Scholar]
- 138. Yang H, Yu Y, Li WG, Yu F, Cao H, Xu TL, Jiang H. Inherent dynamics of the acid-sensing ion channel 1 correlates with the gating mechanism. PLoS Biol 7: e1000151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yang Y, Yu Y, Cheng J, Liu Y, Liu DS, Wang J, Zhu MX, Wang R, Xu TL. Highly conserved salt bridge stabilizes rigid signal patch at extracellular loop critical for surface expression of acid-sensing ion channels. J Biol Chem 287: 14443–14455, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yokokawa M, Carnally SM, Henderson RM, Takeyasu K, Edwardson JM. Acid-sensing ion channel (ASIC) 1a undergoes a height transition in response to acidification. FEBS Lett 584: 3107–3110, 2010 [DOI] [PubMed] [Google Scholar]
- 141. Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68: 61–72, 2010 [DOI] [PubMed] [Google Scholar]
- 142. Yu Y, Li WG, Chen Z, Cao H, Yang H, Jiang H, Xu TL. Atomic level characterization of the nonproton ligand-sensing domain of ASIC3 channels. J Biol Chem 286: 24996–25006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang P, Sigworth FJ, Canessa CM. Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism. J Gen Physiol 127: 109–117, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139: 1012–1021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci 11: 816–822, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]