Abstract
Hyperammonemia and sarcopenia (loss of skeletal muscle) are consistent abnormalities in cirrhosis and portosystemic shunting. We have shown that muscle ubiquitin-proteasome components are not increased with hyperammonemia despite sarcopenia. This suggests that an alternative mechanism of proteolysis contributes to sarcopenia in cirrhosis. We hypothesized that autophagy could be this alternative pathway since we observed increases in classic autophagy markers, increased LC3 lipidation, beclin-1 expression, and p62 degradation in immunoblots of skeletal muscle protein in cirrhotic patients. We observed similar changes in these autophagy markers in the portacaval anastamosis (PCA) rat model. To determine the mechanistic relationship between hyperammonemia and autophagy, we exposed murine C2C12 myotubes to ammonium acetate. Significant increases in LC3 lipidation, beclin-1 expression, and p62 degradation occurred by 1 h, whereas autophagy gene expression (LC3, Atg5, Atg7, beclin-1) increased at 24 h. C2C12 cells stably expressing GFP-LC3 or GFP-mCherry-LC3 constructs showed increased formation of mature autophagosomes supported by electron microscopic studies. Hyperammonemia also increased autophagic flux in mice, as quantified by an in vivo autophagometer. Because hyperammonemia induces nitration of proteins in astrocytes, we quantified global muscle protein nitration in cirrhotic patients, in the PCA rat, and in C2C12 cells treated with ammonium acetate. Increased protein nitration was observed in all of these systems. Furthermore, colocalization of nitrated proteins with GFP-LC3-positive puncta in hyperammonemic C2C12 cells suggested that autophagy is involved in degradation of nitrated proteins. These observations show that increased skeletal muscle autophagy in cirrhosis is mediated by hyperammonemia and may contribute to sarcopenia of cirrhosis.
Keywords: autophagometer, human, tyrosine nitration
sarcopenia or loss of skeletal muscle mass in cirrhosis is nearly universal in cirrhosis and adversely affects survival, enhances development of other complications of cirrhosis, negatively affects outcome after liver transplantation, and decreases quality of life (17, 32, 36). There are no effective therapeutic modalities for sarcopenia associated with cirrhosis because its pathogenesis is poorly understood (36). One of the potential mediators of sarcopenia in cirrhosis is hyperammonemia (13). Since the liver is the major organ responsible for ammonia detoxification, hepatocellular dysfunction and portosystemic shunting in cirrhosis contribute to elevated blood ammonia concentrations (35). Our preliminary studies have shown that hyperammonemia-induced, myostatin-mediated impaired protein synthesis is a consistent abnormality in animal models and in vitro systems (10, 13a). However, a reduction in skeletal muscle protein synthesis alone is not sufficient to account for continued reduction in muscle mass in cirrhosis, and an increase in proteolysis is necessary (18). Since components of the ubiquitin-proteasome proteolysis pathway are not altered in the portacaval anastamosis (PCA) rat with sarcopenia (9–11), there must be alternative mechanisms of protein breakdown contributing to the loss of muscle mass in cirrhosis (28, 29). In this regard, the lysosomal proteolytic pathway of autophagy is a highly conserved process. It is responsible for controlled degradation of cytoplasmic components, including damaged organelles/toxic protein aggregates, as well as providing essential nutrients during starvation and stress to maintain cellular homeostasis (2). Since cirrhosis is a state of accelerated starvation (6, 47), enhanced muscle autophagy may serve as a source of essential amino acids for critical cellular function.
In addition to providing essential nutrients, autophagy plays a critical role in the removal of damaged and abnormal proteins (21). Hyperammonemia has been identified recently to be a potential inducer of autophagy (7, 15, 16, 30), and significant hyperammonemia is a consistent abnormality in cirrhosis (34, 35). Hyperammonemia induces nitration of proteins in astrocytes (20, 41), and nitration has been reported to cause structural changes and impair protein function (1, 3, 39, 46). Therefore, increased autophagy may be a response to degrade these abnormal proteins in the skeletal muscle during hyperammonemia of cirrhosis. Autophagy is dysregulated in pathophysiological states, and an understanding of the mechanisms regulating this pathway can provide novel therapeutic options (21). In this study, we examined the induction of autophagy by hyperammonemia as a potential cause of skeletal muscle loss in cirrhosis.
MATERIALS AND METHODS
Reagents.
Antibodies were obtained as follows: LC3 (Novus Biological, Littleton, CO), p62 (Progen Biotechnical, Heidelberg, Germany), beclin-1 (Cell Signaling Technology, Danvers, MA), nitrotyrosine (Millipore, Billerica, MA), β-actin, and α-tubulin, as well as the secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Ammonium acetate, ammonium chloride, probenecid, potassium ionophore nigericin, tyrosine, nitrotyrosine, and other reagents for making buffers were obtained from Sigma-Aldrich (St. Louis, MO). Free 2′,7′-biscarboxyl-ethyl-5(6)-carboxyfluorescein (BCECF) and BCECF acetomethyl ester (BCECF-AM) were obtained from Life Technologies (Grand Island, NY).
Human studies.
Autophagy was studied in human skeletal muscle from patients with cirrhosis undergoing liver transplantation (n = 13). All patients had histologically confirmed cirrhosis in the explanted liver. Subjects undergoing elective abdominal surgery without disorders or medications that affect skeletal muscle synthesis or breakdown formed the control group (n = 13; hernia repair = 3; donors for liver transplantation, n = 10). The clinical and biochemical characteristics of the two groups are shown in Table 1. Muscle mass was measured by quantifying psoas and paraspinal and abdominal wall areas on CT scans of the abdomen at L4 vertebra (48). This was significantly lower (P < 0.01) in cirrhotics compared with that in control subjects. The studies were approved by the Institutional Review Board of the Cleveland Clinic, and written informed consent was obtained from all subjects. The rectus abdominis muscle was obtained from these subjects at the initiation of surgery, flash-frozen in liquid nitrogen, and stored at −80°C for subsequent assays.
Table 1.
Clinical and demographic features of subjects
| Characteristic | Control | Cirrhosis |
|---|---|---|
| No. | 13 | 13 |
| Sex (males/females) | 7/6 | 7/6 |
| Age, yr | 51.2 ± 12.1 | 54.0 ± 10.3 |
| BMI, kg/m2 | 29.3 ± 6.5 | 29.7 ± 4 |
| Bilirubin, mg/dl | 0.5 ± 0.2 | 11.0 ± 17.8*** |
| Alanine amino transferase, IU/dl | 28.5 ± 20.5 | 75.6 ± 11.9*** |
| Aspartate aminotransferase, IU/dl | 35.6 ± 21.9 | 134.0 ± 240.1*** |
| Serum creatinine, mg/dl | 1.0 ± 0.3 | 1.4 ± 0.4* |
| Blood urea nitrogen, mg/dl | 13.5 ± 4.4 | 24.8 ± 19.4* |
| International normalized ratio | 1.2 ± 0.5 | 1.6 ± 0.5** |
| Serum albumin, g/dl | 3.9 ± 0.6 | 3.4 ± 0.9* |
Values are means ± SE. BMI, body mass index.
P < 0.05;
P < 0.01;
P < 0.001, cirrhosis vs. controls.
PCA rat.
Male Sprague-Dawley rats (weight: 200–260 g) with an end-to-side PCA (n = 8) or sham surgery (n = 8) were obtained from Charles River Laboratories (Wilmington, MA). The pair-fed sham-operated rats were compared with the the PCA rats, as described earlier (9, 11). The gastrocnemius muscle was harvested at 4 wk after surgery, flash-frozen in liquid nitrogen, and stored at −80°C for later assays. These animals were part of earlier studies on characterization of the skeletal muscle response in portosystemic shunting of cirrhosis, and we have reported previously that components of the ubiquitin-proteasome pathway are not activated (9, 11). The studies were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Autophagometer.
Postpubertal C57/bl mice were obtained from Jackson Laboratories and at 8 wk of age. They were injected intraperitoneally with 2.5 mmol·kg−1·day−1 ammonium acetate for 7 days, followed by 2 days of colchicine (0.4 mg·kg−1·day−1) to determine autophagic flux (26). Appropriate controls included vehicle alone for the ammonium acetate and colchicine. There were four groups of animals: vehicle alone, colchicine alone, ammonium acetate alone, and ammonium acetate with colchicine. A total of six animals in each group were studied. The gastrocnemius muscle was harvested, protein was extracted, and immunoblots for LC3 lipidation and α-tubulin as loading control were performed, followed by densitometry for quantification of the blots. These studies were performed at Washington University (St. Louis, MO), as described previously (26).
Cell culture.
Murine C2C12 cell lines were obtained from ATCC (Manassas, VA) and grown to confluence in proliferation medium consisting of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum. After confluence, the medium was changed to differentiation medium (DMEM with 2% horse serum) for the cells to differentiate. Following 48 h of differentiation, cells were exposed to ammonium acetate (10 mM) for various time points to 24 h. Control and treated cells were incubated with 0.4% trypan blue in sterile phosphate-buffered saline (PBS) and immediate counting of cells. Since the trypan blue exclusion suffers from the limitations of subjectivity between multiple shades of blue or blue-gray as well as overestimation of cell viability by this method, a fluorescent cell viability assay (CellTiter-Blue cell viability assay; Promega, Madison, WI) was also performed using the manufacturer protocol prior to the detailed molecular studies. This provided a homogeneous, fluorometric method for estimating viable cells. Viable cells reduce a redox dye (resazurin) into a fluorescent end product (resorufin), whereas nonviable cells do not reduce the indicator dye and fail to generate a fluorescent signal. Since the trypan blue and the CellTiter blue use different chemistries to determine cell viability, both of these methods were used to show high cell viability in response to hyperammonemia.
Muscle tissue protein and RNA extraction.
Protocols for muscle protein and total RNA extraction have been established in our laboratory and described earlier (9, 11). In brief, for total RNA extraction, ∼15 mg of the frozen muscle sample was used. RNA was isolated by using RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol, using chloroform extraction. RNA concentration was determined using the NanoDrop 1,000 UV/Vis Spectrophotometer (Thermo Scientific, Rockford, IL). The quality of RNA was evaluated by gel electrophoresis on a formaldehyde RNA gel.
Total muscle protein was extracted from a precisely weighed amount (∼40 mg) of frozen skeletal muscle samples. Samples were added to the Lysing Matrix D (MP Biomedicals, Solon, OH) tubes with ice-cold RIPA buffer (Thermo Scientific) supplemented with protease and phosphatase inhibitors (Thermo Scientific) for extracting protein. Using the FastPrep 120 (Q-BIOgene, Irvine, CA), the tissue was homogenized, followed by centrifugation at 12,000 g for 5 min at 4°C. The supernatant containing the protein extract was transferred into a new microcentrifuge tube, and protein content was quantified using the bicinchoninic acid assay (Thermo Fisher Scientific), aliquoted, and stored at −80°C for subsequent assays.
Western blots.
Immunoblots were performed as described previously (9). In brief, protein samples were diluted in SDS-PAGE sample buffer and denatured, followed by loading of equal amounts of protein in a 4–12% gradient gel. Following electrophoresis, the proteins were electrotransferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA) that were blocked using 5% nonfat milk in Tris-buffered saline (TBS). The membranes were incubated overnight at 4°C in primary antibodies in TBS with 0.05% Tween-20 (TBST) with either 3% BSA (LC3, beclin-1) or 5% nonfat milk (p62, β-actin). The membranes were washed in TBST, followed by incubation with secondary antibodies. Immunoreactivity was detected by chemiluminescent horseradish peroxidase substrate (Millipore, Billerica, MA). Densitometry was performed on the blots using the Image J program (40). For the nitrotyrosine immunoblots, all the stained bands were included.
Quantitative real-time PCR.
Real-time polymerase chain reaction for quantification of mRNA was performed on a Stratagene Mx 3000P (Stratagene, La Jolla, CA), using a SYBR protocol on the fluorescence temperature cycler by methods as described previously (9, 11). Relative differences were normalized to the expression of β-actin. Expression of autophagy genes was quantified, and the primer sequences are shown in Table 2. Real-time PCR products were then separated by gel electrophoresis to confirm specific product presence and size.
Table 2.
Primer sequences
| Gene | Accession No. | Upper (5′-3′) | Lower (5′-3′) |
|---|---|---|---|
| Human | |||
| Proteasome C3 | NM_002787.4 | TGGAATCTGCAATGAAGCTG | GCTGGAAGGTGGGTTTAACA |
| Proteasome C5 | NM_002793.3 | AAGAAGGAAAGGGGGCTGTA | AGCCGCAGAAATGAAGACAT |
| Murf1 | NM_032588.2 | TGAGCCAGAAGTTTGACACG | TGATGAGTTGCTTGGCAGTC |
| Atrogin | NM_148177.2 | CGAAGCAGGTGTTTCCTCTC | ATGGAATAATCCCCCTTTGC |
| Mouse | |||
| Atg5 | NM_053069.5 | AGATGGACAGCTGCACACAC | GCTGGGGGACAATGCTAATA |
| Atg7 | NM_028835.3 | TCCGTTGAAGTCCTCTGCTT | CCACTGAGGTTCACCATCCT |
| GABARAPL1 | NM_020590.4 | TCGTGGAGAAGGCTCCTAAA | ATACAGCTGGCCCATGGTAG |
| ULK2 | NM_013881.4 | GCAGACAGGAGGACGAAGAC | GCACTGGAGAACCTGAGGAG |
| Beclin-1 | NM_019584.3 | GGCCAATAAGATGGGTCTGA | GCTGCACACAGTCCAGAAAA |
| LC3b | NM_026160.4 | CGGCTTCCTGTACATGGTTT | ATGTGGGTGCCTACGTTCTC |
| mVps34/Pik3C3 | NM_181414.5 | TCAAGTGCGATGACAAGGAG | CTTGTGGTAGCGTTGGGTTT |
| Atg4b | NM_174874.3 | CATGAATTTCTGGGGCACTT | AGCCTTGGGATTCTCTCCAT |
| LAMP2a | NM_010685.3 | CACCCACTCCAACTCCAACT | TTGTGGCAGGGTTGATGTTA |
| β-Actin | NM_007393.3 | ATCGTGCGTGACATCAAAGA | ATGCCACAGGATTCCATACC |
Murf1, muscle RING finger 1.
20S proteasome activity assay.
The 20S proteasome activity assay in whole muscle homogenate quantified degradation via the ubiquitin proteasome pathway, as described previously. The Chemicon Proteasome 20S activity assay kit (Chemicon International, Temecula, CA) based on detection of the fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate LLVY-AMC was used for these studies (10). Enzyme activity of the 20S proteasome was expressed as relative fluorescence units per microgram of protein. For C2C12 cells, whole cell lysates were prepared according to the manufacturer's instructions, and the assay was performed with the fluorescence units normalized to the protein content. All experiments were performed in triplicate, and the values are expressed as means ± SD.
Generation of stably transfected C2C12 cells.
Stable lines expressing GFP-LC3 and GFP-mCherry-LC3 were generated by retrovirus and lentivirus transduction, respectively, using protocols approved by the Institutional Biosafety Committee at the Cleveland Clinic (43). The GFP-mCherry-LC3 plasmid was a kind gift from Dr. Jayanta Debnath (University of California San Fransisco). In brief, the constructs were packaged in human embryonic kindey (HEK)-293T cells that were transfected with GFP-mCherry-LC3 constructs and pCL10 plasmid in a 3:1 ratio using the Fugene transfection reagent. The GFP-LC3 retrovirus was produced in HEK-293T transfected with GFP-LC3 (gift of Toshihiko Suzuki, University of the Ryukyus, Japan) (44), VSV-G, and gag/pol plasmids using Polyfect (Qiagen, Valencia, CA) according to the manufacturer's instructions. Medium containing viral particles was then collected after 24 and 48 h of transfection, passed through a 0.8-μm filter, and added to C2C12 cells along with polybrene (10 μg/ml). Stable cell lines were generated through selection with puromycin resistance (1.5 μg/ml) for 10 days.
Autophagic flux.
This was determined in C2C12 cells stably expressing GFP-mCherry-LC3, as described previously (43). Cells with stable expression of GFP-mCherry-LC3 after treatment were fixed with 4% paraformaldehyde at the indicated times, washed several times with PBS, mounted using Vectashield, and analyzed using an HCX Plan Apo ×63/1.4 NA oil immersion objective on a Leica TCS-SP2 confocal microscope (Leica Microsystems). Quantification of the LC3 puncta was performed using the Red and Green Puncta Colocalization Macro with the Image J Program (43). Increased autophagic flux by hyperammonemia was also quantified on immunoblots of protein from C2C12 myotubes exposed to 5 μM chloroquine (23).
Immunofluorescence and confocal microscopy.
Semiconfluent (70–80%) monolayers of stably transfected GFP-LC3 or GFP-mCherry-LC3 tandem reporter C2C12 cells on glass slides grown in 10% fetal calf serum were incubated in differentiation medium with 2% horse serum for 48 h, followed by exposure to freshly prepared 10 mM ammonium acetate in 2% differentiation medium. After ammonium acetate treatment, cells were washed with PBS and then fixed by 4% paraformaldehyde at room temperature for 30 min. After another three PBS washes, samples were mounted in Vectashield mounting medium for fluorescence with 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were acquired on a Leica TCS SP2 confocal laser scanning microscope. The 488-nm line of an argon/argon-krypton laser and the 561-nm line of a solid-state laser were used to excite the samples. Cells were imaged through a ×40/1.4 NA oil immersion objective. Images were collected and saved using Leica Confocal software and exported to Microsoft Picture Manager for digital processing.
Analysis of GFP-LC3 puncta.
The GFP-LC3 puncta were counted and expressed as number of cells with >5 green puncta/cell, as described previously (22). GFP-LC3 puncta were scored in z-stack overlays from at least four separate fields with at least 100 nuclei, and autophagosome analysis was performed using a customized virtual basic Image-Pro macro.
Electron microscopy.
C2C12 cells in 2% horse serum DMEM were treated with 10 mM ammonium acetate for 6 h or ammonium acetate for 6 h with 100 μM chloroquine to block lysosomal degradation and a combination of chloroquine and ammonium acetate to examine autophagic flux. The results were compared with control untreated cells. After appropriate washing, cells were fixed in 2.5% glutaraldehyde-4% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.3, for 24 h. This was followed by postfixation with 1% osmium tetraoxide for 1 h. After en bloc staining and ethanol dehydration, samples were embedded with eponate 12 medium. Sections (85 nm) were cut using a diamond knife, followed by double staining using uranyl acetate and lead citrate. Electron microscopy was performed at the Imaging Core at the Lerner Research Institute at the Cleveland Clinic on a Philips CM12 electron microscope (FEI, Hillsboro, OR) operated at 10 kv. The autophagosome area was quantified in ≥10 cells/sample and expressed as a proportion of the total cytoplasmic area. The analysis was performed on a PC computer using the public domain NIH Image program (developed at the US National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Intracellular pH measurement.
The pH-sensitive dye BCECF was used for the recording of intracellular pH, using a spectrofluorometric method that measures the fluorescence intensity ratio of intracellular dye (24, 42). In brief, after differentiation for 48 h in six-well plates, cells were loaded with BCECF-AM (1 μM in DMSO). Probenecid, an inhibitor of organic anion transporter, was added to a final concentration of 2.5 mM to reduce leakage of free BCECF from the intracellular compartment. Cells were incubated for 30 min at 37°C with the dye in HEPES buffered balanced salt solution (130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 25 mM HEPES, 5 mM glucose, 0.1% bovine serum albumin, pH 7.5, ∼290 osmolality). Cells were then washed with calcium-balanced salt solution buffer, and the ratio of fluorescence with 440 (isobestic point for BCECF) and 480 nm excitation, 530-nm emission, was corrected for background fluorescence obtained from the cells without dye. Measurements were done at 37°C on a BioTek Synergy II plate reader (BioTek US, Winooski, VT). Response to ammonium acetate was compared with untreated control cells. After 20 min of incubation, cells were then washed with calcium-balanced salt solution to remove the extracellular ammonium acetate, and the fluorescence was recorded again for 10 min to demonstrate that removal of extracellular ammonium acetate resulted in the intracellular release of ammonia that diffuses out with subsequent reduction in intracellular pH. This showed that there was intracellular transport of ammonium ion. The calibration of BCECF was determined using the high K+/nigericin technique. Briefly, cells loaded with BCECF were transferred into calibration solutions containing 120 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 30 mM HEPES, and 10 nM nigericin (a K/H antiporter), with the extracellular pH adjusted to 7.4, 8.3, and 6.7 and the fluorescence read at 37°C for 4 min each. Additionally, 10 μM free BCECF in PBS was used to determine the fluorescence of buffer solutions ranging from pH 5.8 to pH 8.0. This was used to show that the extracellular pH measurements were precise to values of ±5%. Simultaneously, differentiated C2C12 cells were exposed to 10 mM ammonium chloride to demonstrate the anticipated changes in intracellular pH (4). All experiments were done in quadruplicate.
Quantification of nitrotyrosine and tyrosine in C2C12 cells.
Nitrotyrosine content in control and ammonium acetate-treated differentiated C2C12 cells was quantified by stable isotope dilution, LC-MS/MS, as described earlier (5). In brief, cells were washed with ice-cold PBS and harvested by trypsinization. After sonication of the cells, protein was precipitated and protein-bound 3-nitrotyrosine content in the lysates analyzed by HPLC with an on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) on a triple quadrupole mass spectrometer (API 365; Applied Biosystems, Foster City, CA) with an Ionics EP 10+ upgrade (Ionics; Concord, Ontario, CA) interfaced to a Cohesive Technologies (Franklin, MA) Aria LX Series HPLC multiplexing system. Synthetic [13C6]-labeled standard was used as an internal standard to quantify the natural abundance 3-nitrotyrosine. Universal label tyrosine [13C9,15N1] was used to quantify tyrosine and to monitor for the presence of potential artifactual nitration (which was <5% total), as monitored by the universally labeled 3-nitrotyrosine isotopologues. Results presented are normalized to the content of the precursor amino acid tyrosine measured during the same injection.
Statistical analysis.
All data are expressed as means ± SD unless specified. All experiments were done in triplicate. Qualitative variables were compared by the Chi squared test and quantitative and rating variables compared using the analysis of variance with SPSS 20.0 (IBM, Armonk, NY).
RESULTS
Autophagy is increased in skeletal muscle from cirrhotic patients.
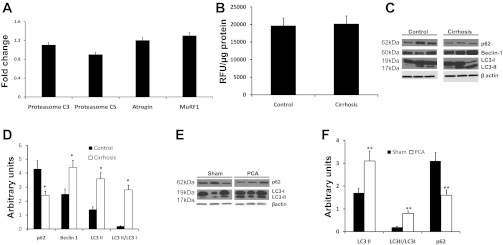
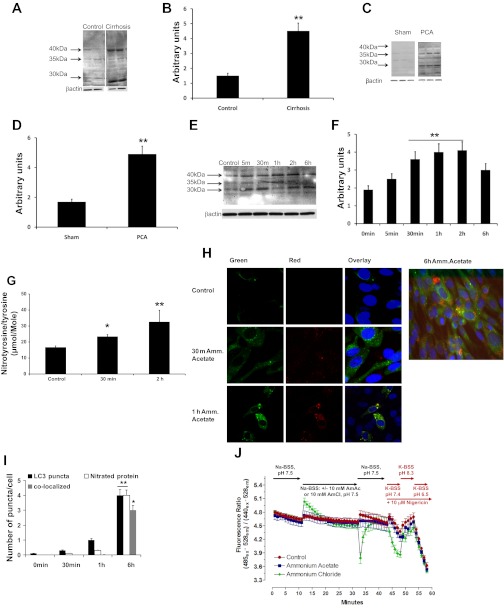
As mentioned earlier, since impaired protein synthesis alone is not adequate to result in a reduction in muscle mass, an increase in proteolysis is necessary for sarcopenia. An increase in ubiquitin-proteasome-mediated proteolysis has been reported in other disorders with significant loss of muscle mass (18). In the present study, components of the ubiquitin-proteasome pathway were quantified by real-time PCR (Fig. 1A), and no significant differences (P > 0.1) were observed between well-characterized patients with cirrhosis and controls. The 20S proteasome activity assay also did not show a difference (P > 0.1) between cirrhotics and controls (Fig. 1B). Since the ubiquitin-proteasome pathway was unaltered in cirrhosis, we examined skeletal muscle autophagic flux since it is another proteolytic pathway that is active during stress and starvation (2). Key components of this pathway include a component (beclin-1) of the phosphatidylinositol 3-kinase complex, a cytosolic protein (LC3-I) that is lipidated (LC3 II) to facilitate autophagosome membrane formation, and p62/SQSTM1 that targets cargo to the autophagosome for degradation. We measured autophagy using standard readouts, including LC3 lipidation, beclin-1 expression, and p62 degradation in cirrhotic patients. Expression of these autophagy markers was enhanced in the skeletal muscle of cirrhotic patients undergoing liver transplantation but not in noncirrhotic control subjects (Fig. 1C). Densitometric analysis from cirrhotics (n = 13) and controls (n = 13) showed significant increases in both LC3-II levels and the ratio of LC3-II/LC3-I, thus supporting increased autophagy in the muscle of patients with cirrhosis (Fig. 1D).
Fig. 1.
Skeletal muscle autophagy but not proteasome-mediated proteolysis is increased in cirrhosis and portosystemic shunting. A: real-time PCR quantification of critical components of the ubiquitin-proteasome pathway in the rectus abdominis skeletal muscle of patients with cirrhosis (n = 13) compared with controls (n = 13). No significant differences were observed between the 2 groups. B: proteasome 20S activity assay in skeletal muscle lysate from cirrhotics (n = 10) and controls (n = 10) showed no significant differences between the 2 groups. C: representative immunoblots performed on total protein extracted from skeletal muscle biopsies from cirrhotic patients (n = 13) and control subjects of p62, beclin-1, and LC3 lipidation (each lane represents a separate subject). D: densitometry with error bars of the blots normalized to β-actin. Increased LC3 lipidation, p62 degradation, and beclin-1 expression in cirrhosis compared with controls. *P < 0.01. E: representative immunoblots performed on total protein extracted from gastrocnemius muscle of portacaval anastamosis (PCA) rat and pair-fed sham-operated control rats showed increased LC3 lipidation and p62 degradation in sham control and PCA rats (each lane is a separate animal). F: densitometry with error bars for the blots normalized to β-actin. **P < 0.01 PCA compared with sham controls.
Hyperammonemia contributes to enhanced autophagy in PCA rat model.
The PCA rat is a well-characterized model of sarcopenia in cirrhosis with portosystemic shunting and is associated with hyperammonemia (9, 10, 12, 13). Plasma concentrations of ammonia were significantly (P < 0.01) elevated in the PCA compared with the sham rats (239.4 ± 63.1 μmol/l in PCA and 79.4 ± 36.2 μmol/l in sham-operated controls). Previously, we have reported unaltered ubiquitin-proteasome-mediated proteolysis in this model despite loss of muscle mass (10). Analysis of autophagy markers in the muscle of PCA and sham control rats showed significant increases in LC3-II lipidation and beclin-1 expression as well as p62 degradation (Fig. 1, E and F). Together with our studies in the muscle from human cirrhotics, these data support the idea that hyperammonemia mediates an increase in autophagy, contributing to the loss of muscle mass in cirrhosis.
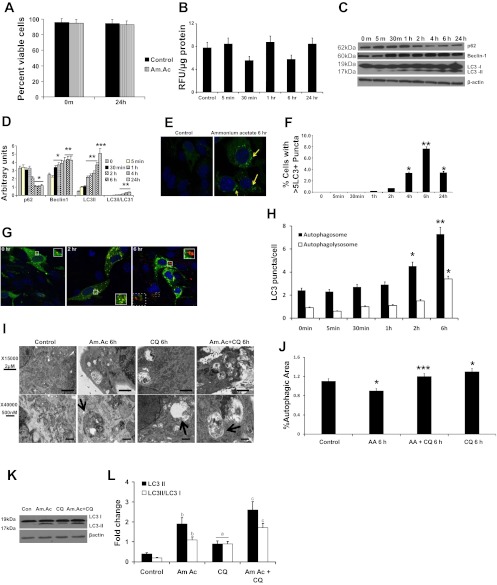
Hyperammonemia induces autophagy in C2C12 myotubes.
Cell viability quantified by the fluorescent assay (Promega) as well as by trypan blue exclusion consistently showed ≥94% cell viability in response to ammonium acetate (Fig. 2A). Similar to our observations in the muscle from human cirrhosis (Fig. 1B) and published data in the PCA rat (10), hyperammonemia did not alter the proteasome activity in the murine myotubes (Fig. 2B). To demonstrate that hyperammonemia mediates increased autophagy, C2C12 myotubes were treated with ammonium acetate for different times. A significant increase in beclin-1 expression and LC3 lipidation was observed by 1 h of treatment, and these were associated with p62 degradation (Fig. 2C). Densitometric analysis showed a significant increase in the LC3-II/LC3–1 ratio in response to hyperammonemia (Fig. 2D). Further analysis examined the formation of LC3+ vesicles in response to ammonium acetate in C2C12 cell lines stably expressing GFP-LC3. Hyperammonemia induced the time-dependent formation of GFP-LC3 positive vesicles (autophagosomes) in these cells, which peaked at 6 h (P = 0.03) (Fig. 2, E and F). To complement these observations, we demonstrated increased autophagy in C2C12 myotubes stably expressing the GFP-mCherry-LC3 tandem reporter. In these assays, the formation and maturation of autophagosomes can be monitored by the formation of dual fluorescent vesicles (autophagosomes), which mature through fusion with the lysosome. As the GFP-mCherry+ autophagosome matures and becomes acidified it leads to quenching of the GFP fluorescence, resulting in mCherry+ vesicles (Fig. 2G). Consistent with this process, we observed an initial increase in green puncta due to the formation of the autophagosome that transitioned to yellow (combination of red and green) and finally the red puncta, demonstrating the formation of the autophagolysosome during hyperammonemia (Fig. 2H).
Fig. 2.
Hyperammonemia induces autophagy in murine myotubes. Differentiated C2C12 murine myotubes incubated in ammonium acetate (Am.Ac) for different time points to measure autophagy response. A: cell viability showed >94% viable C2C12 murine myotubes exposed to Am.Ac. B: proteasome 20S activity assay in C2C12 murine myotubes showed no significant effect of hyperammonemia. C: representative immunoblots of p62 degradation, beclin-1 overexpression, and LC3 lipidation in response to 10 mM Am.Ac over time. D: densitometry for the blots normalized to β-actin is shown. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with controls. All experiments were done in triplicate. E: representative confocal images of immunofluorescence of green fluorescent protein (GFP)-LC3 stably transfected C2C12 murine myotubes treated with Am.Ac and controls. F: quantification of GFP-LC3+ cells with >5 puncta (means ± SE) showed significant increase at 4 h and beyond. *P < 0.05 and **P < 0.01 compared with controls. G: representative confocal images of time course of immunofluorescence of GFP-mCherry-LC3 tandem reporter transfected C2C12 murine myotubes treated with Am.Ac. Initial green fluorescence transforms to yellow (red + green) with activation of autophagy, and the quenching of the green fluorescence in the lysosomes results in red puncta demonstrating the autophagolyosome formation with subsequent degradation of the vesicular contents. All experiments were done in triplicate. H: quantification of yellow and red punctae in GFP-mCherry-LC3 transfected cells treated with Am.Ac. *P < 0.05 and **P < 0.01 compared with controls. I: representative electron microscopy images (×15,000 and ×40,000 magnification) of C2C12 murine myotubes treated with Am.Ac alone, chloroquine (CQ) alone, and Am.Ac and CQ for 6 h and controls. Arrows show the double-membrane-enclosed autophagosomes. J: autophagosome area as a proportion of the total cytoplasmic area was significantly higher in cells treated with Am.Ac compared with controls. Increased autophagic flux was demonstrated using CQ with Am.Ac. *P < 0.05 compared with controls; ***P < 0.01 compared with controls and P < 0.05 compared to CQ alone and Am.Ac alone. All studies were done in triplicate. MuRF1, muscle RING finger 1. K: representative immunoblots for LC3 lipidation in protein extracts from murine myotubes treated with Am.Ac alone, CQ alone, and Am.Ac and chloroquine. L: densitometry of the immunoblots showed increased autophagic flux with hyperammonemia. AA, autophagic area.
Finally, to further demonstrate formation and maturation of the autophagosomes in response to hyperammonemia and increased autophagic flux, electron microscopy was carried out. We observed formation of double-lamellar vesicles representing the autophagosomes in C2C12 cells during hyperammonemia (Fig. 2I). Increased autophagic area and flux were observed in response to ammonium acetate and a combination of ammonium acetate and chloroquine (Fig. 2J). Consistent with the electron microscopy data, immunoblots of cells treated with ammonium acetate and chloroquine also showed increased autophagic flux (Fig. 2, K and L). These studies demonstrate that autophagy is increased in response to hyperammonemia in muscle cells.
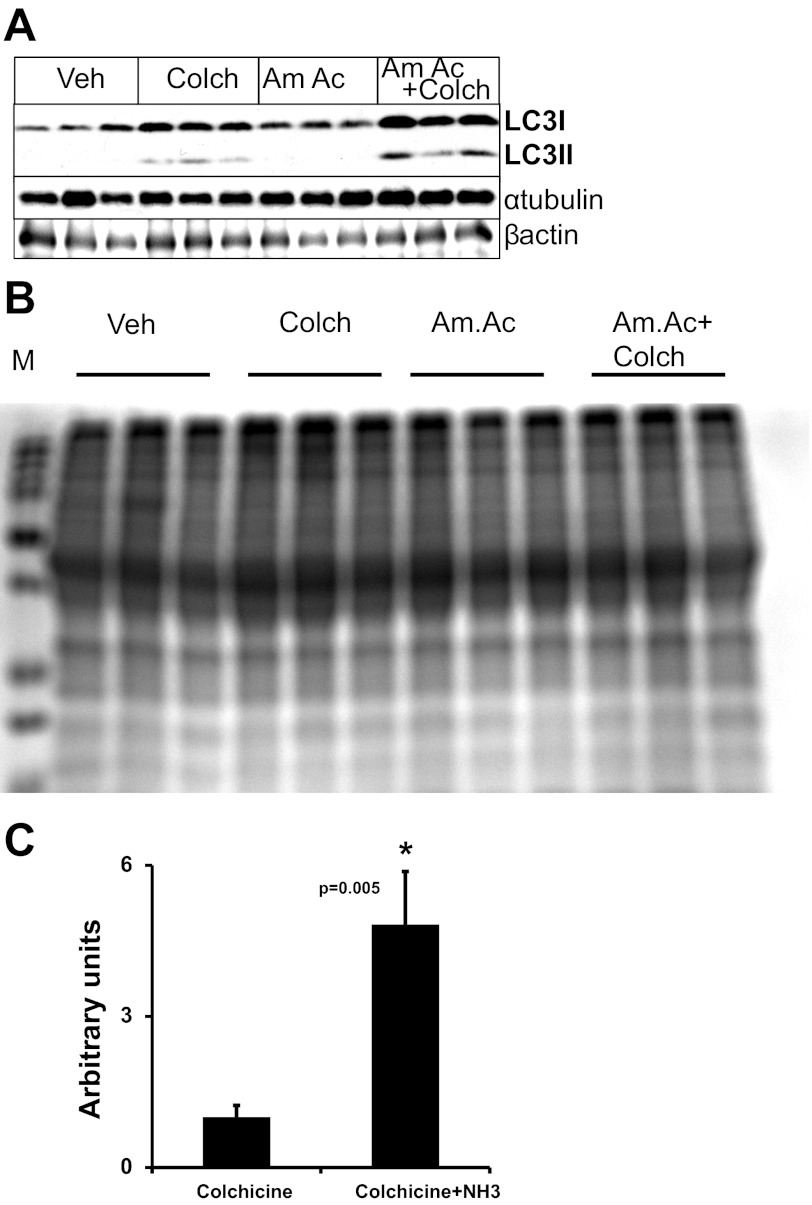
An in vivo autophagometer in mice measures autophagic flux in response to hyperammonemia.
Typically, in vivo autophagic studies are limited to static measurements of autophagy markers, which do not clearly distinguish between the accumulation of an autophagy marker because of an enhancement of autophagy activation or blockade of autophagic flux. Using a novel method for evaluating autophagic flux in animal models designed by Ju et al. (the “autophagometer”) (26), we examined whether hyperammonemia in mice activates autophagy in the skeletal muscle. In these studies, hyperammonemia was induced in mice by treatment with ammonium acetate intraperitoneally and autophagic flux determined by blocking lysosomal fusion with the autophagosome, using colchicine to inhibit degradation of autophagy proteins. Surprisingly, we observed only minimal LC3 lipidation in response to ammonium acetate alone. A potential explanation for this is that ammonium acetate increased autophagic flux, with a rapid degradation of LC3-II. To study autophagic flux, mice were treated with ammonium acetate for 7 days, followed by a 2-day treatment with intraperitoneal colchicine to block autophagy to measure the flux. Consistently, mice treated with ammonium acetate and colchicine showed a significant (P = 0.005) accumulation of LC3-II (Fig. 3, A–C). These studies demonstrated that hyperammonemia significantly increases autophagic flux in vivo.
Fig. 3.
Hyperammonemia induces skeletal muscle autophagic flux in vivo in mice. Hyperammonemia was induced in mice treated with 2.5 mmol·kg−1·day−1 Am.Ac for 7 days, followed by 0.4 mg colchicine (Colch)·kg−1·day−1 ip to block autophagy. This method permits us to quantify autophagic flux in vivo instead of only the static measurements of autophagy using immunoblots. Treatment of mice with Am.Ac enhances autophagic flux by ∼5× (n = 6 animals/treatment group). A: representative immunoblots. B: coumassie stain of the gel to demonstrate equal loading. C: densitometry data of LC3-II normalized to tubulin are shown. *P = 0.005 compared with controls. Veh, vehicle.
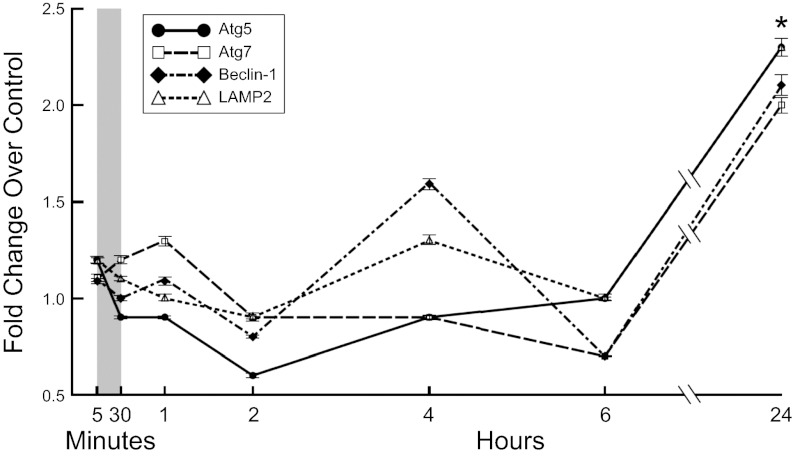
Increased skeletal muscle autophagy in hyperammonemia is not transcriptionally regulated.
To determine whether the increased autophagy during hyperammonemia is transcriptionally regulated, we carried out real-time PCR on critical genes that regulate different stages of autophagy, including ULK1, GABARAP, Atg5, Atg7, Atg4b, beclin-1, mVps34, and LAMP2. We observed a significant increase in the expression of beclin-1, Atg5, and Atg7 after 24 h of ammonium acetate treatment (Fig. 4). Similar changes were seen in the expression of ULK1, GABARAP, Atg4b, mVps34, and LAMP2 (data not shown). Because the transcriptional upregulation of these genes occurs after autophagy is induced by ammonium acetate, these data suggest that transcription of these genes is not required for initiation of autophagy, but rather, transcription is stimulated to replenish autophagy gene products that are depleted with increased autophagy in response to hyperammonemia.
Fig. 4.
Hyperammonemia induces delayed transcription of autophagy regulatory genes in murine myotubes. Fold change in expression of genes regulating autophagy and the ubiquitin proteasome components in differentiated murine C2C12 myotubes treated with Am.Ac. Ubiquitin proteasome components were not increased in response to hyperammonemia in differentiated C2C12 myotubes, whereas the autophagy genes Atg5 and -7, beclin-1, and LAMP2 were increased only at 24 h. All experiments were in triplicate. Gray vertical bar denotes the early time points in minutes. *P < 0.05 compared with controls.
Nitrated protein expression is increased in cirrhosis and colocalized to the autophagosomes.
In astrocytes, hyperammonemia has been reported to induce nitration of proteins with resultant structural and functional alterations (3, 39, 41), and removal of these modified proteins may require increased autophagic degradation. To determine the mechanism by which hyperammonemia induces autophagy, we quantified tyrosine nitration of proteins using immunoblots in the skeletal muscle from human cirrhosis and the PCA rat model as well as in the C2C12 myotubes exposed to hyperammonemia. We found increased levels of nitrated proteins by immunoblots in 1) the muscle from human cirrhosis compared with controls (Fig. 5, A and B), 2) the muscle of the hyperammonemic PCA rat compared with sham-operated controls (Fig. 5, C and D), and 3) differentiated C2C12 murine myotubes exposed to hyperammonemia (Fig. 5, E and F). Quantification of nitrotyrosine/tyrosine ratios using LC-MS/MS (49) also confirmed these observations that intracellular nitrotyrosine/tyrosine ratio was increased significantly in response to ammonium acetate (Fig. 5G). We also observed the colocalization of nitrated proteins with GFP-LC3+ vesicles by immunostaining with 3-nitrotyrosine antibody in C2C12 cells stably expressing GFP-LC3 when the cells were treated with ammonium acetate (Fig. 5, H and I). These consequences of hyperammonemia were not due to changes in intracellular pH since ammonium acetate did not result in a significant alteration in pH (Fig. 5J). Together, these data show that hyperammonemia induces nitration of proteins and targets them to the autophagosome.
Fig. 5.
Hyperammonemia increases tyrosine nitration of skeletal muscle proteins that localize to the autophagosome. Tyrosine nitration of muscle proteins was quantified on immunoblots. A: representative immunoblots from controls and cirrhotic patients (n = 12 each). B: densitometry of all of the bands in each lane showed significant increase (**P < 0.01) in nitration of muscle proteins in cirrhosis. C: representative immunoblots from PCA and sham rats (n = 6 each). D: densitometric quantification of all of the bands in each lane showed significant increase (**P < 0.01) in nitrated proteins in the muscle of the PCA rat compared with the sham-operated control animals. Murine C2C12 myotubes exposed to Am.Ac for different times (experiments in triplicate). E: representative immunoblots at different times probed using 3-nitrotyrosine antibody. F: densitometric quantification of all of the bands in each lane showed increased protein nitration by hyperammonemia compared with controls. **P < 0.01 compared with controls. G: significant increase in concentration of nitrotyrosine/tyrosine ratio in cell lysates in response to Am.Ac. *P < 0.01 compared with controls; **P < 0.001 compared with controls and P < 0.05 compared with 30-m sample. H: murine C2C12 myotubes stably transfected with GFP-LC3 were exposed to Am.Ac for different time points. Representative immunostain for 3-nitrotyrosine (red) in these GFP-LC3 stably transfected C2C12 myotubes showed an initial rapid red staining of nitrated proteins that subsequently begin to colocalize with green LC3 puncta in the autophagosomes (yellow). I: quantification of the colocalization of the nitrated proteins to the GFP-LC3+ puncta. *P < 0.01 and **P < 0.001 compared with control. J: no significant change in intracellular pH in murine myotubes exposed to Am.Ac compared with controls. Nigericin exposure in the presence of a high-potassium buffer with alteration of extracellular pH was used to calibrate the system and demonstrate the appropriate fluorescence responses. A separate set of C2C12 murine myotubes was exposed to 10 mM ammonium chloride to demonstrate the anticipated changes in intracellular pH that have been published by others (4) and are different from the response to Am.Ac used in the present studies. BSS, balanced salt solution.
DISCUSSION
Cirrhosis is a state of accelerated starvation and is associated with a number of metabolic perturbations, including increased blood ammonia concentration (47). The present studies provide a potential mechanistic link involving autophagy between two features of cirrhosis, hyperammonemia and sarcopenia. Autophagy not only serves to degrade dysfunctional proteins and damaged organelles but also provides essential nutrients during states of starvation (21). We show an increased skeletal muscle autophagy in cirrhosis using three different measurements (LC3 lipidation, beclin-1 expression, and p62 degradation). Similar observations were made in the skeletal muscle of the PCA rat, a model that has been used to examine the mechanisms of sarcopenia in cirrhosis with portosystemic shunting. A consistent biochemical abnormality in both of these systems is hyperammonemia, and studies using in vivo mice injected with both ammonium acetate and differentiated murine C2C12 myotubes demonstrated that hyperammonemia induces skeletal muscle autophagy.
Previously, we have reported that the ubiquitin-mediated proteolysis in the PCA rat was unaltered, and this was accompanied by impaired muscle protein synthesis (10). Consistently, in the present study, we show that in patients with cirrhosis expression of the ubiquitin-proteasome components was unaltered in the skeletal muscle. Our in vitro model of murine myotubes exposed to ammonium acetate also showed that the proteasome activity was not altered, whereas markers of autophagy were increased. Similarly, markers of autophagy were increased in the skeletal muscle of the PCA rat model of sarcopenia of cirrhosis with portosystemic shunting. However, studies on static models of autophagy have been identified to have significant limitations (26, 43). Therefore, we used an in vivo autophagometer mouse model to demonstrate that hyperammonemia increases autophagic flux in vivo. Autophagic flux was determined by blocking lysosomal fusion with the autophagosome, using colchicine to inhibit the degradation of the autophagy regulatory proteins. In these studies, we observed that hyperammonemia resulted in an about fivefold increase in autophagic flux. To place this in perspective, rapamycin increases autophagic flux about twofold, and starvation, a well-known inducer of muscle autophagy, increases autophagic flux threefold (25, 26). Our studies on autophagic flux in vivo in mice show that hyperammonemia is a potent inducer of skeletal muscle autophagic flux. These findings, combined with our supporting observations in differentiated C2C12 myotubes and our in vitro data, are consistent with our in vivo observations in cirrhotic patients and the PCA rat model. Thus, our studies suggest that the liver-muscle axis of disease in cirrhosis is mediated through hyperammonemia and stimulation of autophagy.
In cirrhosis, impaired hepatic disposal of ammonia results in its increased skeletal muscle uptake for disposal by nonureagenic pathways (34, 35). Ammonia increases tyrosine nitration of proteins in astrocytes, and nitration of proteins has been suggested to increase autophagy (20, 33, 41, 45). Hyperammonemia may induce skeletal muscle protein nitration, with the increase in autophagy responsible for removing the nitrated proteins. Our studies showed an increased global muscle protein tyrosine nitration in response to hyperammonemia. It was interesting that in the GFP-LC3-expressing cells exposed to hyperammonemia there was a relatively rapid induction of tyrosine nitration, followed by the formation of the green autophagosome puncta and subsequent entry of the nitrated proteins into the autophagosomes. Our studies also suggest that degradation of nitrated proteins may be one of the functions of autophagy in the skeletal muscle in cirrhosis and hyperammonemia. However, this interpretation must be made cautiously since it is not clear from our observations whether the tyrosine-nitrated proteins are responsible for inducing autophagy or whether the nitrated proteins are a consequence of the ammonia-induced nitration stress placed on these cells. Although others have reported that S-nitrosylation of cysteine residues in proteins induces autophagy (19), to the best of our knowledge this is the first report that hyperammonemia-induced tyrosine nitration is associated with increased skeletal muscle autophagy. Whether nitration of autophagy-specific proteins occurs and whether it alters their function is a possibility that needs to be explored in the future since nitration of proteins have been reported to impair their activity (3, 46).
Others have shown that ammonia induces autophagy in human cells as well as in mouse embryonic fibroblasts by a ULK1/2 independent mechanism (8, 16, 30). Hyperammonemia also induces nitration of proteins by inducing cell stress and may be responsible for increased autophagy in the skeletal muscle (14). Our studies showed that the effects of ammonium acetate were not due to the alteration in intracellular pH; rather, they were due directly to the ammonia on the induction of autophagy. Further studies examining the molecular signals required for hyperammonemia-induced autophagy in muscle will be useful in determining how to influence this process clinically.
Few studies have examined the transcriptional response of autophagy components (21, 27). Our systematic studies on the expression of mRNA of the autophagy genes showed that the initial increase in autophagy in response to hyperammonemia was not transcriptional. These observations are novel and appear to be unique to hyperammonemia-induced autophagy in muscle cells. Importantly, our observations suggest that the initial increase in autophagy is due to activation of the preexisting autophagy proteins. Delayed transcriptional upregulation of specific autophagy genes potentially occurs to replenish the proteins cleared by autophagy. Currently, the mechanism(s) responsible for the transcriptional upregulation of these genes during hyperammonemia are not known and are beyond the scope of the present studies.
Our observations show for the first time that skeletal muscle autophagy in cirrhosis is increased and suggest that the liver-muscle axis in cirrhosis is mediated by hyperammonemia. These observations suggest that studies on understanding the mechanisms by which hyperammonemia induces skeletal muscle autophagy will be of significant clinical relevance. Since ammonia lowering therapy is used in clinical practice (14, 31, 37), understanding autophagy in this context may provide novel strategies to reverse sarcopenia of cirrhosis.
GRANTS
S. Dasarathy was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants RO1-DK-083414 and UO1-DK-061732. A. Almasan was supported in part by the National Cancer Institute (CA127264) and K. Singh by a US Department of Defense (PC094405) Postdoctoral Training Award. C. McDonald was supported in part by NIDDK Grant RO1-DK-082437. C. C. Weihl was supported in part by National Institute on Aging Grant RO12-AG-031867 and the Muscular Dystrophy Association. S. L. Hazen was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-076491.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Q., C.T., C.C.W., C.M., A.A., S.V.N.P., and S.D. did the conception and design of the research; J.Q., C.T., S.T., A.N., C.C.W., J.K.C., B.E., K.S., X.F., G.R.D., C.M., A.A., S.L.H., and S.D. performed the experiments; J.Q., S.T., C.C.W., J.K.C., K.S., G.R.D., C.M., S.L.H., and S.D. analyzed the data; J.Q., C.T., A.N., C.C.W., B.E., K.S., X.F., G.R.D., C.M., A.A., S.L.H., S.V.N.P., and S.D. interpreted the results of the experiments; J.Q., S.T., C.C.W., J.K.C., K.S., G.R.D., C.M., and S.D. prepared the figures; J.Q., C.T., S.T., A.N., C.C.W., B.E., K.S., G.R.D., C.M., A.A., S.V.N.P., and S.D. edited and revised the manuscript; J.Q., S.T., A.N., C.C.W., J.K.C., B.E., K.S., X.F., G.R.D., C.M., A.A., S.L.H., and S.V.N.P. approved the final version of the manuscript; C.T., C.M., and S.D. drafted the manuscript.
REFERENCES
- 1. Ascoli M, Ward DN, Jirgensons B. On the optical activity of ionized tyrosyl residues in ovine lutropin. Eur J Biochem 72: 157–165, 1977 [DOI] [PubMed] [Google Scholar]
- 2. Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 37: 2098–2114, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bidmon HJ, Gorg B, Palomero-Gallagher N, Schleicher A, Haussinger D, Speckmann EJ, Zilles K. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia 49: 1733–1748, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Boron WF, De WP. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 277: 17415–17427, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bugianesi E, Kalhan S, Burkett E, Marchesini G, McCullough A. Quantification of gluconeogenesis in cirrhosis: response to glucagon. Gastroenterology 115: 1530–1540, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Cheong H, Lindsten T, Thompson CB. Autophagy and ammonia. Autophagy 8: 122–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci USA 108: 11121–11126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dasarathy S, Dodig M, Muc SM, Kalhan SC, McCullough AJ. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol 287: G1124–G1130, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, Kalhan SC. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol 54: 915–921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dasarathy S, Muc S, Hisamuddin K, Edmison JM, Dodig M, McCullough AJ, Kalhan SC. Altered expression of genes regulating skeletal muscle mass in the portacaval anastomosis rat. Am J Physiol Gastrointest Liver Physiol 292: G1105–G1113, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Dasarathy S, Muc S, Runkana A, Mullen KD, Kaminsky-Russ K, McCullough AJ. Alteration in body composition in the portacaval anastamosis rat is mediated by increased expression of myostatin. Am J Physiol Gastrointest Liver Physiol 301: G731–G738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dasarathy S, Mullen KD, Conjeevaram HS, Kaminsky-Russ K, Wills LA, McCullough AJ. Preservation of portal pressure improves growth and metabolic profile in the male portacaval-shunted rat. Dig Dis Sci 47: 1936–1942, 2002 [DOI] [PubMed] [Google Scholar]
- 13a. Dasarathy S, Yang Y, Runkana A, Prasad Sathyamangala V, McCullough AJ. Hyperammonemia induced myostatin mediated sarcopenia in cirrhosis is transcritpionally regulated by nuclear translocation of p65NFkB (Abstract). Hepatology 54: 713A 2011 [Google Scholar]
- 14. Ecker N, Mor A, Journo D, Abeliovich H. Induction of autophagic flux by amino acid deprivation is distinct from nitrogen starvation-induced macroautophagy. Autophagy 6: 879–890, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Eng CH, Abraham RT. Glutaminolysis yields a metabolic by-product that stimulates autophagy. Autophagy 6: 968–970, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal 3: ra31, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 211: 271–278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care 13: 225–229, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Haldar SM, Stamler JS. S-Nitrosylation at the interface of autophagy and disease. Mol Cell 43: 1–3, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Haussinger D, Schliess F. Astrocyte swelling and protein tyrosine nitration in hepatic encephalopathy. Neurochem Int 47: 64–70, 2005 [DOI] [PubMed] [Google Scholar]
- 21. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis. Gastroenterology 139: 1630–1641, 1641.e1–2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy 4: 322–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James-Kracke MR. Quick and accurate method to convert BCECF fluorescence to pHi: calibration in three different types of cell preparations. J Cell Physiol 151: 596–603, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Ju JS, Miller SE, Jackson E, Cadwell K, Piwnica-Worms D, Weihl CC. Quantitation of selective autophagic protein aggregate degradation in vitro and in vivo using luciferase reporters. Autophagy 5: 511–519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ju JS, Varadhachary AS, Miller SE, Weihl CC. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy 6: 929–935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimura N, Kumamoto T, Kawamura Y, Himeno T, Nakamura KI, Ueyama H, Arakawa R. Expression of autophagy-associated genes in skeletal muscle: an experimental model of chloroquine-induced myopathy. Pathobiology 74: 169–176, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del PP, Burden SJ, Di LR, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4: 524–526, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Mariño G, Kroemer G. Ammonia: a diffusible factor released by proliferating cells that induces autophagy. Sci Signal 3: pe19, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Meijer AJ. Amino acid regulation of autophagosome formation. Methods Mol Biol 445: 89–109, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 10: 166–173, 173.e1, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Oberley TD, Swanlund JM, Zhang HJ, Kregel KC. Aging results in increased autophagy of mitochondria and protein nitration in rat hepatocytes following heat stress. J Histochem Cytochem 56: 615–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olde Damink SW, Deutz NE, Dejong CH, Soeters PB, Jalan R. Interorgan ammonia metabolism in liver failure. Neurochem Int 41: 177–188, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis 24: 169–181, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis 16: 95–131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Phongsamran PV, Kim JW, Cupo AJ, Rosenblatt A. Pharmacotherapy for hepatic encephalopathy. Drugs 70: 1131–1148, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Quint P, Reutzel R, Mikulski R, McKenna R, Silverman DN. Crystal structure of nitrated human manganese superoxide dismutase: mechanism of inactivation. Free Radic Biol Med 40: 453–458, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Rasband WS. ImageJ [Online] Bethesda, MD: US National Institutes of Health; http://imagej.nih.gov/ij/ [2012] [Google Scholar]
- 41. Schliess F, Gorg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Haussinger D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J 16: 739–741, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Shimada Y, Kobayashi H, Kawagoe S, Aoki K, Kaneshiro E, Shimizu H, Eto Y, Ida H, Ohashi T. Endoplasmic reticulum stress induces autophagy through activation of p38 MAPK in fibroblasts from Pompe disease patients carrying c.546G>T mutation. Mol Genet Metab 104: 566–573, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Singh K, Matsuyama S, Drazba JA, Almasan A. Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress. Autophagy 8: 236–251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nuñez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog 3: e111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swanlund JM, Kregel KC, Oberley TD. Autophagy following heat stress: the role of aging and protein nitration. Autophagy 4: 936–939, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takakusa H, Mohar I, Kavanagh TJ, Kelly EJ, Kaspera R, Nelson SD. Protein tyrosine nitration of mitochondrial carbamoyl phosphate synthetase 1 and its functional consequences. Biochem Biophys Res Commun 420: 54–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 27: 430–441, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Tsien CD, Shah S, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after transjugular intrahepatic portosystemic stent. Eur J Gastroenterol. In press [DOI] [PubMed] [Google Scholar]
- 49. Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, 3rd, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol 14: 861–868, 2007 [DOI] [PubMed] [Google Scholar]