Abstract
Vertical sleeve gastrectomy (VSG) is a restrictive procedure that reduces food intake to produce weight loss. Here we assess volume and nutrient effects on the ingestive behavior of VSG and sham surgery animals. Rats given access to Ensure or pelleted chow were used to determine if liquid foods would adversely affect weight loss after surgery. Volume effects were studied by altering the caloric density of Ensure, and dietary preferences for fat and carbohydrate (sucrose) were assessed using a two-bottle test. c-Fos was used to measure neuronal activation in the nucleus of the solitary tract and area postrema in response to intragastric infusions of water, sucrose, or Intralipid. The degree of colocalization with catecholaminergic neurons was also assessed. VSG rats did not show the expected preference for a liquid diet over chow and lacked dietary preferences for fat seen in shams. Preferences for carbohydrate/sucrose solutions were unaffected by surgery. Meal size was reduced by VSG; however, VSG rats were able to alter their volume of intake to compensate for changes in caloric density, and intragastric infusions of water produced similar levels of neuronal activation among VSG, sham, and pair-fed rats. In comparison, nutrient-induced c-Fos activation was substantially increased by VSG. Colocalization between c-Fos and catecholaminergic-expressing neurons was similar among rats treated with water, sucrose, or Intralipid. VSG alters nutrient sensing in a manner that lowers the threshold for satiety and reduces fat preference to induce and maintain weight loss.
Keywords: bariatric surgery, c-Fos, fat preference, nucleus of the solitary tract
bariatric surgery is currently the most effective treatment for obesity and many obesity-related comorbidities (9). In purely restrictive procedures, such as vertical-band gastroplasty and the adjustable gastric band, a restrictive pouch is created in the forestomach that limits meal size to induce weight loss. In other procedures, such as Roux-en-y gastric bypass surgery (RYGB), restriction is combined with malabsorption and changes in nutrient delivery. Compared with banding, RYGB results in superior weight loss and significant changes in dietary preferences, including a reduced preference for foods that are high in fat (22) and the avoidance of high-calorie liquids, milk, ice cream, and other sweets (7, 8, 13, 18, 22, 33, 34). In other restrictive procedures, postsurgical increases in the consumption of high-energy soft foods and liquids is associated with poorer-than-expected weight loss (7, 8, 38). Because both banding procedures and RYGB cause considerable gastric restriction, the superior effect of RYGB surgery on food choice (7, 8, 13, 15, 38) has been hypothesized to result from surgical alterations of the gastrointestinal tract.
More recently, a novel bariatric procedure, the vertical sleeve gastrectomy (VSG), has emerged as a third alternative. In VSG, the majority of the stomach is resected along the greater curvature leaving a tubular gastric remnant of reduced size, but intestinal anatomy is left intact. In several small clinical studies, VSG was found to improve glucose tolerance and produce similar weight loss as RYGB surgery (12, 15, 17, 21, 26–28, 32). However, the mechanism by which VSG exerts these metabolic benefits is not clear, and there has been some debate as to whether it is simply another restrictive procedure.
The aim of the present study was to determine if VSG alters dietary preferences in a way we would expect based on other purely restrictive procedures. Preferences for a liquid diet (Ensure) over pelleted chow, as well as preferences for carbohydrate and fat, were used to determine how VSG alters food choice. Volume effects were studied in rats given access to two different caloric densities of Ensure, and c-Fos was used to measure the degree of neuronal activation produced by an intragastric infusion of water, sucrose, or Intralipid. Because catecholaminergic neurons in the brain stem respond markedly to distention, the degree of colocalization of c-Fos and dopamine β-hyroxylase (DBH) was assessed in each treatment.
METHODS
Animals.
Adult male Long-Evans rats (n = 96, 250–300 g) (Harlan Laboratories, Indianapolis, IN) were individually housed and maintained in a room on a 12:12-h light-dark cycle (lights off at 1400) at 25°C and 50–60% humidity. All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Before surgery, rats were given ad libitum access to water and a high-fat butter diet (HFD, 4.54 kcal/g; 41% fat; Research Diets, New Brunswick, NJ) previously documented to produce metabolic impairments (42). After 8 wk on the HFD, the rats were assigned to surgical groups (RYGB, VSG, ad libitum-fed sham, or sham-operated pair fed) that were counterbalanced based for lean and fat tissue mass.
Surgery.
VSG was performed as previously described (12, 36). A laparotomy was made, and ∼80% of the stomach, including all of the fundus and a portion of the antrum, was excised along the greater curvature using an ETS 35-mm staple gun. The remaining gastric sleeve was reintegrated into the abdominal cavity, and the laparotomy was closed in layers. In the VSG sham procedure, the stomach was isolated in the same way, but no cut was made. Subcutaneous injections of Buprenex (0.3 ml), Metacam (0.25 mg/100 g body wt), saline (10 ml), and gentamicin were given on the day of surgery. Buprenex and saline (5–10 ml) were given twice daily over the next 2–3 days as needed.
Food intake, body weight, and body composition.
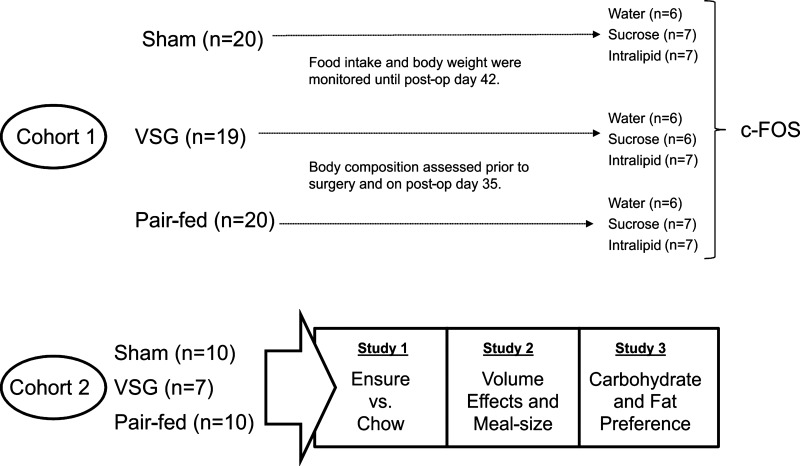
Body composition was assessed using an EchoMRI analyzer (Houston, TX). Beginning 3 days preoperatively, the high-fat diet was replaced with Ensure Plus liquid diet (Abbott Nutrition, Columbus, OH). Two cohorts of rats were used. In the first, the HFD was returned on postoperative day 3. This cohort was used to assess the early effect of the surgery on food intake and body weight, as well as on nutrient-induced c-Fos activation in the brain stem. Sham-operated rats in the pair-fed group were given access to the amount of food eaten by the VSG animals on the previous day. In a second cohort, rats were maintained on Ensure except where indicated. These animals had previously been used in another study that examined the effect of VSG on blood glucose parameters (12); part of the food intake data in that manuscript (postoperative days 100–120) overlap with data reported in the present manuscript. The experimental progression of each cohort is outlined in Fig. 1.
Fig. 1.
The two cohorts of rats used in the present study and details of the experiments each group was assigned to. VSG, vertical sleeve gastrectomy.
Liquid vs. solid diet.
To determine if liquid diets negatively affect weight loss after VSG, we assessed preferences for Ensure (1.41 kcal/g; fat 29%, carbohydrate 56%, protein 15%) relative to solid chow (3.1 kcal/g; fat 17%, carbohydrate 58%, protein 25%). On postoperative day 123, sham and VSG rats were given simultaneous access to Ensure Plus liquid diet and a standard pelleted rat chow. Rats were allowed to acclimate to the presence of both diets for 4 days. The ratio of Ensure/chow consumed over the last 3 days was averaged as an indication of dietary preference. The long-term effect of consuming each diet separately was studied on postoperative days 100–129 and 183–210 for the Ensure diet and on postoperative days 130–182 for the chow diet.
Volume effects and meal size after VSG.
Six-hour-fasted rats were given access to Ensure Plus or Ensure Plus diluted with water (1:2) for 60 min via a repeated-measures design that was counterbalanced across days. Thus, rats were already familiar with this diet, and the only adjustment made by the animals was in response to the different caloric densities of the Ensure. The volume of intake and kilocalories were used to indicate the effect of restriction on meal size. Sham rats, and sham-operated pair-fed rats, were used for comparison.
Carbohydrate and fat preference.
Beginning at 1000, food was replaced with a bottle containing plain water (control), as well as a test bottle containing various concentrations of sucrose (0.1–30%). The intake of each bottle was monitored over the next 4 h. Increasing concentrations of sucrose were presented over consecutive days. Preference is indicated by the relative consumption of sucrose over the control bottle, i.e., >50% of the control value. To determine if VSG alters dietary preferences for fat, the experiment was repeated 1 wk later using 0.2% methyl cellulose and various concentrations of corn oil (1–100%).
Water- and nutrient-induced c-Fos activation.
Six-hour-fasted VSG, sham, or sham-operated pair-fed rats were gavaged with isocaloric amounts (4.0 kcal) of sucrose (50%), Intralipid (20%), or water delivered in a volume of 2 ml. The volume and the caloric load were based upon the mean 15-min food intake by rats following a 6-h fast. Two hours later, rats were given a lethal dose of phenobarbitol and perfused with saline followed by 4% paraformaldehyde. Brains were rapidly removed, postfixed overnight at 4°C, washed 3 × 10 min in PBS, and stored until further processing.
Immunohistochemistry.
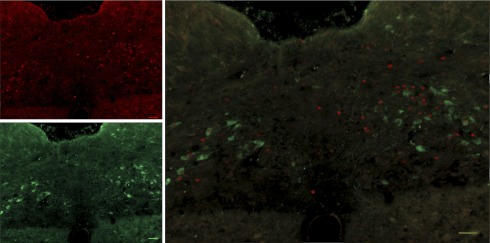
The brain stem was removed and placed into cryoprotectant (30% sucrose in PBS) overnight. The next day, serial 35-μm floating sections were cut using a cryostat beginning −13.3 mm and ending −14.0 mm caudal to bregma. Sections were stored at 4°C in PBS until representative sections from the rostral (−13.3 mm), mid (−13.6 mm), and caudal (−14.0 mm) nucleus of the solitary tract (NTS) were selected and stained for c-Fos. Free-floating sections were incubated for 20 min in 1% H2O2 in methanol solution before being washed 5 × 5 min in PBS + Triton (0.1%) and placed into 10% normal donkey serum for 1 h. Sections were then incubated in c-Fos primary antibody (1:5,000, SC-52 rabbit polyclonal antibody) (Santa Cruz Biotechnology) diluted in blocking solution overnight at 4°C before being placed into a secondary antibody (Biotinylated donkey α-rabbit; 1:200) for 1 h at room temperature. Sections were then placed into aviden-biotynlated complex (Vector ABC) 1 h before being exposed to a nickel-diaminobenzidine (DAB) solution for 8 min. Sections were rinsed 3 × 10 min in between each stage with PBS and washed 5 × 10 min in PBS sections after undergoing DAB. Sections were mounted onto Superfrost slides and covered with a cover slip, and areas of interest were photographed at ×5 using a Zeiss 510 Meta microscope under white light. Bilateral counts were made using Axiovision4.4 software under identical conditions by persons blind to each treatment. Representative unilateral sections of the NTS and area postrema (AP) of rats treated with Intralipid were photographed at ×10 magnification and presented in Fig. 7. Cumulative totals were used to indicate c-Fos activation in the NTS, and c-Fos expression in the AP (bregma −13.6 mm) was counted separately. In a second experiment, dual labeling was used to determine if c-Fos (1:1,000) colocalized with DBH (1:1,000) (MAB308; Chemicon)-expressing neurons. Sections were blocked for 1 h in 5% normal donkey serum before being incubated overnight at 4°C in both antibodies. The following day, sections were rinsed and incubated at room temperature in donkey anti-rabbit CY3 (1:100) and donkey anti-mouse fluorescein isothiocyanate (1:100) (Jackson ImmunoResearch) antibodies on an orbital shaker for 1 h at room temperature. Unilateral sections were visualized as before and photographed under 550 and 488 nm light under ×10 magnification. c-Fos- and DBH-expressing neurons and neurons expressing both were counted by hand.
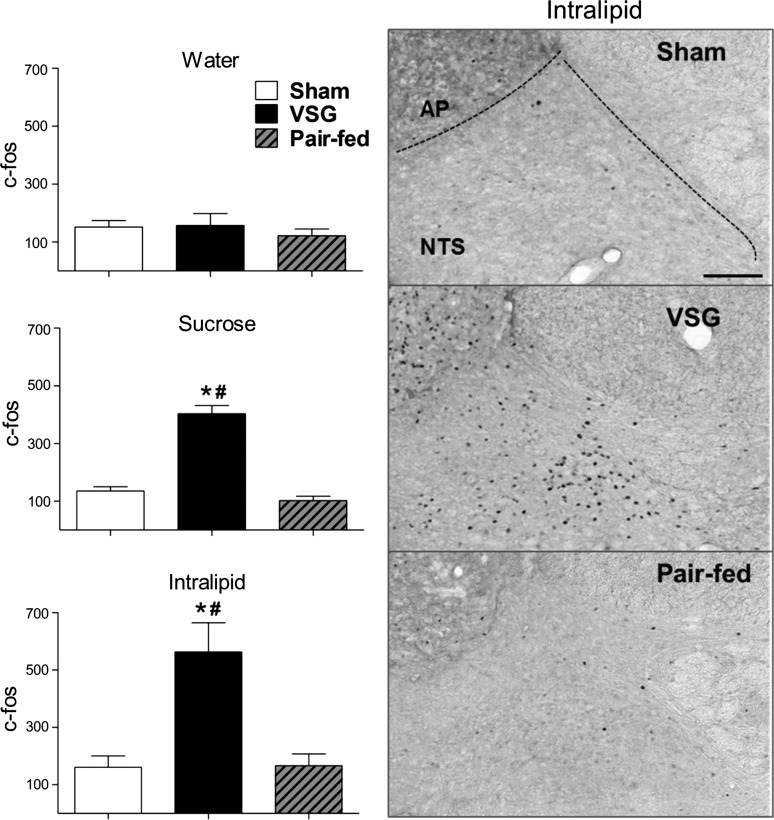
Fig. 7.
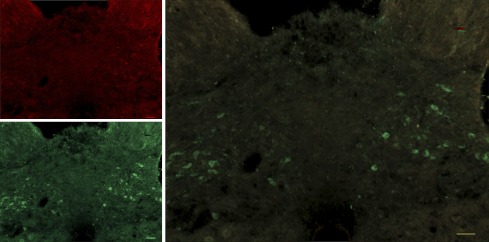
Left, quantification of c-Fos induced by water (2 ml), sucrose (4 kcal), or Intralipid (4 kcal). c-Fos levels were similar among groups treated with water but significantly higher in VSG rats treated with sucrose or Intralipid, P < 0.05. Right, micrographs (x10) showing c-Fos in the nucleus of the solitary tract (NTS) and area postrema (AP) of sham, VSG, and pair-fed rats treated with Intralipid (4 kcal). Differences between treatments in sham and pair-fed rats were nonsignificant, P > 0.05, n = 6–7/group. *P < 0.05 compared with sham. #P < 0.05 compared with pair fed (scale bar = 100 μm).
Statistics.
Data were analyzed using one-way and two-way independent and repeated-measures ANOVAs where appropriate and expressed as means ± SE. Differences between treatments were followed up using Bonferroni's Multiple Comparisons test, with the α-level set at P < 0.05.
RESULTS
Food intake, body weight, and body composition.
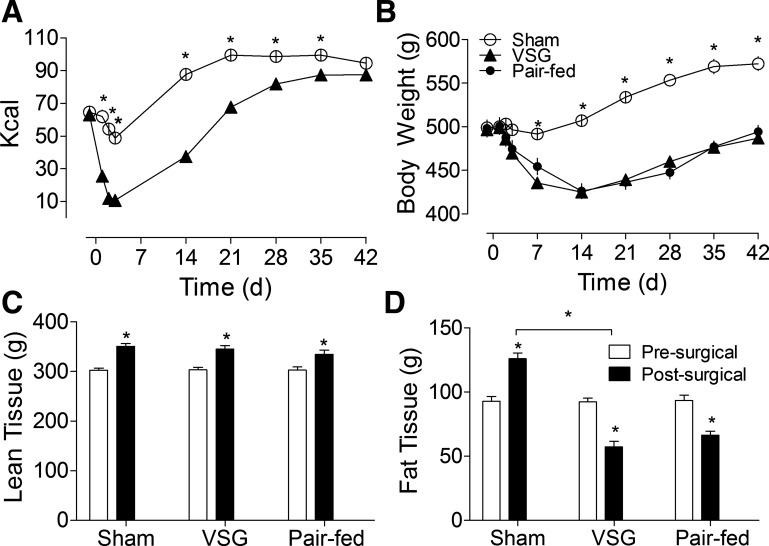
Daily food intake was significantly reduced in VSG rats relative to sham-operated controls for the first several weeks after surgery (P < 0.05; Fig. 2A), and pair feeding produced comparable weight loss (Fig. 2B). As food intake gradually approached that of sham-operated animals, differences in body weight among treatments stabilized. At this time, sham-operated rats were in an anabolic rather than a stable metabolic state. By 5 wk after surgery, lean tissue mass had increased to a similar extent in each group (P < 0.05, Bonferroni repeated-measures posttest; Fig. 3C), whereas postsurgical fat mass was reduced by approximately half in VSG and pair-fed animals relative to ad libitum-fed sham rats (P < 0.05).
Fig. 2.
A: mean daily food intake (kcal) of sham (open circles, n = 20) and VSG (filled triangles, n = 19) rats before and after surgery. Food intake was significantly reduced by VSG rats up until postoperative day 35 (P < 0.05). B: body weight of VSG, pair-fed (filled circles, n = 20), and ad libitum-fed sham animals over the same period (*P < 0.05). C: lean tissue mass 3 days before (open bars) and 4 wk after (filled bars) surgery. D: fat mass before and after surgery (*P < 0.05). Differences between VSG and pair-fed animals were nonsignificant, P > 0.05.
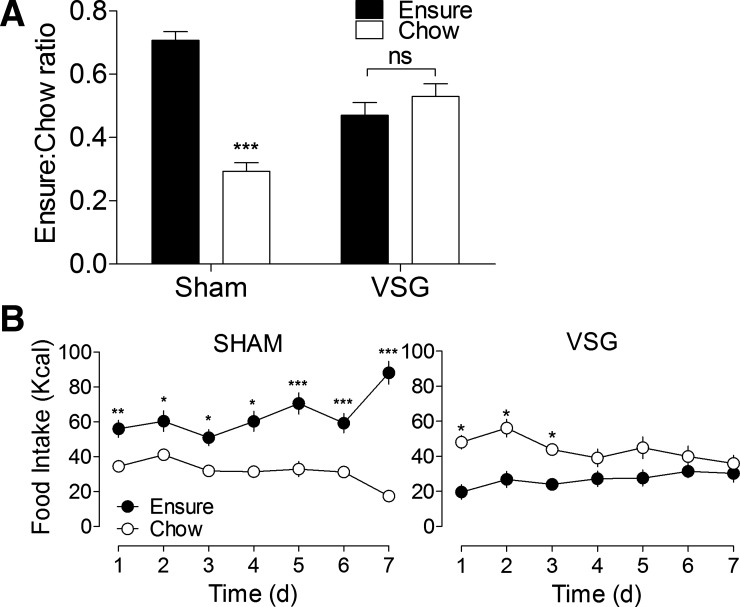
Fig. 3.
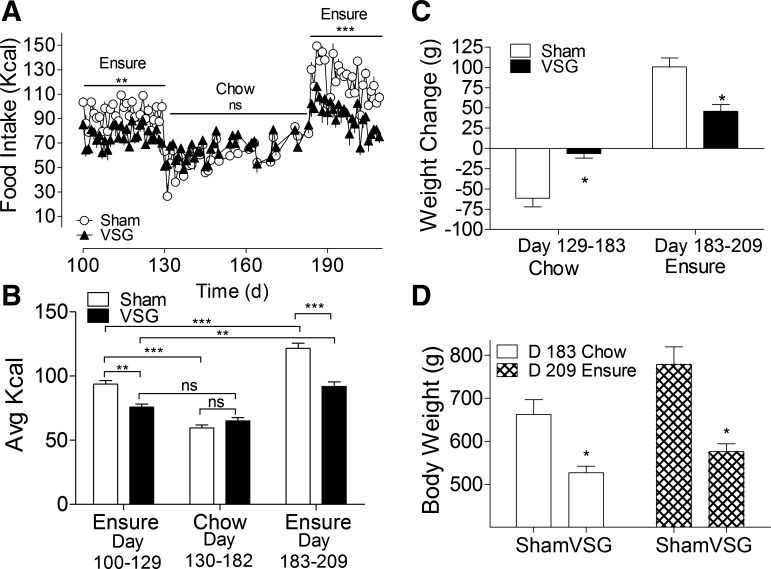
A: the ratio of Ensure liquid diet (filled bars) to solid rat chow (open bars) consumed over the last 3 days of a food preference study. Sham-operated rats (n = 10) consumed more of their daily calories from Ensure, as expected (P < 0.001). However, contrary to our initial hypothesis, VSG rats (n = 7) showed no preference for the liquid diet over pelleted chow (P > 0.05). ns, Not significant. B: daily food intake shows that sham-operated rats exhibited this preference throughout the experiment, whereas VSG rats initially preferred chow to Ensure (P < 0.05), before eventually consuming similar amounts of each diet (P > 0.05). *P < 0.05, **P < 0.01, and ***P < 0.001.
Liquid diet vs. solid chow diet.
At the time of the diet preference study (postoperative day 100), sham rats weighed more than VSG rats (699 ± 23 vs. 525 ± 17 g, P < 0.05). VSG rats lacked the baseline preference for the palatable liquid diet that was apparent in sham rats (P < 0.001; Fig. 3, A and B) and consumed a similar number of calories from each diet during the final 3 days of the study (P > 0.05; Fig. 3A). Results from the second half of the study, in which the effect of each diet on long-term food intake and body weight was assessed (Fig. 4, A–D), show that exposure to a liquid diet does not adversely affect weight loss after VSG. Sham-operated rats ate significantly more liquid diet than VSG rats (P < 0.05), whereas daily food intake on chow was similar among groups (P > 0.05).
Fig. 4.
A and B: daily food intake in rats maintained on Ensure liquid diet (days 100–129), chow diet (days 130–183), and then Ensure again (days 183–209). On the Ensure diet, average daily food intake was significantly lower in VSG (n = 5) rats (filled triangles) relative to sham (n = 8) (open circles) (P < 0.05). In animals fed solid chow, differences in food intake were nonsignificant (P > 0.05). C: sham-operated rats (open bars) lost more weight on chow (P < 0.05) and were more susceptible to diet-induced weight gain on the Ensure diet than VSG rats (filled bars, P < 0.05). D: differences in body weight between sham and VSG rats were maintained on each diet, P < 0.05. D, day. *P < 0.05, **P < 0.01, and ***P < 0.001.
Volume effects and meal size.
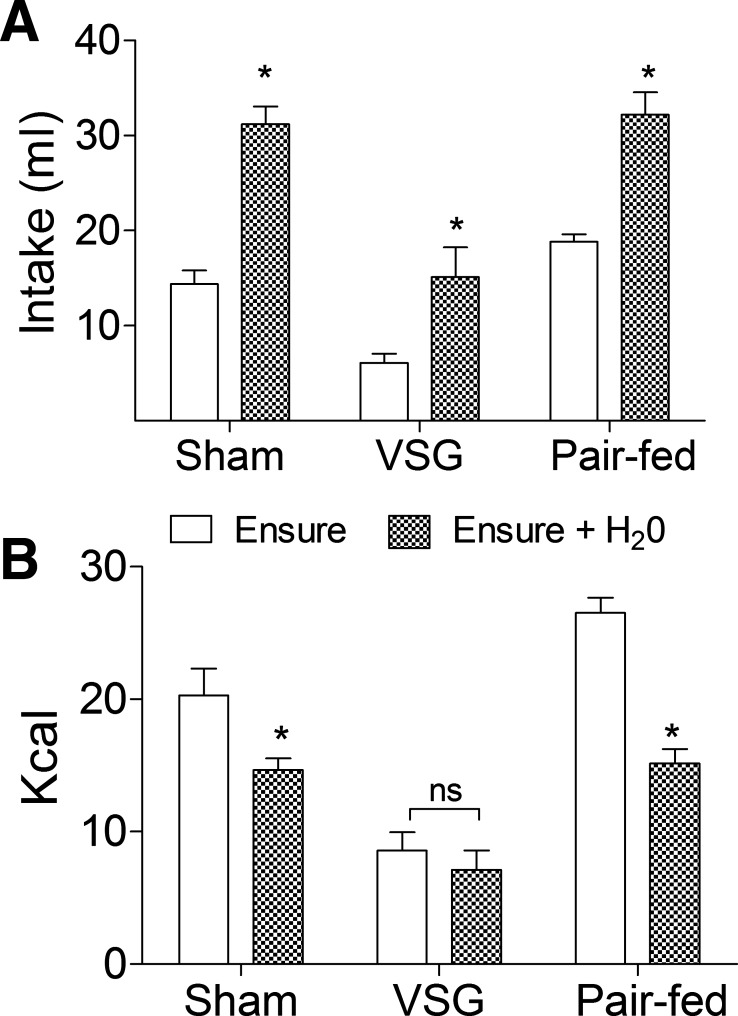
To test the hypothesis that meal size was directly altered by mechanical restriction, 6-h-fasted rats were given access to Ensure or Ensure diluted with water (Fig. 5A). Each group compensated for reductions in caloric density by increasing their volume of intake (P < 0.05; Fig. 5A). VSG rats were able to maintain the same caloric intake in each condition (P > 0.05; Fig. 5B), whereas ad libitum and pair-fed sham rats were unable to fully compensate when exposed to the diluted diet (P < 0.05; Fig. 5B), implying that they could not accommodate the volume necessary to make up for reductions in caloric density. The results demonstrate that rats are able to increase the volume of nutrients ingested when necessary for weight maintenance after VSG and imply that caloric density, rather than gastric restriction per se, may be the primary determinant of meal size following VSG.
Fig. 5.
A: rats compensated for a decrease in calories by volume by increasing their volume of intake during the Ensure + H2O condition (hatched bars) compared with Ensure alone (open bars), P < 0.05. B: VSG rats (n = 7) compensated almost perfectly by eating a similar amount of calories in each condition, P > 0.05. Ad libitum (n = 10) and pair-fed (n = 10) rats increased their volume of intake in the Ensure + H2O condition but ate significantly fewer calories compared with Ensure alone, P < 0.05. *P < 0.05 compared with Ensure.
Carbohydrate and fat preference.
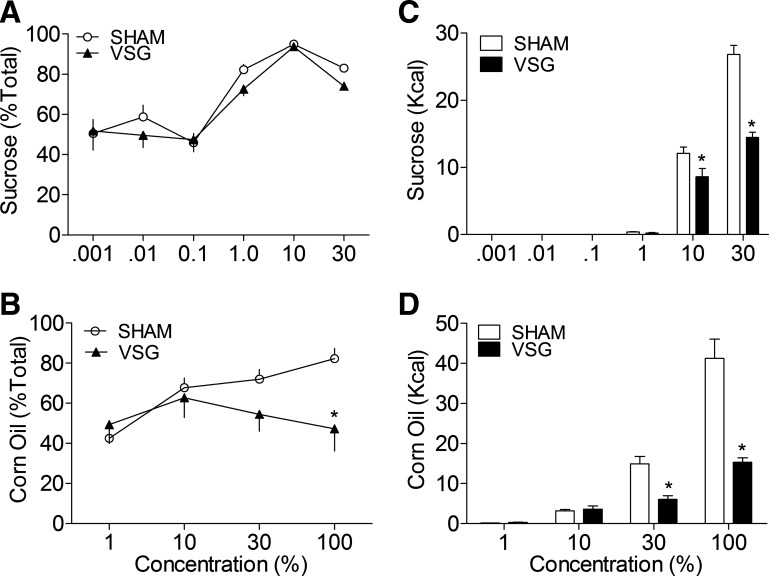
The effect of VSG on dietary preferences for sucrose and Intralipid are shown in Fig. 6, A and B. Differences in sucrose preference among groups were nonsignificant (P > 0.05). At concentrations of 1% or higher, VSG and sham-operated rats consumed significantly more sucrose relative to the control bottle (P < 0.05). Figure 6B shows that, in sham-operated rats, preferences for corn oil also increased in a concentration-dependent manner (P < 0.05). Remarkably, however, VSG rats ate similar amounts from each bottle at each concentration tested (P > 0.05), indicating that dietary preferences for fat were abolished by VSG.
Fig. 6.
Preferences for sucrose were unaltered by VSG (filled triangles, n = 7, P < 0.05) (A); however, preferences for corn oil were significantly reduced compared with ad libitum sham rats (open circles, n = 10, P < 0.05) (B). At the highest concentrations tested, the total intake (kcal) of both sucrose (C) and corn oil (D) was also reduced by VSG (filled bars) compared with sham rats (open bars), P < 0.05. *P < 0.05 compared with sham.
Nutrient-induced c-Fos activation.
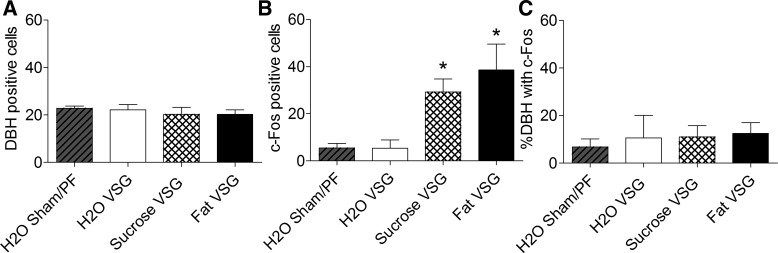
To assess neuronal activation in areas of the brain that have been implicated in satiety, c-Fos activation in response to distention induced by water vs. carbohydrate (sucrose) or fat (Intralipid) was studied in sham, VSG, and sham-operated pair-fed rats (Fig. 7). The volume and number of calories gavaged were consistent across the lipid and carbohydrate conditions and were based upon the mean voluntary intake of Ensure in VSG rats following 6 h of fasting. c-Fos expression was similar among groups in rats administered water intragastically (P > 0.05). However, sucrose and Intralipid induced significantly more c-Fos-like immunoreactivity in the NTS and AP of VSG rats (P < 0.05), implying that VSG enhanced the sensitivity to nutrients, but not to simply volume effects. Dual labeling with an antibody raised against DBH showed that c-Fos-expressing neurons were infrequently colocalized with catecholaminergic neurons (P < 0.05; Figs. 8 and 9). The number of DBH-containing neurons was similar across groups and conditions (P > 0.05), and increases in c-Fos expression in VSG rats given intragastric infusions of sucrose and Intralipid did not result in a higher percentage of c-Fos colocalization with DBH-expressing neurons (P > 0.05; Fig. 10). In ad libitum and pair-fed sham rats, the caloric content of the sucrose and Intralipid was insufficient to induce significantly more c-Fos than water alone (P > 0.05; Fig. 10). Table 1 summarizes levels of c-Fos activation in all regions studied.
Fig. 8.
Micrograph (x10) showing c-Fos (red)- and dopamine β-hydroxylase (DBH) (green)-expressing neurons in the NTS just rostral to obex in a VSG rat given an intragastric infusion of water (scale bar 100 μm).
Fig. 9.
Micrograph (x10) showing c-Fos (red)- and DBH (green)-expressing neurons in the NTS just rostral to obex in a VSG rat given an intragastric infusion of sucrose (scale bar 100 μm).
Fig. 10.
The number of DBH-positive (A) and c-Fos-positive (B) cells and the percentage of DBH-containing neurons expressing c-Fos (C) in sham and pair-fed (PF) rats given an intragastric infusion of water (n = 3/3) and VSG rats given an intragastric infusion of water (n = 5), sucrose (n = 5), or Intralipid (n = 5). *P < 0.05 compared with rats given water.
Table 1.
The expression of c-Fos in different regions of the NTS and AP following exposure to water, sucrose, or Intralipid
| Water | Sucrose | Intralipid | |
|---|---|---|---|
| Sham | |||
| R NTS | 52 ± 11 | 42 ± 7 | 57 ± 17 |
| C NTS | 46 ± 11 | 47 ± 8 | 36 ± 7 |
| M NTS | 25 ± 7 | 29 ± 4 | 40 ± 11 |
| AP | 22 ± 5 | 19 ± 4 | 19 ± 4 |
| VSG | |||
| R NTS | 44 ± 16 | 100 ± 18*# | 127 ± 24*# |
| C NTS | 45 ± 13 | 105 ± 9*# | 157 ± 27*# |
| M NTS | 47 ± 11 | 124 ± 8*# | 167 ± 40*# |
| AP | 10 ± 3 | 58 ± 6*# | 95 ± 13*# |
| Pair fed | |||
| R NTS | 55 ± 15 | 41 ± 5 | 56 ± 15 |
| C NTS | 26 ± 6 | 27 ± 4 | 43 ± 12 |
| M NTS | 20 ± 4 | 22 ± 3 | 49 ± 19 |
| AP | 13 ± 4 | 10 ± 3 | 12 ± 3 |
Values are means ± SE. NTS, nucleus of the solitary tract; AP, area postrema; R, rostral; C, caudal; M, medial; VSG, vertical sleeve gastrectomy.
P < 0.05 compared with sham.
P < 0.05 compared with pair fed.
DISCUSSION
We used a rat model of VSG to study how meal-related stimuli are processed after surgery relative to animals that remain obese, or in rats that lost a similar amount of weight through caloric restriction. VSG's effects were inconsistent with the profile produced by purely restrictive procedures in that preferences for calorically dense liquids were not observed, and weight loss was not adversely affected by access to a high-calorie liquid diet. Surgically induced absences in fat preference and changes in c-Fos expression in response to intragastric infusions of sucrose and Intralipid imply that the effect of VSG on meal size results from increases in sensitivity to the caloric content of the food, rather than being a product of a small stomach resulting in increased distension-related signals.
We examined the responsiveness of neurons in the NTS and AP after VSG because they receive direct input from vagal afferent fibers that innervate the gut and code for key aspects of meal-related stimuli, including mechanical distention, caloric content, and changes in postprandial glucose and hormone levels. Distention of the stomach wall activates mechanoreceptors located in the gastric mucosa that generate impulses carried by vagal afferent neurons and interneurons of the myenteric plexus (1). Most of the vagal afferent fibers that innervate the stomach project to the NTS, rostral to the level of obex. Many of these neurons are adrenergic (31). These neurons are unique in that their activity correlates with gastric distention but not with caloric or osmotic intake (31). We found that the percentage of adrenergic positive cells in the NTS expressing c-Fos in our study was <15% and similar among groups.
Activity in this region is a good correlate for meal termination (30). However, preference and reward pathways are likely the product of a more complex pathway that includes higher forebrain circuits. We postulate that reductions in fat preference are one reason that VSG rats eat less, and gain less weight, than sham-operated animals when exposed to diets that are high in fat. In contrast, the higher levels of c-Fos activation in the brain stem of VSG rats in our study reflect increased sensitivity to nutrients in areas of the brain that convey sensations of fullness and satiety. It seems likely that these effects work in concert to lower body weight and fat mass in VSG animals relative to sham-operated rats.
It should be noted that many of the mechanoreceptors in the gastric mucosa are removed during VSG, which could potentially limit the representation of activity by mechanosensitive neurons in VSG rats in our study. However, the hepatic and gastric branches that innervate the stomach and duodenum are spared, as are the celiac branches that innervate the rest of the alimentary tract. In addition to receiving input from the gut, the NTS has reciprocal connections with the hypothalamus and ventral tegmental area that make it responsive to both homeostatic and nonhomeostatic inputs on meal size (5). Apart from a reduction in activity in catecholaminergic neurons in the NTS, the pattern of c-Fos expression in VSG rats given sucrose or Intralipid in our study is similar to that reported in others (14, 31).
In those studies, the activation of c-Fos in the NTS and AP was contingent upon the animals having access to the same number of calories they would eat voluntarily. c-Fos expression in rats given one-third to one-half of what they normally eat was not significantly different from levels seen in unfed rats (14, 31). The all-or-nothing nature of this response is consistent with the results of our current study in which sucrose and Intralipid had no effect on c-Fos expression in ad libitum and pair-fed animals. This is likely related to the fact that the stimulus was only 20% of what ad libitum and pair-fed sham animals eat voluntarily under similar conditions (Fig. 5), indicating that the size of the caloric stimulus was insufficient to elicit a response in these animals. The lower threshold for activation in our VSG rats, combined with reductions in fat preference after surgery, provide a neural correlate for clinical data showing that VSG results in greater weight loss, significant reduction in hunger ratings, and alterations in food choice when compared with purely restrictive procedures such as the adjustable gastric band (15). These effects are consistent with observations made in rat models of RYGB surgery that have previously been shown to produce a similar effect on meal-induced c-Fos (34) expression and fat preference (10, 33).
Although the physiology that underlies the effects on satiation and food preference following these procedures has not been identified, it is tempting to speculate that it is related to changes in postprandial hormone profiles. Glucagon-like peptide (GLP)-1, peptide YY (PYY), and insulin are all greatly increased after VSG and RYGB (3, 12, 17, 28, 39), and these three hormones modulate intake of certain kinds of foods or the choice among foods in a manner consistent with the observed food choices evident after surgery. The GLP-1 receptor agonist exendin-4 caused a relative increase in carbohydrate intake in rats (29) and was more effective at reducing food intake following an intragastric preload of fat than of carbohydrate (2); PYY injected into the paraventricular nucleus of the hypothalamus stimulates carbohydrate intake to a greater degree than fat intake (35); and insulin injected directly into the arcuate nucleus reduces preferences for fat in rats (20, 40). Losses in preferences for dietary fat may explain why VSG rats gained less weight than sham animals maintained on Ensure (29% fat) but ate similar amounts when the animals were maintained on chow (17% fat) (Fig. 4). The results are consistent with data from our previous study in which rats increased their intake of carbohydrate relative to fat during a food selection test (41) and imply that patients that undergo VSG will be less likely to select foods that are high in fat. However, even when rats are maintained on the same high-fat diet that is used to make them obese before the surgery, VSG still results in large reductions in body fat. Such results imply that changes in food choice are not necessary to reduce body weight after VSG, but it is possible that the same mechanisms that reduce body weight also act to alter food choice as well.
Altered postprandial hormone profiles after VSG may be more broadly related to changes in nutrient delivery to the duodenum. Data from humans indicate that VSG significantly increases the rate of gastric emptying (4, 6, 24) and transit (24), and, in RYGB, transit is increased by the surgical creation of a gastric-jejunal anastomosis (23). This may provide a physiological basis for VSG- and RYGB-mediated increases in postprandial GLP-1 release. As lipid reaches the distal small intestine, GLP-1 is secreted in a fat-dependent manner to reduce intestinal motility and enhance proximal fat absorption (43). Accelerating the appearance of lipids into the distal gut could stimulate the release of supraphysiological levels of GLP-1, PYY, and insulin after these surgeries (12, 17, 28, 39). It could also limit the exposure of fat to lipases and the emulsification process, providing a potential reason for the observed increase in plasma bile acids after VSG (37) and RYGB (16) and reductions in fat preference. This hypothesis could provide an explanation for the differences between VSG and restrictive procedures such as adjustable gastric banding where gastric emptying rates are not increased (11, 25) and postprandial hormone profiles are not changed (19, 20).
Previously, effects of RYGB on food preferences have been attributed to the exclusion of the duodenum and proximal bowel. The profile produced by VSG on dietary preferences for high-energy liquids and dietary fat demonstrates that similar effects can be achieved without bypassing the intestine. We present evidence that reductions in meal size after VSG do not result from volume effects, but rather from the activation of satiation pathways in response to nutrients, and especially to fat content. These data support the hypothesis that, unlike other purely restrictive procedures, the satiating effects of VSG are based upon altered perceptions and actions of calories rather than altered perception of the volume induced by mechanical restriction.
GRANTS
This work was supported by a grant from Ethicon Endo-Surgery. A. P. Chambers is supported by a Canadian Institutes of Health Research fellowship.
DISCLOSURES
The University of Cincinnati receives funding from Ethicon Endosurgery, Pfizer and MannKind. RJS is a paid consultant for Ethicon Endo-Surgery and Novo Nordisk. He has board membership at Novo Nordisk, Angiochem, Takeda, and has grants pending at Novo Nordisk, Pfizer, Ablaris, and stock options at Zafgen. He has received payment for lectures by Novo Nordisk, Merck and Pfizer. DAS has received payment for lectures by Novo Nordisk, and has grants pending at Novo Nordisk, Ethicon Endosurgery, and Pfizer. DAD is a paid consultant for Amylin, Merck, Eli Lilly, Novo Nordisc, Takeda and Zealand Pharmaceuticals. BEG has received money from Ethicon Endosurgery for travel to meetings for purposes other than this study. No other authors have a conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Author contributions: A.P.C., H.E.W.-P., and R.J.S. conception and design of research; A.P.C., H.E.W.-P., S.M., B.E.G., and K.K.R. performed experiments; A.P.C., H.E.W.-P., and S.M. analyzed data; A.P.C., H.E.W.-P., D.A.D., S.C.W., D.A.S., and R.J.S. interpreted results of experiments; A.P.C. and H.E.W.-P. prepared figures; A.P.C. and H.E.W.-P. drafted manuscript; A.P.C., H.E.W.-P., B.E.G., K.K.R., D.A.D., S.C.W., D.A.S., and R.J.S. edited and revised manuscript; A.P.C., H.E.W.-P., S.M., B.E.G., K.K.R., D.A.D., S.C.W., D.A.S., and R.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Maureen Fitzgerald for excellent technical expertise in immunohistochemistry.
REFERENCES
- 1. Arakawa T, Uno H, Fukuda T, Higuchi K, Kobayashi K, Kuroki T. New aspects of gastric adaptive relaxation, reflex after food intake for more food: involvement of capsaicin-sensitive sensory nerves and nitric oxide. J Smooth Muscle Res 33: 81–88, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Aziz A, Anderson GH. Exendin-4, a GLP-1 receptor agonist, modulates the effect of macronutrients on food intake by rats. J Nutr 132: 990–995, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Basso N, Capoccia D, Rizzello M, Abbatini F, Mariani P, Maglio C, Coccia F, Borgonuovo G, De Luca ML, Asprino R, Alessandri G, Casella G, Leonetti F. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc 25: 3540–3550, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer AO, Hopt UT, Langer M, Karcz WK. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy–preliminary results. Obes Surg 21: 95–101, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20, Suppl 1: 64–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg 19: 1515–1521, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Brolin RE, Kenler HA, Clemow LP, Kasnetz KA, Ebert EC, Greenfield DP. Gastric reduction surgery for morbid obesity. N J Med 84: 481–487, 1987 [PubMed] [Google Scholar]
- 8. Brolin RL, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg 220: 782–790, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg 19: 1605–1611, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 104: 709–721, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Burton PR, Yap K, Brown WA, Laurie C, O'Donnell M, Hebbard G, Kalff V, O'Brien PE. Changes in satiety, supra- and infraband transit, and gastric emptying following laparoscopic adjustable gastric banding: a prospective follow-up study. Obes Surg 21: 217–223, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Chambers A, Jessen L, Ryan K, Sisley S, Wilson-Pérez H, Stefater M, Gaitonde S, Sorrell J, Toure M, Berger J, D'Alessio D, Woods S, Seeley R, Sandoval D. Weight-independent changes in blood glucose homeostasis after Roux-en-Y gastric bypass and vertical sleeve gastrectomy surgeries in rats. Gastroenterology 141: 950–958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colles SL, Dixon JB, O'Brien PE. Hunger control and regular physical activity facilitate weight loss after laparoscopic adjustable gastric banding. Obes Surg 18: 833–840, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Emond M, Schwartz GJ, Moran TH. Meal-related stimuli differentially induce c-Fos activation in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 280: R1315–R1321, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg 16: 1450–1456, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, Schaap FG. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis 29: 48–51, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247: 401–407, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr 52: 87–92, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 14: 1553–1561, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 33: 786–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakdawala MA, Bhasker A, Mulchandani D, Goel S, Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg 20: 1–6, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301: R1057–R1066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 95: 1851–1855, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, Karkavitsas N. Sleeve gastrectomy: a restrictive procedure? Obes Surg 17: 57–62, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Mistiaen W, Vaneerdeweg W, Blockx P, Van Hee R, Hubens G, Bortier H, Harrisson F. Gastric emptying rate measurement after vertical banded gastroplasty. Obes Surg 10: 245–249, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Moy J, Pomp A, Dakin G, Parikh M, Gagner M. Laparoscopic sleeve gastrectomy for morbid obesity. Am J Surg 196: e56–e59, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Pereferrer FS, Gonzalez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Dejardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg 18: 97–108, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250: 234–241, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Peters CT, Choi YH, Brubaker PL, Anderson GH. A glucagon-like peptide-1 receptor agonist and an antagonist modify macronutrient selection by rats. J Nutr 131: 2164–2170, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol Regul Integr Comp Physiol 275: R262–R268, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, Basso N. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg 20: 50–55, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35: 642–651, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides 6: 1205–1211, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Stefater M, Perez-Tilve D, Chambers A, Wilson-Perez H, Sandoval D, Berger J, Toure M, Tscoep M, Woods S, Seeley R. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138: 2426–2436, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stefater MA, Sandoval DA, Chambers AP, Wilson-Perez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves post-prandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141: 939–949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg 205: 613–624, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valderas JP, Irribarra V, Boza C, de la Cruz R, Liberona Y, Acosta AM, Yolito M, Maiz A. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab 95: 1069–1075, 2010 [DOI] [PubMed] [Google Scholar]
- 40. van Dijk G, de Groote C, Chavez M, van der Werf Y, Steffens AB, Strubbe JH. Insulin in the arcuate nucleus reduces fat consumption in rats. Brain Res 777: 147–152, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Wilson-Perez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woods SC, Seeley RJ, Rushing PA, D'Alessio DA, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol 297: G299–G305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]