Abstract
Glucagon-like peptide 2 (GLP-2) is an enteroendocrine hormone trophic for intestinal mucosa; it has been shown to increase enteric neuronal expression of vasoactive intestinal polypeptide (VIP) in vivo. We hypothesized that GLP-2 would regulate VIP expression in enteric neurons via a phosphatidylinositol-3 kinase-γ (PI3Kγ) pathway. The mechanism of action of GLP-2 was investigated using primary cultures derived from the submucosal plexus (SMP) of the rat and mouse colon. GLP-2 (10−8 M) stimulation for 24 h increased the proportion of enteric neurons expressing VIP (GLP-2: 40 ± 6% vs. control: 22 ± 5%). GLP-2 receptor expression was identified by immunohistochemistry on neurons (HuC/D+) and glial cells (GFAP+) but not on smooth muscle or fibroblasts in culture. Over 1–4 h, GLP-2 stimulation of SMP increased phosphorylated Akt/Akt ratios 6.1-fold, phosphorylated ERK/ERK 2.5-fold, and p70S6K 2.2-fold but did not affect intracellular cAMP. PI3Kγ gene deletion or pharmacological blockade of PI3Kγ, mammalian target of rapamycin (mTOR), and MEK/ERK pathways blocked the increase in VIP expression by GLP-2. GLP-2 increased the expression of growth factors and their receptors in SMP cells in culture [IGF-1r (3.2-fold increase), EGFr (5-fold), and ErbB-2–4r (6- to 7-fold)] and ligands [IGF-I (1.5-fold), amphiregulin (2.5-fold), epiregulin (3.2-fold), EGF (7.5-fold), heparin-bound EGF (2.0-fold), β-cellulin (50-fold increase), and neuregulins 2–4 (300-fold increase) (by qRT-PCR)]. We conclude that GLP-2 acts on enteric neurons and glial cells in culture via a PI3Kγ/Akt pathway, stimulating neuronal differentiation via mTOR and ERK pathways, and expression of receptors and ligands for the IGF-I and ErbB pathways.
Keywords: differentiation, extracellular signal-regulated kinase, ErbB, epidermal growth factor receptor, insulin-like growth factor I, neuregulin
the enteric nervous system (ENS) is an important regulator of intestinal function and is understood primarily in terms of the control and integration of gastrointestinal motility, blood flow, and secretion (10, 11, 20). The role of the ENS in regulating other aspects of intestinal function, such as nutrient absorption and response to inflammation and injury, is becoming increasingly appreciated (18, 23, 32). The ENS is able to alter neuronal morphology, function, and the pattern of neurotransmitter expression in response to inflammation (17, 33). Enteric neurons also undergo changes in neurochemical phenotype in pathophysiological states such as inflammation (22, 29). The mechanisms that may regulate the phenotype and pattern of transmitter expression in the ENS in vivo are not well understood (5). In recent work, we have shown that glucagon-like peptide 2 (GLP-2), known as a trophic factor regulating intestinal epithelial proliferation and nutrient-absorptive capacity, also influences the number and proportion of vasoactive intestinal polypeptide (VIP)-expressing neurons within the colonic submucosal plexus in vivo (33).
GLP-2 is a unique enteroendocrine hormone; it is produced (with GLP-1) by specialized posttranslational processing of the proglucagon gene transcript in enteroendocrine L cells (24). Undigested nutrients trigger the release of GLP-2; GLP-2 acts via a single receptor located specifically on enteric neurons, enteroendocrine cells, and subepithelial myofibroblasts (6, 21, 26, 34). The epithelial effects of GLP-2 are mediated by second messengers: the trophic effect by IGF-I from myofibroblasts (6, 7, 25) and the anti-inflammatory actions (at least in part) by activation of VIP-expressing neurons (33, 34). Definitive studies of the intracellular mechanisms of action of GLP-2 have been difficult because relatively few cells express the receptor (15, 42). The GLP-2 receptor (GLP-2R) is a typical G protein-coupled receptor of the glucagon/VIP family; it is uniquely expressed in the intestine and the hypothalamic regions of the central nervous system (CNS) (21). G protein-coupled receptor systems may act via variety of intracellular signaling pathways; previous studies used stable cell culture systems transfected with the receptor and suggested a GLP-2-coupled activation of adenylate cyclase (27, 35, 43). However, recent reports using cells that naturally express the receptor suggest that phosphatidylinositol 3-kinase-γ (PI3Kγ) and subsequent Akt phosphorylation are the intracellular pathways activated by GLP-2 (15, 31). GLP-2 has also been reported to induce effects in the ErbB and neuregulin pathways, which also commonly regulate differentiation in neurons (8, 39, 41). The present studies were conducted to determine the mechanism(s) underlying the observed GLP-2-induced increase in the number of VIP-expressing neurons within the rat colonic submucosal plexus (33). We hypothesized that GLP-2 acts on enteric neurons to regulate differentiation of VIP expression by GLP-2 receptor-coupled PI3Kγ phosphorylation of Akt. We performed experiments using a model of primary cultures of cells from the colonic submucosal plexus to establish the mechanism of action of GLP-2. Our results show that GLP-2 stimulation increases the proportion of neurons expressing VIP, similar to what was observed in vivo (33); these effects are mediated by a specific PI3Kγ/Akt pathway with activation of mammalian target of rapamycin (mTOR) and ERK1/2. GLP-2 also stimulated significant increases in gene expression for ErbB and neuregulin ligand and receptors.
METHODS
Experimental plan.
The effects of GLP-2 were studied using cells derived from primary cultures of submucosal enteric neurons, which exhibited a definitive increase in VIP expression following GLP-2 stimulation. The primary end points were VIP expression and detection of the activation of intracellular signaling pathways; studies were done primarily in cultures derived from the submucosal plexus of the colon to model the effects of GLP-2 in vivo (33). To determine the signaling pathways activated by GLP-2, short-term stimulation studies were done, detecting intracellular Akt/phosphorylated (phospho) Akt, ERK/phospho-ERK, and P70S6K by Western blot. To demonstrate the relative effects of signaling pathways activated by the GLP-2 receptor, studies were done using cells isolated from mice lacking PI3Kγ or from wild-type animals subjected to pharmacological blockade, using specific blockers of PI3Kγ (AS-605240), mTOR, and MEK/ERK pathways (PD-98059). The actions of GLP-2 on growth factor receptors [epidermal growth factor (EGF) Erb-1, IGF-I, and GLP-2 itself] and ligands (IGF-I, amphiregulin, epiregulin, and heparin-bound EGF) were quantified using quantitative RT-PCR.
Enteric neuronal and glial cultures.
Cells were isolated from rat colon or mouse ileum; protocols were approved by the University of Calgary Animal Care Committee, following the Canadian Council on Animal Care guidelines. Using previously described methods, primary cultures of submucosal neurons were prepared (5, 16, 31). In brief, animals (rats and mice aged 2–3 mo) were anesthetized using halothane by inhalation (Halocarbon Products, River Edge, NJ) and then exsanguinated. The colon or ileum was removed and placed in iced Krebs solution (133 mM NaCl, 4.7 mM KCl, 1.0 mM MgCl2, 1.4 mM NaHCO3, 2.5 mM CaCl2-2H2O, and 7.8 mM glucose, pH 7.2). The segment was opened along the mesenteric border and gently pinned mucosal side up to a solid Sylgard-coated dish. The mucosa was removed with a pair of fine forceps under a dissecting microscope; the exposed submucosa was then removed, separating it from the underlying tissue. The plexus was minced and incubated at 37°C in calcium- and magnesium-free Hanks' balanced salt solution (Gibco Invitrogen, Grand Island, NY) with 1.5 mg/ml collagenase type II and 0.125% trypsin (Sigma, St. Louis, MO). After 40 min, the digested tissue was triturated and then centrifuged at 120 g, 4°C, and suspended with culture medium [neurobasal-A with 10% fetal calf serum, 0.5 mM l-glutamine, and 0.5% Pen-Strep (Gibco)]. This was repeated, and the pellet was resuspended. Aliquots of dissociated cells (5–10 × 104 cells/well) were plated into 24 multiwell plates containing poly-l-lysine-coated glass coverslips (Fisher Scientific, Ottawa, ON, Canada) (5, 36). Cultures were grown at 37°C and 5% CO2. On days 3 and 5 the culture medium was replaced, and on day 5 cytosine arabinoside (5 μM; Sigma) was added to eliminate the growth of rapidly dividing cells such as fibroblasts or glial cells. After 3 days, cells were recovered in primary culture medium with 10% fetal bovine serum for 24 h, changed to primary culture medium containing 0.1% fetal calf serum for 24 h, and then studied. The standard group studied was eight wells for each treatment condition, unless otherwise specified.
Regulation of differentiation.
The primary stimulus throughout the experiments was the addition of exogenous GLP-2 to the medium. GLP-2 (1–33 human; American Peptide, Sunnyvale, CA) was dissolved in sterile water and diluted to a final concentration in medium of 10−8 M [optimal concentration for proliferation (15, 38)]. On days 10 and 11, in the 0.1% FBS medium, cells were treated with GLP-2 10−8 M or control medium for 1–28 days. Preliminary studies that demonstrated a robust increase in VIP expression by 24 h were done (data not shown).
Immunohistochemistry and identification of cell populations.
After completion of the stimulation phase, cells were fixed in 0.1 M PBS containing 4% paraformaldehyde for 30 min at room temperature and then washed three times with PBS. To control for variations in plating cell numbers, comparisons were always made between wells grown from the same tissue preparation with and without added GLP-2. Typically, cells were labeled with primary antiserum, washed three times with PBS, and incubated for 1 h with the secondary antibodies. Neurons were detected using either mouse anti-β-III-tubulin (1:500; R & D Systems, Minneapolis, MN) or mouse anti-HuC/D (1:200; Molecular Probes, Eugene, OR), and glial cells were detected with mouse anti S-100 (1:500; Sigma) and anti-glial fibrillary acidic protein (GFAP) (1:250; Chemicon, Billerica, MA). Smooth muscle cells were detected with rabbit anti-α-smooth muscle actin (1:100; Abcam, Cambridge, MA) antibody and fibroblasts with mouse anti-TE-7 antibody (1:1,000; Millipore, Billerica, MA). VIP expression was determined by double-labeling with anti-HuC/D or β-tubulin, and VIP-expressing neurons were detected with rabbit anti-vasoactive intestinal peptide (1:600; EuroDiagnostica, Malmo, Sweden), neuronal nitric oxide synthase (nNOS) with rabbit anti-nNOS (1:250; EuroDiagnostica). GLP-2 receptor was detected using goat anti-GLP2R (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) antibody. Donkey anti-mouse IgG conjugated to Alexa 488 (1:100; Jackson ImmunoResearch Laboratories, West Grove, PA) or goat anti-rabbit CY3 (1:1,000; Jackson ImmunoResearch Laboratories) was used to detect the primary antibodies. Cells were washed with PBS and mounted onto slides with Fluorosave mounting medium.
Cell signaling studies.
To determine the requirement for PI3Kγ-induced activation of Akt and downstream activation of mTOR and MEK/ERK by the GLP-2 receptor, cells isolated from the submucosal plexus were prepared as described above and treated with 10−8 M GLP-2 or control medium. After 0, 15, 30, 60, or 120 min, cells were collected on ice in immunoprecipitation buffer (RIPA or Tris buffer containing 1% Triton X-100). Cells were lysed, mixed, and centrifuged (120 g for 5 min at 4°C). Protein content was determined using the Lowry assay. Samples were denatured by boiling for 5 min. Equivalent amounts of protein were loaded on 10% SDS polyacrylamide gel and separated at 100 V × 1.5 h at 4°C. Membranes were blocked with 5% BSA for 1 h and then probed with the following primary antibodies: phospho-Akt (Ser473, 1:750), total Akt (1:1,000), mTOR (phosphorylated mTOR; Ser2448, 1:500), total mTOR (1:1,000), phospho-70SK6 (Thr389, 1:1,000), total p70S6K1 (1:500), phospho-ERK1/ERK2 (1:3,000), total ERK1/2 (1:1,000), and actin (1:2,000) (all antibodies raised in rabbit; Cell Signaling, Danvers, MA). The membrane was washed with PBS and then incubated with anti-rabbit horseradish peroxidase IgG secondary (1:2,000). This was then developed with a commercial horseradish peroxidase detection system (Amersham Biosciences, Piscataway, NJ), scanned, and analyzed using Quantity One software (Bio-Rad).
The functional effects of the blockade of these pathways were determined by preparing identical sets of cultures from rat colonic tissue pretreated with a nonselective PI3K inhibitor (LY-294002, 50 μM; Calbiochem, Billerica, MA), the selective PI3Kγ inhibitor AS-605240 (50 μM; Calbiochem), the ERK1/2 inhibitor PD-98059 (50 μM, Calbiochem) or mTOR inhibitor rapamycin (50 μM, Sigma), a or control carrier for 1 h and then stimulated with GLP-2 (10−8 M) or control medium for 24 h, and VIP expression was quantified as described above (27). To further demonstrate the role of PI3Kγ in mediating GLP-2 activity, similar submucosal plexus cell preparations were made from PI3Kγ−/− [deletion of p110 subunit (2, 28)] or wild-type mice and stimulated for 7 days with control medium or medium + GLP-2 (10−8 M), quantifying VIP expression in HuC/D expressing neurons by immunohistochemistry. To examine the effects of GLP-2 on other regulatory signaling pathways, short-term stimulation studies were done using cells from rat colon stimulated with GLP-2 (10−8 M) or control protein; to follow the time course of genes induced, cells were lysed at 30 min, 60 min, 2 h, 6 h, and 24 h (n = 6/time point). Real-time quantitative reverse-transcribed PCR (qRT-PCR) was done on the mRNA isolates from each time point for the expression of the genes for GLP-2R, IGF-I receptor, EGF receptor, ErbB2, ErbB3, and ErbB4 receptors, and the ligands EGF, IGF-I, amphiregulin, epiregulin, β-cellulin, heparin-binding EGF-like factor (HB-EGF), and neuroregulins 1–4. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA), following the manufacturer's instructions, and then reverse transcribed using Superscript-II reverse transcriptase (Invitrogen). Sequence amplification was performed using Express SYBR Green master mix with ROX (Invitrogen) in an ABI 7900 real-time PCR thermocycler detection system. All primer sequences were obtained from the University of Calgary DNA Core, Calgary, AB, Canada (Table 1). Samples were run in duplicate with a resulting threshold cycle (CT) being determined, using 18S ribosomal RNA as the endogenous control. Data are expressed as a relative fold change that was calculated using the ΔΔCT method.
Table 1.
Primer sequences
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| EGF | TGCCTTGCCCTGACTCTAC | AGCCAATGACACAGTTGCAC |
| IGF-I | AAGCCTACAAAGTCAGCTCG | GGTCTTGTTTCCTGCACTTC |
| Ampiregulin | TTCGCTGAACCTCTCAGT | CCAACCCGACTGCATATTGG |
| Epiregulin | CCGAGAGAAGGATGGAGACTTTC | GGGAACCAAGGCAAAGCA |
| HB-EGF | ACTTGGAAGGGACCGATCTGGA | TAGGGTCAGCCCATGACACCTC |
| TGFα | GGAATTCCTAGCGCTGGGTATCCTGTTA | CAAGCTTACCACCACCAGGGCAGTGATG |
| β-Cellulin | TGGACGAACAAACTCCCTCCT | GACGCCTATCAAGCAGACCAC |
| NRG1 | CATTACACTTCCACAGCCCATC | CTCTCCGTGTGCCCATTACTC |
| NRG2 | GGAGATCCAGATGGCAGATTACA | GTGGTGAGAGGGTGAACAGGA |
| EGF | CACCCCAGAAACTAGCACCA | CAGGGTCTCAATCACAAAGCA |
| NRG4 | GTCCTCGTCACTCTTGCCATC | CACACTGGACTGAACTGGCTCT |
| GLP-2R | ACCTTGCAGCTGATGTACAC | CAGCCAGAACTTCAGGATG |
| EGF-R | TCCCTTTGGAGAACCTGCAGATCA | GTTGCTAAATCGCACAGCACCGAT |
| ErbB2 | GCTCAGAGACCTGCTTTGGA | AGGAGGACGAGTCCTTGTAGTG |
| ErbB3 | GGCCTCAACGTTGACCCCTGGCAT | TGCCAAGCATTCTCCCAGAGGCCG |
| ErbB4 | GTGAAATTGGACACAGCCCTCC | CAAAAGGGTTCTCTTCCACAG |
| IGF-IR | CACAGGCTATGGCTCCAGCAT | TCTCCAGCCTCCTCAGATCACA |
| 18S | CAGAAGGACGTGAAGGATGG | CAGTGGTCTTGGTGTGCTGA |
EGF, epidermal growth factor; HB-EGF, heparin-binding EGF-like factor; TGFα, transforming growth factor-α; GLP-2R, glucagon-like peptide-2 receptor; EGF-R, EGF receptor; IGF-IR, IGF-I receptor.
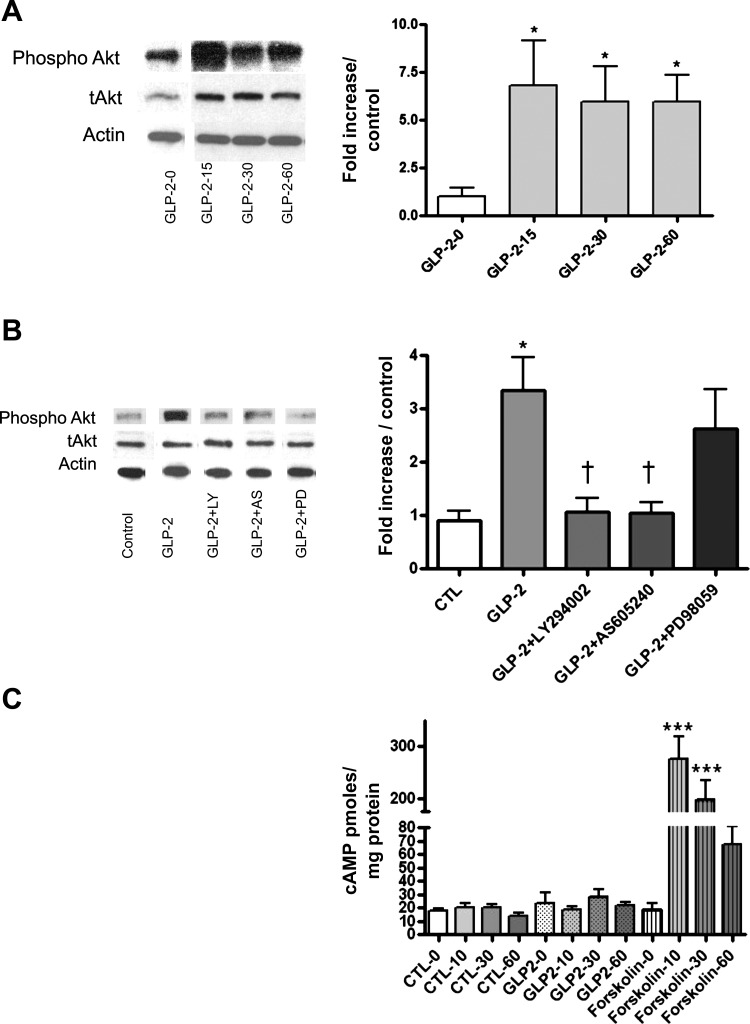
To examine the involvement of cAMP in the actions of GLP-2, the effect of GLP-2 stimulation on intracellular cAMP levels was quantified. Using the methods described above, with medium containing 10 μM 3-isobutyl-1-methylxanthine (Sigma), cells were treated with GLP-2 (10−8 M) or control; 20 μM forskolin (Calbiochem) was used as a positive control (n = 8 wells/group). Cells were treated for 0–10-30–60 min, washed with Hanks' solution, and lysed in 0.1 N HCl, and the lysates were frozen and centrifuged, with the supernatant for cAMP analyzed using a commercial kit (Biomedical Technologies). Briefly, this is an enzyme immunoassay that measures the amount of cAMP using a competitive binding of cAMP and an alkaline phosphate derivative of cAMP to a specific bound amount of antibody on a microplate. The cell culture medium containing cAMP was mixed with cAMP-alkaline phosphatase tracer and cAMP antiserum and added to the wells already coated with cAMP-specific antibody. After incubation overnight, the wells were washed and reagents added to produce a enzymatic color reaction with the materials bound to the plates read at 405–410 nm absorbance. The relative bindings were calculated against a standard curve to determine picomoles per milliliter of cAMP that was then normalized to total protein in each sample.
Statistics.
All data are means ± SE. Data were analyzed using Prism Software (version 4.1). Comparisons were made using Student's t-test or ANOVA, followed by Bonferroni's post hoc testing, as appropriate for parametric data . Kruskal-Wallis testing was used for non parametric data, with a P value of <0.05 considered significant.
RESULTS
GLP-2 induces enteric neuronal expression of VIP.
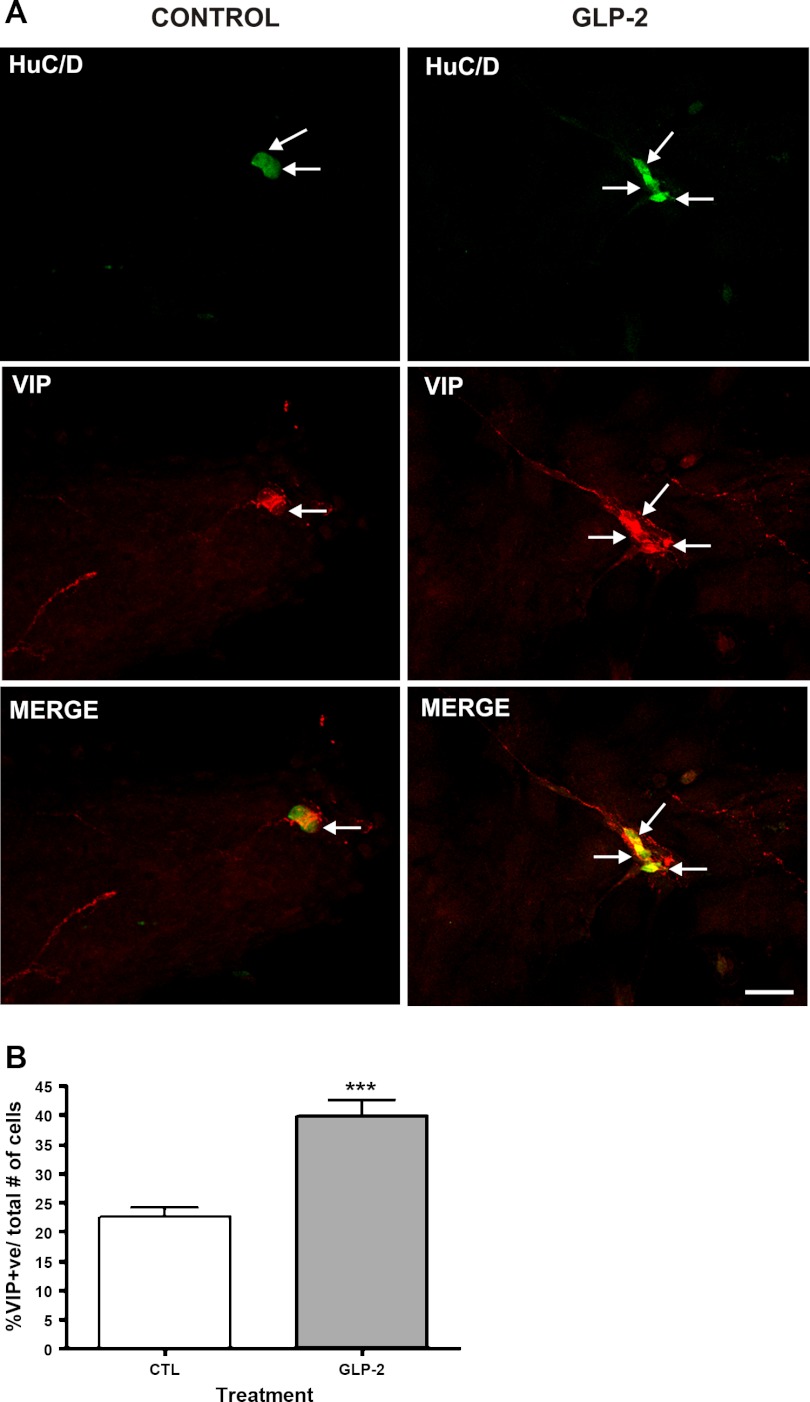
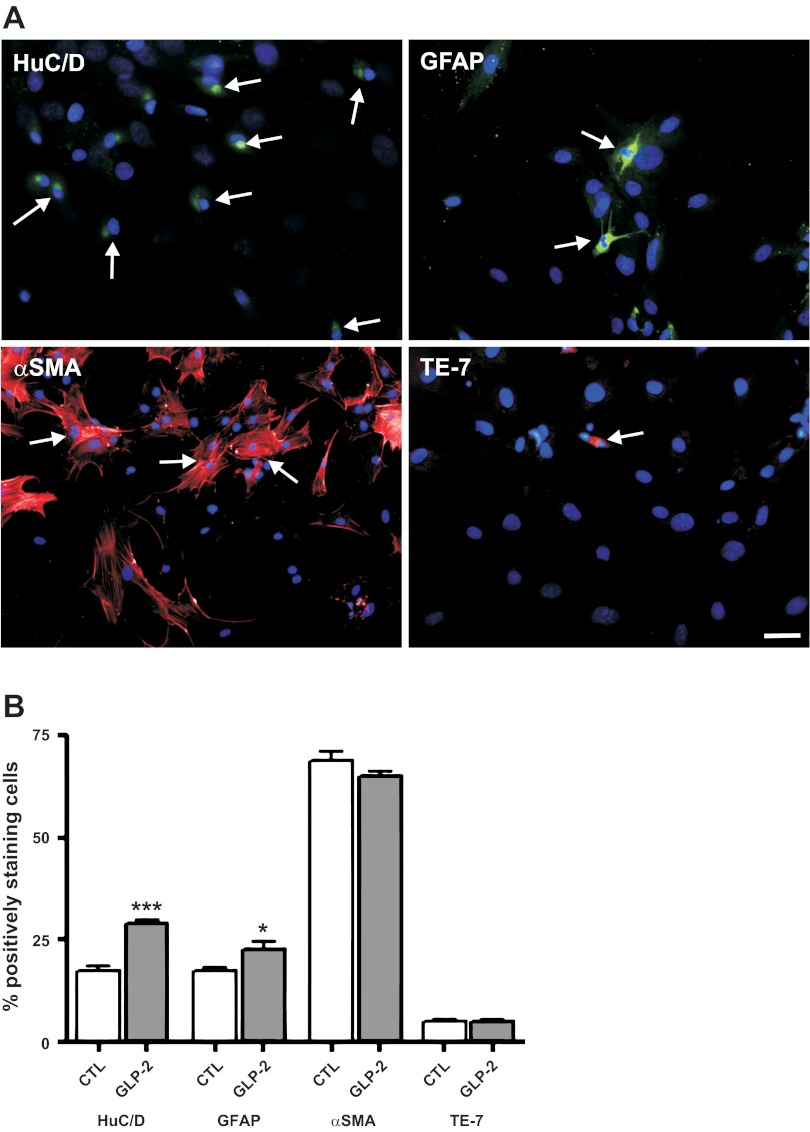
To establish the ability of this model of cells isolated from the submucosal plexus to replicate the actions of GLP-2 in vivo in inducing the expression of VIP, we first examined the effects of exogenous GLP-2 on the neuronal cell phenotype. GLP-2 significantly increased the proportion of cells colabeling with the neuronal markers HuC/D and VIP (controls: 22 ± 5% vs. GLP-2 treated: 40 ± 6%, coexpressed HuC/D and VIP; Fig. 1, A and B). As a comparator, GLP-2 treatment also affected nNOS expression (controls: 9 ± 4% vs. GLP-2: 18 ± 2% coexpressed HuC/D and nNOS; n = 6 preparations, P < 0.05). Having established the ability to replicate the effects of GLP-2 seen in vivo to increase the expression of VIP in neurons, the identity of the responding cells in this system was probed (Fig. 2A). The initial preparation of cells isolated and dissociated from the submucosal plexus consisted of 17% neurons, as detected by HuC/D labeling, 14% enteric glial cells (GFAP+), 62% smooth muscle cells (α-smooth muscle actin+), and 5% fibroblasts (TE-7+). The proportion of cells expressing neuronal and glial markers was unchanged by treatment with control serum but increased after 24 h of treatment with GLP-2 (Fig. 2B). Longer-term studies out to 28 days, which showed a continued increase in the proportion of cells expressing neuronal markers (HuC/D) vs. glial markers (GFAP), were done (day 14; ratio of neurons to glial cells, 14:1).
Fig. 1.
Vasoactive intestinal polypeptide (VIP) expression in cells derived from the submucosal plexus following glucagon-like peptide-2 (GLP-2) stimulation. The effects of GLP-2 on cells isolated from submucosal ganglia prepared from rat colon were studied in vitro. The submucosal neurons were isolated by microdissection, separated, and cultured for 8 days, being treated with cytosine arabinoside over the last 3 days. Medium was replaced with 0.1% FCS with or without added GLP-2 (10−8 M), and cells were studied by immunohistochemistry after 24 h. Labeled cells were imaged using a confocal microscope (Olympus FV1000) and Olympus Fluoview version 3.0 software. A, top: staining for HuC/D (in green). A, middle: staining for VIP (red). A, bottom: merged images. Arrows indicate nuclei. Scale bar, 50 μm. B: effects of GLP-2 on the proportion of cells expressing VIP. Nos. of cells costaining for VIP, as a proportion of all nuclei detected by 4,6-diamidino-2-phenylindole (DAPI) staining, in 10 high-powered fields from 8 coverslips/determination. Data are means ± SE; n = 8. –––P < 0.01 vs. controls (CTL).
Fig. 2.
Identity of cells in primary cultures derived from the submucosal plexus and the effects of GLP-2 stimulation. Primary cultures of cells from the submucosal neuronal plexus were prepared from rat colon, as outlined in Fig. 1. A: cell identification by immunohistochemistry. Coverslips were stained with antibody against the neuronal nuclear marker HuC/D (green), the glial marker glial fibrillary acidic protein (GFAP; green), α-smooth muscle actin (α-SMA; red), or the fibroblast marker TE-7 (red) and costained with the nuclear stain DAPI (blue). Double-labeled cells are marked by arrows. Scale bar, 100 μm. B: effects of GLP-2 on cells derived from the submucosal plexus in culture. Cell types detected by immunohistochemical labeling after 24 h of stimulation with 0.1% FCS (controls) or 0.1% FCS + GLP-2 (10−8 M). Values are the proportion of cells labeled by the antibody as %total cells (detected by DAPI staining) from 10 high-powered fields from 8 coverslips/determination. Data are means ± SE; n = 8. –P < 0.05 vs. controls; –––P < 0.001 vs. controls.
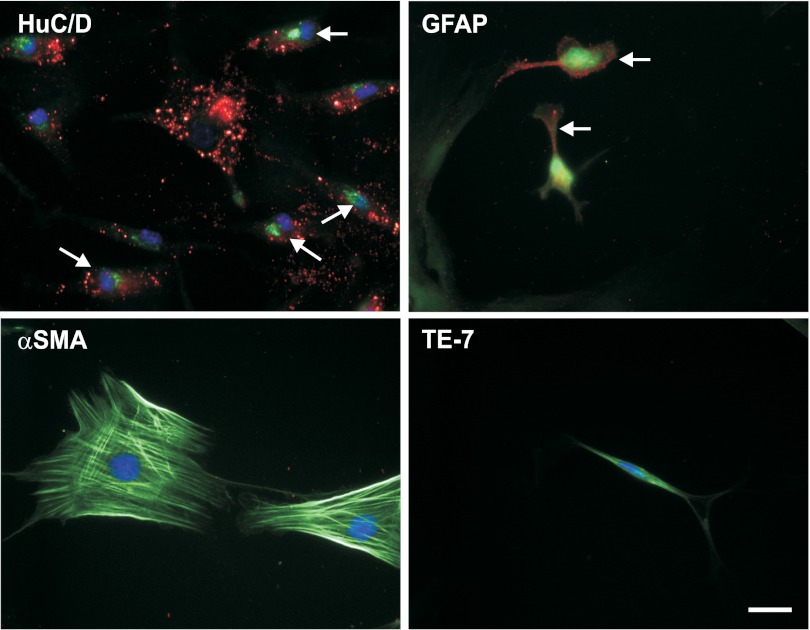
The identity of the cells that are capable of responding to GLP-2 was further defined, examining for the presence of the GLP-2 receptor, as detected by immunohistochemistry (Fig. 3). The typical surface staining pattern for the GLP-2 receptor was seen in 55% of neurons (identified by costaining with HuC/D) and 24% of glial cells (costaining for GFAP), but not in smooth muscle cells (α-SMA) or fibroblasts (TE-7). Similar results were seen when double-labeling for neurons was done with anti-β-tubulin and for glial cells with anti S-100 (38%).
Fig. 3.
Expression of the GLP-2 receptor on primary cell cultures derived from the submucosal plexus. Primary cell cultures derived from the submucosal enteric plexus were prepared as outlined in Fig. 1. Coverslips were stained with antibody against the neuronal nuclear marker HuC/D (green), the glial marker GFAP (green), α-SMA (green), or the fibroblast marker TE-7 (green) and colabeled with antibody against the GLP-2 receptor (red) and the nuclear stain DAPI (blue). Double-labeled cells are marked by arrows. Scale bar, 25 μm.
Signaling pathways activated by endogenous GLP-2 receptor stimulation.
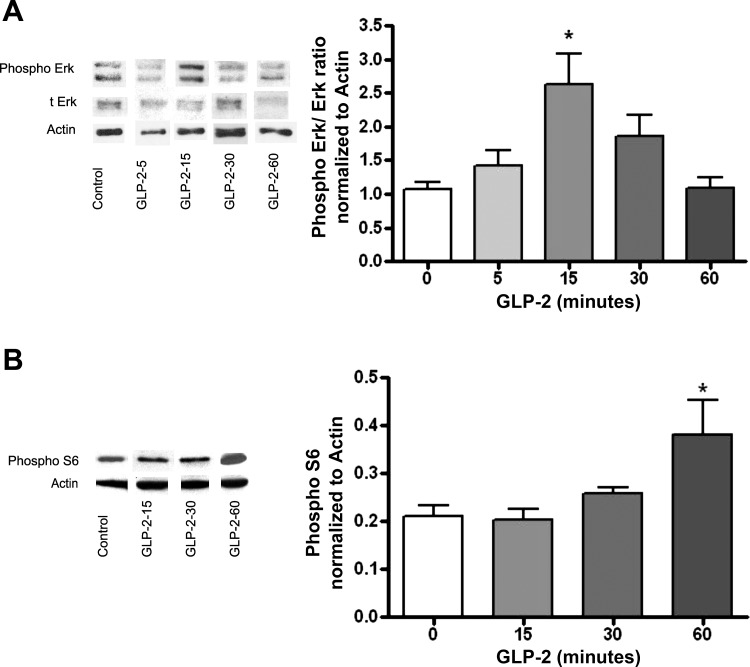
Having recapitulated our in vivo findings in this cell culture model, we wanted to establish the mechanism. The cellular signaling pathways predicted to be activated by the GLP-2 receptor are intracellular PI3Kγ, recruited with kinase activation at the inner cell membrane, which then acts via phosphatidylinositol-3,4,5-triphosphate phosphorylation to activate Akt. This was examined by performing Western blots for p-Akt and total Akt following the addition of GLP-2 (10−8 M) normalized to actin. These experiments demonstrated that exogenous GLP-2 increased the ratio of p-Akt/total Akt significantly, with maximal stimulation at 15 min (Fig. 4A). This was not seen in cells pretreated with the PI3K inhibitors LY2–94002 (nonselective) or AS-605240 (PI3Kγ selective) (Fig. 4B). Downstream activation of ERK pathways was demonstrated by detection of p-ERK/total ERK; treatment with GLP-2 resulted in a 2.5-fold increase in the p-ERK/total ERK ratio (Fig. 5A), with the maximal increase at 15 min after stimulation with GLP-2. The involvement of the mTOR was demonstrated by the specific 2.2-fold increase in p70S6K in GLP-2-treated cells (Fig. 5B), and the increase was maximal at 60 min after GLP-2 treatment. Finally, in these enteric neurons, which express the native GLP-2 receptor, there was no cyclic AMP response after incubation with GLP-2, although they demonstrated a robust response to forskolin (Fig. 4C).
Fig. 4.
GLP-2 effects on Akt phosphorylation and cAMP accumulation. Primary cultures of cells from the submucosal enteric neuronal plexus were prepared from rat colon, as outlined in Fig. 1, and treated with control medium or GLP-2 (10−8 M) for the times indicated. A: time course of phospho-Akt expression; Western blots of representative gels taken from lysates of cells exposed to GLP-2 for the times indicated and probed sequentially with antibodies against phospho-Akt, total Akt (t-Akt), and actin, with stripping and restaining of the original gel. Blots from a single gel are rearranged for clarity. Graph represents phospho-Akt/t-Akt normalized to controls, with actin used a loading control. B: effects of phosphatidylinositol 3-kinase (PI3K) inhibition. Cultured cells were pretreated for 1 h with the nonselective PI3K inhibitor LY-294002 (50 μM), the selective PI3Kγ inhibitor AS-605240 (50 μM), or the ERK1/2 inhibitor PD-98059 (50 μM) and then stimulated with GLP-2 (10−8 M) for 15 min. Representative Western blots of phospho-Akt, t-Akt, and actin staining are presented, and blots from a single gel are arranged for clarity. Results of phospho-Akt/t-Akt normalized to controls are presented. Methods are as outlined in A; n = 6. C: cultured cells were treated with GLP-2 or control solvent vehicle for the times indicated. Positive controls were treated with forskolin (20 μM), cells lysed at the times indicated, and cAMP levels determined. Data are means ± SE; n = 8/condition. –P < 0.05 vs. controls; –––P < 0.0001 vs. controls; †P < 0.05 vs. GLP-2 treated.
Fig. 5.
GLP-2 effects on ERK and mammalian target of rapamycin (mTOR) pathways. Primary cultures of cells from the submucosal enteric neuronal plexus were prepared from rat colon, as outlined in Fig. 1, and treated with control medium or GLP-2 (10−8 M) for the times indicated. A: time course of ERK/phospho-ERK expression. Western blots of representative gels taken from lysates of cells exposed to GLP-2 for the times indicated, probed with antibodies against phospho-ERK, t-ERK, and actin; blots are from a single gel and arranged for clarity. Graph represents phospho-ERK/t-ERK normalized to controls, with actin used a loading control. B: time course of mTOR activity. Using methods similar as in A, gels were probed with antibodies against p70S6K and actin. Graph represents densiometer readings of p70S6K/actin, with actin used as the loading control. Data are means ± SE. –P < 0.05 vs. controls.
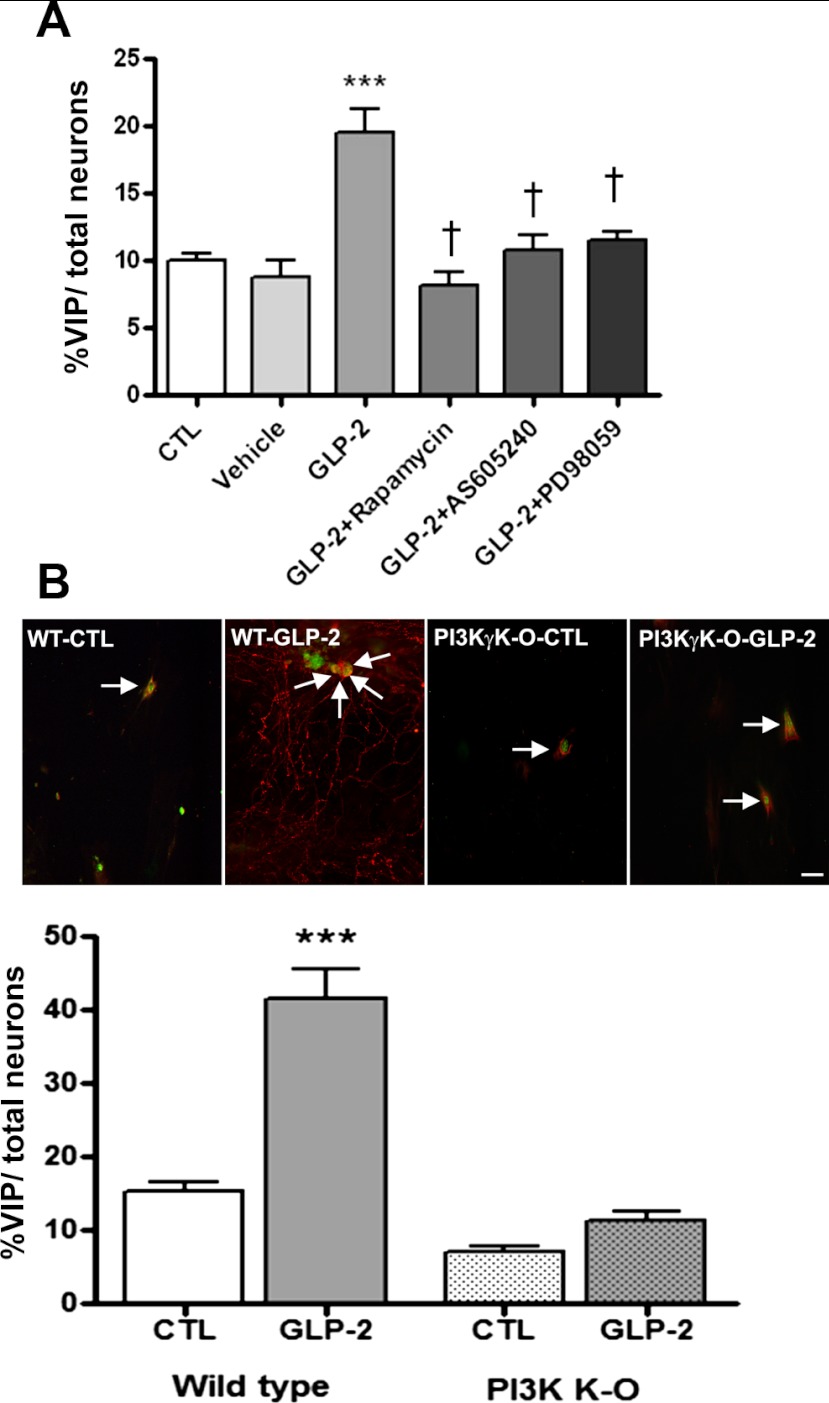
The involvement of the PI3Kγ, p-Akt, MEK/ERK, and mTOR pathways in the GLP-2-mediated effects was further explored using pharmacological techniques with the end point of VIP expression by the responding cells. Cotreatment of cells with the specific PI3Kγ inhibitor AS-65240 rapamycin (10−4 M) or the ERK1/2 inhibitor PD-98059 (10−6 M) and stimulation with GLP-2 (10−8 M) blocked the induction of VIP-expressing neurons (Fig. 6A). Similarly, when mice with a specific PI3Kγ deletion were used as the source for the enteric submucosal preparations and stimulated with GLP-2, no effect on the proportion of neurons expressing VIP was seen following stimulation by GLP-2 at 10−8 M for 7 days (Fig. 6B).
Fig. 6.
Effects of pharmacological antagonists or gene knockout of intracellular signaling pathways on GLP-2-induced VIP expression. A: effect of pharmacological blockers of intracellular signaling. Primary cultures of cells from the submucosal enteric neuronal plexus derived from rat colon were grown on coverslips and pretreated for 1 h with the selective PI3Kγ inhibitor AS-605240 (50 μM), the ERK1/2 inhibitor PD-98059 (50 μM) or the mTOR inhibitor rapamycin (50 μM) or solvent vehicle, followed by additional GLP-2 (10−8 M) or CTL (GLP-2) for 24 h. The proportion of cells costaining for VIP and HuC/D with each condition is shown. B: effect of PI3Kγ knockout (KO). Primary cultures of cells from submucosal ganglia from mouse ileum from wild-type and PI3Kγ−/− animals were isolated and grown on coverslips, as outlined in Fig. 1, culturing cells for 14 days prior to study. Wells were treated with control medium or medium + GLP-2 (10−8 M) for 7 days. The images and the corresponding counts of cells costaining for VIP and HuC/D with each condition is shown. Scale bar, 50 μm. Data are means ± SE; n = 6–8/group. –––P < 0.001 vs. controls; †P < 0.001 vs. GLP-2 treated.
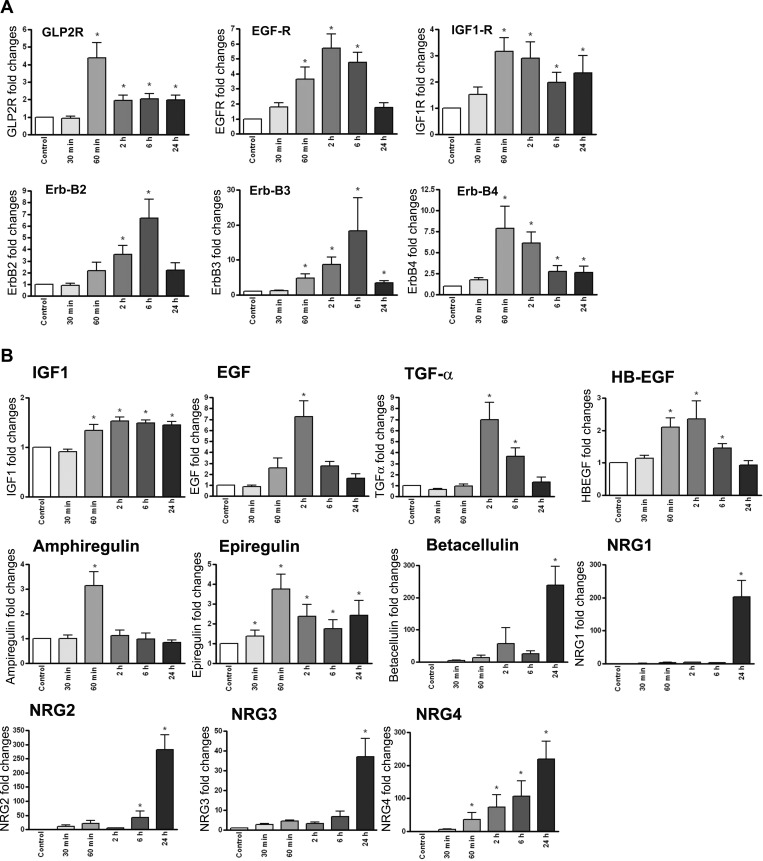
The secondary signals that may have a role in regulating proliferation and differentiation of enteric neurons and glia were studied by quantifying the effects of GLP-2 on receptors and ligands of the EGF and IGF-I family, which have been implicated previously in the trophic effects of GLP-2. As shown in Fig. 7A, there was an increase in the expression of the gene for the GLP-2 receptor itself and the IGF-I receptor, with greater effects on the expression of the ERB family of receptors, with a fivefold increase in the EGF receptor and a six- to sevenfold increase in the Erb-2, Erb-3, and Erb-4 receptors. For the ligands of the ErbB family, there were two- to threefold increases in mRNA expression for ampiregulin, epiregulin, and HB-EGF ligands at the early time points of 30 and 60 min after GLP-2 was added (Fig. 7B). There were more significant seven- to 50-fold increases in the expression of EGF, TNFα, and β-cellulin mRNAs, which occurred over the 1- to 2-h time point post-GLP-2 stimulation. Finally, a truly remarkable 300-fold increase in neuregulin 4 expression beginning at 60 min after GLP-2 exposure and peaking at 24 h was noted. There were equally dramatic increases in expression of the neuregulins 1 and 3, but only at the 24-h time point (Fig. 7B).
Fig. 7.
GLP-2 treatment of isolated enteric neurons increases expression of growth factor and receptors. Submucosal enteric neurons isolated from rat colon were cultured and treated with GLP-2 (10−8 M) for the times indicated, with extraction of total RNA and analysis for the receptor (A) or ligand systems (B) by qRT-PCR. Data are expressed as the fold increase from baseline relative to 18S rRNA. Data are means ± SE; n = 6 at each time point. –P < 0.05 vs. basal expression. GLP-2R, GLP-2 receptor; EGF-R, epidermal growth factor receptor.
DISCUSSION
The findings of the present study describe a mechanism for the previously observed GLP-2-induced differentiation of a VIP-expressing phenotype in enteric submucosal neurons in vivo. Using a model of a primary culture of cells derived from the submucosal plexus, GLP-2 stimulated primarily a neuronal phenotype, increasing VIP expression and acting via a specific PI3Kγ pathway activating Akt and subsequently mTOR and MEK/ERK. The activation of both the mTOR and MEK/ERK pathways appears to be required to induce the VIP-expressing phenotype. These findings extend the role of GLP-2 as a mediator of intestinal function beyond the previously known mucosal effects and suggest that GLP-2 may influence transmitter expression and function of the enteric nervous system.
The trophic effects of GLP-2 on the intestine generally, and the small intestinal mucosa specifically, have been well described. However, it has been a challenge to determine the specific cell types GLP-2 stimulates and the associated intracellular signaling pathways involved. Initial work following the identification of the receptor focused on studies using transfected GLP-2 receptor expression (21, 40). The present results are the first to examine the actions of GLP-2 on isolated enteric neurons, a major effector pathway for GLP-2 in the intestine (33, 34). The refinement of this model of primary culture of cells from the submucosal plexus greatly facilitated the examination of the pathways involved in GLP-2 activity. The diversity and robustness of the response to GLP-2 of this cell population was somewhat surprising. It supports the notion that the ENS is plastic; in this system the cells responded by rapidly changing phenotype (expressing VIP). There may also have been a proliferative effect; this will be the subject of further investigation. The identity of the majority of the cells that expressed VIP after responding to the GLP-2 stimulus as neurons is clear; this was confirmed using antibodies for the pan-neuronal markers HuC/D and β-tubulin at each step of the effects of GLP-2 on differentiation (9, 11). Similar demonstrations of GLP-2 activation and regulation of neuronal phenotype have been shown in vivo in the ENS and in cultures of embryonic hippocampal neurons (31, 33). However, a similar GLP-2-linked Akt-activated pathway was demonstrated in cultured astrocytes from the CNS (37, 38). In the present study, enteric glial cells were also shown to specifically express the GLP-2 receptor, whereas the other main constituents of the cell preparation, smooth muscle cells and the fibroblasts, did not. Thus, enteric glial cells, or possibly a pluripotential progenitor cell, which can differentiate into a neuronal phenotype (13, 14), likely respond to GLP-2 and may have undergone phenotypic changes with some expression of VIP. However, the majority of the cells responding and expressing VIP were of a classical neuronal phenotype identified by immunohistochemistry and morphology. Prolonged stimulation with GLP-2 did not drive an increase in the proportion of enteric glial cells in culture, even out to 28 days, but rather increased the proportion of cells with a neuronal phenotype, some of which are nNOS immunoreactive. Further studies are required to determine the extent to which the GLP-2-induced enteric neuronal differentiation seen in the present study may occur in vivo, how this might be affected by inflammation, and how enteric glial and neuronal progenitor cells may contribute to this (14). The fact that nNOS expression was enhanced in these cultures, but not in vivo, suggests that some changes may be specific to the culture model, and their validity would need to be assessed by direct comparison, as we have done for VIP.
Focusing on the intracellular signaling pathways involved, the present studies confirm the signaling events suggested in previous work done using intestinal myofibroblasts, CNS-derived astrocytes, and hippocampal cell models (15, 31, 37). In these systems, increased cAMP was not a constitutive element in the GLP-2 response (15, 31, 37), which was confirmed in the present study. Collectively, these results show that stimulation of the GLP-2 receptor activates PI3Kγ, which then leads to the phosphorylation of Akt. In the cells stimulated in the present study, the phosphorylation of Akt led to activation of mTOR and ERK1/2, as shown by the increased production of P70S6K following GLP-2 stimulation and the phospho-ERK/ERK ratio, which is similar to observations in CNS astrocyte and hippocampal neuronal cell systems (31, 37). In the present study, the specific involvement of these pathways in regulating differentiation was further confirmed using pharmacological blockers and a knockout model. In enteric neurons treated with PI3Kγ, ERK, or mTOR inhibitors and in cells derived from animals with a specific deletion of PI3Kγ, the increase in the proportion of cells expressing VIP with GLP-2 activation was eliminated. Blockade of PI3Kγ also eliminated the downstream increase in p-Akt, p-ERK, and P70S6K. Both ERK/MEK and mTOR pathways appear to be required for differentiation to occur; blockade of either ERK phosphorylation or mTOR resulted in a loss of the differentiation effects of GLP-2 stimulation (Fig. 8). The present results contrast somewhat with the previous description of the role of PI3Kγ in the trophic actions of GLP-2 in the murine small intestine; in this study, Anini et al. (2) used mice with a knockout of PI3Kγ activity and found that in the knockout animals there was increase in basal intestinal epithelial crypt proliferation rates, with a further GLP-2-induced increase. However, there was a loss of GLP-2-induced mucous cell enhancement and of GLP-2-induced cAMP production by the intact jejunum in the knockout animals. The suggestion from this was that PI3Kγ was not required for GLP-2 stimulation of mucosal growth but may be required in other aspects of signaling. This same group subsequently showed that PI3Kγ activity is required for GLP-2 to stimulate increased IGF-I transcription in the myofibroblast, which is thought to be the major regulator of the proliferative effects of GLP-2 in the intestine (15). It is not clear how the GLP-2-induced increase in crypt proliferation seen in vivo was mediated in the PI3Kγ-knockout animals; however, in the present study, we used the same model to examine the requirement for PI3Kγ signaling in mediating the GLP-2-induced expression of VIP in the ENS-derived cells and found that the PI3Kγ−/− animals showed no response. Further direct study of the effects of this pathway specifically on enteric neuronal and myofibroblast signaling and the more global effects of GLP-2 is required to clarify the links between the intracellular signaling by GLP-2 and the physiological responses of the intestine.
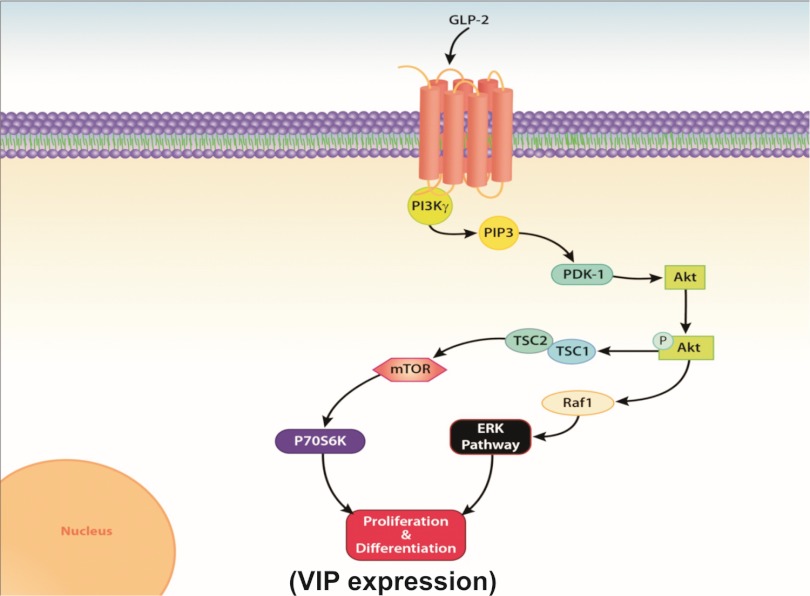
Fig. 8.
Schematic of GLP-2 activation pathways in enteric neurons. GLP-2 binds to the G protein-coupled receptor, which activates the PI3Kγ moiety, leading to phosphorylation of Akt and downstream stimulation of the mTOR and ERK pathways, which leads to the observed increase in neuronal VIP expression. PIP3, phosphatidylinositol-3,4,5-triphosphate; PDK-1, phosphoinositide-dependent kinase-1; TSC, tuberous sclerosis complex.
Interestingly, the results from these different studies suggest that the intracellular pathways stimulated by GLP-2 may diverge after Akt activation. From the foregoing, in enteric neurons and CNS astrocytes and hippocampal neurons, ERK/MEK and mTOR are implicated as important intracellular signaling pathways following GLP-2 stimulation. In contrast, in isolated intestinal myofibroblasts in culture, the ratio of p-ERK1/2 to t-ERK1/2 did not change with GLP-2 stimulation, and the primary action was an increase in IGF-I mRNA expression (15). In the present study, both the proximal signals of mTOR and ERK1/2 and the more downstream IGF-I and ErbB systems were activated with GLP-2 stimulation; in addition, the degree of GLP-2-increased IGF-I ligand transcription and the time course were similar to that described in previous studies using myofibroblasts and neurons (15, 31). The increase in the IGF-I receptor expression is greater than seen previously in astrocytes, whereas in contrast myofibroblasts showed a reduction in IGF-I receptor expression with GLP-2 stimulation. Overall, the results suggest that the IGF-I axis is likely relevant in controlling some aspects of the proliferative and differentiation response seen with GLP-2 stimulation in both neurons and myofibroblast signaling but may be regulated differently in the different cell types.
Further evidence of the specificity of the GLP-2 response in enteric neurons is shown by the remarkable increases in the ErbB family of receptors and ligands following GLP-2 stimulation. The observed increases in amphiregulin, EGF, HB-EGF, and epiregulin are similar to previously described effects of GLP-2 in intact intestinal tissues (41). However, the increases in the Erb2–4 receptor, and especially the very high levels of expression of β-cellulin and the Nrg family of ligands, are novel. These findings, coupled with the observed GLP-2 effects on cell phenotype, suggest a link between the Akt, ERK/MEK, and mTOR signaling initiated by GLP-2 and ongoing modulation by ErbB-mediated effects on neuronal function (3, 39). ErbB-Nrg signaling has been shown to be involved in the regulation of neural crest cell development, which ultimately forms the ENS (4); a corollary of this would be that GLP-2 might be involved in regulating the development of the ENS. In support of this idea, GLP-2 has been shown to have a high level of expression in the immature gut; indeed, in human infants and mouse models, levels of both the hormone and receptor peak in the later phases of gestation, when intestinal development is maximal (1, 3, 19). This will require more direct study to validate.
Of considerable potential importance is that the potent effects of GLP-2 on the growth and neurotransmitter profile of the enteric neurons in vitro suggest a role for GLP-2 in regulating the ENS phenotype in the mature state in vivo. The relative role of the different receptor/ligand combinations stimulated in the present study is unclear, but the early responses of the EGF-related ligands and the EGF receptor are likely important; similar patterns of expression have been described with increases in cell division and differentiation in the intestinal mucosa (30). GLP-2 may be an intermediary induced by nutrient availability and regulating the function and phenotype of the enteric neuronal system. Further work is needed to explore these relationships and the potential role these systems may have in regulating ENS form and function.
The implications for this will depend on the cumulative effect of GLP-2 stimulation within the different plexuses of the bowel. In the present study, we have focused on the submucosal plexus. The present evidence would suggest that high levels of endogenous nutrients would increase the proportion of neurons expressing VIP over time. Given the normal functions of VIP as a promoter of secretion and as an anti-inflammatory mediator, the net effect might be anticipated to be an increase in some aspects of basal secretion, reduction in permeability, and a decrease in the inflammatory status of the bowel. These observations fit at least in part the observed effects of GLP-2 in inflammatory states (12, 34). The converse may also be true; many patients with intestinal inflammation are deprived of nutrients either because of general malaise and dyspepsia or because of therapeutic interventions such as parenteral nutritional support. Over time it has been observed that patients with intestinal inflammation have changes in the pattern of neurons encoded (22, 29). Given the present evidence, it may be that this change in transmitter expression may be signaled by a reduction in ongoing endogenous GLP-2 levels as a consequence of the lack of enteral stimulation. These results are relevant in linking the activity of the nutrient-related signaling of GLP-2 with the activity and overall function of the ENS, especially in the submucosal plexus. This may be important in both physiological as well as pathophysiological states of intestinal function.
GRANTS
Funding was provided by operating grants from the Crohn's and Colitis Foundation of Canada (to D. L. Sigalet) and the Canadian Institutes of Health Research (to K. A. Sharkey). Infrastructure support for the Sigalet laboratory comes from the Professorship in Pediatric Surgical Research, funded by the Alberta Children's Hospital Research Foundation. Confocal microscopy facilities were supported by the Live Cell Imaging Facility of the Calvin, Phoebe, and Joan Snyder Institute of Chronic Diseases (University of Calgary, Calgary, AB, Canada), funded by an equipment and infrastructure grant from the Canadian Foundation for Innovation.
DISCLOSURES
D. L. Sigalet has acted as a paid consultant regarding the development of a GLP-2 ligand for Nycomed. K. A. Sharkey has acted as a paid consultant for NPS Pharmaceuticals. The remaining authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
E.d.H., L.E.W., K.A.S., and D.L.S. did the conception and design of the research; E.d.H., L.E.W., and D.L.S. performed the experiments; E.d.H., L.E.W., K.A.S., and D.L.S. analyzed the data; E.d.H., L.E.W., K.A.S., and D.L.S. interpreted the results of the experiments; E.d.H., L.E.W., K.A.S., and D.L.S. prepared the figures; E.d.H., L.E.W., and D.L.S. drafted the manuscript; E.d.H., L.E.W., K.A.S., and D.L.S. edited and revised the manuscript; E.d.H., L.E.W., K.A.S., and D.L.S. approved the final version of the manuscript.
REFERENCES
- 1.Amin H, Holst JJ, Hartmann B, Wallace L, Sigalet D. Functional ontogeny of the proglucagon derived peptide axis in human neonates. Pediatrics 121: e180–e186, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Anini Y, Izzo A, Oudit GY, Backx PH, Brubaker PL. Role of phosphatidylinositol-3 kinase-γ in the actions of glucagon-like peptide-2 on the murine small intestine. Am J Physiol Endocrinol Metab 292: E1599–E1606, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res 315: 611–618, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev 12: 1825–1836, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevalier J, Derkinderen P, Gomes P, Thinard R, Naveilhan P, Vanden Berghe P, Neunlist M. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol 586: 1963–1975, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131: 589–605, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Dubé PE, Rowland KJ, Brubaker PL. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149: 291–301, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Esper RM, Pankonin MS, Loeb JA. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev 51: 161–175, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Furness JB. Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. Auton Neurosci 125: 81–85, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Furness JB, Alex G, Clark MJ, Lal VV. Morphologies and projections of defined classes of neurons in the submucosa of the guinea-pig small intestine. Anat Rec A Discov Mol Cell Evol Biol 272: 475–483, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ivory CP, Wallace LE, McCafferty DM, Sigalet DL. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 295: G1202–G1210, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Joseph NM, He S, Quintana E, Kim YG, Núñez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest 121: 3398–3411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden BP, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121: 3412–3424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leen JL, Izzo A, Upadhyay C, Rowland KJ, Dube PE, Gu S, Heximer SP, Rhodes CJ, Storm DR, Lund PK, Brubaker PL. Mechanism of action of glucagon-like peptide-2 to increase IGF-I mRNA in intestinal subepithelial fibroblasts. Endocrinology 152: 436–446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Z, Sandgren K, Ekblad E. Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton Neurosci 114: 29–38, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci 126–127: 250–257, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lomax AE, O'Hara JR, Hyland NP, Mawe GM, Sharkey KA. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am J Physiol Gastrointest Liver Physiol 292: G482–G491, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lovshin J, Yusta B, Iliopoulos I, Migirdicyan A, Dableh L, Brubaker PL, Drucker DJ. Ontogeny of the glucagon-like peptide-2 receptor axis in the developing rat intestine. Endocrinology 141: 4194–4201, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Mawe GM, Collins SM, Shea-Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol Motil 16, Suppl 1: 133–136, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96: 1569–1573, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 52: 84–90, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119: 1467–1475, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124: 105–112, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Roberge JN, Brubaker PL. Secretion of proglucagon-derived peptides in response to intestinal luminal nutrients. Endocrinology 128: 3169–3174, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7: 191–201, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287: 1040–1046, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn's disease. Neurogastroenterol Motil 13: 255–264, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Sheng G, Guo J, Warner BW. Epidermal growth factor receptor signaling modulates apoptosis via p38α MAPK-dependent activation of Bax in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 293: G599–G606, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Shi X, Li X, Wang Y, Zhang K, Zhou F, Chan L, Li D, Guan X. Glucagon-like peptide-2-stimulated protein synthesis through the PI 3-kinase-dependent Akt-mTOR signaling pathway. Am J Physiol Endocrinol Metab 300: E554–E563, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc 70: 185–193, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Sigalet DL, Wallace L, De Heuval E, Sharkey KA. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterol Motil 22: 1318–e350, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293: G211–G221, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nürnberg B, Gierschik P, Seedorf K, Hsuan JJ, Waterfield MD, Wetzker R, Dhand R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269: 690–693, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Svenningsen AF, Shan WS, Colman DR, Pedraza L. Rapid method for culturing embryonic neuron-glial cell cocultures. J Neurosci Res 72: 565–573, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Velázquez E, Blázquez E, Ruiz-Albusac JM. Synergistic effect of glucagon-like peptide 2 (GLP-2) and of key growth factors on the proliferation of cultured rat astrocytes. Evidence for reciprocal upregulation of the mRNAs for GLP-2 and IGF-I receptors. Mol Neurobiol 40: 183–193, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Velázquez E, Ruiz-Albusac JM, Blázquez E. Glucagon-like peptide-2 stimulates the proliferation of cultured rat astrocytes. Eur J Biochem 270: 3001–3009, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Yusta B, Boushey RP, Drucker DJ. The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J Biol Chem 275: 35345–35352, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 137: 986–996, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744–755, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Yusta B, Somwar R, Wang F, Munroe D, Grinstein S, Klip A, Drucker DJ. Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibroblasts expressing the rat GLP-2 receptor. J Biol Chem 274: 30459–30467, 1999 [DOI] [PubMed] [Google Scholar]