Abstract
Previously, we reported that fenvalerate (Fen) promotes proliferation of human uterine leiomyoma (UtLM) cells by enhancing progression of cells from G0-G1 to S phase through molecular mechanisms independent of estrogen receptor-α and -β. The cyclin-dependent kinase (CDK) inhibitor p27, which blocks G1 to S phase transitions and is an important regulator of CDK2, is often decreased in hormonally regulated diseases, including uterine leiomyomas. Therefore, we were interested in whether Fen could regulate the expression of p27 and whether p27 might play a role in Fen-induced cell proliferation. Expression of p27 in Fen-treated UtLM and uterine smooth muscle cells (UtSMCs) was examined. We found that p27 mRNA was significantly downregulated and that protein levels were decreased in both cell types treated with 10 μM Fen for 24 h compared with respective controls. Overexpression of p27 in UtLM cells and UtSMCs using an adenovirus doxycycline (Dox)-regulated Tet-off system abrogated the proliferative effects of Fen, as evidenced by decreased total cell numbers and BrdU incorporation. Fen treatment increased CDK2 mRNA expression levels; however, overexpression of p27 also abolished this effect. In contrast, Dox treatment dramatically restored the above muted responses. Finally, we utilized siRNA to knock down p27 expression. After transfection, mRNA levels of p27 were downregulated in UtLM cells and UtSMCs and total cell numbers and BrdU incorporation increased significantly compared with nontransfected cells. Fen treatment in the presence of p27 silencing enhanced the increased cell counts and BrdU labeling in UtLM cells and UtSMCs. Taken together, these results indicate that p27 downregulation is critical for Fen-induced cell proliferation.
Keywords: fibroids, endocrine-disrupting chemical
uterine leiomyoma is one of the most common benign neoplasms and a major cause of hysterectomy in the US (22). Great efforts have been invested to screen and identify potential risk factors for this disorder. Among these, we reported recently that fenvalerate (Fen), an insecticide, can induce fibroid cell proliferation and overproduction of extracellular matrix components (10), suggesting its potential role as a candidate environmental endocrine-disrupting chemical (EDC) for women's health. Given that Fen has been widely used in industrial and household settings (17a), it is of importance to explore and understand the underlying mechanisms involved in the proliferative and collagen-inducing effects of Fen on fibroid cells.
Fen was shown initially to be an estrogen analog, exhibiting weak estrogenic activity in various types of tumor cell lines (11). Therefore, it was assumed that the estrogen receptor (ER) could serve as a mediator of Fen-induced effects. Inconsistent with this notion was an earlier study by Go et al. (14), which documented that Fen-induced proliferation of MCF-7 cells was resistant to antiestrogen treatment. Additionally, our earlier findings showed that Fen was less likely to act through the estrogen receptor due to its lack of binding or significant transactivation of either α- or β-isoforms of the ER (10). Collectively, current available data add more complexities to how Fen exhibits estrogen-like activity via an ER-independent manner. It leans toward an alternative approach to explore the intracellular target molecules of Fen apart from screening its potential ability to bind the ER.
The cyclin-dependent kinase inhibitor p27 is important in blocking the progression of cells from G1 to S phase. Reduced p27 has been observed in tumors of many cell types and tissues, and associated with poor prognosis, as well as a predictor of treatment response (1). Lahav-Baratz et al. (21) demonstrated that p27 is significantly lower in endometrial carcinoma compared with normal endometrium. Furthermore, upregulation of p27 by progestin can suppress growth of human endometrial cells (27). In contrast, downregulation of p27 abrogates anti-estrogen-mediated cell cycle arrest in human breast cancer cells (7). These data suggest a role for p27 in neoplasia of the female reproductive tract as a possible tumor suppressor.
Moreover, accumulating evidence suggests that p27 is likely to be modified in tumors and other disease processes. Translational, posttranslational, and epigenetic mechanisms have all been documented to contribute to the regulation of p27 expression (6). This diversity in regulatory properties also positions this molecule to be susceptible to various stimuli. In other words, p27 holds the potential to be a target for tumorigenesis as well as prevention and therapeutic intervention strategies.
In this study, we hypothesize that reduction of p27 plays an essential role in mediating Fen-induced cell proliferation in uterine fibroid and myometrial cells. To test this hypothesis, we utilized human uterine leiomyoma (UtLM) cells and uterine smooth muscle cells (UtSMCs) as our in vitro modeling system. We first aimed to test whether Fen could impact p27 expression. Next, we sought to examine whether overexpression of p27 abrogates Fen-induced cell proliferation and whether silencing p27 results in cell proliferation in both cell types. Our study provides evidence that p27 is an intracellular target molecule for Fen and offers a potential mechanism for Fen-induced effects in human uterine fibroid and myometrial cells.
MATERIALS AND METHODS
Materials
The materials for immunoblotting and small interfering RNA (siRNA) were purchased from Invitrogen (Carlsbad, CA), real-time PCR-related reagents were from Applied Biosystems (Foster City, CA), materials for immunofluorescence were purchased from Vector Laboratories (Burlingame, CA), and materials for adenovirus infections were gifted (please see below) or purchased from Clontech (Mountain View, CA). Unless specified, all other materials were purchased from Sigma (St. Louis, MO).
Cell lines, Reagents, and Antibodies
Commercially available (established) human UtLM cells (GM10964; Coriell Institute for Medical Research, Camden, NJ) and UtSMCs (Clonetics, San Diego, CA) were maintained in a standard tissue culture incubator at 37°C with 5% carbon dioxide and maintained with media, as described previously (10). Prior to treatment, the media were changed to DMEM-F-12 with charcoal/dextran-treated fetal bovine serum (HyClone, Logan, UT) for both cell types. Fen (CAS no. 51630-58-1) was dissolved in DMSO to make a stock solution, and the final concentration of DMSO in culture medium was calculated to be 0.01%. Based on our previous findings (10), 10 μM Fen was selected as the optimal dose for the study.
Confocal Immunofluorescence Staining
UtLM cells and UtSMCs were cultured in 35-mm glass bottom culture dishes (MatTek, Ashland, MA) and treated with 10 μM Fen or vehicle for 24 h. Cells were then fixed in 4% paraformaldehyde in PBS for 20 min. Fixed cells were then incubated with a monoclonal mouse anti-p27 primary antibody (Cell Signaling Technology, Danvers, MA) at 4°C overnight, followed by incubation with Alexa fluor 594 donkey anti-mouse (red fluorescence) secondary antibody (Invitrogen) at room temperature for 1 h. Next, cells were counterstained with 100 ng/ml 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes) for 30 min. All samples were stained and observed on the same day using identical imaging conditions. Confocal images were taken on a Zeiss LSM510-UV meta (Carl Zeiss, Oberkochen, Germany) using a Plan-Apochromat ×40/1.2 Water DIC objective. The 543-nm laser line from a helium neon laser at 99% power was used for excitation of the Alexa fluor 594. Subsequently, a 560-nm long-pass emission filter was used to collect the images of the Alexa fluor 594 with a pinhole setting of one airy unit. The 364-nm laser line from an Enterprise laser at 100% power was used for excitation of DAPI, whereas a band pass 385–470 filter was used for collection of the emission signal. The PMT voltage gain was held constant for all images, with a setting of 660 V for the DAPI channel and 897 V for the Alexa fluor 594. Furthermore, all images were taken with a zoom of 1.5, a 3.2-μs pixel dwell time, and a 0.29-μm pixel size, with line averaging set to 4.
Overexpression of p27 by an Inducible Adenovirus System
Preparation of inducible adenovirus-p27.
An inducible adenovirus Tet-off system was used (Clontech). Adenoviral plasmid containing human p27-adenvirus-p27 (Adp27; kindly gifted by Dr. Francois X. Claret, The University of Texas M. D. Anderson Cancer Center) was introduced in UtLM cells and UtSMCs to overexpress p27. The preparation of recombinant adenovirus vector was done according to previously published protocols (23). Briefly, human embryonic kidney-293 cells were infected with adenovirus at a multiplicity of 5 pfu/cell. After incubation for 90 min at 37°C and 5% CO2, fresh medium was added into the flask. When ∼50% of the cells had detached, the suspension was transferred to a 15-ml conical centrifuge tube. Viruses were isolated by a freeze-thaw method (20). Adenoviral titers were determined by Adeno-X Rapid Titer Kit (Clontech).
Infection with Adp27.
A count of 2 × 105 UtLM cells or UtSMCs was seeded in 10-cm cell culture dishes and incubated at 37°C overnight. Then, both cell lines were coinfected by a method described previously by Kouvaraki et al. (20) and Zhang et al. (31). We found that our optimal multiplicity of infection was 10. The cells were then cultured in media containing 10% Tet System-approved FBS (Clontech) for 48 h, with or without the presence of doxycycline (Dox; 1 μg/ml; Clontech). Of note, media for both cell lines were replaced every 48 h. After 48–72 h, cells were harvested for verification of the expression of p27 by Western blot or real-time PCR analysis.
Fen intervention.
After infection with Adp27, UtLM cells and UtSMCs were pretreated with phenol-red free DMEM-F-12 with charcoal/dextran-treated FBS (HyClone) for 24 h and then exposed to medium with or without 10 μM Fen for another 24 h. Cells were then harvested for further analysis, including cell counts, bromodeoxyuridine (BrdU) incorporation, immunoblotting analysis, and real-time PCR.
Suppression of p27 Expression by siRNA
To knock down expression of p27, a synthesized double-stranded RNA interference system was used according to the manufacturer's instructions. Briefly, stealth RNAi siRNA targeting the human p27 gene (5′ to 3′ sequence CGG CUA ACU CUG AGG ACA CGC AUU U) and a control scrambled siRNA containing a nonsense sequence (5′ to 3′ sequence CGG UCA AAG UCC AGG CGC AAU CUU U) were produced by Invitrogen and transfected into prepared UtLM cells and UtSMCs with Lipofectamine (Lipo) RNA iMAX Transfection Reagent (Invitrogen). To determine the optimal working conditions of siRNA, cellular RNA was harvested for assessing efficacy of specific knockdown by real-time PCR.
After transfection with siRNA overnight, the transfection medium was replaced by phenol-red free DMEM-F-12 with charcoal/dextran-treated FBS (HyClone) for 24 h, and cells were then subjected to treatment with media with or without 10 μM Fen for another 24 h. At the end of 24 h, cells were harvested for further analysis, including cell counts, BrdU incorporation, and real-time PCR.
Cell Counts
After cells reached 70–80% confluency, Fen (10 μM) or control reagent (DMSO) was supplemented into culture medium for 24 h. After treatments, cells were harvested using trypsin for enumeration with a Cellometer (Cellometer Auto T4; Nexcelom Bioscience, Lawrence, MA). Mean cell count numbers for each group were obtained by averaging nine dishes from three independent experiments. Cell number changes were interpreted as mean cell number of the treated group divided by that of the respective vehicle control group.
BrdU Incorporation Analysis
To analyze cell proliferation, BrdU incorporation was measured by using a commercially available kit (Roche, Indianapolis, IN). Various groups of UtLM cells or UtSMCs were first incubated with 0 μM (0.01% DMSO, control) or 10 μM Fen for 18 h. After that, BrdU was supplemented into the medium for 6 h. During this incubation, BrdU was covalently incorporated into the 3′ DNA ends. Following the BrdU incorporation period, the labeled cells were rinsed thoroughly and incubated with a FITC-labeled antibody against BrdU for 30 min. The stained cells were then harvested and analyzed by a fluorescence microplate reader (SpectraMax M5; Molecular Devices). Fold changes of BrdU incorporation of individual samples were normalized by the mean fluorescence intensity of the control group.
Real-Time Reverse Transcriptase-PCR Analyses
Total RNA was extracted from cells with TRIzol Reagent (Invitrogen) and purified further by the RNeasy Mini Kit from Qiagen (Valencia, CA). Reverse transcriptase was executed with 2 μg RNA by using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real time PCR was performed using the ABI Prism 7900HT Sequence Detection System with SYBR Green PCR Master Mix reagents (Applied Biosystems) according to the manufacturer's protocol. The sequences for each primer set were generated by Primer Express 3.0 (Applied BioSystems). The following is a detailed description of each primer set: p27, 5′-GCA CAC TTG TAG GAT AAG TGA AAT GG-3′ (forward) and 5′-CCT ATT CTA CCC AAC ACA GCA TTT AC-3′ (reverse); cyclin-dependent kinase-2 (CDK2), 5′-GAC TCG CTG GCG CTT CA-3′ (forward) and 5′-CGT GCC CTC TCC GAT CTT T-3′ (reverse); GAPDH, 5′-GAG TCA ACG GAT TTG GTC GT-3′ (forward) and 5′-TTG ATT TTG GAG GGA TAT CG-3′ (reverse). GAPDH was used as the housekeeping gene. Data were obtained as CT (cycle no. at which PCR plots cross a calculated threshold line) values. Visual representation of data was carried out as reported previously (10) (method of 2−ΔΔCT).
Western Blot Analysis
To determine expressional changes at the protein level, Western blot analysis was done as described previously (10). Briefly, whole cell lysates were prepared by using a radioimmunoprecipitation assay buffer as reported previously (30). Protein concentrations were measured by Pierce BCA Protein Assay (Thermo Scientific, Rockford, IL). Aliquots of the protein underwent electrophoreses on a NuPAGE 4–12% Bis-Tris Gel (Life Technology, Carlsbad, CA) and were electrotransferred onto 0.22-μM PVDF membranes (Millipore, Billerica, MA). After blocking with 5% BSA (wt/vol) for 1 h, blots were incubated with a monoclonal rabbit anti-p27 primary antibody (1:1,000; Cell Signaling Technology). Horseradish peroxidase-conjugated secondary antibodies (1:5,000, ECL; GE Healthcare) were used for detection. Horseradish peroxidase-labeled anti-human hypoxanthine phosphoribosyltransferase antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) served as an internal control protein. The blots were detected with ECL Western Blotting Detection Reagents (GE Healthcare). The membranes were exposed to X-ray film (Kodak, Rochester, NY) and went through a Konica Minolta SRX-101A tabletop processor (Wayne, NJ). The density of the respective band intensities was quantitated by using a densitometer with AlphaView software (FluorChem SP; Alpha Innotech, San Leandro, CA). The signal ratio of each target protein over internal control was generated from at least three independent experiments.
Statistical Analysis
All of the experiments were performed at least three times in duplicate, using independent cell cultures. Results were expressed as means ± SE and were analyzed statistically with two-tailed Student's t-test. P values <0.05 were considered significantly different.
RESULTS
Fen Decreased Expression of p27 in Both UtLM Cells and UtSMCs
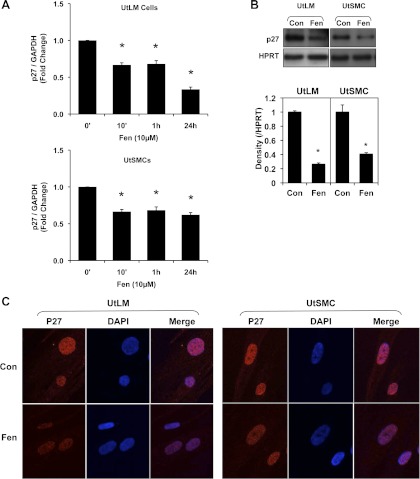
The first question we sought to answer was whether expression of p27 is impacted by Fen. By real-time PCR, we found that p27 mRNA expression in UtLM cells was decreased after treatment with Fen at 10 min and reached its lowest level, which was less than one-half that of control (0.335 ± 0.031, P < 0.05, vs. 0 h), at 24 h (Fig. 1A). A similar trend was observed in UtSMCs, but to a lesser extent at 24 h (0.620 ± 0.021, P < 0.05, vs. 0 h; Fig. 1A). To determine whether Fen decreased protein expression of p27 in addition to mRNA level, immunoblots were performed on cell lysis samples from cells treated with or without Fen for 24 h. As shown in Fig. 1B, bands of p27 in UtLM cells or UtSMCs incubated with Fen were less intense compared with respective vehicle controls. It has been reported that p27 is located in the nucleus of cells (5). We were further interested as to whether Fen impacts the localization of p27 in the above two cell types. Using confocal microscopy, we found that p27 was expressed (red fluorescence) within nuclei of both cell types treated with or without Fen; however, as evidenced by nuclear staining with DAPI (blue fluorescence), the intensity of p27 appeared to be less intense in Fen-treated cells compared with those without Fen treatment (Fig. 1C).
Fig. 1.
Fenvalerate (Fen) decreased p27 expression in uterine leiomyoma (UtLM) and uterine smooth muscle cells (UtSMCs). A: p27 mRNA expression was downregulated by 10 μM Fen in UtLM cells and UtSMCs. Fold changes were standardized by control (0). B: protein expression of p27 was decreased following treatment with 10 μM Fen for 24 h in UtLM cells and UtSMCs. C: localization of p27 in UtLM cells and UtSMCs. The intensity of p27 fluorescence was decreased by 10 μM Fen treatment at 24 h. Note that p27 is shown as red fluorescence by Alexa fluor secondary antibody, and nuclei are shown as blue fluorescence by 4,6-diamidino-2-phenylindole (DAPI). All of the experiments were repeated at least 3 independent times. *P < 0.05 vs. control. Error bars represent SE; n ≥ 3. HPRT, hypoxanthine phosphoribosyltransferase. Con, control.
Proliferative Response to Fen was Abrogated by Overexpression of p27 by Adenovirus in UtLM Cells and UtSMCs
To determine whether there was an association between the Fen-induced decrease in p27 expression observed and Fen-induced proliferation previously observed in UtLM cells and UtSMCs, we introduced an inducible adenovirus Dox-regulated Tet-off system (Adenovirus-X Tet-off System) to overexpress p27 in UtLM cells and UtSMCs. We successfully confirmed that the Tet-off system resulted in overwhelming stable expression of p27 from 48 to 96 h after induction (data not shown). Thus, we chose this period as our interventional window for Fen.
UtLM cells.
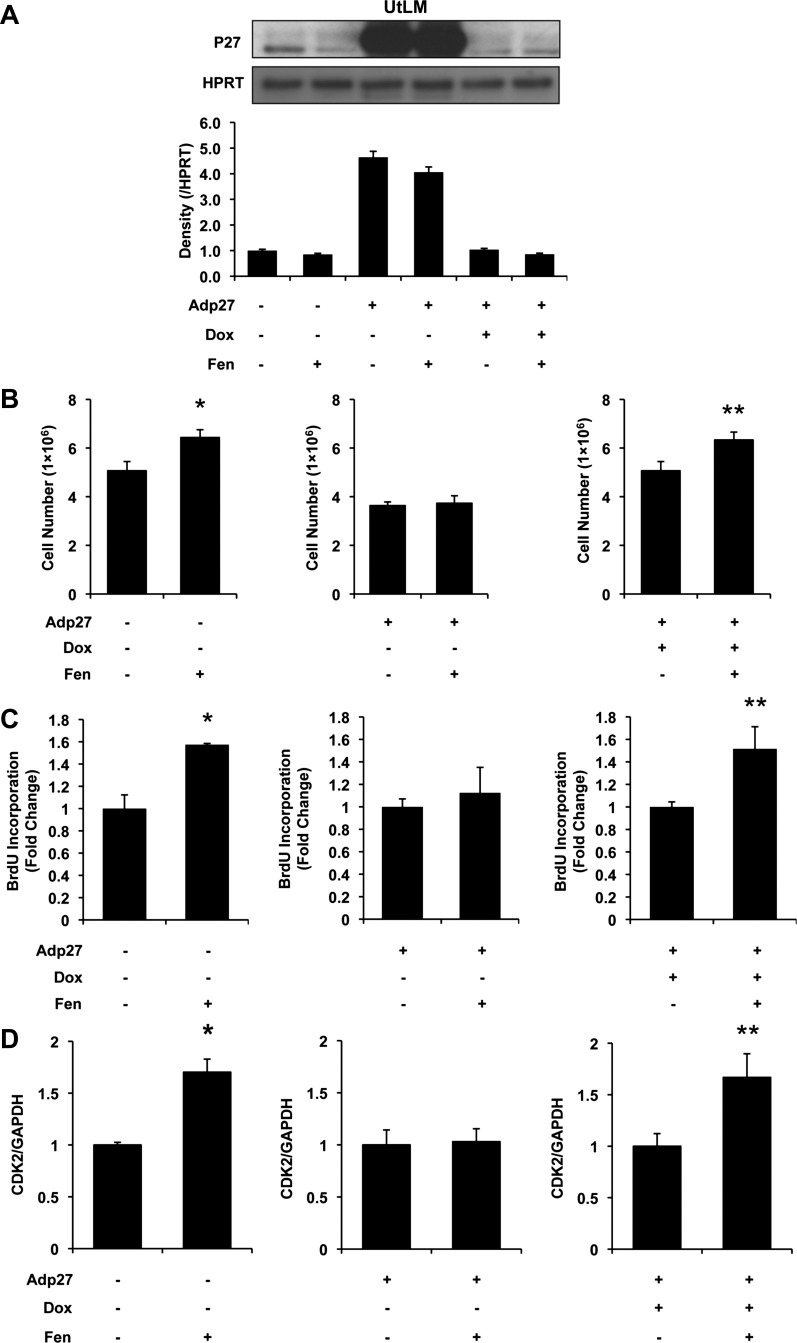
As indicated in Fig. 2A, protein expression of p27 was detectable in noninfected vehicle control UtLM cells, and Fen was shown to decrease p27 expression at 24 h. In contrast, overexpression of p27 showed increased p27 protein expression, and this did not change significantly in UtLM cells following Fen treatment. When p27 overexpression was “shut off” by Dox treatment, p27 levels were minimal. Similar results were seen when Dox treatment was followed by Fen administration. Under these conditions, we next compared Fen-induced proliferative effects with the presence or absence of p27 overexpression. By directly counting cell numbers (Fig. 2B), we found that Fen treatment resulted in an increase in numbers of noninfected UtLM cells compared with controls; however, Fen was unable to induce a proliferative response in UtLM cells that overexpressed p27. As to be expected, the proliferative effects of Fen were restored when overexpressed p27 was suppressed in the “Tet-off” system in the presence of Dox.
Fig. 2.
Influence of human adenovirus-p27 (Adp27) on Fen-induced cell proliferation and cyclin-dependent kinase-2 (CDK2) mRNA expression in UtLM cells at 24 h. A: p27 expression measured by Western blot analysis. Bars from left to right: control, Fen, Adp27, Adp27 + Fen, Adp27 + doxycycline (Dox), and Adp27 + Dox + Fen. Note that p27 in noninfected cells was decreased by Fen. In Adp27-infected cells, p27 was overexpressed. In the presence of Dox, exogenous expression of p27 was turned off. B: cell counts. Cell numbers were increased in noninfected cells treated with 10 μM Fen. In Adp27-infected cells, this effect was abolished. In the presence of Dox, Fen-induced increased cell numbers were restored. C: bromodeoxyuridine (BrdU) incorporation. BrdU incorporation was increased after treatment with 10 μM Fen in noninfected cells. In Adp27-infected cells, this effect was abolished. In the presence of Dox, Fen-induced increased BrdU incorporation was restored. D: CDK2 mRNA expression. CDK2 mRNA expression was increased after 10 μM Fen treatment in noninfected cells. In Adp27-infected cells, this effect was abolished. In the presence of Dox, Fen-induced increased CDK2 mRNA levels were restored. All experiments were repeated at least 3 times with independent cell cultures. Fold changes were standardized to controls. *P < 0.05 vs. control group; **P < 0.05 vs. Adp27 + Dox group. Error bars represent SE; n ≥ 3.
BrdU incorporation is a classic method to measure DNA synthesis and considered to be a surrogate procedure to evaluate proliferation. Therefore, we tested whether p27 plays a role in Fen-induced DNA synthesis. Not surprisingly, Fen was found to facilitate BrdU incorporation significantly in noninfected UtLM cells compared with controls (Fig. 2C). Interestingly, overexpressed p27 substantially disrupted BrdU incorporation in UtLM cells, whereas Dox treatment resulted in significantly increased BrdU incorporation in the presence of Fen.
CDK2 is a molecule associated with G1/S-dependent cell proliferation and has been shown to function as a complex of cyclin-CDK2, which can be inhibited by p27. We hypothesized that p27 levels might have an affect on modulating expression of CDK2. As indicated in Fig. 2D, 10 μM Fen significantly elevated CDK2 mRNA levels nearly twofold (P < 0.05). In contrast, Fen was not able to induce significant changes in CDK2 mRNA following overexpression of p27 in UtLM cells; however, Dox treatment successfully restored Fen-induced CDK2 mRNA levels.
UtSMCs.
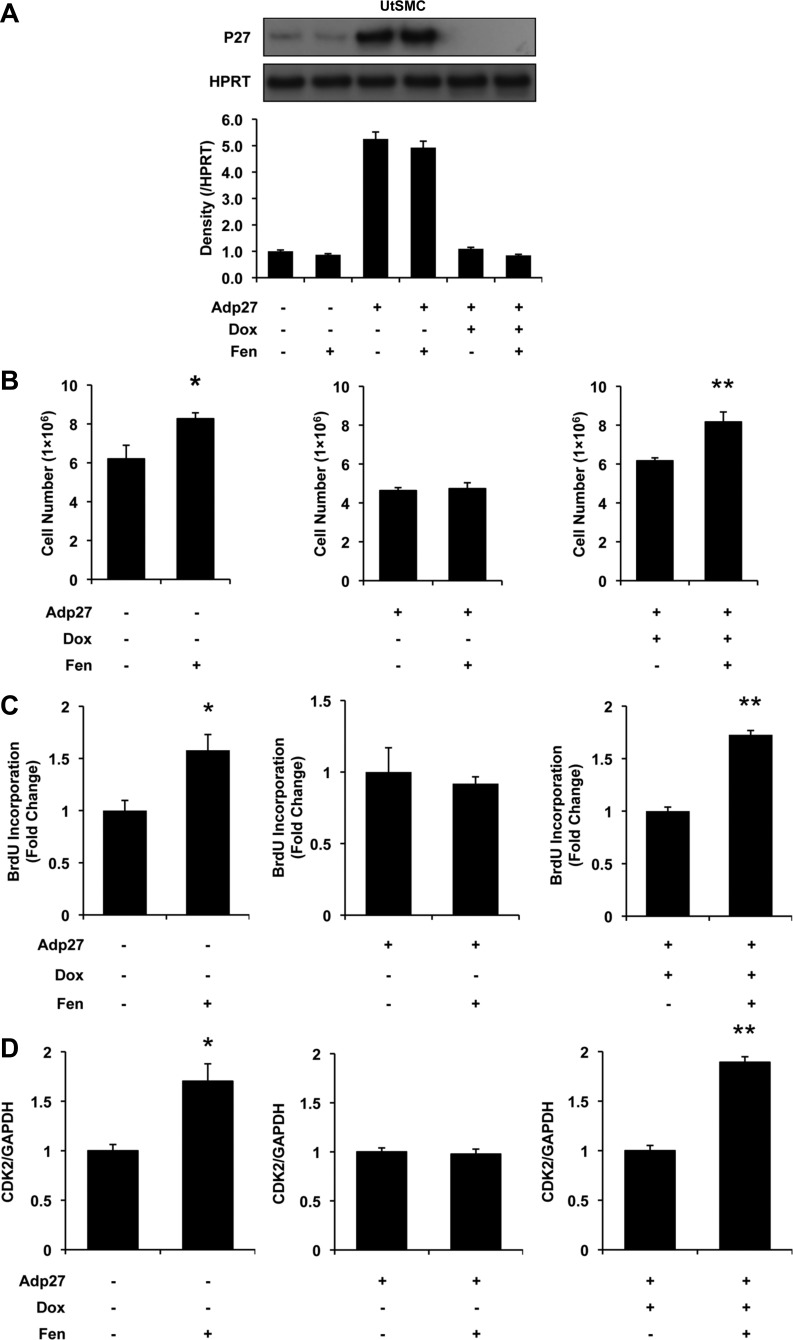
To test whether the effects observed in the UtLM cells would be present in UtSMCs, we conducted similar experiments in the UtSMCs accordingly. In noninfected UtSMCs, 10 μM Fen decreased the protein level of p27 significantly, as detected by Western blotting (Fig. 3A). Also, 10 μM Fen increased cell counts significantly (P < 0.05; Fig. 3B) with accompanying elevated BrdU incorporation rates (P < 0.05; Fig. 3C). Moreover, CDK2 mRNA levels were increased (P < 0.05; Fig. 3D) after 10 μM Fen treatment for 24 h.
Fig. 3.
Influence of Adp27 on Fen-induced cell proliferation and CDK2 mRNA expression in UtSMCs at 24 h. A: p27 expression measured by Western blot analysis. Bars from left to right: control, Fen, Adp27, Adp27 + Fen, Adp27 + Dox, and Adp27 + Dox + Fen. Note that p27 expression was decreased by 10 μM Fen in noninfected cells. p27 was overexpressed in Adp27-infected cells. In the presence of Dox, exogenous expression of p27 was turned off. B: cell counts. Cell numbers were increased after 10 μM Fen in noninfected cells. In Adp27-infected cells, this effect was abolished. In the presence of Dox, Fen-induced increased cell numbers were restored. C: BrdU incorporation. incorporation of BrdU was increased after 10 μM Fen in noninfected cells. In Adp27-infected cells, this effect was abolished. In the presence of Dox, Fen-induced increased BrdU incorporation was restored. D: CDK2 mRNA expression. CDK2 mRNA expression was increased after 10 μM Fen in noninfected cells. In Adp27-infected cells, this effect was abolished. In the presence of Dox, Fen-induced increased CDK2 mRNA levels were restored. All experiments were repeated at least 3 times with independent cell cultures. Fold changes were standardized to controls. *P < 0.05 vs. control group; **P < 0.05 vs. Adp27 + Dox group. Error bars represent SE; n ≥ 3.
In contrast, when p27 was overexpressed in UtSMCs, the proliferative effects induced by Fen were eliminated, as shown in Fig. 3B. Similar results were detected in BrdU incorporation and CDK2 mRNA expression levels (Fig. 3, C and D, respectively).
However, in the presence of Dox, which terminated overexpression of p27 in UtSMC cells, cellular responses to Fen were brought back to what we observed originally in the absence of overexpression for cell proliferation, BrdU incorporation, and CDK2 mRNA levels (P < 0.05; Fig. 3, B–D).
These results suggest that downregulation of p27 plays a critical role in Fen-induced proliferation in UtLM cells and UtSMCs, which may be associated partially with cell cycle molecules like CDK2.
Knockdown of p27 by siRNA was Sufficient to Induce Proliferative Response in UtLM Cells and UtSMCs
We hypothesized that downregulation of p27 alone is sufficient to induce proliferative responses in UtLM cells and UtSMCs. Moreover, downregulation of p27 has an “add-on” effect to the proliferative effect of Fen. To address this hypothesis, we used specifically synthesized siRNA to knock down p27 expression. By real-time PCR, we were able to optimize our siRNA concentration to 250 pmol/l and demonstrated that a >70% downregulation of p27 could be obtained for 24–72 h after transfection (data not shown).
UtLM cells.
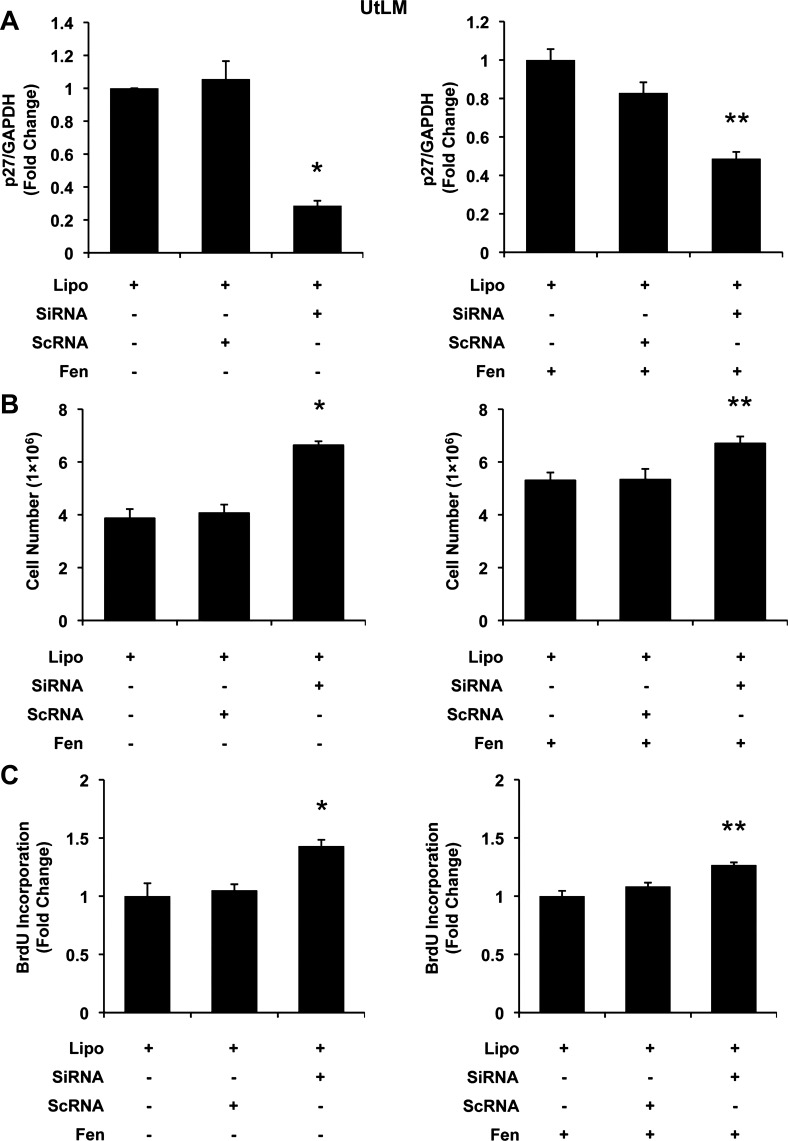
As shown in Fig. 4A, siRNA resulted in downregulation of p27 mRNA levels in UtLM cells to 0.286 ± 0.030 (P < 0.05). This silencing resulted in significantly increased proliferation (P < 0.05; Fig. 4B) and accompanying increased BrdU incorporation (1.431 ± 0.054, vs. Lipo, P < 0.05; Fig. 4C) and suggests that knockdown of p27 alone is sufficient to induce cellular proliferation in UtLM cells.
Fig. 4.
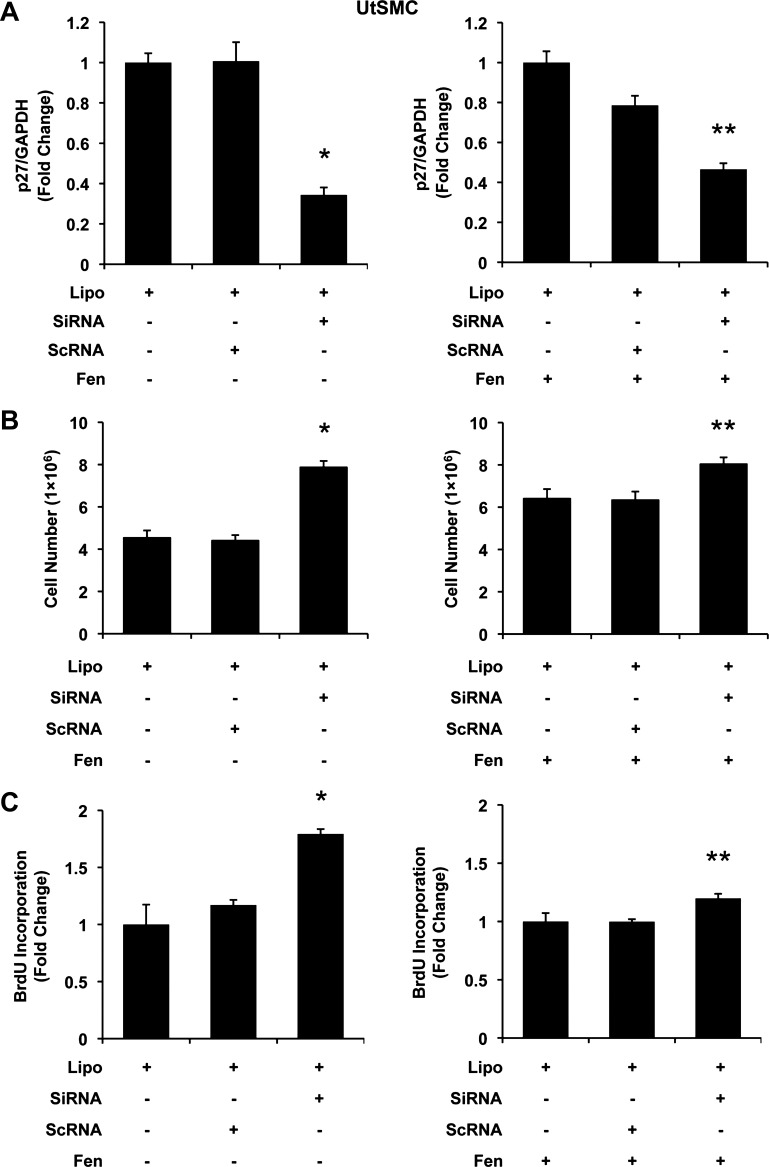
The effects of p27 small interfering RNA (siRNA) on Fen-induced cell proliferation in UtLM cells. A: p27 mRNA expression. Left (bars from left to right): Lipofectamine (Lipo; control), Lipo + scrambled siRNA (scRNA), and Lipo + siRNA targeting human p27 gene (siRNA). Right (bars from left to right): Lipo + Fen (control), Lipo + Fen + scRNA, and Lipo + Fen + siRNA. In the presence of siRNA, p27 mRNA was largely diminished. B: cell counts. Cell numbers were increased in siRNA-treated groups. C: BrdU incorporation. Incorporation of BrdU was increased in siRNA-treated groups. All experiments were repeated at least 3 times with independent cell cultures. Fold changes were standardized to controls. *P < 0.05 vs. Lipo; **P < 0.05 vs. Lipo + Fen. Error bars represent SE; n ≥ 3.
Moreover, in the presence of 10 μM Fen, similar doses of siRNA were able to decrease p27 mRNA to 0.487 ± 0.035. However, within this degree of further reduction in p27, an additional proliferative effect was detected from 5.3 × 106 to 6.7 × 106 cells by cell counting (P < 0.05; Fig. 4B) as well as 1.267 ± 0.023 by measuring BrdU incorporation rate (P < 0.05; Fig. 4C). Instructively, these pieces of data suggested that further downregulation of p27 may enhance the proliferative effects of Fen in UtLM cells.
UtSMCs.
To confirm that the effects observed in UtLM cells would also exist in UtSMC cells, we performed similar experiments in UtSMC accordingly. Not surprisingly, siRNA p27 levels of 0.344 ± 0.037 reduction in p27 mRNA levels were found in p27-silenced UtSMCs (vs. Lipo, P < 0.05; Fig. 5A). Silencing of p27 in UtSMCs resulted in significant increases in cell proliferation in UtSMCs, as evidenced by direct cell counts (P < 0.05; Fig. 5B) and by measuring BrdU incorporation (P < 0.05; Fig. 5C). Additionally, in UtSMC cells with silenced p27 in the presence of 10 μM Fen, an additional proliferative effect was detected (6.40 × 106 to 8.03 × 106 cells) in cell numbers (P < 0.05; Fig. 5B) as well as in BrdU incorporation rate (1.197 ± 0.040, P < 0.05; Fig. 5C).
Fig. 5.
The effects of p27 siRNA on Fen-induced cell proliferation in USMCs. A: p27 mRNA expression. Left (bars from left to right): Lipo (control), Lipo + ScRNA, and Lipo + siRNA targeting human p27 gene (SiRNA). Right (bars from left to right): Lipo + Fen (control), Lipo + Fen + scRNA, and Lipo + Fen + siRNA. p27 mRNA was largely diminished by presence of siRNA. B: cell counts. Cell numbers were increased in siRNA-treated groups. C: BrdU incorporation. Incorporation of BrdU was increased in siRNA-treated groups. All experiments were repeated at least 3 times with independent cell cultures. Fold changes were standardized to controls. *P < 0.05 vs. Lipo; **P < 0.05 vs. Lipo + Fen. Error bars represent SE; n ≥ 3.
Collectively, these data indicate that silencing p27 is sufficient to induce cell proliferation, suggesting an essential role of p27 downregulation in Fen-induced proliferation observed in UtLM cells and UtSMCs.
DISCUSSION
Cell proliferation is a hallmark event in neoplasia, including uterine leiomyomas. A large proportion of abnormal cell growth is cell cycle dependent, whereas cell cycle kinase inhibitors such as p27 function internally to suppress inappropriate responses to tumorigenic stimuli (18). In the present study, we first observed that both mRNA and protein levels of p27 were reduced significantly by Fen in UtLM cells and UtSMCs. Proliferative responses to Fen were abrogated by overexpression of p27 in UtLM cells and UtSMCs. In contrast, reduced expression of p27 was sufficient to induce cell proliferation in the two cell lines and even further enhance proliferation in the presence of Fen.
Previously, we reported that Fen promoted cell cycle progression from the G1 phase to S phase, and in this study we detected a substantial reduction in the expression of a “checkpoint” molecule, p27, which is important in inhibiting G1 to S phase transitions. Whereas p27 is ubiquitously expressed at a high level and regulates cell proliferation in normal quiescent mammalian cells, diminished expression of p27 protein has been documented in many tumors, including 80% of human uterine leiomyomas (16). Thus, the current finding provides additional evidence at a molecular level that Fen may be considered a risk factor for fibroids. Moreover, accumulating data suggest that reduced p27 is correlated with poor survival outcomes and insensitive responses to certain chemotherapies (29). Therefore, it raises concern about chronic exposures or even early exposures of the developing organisms to Fen and how it may adversely contribute to the initiation and progression of other tumors in addition to uterine leiomyomas.
Sequentially, our results show that Fen-induced cell proliferation is fully abrogated by overexpression of p27. This is consistent with other studies utilizing a similar inducible p27 transfection system. Goukassian et al. (15) found that overexpression of p27 results in marked inhibition of DNA replication in endothelial cells. Moreover, other experiments have shown that overexpressed p27 rescues tumor cells from unlimited growth by inducing G0/G1 arrest as well as promoting apoptosis (24).
Several pieces of information can be interpreted from our findings. First, it sheds light on Fen as a preventable risk factor for uterine fibroids. A few clinical and preclinical pharmacological reagents such as nonsteroidal anti-inflammatory drugs, rapamycins, and astrozole were reported to increase/stabilize p27 expression in some tumors with accompanying regressive phenotypes (4, 17, 19); therefore, it may be of interest to explore whether nonsteroidal anti-inflammatory drugs are able to inhibit p27 effects due to exposure to environmental risk factors such as Fen.
Second, it appears that downregulation of p27 mediates Fen-induced cell proliferation in leiomyoma cells. In support of this, stabilization of p27 has been proposed to be clinically beneficial for various tumors by counterregulating proliferation and promoting undifferentiated cells to redifferentiate (26). Similarly in vivo, genetically engineered mice deficient in p27 were determined to have increased susceptibility to cancers after exposure to chemicals or irradiation risk factors (13, 28). Thereafter, this concept brings a new question to mind: how does Fen regulate p27? It is also interesting that estrogen has been reported to decrease p27 expression in several tumor cells (3, 9). Results from our previous studies showed that Fen-induced effects are independent of binding to estrogen receptors but may not preclude nonclassical and nongenomic estrogen-related pathways such as signaling through MAPK and S phase kinase protein 2 (9). Recently, a new miRNA-mediated translational regulation of p27 has emerged as a novel mechanism in various human tumors (12, 25) and may be important in the Fen-induced regulation of p27 observed in our study. It should be encouraged to explore the potential role of miRNAs in modulating p27 expression in the setting of Fen-induced proliferation in fibroid cells.
If one takes this concept that reduction in p27 is a mechanism of Fen-induced abnormal growth in uterine fibroid cells, the next question might be whether loss of p27 alone is sufficient enough to mimic Fen-induced proliferation and whether additional reduction in p27 would be obtained to further enhance proliferative cellular effects observed following Fen treatment. In line with earlier findings in human breast cancer (1–3, 6, 7), our data show that silencing of p27 alone conferred significantly enhanced proliferation in UtLM cells and UtSMCs. Additionally, Fen-treated cells in the presence of p27 silencing showed accentuation of the proliferative responses compared with Fen-only treated cells in both cell lines. This clearly indicates a new scenario for investigators to consider: a potentially synergistic risk of Fen exposure in premenopausal women with fibroids and in women who are receiving hormone replacement therapy. It may be that combinational exposures to Fen and environmental or exogenous estrogens may render women at greater risk for cellular proliferation of myometrial, leiomyoma, and/or other hormonally regulated tissues and cells.
In general, this study shows an essential role for p27 in Fen-induced proliferation in UtLM cells and UtSMCs, as depicted in Fig. 6. It proposes downregulation of p27 as a potential mechanism of the Fen-related effects observed in UtLM cells and UtSMCs. Moreover, it suggests a non-ER-binding mechanism by which Fen induces proliferation in fibroid cells. However, a better understanding of the mechanisms of how Fen regulates expression of p27 may confer new hope in managing these risks and developing prevention and intervention strategies for managing fibroids.
Fig. 6.
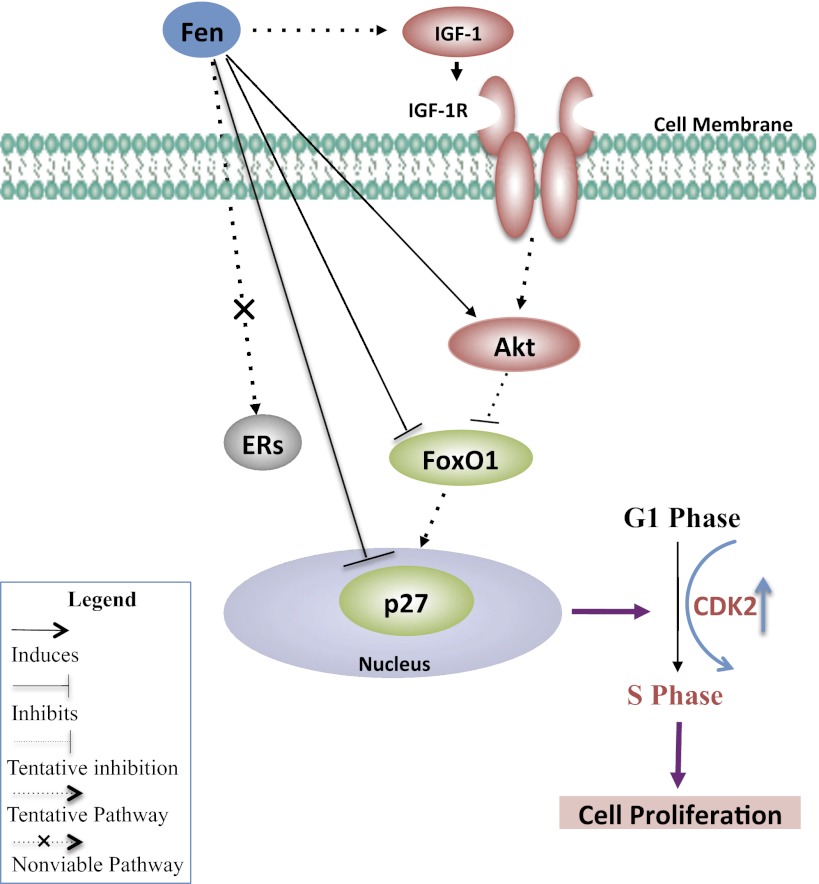
Schematic diagram of proposed molecular mechanism of p27 downregulation in Fen-induced cell growth in fibroids. As reported previously, estrogen receptors (ERs) are not involved in Fen-induced cell proliferation in UtLM cells and UtSMCs (see Ref. 10). We propose that downregulation of p27 plays an essential role in Fen-induced proliferation in UtLM cells and UtSMCs. We speculate that Fen may also stimulate growth factors and their receptors, such as IGF-I and IGF-I receptor (IGF-IR), which are known regulators of uterine leiomyoma growth (see Ref. 30). Preliminary data suggest that Fen can increase the expression of Akt while downregulating the expression of forkhead box O1 (FoxO1; data not shown). Thus, Fen-induced p27 downregulation may be mediated in part by the Akt/FoxO1 signaling pathway. The downregulation of p27 leads to G1/S phase transition with accompanying increases in cyclin-dependent kinase 2 (CDK2) expression. The promotion of G1 to S phase cell cycle progression results in cell proliferation, which can contribute to tumor growth and expansion. Green shaded molecules are downregulated, whereas red shaded molecules are upregulated. Gray shaded molecule is unaffected. Akt, also known as protein kinase B, is a serine/threonine-specific protein kinase. p27, also known as p27kip1, is a CDK inhibitor.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences, and the National Toxicology Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.G., H.X., and D.D. did the conception and design of the research; X.G., L.Y., L.C., and C.J.T. performed the experiments; X.G., L.Y., L.C., C.J.T., and D.D. analyzed the data; X.G., C.J.T., and D.D. interpreted the results of the experiments; X.G. and A.B.M. prepared the figures; X.G. drafted the manuscript; A.B.M., H.X., and D.D. edited and revised the manuscript; D.D. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Abraham Nyska and Wendy Jefferson for their critical review of the manuscript.
REFERENCES
- 1. Alkarain A, Jordan R, Slingerland J. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J Mammary Gland Biol Neoplasia 9: 67–80, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Alkarain A, Slingerland J. Deregulation of p27 by oncogenic signaling and its prognostic significance in breast cancer. Breast Cancer Res 6: 13–21, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cariou S, Donovan JC, Flanagan WM, Milic A, Bhattacharya N, Slingerland JM. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc Natl Acad Sci USA 97: 9042–9046, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Guggisberg N, Jorda M, Gonzalez-Angulo A, Hennessy B, Mills GB, Tan CK, Slingerland JM. Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin Cancer Res 15: 3396–3405, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cheng J, Wang Y, Ma Y, Chan BT, Yang M, Liang A, Zhang L, Li H, Du J. The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circ Res 107: 1265–1274, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, Sun P, Tan CK, Hengst L, Slingerland J. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128: 281–294, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 8: 253–267, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem 278: 41355–41366, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gao X, Yu L, Castro L, Moore AB, Hermon T, Bortner C, Sifre M, Dixon D. An endocrine-disrupting chemical, fenvalerate, induces cell cycle progression and collagen type I expression in human uterine leiomyoma and myometrial cells. Toxicol Lett 196: 133–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garey J, Wolff MS. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem Biophys Res Commun 251: 855–859, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Gillies JK, Lorimer IA. Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 6: 2005–2009, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Glover CE, Gurley KE, Kim KH, Storer B, Fero ML, Kemp CJ. Endocrine dysfunction in p27Kip1 deficient mice and susceptibility to Wnt-1 driven breast cancer. Carcinogenesis 30: 1058–1063, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Go V, Garey J, Wolff MS, Pogo BG. Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environ Health Perspect 107: 173–177, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goukassian D, Díez-Juan A, Asahara T, Schratzberger P, Silver M, Murayama T, Isner JM, Andrés V. Overexpression of p27(Kip1) by doxycycline-regulated adenoviral vectors inhibits endothelial cell proliferation and migration and impairs angiogenesis. FASEB J 15: 1877–1885, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Houston KD. Convergent Etiology of Eker Rat and Human Uterine Leiomyoma (Texas Medical Center Dissertations). Ann Arbor, MI: ProQuest, 2004 [Google Scholar]
- 17. Huang YC, Chuang LY, Hung WC. Mechanisms underlying nonsteroidal anti-inflammatory drug-induced p27(Kip1) expression. Mol Pharmacol 62: 1515–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 17a. International Programme on Chemical Safety Environmental Health Criteria 95, Fenvalerate. Geneva: United Nations Environment Programme, International Labour Organisation, and World Health Organization, 1990. [The data are available at http://www.inchem.org/documents/ehc/ehc/ehc95.htm]. [Google Scholar]
- 18. Kaldis P. Another piece of the p27Kip1 puzzle. Cell 128: 241–244, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Kawamata S, Sakaida H, Hori T, Maeda M, Uchiyama T. The upregulation of p27Kip1 by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood 91: 561–569, 1998 [PubMed] [Google Scholar]
- 20. Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX. Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1). Cancer Res 63: 2977–2981, 2003 [PubMed] [Google Scholar]
- 21. Lahav-Baratz S, Ben-Izhak O, Sabo E, Ben-Eliezer S, Lavie O, Ishai D, Ciechanover A, Dirnfeld M. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol Hum Reprod 10: 567–572, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med 28: 204–217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPushin R, Tari AM, Bast RC., Jr The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem 278: 23441–23450, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9: 218–224, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem 283: 29897–29903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nickeleit I, Zender S, Kossatz U, Malek NP. p27kip1: a target for tumor therapies? Cell Div 2: 13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shiozawa T, Horiuchi A, Kato K, Obinata M, Konishi I, Fujii S, Nikaido T. Up-regulation of p27Kip1 by progestins is involved in the growth suppression of the normal and malignant human endometrial glandular cells. Endocrinology 142: 4182–4188, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Teixeira LT, Kiyokawa H, Peng XD, Christov KT, Frohman LA, Kineman RD. p27Kip1-deficient mice exhibit accelerated growth hormone-releasing hormone (GHRH)-induced somatotrope proliferation and adenoma formation. Oncogene 19: 1875–1884, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Wander SA, Zhao D, Slingerland JM. p27: a barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res 17: 12–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, Di X, Lucas S, Robboy SJ, Dixon D. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med 14: 264–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Q, Tian L, Mansouri A, Korapati AL, Johnson TJ, Claret FX. Inducible expression of a degradation-resistant form of p27Kip1 causes growth arrest and apoptosis in breast cancer cells. FEBS Lett 579: 3932–3940, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]