Abstract
Glucagon-like peptides (GLP-1/2) are cosecreted from endocrine L cells in the gut and preproglucagonergic neurons in the brain. Peripheral GLP-2 action is essential for maintaining intestinal homeostasis, improving absorption efficiency and blood flow, promoting immune defense, and producing efficacy in treatment of gastrointestinal diseases. However, it is unknown if CNS GLP-2 plays a physiological role in the control of energy homeostasis. Since GLP-1/2 are cotranslated from preproglucagongene and coproduced by prohormone convertase-1, it is challenging to knockout GLP-2 only. Instead, our laboratory has generated a Glp2r-floxed mouse line to dissect cell-specific GLP-2 receptor GLP-2R) action in the regulation of energy balance. Our objective was to determine if GLP-2R in the hypothalamus modulates feeding behavior and gastric emptying. We show that Glp2r mRNA and protein are highly expressed in the arcuate nucleus and dorsomedial nucleus of the mouse hypothalamus. Using the Cre-LoxP system, we generated mice that lack Glp2r expression in POMC neurons (KO; mainly in the hypothalamus). The KO mice showed hyperphagic behavior (such as increases in food intake and meal frequency), accelerated gastric emptying (assessed by [13C]octanoic acid breath test), and late-onset obesity, yet there was no decrease in basal metabolic rate. Infusion of GLP-2 (2.5 nmol into the 4th ventricle) suppressed food intake and gastric emptying, while GLP-2-mediated effects were abolished in the melanocortin receptor-4 (MC4R) KO mice. We conclude that Glp2r deletion in POMC neurons enhances feeding behavior and gastric motility, whereas icv GLP-2R activation suppresses food intake and gastric emptying through the MC4R signaling pathway. This study indicates that CNS GLP-2R plays a physiological role in the control of feeding behavior and gastric emptying and that this is mediated probably through the melanocortin system.
Keywords: glucagon-like peptides, proopiomelanocortin, energy homeostasis, food intake, gastric emptying
obesity and type 2 diabetes affect millions of people and are major health problems in the United States. The hypothalamus, a primary site of convergence and integration in the brain for hormonal and nutritional signals, plays pivotal roles in the regulation of energy balance (42). In the arcuate nucleus (ARC) of the hypothalamus, neurons expressing proopiomelanocortin (POMC neurons) are perfectly positioned to integrate long-term adiposity and short-term satiety signals for energy homeostasis (12). POMC neuronal activation enhances energy expenditure and suppresses food intake, yet neuroendocrine mechanisms are not fully established. Notably, the gastrointestinal (GI) tract has a crucial role in digestion, absorption, and assimilation of nutrients (15), thus controlling energy intake at the first pass. Through the gut-brain axis, gut hormones play a key role in the regulation of energy balance (including food intake) (11, 13, 22). Glucagon-like peptide-1/2 (GLP-1/2) are cosecreted from endocrine L cells in the gut and coreleased from preproglucagonergic (PPG) neurons in the brain. GLP-1/2 receptor agonists and dipeptidyl peptidase IV (DPP-IV) antagonists are clinically tested for the prevention and treatment of obesity, diabetes, inflammatory bowel disease, and short bowel syndrome (34). GLP-1/2 are key signals for the brain and pancreas to control energy balance and glucose homeostasis in rodents and humans (34) and are proposed as key mediators for bariatric surgery-improved energy balance (28, 29, 39). Thus, elucidation of GLP-1/2 signaling and action in the control of energy balance may reveal novel targets for the treatment of intestinal dysfunctions, obesity, and diabetes (34).
Through a specific G protein-coupled receptor, GLP-2R, GLP-2 action is essential for maintaining intestinal homeostasis, improving absorption efficiency and blood flow, promoting immune defense, and producing efficacy in treatment of GI diseases such as short bowel syndrome and inflammatory bowel disease (4, 21, 24). The GLP-2R is not only expressed in endocrine cells (such as enteroendocrine cells and pancreatic α-cells) but also in neurons (such as enteric neurons, vagal sensory neurons, and central neurons) (14, 20, 33, 37, 48, 53). GLP-2 acutely potentiates L-type voltage-gated Ca2+ channel activity in hippocampal neurons (48). Importantly, GLP-2 (as a neurotransmitter) may mediate PPG neuron-induced synaptic transmission linking the hypothalamus and the brain stem (44). Central infusion of GLP-2 suppresses food intake (44, 45). By means of neural and endocrine pathways, GLP-2 may act as a key satiation signal (for short-term energy availability) to the hypothalamus in the control of feeding behavior (food intake). However, it is unknown whether GLP-2 plays a physiological role in the homeostatic control of energy balance and body weight. GLP-2 is cotranslated with GLP-1 from preproglucagongene and coproduced by prohormone convertase-1 in enteroendocrine L cells and neurons. Thus, it is challenging to knock out GLP-2 only. We cannot interpret tissue-specific metabolic phenotypes from GLP-2R global knockout mice. Therefore, our laboratory has generated a Glp2r-floxed mouse line that would provide a powerful genetic tool to dissect cell-specific GLP-2R action in the regulation of energy balance.
Gastric emptying (GE) is a key process for the short-term control of feeding behavior and contributes to the long-term homeostatic regulation of energy balance. GE is a key mediator of hunger, satiation, and satiety (25). Faster GE results in shorter satiety period and overeating (23) and is considered a major cause of obesity (16). GE is enhanced in obese subjects (8, 52). Orexigenic peptides (e.g., ghrelin) increase GE, whereas anorexigenic peptides (e.g., leptin) decrease it (7, 9). Young mice with leptin deficiency show rapid GE; in contrast, old mice display gastroparesis (3). The central melanocortin system provides a potential substrate for integration of long-term adipostatic and short-term satiety signals. For example, activation of the neuronal melanocortin-4 receptor (MC4R) is required for cholecystokinin-induced suppression of feeding (17). It is plausible that POMC neuron-suppressed food intake might be executed through fine tuning gastric emptying. Thus, we hypothesized that GLP-2R in POMC neurons plays an important physiological role in the control of energy balance, specifically feeding behavior and gastric emptying. Our objective was to determine the physiological significance of GLP-2R in POMC neurons in the control of feeding behavior (including gastric emptying).
In the present study, we show that mice with Glp2r deletion in POMC neurons have enhanced feeding behavior and gastric emptying, whereas activation of GLP-2R signaling in the brain suppresses food intake and gastric emptying and that these effects are blocked in the MC4R KO mice. Our findings suggest that central nervous system (CNS) GLP-2R plays an important physiological role in the control of food intake and gastric emptying and that the CNS regulation of food intake by POMC neurons is linked to the control of gastric emptying.
MATERIALS AND METHODS
Animals
The protocols of this study were approved by the Animal Care and Use Committee of Baylor College of Medicine and carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Bethesda, MD). C57BL/6J and transgenic mice [Tg (Pomc1-cre)16Lowl/J (POMC-cre); Tg (Pomc1-hrGFP)1Lowl/J (POMC-GFP)] were obtained from the Jackson Laboratory. Mc4r-null mice were provided by Dr. Yong Xu at Baylor College of Medicine. All the mice were maintained under a 12:12-h light-dark cycle with room temperature (∼22°C) and humidity (∼50%) conditions and provided ad libitum access to water and food. Mice at the age of 10–12 wk (or at a specified age) were measured for body composition, food intake, energy expenditure, physical activity, and gastric emptying. Mice were euthanized under isoflurane anesthesia and dissected for tissue samples fixed in 10% neutral buffered formalin for immunohistochemistry or frozen immediately in liquid nitrogen and stored at −80°C for molecular analysis.
Generation of the Glp2r-flox/flox Mouse Line
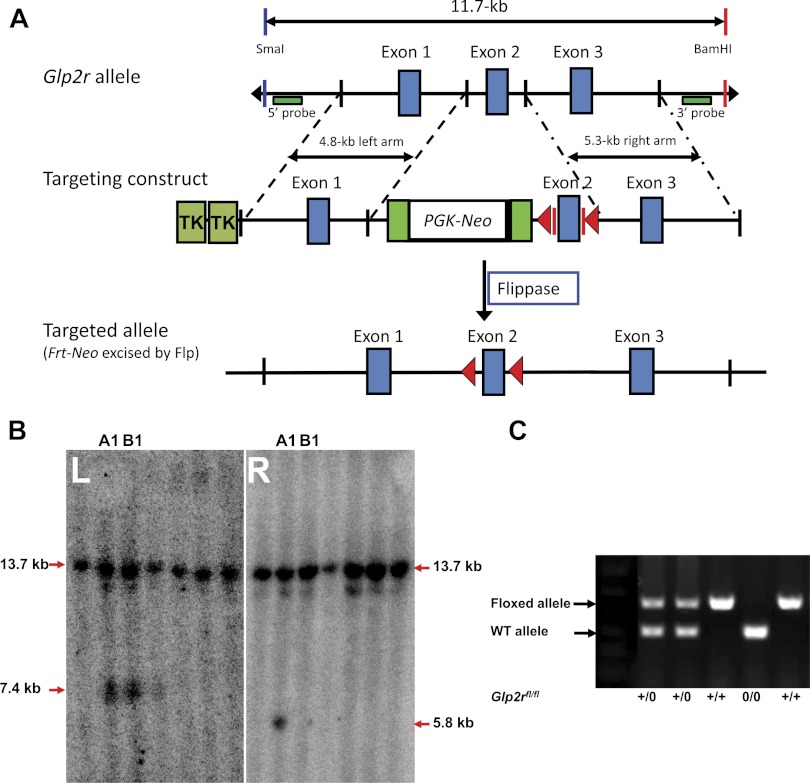
To determine the physiological significance of GLP-2R in a tissue-specific manner, we generated the conditional Glp2rfl/fl mouse line. Using the BAC recombineering technique (30), we made the Glp2r-targeting construct. In brief, a BAC clone (435L24) that contains the mouse Glp2r gene was purchased (Invitrogen) and confirmed by PCR analysis. Two DNA fragments (i.e., 4.8-kb left arm and 5.3-kb right arm; see primers in Table 1) were selected and cloned by BAC recombineering and used for homologous recombination. A 0.5-kb DNA fragment containing the targeted exon 2 with its immediate 5′ and 3′ introns (partial) of the Glp2r gene was amplified by PCR and inserted in between two loxP sites of the Frt-Neo vector. Two TK cassettes were inserted into the 5′ end of the targeting vector. After the targeting construct was linearized and electroporated into R1 mouse ES cells (36), positive ES cells (of ∼400) were selected under G418 and ganciclovir and screened by mini Southern blotting. The protocol for mini Southern blotting is available on line (http://www.bcm.edu/dtmc/index.cfm?PMID=7807). We chose three independent ES clones containing the targeted Glp2r allele for injection into blastocysts derived from C57BL/6J mice. The chimeric founders were crossed to C57BL/6J mice, and germ-line transmission of F1 offspring was confirmed by PCR analysis. The F1 mice were then crossed with flippase transgenic mice to delete the Neo cassette flanked by two Frt recombination sites. Finally, a conditional Glp2r-flox/flox (Glp2rfl/fl) mouse line was generated and confirmed by DNA sequencing. Genotyping of tail DNA was performed by PCR using forward primer 5′-CTTGCTCGAGAAACCTTCTG and reverse primer 5′-GCTGTGTCTCTACAGAGAGG, resulting in the 321-bp band for the wide-type allele and the 473-bp band for the floxed allele.
Table 1.
Primers for generating Glp2r-targeting construct and genotyping
| DNA Fragment | Primer ID | Engineered Enzyme Restriction Site | Sequence |
|---|---|---|---|
| Exon 2 | E5′ primer | BamHI | GGATCCACCCTTTCTTAGTCACACTG |
| Exon 2 | E3′ primer | BamHI | GGATCCAGAACTAGTCATCCAGCAGA |
| Left arm, left | LL5′ primer | BamHI | GGATCCGTCCTCATTTGCATCTTCAG |
| Left arm, left | LL3′ primer | EcoRI | GAATTC TGCCAACTCTCCCTACAGTG |
| Left arm, right | LR5′ primer | EcoRI | GAATTCATCCCTTTCTGCTGCATCTG |
| Left arm, right | LR3′ primer | NotI | GCGGCCGCGGGTGTGATTGGAACTGGTG |
| Right arm, left | RL5′ primer | MulI | ACGCGTTAGAGTAAAGTAGTGACTGG |
| Right arm, left | RL3′ primer | EcoRI | GAATTCCAGTATGTGTGCCAAGACAG |
| Right arm, right | RR5′ primer | EcoRI | GAATTC AGGAGAATGAAGCCACCATG |
| Right arm, right | RR3′ primer | AscI | GGCGCGCCAATGCTAAGAGAGGCCTCAG |
| Selection probe 5′ | Glp2r-L5′ | ACCAGATCCAAAGGCACATC | |
| Selection probe 5′ | Glp2r-L3′ | ACCGAGATGAGTGACACTAG | |
| Selection probe 3′ | Glp2r-R5′ | TGTGCACTCTCCTAGCTCAG | |
| Selection probe 3′ | Glp2r-R3′ | GTCTCTGCGCTCACCTCATG | |
| Glp2r-floxed allele | Glp2rcko5F | 5′-CTTGCTCGAGAAACCTTCTG | |
| Glp2rcko3R | 5′-GCTGTGTCTCTACAGAGAGG | ||
| Glp2r deletion | Glp2r-LR5N | 5′-ATCCCTTTCTGCTGCATCTG | |
| Glp2r-RL3NB | 5′-ACAGCGGTCACTTATGTCAG | ||
| Cre | Cre-F5 | 5′-CGGTCGATGCAACGAGTGAT | |
| Cre-R3 | 5′-CCACCGTCAGTACGTGAGAT | ||
| Gfp | Gfp-F5 | 5′-GGT GCG GTT GCC GTA CTG GA | |
| Gfp-R3 | 5′-TGG CTC AAT GTC CTT CCT GG |
Generation of Glp2r Global Knockout Mice
We used the Cre-loxP system for generating Glp2r KO mice. In brief, Glp2rfl/fl mice were bred with protamine-Cre transgenic mice (PrmCre+/0), which express Cre in the sperm (38). Then Glp2rfl/+/PrmCre+/0 males were bred with C57BL/6J females to generate Glp2r+/−/PrmCre0/0 mice, i.e., essentially heterozygous mutant mice (Glp2r+/−). Genotyping of tail DNA was performed by PCR using forward primer 5′-ATCCCTTTCTGCTGCATCTG and reverse primer 5′-ACAGCGGTCACTTATGTCAG, resulting in the 1219-bp band for the wide-type allele and the 843-bp band for the deleted allele. To confirm the efficiency of Glp2r deletion in mutant mice, the Glp2r mRNA abundance was determined by RT-PCR using forward primer 5′-ATGCGTCGGCTCTGGGGCCCTG and reverse primer 5′-TGCTTCTGCCAAGTCCCCTGAG, in which first-strand cDNA was generated from total RNA isolated from various tissues using Superscript II kits (Invitrogen). After crossing back with C57BL/6J mice for eight generations, the Glp2r mutant mice were used throughout this study.
Generation of POMC-Glp2r Conditional Knockout Mice
We generated POMC neuron-specific Glp2r knockout mice by using the Cre/loxP system. In brief, Glp2rfl/fl mice were crossed with POMC-Cre transgenic mice, which express Cre in POMC neurons. To confirm the efficiency of Glp2r deletion, the expression of Glp2r protein in POMC neurons were determined by immunostaining. The mice were genotyped using primers (Table 1) for the Glp2r-floxed allele and for the POMC-cre allele. Homozygous male mice (littermates) (Glp2rfl/fl; POMC-cre0/0 = POMC-Glp2r WT, and Glp2rfl/fl, POMC-cre+/0 = POMC-Glp2r KO) were used in the study. All mice will be genotyped for ubiquitous deletion of the floxed gene; any such “delta” mice (the result of spontaneous postmeiotic Cre activation) were excluded from the studies. (Delta deletion was checked by genotyping of tail DNA using forward primers 5′-ATCCCTTTCTGCTGCATCTG and reverse primer 5′-ACAGCGGTCACTTATGTCAG, resulting in the 1496-bp band for the Glp2r-floxed allele, the 1219-bp band for the WT allele, and the 815-bp band for the deleted allele.)
Intracerebroventricular Infusion
Lateral ventricle chronic infusion of GLP-2.
C57BJ/6J mice (12 wk old, male) were fasted overnight, anesthetized under 1.5% isoflurane, and implanted with ALZET osmotic pumps (DURECT) connected to a stainless steel cannula into the left lateral ventricle (stereotaxic coordinates: 0.34 mm posterior to bregma, 1.0 mm lateral from midline, 2.3 mm depth from skull surface). Synthetic human GLP-2 (250 μM, American Peptide) or vehicle (sterile artificial cerebrospinal fluid) was delivered at a rate of 0.5 μl/h for 2 wk via this microosmotic pump. During the second week, mice were assessed for food intake and meal pattern ad libitum using the Comprehensive Laboratory Animal Monitoring System (CLAMS), and the brain samples were obtained for frozen sections.
Fourth ventricle acute infusion of MC4R antagonist or GLP-2.
MC4R-null mice and WT mice (5 wk old, male) were fasted overnight, anesthetized under 1.5% isoflurane, and implanted with a 28-G stainless steel guide cannula into the fourth ventricle (stereotaxic coordinates: 6 mm posterior to bregma, 0 mm from midline, 3.8 mm depth from skull surface). After a 7-day recovery and a 3-day adaptation in metabolic cages, mice were fasted overnight and infused icv with 1 μl of MC4R antagonist (SHU9119, 0.5 nmol; Tocris Bioscience) or GLP-2 (2.5 nmol) over 5 min. Then, mice were assessed for food intake and meal pattern using CLAMS and/or gastric emptying using a [13C]octanoic acid breath test.
Immunohistochemistry and In Situ Hybridization
Mice were anesthetized using pentobarbital sodium and transcardially perfused with 5 mM EDTA, followed by 4% paraformaldehyde in PBS. The whole brains were fixed in 10% neutral buffered formalin, stored at 4°C in 20% sucrose in PBS, and cut at 25 μm for coronal sections.
Immunohistochemistry.
Double immunostaining was performed to colocalize the expression of GLP-2R protein with GFP in the POMC-GFP mice in the absence or presence of POMC-Cre expression. To define whether Glp2r protein was expressed in POMC neurons, Glp2rfl/fl mice were crossed with POMC-GFP transgenic mice. To determine whether Glp2r was deleted in POMC neurons, POMC-Glp2r KO mice (i.e., Glp2rfl/fl; POMC-cre+/0) were crossed with POMC-GFP transgenic mice. To determine whether GLP-2R protein is expressed in the hypothalamic ARC, coronal sections of Glp2r WT and KO mice were immunostained for GLP-2R. After 1 μl of colchicine (of 20 μg/μl) was injected icv, 12-wk-old mice were deeply anesthetized using pentobarbital sodium and transcardially perfused with 5 mM EDTA in saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). The whole brains were removed, fixed for 24 h in 10% neutral buffered formalin, and stored at 4°C in 20% sucrose in PBS. The brain tissues on dry ice were cut at 25 μm for coronal sections using a sliding microtome (Leica SM2010R; Leica Microsystems, Bannockburn, IL) and fixed in the buffered formalin for 30 min for immunohistochemistry or in situ hybridization as follows. After being permeabilized in 0.5% Triton X-100-PBS (PBS-T) and blocked in 10% normal donkey serum in PBS-T, sections were incubated with primary antibodies (rabbit GLP-2R from Alpha Diagnostic Intl. and chicken GFP from Aves Laboratories) at 4°C for 1 wk. After a washing in PBS-T, sections were incubated with secondary donkey anti-rabbit IgG Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) and donkey anti-chicken IgG 488 (Invitrogen, Carlsbad, CA) with TOPRO-3 (Invitrogen) for nucleus counterstaining and mounted with 30% glycerol in PBS. Images were captured using a confocal laser-scanning microscope (Zeiss LSM 510, Carl Zeiss).
In situ hybridization.
Glp2r mRNA in mouse brain was detected by in situ hybridization (ISH) as previously reported (48).
Glp2r cDNA probes.
Total RNA was isolated from the WT mouse brain using RNeasy Mini kit (Qiagen) and used to generate cDNA with a Superscript III First-Strand cDNA Synthesis kit (Invitrogen). The cDNA was amplified by PCR using primers (forward 5′-ATGCGTCGGCTCTGGGGCCCTG, reverse 5′-TGCTTCTGCCAAGTCCCCTGAG) based on the mouse Glp2r cDNA sequence (BC044746) and subcloned into pGEM-T easy vector (Promega). After the cDNA clone was digested by restriction endonucleases (SalI/SacII), Glp2r cRNA antisense and sense probes were labeled with digoxigenin (DIG)-UTP by in vitro transcription (Roche) with T7 and SP6 RNA polymerase, respectively.
in situ hybridization.
The brain coronal sections (25 μm) were fixed with 4% neutral buffered paraformaldehyde, acetylated in triethanolamine, dehydrated gradually, and quenched for endogenous peroxidase activity. After being permeabilized with proteinase K, the sections were hybridized with the DIG-labeled cRNA sense (as a negative control) or antisense probe (100–300 ng/ml) in ultrasenstive hybridization buffer (Invitrogen) at 63.5°C for 12 h and washed with saline-sodium citrate buffer.
detection with tyramide signal amplification.
The bound probe was incubated with sheep anti-DIG antibody conjugated with horseradish perosidase (1:250, Roche) and detected with tyramide signal amplification (TSA) plus Cy3 fluorescence system (PerkinElmer NEL744, Life Science Products). Images were captured using a confocal laser-scanning microscope (Zeiss LSM 510, Carl Zeiss). To show if POMC-Glp2r deletion is neuron specific, we also performed ISH on frozen sections of the mouse jejunum as previously reported (20). However, the bound probes were detected by alkaline phosphatase reaction (20).
Indirect Calorimetry, Feeding Behavior, and Body Composition
Indirect calorimetry.
To avoid the confounding effects of body weight and body composition on energy balance, calorimetry was performed prior to the onset of obesity. Thus, 12-wk-old mice were housed in individual chambers at 22 ± 1°C with free access to food and water. After a 3-day adaptation, food intake was automatically recorded. Physical (locomotor) activity, feeding behavior, oxygen consumption (V̇o2), and CO2 production (V̇co2) were measured individually over 72 h using the the Oxymax/CLAMS (Comprehensive Lab Animal Monitoring System; Columbus Instruments, Columbus, OH). Heat production (HP) was calculated using the formula provided by the manufacturer: HP (kcal) = (3.815 + 1.232 V̇o2/V̇co2)·V̇o2. Energy metabolism (V̇o2, V̇co2, and HP) were normalized by metabolic body weight (BW0.75). In addition, after a 4-h withdrawal of food, basal metabolic rates of mice (without access to food) were then estimated from 8:00 AM to 12:00 noon.
Body composition.
Twelve- or 32-wk-old mice in the fed status were anesthetized briefly with a 1–2% isoflurane flow, and body composition was assessed in vivo by dual-energy X-ray absorptiometry (DEXA; Lunar, Madison, WI). Body fat mass, lean mass, and bone density were analyzed using GE Lunar PIXImus software version 1.45 (Lunar, Madison, WI).
Gastric Emptying by [13C]Octanoic Acid Breath Test
Mice (5 or 12 wk old, n = 8 for each genotype) were fasted overnight under the conscious condition for assessing gastric emptying rate (GER) using the [13C]octanoic acid breath test (43, 49), which was modified and coupled with real-time measurement of V̇co2 using CLAMS (49). In brief, mice were transferred and adapted to the calorimetric chamber for 1 h prior to gastric administration of a [13C]octanoic acid-containing meal. To minimize effects of circadian rhythm and gastric volume on the GER, mice were tested between 09:00 and 11:00 AM. A baseline breath sample was taken prior to the gastric tracer administration. The test meal was composed of 5% (vol/vol) [1-13C]octanoic acid (99% enrichment; Cambridge Isotope Laboratories, Andover, MA) in milk (containing 20% dry matter), which produced a reasonable abundance of 13C isotopic enrichment in breath CO2. This test meal was administered at 8 μl/g BW into the stomach via orogastric gavage. After the gastric administration, breath CO2 was sampled every 5 min during the first hour and ever 15 min during the second hour from the chamber and injected into evacuated 10-ml Exetainer tubes. During the test period, mice were provided water (without food) and monitored for V̇co2and V̇o2. The 13C enrichment of breath samples was measured by isotope ratio mass spectrometry (Thermo Finnigan Deltaplus XL, San Jose, CA) and expressed as atom percentage. The measured 13CO2 recovery in the breath was expressed as percent excretion per hour of the given [1-13C]octanoic acid dose (%dose/h). Data for the 13CO2 excretion rate were fitted by a nonlinear regression model (19, 49) using the NLIN Procedure (SAS version 9.1; SAS Institute, Cary, NC): y = atbe−ct, where y is the percentage of 13CO2 excretion in breath per hour, t is time in hours, and a, b, and c are regression-estimated constants. The following parameters were computed (19, 49): half-excretion time (T1/2, min) = gamma inv (0.5, b+1, 1/c); lag phase (Tlag, min) = b/c; and gastric emptying coefficient (GEC) = In (a).
qRT-PCR
Total RNA was extracted from the hypothalamus samples using an RNeasy Mini kit (Qiagen, Valencia, CA) and from the hypothalamic ARC (on frozen sections acquired by laser capture microdissection) (20) using an Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Foster City, CA). RNA concentrations were determined by NanoDrop 1000 (NanoDrop Products, Wilmington, DE). Reverse transcription was performed with the total RNA, random hexamers, and oligo(dT)12–18 using a SuperScript-III-First-Strand-Synthesis-SuperMix kit (Invitrogen). mRNA abundance was quantified by real-time qRT-PCR. Primers and probes (for Preproglucagon, Pomc, and Agrp) were designed according to the GenBank database (Table 2). The housekeeping gene Gapdh or 18S ribosomal RNA were not altered and were used as an internal control in the study. Assays were performed in triplicate with an ABI 7900HT Fast Real-time PCR System (Applied Biosystems). qPCR conditions were as follows: 10 min at 95°C, 40 cycles of 30 s at 95°C, and 60 s at 60°C. Data were normalized to Gapdh or 18S ribosomal RNA (ΔΔCT analysis) as indicated.
Table 2.
Primers for RT-PCR
| Gene | GenBank Sequence | Primer | Note |
|---|---|---|---|
| Agrp | NM_007427.2 | 5′-CTTTGGCGGAGGTGCTAGA | 5′-FAM-TCCACAGAACCGCGAGTCTCGTTC-TAMRA |
| 5′-GGACTCGTGCAGCCTTACACA | |||
| Pomc | NM_008895.3 | 5′-GACACGTGGAAGATGCCGAG | 5′-FAM-CAACCTGCTGGCTTGCATCCGG-TAMRA |
| 5′-CAGCGAGAGGTCGAGTTTGC | |||
| PPG | AF276754 | 5′-ATTGCCAAACGTCATGATGA | Syber Green (for qPCR) |
| 5′-GGCGACTTCTTCTGGGAAGT | |||
| Glp2r | NM_175681.3 | 5′-ATGCGTCGGCTCTGGGGCCCTG | Glp2r mRNA (probe for ISH) |
| 5′-TGCTTCTGCCAAGTCCCCTGAG | |||
| Lepr | NM-146146.2 | 5′-CTTTCCTGTGGACAGAACCAGC | Lepr mRNA (for single cell-RT PCR) |
| 5′-AGCACTGAGTGACTCCACAGCA | |||
| Insr | NM-010568.2 | 5′-GCTTAAATTTTCTTTCATTCGGACAT | Insr mRNA (for single cell-RT PCR) |
| 5′-TAGAACAACATGAATCCCAGGAGAT | |||
| Glp2r | NM_175681.3 | 5′-TGTCTGAAAGACTTGCTCGAGAA | Glp2r mRNA (for single cell-RT PCR) |
| 5′-GGCCAGCACACGTACTTATCAA | |||
| Gapdh | NM_008084.2 | 5′-GCACAGTCAAGGCCGAGAAT | Syber Green (for qPCR) |
| 5′-GCCTTCTCCATGGTGGTGAA | |||
| 18S rRNA | VIC/TAMRA Probe, Primer Limited (ABI) |
Statistical Analysis
Using the mixed procedure (SAS 9.2), we analyzed data with fixed effects (including genotype, zeitgeber, and treatment), random effect (including individual mouse), and repeated measures (at different time points in the same mouse). Thus, a full model for analysis of covariance (ANCOVA) include BW, genotype (WT vs. KO), zeitgeber (light cycle vs. dark cycle), treatment (vehicle vs. GLP-2 or SHU9119), and their interactions. If applicable, repeated measures were included in a repeated statement. Least squares mean of each variable was reported, and differences were considered significant at P < 0.05.
RESULTS
GLP-2R is Expressed in the Hypothalamus, and icv GLP-2 Suppresses Food Intake
The rodent Glp2r mRNA is not only expressed in the hypothalamus (DMH and VMH) (44) but is also localized in the hippocampus and the brain stem (NTS and PBN) (32, 48). However, GLP-2R expression in the mouse brain has not been fully examined. To address whether Glp2r mRNA is expressed in the mouse ARC neurons, fluorescence in situ hybridization was performed in floating coronal sections. Glp2r mRNA (in red in Fig. 1A) was expressed in neurons (white arrows) but not in non-neurons (green arrows). Note that Glp2r mRNA in the ARC was not detected in the Glp2r KO mouse (Fig. 1B) or in the WT mouse, using the sense cRNA probe (not shown). Consistently, the GLP-2R protein (in red in Fig. 1C) was expressed in the ARC in the WT mouse, whereas it was not in the Glp2r KO mouse (Fig. 1D). Of note, the coronal sections of the Glp2r global KO mouse brain were negative for Glp2r mRNA or protein. The antibody against GLP-2R has been validated previously (20). As reported in the rat hypothalamus (44), the GLP-2R protein (in red in Fig. 1E) was also expressed in the DMH in the WT mouse, and the selected region (in the white square) was enlarged for detail (in Fig. 1F).
Fig. 1.
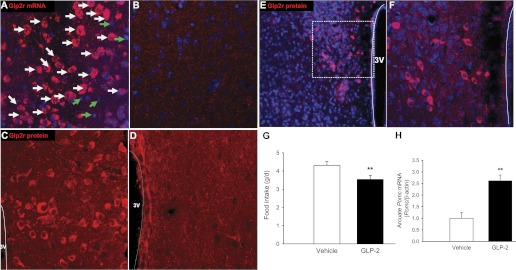
Glucagon-like peptide-2 receptor (GLP-2R) is expressed in the hypothalamus, and icv GLP-2 suppresses food intake. A: Glp2r mRNA (in red) is expressed in the arcuate nucleus (ARC) of the Glp2r WT mouse hypothalamus by fluorescence in situ hybridization. Glp2r-positive cells are indicated by white arrows; Glp2r-negative cells by green arrows. C: GLP-2R protein (in red) is expressed in ARC of Glp2r WT mouse hypothalamus, by immunohistochemistry. E: GLP-2R protein (in red) is also expressed in the dorsomedial nucleus (DMH) of Glp2r WT mouse hypothalamus; white square is enlarged for details (shown in F). Neither Glp2r mRNA (B) nor GLP-2R protein (D) is detected in hypothalamus of the Glp2r KO mouse. 3V, 3rd ventricle; nuclear DNA was counterstained with TOPOR3 (in blue). icv Infusion of GLP-2 in mice suppressed food intake (G) and increased Pomc mRNA abundance in ARC (H). GLP-2 (250 pmol/μl) was infused into the lateral ventricle through an ALZET osmotic pump at 0.5 μl/h for 2 wk. Food intake was determined during the 2nd week, and Pomc mRNA was quantified by qRT-PCR in ARC samples. Data are expressed as means ± SE (n = 8 per group), **P < 0.01, denoting significance between GLP-2 and vehicle.
To confirm whether activation of GLP-2R signaling in the mouse brain suppresses food intake, icv infusion of GLP-2 (for 2 wk) suppressed food intake in 12-wk-old mice (Fig. 1G), as reported in rodents (31, 44, 45), and increased Pomc mRNA abundance in the ARC (Fig. 1H), suggesting that CNS GLP-2 may play a direct role in the regulation of satiety involving the melanocortin system.
Generation of POMC-Glp2r KO Mice
To address tissue-specific action of GLP-2R, we generated the Glp2r-floxed mice. Using the BAC recombineering technique, we cloned the glp2r genomic DNA to make Glp2r conditional targeting construct (Fig. 2A). After the construct DNA was electroporated into the mouse ES cells, those single clones [containing the targeted Glp2r allele screened by mini Southern blotting (Fig. 2B)] were microinjected into blastocysts. After the chimeric mice were bred to C57BL/6J mice, germ-line transmission in F1 offspring was confirmed by PCR analysis. The F1 mice were then crossed with flippase transgenic mice to delete the Frt-Neo cassette. Finally, a conditional Glp2r-flox/flox (Glp2rfl/fl) mouse line was generated and confirmed by DNA sequencing. Genotyping of tail DNA shows the 321-bp band for the WT allele and the 473-bp band for the floxed allele (Fig. 2C).
Fig. 2.
Generation of the Glp2r-flox/flox (Glp2rfl/fl) mouse line. A: strategy for targeting construct. Exon 2 encoding Glp2r was targeted. Frt-Neo box denotes a cassette flanked by Frt recombination sites, driven by PGK promoter and selected by neomycin and TK. After the Frt-Neo cassette was excised in germ-line mice by crossing with flippase mice, the Glp2rfl/fl mouse line was confirmed by DNA sequencing. B: Glp2r-targeted embryonic stem (ES) cell colonies screened by mini Southern blotting. Representative images of mini Southern blotting are shown. Negative ES cell colonies are indicated by one 15-kb band. In addition to this band, positive ES cell colonies (A1 and B1) are indicated by another band of 7.3 kb when hybridized with 5′ probe (in L); or 6.7 kb when hybridized with 3′ probe (in R). ES cells (e.g., A1) positively indicated by both 5′ and 3′ probes were used for microinjection into blastocysts. The protocol for mini Southern blotting is available on line (http://www.bcm.edu/dtmc/index.cfm?PMID=7807). C: The Glp2r floxed allele was genotyped with tail DNA. The 321-bp band was for the WT allele and the 473-bp band for the Glp2r floxed allele.
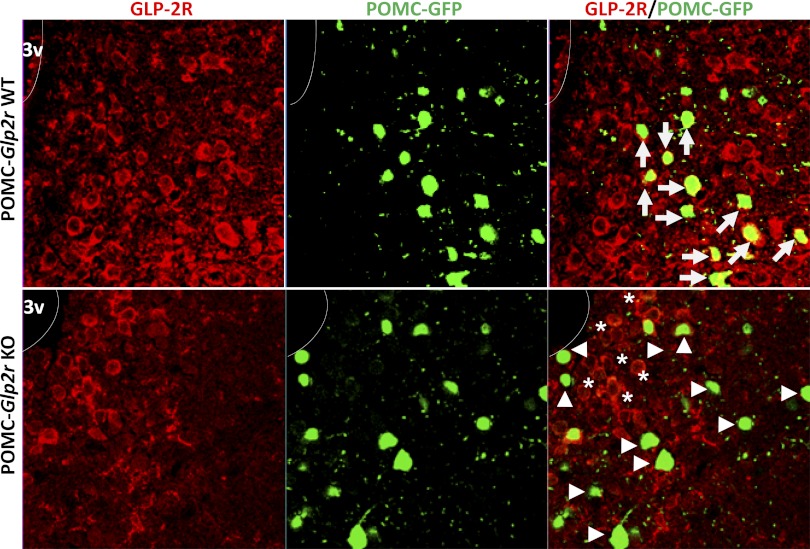
Then, we decided to generate POMC neuron-specific Glp2r KO mice by using the Cre-loxP system. Glp2rfl/fl mice were bred with POMC-Cre transgenic mice, which express Cre in POMC neurons. Of note, Pomc1 promoter-driven Cre is expressed in POMC neurons in the ARC of the hypothalamus and nucleus of the solitary tract in the hindbrain (5). The POMC-cre transgenic mice do not display any physical, physiological, or behavioral abnormalities and have identical body weights to WT littermates (5); therefore, we did not include C57BL/6J or POMC-cre mice as control groups in this study. To confirm the functional deletion of Glp2r in the mouse hypothalamus, Glp2r protein expression was determined by immunohistochemistry (Fig. 3). In the POMC-Glp2r WT mice, the Glp2r protein (in red) was expressed in POMC-GFP neurons (in green, indicated by arrows). In the POMC-Glp2r KO mice, however, the Glp2r protein was not shown in POMC-GFP neurons (indicated by arrow heads), but were still expressed in adjacent cells (indicated by stars). The POMC neurons were identified by POMC promoter-driven GFP (in POMC-GFP reporter mice). Neither the number nor the size of POMC neurons in the hypothalamus was altered in POMC-Glp2r KO mice compared with their WT littermates. Importantly, Glp2r mRNA was still expressed in the intestine of POMC-Glp2r KO mice (data not shown), indicating that Glp2r was deleted only in POMC neurons.
Fig. 3.
Glp2r deletion in hypothalamic proopiomelanocortin (POMC) neurons. POMC-Glp2r KO mice were generated by crossing Glp2rfl/fl mice with POMC-Cre mice. Immunohistochemistry was performed on brain slices. Top: GLP-2R protein (in red) was colocalized in POMC neurons (in green, white arrows) in the ARC of POMC-eGFP, Glp2rfl/fl (WT) mouse hypothalamus. Bottom: efficiency of Glp2r deletion in POMC neurons was confirmed in POMC-Cre+/0, POMC-eGFP, and Glp2rfl/fl KO mouse hypothalamus. Note that GLP-2R protein (in red) is still expressed in non-POMC neurons (stars) but not expressed in POMC neurons (arrowheads), indicating that Glp2r was deleted specifically in POMC neurons.
POMC-Glp2r KO Mice Show Late-Onset Obesity
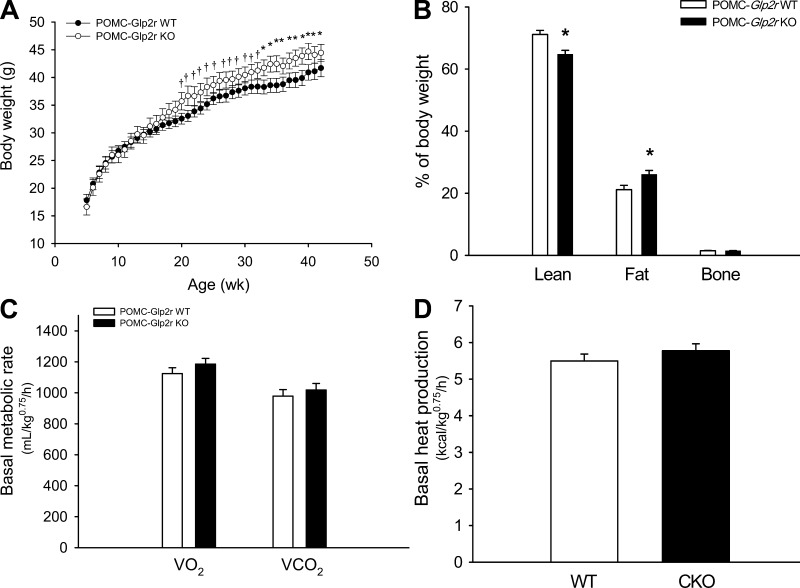
Body weights of POMC-Glp2r KO mice were similar prior to the age of 22 wk but were heavier after the age of 33 wk compared with Glp2rfl/fl (WT) littermates (Fig. 4A). There were no differences in body composition at the age of 12 wk between the two genotypes (data not shown). When the age was ≥32 wk, however, fat mass (%BW) was higher, whereas lean mass (%BW) was lower in the POMC-Glp2r KO mice (Fig. 4B).
Fig. 4.
POMC-Glp2r KO mice show late-onset obesity not due to decrease in energy expenditure. A: body weight curves show that POMC-Glp2r KO mice displayed late-onset obesity. B: body composition of 32-wk-old mice. C and D: basal metabolic rates of 12-wk-old mice. POMC-Glp2r KO mice showed identical basal metabolic rates to their WT littermates. Basal energy metabolism was assessed using Comprehensive Laboratory Animal Monitoring System (CLAMS) in mice after 4-h withdrawal of food under the light phase. Data were analyzed by repeated-measures ANOVA and expressed as means ± SE (n = 10 per group). †P < 0.10, *P < 0.05, denoting difference at the same time point (or phase) between the 2 genotypes.
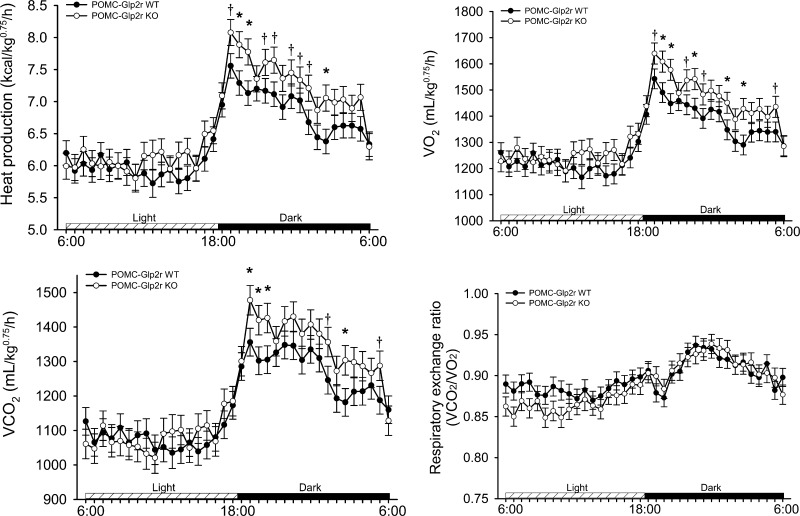
We wanted to determine whether Glp2r deletion in POMC neurons decreases energy expenditure or locomotor activity, which might account for the late-onset obesity. POMC-Glp2r KO mice had higher vertical activity (data not shown) during the dark cycle but not during the light cycle. This hyperactivity might represent their effort to seek food. However, there were no differences between the two genotypes in counts of horizontal activity. Both V̇o2 and heat production (HP) under the fed status were higher in the KO mice during the dark cycle (V̇o2 : 1,524 ± 55 vs. 1,368 ± 50 ml/BW0.75/h, P < 0.05; HP: 7.55 ± 0.26 vs. 6.76 ± 0.24 kcal/BW0.75/h, P < 0.05, for KO and WT mice, respectively). Note that body weight was similar during the CLAMS (26.2 ± 0.29 vs. 26.5 ± 0.28 g BW for the KO and WT mice, respectively). Importantly, differences in V̇o2 and HP occurred only during the dark cycle (Fig. 5), suggesting that increased HP in the KO mice might have resulted from their increased food-seeking activity.
Fig. 5.
POMC-Glp2r KO mice show increased nocturnal energy expenditure. POMC-Glp2r KO mice had slightly higher oxygen consumption (V̇o2), CO2 production (V̇co2), and heat production (HP) during the dark phase. Indirect calorimetry was monitored for 72 h in 12-wk-old POMC-Glp2r KO mice (fed ad libitum) using CLAMS. Data were analyzed by repeated-measures ANOVA using the MIXED procedure and expressed as means ± SE (n = 10 per group), †P < 0.10, *P < 0.05 denoting difference at the same phase between the 2 genotypes.
We further defined whether Glp2r deletion in POMC neurons impaired basal metabolic rates prior to obesity. Energy metabolism during the light cycle was assessed in 12-wk-old mice after a 4-h withdrawal of food, which would approximately represent the basal metabolic rate, i.e., under the condition of little activity (inactive), thermoneutral temperature, and postabsorptive state. There were no differences in V̇o2, V̇co2, or HP over a 4-h measurement between the two genotypes (Fig. 4), suggesting that Glp2r deletion in POMC neurons did not impact basal energy expenditure. Therefore, the KO late-onset obesity did not result from decreases in energy expenditure or locomotor activity.
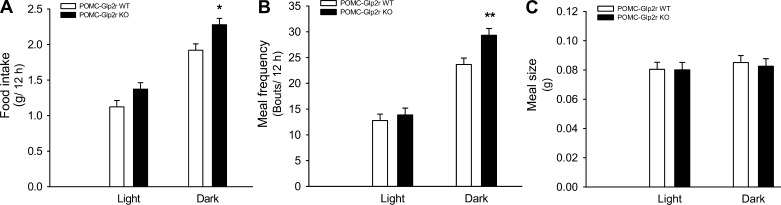
POMC-Glp2r KO Mice Show Hyperphagic Behavior
We wanted to determine whether GLP-2R in POMC neurons controls feeding behavior (Fig. 6), which might be a driving force for the late-onset obesity. The 12-wk-old mice with Glp2r deletion in POMC neurons had higher food intake during the dark cycle, and this was associated with increased meal frequency, indicating that they were hungry. In contrast, no increase in meal size was observed in the POMC-Glp2r KO mice. This hyperphagic behavior indicates that GLP-2R in POMC neurons plays an important physiological role in the control of feeding behavior, which agrees with a notion of the CNS GLP-2 as a satiety signal.
Fig. 6.
POMC-Glp2r KO mice show hyperphagic behavior. POMC-Glp2r KO mice showed increased food intake and meal frequency during the dark phase. However, meal size was similar between the 2 genotypes. Feeding behavior was monitored using CLAMS for ∼12-wk-old male mice fed ad libitum after 3-day adaptation in metabolic cages. *P < 0.05, **P < 0.01, denoting significance at the same phase between the 2 genotypes.
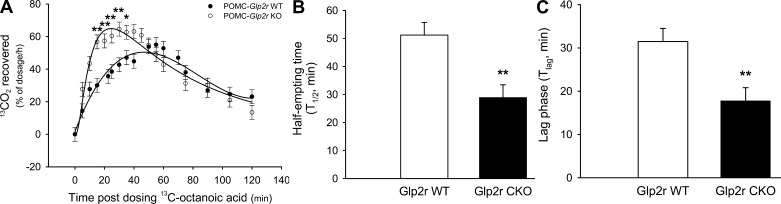
POMC-Glp2r KO Mice Show Accelerated Gastric Emptying
Digestion and absorption of food affect net intake of food energy, which is mainly regulated by gastric emptying and intestinal motility. Mice with Glp2r deletion in POMC neurons might have defects in digestion and absorption. We hypothesized that GLP-2R in POMC neurons modulates gastric motility to control net intake (input) of food energy. Thus, we wanted to quantify the rate of gastric emptying in the KO mice in the conscious condition by using a [13C]octanoic acid breath test (Fig. 7). Gastric emptying (GE; indicated by the rate of 13CO2 recovered in breath) increased during the first 40 min in the 12-wk-old KO mice. To derive key parameters for GE, the data were fitted by a nonlinear regression model. Both half-excretion time (T1/2) and lag phase (Tlag) for liquid meal were dramatically shorter, whereas gastric emptying coefficient was higher in the KO mice (1.18 ± 0.13) than those in the WT mice (0.62 ± 0.13). T1/2 was 29.0 ± 4.5 and 51.2 ± 4.5 min; and Tlag was 17.8 ± 3.0 and 31.5 ± 3.0 min for the KO and WT mice, respectively. This is consistent with the notion that GLP-1/2 secreted from the endocrine L cells in the ileum slow down GE to suppress food intake. However, the meal frequency was enhanced in the KO mice compared with that in the WT, suggesting that the KO mice were hungry. These data suggest that hyperphagic behavior with accelerated GE in POMC-Glp2r KO mice partially accounts for the late-onset obesity.
Fig. 7.
POMC-Glp2r KO mice show accelerated gastric emptying (GE). Rates of GE (indicated by rate of 13CO2 recovered in breath) were higher during the first 40 min in 12-wk-old POMC-Glp2r KO mice. KO mice had shorter half-empting time and lag phase but higher GE coefficient than WT mice. After an overnight fast, 12-wk-old male mice were assessed for GE rate of the test meal using a [13C]octanoic acid breath test. Data for the 13CO2 excretion rate were fitted by a nonlinear regression model. Data are expressed as means ± SE (n = 8–10 per group), *P < 0.05, **P < 0.01, denoting significance at the same time between the 2 genotypes.
Glp2r Deletion in POMC Neurons Increases Preproglucagon Expression
To determine whether Glp2r deletion in POMC neurons impacts expression of the neuropeptide (GLP-2), we quantified mRNA abundance of preproglucagon in the hypothalamus. POMC-Glp2r KO mice showed higher abundance of preproglucagon mRNA in the brain than the WT mice (Preproglucagon mRNA: 1.52 ± 0.14- vs. 1.01 ± 0.15-fold, P < 0.05, for KO and WT mice, respectively), suggesting an inverse relationship between GLP-2 and GLP-2R in this neural circuit. Interestingly, the abundance of Pomc mRNA in the hypothalamus was increased in the KO mice compared with the WT mice (Pomc mRNA: 2.28 ± 0.17- vs. 1.01 ± 0.16-fold, P < 0.01, for KO and WT mice, respectively), which might have been due to an increased adipostatic leptin level. The abundance of Agrp mRNA in the hypothalamus was similar between the two genotypes (Agrp mRNA: 0.98 ± 0.14- vs. 1.01 ± 0.15-fold, P > 0.1, for KO and WT mice, respectively).
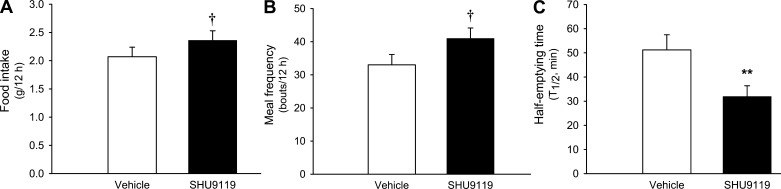
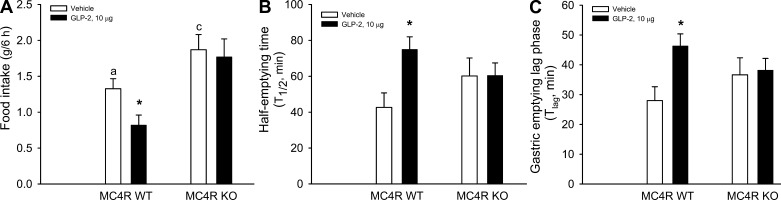
MC4R Signaling is Required for icv GLP-2-Mediated Suppression of Food Intake and GE
The POMC-derived melanocortin system (α-MSH) in the hypothalamus promotes anorexia. As the major α-MSH receptor, MC4R plays a pivotal role in maintaining energy homeostasis. The brain stem's NTS is a critical site of MC4R signaling for hypothalamic α-MSH input and GI signals to suppress food intake (6, 12, 41). We were wondering whether Glp2r-initiated action on POMC neurons enhances MC4R signaling in the brain stem, which suppresses feeding and GE. To define if suppression of MC4R signaling in the brain stem increases food intake and GE, we found that acute infusion of an MC4R antagonist (SHU9119, 0.5 nmol into the 4th ventricle in C57BL/6 mice) not only increased food intake and meal frequency but also accelerated GE and GE coefficient (vehicle vs. SHU9119: 0.69 ± 0.13 vs. 1.19 ± 0.14; Fig. 8). To address whether MC4R signing is required for icv GLP-2 action, acute infusion of GLP-2 (2.5 nmol into the 4th ventricle) suppressed food intake and GE in 5-wk-old MC4R WT mice, but these effects were diminished in MC4R KO mice (Fig. 9) when their body weight was similar to that of their WT littermates. These data suggest that MC4R signaling (in the brain) is important for the tonic control of GE but is also required partially for icv GLP-2-mediated suppression of food intake.
Fig. 8.
icv melanocortin receptor-4 (MC4R) antagonist enhances food intake and GE. After 7-day surgical recovery, acute infusion of MC4R antagonist SHU9119 (0.5 nmol into the 4th ventricle in 12-wk-old C57BL/6 mice) not only increased food intake and meal frequency but also accelerated GE. Feeding behavior was monitored by CLAMS and GE by [13C]octanoic acid breath test. Data are expressed as means ± SE (n = 6∼8 per group), †P < 0.06, **P < 0.01, denoting significance between vehicle and MC4R antagonist.
Fig. 9.
MC4R signaling is required for icv GLP-2-mediated suppression of food intake and GE. After 7-day surgical recovery, acute infusion of GLP-2 (10 μg into the 4th ventricle) suppressed food intake and GE in MC4R WT mice; but these effects were diminished in MC4R KO mice (at 5 wk), when they were not heavier than WT littermates. Feeding behavior was monitored by CLAMS and GE by [13C]octanoic acid breath test. Data are expressed as means ± SE (n = 6∼8 per group), *P < 0.05, denoting significance between vehicle and GLP-2; a,cP < 0.01 between WT and KO mice under control (vehicle).
DISCUSSION
In this study, we demonstrate that mice lacking Glp2r in POMC neurons exhibit impaired energy balance (such as late-onset obesity, hyperphagic behavior, and food-seeking activity) and accelerated gastric emptying. Moreover, icv activation of GLP-2R signaling suppresses food intake and gastric emptying in an MC4R-dependent manner. Thus, Glp2r in POMC neurons is essential for the physiological short-term control of feeding behavior (such as meal frequency and gastric emptying), which contributes to the homeostatic long-term control of energy balance and body weight.
GLP-1/2 are cosecreted from endocrine L cells in the gut in response to food intake and coreleased from preproglucagonergic (PPG) neurons in the brain as well. Besides their local production in the brain, gut-derived GLP-1/2 may cross the semipermeable blood-brain barrier in the hypothalamic ARC. Thus, GLP-1/2 as satiety signals are proposed to modulate short-term feeding behavior and may promote long-term energy balance. DPP-IV inhibitors (to suppress GLP-1/2 degradation) have been tested clinically in the treatment of diabetes and obesity. GLP-2 has been proposed as a neurotransmitter in the control of feeding behavior (44, 45); however, little is known about its chronic efficacy and physiological significance in the homeostatic control of energy balance (and body weight). GLP-2R is expressed not only in nutrient-sensing enteroendocrine cells but also in peripheral nervous system (such as enteric neurons and sensory neurons) and CNS (including hippocampus, hypothalamus, and brain stem) (20, 32, 33, 37, 44, 48, 53). To determine the neuron-specific action of GLP-2R, we generated a Glp2r-floxed mouse line. In addition to Glp2r mRNA expression in the DMH, we show that Glp2r mRNA and protein are highly expressed in the mouse ARC neurons in the present study. Since POMC neurons play a critical role in the homeostatic control of energy balance, we wanted to determine whether GLP-2R in POMC neurons plays an important physiological role in energy balance.
One major finding in this study is that mice with Glp2r deletion in POMC neurons show hyperphagic behavior and faster gastric emptying. Gastric emptying may serve as a key factor in the control of energy homeostasis. As a quantitative determinator of food intake (16, 23), gastric emptying is fine-tuned by metabolic, neuronal, and hormonal signals. Gastric emptying is positively associated with overeating (16) and is enhanced in obese subjects (8, 52). Peripheral GLP-2 inhibits (−75%) gastric antral motility in humans (35) and pigs (51). GLP-2R is expressed in both excitatory (SP- and ChAT-positive) and inhibitory (VIP- and nNOS-positive) enteric neurons (10, 20). Thus, peripheral GLP-2 inhibits GI smooth muscle contractility by decreasing cholinergic neurotransmission and increasing NO or VIP release (1, 2, 10). However, it is unknown whether CNS GLP-2 plays a physiological role in the control of gastric emptying. Here, we show that the CNS GLP-2 suppresses gastric emptying, linking to feeding behavior. Mice with Glp2r deletion in POMC neurons not only show hyperphagic behavior but also have accelerated gastric emptying. It is conceivable that the overall impact of faster gastric emptying results in a shorter satiety period, increasing meal frequency and energy intake (23). Thus, our evidence that Glp2r deletion in POMC neurons accelerated rates of gastric emptying not only explains the hyperphagic behavior but also points to the physiological role of CNS GLP-2 in the regulation of satiety.
Another major finding in this study is that icv activation of GLP-2R signaling suppresses food intake and gastric emptying and that this is mediated by the MC4R signaling pathway. A brain stem NTS-DMV (dorsal motor nucleus of the vagus) neural circuit integrates hypothalamic metabolic and nutritional inputs and gut-derived satiety signals to regulate autonomic outflow (feeding behavior, glucose homeostasis, and energy intake) (27, 46). MC4R are heavily expressed in the brain stem dorsal vagal complex (DVC, including NTS plus DMV) (18, 26). MC4R-GFP-positive efferents (coexpressing ChAT) terminate in the GI myenteric plexus, suggesting that MC4R signaling in vagal efferents may contribute to the control of GI function (18, 47). In this study, we demonstrate that acute suppression of MC4R signaling in the brain stem increases food intake and meal frequency and accelerates gastric emptying; yet we cannot exclude the possibility that the MC4R antagonist may act on sites other than the brain stem. These results are not only consistent with previous reports that MC4R signaling in the brain stem mediates food intake (50, 54) but also suggest a novel function of MC4R signaling in the control of gastric motility (emptying). Inhibition of MC4R-positive cholinergic neurons in the DMV would alleviate excitatory input to the GI, decreasing gastric emptying (18, 41). Moreover, icv infusion of GLP-2 (1 μl into the 4th ventricle) inhibits food intake and gastric emptying, and this is blocked in the MC4R KO mice. Thus, MC4R signaling is required for CNS GLP-2-mediated suppression of food intake and gastric emptying. Further studies are warranted to determine whether GLP-2 directly acts on MC4R-positive neurons or indirectly modulates them through presynaptic input (e.g., from POMC neurons). It will also be important to determine how MC4R signing modulates the DMV and thus controls autonomic outputs (46).
POMC neurons are not only localized in the hypothalamic ARC but also in the brain stem's NTS. Of note, this POMC promoter-driven cre is expressed mainly in the ARC (5). Thus, the metabolic phenotype of POMC-Glp2r KO mice might result from Glp2r deletion in POMC neurons of the ARC. As a neurotransmitter, GLP-2 would act on GLP-2R on postsynaptic dendrites. It will be important to define if the 4th-ventricular GLP-2-mediated action is due to presynaptic effects of GLP-2R on axon terminals (arising from the ARC POMC neurons) and/or postsynaptic effects of GLP-2R on dendrites (of the NTS POMC neurons). We note that POMC-cre could be transiently expressed in embryonic non-POMC neurons in the brain (40), which might mediate recombination of GLP-2R in off-target cells. However, the POMC-Cre-mediated recombination of each floxed allele is unique, depending on endogenous recombination efficiency. It is important to note that endogenous Glp2r is not expressed in the brain regions where POMC-Cre is expressed highly transiently (40). Our data suggest that GLP-2R in Pomc-expressing progenitors is required for the control of feeding behavior and gastric emptying; yet it is unknown about the extent to which the Cre-mediated recombination in off-target sites (if any) may contribute to the observed metabolic phenotype. Further studies are warranted to confirm the physiological significance of GLP-2R in POMC neurons through inactivating Glp2r specifically in mature POMC neurons using inducible POMC-Cre mice.
In summary, the CNS GLP-2R is essential for the control of feeding behavior. Glp2r deletion in POMC neurons increases food intake with amplified meal frequency and accelerates gastric emptying, suggesting that CNS GLP-2 is a key satiety signal for the physiological short-term control of feeding behavior and gastric motility and contributes to the long-term homeostatic control of energy balance (or body weight). Moreover, activation of GLP-2R signaling suppresses food intake and gastric emptying through the MC4R signaling pathway. Our findings suggest that gastric emptying is a key process for the short-term control of feeding behavior (satiation and satiety), and POMC neuron-mediated suppression of food intake may be executed through decelerating gastric emptying.
GRANTS
This work is supported by the United States Department of Agriculture, Agricultural Research Service (USDA/ARS) under Cooperative Agreement No. 6250-51000-043, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-075489 and DK-084125, and the National Natural Science Foundation of China Grant 30728016 (X. Guan); and NIDDK P30 DK-079638 (L. Chan). This work is a publication of the USDA/ARS Children's Nutrition Research Center, Departments of Pediatrics and Medicine, Baylor College of Medicine, and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.G. and L.C. conception and design of research; X.G., X.S., X.L., and Y.W. performed experiments; X.G., X.S., X.L., and Y.W. analyzed data; X.G., X.S., X.L., B.C., Y.W., D.L., and L.C. interpreted results of experiments; X.G. and X.S. prepared figures; X.G. and X.S. drafted manuscript; X.G. and L.C. edited and revised manuscript; X.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yong Xu, Zhuo Yang, Maria Truong, Shixiang Wen, Fuguo Zhou, Jian Qi, Wei Wang, Aijun Zhang, Yisheng Yang, Lan Li, Qiang Tong, Marta Fiorotto, Douglas Burrin, Firoz Vohra, and Shaji Chacko at Baylor College of Medicine for scientific and technical support. We also thank Qingchun Tong at the University of Texas Health Science Center at Houston for technical support.
REFERENCES
- 1. Amato A, Baldassano S, Serio R, Mule F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol 296: G678– G684, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Amato A, Rotondo A, Cinci L, Baldassano S, Vannucchi MG, Mule F. Role of cholinergic neurons in the motor effects of glucagon-like peptide-2 in mouse colon. Am J Physiol Gastrointest Liver Physiol 299: G1038– G1044, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Asakawa A, Inui A, Ueno N, Makino S, Uemoto M, Fujino MA, Kasuga M. Ob/ob mice as a model of delayed gastric emptying. J Diabetes Complications 17: 27– 28, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138: 2447– 2456, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983– 991, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493– 505, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cakir B, Kasimay O, Devseren E, Yegen BC. Leptin inhibits gastric emptying in rats: role of CCK receptors and vagal afferent fibers. Physiol Res 56: 315– 322, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Cardoso-Junior A, Coelho LG, Savassi-Rocha PR, Vignolo MC, Abrantes MM, de Almeida AM, Dias EE, Vieira JG, de Castro MM, Lemos YV. Gastric emptying of solids and semi-solids in morbidly obese and non-obese subjects: an assessment using the 13C-octanoic acid and 13C-acetic acid breath tests. Obes Surg 17: 236– 241, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chen CY, Chao Y, Chang FY, Chien EJ, Lee SD, Doong ML. Intracisternal des-acyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. Int J Mol Med 16: 695– 699, 2005 [PubMed] [Google Scholar]
- 10. Cinci L, Faussone-Pellegrini MS, Rotondo A, Mule F, Vannucchi MG. GLP-2 receptor expression in excitatory and inhibitory enteric neurons and its role in mouse duodenum contractility. Neurogastroenterol Motil 23: e383– e392, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell 129: 251– 262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571– 578, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13– 23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Heer J, Pedersen J, Orskov C, Holst JJ. The alpha cell expresses glucagon-like peptide-2 receptors and glucagon-like peptide-2 stimulates glucagon secretion from the rat pancreas. Diabetologia 50: 2007 [DOI] [PubMed] [Google Scholar]
- 15. Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24– 32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duggan JP, Booth DA. Obesity, overeating, and rapid gastric emptying in rats with ventromedial hypothalamic lesions. Science 231: 609– 611, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7: 335– 336, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol 518: 6– 24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 104: 1640– 1647, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150– 164, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology 125: 136– 147, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Heijboer AC, Pijl H, Van den Hoek AM, Havekes LM, Romijn JA, Corssmit EP. Gut-brain axis: regulation of glucose metabolism. J Neuroendocrinol 18: 883– 894, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Horner KM, Byrne NM, Cleghorn GJ, Naslund E, King NA. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev 12: 935– 951, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137: 997– 1005, 1005, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Janssen P, Vanden BP, Verschueren S, Lehmann A, Depoortere I, Tack J. Review: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther 33: 880– 894, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213– 235, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes 60: 107– 113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJ. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg 252: 50– 56, 2010 [DOI] [PubMed] [Google Scholar]
- 29. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780– 785, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lee EC, Yu D, Martinez-De Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73: 56– 65, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem 276: 21489– 21499, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Lovshin JA, Huang Q, Seaberg R, Brubaker PL, Drucker DJ. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology 145: 3495– 3506, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96: 1569– 1573, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature 444: 854– 859, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Nagell CF, Wettergren A, Pedersen JF, Mortensen D, Holst JJ. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand J Gastroenterol 39: 353– 358, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Nagy A, Rossant J, Nagy R, Bramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90: 8424– 8428, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 148: 1954– 1962, 2007 [DOI] [PubMed] [Google Scholar]
- 38. O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci USA 94: 14602– 14607, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes (Lond) 35: 153– 166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 153: 1219– 1231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13: 195– 204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science 307: 375– 379, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Symonds E, Butler R, Omari T. Noninvasive breath tests can detect alterations in gastric emptying in the mouse. Eur J Clin Invest 32: 341– 344, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med 6: 802– 807, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord 25, Suppl 5: 2001 [DOI] [PubMed] [Google Scholar]
- 46. Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279– 305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 28: 4957– 4966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Guan X. GLP-2 potentiates L-type Ca2+ channel activity associated with stimulated glucose uptake in hippocampal neurons. Am J Physiol Endocrinol Metab 298: E156– E166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y, Shi X, Qi J, Li X, Uray K, Guan X. SIRT1 inhibits the mouse intestinal motility and epithelial proliferation. Am J Physiol Gastrointest Liver Physiol 302: G207– G217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141: 1332– 1337, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Wojdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol 33: 828– 832, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology 84: 747– 751, 1983 [PubMed] [Google Scholar]
- 53. Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744– 755, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289: R247– R258, 2005 [DOI] [PubMed] [Google Scholar]