Abstract
We have suggested previously that Tribbles homolog 3 (TRIB3), a negative regulator of Akt activity in insulin-sensitive tissues, could mediate glucose-induced insulin resistance in muscle under conditions of chronic hyperglycemia (Liu J, Wu X, Franklin JL, Messina JL, Hill HS, Moellering DR, Walton RG, Martin M, Garvey WT. Am J Physiol Endocrinol Metab 298: E565–E576, 2010). In the current study, we have assessed short-term physiological regulation of TRIB3 in skeletal muscle and adipose tissues by nutrient excess and fasting as well as TRIB3's ability to modulate glucose transport and mitochondrial oxidation. In Sprague-Dawley rats, we found that short-term fasting enhanced insulin sensitivity concomitantly with decrements in TRIB3 mRNA (66%, P < 0.05) and protein (81%, P < 0.05) in muscle and increments in TRIB3 mRNA (96%, P < 0.05) and protein (∼10-fold, P < 0.05) in adipose tissue compared with nonfasted controls. On the other hand, rats fed a Western diet for 7 days became insulin resistant concomitantly with increments in TRIB3 mRNA (155%, P < 0.05) and protein (69%, P = 0.0567) in muscle and a decrease in the mRNA (76%, P < 0.05) and protein (70%, P < 0.05) in adipose. In glucose transport and mitochondria oxidation studies using skeletal muscle cells, we found that stable TRIB3 overexpression impaired insulin-stimulated glucose uptake without affecting basal glucose transport and increased both basal glucose oxidation and the maximal uncoupled oxygen consumption rate. With stable knockdown of TRIB3, basal and insulin-stimulated glucose transport rates were increased, whereas basal glucose oxidation and the maximal uncoupled oxygen consumption rate were decreased. In conclusion, TRIB3 impacts glucose uptake and oxidation oppositely in muscle and fat according to levels of nutrient availability. The above data for the first time implicate TRIB3 as a potent physiological regulator of insulin sensitivity and mitochondrial glucose oxidation under conditions of nutrient deprivation and excess.
Keywords: tribbles 3, glucose oxidation, nutrient sensor, skeletal muscle, adipose tissue, adipocyte, mitochondria, glucose transport, glucose oxidation
tribbles 3 (TRIB3; also known as NIPK and SIKP3), TRIB1, and TRIB2 are the three mammalian homologues of tribbles in Drosophila and were first identified as mitosis blockers in embryo and germ cell development (12, 26, 41). The tribbles family is comprised of pseudokinases because all members share an evolutionarily conserved kinase domain without a critical ATP binding site (13); as a result, TRIBs have no detectable kinase catalytic function. Through binding with various proteins, TRIBs are involved in regulating biological functions such as cell proliferation, differentiation, and metabolism. TRIB3 is the most studied homolog, and its expression is subject to various signals such as endoplasmic reticulum (ER) stress, nutrient availability, and insulin (9). By interacting with transcoactivators such as activating transcription factor 4 (ATF4) and CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) (33–37), it can regulate apoptotic pathways in tumor cell lines. In studies of metabolism and insulin action, TRIB3 directly binds to unphosphorylated Akt and blocks its phosphorylation, resulting in impaired insulin signaling in skeletal muscle, liver, fat, and pancreas (10, 21–23, 25, 27, 39). TRIB3 expression is also regulated by nutrient availability in skeletal muscle cells, β-cells, adipocytes, and tumor cells (8, 9, 23, 25, 50).
Previously, we reported a role for TIRB3 in glucose-induced insulin resistance in skeletal muscle (25). TRIB3 mRNA and protein levels were elevated in skeletal muscle from patients with type 2 diabetes compared with insulin-sensitive individuals, and TRIB3 muscle content was positively correlated with fasting blood glucose levels and inversely correlated with glucose disposal rates. High TRIB3 muscle expression during hyperglycemia was observed in multiple rodent models of insulin resistance, such as streptozotocin-treated rats, Zucker fatty rats, and db/db mice compared with insulin-sensitive controls. Moreover, high glucose levels in L6 cells can induce TRIB3 expression, and overexpression of TRIB3 impaired insulin's ability to stimulate Akt phosphorylation and glucose uptake (25). These observations regarding upregulation of TRIB3 by high glucose, combined with the demonstrated ability of stable TRIB3 overexpression to impair insulin-stimulated glucose transport in muscle cells, led us to hypothesize that TRIB3 was an important mediator of glucose toxicity (25).
Defects in insulin-stimulated glucose oxidation and glycogen synthesis are the other major features of insulin resistance that have been demonstrated in normal glucose tolerance offspring of type 2 diabetic patients, patients with overt type 2 diabetes, and obese individuals (40, 42). An expanding body of literature has linked mitochondria dysfunction to defects in substrate oxidation as well as insulin resistance in skeletal muscle (4, 30, 45) and in other tissues. including liver, fat, heart, and β-cells (1, 5, 6, 32, 47). In these studies, mitochondrial defects have involved decrements in mitochondrial mass and impaired oxidative function in the skeletal muscle in type 2 diabetic and obese individuals (14, 20, 29, 38, 44). Recently, in a study to determine gene expression resulting from pharmacological inhibitions of the respiratory chain in mitochondria, TRIB3 was found to be upregulated in a CHOP-10/C/EBPβ-dependent manner in C2C12 cells (16). This led us to explore the possibility that TRIB3 plays a role in regulating mitochondrial substrate oxidation. To address this issue, we have combined studies in L6/L6-GLUT4myc muscle cells assessing mitochondrial function following manipulation of TRIB3 expression together with studies in intact mice examining the regulated expression of TRIB3 in muscle and adipose tissue during short-term fasting and high-fat feeding. We have elucidated a novel physiological role for TRIB3 to regulate glucose transport and mitochondrial substrate oxidation in muscle and in adipose tissue in response to nutrient deprivation and excess.
MATERIALS AND METHODS
Animal care, treatment, and assays.
All experimental plans were approved by the Animal Care Committee of the University of Alabama at Birmingham, Alabama. Five-week-old male Sprague-Dawley rats (Charles River, Wilmington, MA) were housed two per cage with a 12-h light cycle. Animals were randomized to be placed on 1) an ad libitum normal chow control diet (12% kcal fat/19% protein/69% carbohydrate, 3.87 kcal/g, n = 6), 2) an ad libitum Western diet (40% kcal fat/15% protein/45% carbohydrate, 4.62 kcal/g) prepared from TestDiet (Richmond, IN) for 7 days (n = 6), or 3) fasting consisting of withdrawal from food but not water for 48 h (n = 6). Serum glucose and triglyceride levels were measured using a Sirrus chemistry auto analyzer (Stanbio Laboratory, Boerne, TX) with a Stanbio Glucose Liquicolor (Stanbio Laboratory) and Stanbio Triglyceride Liquicolor (Stanbio Laboratory), respectively; serum insulin level was measured with the Rat Insulin RIA kit (Millipore, St. Charles, MO), and the level of free fatty acids was measured using an HR Series NEFA-HR kit (Wako Diagnostics, Richmond, VA).
Cell culture.
Rat L6 myoblasts (ATCC, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 U/ml penicillin, and 100 mg/ml streptomycin in a 5% CO2 atmosphere at 37°C. L6-GLUT4myc myoblasts expressing an exofacial myc epitope (a kind gift from Dr. Amira Klip, University of Toronto, Hospital for Sick Children) were cultured as reported previously (46). To activate differentiation, cells were cultured to reach 100% confluence, and medium was changed to DMEM with 2% horse serum (Invitrogen) and changed every 2 days. After 6 days, fully differentiated L6/L6-GLUT4myc myotubes were used in experiments.
Lentiviral-mediated overexpression and knockdown of TRIB3.
Wild-type TRIB3 gene was kindly provided by Dr. E. Kiss-Toth (University of Sheffield, Sheffield, UK). Lentivirus vector was constructed and packaged by ADV Bioscience (Birmingham, AL). The stop codon in the TRIB3 cDNA was removed, and c-Myc was attached to the COOH terminus. The modified cDNA was ligated into lentivector (pHR-EF-IRES-Bla) at the BamHI and XhoI sites. DNA sequencing was used to ensure proper insertion. Lentiviral short-hairpin RNA (shRNA; sense: 5′-GAT CCA GGA AGA AAC CGT TGG AGT TTG TCA AGA GCA AAC TCC AAC GGT TTC TTC CTT TTT GG-3′; antisense: 5′-AAT TCC AAA AA GGA AGA AAC CGT TGG AGT TTG CTC TTG ACA AAC TCC AAC GGT TTC TTC CTG-3′) locates at rat TRIB3 cDNA position 212 (NM_144755). To establish stably transfected cell lines, procedures were performed as described previously (25).
Extracellular flux bioenergetic assays and test compounds.
L6 cells and L6 cells with stable TRIB3 overexpression or knockdown were seeded in extracellular flux (XF) 24-cell culture plates (Seahorse Bioscience, Billerica, MA) at 4 × 104 cells/well in DMEM with 10% FBS and then incubated at 37°C/5% CO2 the night before the assays. On the morning of the assay, even cell distribution was assured in each well by microscopy, and media were refreshed with warmed, freshly prepared, unbuffered DMEM (Seahorse Bioscience) plus 5/25 mM glucose, 4 mM glutamine, 82 mM sodium chloride, and 15 mg/l phenol red adjusted to pH 7.4. Cells were incubated at 37°C without CO2 for 60 min to allow media temperature and pH to reach equilibrium. A Seahorse XF24 analyzer (Seahorse Bioscience) was used to measure oxygen consumption and extracellular acid flux simultaneously in a 500-μl chamber (48) with measure/mix/wait cycles of 3:2:3 min. All measurements were normalized to protein quantification in each well assessed by Bicinchoninic Acid assays.
Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), antimycin, rotenone, and oligomycin were all purchased from Sigma (St. Louis, MO). Concentrated stocks of 30 mM FCCP were prepared in unbuffered DMEM and then diluted to 900 nM as final concentration in assay medium. Concentrated 5 mM antimycin, 50 mM rotenone, and 50 mM oligomycin were prepared in ethanol and diluted to 4 μM, 1 μM, and 500 nM, respectively, as final concentration in assay medium.
Glucose transport assays.
Glucose transport rates were assayed in monolayers of L6-GLUT4myc cells following previously described procedures (28). Same numbers of L6-GLUT4myc cells were plated in each cell culture well. Cells were incubated in serum-free DMEM for 3 h before the experiments and in the absence and presence of 100 nM insulin stimulation for an additional 45 min. Then, basal and the maximally insulin-stimulated glucose transport rates were measured as described previously (28).
RNA isolation and expression analysis.
Total RNA was isolated from L6 or L6-GLUT4myc cells using RNeasy columns with DNase I treatment (Qiagen, Valenica, CA). Rat tissue samples were flash-frozen by liquid nitrogen after being harvested, and total RNA was isolated using Trizol reagent (Invitrogen). cDNA synthesis was carried out by VILO kit (Invitrogen), following the manufacturer's instructions. Real-time quantitative PCR analysis was performed on a StepOnePlus 96-well machine (Applied Biosystems, Foster City, CA). PCR products were detected using SYBR Green and normalized to 18S ribosomal RNA by using specific oligonucleotide primers indicated as follows: for rat 18S, 5′-GGAGGATGAGGTGGAGCGAGT-3′ (5′ primer) and 5′-GCCTCTCCAGGTCCTCACGC-3′ (3′ primer); for rat TRIB3, 5′-AGAGTCCTGGAACGGGTATC-3′ (5′ primer) and 5′-AGTTGCGTCGATTTGTCTTC-3′ (3′ primer).
Protein isolation and immunoblot analysis.
Proteins were harvested from rat tissues or cells in lysis buffer containing 50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1% Na deoxycholate, 1% NP-40, 10 mM NaF, 5 mM Na3VO4, 2 mg/ml pepstatin, 2 mM PMSF, 1 mM DTT, 20 μg/ml leupeptin, and 10 μg/ml aprotinin. The Bicinchoninic Acid kit (Sigma) was used to determine protein concentrations. Proteins were separated by SDS-PAGE for subsequent Western blotting. Membrane filters were incubated with TRIB3 antibody (Calbiochem, Damstadt, Germany) and followed by horseradish peroxidase-conjugated anti-rabbit IgG (Pierce, Rockford, IL). Cytochrome c oxidase IV (COX IV; Cell Signaling Technology) antibody was used to detect the total COX IV protein as an indicator of mitochondrial mass. Images were captured using enhanced chemiluminescence (Pierce) on a ChemiDoc XRS imager (BioRad, Hercules, CA) and were subsequently quantified using Image Lab software (Bio-Rad).
Statistical analysis.
Experimental results are shown as the mean ± SD. Comparisons between means were performed by Student's t-tests or ANOVA using Prism version 4 (GraphPad, San Diego, CA). Significance was defined as P < 0.05 or P < 0.01.
RESULTS
Effects of TRIB3 knockdown on insulin-stimulated glucose transport activity and insulin signal transduction.
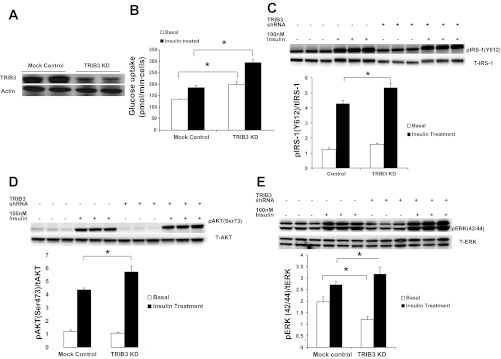
As we reported previously (25), TRIB3 overexpression in L6-GLUT4myc cells reduced insulin-stimulated glucose uptake rate without affecting the basal rate. To determine whether suppression of TRIB3 could impact insulin sensitivity, we established stable lentiviral TRIB3 shRNA-transfected L6/L6-GLUT4myc cell lines, which exhibited an ∼70% reduction of the TRIB3 protein compared with empty lentiviral vector control cells without compensatory upregluation of TRIB1 and TRIB2 (Fig. 1A). As shown in Fig. 1B, TRIB3 knockdown in L6-GLUT4myc cells led to significant increments in both basal (1.4-fold, P < 0.05) and insulin-stimulated (1.6-fold, P < 0.05) glucose uptake rates compared with control cells.
Fig. 1.
L6-GLUT4myc cells were stably transfected with either an empty lentiviral vector or a tribbles 3 (TRIB3) short-hairpin RNA (shRNA) lentiviral vector. A: knockdown (KD) of TRIB3 protein in L6 muscle cells. B: basal (open bars) and insulin-stimulated (filled bars) glucose uptake rates were measured; values are means ± SD. C–E: L6 cells were stably transfected with either an empty lentiviral vector or a TRIB3 shRNA lentiviral vector and analyzed by Western blot for phosphorylated insulin receptor substrate-1 (p-IRS-1) at the Tyr612 site (C), p-Akt at the Ser473 site (D), and p-ERK (E) with or without 45 min of 100 nM insulin stimulation. *P < 0.05. Values are means ± SD for 3 repeated experiments.
This resulting increase in the activity of the glucose transport system was explained partially by enhanced insulin signal transduction. As can be seen in Fig. 1C, phosphorylation of insulin receptor substrate-1 (IRS-1) at Tyr612 in the TRIB3 knockdown L6 myocytes was increased significantly in basal (1.2-fold, P < 0.05) and in insulin-stimulated cells (1.3-fold, P < 0.05) when compared with controls. Downstream of IRS-1, insulin-stimulated phosphorylation of Akt on Ser473 was increased 1.3-fold in the TRIB3 knockdown cells without effect on basal Akt phosphorylation (P < 0.05; Fig. 1D). Finally, TRIB3 suppression in L6 cells increased phosphorylation of ERK1/2 after insulin stimulation significantly (1.2-fold, P < 0.05) but decreased basal ERK1/2 phosphorylation 1.6-fold (P < 0.05; Fig. 1E).
Acute effects of TRIB3 on mitochondrial oxidation.
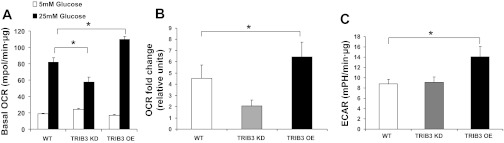
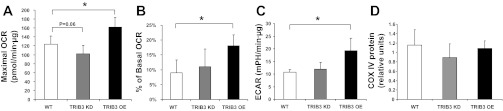
Skeletal muscle is the major tissue contributing to glucose uptake as well as glucose oxidation. Therefore, we also examined whether TRIB3 modulated intracellular glucose oxidation in intact L6 myocytes by using an XF bioenergetic analyzer. At a physiologically normal glucose concentration (i.e., 5 mM d-glucose), the basal cellular oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were not changed when wild-type cells were compared with L6 cell lines with stable TRIB3 overexpression or TRIB3 knockdown (Fig. 2A). However, in the presence of high glucose (i.e., 25 mM d-glucose), basal cellular OCR was decreased by 39% (P < 0.05) in TRIB3 knockdown L6 cells, whereas TRIB3 overexpression increased OCR by 34% (P < 0.05) compared with L6 wild-type cells, as shown in Fig. 2A. The fold increase in OCR when glucose was increased from 5 to 25 mM was similarly reduced in TRIB3 knockdown and increased in TRIB3-overexpressing cells when compared with controls (Fig. 2B), suggesting that manipulation of TRIB3 expression could be affecting mitochondrial respiration capacity in the presence of a high-glucose environment. Along these same lines, TRIB3 overexpression led to a 69% increase in ECAR (P < 0.05) compared with controls, without any significant effect observed following TRIB3 knockdown (Fig. 2C), suggesting that TRIB3-overexpressing L6 cells were more glycolytic than TRIB3 knockdown and wild-type L6 cells. To further define the above observations, we measured the maximal mitochondrial respiration capacity in the three cell lines by using the proton ionophore FCCP to dissipate the mitochondrial proton gradient and allow for fully uncoupled respiration. As we expected, maximal OCR of TRIB3 knockdown was decreased (18 ± 2%, P = 0.06) in TRIB3 knockdown and increased (31 ± 8%, P < 0.05) in TRIB3-overexpressing cells compared with controls (Fig. 3A).
Fig. 2.
Mitochondrial respiration and extracellular acidification rate (ECAR) in wild-type (WT), TRIB3 KD, and TRIB3 overexpression (OE) L6 cells. Basal cellular oxygen consumption rate (OCR) (A and B) and ECAR (C) are shown for all 3 cell lines under high-glucose (25 mM) culture conditions. OCR fold change in B was calculated as basal cellular OCR under the 25-mM glucose condition divided by basal cellular OCR under conditions of 5 mM glucose. *P < 0.05. Values were expressed as means ± SD; n = 9.
Fig. 3.
Real-time bioenergetic analyses in WT, TRIB3 KD, and OE L6 cells using various small-molecule metabolic modulators. Maximal OCR and proton leak as %basal mitochondrial OCR (% of basal OCR) were measured sequentially after injection of 0.9 μM FCCP (A) and 0.5 μM oligomycin (B) following baseline rate measurements. C: ECAR was measured after injection of 0.5 μM oligomycin. D: total cytochrome c oxidase IV (COX IV) protein levels were measured as a marker of mitochondrial mass. *P < 0.05. Results are expressed as means ± SD; n = 9.
To determine the fraction of mitochondria leak that contributes to total cellular oxygen consumption, we used the F1F0-ATPase inhibitor oligomycin to inhibit ATP synthesis and determine the proton leak associated with mitochondrial respiration. In the presence of 0.5 μM oligomycin, proton leak was then calculated as the mitochondrial rate nonsensitive to oligomycin minus the mitochondrial rate nonsensitive to antimycin/rotenone. As shown in Fig. 3B, proton leak as a percent of basal mitochondrial OCR was somewhat increased in TRIB3-overexpressing L6 cells at 18% (P < 0.05) compared with values of 11 and 9% in TRIB3 knockdown and wild-type L6 cells, respectively. When ATP synthase is inhibited by oligomycin, cells will increase ATP production from glycolysis and augment lactate production (48). As shown in Fig. 3C, the observed ECAR in oligomycin-treated TRIB3-overexpressing L6 cells increased (82 ± 23%, P < 0.05) compared with control cells, suggesting that TRIB3 enhanced flexibility in switching from mitochondrial respiration to glycolysis under these conditions. Finally, COX IV expression was measured as a marker for mitochondrial mass, as shown in Fig. 3D. COX IV levels are statistically similar among control cells and stably transfected cell lines exhibiting TRIB3 overexpression and knockdown, indicating that TRIB3 affects mitochondrial function and not mitogenesis.
Effects of short-term nutrient excess and fasting on TRIB3 regulation in skeletal muscle and adipose tissue.
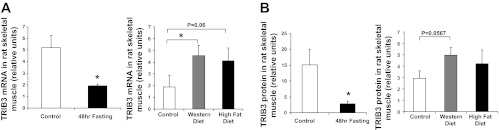
Previously, we reported (25) that TRIB3 expression was increased in skeletal muscle of patients with type 2 diabetes and in various rodent models of chronic hyperglycemia and insulin resistance, including db/db mice, Zucker fatty rats, and streptozotocin-treated rats. Furthermore, based on the ability of TRIB3 to impair insulin-stimulated glucose transport and its upregulation in a high-glucose environment, we proposed that TRIB3 played a pathophysiological role in the setting of chronic hyperglycemia by mediating glucose-induced insulin resistance. The current demonstration of TRIB3's ability to augment glucose oxidation and mitochondrial respiration while at the same time reducing rates of insulin-stimulated glucose transport led us to hypothesize that muscle TRIB3 could also be regulated under conditions of short-term nutrient excess and fasting in vivo. These latter actions would relate to a physiological role for TRIB3 to regulate metabolism of extracellularly vs. intracellularly derived glucose in muscle and fat as a function of nutrient availability. As shown in Table 1, neither short-term “Western diet” (7 days) nor fasting (48 h) affected body weight significantly compared with rats fed normal chow. However, these conditions of nutrient excess and deprivation had opposite effects on blood glucose, insulin, and triglyceride levels, whereas circulating free fatty acids were elevated by both perturbations. These data are indicative of relative insulin resistance and hyperglycemia in the Western diet-fed rats and enhanced insulin sensitivity and reduced glycemia in the fasted rats. As shown in Fig. 4, Western diet led to pronounced increments in TRIB3 mRNA (155.5%, P < 0.05) and protein levels (69%, P = 0.0567) in skeletal muscle, whereas fasting reduced TRIB3 expression when compared with controls.
Table 1.
Metabolic characteristics of fed and fasted rats
| Nutrient Excess |
48-h Fasting |
|||||
|---|---|---|---|---|---|---|
| Control | High-fat diet | Western diet | Control | High-fat diet | Western diet | |
| Body weight, g | 371.83 ± 11.37 | 373.33 ± 19.10 | 384.67 ± 13.11 | 379.67 ± 11.06 | 381.5 ± 16.1 | 383.0 ± 12.39 |
| Glucose, mg/dl | 234.5 ± 13.48 | 261.75 ± 12.28 | 272.33 ± 19.87† | 169.33 ± 18.13‡ | 134.0 ± 22.91‡ | 163.17 ± 16.04‡ |
| Serum insulin, ng/ml | 6.51 ± 1.51 | 7.0 ± 3.15 | 9.64 ± 1.95* | 0.93 ± 0.48‡ | 0.48 ± 0.19‡ | 0.43 ± 0.08‡ |
| Cholesterol, mg/dl | 89.0 ± 16.60 | 83.17 ± 9.00 | 102.17 ± 7.39* | 60.33 ± 10.44‡ | 38.83 ± 14.72‡ | 49.33 ± 6.41‡ |
| Triglyceride, mg/dl | 139.75 ± 42.24 | 119.75 ± 12.66 | 379.17 ± 116.73† | 50.67 ± 13.79‡ | 40.5 ± 19.87‡ | 44.67 ± 9.54‡ |
| Free fatty acid, mEq/l | 0.22 ± 0.01 | 0.46 ± 0.16* | 0.52 ± 0.11† | 0.46 ± 0.13‡ | 0.44 ± 0.08 | 0.45 ± 0.12 |
Results are expressed as means ± SD; n = 6. Metabolic impact of short-term fasting and nutrient excess in rats. Male Sprague-Dawley rats were subjected to 48-h fasting or 7-day high-fat diet or Western diet feeding. Body weight, fasting blood glucose level, insulin level, cholesterol, trigyceride, and serum free fatty acid level were measured.
P < 0.05;
P < 0.01 compared with control group under nutrient excess condition;
P < 0.05, each group under 48-h fasting compared with its corresponding nutrient excess group.
Fig. 4.
Fasting and short-term nutrient excess have opposite effects on TRIB3 expression in rat skeletal muscle. RNA and protein were isolated from rat skeletal muscle samples from control (normal chow), fasted, and Western diet-fed groups. Real-time RT-PCR analysis of TRIB3 mRNA level (A) and Western blot analysis of TRIB3 protein levels (B) were measured in all groups. *P < 0.05. Results are expressed as means ± SD; n = 6.
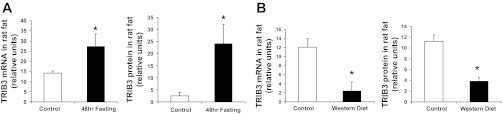
Interestingly, in adipose tissue, the effects of nutrient excess and deprivation on TRIB3 expression were opposite of those in skeletal muscle. We found that short-term nutrient excess in Western diet-fed animals decreased TRIB3 mRNA levels (76.6%, P < 0.05) and protein expression (70%, P < 0.05) in adipose tissue, whereas fasting led to an increase in TRIB3 mRNA (96.3%, P < 0.05) and protein (∼10-fold, P < 0.05) levels compared with controls (Fig. 5). In rat liver, we found increased TRIB3 mRNA levels after fasting (data not shown), which is consistent with previous literature (10); however, this did not translate into changes in levels of the encoded protein, which were not altered. These data suggest that, under conditions of nutrient excess and nutrient lack, TRIB3 is oppositely regulated in skeletal muscle, an energy-utilizing tissue, and adipose tissue, which is primarily responsible for energy storage.
Fig. 5.
Fasting and short-term nutrient excess have opposite effects on TRIB3 expression in rat adipose tissue. RNA was isolated from rat mesenteric fat samples from control (normal chow), fasted, and Western diet-fed groups. Real-time RT-PCR analysis of TRIB3 mRNA level and Western blot analysis of TRIB3 protein level were measured. A: effects of fasting. B: effects of Western diet feeding. *P < 0.05. Results were expressed as means ± SD; n = 6.
DISCUSSION
TRIB3 is a pseudokinase that has previously been shown to act as an inhibitor of several signal transduction cascades. Previously, we reported that overexpression of TRIB3 in L6-GLUT4 myotubes reduced insulin-stimulated glucose uptake significantly and that expression was increased in muscle from type 2 diabetic humans and in rodent models of insulin resistance and hyperglycemia (25). Because TRIB3 expression was increased by high glucose in cultured L6 myotubes, we further proposed that TRIB3 mediates glucose-induced insulin resistance under conditions of chronic hyperglycemia. In the present study, we found that knockdown of TRIB3 in L6-GLUT4 muscle cells results in an increase in both basal and insulin-stimulated glucose transport rates associated with an increase in IRS-1/Akt and ERK-1/2 signaling. When combined with the previous data (25), it is evident that manipulations of TRIB3 expression both above and below baseline levels exert opposite effects on insulin action, which adds credence to the idea that variations in TRIB3 levels have the potential to be an important regulator of the insulin-responsive glucose transport system.
In addition to the chronic pathophysiological role of TRIB3 in diabetes as a mediator of glucose-induced insulin resistance, we have now examined whether TRIB3 could subserve a more acute physiological role in regulating glucose transport in response to short-term changes in nutrient availability. To achieve a state of nutrient excess, we fed rats a “high-fat diet” or Western diet for 7 days, and according to our data, the short-term Western diet exhibited better “nutrient excess” effects than the short-term high-fat diet, with induced systemic insulin resistance as manifested by increased blood glucose, insulin, free fatty acid, and triglyceride levels. Under these physiological conditions, TRIB3 mRNA and protein levels were increased in skeletal muscle compared with normal chow-fed rats, whereas an opposite effect of decreased TRIB3 mRNA and levels were observed in adipose tissue. Although the effects on glucose transport were not measured directly in these tissues, based on the results in cultured cells (25), the consequence of TRIB3 upregulation would be to contribute to insulin resistance and reduced glucose transport in skeletal muscle. Furthermore, a decrease in TRIB3 expression in adipose could mediate increased glucose uptake, facilitating triglyceride storage in fat.
Effects of nutrient deprivation on TRIB3 stand in stark contrast. Short-term fasting (2 days) led to a state of enhanced insulin sensitivity with lower circulating glucose and insulin values, decreased TRIB3 mRNA and protein levels in muscle, and an increase in TRIB3 mRNA and protein in adipose tissue. Predictably, the decrease in TRIB3 would augment glucose uptake into muscle during nutrient lack, and increased TRIB3 would reduce the uptake in fat under conditions when adipose tissue is releasing fatty acids and is not primed for fuel storage. This scenario would facilitate repletion of muscle glycogen when the next meal becomes available. These data implicate TRIB3 in the short-term physiological regulation of insulin sensitivity, with opposite effects on glucose transport in muscle and fat in response to nutrient excess and deprivation.
Given these results, it is interesting to consider the data showing that TRIB3 influences intracellular glucose oxidation in addition to the effects on glucose uptake in L6 myocytes. Using a bioenergetic extracellular flux analyzer to assess mitochondrial oxidation in intact cells, we found that overexpression of TRIB3 produced an increase in the OCR, the ECAR, and the maximal mitochondrial oxidative capacity in fully uncoupled mitochondria when compared with control cells. In contrast, OCR and maximal mitochondrial oxidative capacity were decreased in L6 cells with stable suppression of TRIB3 expression, whereas ECAR was unaffected. Thus, manipulation of TRIB3 expression above and below baseline in L6 myotubes induces opposite changes in mitochondrial oxygen consumption rates and metabolism. Since COX IV levels were unaffected in TRIB3-overexpressing and knockdown cell lines, it does not appear that TRIB3 alters mitochondrial mass. By inference, TRIB3 regulates mitochondrial function. Although the direct evidence is not available, there might be some interactions between TRIB3 and peroxisome proliferator-activated receptor-γ coactivator-1 (PGC-1) as the mechanisms in modulating glucose oxidation in mitochondria. The nuclear hormone receptor coactivator PGC-1 can be induced by environmental stimuli such as fasting and low temperature, and increased PGC-1 has been identified as a potent pathway in regulating both mitochondria function and number by directly altering gene expression, affecting the respiratory chain and stimulating mitochondria biogenesis (49). Induction of TRIB3 has been reported as one of the mechanisms by which PGC-1 promotes insulin resistance, since PGC-1-deficient mice showed markedly reduced TRIB3 expression, and overexpression of TRIB3 in liver can reverse the insulin-sensitizing phenotype of PGC-1-deficient mice. Also, both TRIB3 and PGC-1 localize in nucleus, and it would be interesting to know whether PGC-1 is involved as part of the mechanisms through which TRIB3 modulates mitochondria oxidation rates (22).
Our data leave us with the paradox that TRIB3 upregulation impairs glucose entry into muscle cells while enhancing glucose oxidation and that TRIB3 suppression augments glucose uptake and reduces glucose oxidation. To explain these data in rodents and muscle cells, we propose the following hypothesis regarding the physiological role for TRIB3 under conditions of altered nutrient availability. With nutrient excess, TRIB3 upregulation would limit excessive uptake of glucose into muscle and facilitate oxidation of intracellular fuel stores (e.g., glycogen) protecting muscle from excessive storage of fuel, including glycogen and intramyocellular triglyceride. At the same time, nutrient excess is associated with decreased TRIB3 expression in adipose, leading to increased glucose uptake and triglyceride storage, thereby redirecting fuel from muscle to adipose tissue for storage. The opposite scenario accompanies nutrient deprivation. Fasting reduces muscle TRIB3 levels, resulting in increased glucose transport and reduced glucose oxidation in a setting where glycogen stores are depleted. When the next meal is eventually consumed and insulin levels rise, these changes would facilitate the restoration of muscle glycogen stores. In adipose tissue, fasting increases TRIB3 and impairs glucose uptake under conditions when fat is geared for lipolysis as opposed to lipogenesis. The finding that TRIB3 expression was increased in adipose tissue during fasting is consistent with a previous report (39) showing that TRIB3 inactivates fatty acid synthesis by promoting acetyl-CoA carboxylase degradation through an association with the E3 ubiquitin ligase constitutive photomorphogenic protein 1. Qi et. al. (39) also showed that transgenic mice with TRIB3 overexpression were protected from high-fat diet-induced weight gain, showed increased oxygen consumption (V̇o2), were more insulin sensitive compared with wild-type littermates through increased fatty acid oxidation, had increased energy expenditure, and had a “persistent increase in core body temperature,” suggesting increased mitochondrial uncoupling and capacity. Here, we show similar results at the cellular level with significantly increased OCR in the TRIB3-overexpressing cells, maximal uncoupled respiratory capacity, and proton leak, all supporting mechanistically that these TRIB3-overexpressing cells have a greater mitochondrial capacity (both coupled and uncoupled), translating into higher energy production/utilization. TRIB3 has also been shown to negatively regulate ATF4, an eukaryotic initiation factor-2α-activated transcription factor (18) that regulates CHOP, which controls many stress-induced genes, including carbonic anhydrase and other ER stress response proteins (43). Metabolic stresses, including energy overload, have been shown to be accompanied by excess production of reactive oxygen species (ROS) (3) and increased ER stress responses (24) and found to initiate Nrf2-mediated antioxidant responses (17). Our findings of decreased TRIB3 in muscle and increased levels in adipose tissue upon fasting with increased TRIB3 expression in muscle after 7 days on a Western diet in the rodents, along with the coordinated decrease in mRNA in the adipose, support the concept that TRIB3 may be activated as a nutrient sensor at the extremes of nutrient deprivation and excess. Of course, these ideas are speculative but are nevertheless consistent with the current data and warrant further study along these lines.
The pathophysiological role of TRIB3 in diabetes should be considered somewhat differently since this involves a persistent increase in TRIB3 expression in muscle in the face of chronic hyperglycemia (25). This would impair insulin-stimulated glucose transport on a chronic basis, and we have suggested that this represents a mechanism contributing to glucose-induced insulin resistance or “glucose toxicity” (25). Previous data have supported a role for hepatic TRIB3 in diabetes, showing that overexpression of TRIB3 in liver promoted hyperglycemia and glucose intolerance and reversed the insulin-sensitive phenotype of PGC-1-deficient mice (10, 22). The current data showing the effects of TRIB3 on glucose oxidation could contribute to pathophysiology in diabetes in an additional way. Although insulin-stimulated glucose oxidation is impaired in type 2 diabetes, the increase in TRIB3 would have the effect of facilitating mitochondrial oxidative capacity under basal conditions. This would exacerbate ROS generation since the highest rate of ROS production occurs when the proton gradient is high and ATP demand is low. Indeed, hyperglycemia has been shown to promote ROS production and increase oxidative stress in various tissues (3). ROS not only damages DNA, proteins, and lipid in membrane components (7, 31) but can also activate stress-sensitive and inflammatory pathways and their intracellular targets, such as NF-κB, c-Jun NH2-terminal kinase (JNK), stress-activated protein kinase (SAPK), and p38 MAPK (11, 15, 19), all of which result in mitochondrial dysfunction and insulin resistance.
Our results are contrary to a recent study finding that transgenic mice with cardiac-specific overexpression of TRIB3 exhibited a decrease in cardiac glucose oxidation rates (2). It is possible that these discrepancies may be explained by different functions of TRIB3 in skeletal muscle cells and cardiac cells and the fact that we measured glucose oxidation acutely and in real time in L6 cell lines, whereas in the mouse model the rate was measured ex vivo after prolonged TRIB3 overexpression (12 wk). Moreover, the effects of TRIB3 on mitochondrial oxidation in L6 cells were observed in the presence of 25 mM glucose, and the myocardial TRIB3 transgenic mice were normoglycemic.
In conclusion, these data implicate for the first time TRIB3 as a potent physiological regulator of insulin sensitivity and mitochondrial glucose oxidation under conditions of nutrient deprivation and excess. TRIB3 impacts glucose uptake and oxidation oppositely according to levels of nutrient availability. With nutrient excess, insulin resistance is associated with TRIB3 elevation in muscle, which has the effect of inhibiting glucose uptake and enhancing mitochondrial glucose oxidation in combination with a decrease in TRIB3 expression in adipose that would enhance the transport of glucose needed for triglyceride storage. In fasting, TRIB3 suppression in muscle tissue increases glucose transport and limits fuel oxidation in a manner that would facilitate glycogen repletion when the next meal is consumed. At the same time, fasting increases TRIB3 expression in adipose tissue, thus reducing glucose uptake and fatty acid synthesis (39) at a time when the fat cell is primed for lipolysis. These physiological roles for TRIB3 are in contradistinction to the pathophysiological role in diabetes where chronic hyperglycemia results in persistent upregulation of muscle TRIB3 (25), thus contributing to glucose-induced insulin resistance. Overall, although more studies are clearly required to elucidate mechanisms by which TRIB3 affects mitochondrial fuel oxidation and insulin signaling, the data point to a new mechanism for the coordinated function of adipose and muscle tissues in nutrient metabolism and identify TRIB3 as novel and interesting potential target for treatment of insulin resistance and metabolic diseases.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-038765, DK-083562) and by the Merit Review Program of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L., W.Z., H.S.H., L.T., Y.F., and W.G. did the conception and design of the research; J.L., W.Z., G.C.C., H.S.H., and D.R.M. performed the experiments; J.L., W.Z., H.S.H., and D.R.M. analyzed the data; J.L., W.Z., Y.F., D.R.M., and W.G. interpreted the results of the experiments; J.L. and W.Z. prepared the figures; J.L. and W.G. drafted the manuscript; J.L., W.Z., Y.F., D.R.M., and W.G. edited and revised the manuscript; W.Z., D.R.M., and W.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge support from core facilities of the University of Alabama at Birmingham Diabetes Research and Training Center (P60 DK-079626).
REFERENCES
- 1.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 116: 434–448, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Avery J, Etzion S, DeBosch B, Jin X, Lupu T, Beitinjaneh B, Grand J, Kovacs A, Sambandam N, Muslin A. TRB3 function in cardiac endoplasmic reticulum stress. Circ Res 106: 1516–1523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56: 1376–1381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. A radical explanation for glucose-induced beta cell dysfunction. J Clin Invest 112: 1788–1790, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta 1688: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Kato S, Du K. PI3K activates negative and positive signals to regulate TRB3 expression in hepatic cells. Exp Cell Res 314: 1566–1574, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Du K, Ding J. Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta. Mol Endocrinol 23: 475–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du K, Herzig S, Kulkarni R, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300: 1574–1577, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101: 523–531, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: novel regulators of cell function; evolutionary aspects. Cell Mol Life Sci 63: 1632–1641, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab 92: 1467–1473, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa F, Akimoto T, Yamamoto H, Araki Y, Yoshie T, Mori K, Hayashi H, Nose K, Shibanuma M. Gene expression profiling identifies a role for CHOP during inhibition of the mitochondrial respiratory chain. J Biochem 146: 123–132, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Jousse C, Deval C, Maurin AC, Parry L, Cherasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 282: 15851–15861, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kaneto H, Xu G, Song KH, Suzuma K, Bonner-Weir S, Sharma A, Weir GC. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem 276: 31099–31104, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Koh H, Arnolds D, Fujii N, Tran T, Rogers M, Jessen N, Li Y, Liew C, Ho R, Hirshman M, Kulkarni R, Kahn C, Goodyear L. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol 26: 8217–8227, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo S, Satoh H, Herzig S, Lee C, Hedrick S, Kulkarni R, Evans R, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 10: 530–534, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Liew C, Bochenski J, Kawamori D, Hu J, Leech C, Wanic K, Malecki M, Warram J, Qi L, Krolewski A, Kulkarni R. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest 120: 2876–2888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis 19: 146–152, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Wu X, Franklin JL, Messina JL, Hill HS, Moellering DR, Walton RG, Martin M, Garvey WT. Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: role in glucose-induced insulin resistance. Am J Physiol Endocrinol Metab 298: E565–E576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mata J, Curado S, Ephrussi A, Rørth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101: 511–522, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Matsushima R, Harada N, Webster N, Tsutsumi Y, Nakaya Y. Effect of TRB3 on insulin and nutrient-stimulated hepatic p70 S6 kinase activity. J Biol Chem 281: 29719–29729, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mayor P, Maianu L, Garvey WT. Glucose and insulin chronically regulate insulin action via different mechanisms in BC3H1 myocytes. Effects on glucose transporter gene expression. Diabetes 41: 274–285, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 31: 1302–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56: 1592–1599, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Murphy MP. Induction of mitochondrial ROS production by electrophilic lipids: a new pathway of redox signaling? Am J Physiol Heart Circ Physiol 290: H1754–H1755, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res 100: 795–806, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Ohoka N, Sakai S, Onozaki K, Nakanishi M, Hayashi H. Anaphase-promoting complex/cyclosome-cdh1 mediates the ubiquitination and degradation of TRB3. Biochem Biophys Res Commun 392: 289–294, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24: 1243–1255, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ord D, Meerits K, Ord T. TRB3 protects cells against the growth inhibitory and cytotoxic effect of ATF4. Exp Cell Res 313: 3556–3567, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Ord D, Ord T. Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem Biophys Res Commun 330: 210–218, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Ord T, Ord D, Kõivomägi M, Juhkam K. Human TRB3 is upregulated in stressed cells by the induction of translationally efficient mRNA containing a truncated 5′-UTR. Gene 444: 24–32, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi L, Heredia J, Altarejos J, Screaton R, Goebel N, Niessen S, Macleod I, Liew C, Kulkarni R, Bain J, Newgard C, Nelson M, Evans R, Yates J, Montminy M. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 312: 1763–1766, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Roden M, Shulman GI. Applications of NMR spectroscopy to study muscle glycogen metabolism in man. Annu Rev Med 50: 277–290, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Rørth P, Szabo K, Texido G. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol Cell 6: 23–30, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322: 223–228, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D. CHOP-Dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol 19: 495–504, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4: e154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomás E, Lin YS, Dagher Z, Saha A, Luo Z, Ido Y, Ruderman NB. Hyperglycemia and insulin resistance: possible mechanisms. Ann NY Acad Sci 967: 43–51, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 19: 4008–4018, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 147: 2643–2649, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Wu M, Neilson A, Swift A, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick D. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292: C125–C136, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124 1999 [DOI] [PubMed] [Google Scholar]
- 50.Yacoub Wasef SZ, Robinson KA, Berkaw MN, Buse MG. Glucose, dexamethasone, and the unfolded protein response regulate TRB3 mRNA expression in 3T3-L1 adipocytes and L6 myotubes. Am J Physiol Endocrinol Metab 291: E1274–E1280, 2006 [DOI] [PubMed] [Google Scholar]