Abstract
There is significant variability in diet-induced obesity (DIO) among humans and rodents, which has been associated with differences in intrinsic spontaneous physical activity (SPA). The orexin neuropeptides positively modulate SPA through multiple brain sites, but the effects of DIO on orexin's activity are not well understood. In this study, we tested the hypothesis that DIO sensitivity is mediated by decreased SPA and changes in the function of the orexins. As a DIO model, we used male Sprague-Dawley rats fed a high-fat (HF; 45% kcal from fat) or a low-fat (LF; 10% kcal from fat) diet for 10 wk. We measured SPA before and after HF or LF feeding and expression of orexin receptors by real-time PCR after dietary treatments. We tested DIO effects on orexin signaling by measuring SPA after injection of orexin A in the rostral lateral hypothalamus (RLH) before and after 10 wk of HF feeding. Finally, we tested whether daily orexin A RLH injections prevent DIO caused by HF feeding. Our results show that resistance to DIO is associated with an increase in SPA, SPA after injection of orexin A in RLH, and orexin receptor expression in sites that mediate orexin's effect on SPA, including RLH. We show that daily injections of orexin peptide in RLH prevent DIO without altering food intake. We estimate that the energetic cost of SPA after orexin A RLH injection accounts for approximately 61% of the extra caloric intake associated with HF intake, suggesting additional effects of orexins. In summary, our results suggest that variability in DIO sensitivity is mediated through adaptations in the activity of the orexin peptides and their receptors.
Keywords: hypothalamus, high-fat diet, neuropeptides, energy balance
there is significant variability among humans in sensitivity to diet-induced obesity (DIO) (4, 11). Similarly, rodents show significant differences in DIO sensitivity within and/or between species and strains (3, 6, 13, 20). This variability is associated with differences in intrinsic spontaneous physical activity (SPAINT) (28, 29, 41). In humans, SPAINT is defined as “physical activity that does not qualify as voluntary exercise” (14). In rodents, SPAINT is modeled as spontaneous activity in an open field after adaptation to the testing conditions (e.g., 24-h testing after 24-h adaptation) to avoid confounding effects of anxiety and exploratory behavior of a novel environment (24). This differentiates SPAINT from wheel running activity, which might represent volitional exercise (10, 45).
The neurobiological bases for control of SPAINT in rodents involve several neuropeptides, including the orexins (24). There are two orexin peptides [also known as hypocretins, orexin A/hypocretin-1 (OXA) and orexin B/hypocretin-2 (OXB)] and two receptors [orexin/hypocretin receptor 1 (OX1R) and orexin/hypocretin receptor 2 (OX2R)] (9, 43). In the brain, the orexin neurons are located in the lateral hypothalamic and perifornical areas and have efferents to multiple brain sites (39, 49) with different orexin receptor (OXR) expression levels (7, 19, 31, 55). OXR activation promotes negative energy balance, likely through increased and/or maintenance of SPAINT (12, 16, 26). Orexin modulation of SPAINT is distributed across several brain sites with site-specific participation of the OXR subtypes (22, 25, 26, 54).
In a polygenic model of obesity, the obesity-prone (OP) and obesity-resistant (OR) rats (27), the OR phenotype shows higher SPAINT and SPA induced by injection of OXA in rostral lateral hypothalamus (RLH) (53). Also, high-fat (HF) feeding decreases SPAINT and the effect of OXA hypothalamic paraventricular nucleus injections on energy expenditure in OP relative to OR rats (36). Finally, higher SPAINT in OR rats predicts lower fat mass gain throughout their lifetime (52). Together, these data suggest that lower SPAINT and changes in orexin function are related to higher DIO sensitivity. However, studies of DIO in nonselectively bred rodents are inconclusive about the effects of DIO in SPAINT (3, 6, 13). This has been attributed to rodent species, strain, duration, and composition of the obesogenic diet used (14).
Together, the previous evidence shows that the relationship between DIO variability and orexin activity remains largely unexplored. Therefore, we undertook studies to explore how individual variability in DIO sensitivity affects the activity of the orexin peptides and their receptors. We show that DIO resistance is reflected by decreased fat mass gain, increased lean mass gain and SPAINT, and altered OXR expression in several brain sites (RLH, caudal lateral hypothalamus, dorsal raphe, and paraventricular thalamic nucleus). Development of severe DIO decreased, whereas development of mild DIO increased OXA behavioral responsivity in RLH. We showed that treatments with daily OXA in RLH prevented DIO without altering food intake. Finally, we estimate that the energetic cost of SPA after OXA RLH injection accounts for ∼61% of the increased caloric intake associated with HF intake. Thus, our results show that DIO sensitivity is mediated through adaptations in the activity of the orexin peptides and their receptors. Our results also show that SPA caused by OXA RLH injections is an important, but not the only, contributor to OXA antiobesity effects.
METHODS
Animals
Male Sprague-Dawley (SD) rats (200–250 g at arrival; Charles River Laboratories, Kingston, NY) were housed individually in hanging-wire cages, with a 12:12-h light-dark cycle (lights on at 0630) at a constant temperature (21–22°C). Animals were given 1 wk of acclimation to housing conditions, with low-fat (LF) diet and water ad libitum. The experiments were approved by the Local Institutional Animal Care and Use Committee at the Minneapolis Veterans Affairs Medical Center.
Diets
HF (D12451; 45% kcal from fat) and LF (D12450B; 10% kcal from fat) diets from Research Diets (New Brunswick, NJ) were used. Food and water were available ad libitum, except where noted.
Peptides
Orexin A (American Peptides, Sunnyvale, CA) was dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA), aliquoted, and kept at −20°C until needed.
Body Weight, Fat and Lean Mass, and Food Intake Measurements
Body weight (BW; g) and food intake corrected by spillage (g) were measured every other day unless otherwise noted. Total fat mass (FM; g) and lean mass (LM; g) were measured by quantitative magnetic resonance (35) using the EchoMRI-900 scan (Echo Medical Systems, Houston, TX).
Cannulations and Injections
Rats were prepared with either unilateral (experiment 3) or bilateral (experiments 4 and 5) cannulae targeting RLH (stereotaxic coordinates: −2.0 mm lateral, −2.1 mm posterior to bregma, and 7.3 mm below the skull surface) (38). Animals recovered for 1 wk before we proceeded with experiments.
Injections were done between 8 and 11 AM in a volume of 0.5 μl injected over 30 s, after which the injector was left inside the cannula for 30 s to ensure diffusion of injectate.
Measurement of SPA
SPA was measured in a 17.0 by 17.0 in. squared acrylic cage surrounded by three 16-beam sets of infrared activity sensors (Med Associates, St. Albans, VT). Two sets of arrays were in the xy plane, and the third set was elevated in 3 in. above the xy plane. Movement was recorded by beam breaking with 100-ms resolution. SPA (min) corresponds to time spent moving lateral plus time spent rearing. SPAINT levels were measured over a 24-h period after 24-h cage habituation. To quantify the pattern of SPA, we binned SPA data using 1-min intervals. A bout of SPA was defined as consecutive 1-min bins, in which SPA was detected in each bin.
In experiments where the effects of OXA RLH injection in SPA were measured, animals were acclimated to the SPA recording chambers for ≥1 h before injection. Time course analysis showed that SPA for 5 min right after injection was related to the injection procedure and was excluded from analysis (Figs. 5A and 7A). For experiment 3, SPA was recorded for 1 h postinjection (Fig. 5A). For experiment 5, SPA was recorded for 2 h postinjection (Fig. 7A).
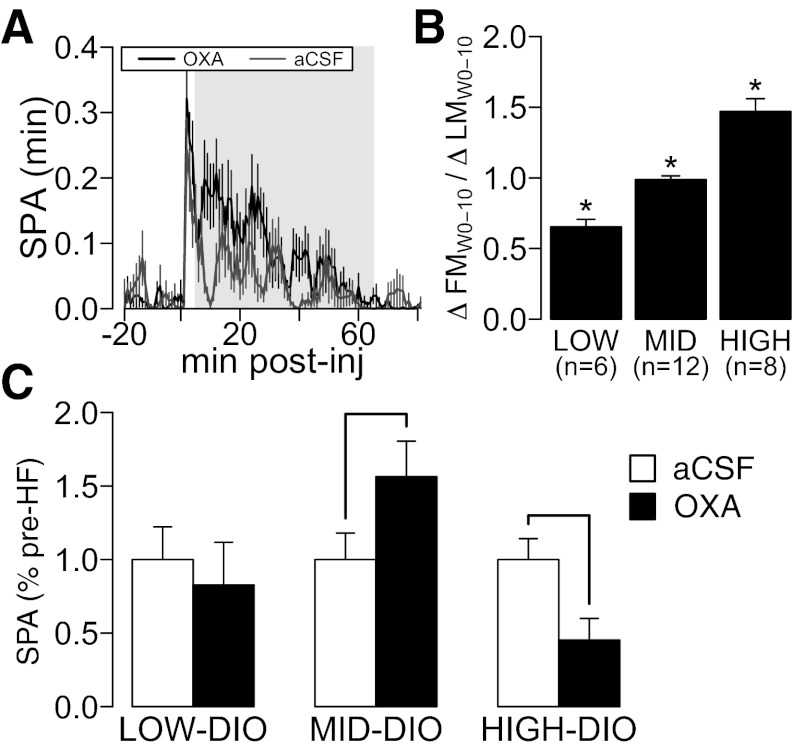
Fig. 5.
Effects of DIO in SPA after orexin A (OXA) injection in RLH. A: time course of SPA after OXA (125 pmol) injection in RLH prior to HF feeding (see methods). The shaded region represents the 1-h time frame used to measure SPA after OXA injection. Note the peak associated with the injection of the drug. B: after 10 wk of HF diet feeding, rats were separated according to DIO severity, measured as ΔFM/ΔLM (see results for details). *P ≤ 0.05 for comparison with all groups after pairwise analysis (see Statistical Analysis for details). C: severe DIO decreases whereas development of mild DIO increases OXA RLH behavioral responses. Line indicates that P ≤ 0.05 for a paired t-test for comparison of SPA after OXA RLH injection before and after HF feeding across different levels of DIO severity. The y-axis represents means ± SE for all plots. aCSF, artificial cerebrospinal fluid.
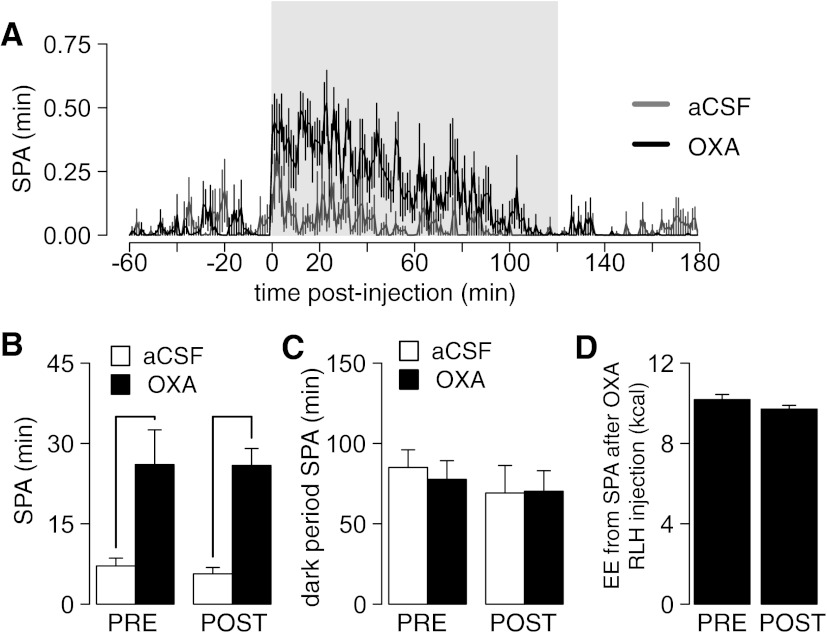
Fig. 7.
Daily repeated OXA injections do not habituate SPA response to OXA injection in RLH. A: time course of SPA after bilateral OXA (500 pmol/side) injections in RLH relative to the effect of an aCSF injection. The shaded region represents the 2-h time frame used to measure SPA. Note the peak associated with the injection of the drug. B: SPA (500 pmol/side) after pre- (PRE) and posttreatment (POST) with 10 daily bilateral injections in RLH with OXA (500 pmol/side). Line indicates that P ≤ 0.05 for pairwise comparison between SPA after OXA and aCSF injection PRE and POST with daily OXA injections. There was no significant effect of daily bilateral OXA in SPA after an acute OXA RLH injection (see results for details). C: SPAINT levels during the lights-off period were not affected by aCSF or OXA injection or by daily OXA injections (see Statistical Analysis for details). D: estimated energy expenditure (EE; kcal/h) associated with SPA in the 2-h window after OXA RLH injection (500 pmol bilateral OXA).
Experimental Design
Experiment 1: effect of HF feeding on DIO development and SPAINT levels.
SPAINT levels were determined in all animals. Next, animals were fed HF (n = 25) or LF (n = 23) diet ad libitum for 10 wk, with animals matched by BW, FM, and LM (Table 1). BW, FM, LM, and food intake were measured as described above in Body Weight, Fat and Lean Mass, and Food Intake Measurements. At the end of the feeding period, SPAINT levels were measured.
Table 1.
Body weight, fat mass, and lean mass prior to HF or LF diet feeding in experiment 1
| Body Weight | Fat Mass | Lean Mass | |
|---|---|---|---|
| Diet | |||
| LF | 346.13 ± 3.70 | 36.95 ± 1.28 | 260.98 ± 3.47 |
| HF | 344.00 ± 3.75 | 37.47 ± 1.64 | 258.15 ± 3.43 |
| Statistical analysis | |||
| t46 | −0.403 | 0.246 | −0.579 |
| P value | 0.689 | 0.807 | 0.566 |
Values are means ± SE. LF, low-fat diet; HF, high-fat diet. A t-test analysis showed no significant differences between animals assigned to HF or LF diet.
Experiment 2: expression of OXR after DIO development.
We randomly selected HF (n = 10) and LF (n = 10) rats from experiment 1 for analysis of OXR expression. One week after completion of experiment 1, animals were euthanized by decapitation between 10 AM and 12 PM. Food was removed 2 h before euthanasia. Samples from nucleus accumbens (NAC), periaquaductal gray, dorsal raphe (DR), substantia nigra (SN), intermediate lateral septal nuclei, arcuate, paraventricular nucleus of the thalamus (PVT), ventral medial hypothalamic nuclei, paraventricular hypothalamic nuclei (PVN), central amygdala, caudal lateral hypothalamic area (CLH), and RLH were collected by using the micropunch technique (23), frozen immediately in liquid nitrogen, and stored at −80°C.
Total RNA was extracted using TRIzol (15596-026; Invitrogen, Carlsbad, CA), cleaned using the RNAeasy micro kit (74034; Qiagen, Germantown, MD), quantified by UV absorption at 260 nm with the ND-1000 spectophotometer (ThermoScientific, Wilmington, DE), and stored at −80°C until use. Equal amounts of RNA were used to measure expression levels of OX1R, OX2R, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with the LightCycler RNA Master SYBR Green I kit (03064760001; Roche, Indianapolis, IN) in a LightCycler 2 thermocycler (Roche). Primer sequences were described previously (53). Efficiency of each PCR reaction was determined using the dilution method (40). Expression levels of OX1R and OX2R were normalized against GAPDH using an efficiency-corrected formula (40).
Experiment 3: effects of DIO development on orexin responsivity of RLH.
SD rats (n = 42) were prepared with unilateral cannulae targeting RLH. Next, SPA after OXA RLH injection was measured (125-pmol OXA dose), and FM and LM were measured before and after 10 wk of HF ad libitum feeding. BW and food intake were recorded throughout the experiment, as described in Body Weight, Fat and Lean Mass, and Food Intake Measurements.
Rats were acclimated to the injection procedure for 3 days by receiving a daily injection of aCSF in the SPA recording chambers. On the 4th day, SPA after OXA RLH injection was recorded. OXA injections in RLH reliably induce SPA (25, 26). Therefore, only rats in which the first OXA injection increased SPA relative to aCSF injection were fed a HF diet (n = 33). After completion of the study, cannula placement was verified by histological analysis (n = 26, Fig. 1A) (26).
Fig. 1.
Cannula placement. Placement of rostral lateral hypothalamus (bregma −1.40 to −2.56) cannulae for all animals used in experiments 3 (A), 4 (B), and 5 (C).
Experiment 4: effects of daily bilateral RLH OXA injections on DIO severity.
SD rats (n = 30) were prepared with bilateral cannulae targeting RLH. Next, rats (n = 10/group) were assigned to experimental groups matched by BW, FM, and LM (Table 2). One group was fed a LF diet and received daily aCSF bilateral injections (LF/aCSF), a second group was fed a HF diet and received daily aCSF bilateral injections (HF/aCSF), and the third group was fed a HF diet and received daily bilateral OXA injections (500 pmol/side; HF/OXA). Animals received injections for 10 consecutive days. BW and food intake were measured daily. FM and LM were measured before and after the injection period. We injected OXA during the light cycle because OXA RLH injections increase SPA during both the light and dark cycles (25). Cannula placement was verified by histological analysis (LF/aCSF, n = 5; HF/aCSF, n = 8; HF/OXA, n = 6; Fig. 1B) (26).
Table 2.
Body weight, fat mass, and lean mass for all groups prior to start of experiment 4
| Injections | Body Weight | Fat Mass | Lean Mass | |
|---|---|---|---|---|
| Diet | ||||
| LF | aCSF | 379.00 ± 7.70 | 46.67 ± 4.11 | 298.58 ± 6.79 |
| HF | aCSF | 395.63 ± 12.61 | 47.48 ± 4.20 | 310.08 ± 8.32 |
| HF | OXA | 393.83 ± 16.60 | 42.96 ± 3.72 | 316.59 ± 12.41 |
| Statistical analysis | ||||
| F2,16 | 0.408 | 0.344 | 0.76 | |
| P value | 0.672 | 0.714 | 0.484 |
Values are means ± SE. aCSF, artificial cerebrospinal fluid; OXA, orexin A. A 1-way ANOVA showed no significant differences between groups.
Experiment 5: effects of repeated OXA RLH injection on SPA after acute OXA RLH injection.
A new set of SD rats (n = 10) was prepared with bilateral cannulae targeting RLH. Next, SPA after bilateral injection of OXA in RLH (500 pmol/side) was measured. On day 1, half of the animals received either bilateral aCSF or OXA injection. SPA was measured for 24 h. On day 3, injection of OXA or aCSF was reversed, and SPA was measured for 24 h. Rats were returned to their home cages between measurements. Starting on day 5, rats received a daily bilateral OXA injection (500 pmol/side) for 10 days. On days 16 and 18, SPA after a bilateral injection of OXA in RLH (500 pmol/side) was measured. Next, cannula placement was verified by histological analysis (n = 8; Fig. 1C) (26).
Experiment 6: measurement of 24-h energy expenditure and SPA.
Energy expenditure (EE) and SPA were recorded simultaneously in airtight SPA cages of the same dimensions and configurations, as described in Measurement of SPA. Oxygen consumption and carbon dioxide production were measured by using a customized, high-precision, single-chamber indirect calorimeter (Columbus Instruments, Columbus, OH). SPA was recorded as described in Measurement of SPA. Thermogenesis was calculated from oxygen consumption and carbon dioxide production, and it is reported as average kilocalories per hour over 24 h. During IDC recordings, animals had food and water available ad libitum. Animals (n = 32) were acclimated for 7 days prior to the beginning of IDC recordings in the IDC SPA cages.
Statistical Analysis
All statistical analyses were done with R software version 2.15.0 (40a). Data are presented as means ± SE. Group effects were analyzed with a two-tailed t-test or one-way ANOVA or analysis of covariance (ANCOVA) when appropriate. The effects of DIO severity on change in SPAINT (experiment 1) were analyzed with a one-way ANCOVA, with pretreatment value of response variable as covariate and group as independent variable. The effects of DIO severity on change in response to OXA injections in RLH (experiment 3) were analyzed with a one-way ANCOVA, with pretreatment OXA response as covariate. For experiments 1 and 3, the effects of diet were analyzed using paired t-tests comparing pre- and postdietary treatment. The effects of repeated OXA injections in RLH in DIO severity or food intake (experiment 4) or effects of OXA RLH injection in SPA (experiment 5) were also analyzed by paired t-tests comparing response variables pre- and post-repeated OXA injections.
For experiment 2, we hypothesized that the pattern of OXR expression across multiple brain sites after 10 wk of either HF or LF diet would reflect DIO severity. Thus, we measured OX1R and OX2R expression in 13 brain sites in rats fed either a HF or LF diet, resulting in a multidimensional data set in which the expression of OX1R and OX2R in each brain site corresponds to a dimension.
To analyze this data set, we used a principal component analysis (PCA), a statistical technique that, starting with a multidimensional data set, generates two or more vectors that describe the major sources of variability in the data set (42). Thus, using a PCA allows us to generate two or more vectors that describe the variability between HF- and LF-fed rats while simultaneously using all of the OXR expression data from all of the brain sites analyzed.
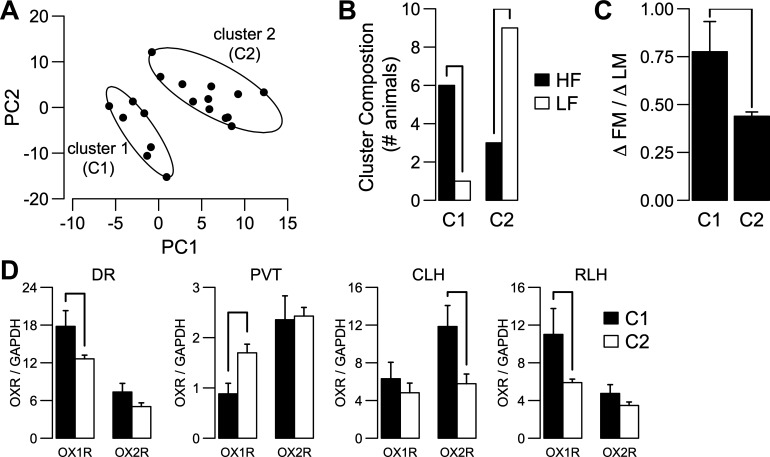
Because of technical problems with PCR reactions, 16.15% of all (animal by site by OXR subtype) combinations were not included in the analysis. Also, samples from one HF-fed animal were excluded due to problems during RNA extraction. To account for incomplete data, we used an implementation of the svdImpute algorithm (48, 56). After PCA, we plotted the data using the first two principal component vectors, which describe 65% of the total variability in the original data set. In this scatterplot (Fig. 4A), each point represents a single animal fed either a HF or a LF diet. Inspection of this plot (Fig. 4A) showed the presence of two possible clusters, which was confirmed by using the K-means clustering algorithm (8). A two-tailed t-test was used to test differences in DIO severity and OXR expression in individual brain sites across clusters.
Fig. 4.
Orexin receptor (OXR) expression pattern mirrors DIO severity. A: scatter plot of the principal component analysis scores for principal component vector 1 (PC1) and principal component vector 2 (PC2) for randomly selected rats from HF (n = 9) and LF (n = 10) groups from experiment 1. The ellipsoids for each cluster were calculated to be the minimal area to include all the points within each cluster, using the 2-dimensional mean of each cluster as center. B: cluster composition based on diet treatments was significantly different (χ2 = 4.32, df = 1, P = 0.037). C: difference in ΔFM/ΔLM between clusters is significant (t17 = 2.77, P = 0.013). D: brain sites with significantly different OXR expression between clusters. Cluster 1 had higher orexin/hypocretin receptor 1 (OX1R) expression than cluster 2 in the dorsal raphe (DR; C1, n = 6; C2, n = 10; t14 = −2.19, P = 0.045) and the rostral lateral hypothalamus (RLH; C1, n = 6; C2, n = 10; t14 = −2.33, P = 0.034). In paraventricular nucleus of the thalamus (PVT), there was a decrease in OX1R expression (C1, n = 6; C2, n = 11; t15 = 2.36, P = 0.032). For orexin/hypocretin receptor 2 (OX2R) expression, only a significant increase in cluster 1 vs. cluster 2 was detected in the caudal lateral hypothalamus (CLH; C1, n = 5; C2, n = 9; t12 = −2.85, P = 0.014). The y-axis represents means ± SE.
Estimation of the energetic cost of SPA was done using SPA and EE data collected over 24 h using a linear regression approach (3). We calculated the change in SPA (ΔSPA) and EE (ΔEE) between the dark and light periods and used those terms in a multiple linear regression model: ΔEE ∼ ΔSPA + BW (Eq. 1). This regression, based on the natural increase in SPA and EE between light and dark periods, estimates the contribution of SPA to the observed change in EE. Including body weight in this model controls for non-SPA effects in EE, such as basal resting metabolism.
For all analyses, pairwise comparisons were done by multiple t-test, with pooled variance corrected for multiple comparisons with the method of Hochberg (21). A P value of <0.05 was considered significant.
RESULTS
Variability in DIO Severity and Changes in SPAINT After HF Feeding
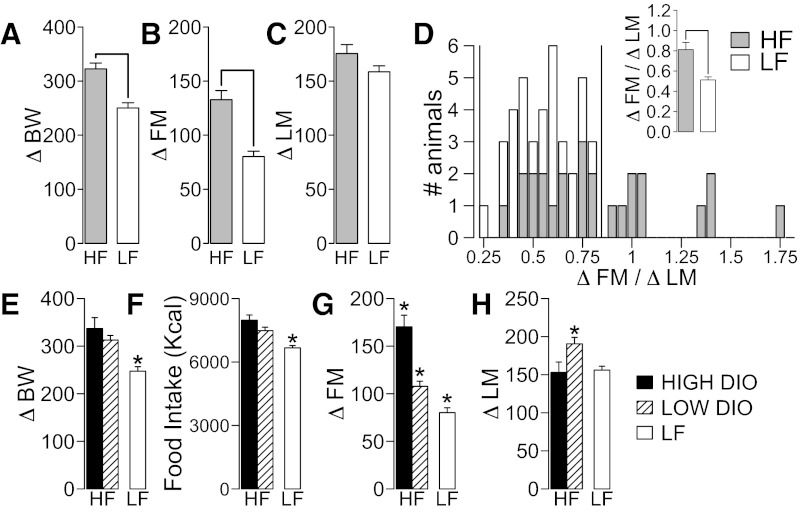
Ten weeks of HF diet (n = 25) feeding caused a significant increase in body weight gain compared with LF diet (ΔBW, t47 = 4.98, P < 0.001; Fig. 2A). HF feeding also caused higher FM gain (ΔFM, t47 = 5.32, P < 0.001; Fig. 2B) and a trend toward higher LM gain (ΔLM, t47 = 1.68, P = 0.099; Fig. 2C) compared with LF-fed animals (n = 24). Because obesity is defined by chronic accumulation of fat mass (2), severity of obesity was measured as accumulation of fat relative to lean mass (ΔFM/ΔLM). ΔFM/ΔLM was higher in HF compared with LF-fed rats (t47 = 3.88, P < 0.001; Fig. 2D, inset). Figure 2D shows individual DIO variability. We classified HF-fed rats as high-DIO (ΔFM/ΔLM > 0.82, n = 10) and low-DIO (ΔFM/ΔLM ≤ 0.82, n = 15), using as limit 1.92 standard deviations above the mean of the ΔFM/ΔLM for the LF rats (the upper limit of a range covering 95% of the data under a normal distribution).
Fig. 2.
Effects of diet-induced obesity (DIO) severity on fat mass (FM) and lean mass (LM) gain. Effect of high-fat (HF; n = 25) and low-fat (LF; n = 23) diet feeding for 10 wk on body weight gain (ΔBW; A), FM gain (ΔFM; B), and LM gain (ΔLM; C). D: histogram of ΔFM/ΔLM for HF and LF rats. Vertical line (1.92 standard deviations plus mean of LF rats) was used to separate HF diet rats in high-DIO and low-DIO. D, inset: effect of diet in ΔFM/ΔLM. For D–G, there were significant differences among groups (high-DIO, n = 10; low-DIO, n = 15; LF, n = 23) measured by a 1-way ANOVA (P ≤ 0.05, see results for details). There was no difference between high- and low-DIO rats in ΔBW, but there was an effect of HF feeding on ΔBW (E) and food intake (in calories; F) after 10 wk of HF or LF feeding. G: ΔFM was significantly different between groups. High-DIO rats showed larger ΔFM than low-DIO rats and LF rats. H: low-DIO rats showed significantly higher LM gain (ΔLM) after 10 wk of HF feeding compared with high-DIO and LF groups. The y-axis indicates means ± SE in all plots, except for C. *P ≤ 0.05 compared with other groups (see Statistical Analysis for details).
Before dietary treatments, there were no differences between the high-DIO, low-DIO, and LF group in BW (F2,46 = 0.84, P = 0.44), FM (F2,46 = 2.01, P = 0.14), and LM (F2,46 = 0.28, P = 0.75). After dietary treatments, there were significant differences between groups in ΔBW (F2,46 = 13.18, P < 0.001; Fig. 2E), food intake (F2,46 = 18.52, P < 0.001; Fig. 2F), ΔFM (F2,46 = 40.23, P < 0.001; Fig. 2G), ΔLM (F2,46 = 5.58, P = 0.006; Fig. 2H), and BW (F2,46 = 8.15, P < 0.001). There were no differences between high-DIO and low-DIO rats in ΔBW, food intake, or BW after HF feeding, but both groups had significantly higher values compared with LF rats. ΔFM was higher in high-DIO compared with low-DIO and LF groups and in low-DIO rats compared with LF rats (Fig. 2G). ΔLM was significantly higher in low-DIO rats compared with both high-DIO and LF rats (Fig. 2H).
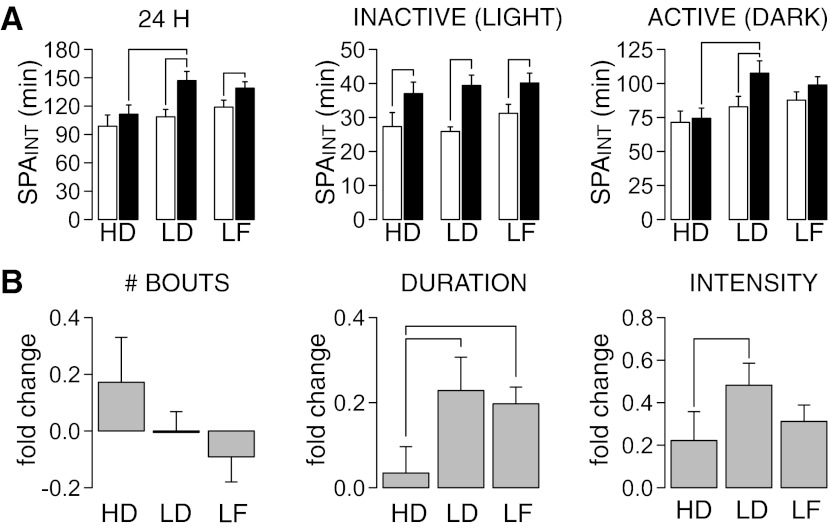
Figure 3A shows the effects of DIO severity on SPAINT over 24-h, inactive (lights on), and active (lights off) periods. These data were analyzed with an ANCOVA using SPAINT measured before dietary treatments as the covariate and group (high-DIO, low-DIO or LF) as the dependent variable. Before dietary treatments, there were no significant differences between groups in SPAINT during the 24-h (F2,46 = 1.28, P = 0.29), active (F2,46 = 1.12, P = 0.34), or inactive periods (F2,46 = 1.17, P = 0.32). After dietary treatments, there were significant differences between groups in SPAINT over the 24-h (group: F2,45 = 3.67, P = 0.035; covariate: F2,45 = 2.58, P = 0.12) and active periods (group: F2,45 = 3.72, P = 0.032; covariate: F2,45 = 0.83, P = 0.36), but not during the inactive period (group: F2,45 = 0.23, P = 0.79; covariate: F2,45 = 3.72, P = 0.06). Pairwise analyses showed that low-DIO rats had significantly higher SPAINT than high-DIO rats measured at the 24-h and active periods (Fig. 3A). LF rats increased their SPAINT measured only at 24 h, whereas low-DIO rats increased their SPAINT during both the 24-h and active periods (Fig. 3A). These data suggest specific adaptations of SPAINT during the active period of the 24-h cycle in low-DIO rats after HF feeding.
Fig. 3.
Effects of DIO severity on intrinsic spontaneous physical activity (SPAINT). A: comparison of SPAINT levels (measured at 24 h; inactive and active periods) at 0 (open bars) and 10 wk (black bars) of HF or LF diet feeding. Low-DIO (LD) rats had significantly higher SPAINT than high-DIO (HD) rats measured at 24 h and during the active period. LF rats increased their SPAINT measured only at 24 h, and LD rats increased their SPA only during the inactive period. B: fold change in number, duration, and intensity (time spent moving over duration of bout) of long (>10 min) SPAINT bouts during the dark period after dietary treatments. In A, the line for comparisons within group indicates that P ≤ 0.05 for a paired t-test. In A and B, lines for comparison between groups indicate that P ≤ 0.05 after pairwise comparisons (see methods for details).
Next, we analyzed whether changes in SPAINT in low-DIO rats were caused by changes in duration, intensity, and/or number of SPA bouts (see methods for definition). Initial analysis of the cumulative frequency distributions suggested an increase in duration of longer bouts (>10 min) in low-DIO rats compared with high-DIO and LF rats (data not shown). Therefore, we classified SPAINT bouts as short (<10 min) and long (>10 min). The results of this analysis are shown in Fig. 3B. These data were analyzed with a one-way ANCOVA, using group as the dependent variable and pretreatment value as the covariate. For short bouts there were no significant differences in duration (group: F2,45 = 0.65, P = 0.53; covariate: F1,45 = 0.45, P = 0.51) or intensity (time spent moving during each bout; group: F2,45 = 0.48, P = 0.62; covariate: F1,45 = 2.09, P = 0.16), but there was a significant difference in number of bouts (group: F2,45 = 3.21, P = 0.049; covariate: F1,45 = 0.48, P = 0.48). However, pairwise analyses failed to find significant differences between groups (data not shown). For long bouts (Fig. 3B) there were significant differences in duration of bouts (group: F2,45 = 3.39, P = 0.042; covariate: F1,45 = 3.73, P = 0.059) and intensity (group: F2,45 = 4.65, P = 0.0015; covariate: F1,45 = 1.37, P = 0.25) but not of number of bouts (group: F2,45 = 1.16, P = 0.32; covariate: F1,45 = 0.37, P = 0.55). Pairwise analysis indicated an increase in the duration of long bouts in LF and low-DIO rats compared with high-DIO rats and an increase in intensity of bouts in low-DIO rats compared with high-DIO rats (Fig. 3B). These results suggest that increased duration and higher intensity of SPA bouts, but not changes in the total number of bouts, contribute to increased SPAINT in low-DIO compared with high-DIO rats.
OXR Expression Mirrors DIO Severity
Orexin modulation of SPAINT involves multiple brain sites. We hypothesized that the pattern of OXR expression across multiple brain sites reflects DIO severity. OXR expression was measured in a random sample of HF (n = 9) and LF (n = 10) rats in 13 different brain sites. PCA of the OXR expression data showed that the first two principal component (PC) vectors explained 65% of the variability. A scatter plot using the first two PC vectors showed two clusters of rats (Fig. 4A). Thus, on the basis of OXR expression alone, the animals could be separated into two groups, which showed a significant difference in ΔFM/ΔLM (t17 = 2.77, P = 0.013; Fig. 4C). The distribution of HF and LF fed rats between clusters was not random (χ2 = 4.32, df = 1, P = 0.037; Fig. 4B). Animals from cluster 1 showed higher OX1R expression in DR and RLH, higher OX2R expression in CLH, and lower OX1R expression in PVT compared with animals from cluster 2 (Fig. 4D). Together, these results suggest that DIO-induced changes in fat and lean mass correlate with OXR expression levels across multiple brain sites, including DR, PVT, CLH, and RLH.
Development of Severe DIO Decreases Whereas Mild DIO Increases Orexin Responsivity in RLH
Data from OP and OR rats suggest that RLH orexin signaling is involved in DIO sensitivity (36, 53), and the present data show differential RLH OX1R expression between animals with different ΔFM/ΔLM (Fig. 4D). This led us to hypothesize that DIO alters orexin RLH signaling.
SPA after OXA RLH injection was measured before and after 10 wk of HF ad libitum feeding. We use a submaximal dose of OXA (125 pmol) (26) to avoid a ceiling response effect. Injection of this dose of OXA pre-HF feeding increased SPA compared with aCSF (aCSF: 1.47 ± 0.61 min; OXA: 5.00 ± 0.46 min; paired t-test: t24 = −5.08, P < 0.001; Fig. 5A).
After HF feeding, rats were classified by DIO severity (Fig. 5B) as low-DIO (ΔFM/ΔLM ≤ 0.82; n = 6), mid-DIO (0.82 < ΔFM/ΔLM ≤ 1.14; n = 12), or high-DIO (ΔFM/ΔLM > 1.14; n = 8). Low-DIO rats were classified using the same criteria as used before (Fig. 2D), which correspond to the 35% quantile of the ΔFM/ΔLM data from this experiment. Mid- and high-DIO rats were separated using the 70% quantile of the ΔFM/ΔLM data from this experiment. Figure 5C shows the effects of DIO severity on SPA after OXA RLH injection. There were no significant differences among groups in SPA after OXA RLH injection pre-HF feeding (F2,23 = 1.96, P = 0.163). A one-way ANCOVA analysis using pre-HF SPA after OXA RLH injection as the covariate and group (low-DIO, mid-DIO, high-DIO) as the dependent variable showed significant differences in responses to OXA after HF feeding (group: F2,22 = 4.203, P = 0.028; covariate: F1,22 = 2.68, P = 0.16). Paired t-tests show no change in orexin responses in low-DIO (t5 = 0.16, P = 0.87), an increase in mid-DIO (t11 = 2.35, P = 0.038), and a decrease in high-DIO rats (t7 = −2.52, P = 0.049). These results show that development of severe DIO decreases whereas mild DIO increases orexin responsivity in RLH. Furthermore, the effects of DIO severity on SPA induced by OXA RLH injections do not follow a linear trend.
Daily Bilateral RLH OXA Injections Prevent Early DIO
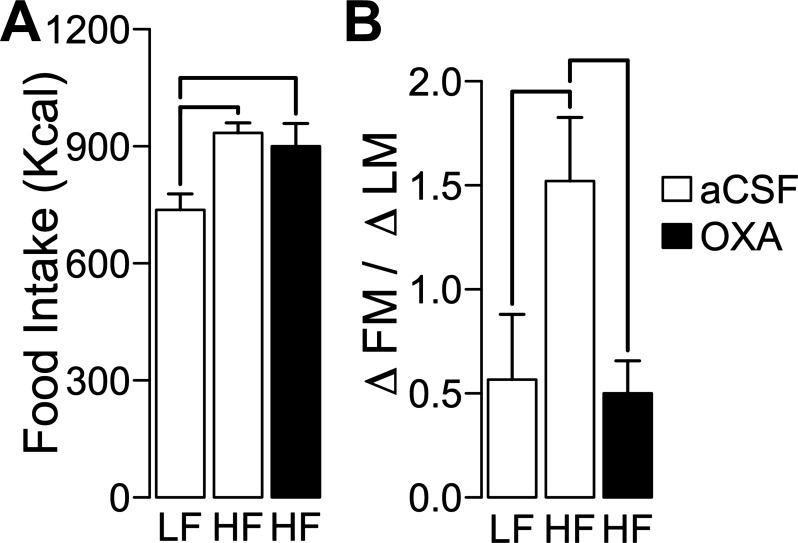
Because OXA RLH injections increase SPA (25, 26) and our results suggest an effect of DIO in OXA RLH signaling, we tested whether daily OXA RLH injections prevent DIO. SD rats were injected daily with OXA (500 pmol/side) or aCSF the in RLH while they were fed a HF or LF diet for 10 days (LF/aCSF, n = 5; HF/aCSF, n = 8; HF/OXA, n = 6). Cumulative food intake was significantly different between groups (F2,16 = 5.88, P = 0.012), but pairwise comparisons showed an effect of diet and not of OXA injections (Fig. 6A). ΔFM/ΔLM was significantly different between groups after treatment (F2,16 = 4.59, P = 0.026), with the HF/aCSF significantly higher than LF/aCSF and HF/OXA and without significant differences between the last two groups (Fig. 6B). This result shows that OXA RLH injections prevent early DIO.
Fig. 6.
Daily OXA injections in RLH prevent DIO. Ten days of daily bilateral injections in RLH of rats with either OXA (500 pmol/side) or aCSF while fed a HF or LF diet for 10 days (LF/aCSF, n = 5; HF/aCSF, n = 8; HF/OXA, n = 6) showed significant effects in cumulative food intake (as calories; A) and ΔFM/ΔLM (B). There was a significant effect of OXA injections in ΔFM/ΔLM without an effect in caloric intake. Lines indicate that P ≤ 0.05 for pairwise comparison between groups (see Statistical Analysis for details). The y-axis represents means ± SE for all plots.
It is possible the increase in SPA after OXA RLH injection contributes to effects of OXA against DIO (Fig. 6B). If this is true, then we reason that SPA after OXA RLH should not habituate throughout repeated OXA injections. In a new set of SD rats, we measured SPA after a single OXA RLH injection before and after 10 daily bilateral OXA RLH injections (n = 8). Figure 7A shows the time course of the response to OXA RLH injections. Pre- and posttreatment, acute bilateral OXA injections in RLH (500 pmol/side) increased SPA compared with aCSF (pretreatment: paired t-test, t7 = 5.70, P < 0.001; posttreatment: paired t-test, t7 = 3.17, P = 0.015; Fig. 7B). There were no differences in fold increase in SPA after OXA relative to aCSF injection pre- and posttreatment (SPAOXA/SPAaCSF, paired t-test; t7 = 0.069, P = 0.94). OXA RLH injections did not affect SPAINT during the lights-off period (Fig. 7C) either pre- (paired t-test; t7 = 0.48, P = 0.64) or posttreatment (paired t-test; t7 = 0.069, P = 0.94). These results show that behavioral responses to OXA in RLH do not habituate over repeated injections.
To quantify the energetic cost of the increase in SPA caused by OXA RLH injections, we used a multiple linear regression model based on 24-h indirect calorimetry recordings in male SD rats (n = 32), with the increase in EE between the active and inactive period as response variable and the change in SPAINT between active/inactive period and body weight as predictors (Eq. 1). Terms of the model are shown in Table 3. Estimates of EE associated with the observed increase in SPA for 2 h after OXA RLH injection are shown in Fig. 7D. The average EE associated with SPA after OXA RLH injection using both pre- and posttreatment responses is 9.95 ± 0.43 kcal. Because there is no habituation of OXA behavioral responses due to multiple orexin injections (Fig. 7, B and C), we estimate that 10 daily OXA RLH injections have an energetic cost of 99.95 ± 4.36 kcal. After 10 days, animals fed a HF diet consumed 163.15 kcal more than animals fed a LF diet (Fig. 6A). Therefore, these data suggest that daily OXA RLH injections account for 60.98% of the extra caloric intake caused by HF consumption over 10 days. Importantly, these data also suggest that OXA RLH injections must have other effects independent of SPA that contribute to a large component of their increased EE, which results in protection against DIO.
Table 3.
Multiple linear regression models for estimating the contribution of SPA to increases in total energy expenditure between active and inactive periods of the 24-h cycle
| Parameter | Estimate | t Value | P value |
|---|---|---|---|
| SPA | 0.132 ± 0.027 | 4.871 | 0.00004 |
| Body weight | 0.018 ± 0.009 | 2.095 | 0.04537 |
| Intercept | −0.699 ± 3.459 | −0.202 | 0.84139 |
Values are means ± SE. SPA, spontaneous physical activity. This regression was based on simultaneous SPA and energy expenditure data collected for 32 male Sprague-Dawley rats with the use of indirect calorimetry.
DISCUSSION
Our results show that DIO influences SPAINT and orexin RLH signaling. The key novel findings are that 1) expression of OXR in brain mirrors DIO severity (Fig. 4), 2) DIO severity selectively affects orexin response through RLH (Fig. 5), 3) daily intra-RLH orexin A injections prevent DIO development through changes in energy expenditure (Fig. 6), 4) there is no habituation of OXA RLH effects on SPA (Fig. 7), and 5) the increase in SPA after OXA RLH injection offsets ∼61% of the extra caloric intake caused by HF feeding (Fig. 7).
Current literature is inconsistent regarding the contribution of SPAINT to DIO susceptibility in rodents (3, 6, 13, 20, 27). Our results show that, in male SD rats, DIO resistance involves lower ΔFM (Fig. 2G) and higher ΔLM (Fig. 2H). In relation to SPA, low-DIO and LF rats increased their SPAINT for 24 h, and we found no change in high-DIO rats. Although all rats increase their SPAINT during the inactive period (Fig. 3B), only low-DIO rats increase their SPAINT significantly during their active period (Fig. 3B). Thus, increased SPAINT during the dark period is a specific adaptation of low-DIO rats and suggest that this mechanism is important for DIO resistance, since higher SPAINT and activity heat correlate with higher energy expenditure (24, 26, 36). Whether the absence of change in SPAINT in high-DIO is a cause or consequence of DIO is still an unresolved issue, and it is technically challenging to test. It is important to notice that high- and low-DIO rats had similar body weights after HF feeding, suggesting that body weight is not a confound for the observed effects on SPAINT. An interesting hypothesis is that SPAINT, orexin activity, and the OXR expression pattern determine DIO sensitivity. Higher SPAINT in OR rats compared with OP rats (53) and lower fat mass throughout the lifetime in OR compared with SD rats support this hypothesis (52). This hypothesis can be tested only with prior knowledge of individual DIO sensitivity. Although our results suggest that SPAINT does not predict DIO (Fig. 3), we interpret these data cautiously; SPAINT levels in OP and OR are ∼98 and 150 min/24 h, respectively (53). In contrast, pre-DIO SPAINT values of high- and low-DIO rats were 98.77 ± 11.9 and 108.72 ± 7.9, respectively; the range of SPAINT in our sample may not have been sufficient to illustrate the effects of SPAINT as a predictor of DIO sensitivity. Thus, future studies on the role of SPAINT and OXA RLH responsivity in DIO sensitivity are needed to explicitly address the difference in SPAINT as causal for differences in DIO susceptibility.
Higher orexin activity is associated with DIO resistance (12, 16), which likely depends on orexin modulation of SPAINT through a brain network (22, 25, 26, 54). Our present data show that OXR expression across several brain sites after HF and LF feeding separates all animals into two clusters with different ΔFM/ΔLM (Fig. 4C), indicating that OXR expression levels mirror DIO severity (Fig. 4). Rats with higher ΔFM/ΔLM showed increased OXR expression levels in RLH, DR, and CLH and a decrease in PVT (Fig. 4D). Injection of OXA in RLH increases SPA (25, 26), orexin peptides activate serotoninergic neurons in DR (5), OXA excites orexigenic neurons in CLH through the OX2R (59), and OXA in PVT decreases SPA (30). One possible interpretation of these data is that they represent a compensatory mechanism to offset DIO development by increasing orexin's behavioral effects.
To explore whether adaptations in RLH orexin signaling occur during DIO, we measured the effects of OXA RLH injection in SPA before and after HF feeding. Our results show that HF feeding effects depend on severity of obesity caused by HF consumption. We found no difference in the responses to orexin between groups before HF feeding but severe DIO decreases, whereas mild DIO was found to increase RLH orexin responsivity (Fig. 4C). This could represent a compensatory effect to offset the caloric surplus and/or the decreased orexin signaling during DIO development; i.e., rats developing mild obesity attempt to increase energy expenditure by increasing SPA, whereas effects of HF consumption in high-DIO rats override this mechanism. Although the pattern of OXR expression after DIO supports this idea (Fig. 4), additional studies are needed to test this hypothesis. The absence of changes in orexin's responsivity in low-DIO rats is at odds with evidence showing higher orexin signaling in DIO resistance (14, 24). However, orexin regulation of SPA is a distributed process in which different brain sites might show nonlinear interactions. Therefore, we suggest that there is no theoretical necessity for adaptations of orexin signaling to follow a linear trend within a single brain site. This highlights the need to investigate the effects DIO on orexin signaling adaptations in other brain sites that regulate SPA, including PVN, NAC, and SN (22, 26, 37).
If the observed changes in orexin RLH responsivity represent a compensatory mechanism against DIO, it follows that OXA RLH injections should prevent DIO. Daily bilateral OXA RLH injections prevented early DIO (Fig. 6B) without altering total caloric intake compared with aCSF (Fig. 6A), showing that orexin RLH signaling promotes DIO resistance through changes in energy expenditure. In support of this idea, a single unilateral OXA RLH injection increases energy expenditure significantly (51). It remains critical to test whether inhibiting orexin RLH signaling increases DIO sensitivity. The lack of effect of repeated OXA injections on cumulative caloric intake agrees with previous studies showing that whereas orexin increases food intake within 1–2 h following injection, overall 24-h food intake following injection is unaffected (18, 43).
The lack of habituation of SPA after OXA RLH injections (Fig. 7, B and C) suggests that SPA caused by OXA RLH injections might contribute to prevention of DIO. Using a multiple linear regression model derived from the changes in energy expenditure and SPAINT between active/inactive periods over 24 h (Table 1), we estimate that the energetic cost of SPA after a single OXA RLH injection is 9.95 ± 0.43 kcal. Linear regression has been used to estimate the contribution of SPAINT to energy expenditure (3). The multiple linear regression model is derived from SPAINT and not from SPA after orexin A injections; thus it allows a better estimation of the energetic contribution of SPA caused by OXA RLH injections.
The lack of habituation of SPA after OXA RLH injections (Fig. 7A) allows us to estimate that, over 10 days of injections, SPA caused by OXA RLH injections has an energetic cost of 99.95 ± 4.36 kcal. This accounts for 60.98% of the extra caloric intake associated with HF consumption over the same time period (Fig. 6A). These experiments do not conclusively quantify the contribution of SPA to exogenous OXA RLH antiobesity effects. Doing so would require preventing OXA effects in SPA without altering other potential effectors of OXA RLH signaling in overall energy expenditure. These data suggest that SPA that is due to OXA in RLH plays an important role in the antiobesity effects of OXA in RLH.
Based on our data, OXA RLH injections should engage mechanisms other than SPA to increase energy expenditure. Other potential effectors include sympathetic outflow (1, 15, 32–34, 46, 47) and thermogenic brown fat activity and abundance (44, 57) and changes in basal metabolic rate. However, repeated injections of orexin A in RLH do not appear to alter gene expression of uncoupling protein 1 or 3 in interscapular brown adipose tissue (Wang CF, personal communication). Although these data suggest that orexin RLH signaling does not alter sympathetic activity, analyses of energy expenditure after OXA RLH injection coupled with manipulations of sympathetic activity are necessary to address this question.
This study does not address whether OX1R or OX2R mediates the observed effects. Current evidence suggests that both OXR subtypes participate in orexin signaling through RLH (12, 53). Future experiments with OXR antagonists (17, 58) are necessary to clarify the specific roles of each OXR subtype in RLH orexin signaling.
Collectively, our results show that DIO alters orexin signaling responsiveness and that variation in this response is related to adaptations of orexin signaling to physiological changes induced by DIO. Furthermore, OXA treatments prevent early development of DIO. In this framework, it is tempting to speculate that decreased orexin system function and decreased SPA levels result in reduced levels of energy expenditure, thus fostering the development of severe DIO.
GRANTS
Funding for this work was provided by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-078985.
DISCLOSURES
The authors declare no conflict of interests, financial or otherwise.
AUTHOR CONTRIBUTIONS
C.E.P.-L., C.B., and C.M.K. did the conception and design of the research; C.E.P.-L., K.B., and J.A.T. performed the experiments; C.E.P.-L. analyzed the data; C.E.P.-L., C.B., and C.M.K. interpreted the results of the experiments; C.E.P.-L. prepared the figures; C.E.P.-L. drafted the manuscript; C.E.P.-L., K.B., C.B., and C.M.K. edited and revised the manuscript; C.E.P.-L., K.B., J.A.T., C.B., and C.M.K. approved the final version of the manuscript.
REFERENCES
- 1. Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281: R1801– R1807, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol 59: 55– 92, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Törnell J, Oscarsson J, Bohlooly-Y M. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab 294: E251– E260, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med 322: 1477– 1482, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40: 457– 459, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav 60: 37– 41, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept 104: 131– 144, 2002 [DOI] [PubMed] [Google Scholar]
- 8. D'Haeseleer P. How does gene expression clustering work? Nat Biotechnol 23: 1499– 1501, 2005 [DOI] [PubMed] [Google Scholar]
- 9. de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322– 327, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eikelboom R. Human parallel to voluntary wheel running: exercise. Anim Behav 57: F11– F12, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr 56: 1– 9, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab 9: 64– 76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funkat A, Massa CM, Jovanovska V, Proietto J, Andrikopoulos S. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J Nutr 134: 3264– 3269, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214: 206– 229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience 122: 541– 550, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30: 345– 354, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96: 45– 51, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JR. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20: 1099– 1105, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103: 777– 797, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hesse D, Dunn M, Heldmaier G, Klingenspor M, Rozman J. Behavioural mechanisms affecting energy regulation in mice prone or resistant to diet- induced obesity. Physiol Behav 99: 370– 380, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75: 800– 802, 1988 [Google Scholar]
- 22. Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab 286: E551– E559, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kotz CM, Levine AS, Billington CJ. Effect of naltrexone on feeding, neuropeptide Y and uncoupling protein gene expression during lactation. Neuroendocrinology 65: 259– 264, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 294: R699– R710, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept 104: 27– 32, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience 142: 29– 36, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725– R730, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283: 212– 214, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584– 586, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Li S, Sui N, Kirouac GJ. Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol Biochem Behav 93: 506– 514, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6– 25, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension 37: 1382– 1387, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Monda M, Viggiano A, De Luca V. Paradoxical [correction of parodoxical] effect of orexin A: hypophagia induced by hyperthermia. Brain Res 961: 220– 228, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Monda M, Viggiano A, Fuccio F, De Luca V. Injection of orexin A into the diagonal band of Broca induces sympathetic and hyperthermic reactions. Brain Res 1018: 265– 271, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 18: 1652– 1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab 290: E396– E403, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Novak CM, Levine JA. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity (Silver Spring) 17: 1493– 1498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam; Boston, MA: Elsevier Academic, 2005 [Google Scholar]
- 39. Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996– 10015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a. R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- 41.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 78: 1568– 1578, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ringner M. What is principal component analysis? Nat Biotechnol 26: 303– 304, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 1 page following 696, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab 14: 478– 490, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav 56: 11– 27, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol Regul Integr Comp Physiol 277: R1780– R1785, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T, Yanagisawa M, Shioda S, Imoto K, Minokoshi Y. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab 10: 466– 480, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Stacklies W, Redestig H, Scholz M, Walther D, Selbig J. pcaMethods—a bioconductor package providing PCA methods for incomplete data. Bioinformatics 23: 1164– 1167, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Taheri S, Gardiner J, Hafizi S, Murphy K, Dakin C, Seal L, Small C, Ghatei M, Bloom S. Orexin A immunoreactivity and preproorexin mRNA in the brain of Zucker and WKY rats. Neuroreport 12: 459– 464, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Teske JA, Billington CJ, Kotz CM. Hypocretin/orexin and energy expenditure. Acta Physiol (Oxf) 198: 303– 312, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Teske JA, Billington CJ, Kuskowski MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. Int J Obes (Lond) 36: 603– 613, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 291: R889– R899, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res 1050: 156– 162, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438: 71– 75, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics 17: 520– 525, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci 31: 15944– 15955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet 25: 52– 61, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci 30: 12642– 12652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]