Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR), a Cl− channel in airway epithelial cells, plays an important role in maintaining the volume of the airway surface liquid and therefore mucociliary clearance of respiratory pathogens. A recent study has shown that the E3 ubiquitin ligase Neural precursor cells expressed developmentally downregulated (Nedd4–2) ubiquitinates ΔF508-CFTR in pancreatic epithelial cells and that siRNA-mediated silencing of Nedd4–2 increases plasma membrane ΔF508-CFTR. Because the role of Nedd4–2 in regulating wild-type (wt)-CFTR in airway epithelial cells is unknown, studies were conducted to test the hypothesis that Nedd4–2 also ubiquitinates wt-CFTR and regulates its plasma membrane abundance. We found that Nedd4–2 did not affect wt-CFTR Cl− currents in Xenopus oocytes. Likewise, overexpression of Nedd4–2 in human airway epithelial cells did not alter the amount of ubiquitinated wt-CFTR. siRNA knockdown of Nedd4–2 in human airway epithelial cells had no effect on ubiquitination or apical plasma membrane abundance of wt-CFTR. Thus Nedd4–2 does not ubiquitinate and thereby regulate wt-CFTR in human airway epithelial cells.
Keywords: cystic fibrosis transmembrane conductance regulator, CFBE41o-, bronchial epithelial cells, siNedd4–2, ubiquitination, mucoviscidosis
the cystic fibrosis transmembrane conductance regulator (CFTR) is a camp-activated Cl− channel expressed in the apical membrane of airway epithelial cells, where it mediates Cl− secretion and regulates the volume and composition of the periciliary layer (6). Mutations in CFTR cause cystic fibrosis (21), the most common genetic disease in Caucasians (12). The most frequent mutation in CFTR, ΔF508, results in the proteasomal degradation of CFTR (15) and therefore an inability of airway cells to secrete Cl−, which leads to dehydration of the airway surface liquid that interferes with mucociliary clearance and results in chronic airway infection and, ultimately, death through respiratory failure (6).
A recent study demonstrated that siRNA-mediated knockdown of Nedd4–2, a E3 ubiquitin ligase, increased the amount of ΔF508-CFTR in the plasma membrane of CF airway epithelial cells (IB3–1 cell line) and CF pancreatic cells (CFPAC-1) (7) and augmented the Cl− permeability of the plasma membrane. However, nothing is known about the role of Nedd4–2 in regulating the ubiquitination and abundance of wild-type (wt)-CFTR in human airway epithelial cells. Therefore, the goal of this study was to test the hypothesis that Nedd4–2 regulates the ubiquitination and degradation of wt-CFTR in human airway epithelial cells.
Nedd4–2 belongs to the family of homologous to E6-AP COOH-terminus (HECT) E3 ligases that catalyze the final step in the ubiquitination cascade, the conjugation of ubiquitin to lysine residues of their target proteins, thus marking them for degradation (9). Nedd4–2 ubiquitinates the epithelial sodium channel ENaC (17), the chloride channel ClC-5 (14), voltage-gated potassium and sodium channels (10, 26), the intestinal apical calcium entry channel TRPV6 (28), and the cardiac potassium channel hERG1 (1), but nothing is known about the effect of Nedd4–2 on wt-CFTR in airway epithelial cells.
Here we report that Nedd4–2 did not affect wt-CFTR Cl− currents in Xenopus oocytes and that overexpression did not alter the amount of ubiquitinated wt-CFTR in human airway epithelial cells. siRNA-mediated silencing of Nedd4–2 in human airway epithelial cells had no effect on the amount of ubiquitinated wt-CFTR or the amount of wt-CFTR in the apical membrane. Together, these results suggest that Nedd4–2 does not ubiquitinate and thereby regulate wt-CFTR abundance in human airway epithelial cells.
MATERIALS AND METHODS
Cell culture.
CFBE-wt (CFBE41o- cells homozygous for the ΔF508 mutation and stably transduced with wt-CFTR; Ref. 3) cells were maintained in culture and grown as polarized monolayers on collagen coated Transwell permeable supports (Costar) as described in detail previously (4).
Antibodies.
CFTR was detected in Western blots using either the COOH-terminus-specific clone 24–1 (R&D Systems, Minneapolis, MN) or clone 596 (Cystic Fibrosis Foundation Therapeutics, Chapel Hill, NC). CFTR was immunoprecipitated using CFTR antibody clone M3A7 (Millipore, Billerica, MA) or COOH-terminus-specific clone 24–1 (R&D Systems). Mono- and polyubiquitinated CFTR were detected with the mouse anti-mono/poly-ubiquitin antibody FK2 (Enzo Life Sciences, Plymouth Meeting, PA). Nedd4–2 was detected with a polyclonal rabbit anti-Nedd4–2 antibody (ab46521; Abcam, Cambridge, MA), which reacts with human and mouse Nedd4–2. Ezrin was a loading control in Western blot studies and was detected with a mouse anti-ezrin antibody (1:1,000; BD Biosciences, San Jose, CA). EBP50 was detected using a mouse monoclonal antibody (no. 611160, BD Biosciences), and SLC26A9 was probed with a mouse polyclonal antibody (H00115019-A01; Abnova, Taipei, Taiwan). USP10 antibody (Bethyl Laboratories, Montgomery, TX) was used as a positive control in coimmunoprecipitation experiments. Mouse IgG1 antibody (Millipore Australia, Boronia, Australia) was used as a negative control in immunoprecipitation (IP) and ubiquitination experiments. Horseradish peroxide-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Bio-Rad, Hercules, CA) were used at a dilution of 1:3,000 for Western blots. All of the antibodies used have been shown in previous studies to be specific for their target protein (4, 5, 8).
Synthesis and expression of cRNAs in Xenopus oocytes and voltage-clamp recordings.
cRNAs were synthesized and expressed in Xenopus oocytes, and CFTR Cl− currents were analyzed as described in detail previously (22). Briefly, oocytes were injected with either 500 pg Nedd4–2 cRNA, 50 pg CFTR cRNA, or 50 pg CFTR cRNA plus 500 pg Nedd4–2 cRNA. CFTR-mediated Cl− currents before and after stimulation with IBMX (1 mM) were measured by the two-electrode voltage-clamp technique 1–3 days after cRNA injection.
Nedd4–2 knockdown with siRNA.
To reduce the amount of endogenous Nedd4–2, CFBE-wt cells were transfected with siRNA (50 nM) against human Nedd4–2 (Dharmacon siGENOME SMARTpool M-007187–02-0005; Thermo Fisher Scientific, Waltham, MA) using HiPerfect transfection reagent (Qiagen, Valencia, CA). AllStars negative control siRNA (Qiagen no. 1027280), hereafter called siNeg (50 nM), was used as a control. For ubiquitination and biotinylation experiments, CFBE-wt cells were seeded at 100,000 cells/filter on collagen coated 24-mm Transwell permeable supports (Costar no. 3412), transfected the next day and cultured at an air-liquid interface at 37°C for three more days. For short-circuit current measurements of CFTR Cl− currents, CFBE-wt cells were seeded at 1 × 106 cells/75-cm2 cell-culture flask (T75, no. 3276; Corningware, Corning, NY) the day before transfection. One day after transfection, cells were seeded at 6 × 105/filter on collagen-coated 12-mm Transwell permeable supports (Costar no. 3407) and grown at an air-liquid interface for 4 more days.
Nedd4–2 construct and overexpression.
Mouse Nedd4–2 cloned into a pCMV-SPORT6 vector was a generous gift from Dr. Ray Frizzell (University of Pittsburgh). A COOH-terminal HA-tag was added onto mouse Nedd4–2 in a three-step process using Taq polymerase (Invitrogen, Carlsbad, CA) and the following sets of primers: set 1 - Forward 5′-CACAGGACGACTTCATGTACCCATACGACGTGCCT-3′, Reverse 5′-AGGCACGTCGTATGGGTACATGAAGTCGTCTCGTG-3′; set 2 - Forward 5′-CGTGCCTGACAGTCACTTACCGACAGAAGATCCAAC-3′, Reverse 5′-GTTGGATCTTCTGTCGGTAAGTGACTGTCAGGCACG-3′; set 3 - Forward 5′-CGTGCCTGACTATGCTTTACCGACAGAAGATCCAAC-3′, Reverse 5′-GTTGGATCTTCTGTCGGTAAAGCATAGTCAGGCACG-3′. The resulting clone was verified by sequencing with T7 and SP6 primers (Invitrogen). For all Nedd4–2 overexpression experiments, CFBE-wt cells were seeded at 3 × 106 cells/T75 flask. Two days after seeding, cells were transfected with Nedd4–2 plasmid (6.25 μg) or pCMV-SPORT β-Gal control vector (GIBCO/Invitrogen) using Effectene transfection reagent (Qiagen). The day after transfection, cells from each flask were seeded on two collagen-coated 75-mm Transwell filters (Costar no. 3419) for ubiquitination experiments, on 24-mm Transwell filters (Costar no. 3412) for biotinylation experiments, or on 12-mm Transwell filters (Costar no. 3407) for CFTR short-circuit current measurements and grown at an air-liquid interface for 3 days.
CFTR ubiquitination.
Ubiquitination assays were performed as described in detail previously (4). Briefly, cells were treated with the lysosomal inhibitor chloroquine (200 μM) for 4 h. CFTR was precipitated from whole cell lysates (WCL) with the CFTR antibody M3A7 or mouse IgG1 as a negative control using 5 μg antibody/25 μl protein G agarose (Thermo Scientific, Rockford, IL). WCL and immunoprecipitated proteins were separated on Western blots and probed with anti-ubiquitin antibody FK2, CFTR 596, or COOH-terminal antibody as indicated and the Nedd4–2 antibody.
Biotinylation of membrane CFTR.
Quantification of apical membrane CFTR by biotinylation with EZ-Link Sulfo-NHS-LC-Biotin (Thermo Scientific) was performed as described in detail previously (19, 20).
CFTR short-circuit current measurements.
Transepithelial Cl− current measurements in Ussing chambers were performed as described in detail previously (25). Amiloride (50 μM, A7410; Sigma-Aldrich, St. Louis, MO) was added to the apical side of the polarized monolayer to inhibit epithelial sodium channel currents. CFTR Cl− currents were stimulated with forskolin (20 μM, Sigma-Aldrich F6886) added to the apical and basolateral sides of the monolayer. CFTR Inhibitor 172 (Thiazolidinone; 5 μM, Sigma-Aldrich C2992) was added to the apical side of the polarized monolayer to inhibit CFTR currents. Data were collected using the Acquire and Analyze software version 2.3 (Physiologic Instruments, San Diego, CA), and data analysis was performed with GraphPad Prism version 5.0c for Mac OS X (GraphPad Software, San Diego, CA). Differences between means were assessed by unpaired t-tests.
Coimmunoprecipitation.
Coimmunoprecipitation experiments were conducted as described in detail previously (7). Briefly, CFBE-wt cells were seeded at 3 × 106 cells/75-cm2 cell-culture flask (T75, Corning no. 3276) 2 days before the experiment. CFTR was immunoprecipitated from WCL with CFTR COOH-terminal antibody or mouse IgG1 as a negative control using 10 μg antibody for 1 mg total protein and 100 μl protein A agarose (Thermo Scientific). WCL and immunoprecipitated proteins were separated by Western blot and probed for CFTR (596 antibody), Nedd4–2, or USP10 (positive control).
RESULTS
Nedd4–2 does not decrease wt-CFTR currents in Xenopus oocytes.
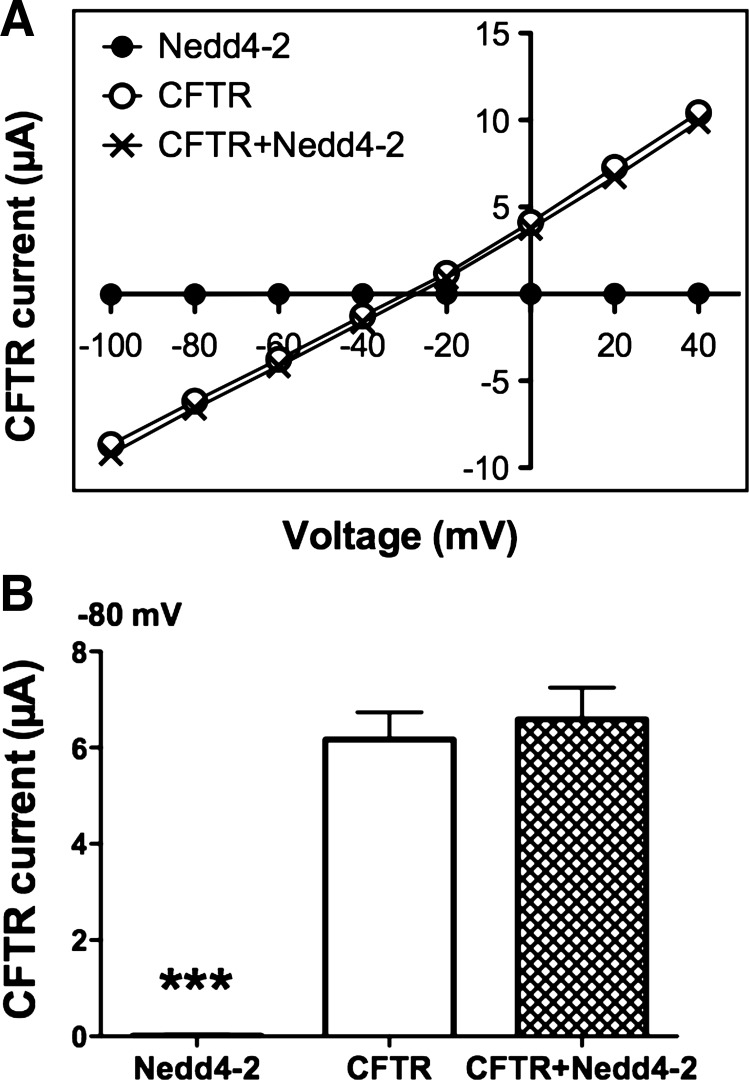
Xenopus oocytes were used as a model to assess whether Nedd4–2 regulates wt-CFTR Cl− currents. If Nedd4–2 ubiquitinates wt-CFTR and thereby enhances its degradation; then coexpression of Nedd4–2 should decrease wt-CFTR Cl− currents. Expression of wt-CFTR alone in oocytes increased IBMX-stimulated currents compared with oocytes expressing only Nedd4–2 (Fig. 1A). Inspection of the current voltage (I/V) plots revealed that wt-CFTR currents had a linear I/V relationship with a reversal potential of −33 mV, characteristics of CFTR Cl− currents, as reported previously (22). Coinjection of Nedd4–2 with wt-CFTR had no effect on the IBMX-stimulated CFTR Cl− currents (Fig. 1A). The I/V plot for oocytes expressing wt-CFTR alone was similar to the I/V plot of oocytes expressing CFTR and Nedd4–2. For example, the IBMX-stimulated Cl− current at −80 mV was 6.2 ± 0.6 μA in oocytes expressing wt-CFTR (Fig. 1B; N = 66) and 6.6 ± 0.7 μA in oocytes expressing wt-CFTR and Nedd4–2 (Fig. 1B; N = 77).
Fig. 1.
Voltage-clamp recordings in Xenopus oocytes. Oocytes were injected with either Nedd4–2 cRNA (500 pg, N = 14), cystic fibrosis transmembrane conductance regulator (CFTR) cRNA (50 pg, N = 66), or CFTR cRNA plus Nedd4–2 cRNA (50 and 500 pg, respectively, N = 77). A: current-voltage (I/V) plots of IBMX-stimulated currents. B: summary of IBMX-stimulated currents at −80 mV. ***P < 0.001.
siRNA silencing of Nedd4–2 in human airway epithelial cells.
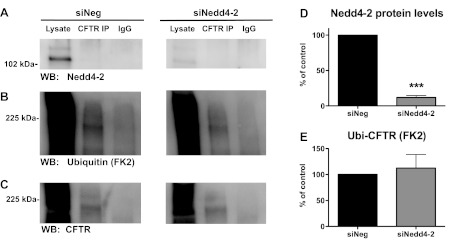
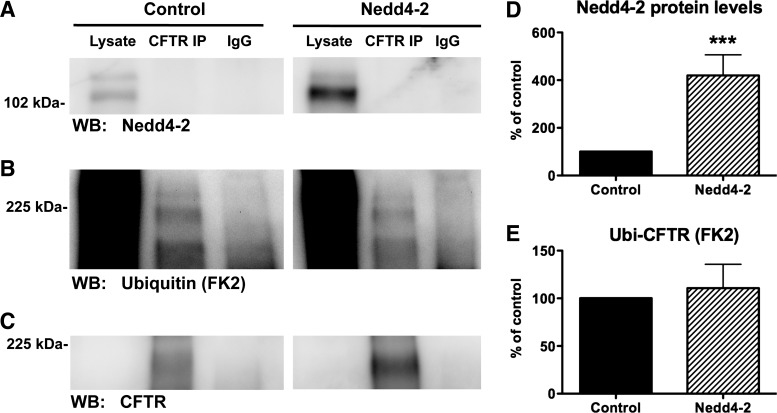
Although Nedd4–2 did not affect CFTR Cl− currents in oocytes, it is possible that Nedd4–2 ubiquitinates wt-CFTR in human airway epithelial cells. To test the hypothesis that Nedd4–2 ubiquitinates wt-CFTR in human airway epithelial cells, we first knocked down Nedd4–2 in CFBE-wt cells using siRNA and then measured ubiquitinated wt-CFTR, as well as plasma membrane wt-CFTR and wt-CFTR Cl− currents. siRNA against Nedd4–2 reduced Nedd4–2 protein abundance by 87%. Two protein bands were identified by Western blot using the Nedd4–2 antibody, and siNedd4–2 reduced both bands (Fig. 2A, compare lysate lanes), which are most likely two previously identified splice variants of Nedd4–2 (9).
Fig. 2.
Ubiquitination assay in CFBE-wild-type (wt) cells transfected with siNeg or siNedd4–2. A–C: immunoprecipitation (IP) was conducted with either an anti-CFTR antibody (M3A7) or a control antibody (IgG), and Western blots (WB) were probed for Nedd4–2 (A), ubiquitin (FK2 antibody, B) or wt-CFTR (596 antibody, C). siNeg and siNedd4–2 samples were run on the same gel but are separated for presentation. D: summary of Nedd4–2 levels in siNeg and siNedd4–2 experiments (N = 3). E: summary of the effects of siNedd4–2 on ubiquitinated CFTR, which is reported as the amount of ubiquitinated CFTR (FK2) divided by the amount of total CFTR determined by Western blot (N = 3). ***P < 0.001.
siRNA knockdown of Nedd4–2 does not affect the amount of ubiquitinated wt-CFTR in human airway epithelial cells.
If Nedd4–2 ubiquitinates wt-CFTR, knockdown of Nedd4–2 with siRNA should reduce the amount of ubiquitinated wt-CFTR (ubi-CFTR). However, knockdown of Nedd4–2 did not decrease the amount of ubi-CFTR in CFBE-wt cells (Fig. 2).
siRNA knockdown of Nedd4–2 does not affect the amount of wt-CFTR in cell lysates or the plasma membrane of human airway epithelial cells.
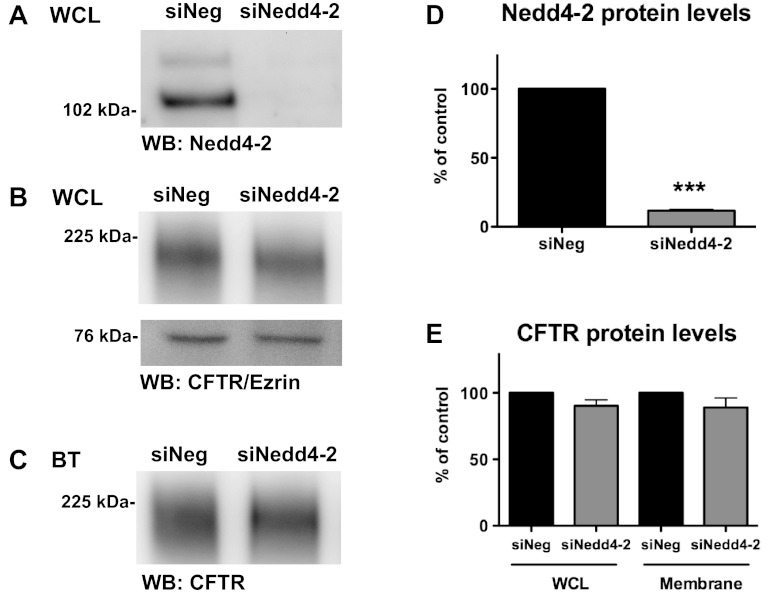
Although siNedd4–2 did not alter the amount of ubi-CFTR, it is possible that Nedd4–2 may affect the amount of wt-CFTR in the plasma membrane or CFTR Cl− currents by other, unknown mechanisms. To test the hypothesis that Nedd4–2 modulates plasma membrane wt-CFTR abundance, cell surface biotinylation experiments were performed to assess the amount of apical membrane wt-CFTR in CFBE-wt cells treated with siNedd4–2 or siNeg. siNedd4–2 did not alter the amount of wt-CFTR in WCLs or at the plasma membrane (Fig. 3).
Fig. 3.
Biotinylation experiments in CFBE-wt cells transfected with siNeg or siNedd4–2. Representative Western blots of Nedd4–2 in whole cell lysates (WCL) (A), wt-CFTR and Ezrin in WCL (B), and biotinylated (BT) membrane CFTR (C). D: summary of Nedd4–2 levels in siNeg and siNedd4–2 experiments (N = 3). E: summary of WCL and membrane wt-CFTR for siNeg and siNedd4–2 (N = 3). ***P < 0.001.
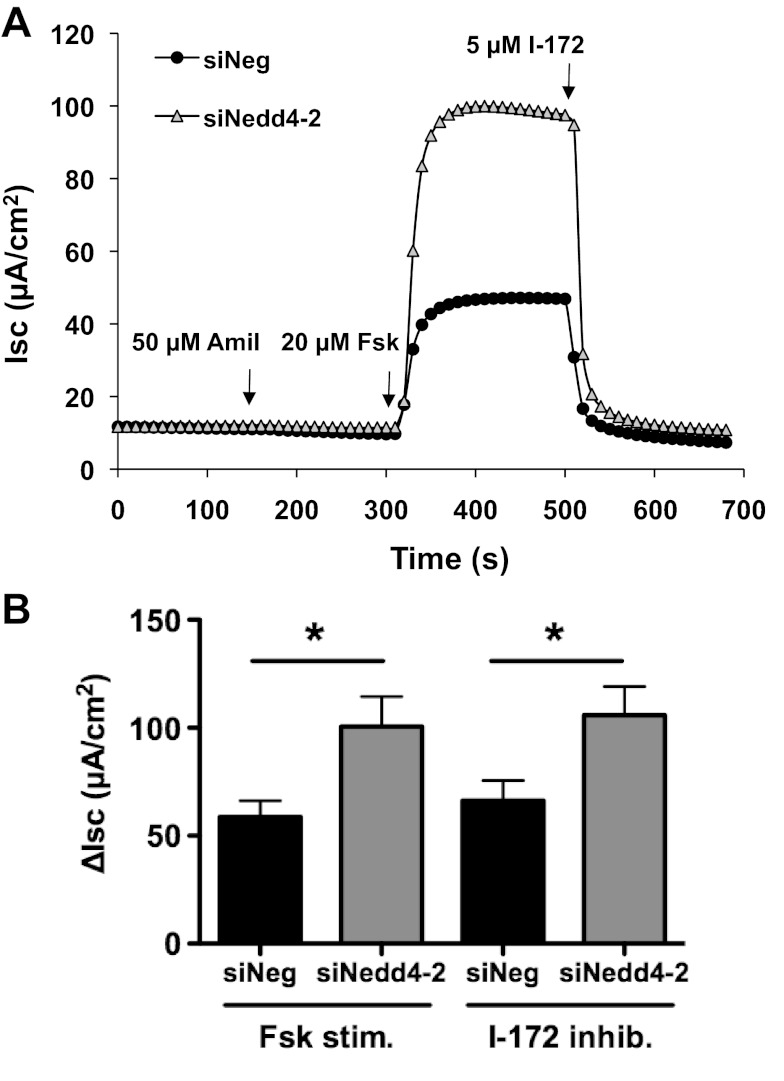
siNedd4–2 increases wt-CFTR Cl− currents in human airway epithelial cells.
To determine whether Nedd4–2 regulates wt-CFTR function, short-circuit currents (Isc) were measured in CFBE-wt cells treated with siNeg or siNedd4–2. siNedd4–2 significantly augmented forskolin-stimulated and I-172-inhibited wt-CFTR Cl− currents in CFBE-wt cells (Fig. 4). Mean forskolin-stimulated Cl− currents were 58.8 ± 7.5 μA/cm2 for siNeg control and 100.6 ± 13.8 μA/cm2 for siNedd4–2, and mean I-172-inhibited Cl− currents were 66.3 ± 9.3 μA/cm2 for siNeg control and 105.8 ± 13.3 μA/cm2 for siNedd4–2 (P < 0.05, N = 9). There was no significant difference between Fsk-stimulated and I-172-inhibited currents in either the siNeg or siNedd4–2 experiments.
Fig. 4.
Short-circuit current (Isc) recordings of CFBE-wt cells transfected with siNeg or siNedd4–2. A: representative current traces. Amil, amiloride; Fsk, forskolin; I-172, CFTR inhibitor Thiazolidinone. B: summary of Fsk-stimulated and I-172-inhibited wt-CFTR ΔIsc (N = 9). Although there was a significant increase in wt-CFTR ΔIsc with siNedd4–2 (*P < 0.05), there was no significant difference between Fsk-stimulated and I-172-inhibited currents in either the siNeg or siNedd4–2 experiments.
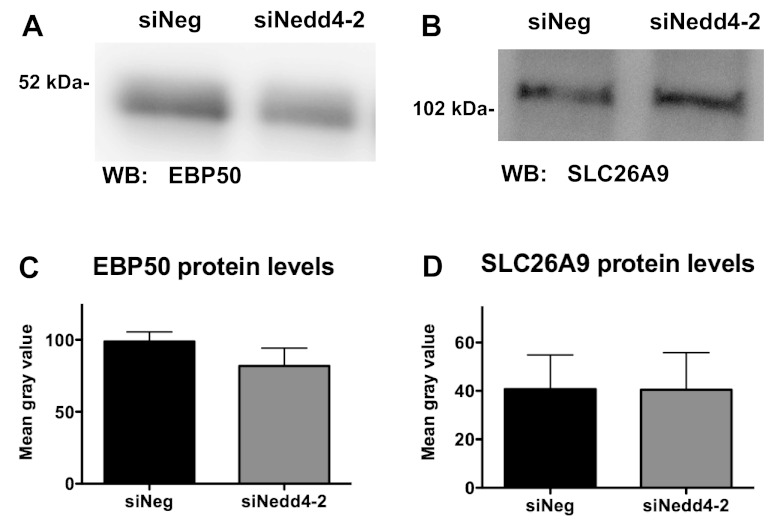
siNedd4–2 does not alter the abundance of EBP50 or SLC26A9.
To explain the increase in wt-CFTR currents that we observed with siNedd4–2, we speculated that siNedd4–2 may reduce the ubiquitination and degradation of positive regulators of CFTR activity such as NHERF1/EBP50 or SLC26A9. EBP50 has been reported to increase CFTR open probability by 2.8-fold (18), and SLC26A9 has been shown to stimulate wt-CFTR currents (2). However, siNedd4–2 did not change the abundance of EBP50 or SLC26A9 (Fig. 5), an observation inconsistent with our hypothesis that a decrease in Nedd4–2 may lead to a reduction in the ubiquitination of EBP50 and SLC26A9, which would increase their abundance and increase the activity of wt-CFTR.
Fig. 5.
Representative Western blots of CFBE-wt cells transfected with 50 nM siNeg or siNedd4–2 were probed with an antibody against EBP50 (A) or SLC26A9 (B). C: summary of EBP50 protein levels in CFBE-wt cells transfected with siNeg or siNedd4–2 (N = 6). D: summary of SLC26A9 protein levels in CFBE-wt cells transfected with siNeg or siNedd4–2 (N = 4).
Nedd4–2 overexpression does not increase ubiquitinated CFTR.
Additional studies were conducted to test the hypothesis that Nedd4–2 ubiquitinates and thereby downregulates wt-CFTR abundance. To this end, Nedd4–2 was overexpressed in CFBE-wt cells, and experiments were performed to examine ubi-CFTR as well as wt-CFTR protein levels and CFTR Cl− currents. Overexpression of Nedd4–2 had no effect on the amount of ubi-CFTR (Fig. 6). This observation confirms the finding with siRNA for Nedd4–2, above, that Nedd4–2 does not ubiquitinate wt-CFTR.
Fig. 6.
Ubiquitination assay of CFBE-wt cells overexpressing Nedd4–2. A–C: IP was conducted with either an anti-CFTR antibody (M3A7) or a control antibody (IgG). WB were probed for Nedd4–2 (A), ubiquitin (FK2 antibody, B), or wt-CFTR (C-term antibody, C). Control and Nedd4–2 samples were run on the same gel, but are separated for presentation. D: summary of Nedd4–2 levels in control and Nedd4–2 experiments (N = 3). E: summary of the effects of Nedd4–2 overexpression on ubiquitinated wt-CFTR, which is reported as the amount of ubiquitinated wt-CFTR (FK2) divided by the amount of total wt-CFTR determined by Western blot (N = 3). ***P < 0.001.
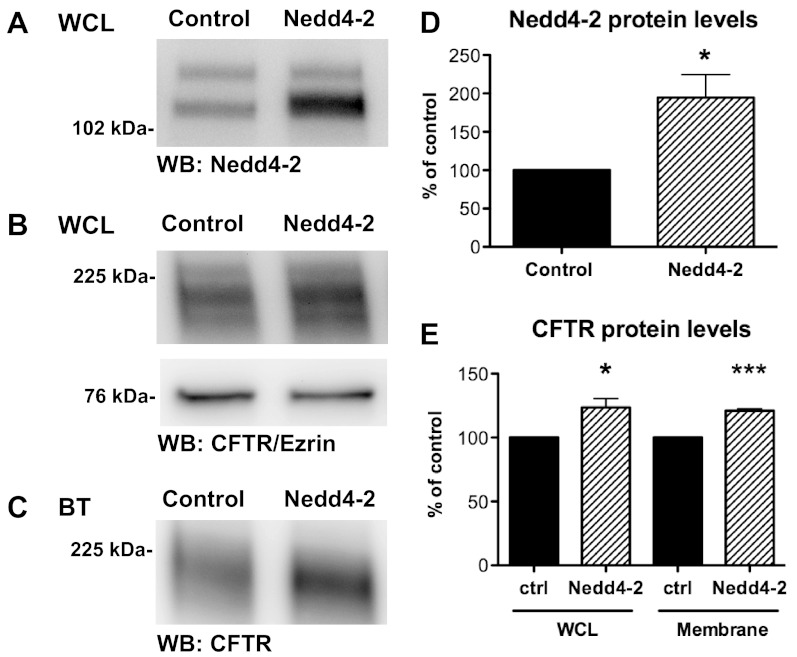
Nedd4–2 overexpression increases whole cell and plasma membrane wt-CFTR.
Paradoxically, overexpression of Nedd4–2 by 195% (Fig. 7, A and D) increased wt-CFTR protein levels in cell lysates (Fig. 7, B and E) and in the plasma membrane (Fig. 7, C and E) by 24 and 21%, respectively. These results were unexpected because Nedd4–2 overexpression did not affect the amount of ubiquitinated wt-CFTR and suggest that overexpression of Nedd4–2 alters wt-CFTR abundance by a mechanism that does not involve ubiquitination of CFTR.
Fig. 7.
Biotinylation experiments in CFBE-wt cells transfected with control vector or Nedd4–2. Representative Western blots of Nedd4–2 (A) and wt-CFTR and Ezrin in WCL (B). Plasma membrane wt-CFTR as determined by membrane biotinylation (C). D: summary of Nedd4–2 levels in control (vector) and Nedd4–2 overexpressing cells (N = 6). E: summary of WCL and membrane wt-CFTR for control and Nedd4–2 overexpressing cells (N = 6). *P < 0.05, ***P < 0.001.
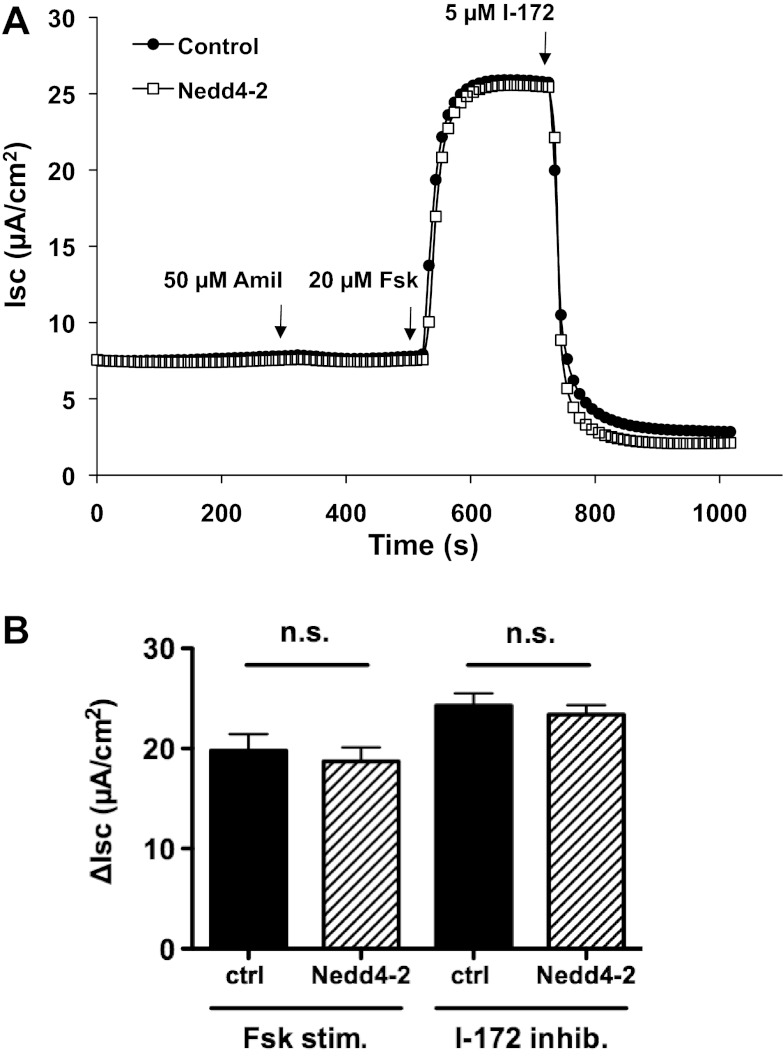
Nedd4–2 overexpression does not decrease CFTR short-circuit currents.
To analyze the effect of Nedd4–2 on CFTR function, short-circuit currents were measured in CFBE-wt cells overexpressing Nedd4–2. Nedd4–2 overexpression did not alter forskolin-stimulated or I-172-inhibited CFTR Cl− currents (Fig. 8). Mean forskolin-stimulated Cl− currents were 19.8 ± 1.6 μA/cm2 for cells transfected with control vector and 18.7 ± 1.4 μA/cm2 for cells transfected with Nedd4–2. Mean I-172-inhibited Cl− currents were 24.3 ± 1.2 μA/cm2 for control vector and 23.4 ± 0.9 μA/cm2 for Nedd4–2 overexpression (N = 6).
Fig. 8.
Isc recordings of CFBE-wt cells transfected with control vector or Nedd4–2. A: representative current traces. B: summary of Fsk-stimulated and I-172-inhibited wt-CFTR ΔIsc (N = 6).
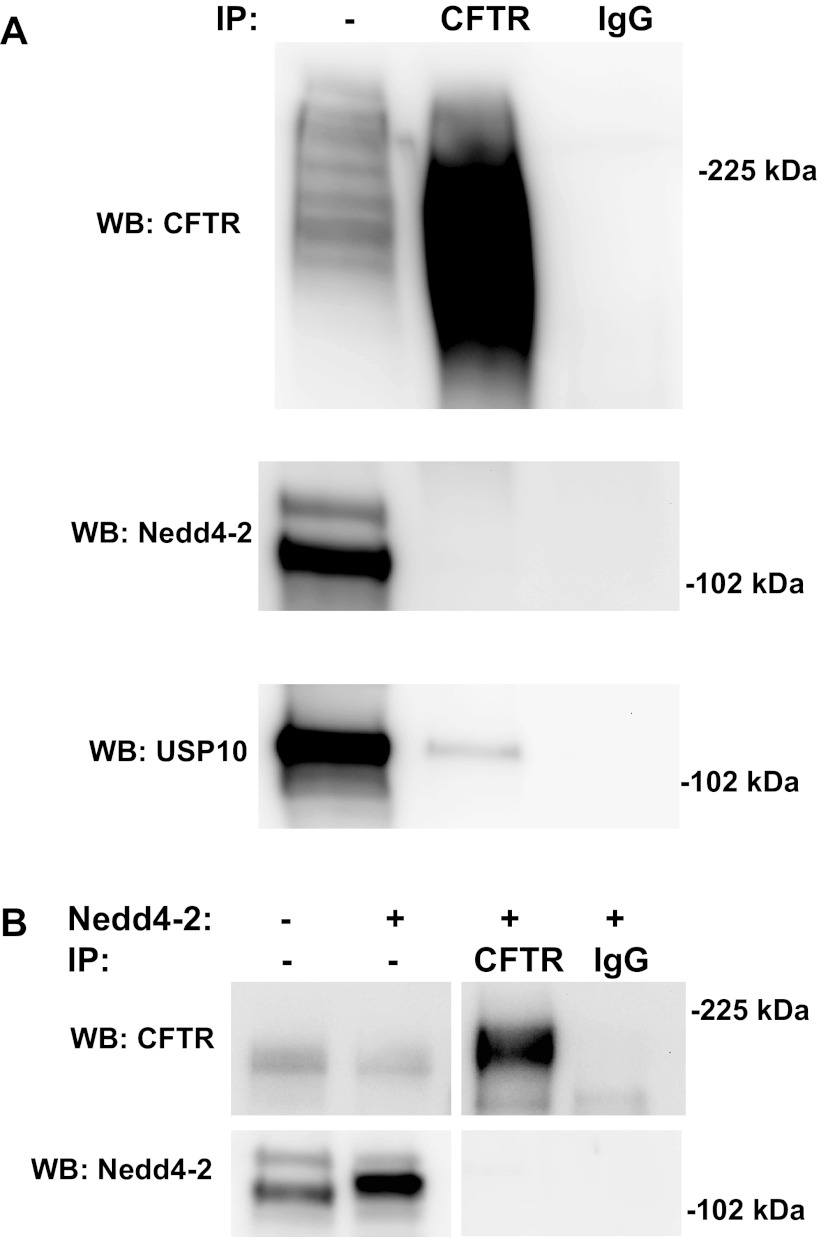
Nedd4–2 does not coimmunoprecipitate with CFTR in CFBE-wt cells.
Because Nedd4–2 coimmunoprecipitates with ΔF508-CFTR in CFPAC cells, studies were performed to determine whether Nedd4–2 coimmunoprecipitates with wt-CFTR in CFBE-wt cells. Western blots of wt-CFTR, which had been immunoprecipitated from CFBE-wt cells, revealed that endogenous Nedd4–2 did not coimmunoprecipitate with wt-CFTR in CFBE-wt cells (Fig. 9A, lane 2). As a positive control, USP10 did coimmunoprecipitate with CFTR, as shown previously (Fig. 9A, bottom) (4). Likewise, in cells overexpressing Nedd4–2, wt-CFTR did not coimmunoprecipitate with Nedd4–2 (Fig. 9B, lane 3). The lack of coimmunoprecipitation suggests that there is either no direct interaction between wt-CFTR and Nedd4–2 in this model system, or, if there is any binding, it is of such low affinity that it is below the detection limit.
Fig. 9.
Coimmunoprecipitation experiments with CFBE-wt cells. IP was conducted with either an anti-CFTR antibody (C-term) or a control antibody (IgG). A: WB were probed for wt-CFTR (596 antibody, top), Nedd4–2 (middle), or USP10 (bottom). B: Western blots of cells overexpressing Nedd4–2 were probed for CFTR (596 antibody, top) and Nedd4–2 (bottom).
DISCUSSION
The goal of this study was to test the hypothesis that Nedd4–2, which ubiquitinates ΔF508-CFTR (7), also ubiquitinates wt-CFTR and regulates its expression. Several lines of evidence in this report demonstrate that Nedd4–2 does not ubiquitinate wt-CFTR. First, Nedd4–2 did not alter wt-CFTR Cl− currents in Xenopus oocytes. Second, overexpression of Nedd4–2 in human airway epithelial cells had no effect on the amount of ubiquitinated wt-CFTR or on CFTR Cl− currents. Third, siRNA knockdown of Nedd4–2 in human airway epithelial cells had no effect on the amount of ubiquitinated wt-CFTR. Taken together, the data in this report are consistent with the conclusion that Nedd4–2 does not ubiquitinate and thereby regulate wt-CFTR abundance in human airway epithelial cells.
However, our data suggest that Nedd4–2 may regulate CFTR indirectly. Because E3 ligases are highly promiscuous, Nedd4–2 is likely to ubiquitinate many targets, even though it does not ubiquitinate CFTR in our model system. Thus knockdown or overexpression of Nedd4–2 may have indirect effects on CFTR membrane density and activity by ubiquitinating positive and negative regulators of CFTR. These indirect effects would explain the small but significant increase in whole cell and plasma membrane wt-CFTR without a concomitant increase in CFTR Cl− currents upon Nedd4–2 overexpression, as well as the increase in wt-CFTR currents without elevation in wt-CFTR membrane density with siNedd4–2. Overexpression of Nedd4–2 may lead to increased ubiquitination and degradation of negative regulators of CFTR abundance as well as positive regulators of CFTR activity, resulting in no net increase in CFTR current. Likewise, siNedd4–2 may reduce the ubiquitination and degradation of positive regulators of CFTR activity, such as NHERF1/EBP50 and SLC26A9, which have been shown to increase wt-CFTR currents (2, 18). However, siNedd4–2 did not change the abundance of EBP50 or SLC26A9, an observation inconsistent with the hypothesis that a reduction in Nedd4–2 led to a reduction in the ubiquitination of EBP50 and SLC26A9, which would increase their abundance. Because the goal of this study was to test whether Nedd4–2 ubiquitinates wt-CFTR in airway epithelial cells, the identification of additional Nedd4–2 target proteins that regulate CFTR abundance and function is beyond the scope of this study.
In contrast to the present study, Caohuy and coworkers demonstrated that decreasing endogenous Nedd4–2 with siRNA led to a fivefold reduction in the amount of ubiquitinated ΔF508-CFTR and a fivefold increase in Cl− transport in CFPAC-1 cells. Moreover, siNedd4–2 increased ΔF508-CFTR membrane density by 2.5-fold in both CFPAC-1 cells and IB3–1 cells and reduced the interaction between Nedd4–2 and ΔF508-CFTR in CFPAC-1 cells, as determined by coimmunoprecipitation studies. These findings led the authors to conclude that ΔF508-CFTR is a target for Nedd4–2-mediated ubiquitination and degradation (7). By contrast, as noted above, we found that, in CFBE-wt cells, siNedd4–2 did not alter the amount of ubiquitinated wt-CFTR or wt-CFTR in the plasma membrane. Furthermore, wt-CFTR did not coimmunoprecipitate with Nedd4–2 in CFBE-wt cells. The most likely explanation for this difference is that the ability of Nedd4–2 to ubiquitinate CFTR requires interaction between wt-CFTR and Nedd4–2 and that interaction is mediated by cell type-specific adaptor protein(s). For example, whereas the WW domain in Nedd4–2 binds directly to PY motifs on ENaC and thereby enhances its ubiquitination (24), CFTR does not have a conventional PY motif that could account for a direct interaction between Nedd4–2 and wt-CFTR (7). However, the NEDD4 family-interacting proteins, Ndfip1 and Ndfip2, assist Nedd4-family proteins in binding to target proteins (13, 16, 23, 27). For example, the binding of the divalent metal-ion transporter DMT1 to the NEDD4-like E3 ligase WWP2 and its subsequent ubiquitination and degradation require Ndfips (11). Thus we propose that an unknown adaptor protein may be present in CFPAC cells but not in CFBE cells. In summary, the present study demonstrates that Nedd4–2 does not regulate the ubiquitination and degradation of wt-CFTR in CFBE-wt airway epithelial cells.
GRANTS
This research was supported by the National Institutes of Health (R01 HL074175 and P20 RR016463) and a Cystic Fibrosis Foundation Research Development Program (STANTO07R0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.K. and B.A.S. conception and design of research; K.K., C.C., and J.D.S. performed experiments; K.K., C.C., J.D.S., and B.A.S. analyzed data; K.K. and B.A.S. interpreted results of experiments; K.K. prepared figures; K.K. and B.A.S. drafted manuscript; K.K., J.D.S., and B.A.S. edited and revised manuscript; K.K., C.C., J.D.S., and B.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. John P. Clancy for the CFBE-wt cells and Dr. Ray Frizzell for the original, untagged mouse Nedd4–2 expression vector.
REFERENCES
- 1. Albesa M, Grilo LS, Gavillet B, Abriel H. Nedd4–2-dependent ubiquitylation and regulation of the cardiac potassium channel hERG1. J Mol Cell Cardiol 51: 90–98, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Avella M, Loriol C, Boulukos K, Borgese F, Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J Cell Physiol 226: 212–223, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, Sorscher EJ, Clancy JP. Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers. J Physiol 569: 601–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem 284: 18778–18789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bomberger JM, Ye S, Maceachran DP, Koeppen K, Barnaby RL, O'Toole GA, Stanton BA. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog 7: e1001325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23: 146–158, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Caohuy H, Jozwik C, Pollard HB. Rescue of DeltaF508-CFTR by the SGK1/Nedd4–2 signaling pathway. J Biol Chem 284: 25241–25253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandran S, Li H, Dong W, Krasinska K, Adams C, Alexandrova L, Chien A, Hallows KR, Bhalla V. Neural Precursor Cell-expressed Developmentally Down-regulated Protein 4–2 (Nedd4–2) Regulation by 14–3-3 Protein Binding at Canonical Serum and Glucocorticoid Kinase 1 (SGK1) Phosphorylation Sites. J Biol Chem 286: 37830–37840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H, Ross CA, Wang N, Huo Y, MacKinnon DF, Potash JB, Simpson SG, McMahon FJ, DePaulo JR, Jr, McInnis MG. NEDD4L on human chromosome 18q21 has multiple forms of transcripts and is a homologue of the mouse Nedd4–2 gene. Eur J Hum Genet 9: 922–930, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Ekberg J, Schuetz F, Boase NA, Conroy SJ, Manning J, Kumar S, Poronnik P, Adams DJ. Regulation of the voltage-gated K(+) channels KCNQ2/3 and KCNQ3/5 by ubiquitination. Novel role for Nedd4–2. J Biol Chem 282: 12135–12142, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Foot NJ, Dalton HE, Shearwin-Whyatt LM, Dorstyn L, Tan SS, Yang B, Kumar S. Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood 112: 4268–4275, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Harvey KF, Shearwin-Whyatt LM, Fotia A, Parton RG, Kumar S. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J Biol Chem 277: 9307–9317, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, Cook DI, Pollock CA, Poronnik P. Nedd4–2 functionally interacts with ClC-5: involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem 279: 54996–55007, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83: 129–135, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Jolliffe CN, Harvey KF, Haines BP, Parasivam G, Kumar S. Identification of multiple proteins expressed in murine embryos as binding partners for the WW domains of the ubiquitin-protein ligase Nedd4. Biochem J 351: 557–565, 2000 [PMC free article] [PubMed] [Google Scholar]
- 17. Kamynina E, Tauxe C, Staub O. Distinct characteristics of two human Nedd4 proteins with respect to epithelial Na(+) channel regulation. Am J Physiol Renal Physiol 281: F469–F477, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Lee JH, Richter W, Namkung W, Kim KH, Kim E, Conti M, Lee MG. Dynamic regulation of cystic fibrosis transmembrane conductance regulator by competitive interactions of molecular adaptors. J Biol Chem 282: 10414–10422, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Lisanti MP, Le Bivic A, Sargiacomo M, Rodriguez-Boulan E. Steady-state distribution and biogenesis of endogenous Madin-Darby canine kidney glycoproteins: evidence for intracellular sorting and polarized cell surface delivery. J Cell Biol 109: 2117–2127, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moyer BD, Loffing J, Schwiebert EM, Loffing-Cueni D, Halpin PA, Karlson KH, Ismailov II, Guggino WB, Langford GM, Stanton BA. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in madin-darby canine kidney cells. J Biol Chem 273: 21759–21768, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Sato JD, Chapline MC, Thibodeau R, Frizzell RA, Stanton BA. Regulation of human cystic fibrosis transmembrane conductance regulator (CFTR) by serum- and glucocorticoid-inducible kinase (SGK1). Cell Physiol Biochem 20: 91–98, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Shearwin-Whyatt LM, Brown DL, Wylie FG, Stow JL, Kumar S. N4WBP5A (Ndfip2), a Nedd4-interacting protein, localizes to multivesicular bodies and the Golgi, and has a potential role in protein trafficking. J Cell Sci 117: 3679–3689, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Sudol M, Hunter T. NeW wrinkles for an old domain. Cell 103: 1001–1004, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Talebian L, Coutermarsh B, Channon JY, Stanton BA. Corr4A and VRT325 do not reduce the inflammatory response to P. aeruginosa in human cystic fibrosis airway epithelial cells. Cell Physiol Biochem 23: 199–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, Rivolta I, Thomas MA, Kass RS, Staub O, Abriel H. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4–2 mediated ubiquitination. Circ Res 95: 284–291, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Yang B, Kumar S. Nedd4 and Nedd4–2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ 17: 68–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang W, Na T, Wu G, Jing H, Peng JB. Down-regulation of intestinal apical calcium entry channel TRPV6 by ubiquitin E3 ligase Nedd4–2. J Biol Chem 285: 36586–36596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]