Abstract
Clinical reports indicate that patients with allergy/asthma commonly have associated symptoms of anxiety/depression. Anxiety/depression can be reduced by 5-hydroxytryptophan (5-HTP) supplementation. However, it is not known whether 5-HTP reduces allergic inflammation. Therefore, we determined whether 5-HTP supplementation reduces allergic inflammation. We also determined whether 5-HTP decreases passage of leukocytes through the endothelial barrier by regulating endothelial cell function. For these studies, C57BL/6 mice were supplemented with 5-HTP, treated with ovalbumin fraction V (OVA), house dust mite (HDM) extract, or IL-4, and examined for allergic lung inflammation and OVA-induced airway responsiveness. To determine whether 5-HTP reduces leukocyte or eosinophil transendothelial migration, endothelial cells were pretreated with 5-HTP, washed and then used in an in vitro transendothelial migration assay under laminar flow. Interestingly, 5-HTP reduced allergic lung inflammation by 70–90% and reduced antigen-induced airway responsiveness without affecting body weight, blood eosinophils, cytokines, or chemokines. 5-HTP reduced allergen-induced transglutaminase 2 (TG2) expression and serotonylation (serotonin conjugation to proteins) in lung endothelial cells. Consistent with the regulation of endothelial serotonylation in vivo, in vitro pretreatment of endothelial cells with 5-HTP reduced TNF-α-induced endothelial cell serotonylation and reduced leukocyte transendothelial migration. Furthermore, eosinophil and leukocyte transendothelial migration was reduced by inhibitors of transglutaminase and by inhibition of endothelial cell serotonin synthesis, suggesting that endothelial cell serotonylation is key for leukocyte transendothelial migration. In summary, 5-HTP supplementation inhibits endothelial serotonylation, leukocyte recruitment, and allergic inflammation. These data identify novel potential targets for intervention in allergy/asthma.
Keywords: 5-hydroxytryptophan metabolites, serotonylation, endothelial cell, leukocyte recruitment, lung
tissue-derived mediators stimulate endothelium to express adhesion molecules and secrete cytokines that then regulate leukocyte recruitment. Leukocyte binding to endothelial cell adhesion molecules activates intracellular signaling in the endothelial cells that then induces localized endothelial cell retraction. This retraction opens narrow passageways through which the leukocytes migrate. Inhibition of signals in endothelial cells blocks recruitment of leukocytes to tissues (2, 9, 25, 94).
Activated endothelium also secretes cytokines, chemokines, and neurotransmitters that regulate immune responses. Endothelial cells synthesize the neurotransmitter serotonin (33, 39, 81, 87) and express stimulatory and inhibitory serotonin receptors (33, 62, 78–80). The serotonin receptors are also expressed by leukocytes, and it is reported that serotonin regulates leukocyte chemotaxis, cytokine production, and dendritic cell activation of T cells (3, 4, 13, 23, 66, 72, 73, 85, 88, 115, 122). Serotonin also regulates cell signaling by covalent linkage of serotonin to proteins through transamidation of glutamines (serotonylation) (30, 65, 75, 93, 119). Therefore, changes in localized levels of metabolites in the serotonin pathway would alter signaling through serotonylation and inhibitory/stimulatory serotonin receptors.
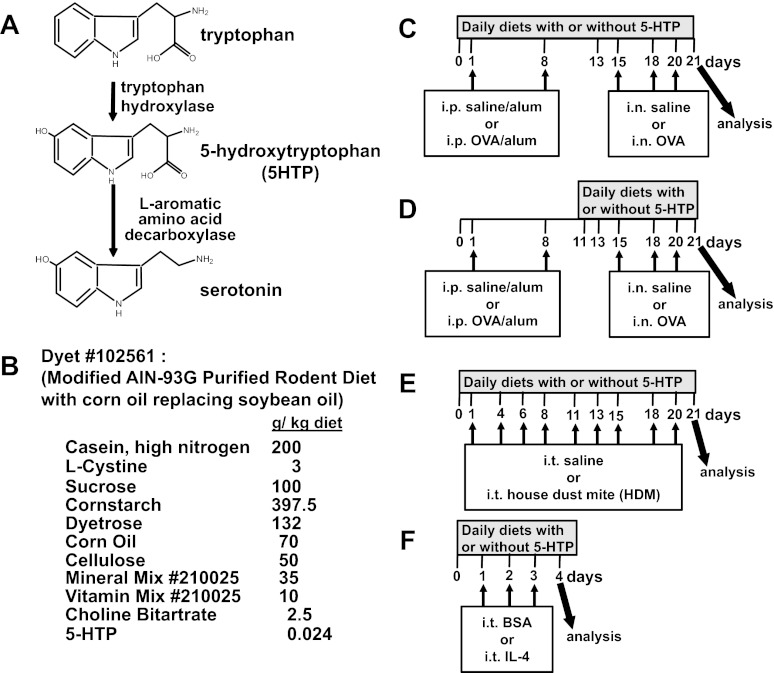
A reduction in serotonin synthesis in vivo results in anxiety/depression (116). Symptoms of anxiety/depression are commonly associated with allergy/asthma in humans and mice (21, 27, 36, 100, 106). Moreover, 46% of patients who have allergies and anxiety/depression do not receive or recognize the need for psychopharmacological treatment (28). The mechanisms for the association of anxiety in individuals with allergy/asthma are not known. Anxiety/depression, which correlates with serotonin deficiencies, are associated with polymorphisms in tryptophan hydroxylase 1 or 2 (Fig. 1A), the rate-limiting enzymes that catalyze the formation of 5-hydroxytryptophan (5-HTP) (44). 5-HTP is further metabolized to serotonin by decarboxylases (Fig. 1A).
Fig. 1.
5-Hydroxytryptophan (5-HTP) supplementation. A: serotonin synthesis. B: purified 5-HTP from Sigma was used to supplement the diets by Dyets. Consumption of this diet (0.024 g 5-HTP/kg diet) at 4 g diet/day/20 g mouse is equivalent to 200 mg 5-HTP/100 pound person/day. This calculation is as follows: 200 mg 5-HTP/100 pound person/day = {[(0.024 g 5-HTP × 1,000 mg/g)/1,000 g diet] × 4 g diet/mouse/day}/[20 g mouse × (1 pound/453 g)]. The control diet was the same except without 5-HTP. We confirmed the concentration of 5-HTP in the diet as 0.024 g 5-HTP/kg diet by HPLC/electrochemical detector (ECD). C: timeline for 5-HTP supplementation during ovalbumin fraction V (OVA) sensitization and OVA challenge treatments. D: timeline for 5-HTP supplementation after OVA sensitization. E: timeline for 5-HTP supplementation during treatments with house dust mite (HDM) extract from Dermatophagoides pteronyssius. F: timeline for 5-HTP supplementation during intratracheal IL-4 treatments. On day of analysis in C–F, the bronchoalveolar lavage (BAL), perfused lungs, blood, brains, and intestines were collected. i.p., intraperitoneal. i.n., intranasal. i.t., intratracheal. n = 6–10 mice/group.
Anxiety/depression has been treated with antidepressant medication, such as selective serotonin reuptake inhibitors (SSRIs), atypical antipsychotics, and monoamine oxidase inhibitors (114). Little research has addressed the impact of antidepressants on asthma (17, 112). In one clinical study of asthmatics, treatment with the antidepressant bupropion is associated with improvements in depression and lung function (15). SSRIs, which are used as antidepressants, block the reuptake of serotonin and thus regulate the function of cells that express selective serotonin reuptake receptors, including neurons, platelets, and smooth muscle cells. However, an undesired effect of SSRIs such as citalopram and fluoxetine is that they increase vasoconstriction of the pulmonary arteries and the aorta (32, 54, 117). In contrast to SSRIs that block uptake of serotonin, the antidepressant Tianeptine stimulates uptake of free serotonin by neurons and platelets, resulting in reduced free serotonin in the plasma (41, 91, 92). Because symptomatic patients with asthma can have elevated free serotonin in plasma and this free serotonin associates with asthma symptoms (71), Tianeptine has been used in clinical studies of asthma. It is reported that Tianeptine reduces free serotonin in plasma and that this associates with reduced asthma symptoms in children (69, 70, 112).
Supplementation with the serotonin precursor 5-HTP is an alternative approach for anxiety/depression and anxiety in panic disorder (12, 101), albeit perhaps 5-HTP is used less frequently than pharmacological agents. Interestingly, 5-HTP and its metabolite serotonin have been reported to have opposing outcomes on some physiological responses. For example, when administered systemically, 5-HTP decreases blood pressure, whereas serotonin can increase vasoconstriction in humans and animal models (37, 39, 81, 105, 109, 118). In another report, 5-HTP did not affect heart rate or mean arterial pressure (11). The opposing functions of 5-HTP and its metabolite serotonin on vasoconstriction may occur as an outcome of changes in local microenvironment concentrations of 5-HTP and its metabolites that would generate a change in the microenvironment balance of serotonylation and the stimulation of the multiple inhibitory serotonin receptors and the multiple stimulatory serotonin receptors (5-HTRs) that are differentially expressed by cells. Because leukocytes and endothelial cells express inhibitory and stimulatory serotonin receptors (5HTRs), recruitment of leukocytes during inflammation may be regulated by a localized balance of the 5-HTP/serotonin pathway in the microenvironment of tissues. Clinical studies with inhibitors of specific serotonin receptors such as 5-HT1A and 5-HT2A have had only marginal benefit for asthma as summarized in a review by Cazzola et al. (17). In contrast to targeting inhibition of individual serotonin receptors during allergies/asthma, we propose that a balance of serotonylation and serotonin inhibitory/stimulatory receptors may be achieved by supplementation with 5-HTP such that 5-HTP is metabolized in local microenvironments.
For these studies, we hypothesized that 5-HTP supplementation reduces allergic inflammation because 5-HTP reduces symptoms of anxiety/depression, anxiety/depression is increased with allergy/asthma, and because 5-HTP administration has regulatory functions that can be opposite to serotonin. Moreover, we focused the studies in this report on 5-HTP rather than SSRIs or individual serotonin receptors because many cells that regulate immune responses do not have selective serotonin reuptake receptors that are the target of SSRIs and because previous studies targeting individual receptors have only marginal effects on asthma (17).
We report that supplementation with 5-HTP reduced allergic inflammation induced by ovalbumin fraction V (OVA), house dust mite (HDM) or IL-4 administration. In addition, 5-HTP supplementation decreased leukocyte recruitment, at least, through inhibition of transglutaminase (TG) expression and serotonylation in endothelial cells. Because 5-HTP is also reported to reduce anxiety/depression, these data have important implications for coordinated regulation of anxiety/depression and allergic inflammation.
MATERIALS AND METHODS
Animals.
C57BL/6J mice (female, 6–8 wk old) were from Jackson Laboratory (Bar Harbor, ME) and the NJ.1638 IL-5 transgenic mice were from James J Lee (Mayo Clinic, AZ). The studies are approved by the Northwestern University Institutional Review Committee for animals.
Cells.
The murine endothelial cell line mHEVa was cultured as previously described (1, 84). Spleen cells were prepared from male BALB/c or 4-mo-old NJ1638 mice (84), and the spleen red blood cells were lysed by hypotonic shock (84). These cells from BALB/c mice were >90% lymphocytes as previously described (110). The NJ1638 spleen cells were >85% eosinophils as determined by cytospin.
5-HTP and control rodent diets.
The diet with 5-HTP (diet no. 102561) and the control diet without 5-HTP (diet no. 101591) were from Dyets; the 5-HTP (catalog no. 9772) added to the diet was from Sigma (St. Louis, MO). C57BL/6J female mice were maintained on the diets with or without 5-HTP at 0.024 g 5-HTP/kg diet (Fig. 1B). Corn oil, which was commonly used in rodent diet in the past, was used in this diet instead of the more recent formulas with soybean oil because we wanted to avoid the proinflammatory contribution of high γ-tocopherol in soybean oil as we recently reported (9, 26). Diets were started on the days indicated in Fig. 1, C–F. The diets were replaced every 3 to 4 days.
OVA, HDM, or IL-4 administration in vivo.
Many mouse strains have polymorphisms in the genes in the serotonin system, and this is thought to contribute to their survival (16, 47, 59, 83, 90, 104, 108). C57BL/6 mice were used because they are commonly used to examine regulation of the serotonin pathway in vivo (16, 47, 59, 83, 90, 104, 108). For the OVA models (Fig. 1, C and D), mice (8–10 mice/group) received intraperitoneal (i.p.) injections (200 μl) of chicken egg OVA (catalog no. 5503, Sigma) (10 μg)/alum or saline/alum on days 1 and 8 (2). OVA grade V was used for sensitization because it contains low endotoxin levels that are required for adequate OVA sensitization (40); in contrast, high levels of endotoxin suppress the OVA response (40). On days 15, 18, and 20, mice received intranasal endotoxin-free OVA fraction VI (catalog no. A2512, Sigma) (150 μg) in saline or saline alone. For the HDM (Dermatophagoides pteronyssius extract containing DerP1 was from Greer Laboratories, Lenoir, NC) model (Fig. 1E), mice (8–10 mice/group) received an intratracheal 10 μg HDM/50 μl saline three times per week for 3 wk. For the IL-4 model (Fig. 1F), mice (4 mice/group) received intratracheal administration of endotoxin-free murine IL-4 (catalog no. 200–18; Shenandoah Biotechnology, Warwick, PA) (4 μg IL-4/mouse/day) or the protein control bovine serum albumin grade VI (BSA, catalog no. A7030, Sigma) on days 1, 2, and 3. For these models, 24 h after the last treatment, tissues were collected. Blood eosinophils were stained and counted (2). Bronchoalveolar lavage (BAL) cells were counted and cytospun for differential counts (2). For platelet-containing plasma, blood was collected in EDTA tubes and centrifuged for 15 min at 1,500 g. Lungs were perfused free of blood, and right lung lobes were collected and weighed. The plasma and lungs were frozen at −80°C until analyzed for neurotransmitters by HPLC/electrochemical detector (ECD). Left lung lobes were collected and frozen in optimal cutting temperature compound for tissue sections.
OVA-induced airway responsiveness.
The mice (8–10 mice/group) were sensitized by intraperitoneal (i.p.) injection (200 μl) of OVA grade V (10 μg)/alum or saline/alum on days 1 and 8 (2). On days 10 and 12, the mice received intranasal endotoxin-free OVA fraction VI (150 μg) in saline or saline alone. On day 13, the mice were anesthetized, tracheostomized, and mechanically ventilated. Exposure to mechanical ventilation and measurements of lung mechanics were performed using a flexiVent mouse ventilator (Scireq, Montreal, Quebec, Canada) according to Scireq (113). A standard ventilation history for each mouse was obtained with two total lung capacity maneuvers before the mice were treated retro-orbitally with 500 μg OVA VI in 50 μl sterile saline to induce a response to antigen (29). The forced oscillation and quasistatic pressure volume curve protocols were used to calculate airway resistance. The data are presented as percentage of baseline lung airway resistance.
In vitro leukocyte association and migration assays in Transwells or under laminar flow.
For Transwell migration assays, spleen leukocytes (>90% lymphocytes) (110) from control and 5-HTP-supplemented mice were added overnight to TNF-α-stimulated confluent monolayers of endothelial cells grown on 8-μm pore Transwell inserts, and the number of migrated leukocytes was determined as we previously described (84). For transendothelial migration under laminar flow, the endothelial cells were stimulated overnight with TNF-α (catalog no. 300–01A; PeproTech, Rocky Hill, NJ) (5 ng TNF-α/ml) in the presence or absence of 5-HTP (catalog no. 9772, Sigma Aldrich) in PBS or the inhibitors 3-hydroxybenzylhydrazine dihydrochloride (NSD1015, catalog no. 54880, Sigma), dansylcadaverine (MDC, catalog no. 30432, Sigma), cystamine dihydrochloride (catalog no. 30050, Sigma), 1-methyl-l-tryptophan (catalog no. 447439, Sigma), and 1-methyl-d-tryptophan (catalog no. sc200313; Santa Cruz Biotechnology; Santa Cruz, CA). The treatments had no effect on cell viability as determined by Trypan blue exclusion and were not cytotoxic to the cells as determined by the Vibrant Cytotoxicity Assay Kit (catalog no. V-23111; Molecular Probes, Eugene, OR). At 18 h after treatment, the endothelial cells were washed, and spleen leukocyte transendothelial migration was examined using a parallel plate flow chamber under conditions of laminar flow of 2 dynes/cm2, as previously described (2, 31). This migration assay is dependent on endothelial cell expression of VCAM-1 and the chemokine monocyte chemoattractant protein (MCP)-1 (84, 96). Spleen leukocytes (>90% lymphocytes) (110) or NJ.1638 spleen eosinophils (>85% eosinophils) were added to the endothelial cell monolayers, and leukocyte migration was examined at 15 min by phase contrast microscopy, whereas initial spleen cell contact with the endothelial cells is determined after 2 min of laminar flow (2, 31).
HPLC measurement of amino acid metabolites.
This method measures free unconjugated serotonin. Dopamine (DA, catalog no. H60255), 5-hydroxyindoleacetic acid (5-HIAA, catalog no. H2255), homovanillic acid (HVA, catalog no. H1252), 5-hydroxytryptamine (5-HT, catalog no. H9523), and the 3,4-dihydroxybenzylamine internal standard, zinc sulfate, sodium hydroxide and the HPLC grade chemicals for HPLC mobile phase including sodium acetate, citric acid, disodium-EDTA (Na2+EDTA), dibutylamine, sodium octyl sulfate (SOS), and methanol were purchased from Sigma. Lung and plasma 5-HTP and dopamine metabolites were analyzed by HPLC according to standard methods (20). Briefly, the diet, frozen plasma (100 μl) or lung tissue (0.1 mg) were thawed and diluted with an equal amount of HPLC-grade water containing 8 ng/ml of the internal standard 3,4-dihydroxybenzylamine. To the diluted samples was added 0.2 ml of 20% ZnSO4. The samples were vortexed and then centrifuged for 10 min at 4°C. The supernatant was collected, treated with 1 μl of 10% NaOH, vortexed, and centrifuged as above. The supernatant from the second centrifugation was filtered through a 0.2-μm syringe filter, and 1–20 μl was injected into the HPLC for separation and quantification. The HPLC Breeze system (Waters, Milford, MA) was equipped with an ECD. The ECD system is comprised of a glassy carbon working electrode, an auxiliary electrode, and an Ag/AgCl reference electrode. Chromatographic separations were performed using a 3.9 × 150 mm stainless steel resolve column packed with octadecylsilane C18 on microparticulate (5 μm) spherical silica gel. The mobile phase contained sodium acetate 0.1 M, citric acid 0.1 M, SOS 0.75 mM, sodium EDTA 0.15 mM, dibutylamine 1.0 mM, and methanol 15%. The solution was adjusted to pH 4.0 and was filtered through a 0.2-mm filter. All separations were performed isocratically at a flow rate of 0.5 ml/min and at a potential of +0.60 V. Standard curves were prepared with mixtures of standard solutions containing various concentrations of 5-HTP, DA, 5-HIAA, HVA, and 5-HT. In analysis of mouse tissues, metabolite concentrations are expressed as ng/ml plasma, ng/mg tissue, or ng/g tissue (n = 8–10 mice/group)
Immunolabeling of tissue sections and cultured endothelial cells.
Lung tissue sections and cultured endothelial cell monolayers were fixed in −20°C methanol for 15 min and rehydrated with PBS for 1 h, which removes free serotonin. The samples were blocked with goat serum in PBS-0.3% BSA, incubated with rabbit antiserotonin antibodies (catalog no. 8250–0004; AbD Serotec, Dusseldorf, Germany) (2 μg Ab/200 μl PBS-0.3% BSA), rabbit anti-TG2 antibodies (catalog no. NB120–421; Novus Biologicals, Littleton, CO) (2 μg Ab/200 μl PBS-0.3% BSA), rat anti-mouse major basic protein (MBP) (kind gift from James J. Lee, Mayo Clinic) (2 μg Ab/200 μl PBS-0.3% BSA) or isotype control antibodies for 1 h at room temperature, washed, then labeled with FITC-conjugated goat anti-rabbit IgG antibodies (catalog no. 554020; BD PharMingen, San Diego, CA) (2 μg Ab/200 μl PBS-0.3% BSA) or peroxidase-conjugated goat anti-rat IgG antibodies (catalog no. 112–035-003, Jackson ImmunoResearch Laboratory, Bar Harbor, ME) (2 μg Ab/200 μl PBS-0.3% BSA), washed, and analyzed by fluorescence microscopy with Slidebook software. Data are presented as the sum of the pixel intensities per μm2 of vessel endothelium, vessel lumen, airway epithelium, or lung tissue. Data for all cell types within three sections from each mouse were collected and averaged to obtain an average for each mouse. Then, the data in the graphs are the means ± SE for 8 mice/group. Immunohistochemistry for MBP was done as previously reported (2).
Cytokines, chemokines, and adhesion molecules.
The BAL supernatants were tested for protein levels of cytokines using the Luminex 20plex or 6plex cytokine kits (catalog no. LMC0006 or LMC0002; Invitrogen, Carlsbad, CA). The expression of those cytokines and chemokines that are not in the Luminex kit were examined by qPCR. Briefly, total RNA was isolated from 10–15 mg of lung tissue or 1 million endothelial cells using the Qiagen RNeasy Mini Kit. cDNA was prepared from 500 ng of mRNA/reaction using qScript cDNA Synthesis Kit (catalog no. 95047–100, Quanta BioSciences, Gaithersburg, MD) and analyzed by real-time PCR on an 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) using Taqman probes and Taqman Universal Master Mix (Applied Biosystems) or using PrimeTime probes and PrimeTime qPCR assays (Integrated DNA Technologies). VCAM-1 and ICAM-1 expression by mHEVa cells was also examined by immunolabeling with antibodies from BD PharMingen (FITC-conjugated hamster anti-mouse ICAM-1, catalog no. 01544D; rat anti-mouse VCAM-1 clone MVCAMa, catalog no. 550547; FITC-conjugated goat anti-rat IgG antibodies, catalog no. 554020; FITC-conjugated control antibody, catalog no. 554679; and FITC-conjugated goat anti-rat IgG, catalog no. 554016). Analysis was by flow cytometry.
In vitro chemotaxis and chemokinesis assays.
Spleen leukocytes isolated from mice supplemented with 5-HTP after OVA sensitization and before OVA challenge (Fig. 1D) were used in Transwell chemotaxis assays with eotaxin (CCL11). Both lymphocytes and eosinophils express the CCL11 receptor CCR3 and respond to CCL11. For chemotaxis, 1 × 106 cells/100 μl were added to the upper chamber of 5.0-μm pore Transwell inserts (Corning COSTAR no. 3421; Corning, NY). The lower chamber contained 600 μl of media with 0.05 ng/μl recombinant murine CCL11 (PeproTech no. 250–01). After 3 or 6 h at 37°C, cells in the lower chamber were counted using a hemocytometer. For chemokinesis, 0.05 ng/μl recombinant murine CCL11 was added to the upper well of the Transwells (n = 6 animals/group).
Statistics.
Data were analyzed by a one-way ANOVA followed by Tukey's or Dunnett's multiple-comparisons test (SigmaStat; Jandel Scientific, San Ramon, CA). P < 0.05 is considered significant. Presented are the means ± SE.
RESULTS
5-HTP reduces lung inflammation in two models of allergy/asthma.
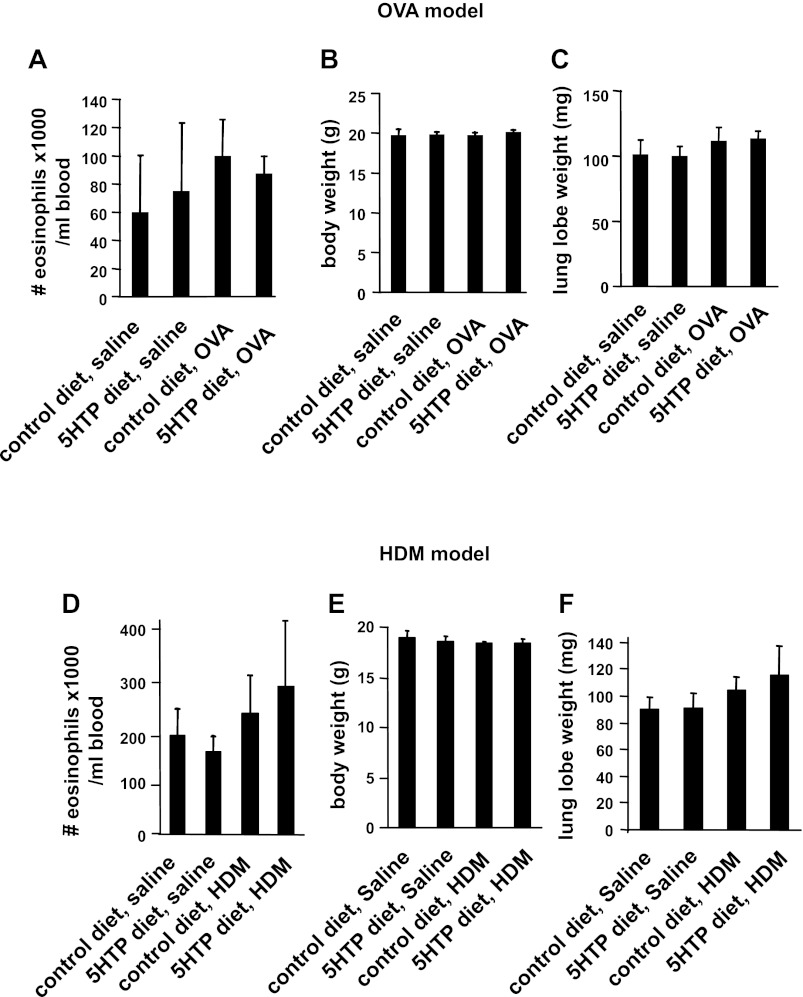
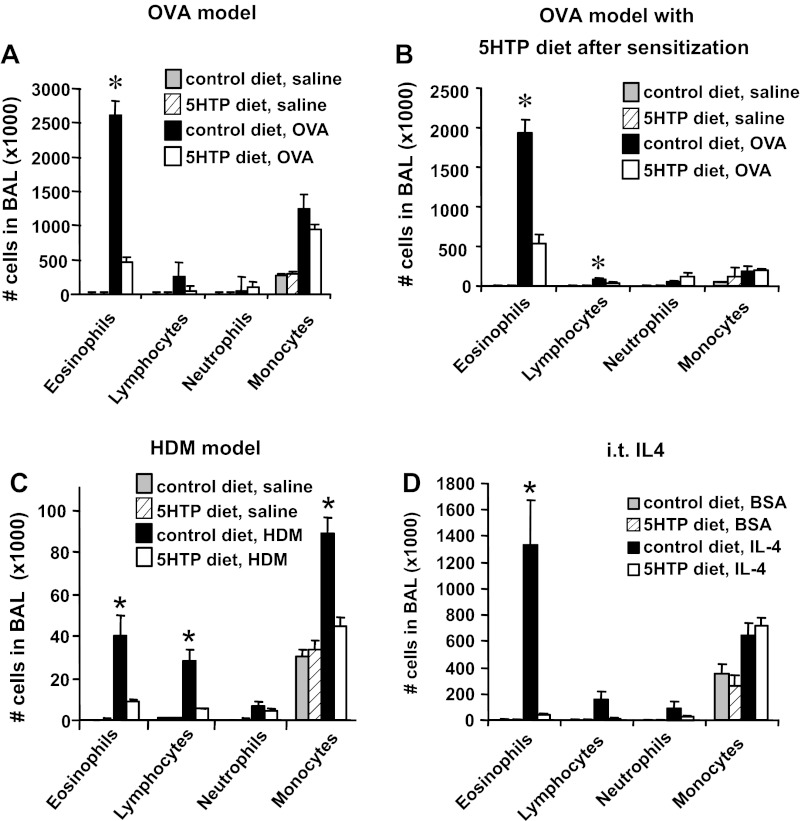
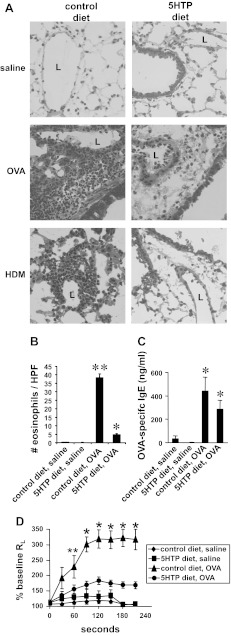
We determined whether dietary supplementation with 5-HTP reduces inflammation in mice challenged with OVA (Fig. 1C) or HDM extract (Fig. 1E). We also determined whether inflammation was reduced by supplementation with 5-HTP during the OVA challenge phase (after OVA sensitization) (Fig. 1D). The level of 5-HTP in the rodent diets (Fig. 1B) was calculated to be equivalent to that for human consumption of 200 mg 5-HTP/100 pound person/day because clinical studies with 5-HTP supplementation use 100–300 mg 5-HTP/person/day (12, 45, 50, 60, 76, 101, 103). We confirmed the 5-HTP concentration in the diet by HPLC/ECD (Fig. 1B). The 5-HTP in this diet is about fivefold lower than the 5-HTP concentrations reported to increase systemic serotonin, increases serotonin-mediated intestinal peristalsis, and induces diarrhea in mice (8, 14, 19, 58, 99). 5-HTP supplementation as in Fig. 1, C and E, did not affect the number of blood eosinophils, body weight, or lung lobe weight of the mice (Fig. 2). 5-HTP supplementation reduced the number of eosinophils in the BAL of OVA-challenged mice and reduced the number of eosinophils, lymphocytes, and monocytes of HDM-challenged mice (Fig. 3, A–C). 5-HTP supplementation also reduced OVA- and HDM-induced lung tissue inflammation (Fig. 4A), OVA-induced lung tissue eosinophils (Fig. 4B), HDM-induced lung tissue eosinophils (data not shown), and OVA-induced lung responsiveness (Fig. 4D), but not OVA-specific IgE levels (Fig. 4C). Because 5-HTP did not alter antigen sensitization (OVA-specific IgE), we determined whether 5-HTP reduced leukocyte recruitment when antigen presentation was bypassed by administration of intratracheal IL-4. 5-HTP reduced IL-4-induced recruitment of BAL eosinophils (Fig. 3D). Thus 5-HTP supplementation reduced allergic inflammation and allergen-induced lung hyperresponsiveness without altering body weight, OVA-specific IgE, or blood eosinophils.
Fig. 2.
5-HTP supplementation did not alter blood eosinophils, body weight, or lung weight. Mice were supplemented with 5-HTP and received the antigens OVA (A–C) or HDM (D–F) as in the timeline Fig. 1, C and E. The mice were weighed, and blood eosinophils were counted. A and D: blood eosinophils. B and E: mouse body weight. C and F: mouse lung lobe weight. n = 8–10 mice/group. The groups are not statistically different.
Fig. 3.
5-HTP supplementation reduced the number of leukocytes in the BAL. A: mice were supplemented with 5-HTP and received OVA as in the timeline Fig. 1C. n = 8–10 mice/group. B: mice were supplemented with 5-HTP after OVA sensitization as in the timeline in Fig. 1D. n = 6–8 mice/group. C: mice were supplemented with 5-HTP and received HDM as in the timeline Fig. 1E. n = 8–10 mice/group. D: mice received intratracheal (i.t.) administration of 4 μg IL-4/mouse/day or the protein control BSA grade VI as in the timeline in Fig. 1F. n = 6 mice/group. The BAL cells were collected and cytospun and then neutrophils, eosinophils, monocytes, and lymphocytes were counted by standard morphological criteria. *P < 0.05 compared with the other groups.
Fig. 4.
5-HTP supplementation reduced allergen-induced lung tissue inflammation and lung responsiveness. A: frozen lung tissue sections from the mice in Fig. 3, A and C, were stained with hematoxylin and eosin. Shown are representative micrographs of perivascular regions in lung tissue. n = 8–10 mice/group. L, vessel lumen. B: number of antimajor basic protein (MBP)-labeled lung perivascular eosinophils per high-powered field (HPF) from mice in Fig. 3A. C: 5-HTP diets did not alter plasma OVA-specific IgE from mice in Fig. 3A as measured by ELISA. n = 8–10 mice/group. D: to examine OVA-induced lung resistance, OVA-treated mice were retro-orbitally challenged with 500 μg OVA VI in 50 μl saline or saline alone (29). Exposure to mechanical ventilation and measurements of lung mechanics were performed using a flexiVent mouse ventilator. Presented are the percentages of baseline lung resistance (RL). **P < 0.05 compared with saline groups. *P < 0.05 compared with all other groups.
5-HTP supplementation does not alter expression of several cytokines, chemokines, or adhesion molecules.
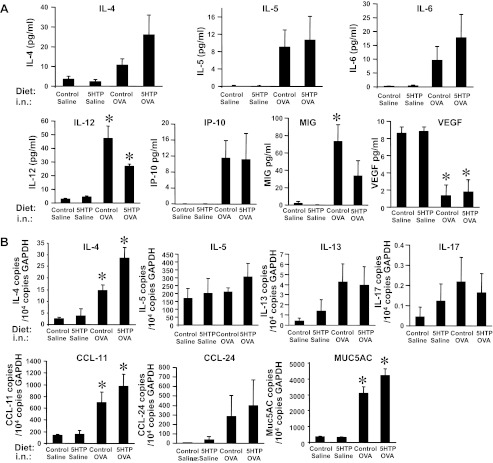
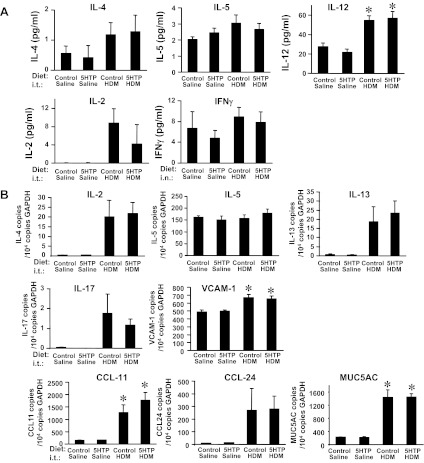
We determined whether 5-HTP supplementation alters lung cytokines, chemokines, or endothelial cell adhesion molecules that regulate allergic inflammation. We measured mRNA expression of cytokines, chemokines, and adhesion molecules in whole lung tissue and examined cytokines and chemokines in BAL. MUC5AC was examined because it is a quantitative measure of goblet cell metaplasia. For the mice from the OVA model and HDM model (Fig. 3, A and C), 5-HTP supplementation of OVA-treated mice did not change BAL or lung tissue protein expression (Figs. 5A and 6A) or mRNA expression (Figs. 5B and 6B) for the cytokines IL-4, IL-5, IL-6, IL-12, IL-13, and IL-17, the chemokines CCL11, CCL24, MIG, and IP-10, or the adhesion molecule VCAM-1 compared with OVA-treated mice on control diet. Consistent with no change in IL-13 mRNA in the lung tissue, there was no change in MUC5AC mRNA. 5-HTP supplementation after OVA sensitization (mice in Fig. 3B) also did not alter lung tissue cytokines (data not shown). The 5-HTP supplementation did not induce a switch of the Th2 cell response to a Th1 response because there was no effect of 5-HTP diets on expression of the Th1 cytokines IFNγ and IL-2 (Fig. 6A and data not shown for OVA model). The antigen-treated lungs and BAL had no significant induction of IL-1α, IL-1β, IL-2, IL-10, KC, MCP-1, macrophage inflammatory protein-1α, TNF-α, granulocyte-macrophage colony-stimulating factor, and fibroblast growth factor basic; this was not altered by 5-HTP diets (data not shown).
Fig. 5.
For OVA-challenged mice, 5-HTP supplementation did not alter expression of lung cytokines, chemokines, growth factors, or mucin. The lungs and BAL were collected from the OVA-challenged mice in Fig. 3A. A: BAL supernatants from lungs in Fig. 3A were tested for levels of cytokines using the Luminex 20plex cytokine kit. Not detected were IFN-γ, IL-1α, IL-1β, IL-2, KC, monocyte chemoattractant protein (MCP)-1, and TNF-α; IL-17 was very low (data not shown). B: lung tissue from mice in Fig. 3A was placed in RNAlater and then examined by quantitative PCR for IL-4, IL-5, IL-13, IL-17, CCL11, CCL24, and MUC5AC expression. n = 8–10 mice/group. *P < 0.05 compared with the saline groups. There is not a significant effect of 5-HTP supplementation of OVA-treated mice compared with control diet OVA-treated mice.
Fig. 6.
For the HDM-challenged mice, 5-HTP supplementation did not alter lung cytokines, chemokines, mucin, or adhesion molecules. The lungs and BAL were collected from the HDM-challenged mice in Fig. 3C. A: BAL supernatants were tested for levels of cytokines using the Luminex 6plex cytokine kit. B: lung tissue was placed in RNAlater and then examined by quantitative PCR for IL-4, IL-5, IL-13, IL-17, VCAM-1, CCL11, CCL24, and MUC5AC. n = 8–10 mice/group. *P < 0.05 compared with the saline groups. There is not a significant effect of 5-HTP supplementation of HDM-treated mice compared with control diet HDM-treated mice.
5-HTP diets did not alter total tissue levels of 5-HTP and its metabolites.
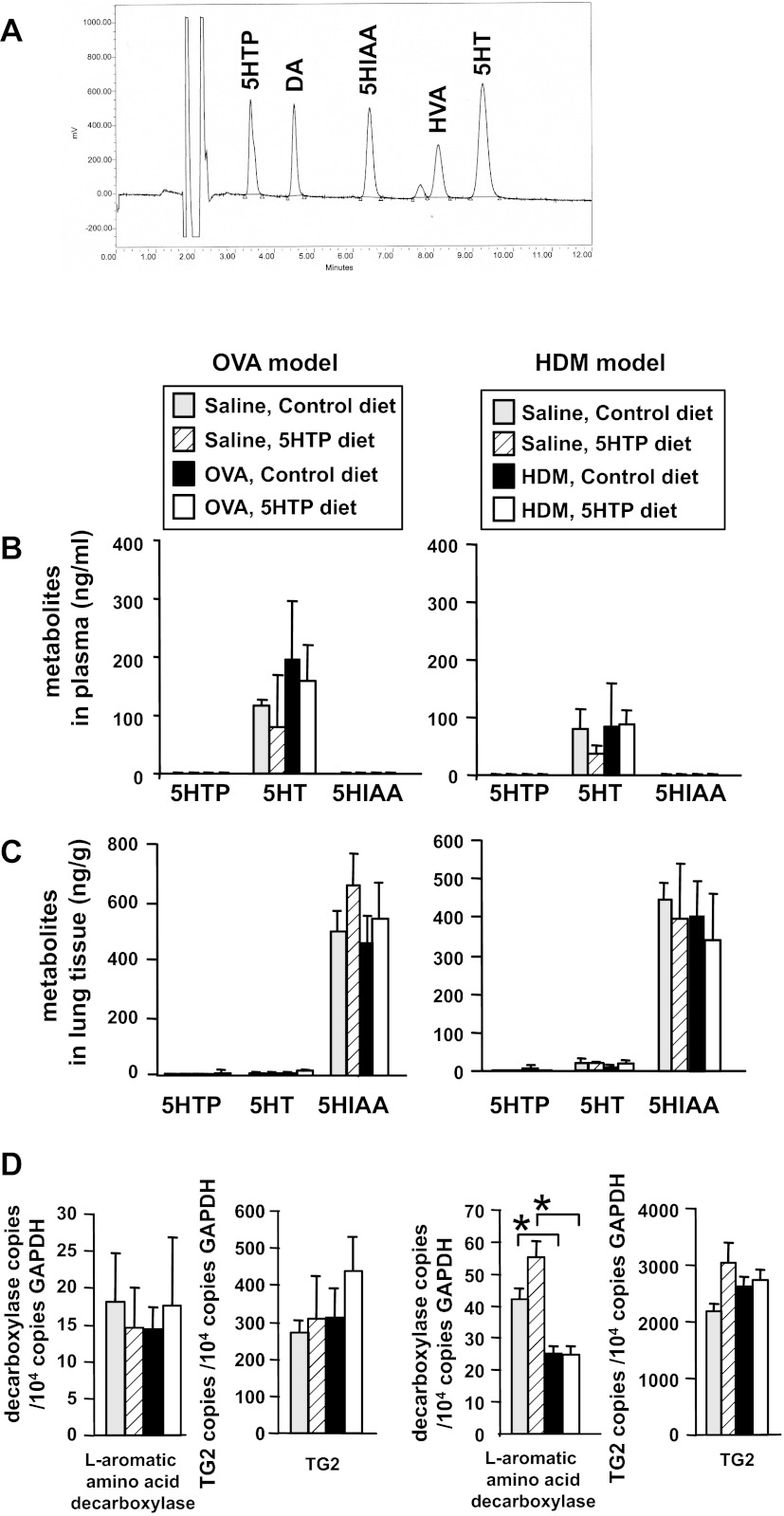
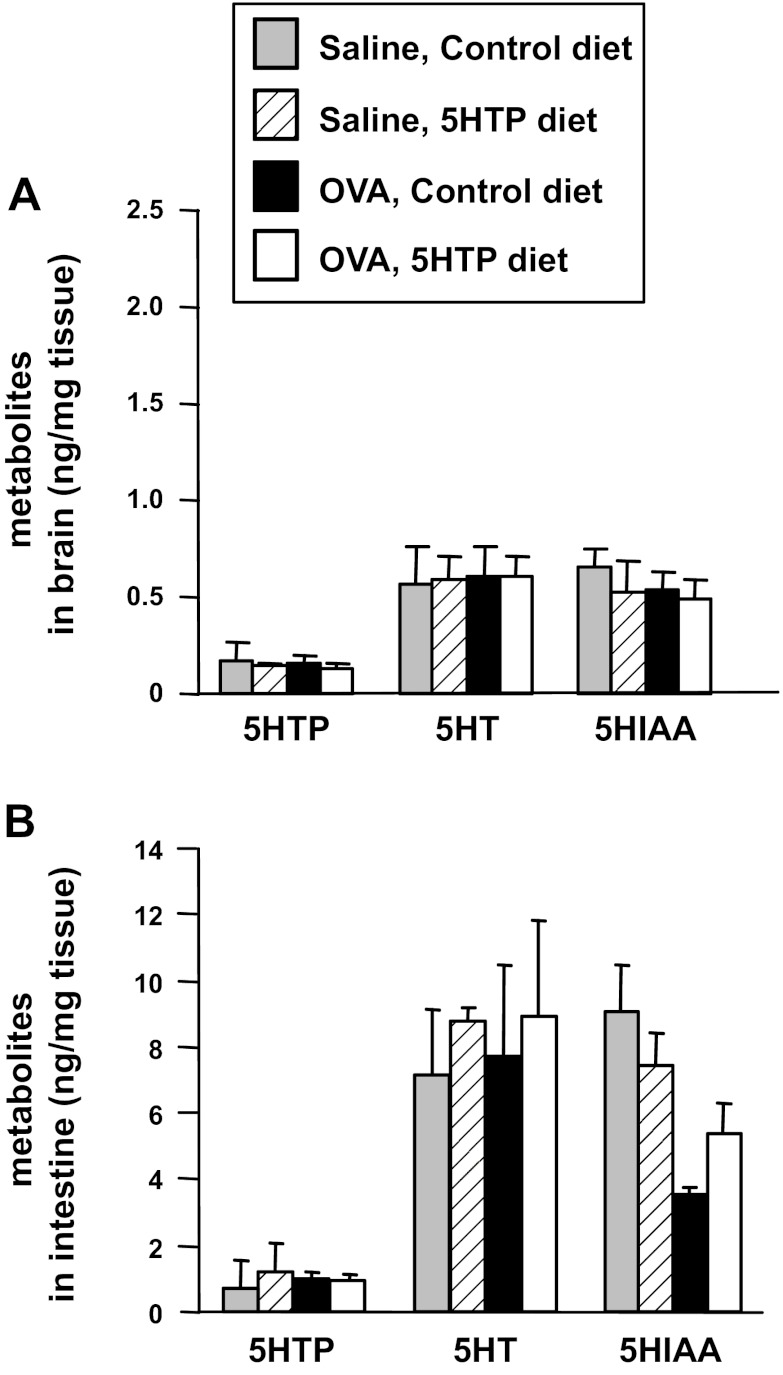
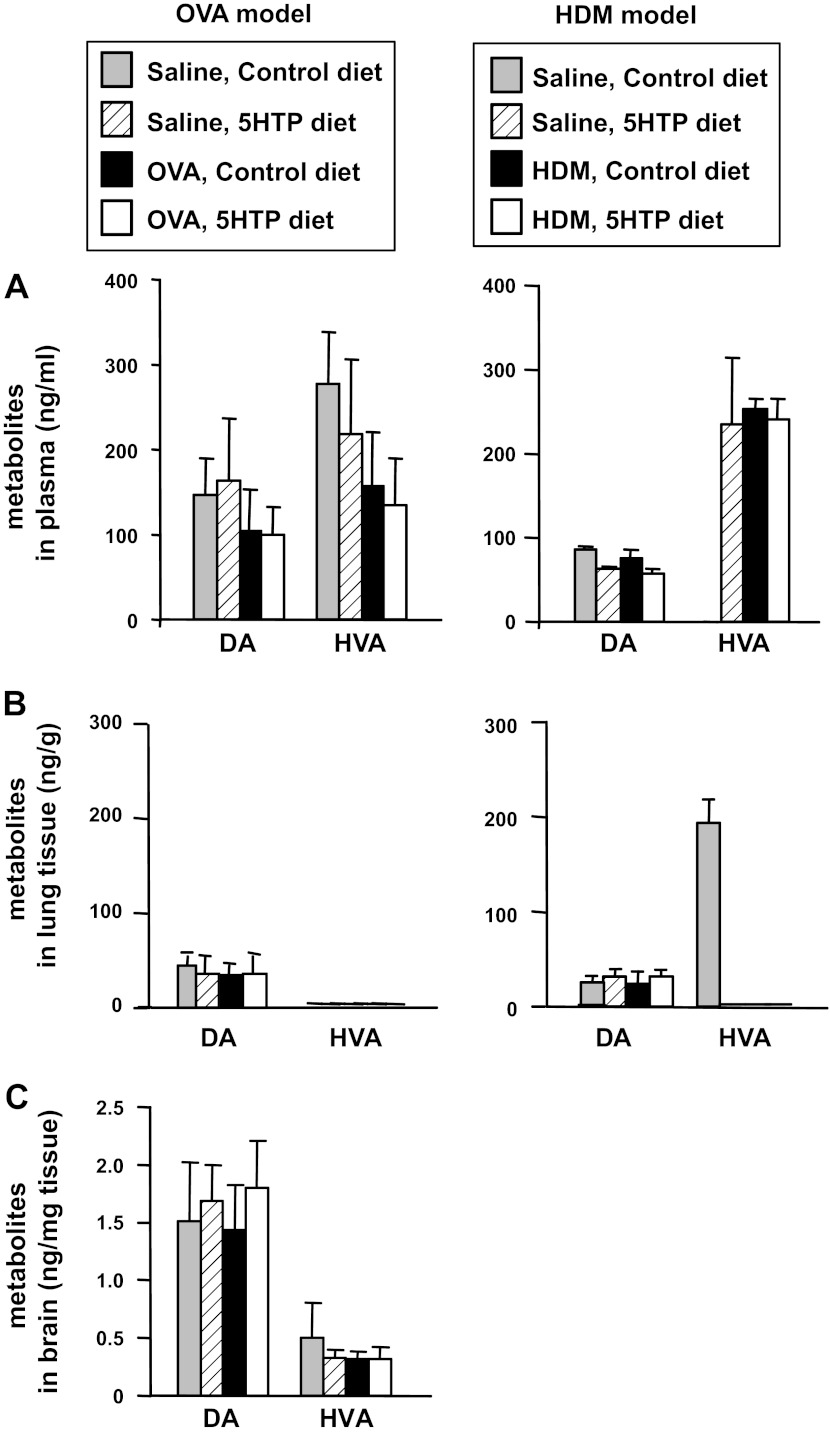
It is reported that low levels of 5-HTP supplementation (≤10 mg 5-HTP/kg body wt/day) does not alter 5-HTP metabolites in blood and tissues (8, 58). Consistent with this, 5-HTP supplementation for mice in Fig. 3, A and C, at the low levels used in our studies (about 5 mg 5-HTP/kg body wt/day), did not alter systemic levels of 5-HTP, free unconjugated serotonin, or the metabolite of serotonin HIAA as determined by HPLC/ECD analysis of lung, plasma, intestine, or brain when tissues were collected midday (Figs. 7 and 8). Mice are nocturnal, and it is reported that plasma serotonin levels are greatest within a couple of hours of feeding (5, 55). Therefore, we also examined 5-HTP metabolites in lung, plasma, intestine, and brain at 2 h after feeding the diets because this is the time of maximal increases in 5-HTP metabolites as determined in a time course in our studies (data not shown) and in a previous report with oral gavage of 5-HTP (77). At 2 h after feeding, there was a fourfold increase in 5-HTP and its metabolites compared with values in Figs. 8 and 9, but there were no differences among the treatment groups for these tissues (data not shown).
Fig. 7.
5-HTP supplementation did not alter 5-HTP or its metabolites in plasma or perfused whole lung tissue. A: HPLC/ECD profile of standards demonstrating separation of 5-HTP and its metabolites serotonin (5-HT) and 5-hydroxyindoleacetic acid (HIAA) as well as dopamine (DA) and its metabolite homovanillic acid (HVA). The solvent peaks come off the column before the metabolites (unlabeled peaks). Peaks are labeled with the metabolite. From mice in Fig. 3, A and C, plasma (B) and lungs (C), which had been perfused free of blood, were examined for 5-HTP, 5-HT, and HIAA by HPLC with electrochemical detection. D: lung tissue was placed in RNAlater and then examined by quantitative PCR for l-aromatic amino acid decarboxylase and transglutaminase (TG)2 in total lung tissue. n = 8–10 mice/group. *P < 0.05 for the indicated groups.
Fig. 8.
5-HTP supplementation did not alter 5-HTP or its metabolites in brain or intestine. Brain and intestines were collected from OVA-challenged mice from Fig. 3A and frozen. Brains (A) and intestines (B) were examined for 5-HTP, 5-HT, and HIAA by HPLC/ECD. n = 8–10 mice/group. There is not a significant difference among the treatment groups.
Fig. 9.
5-HTP supplementation did not alter dopamine or its metabolite HVA in plasma, perfused whole lung tissue, or brain. From mice in Fig. 3, A and C, plasma (A), lungs that had been perfused free of blood (B), and brains (C) were examined for DA and HVA by HPLC/ECD. DA and HVA were not detected in intestines (data not shown). n = 8–10 mice/group. There is not a significant difference among the treatment groups.
It is reported that high levels of 5-HTP reduce serum dopamine and that serotonin counterregulates dopamine (35, 43). It is also reported that dopamine regulates Th17 and mast cell responses (67, 68, 89). Therefore, we determined whether low-level 5-HTP supplementation reduced dopamine concentrations in the mice from Fig. 3, A and C. The 5-HTP supplementation did not alter dopamine or its metabolite HVA as determined by HPLC/ECD analysis of lung, plasma, or brain (Fig. 9). Therefore, the low level of 5-HTP supplementation in these studies did not alter systemic serotonin or dopamine and their metabolites.
5-HTP diets reduced endothelial cell TG2 expression and serotonylation in the lung.
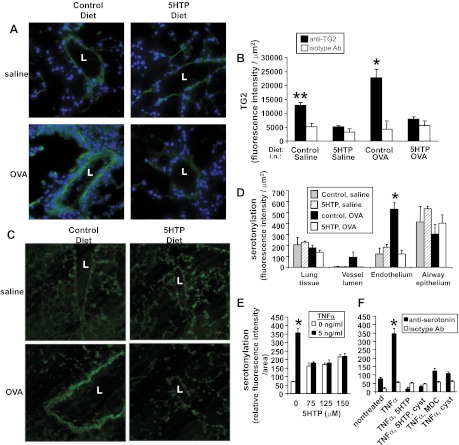
TG is increased in allergic disease, TG catalyzes localized serotonin transamidation of glutamines (serotonylation), and serotonylation regulates cell functions (30, 65, 75, 93, 119). Therefore, we determined whether 5-HTP diets altered localized TG2 expression and serotonylation by immunolabeling lung tissue sections from mice in Fig. 3A with anti-TG2 antibodies and antiserotonin antibodies, respectively. Lung endothelial cell TG2 protein expression was increased after OVA challenge (Fig. 10, A and B). The endothelial TG2 expression in saline and OVA-treated mice was reduced with 5-HTP (Fig. 10, A and B), suggesting a negative feedback regulation of TG2 expression with 5-HTP supplementation. In contrast, total lung expression of TG2 and decarboxylase were not altered (Fig. 7D). To examine serotonylation, lung tissue sections from the OVA model (Fig. 3A) were fixed, which removes free serotonin, and labeled with antiserotonin antibodies to detect bound serotonin (serotonylation). OVA induced a significant increase in lung endothelial cell serotonylation (Fig. 10, C and D), and this was completely blocked in the group with 5-HTP supplementation (Fig. 10, C and D). The airway epithelium was serotonylated, but this was not regulated by OVA challenge or by 5-HTP supplementation (Fig. 10D).
Fig. 10.
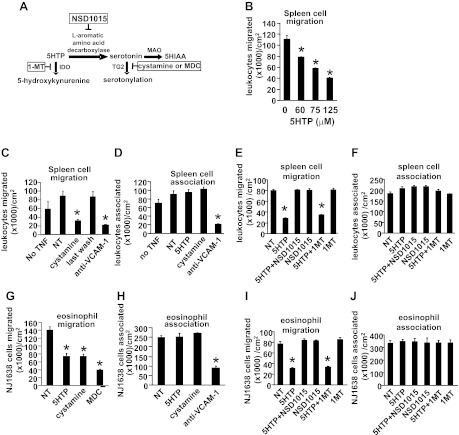
5-HTP supplementation reduced OVA-induced lung vascular TG2 expression and serotonylation in vivo and reduced TNF-α-stimulated endothelial cell serotonylation in vitro. A–D: tissue sections from perfused lungs of mice from Fig. 3A were labeled with anti-TG2 or antiserotonin antibodies and examined by fluorescence microscopy. Labeling with isotype control antibodies was negative such that the fluorescence was lower than the intensity in the vessel lumen of the saline control groups (data not shown). A: anti-TG2 immunofluorescence labeling (green) and DAPI (blue). Representative micrographs with vessels. B: fluorescence intensity of TG2/μm2 of vessel endothelium. C: antiserotonin immunofluorescence labeling. Representative micrographs with vessels. D: fluorescence intensity of serotonylation/μm2 of vessel endothelium, vessel lumen, airway epithelium, or lung tissue. B and D: for each cell type, data from 3 sections per mouse were collected and averaged to obtain an average for each mouse. Then the data in the graphs are from the average ± the SE of 8 mice/group. E–F: in vitro, monolayers of endothelial cells were stimulated with TNF-α in the presence or absence of 125 μM 5-HTP, and/or the TG inhibitors cystamine (Cyst, 100 μM) or dansylcadaverine (MDC, 200 μM). n = 3–5 for in vitro serotonylation. The inhibitors did not affect cell viability (shown in Fig. 14). L, vessel lumen. *P < 0.05 compared with the other treatment groups. **P < 0.05 compared with the other treatment groups.
5-HTP reduced endothelial serotonylation in vitro.
Because 5-HTP regulated serotonylation in vivo in lung endothelium, we also examined 5-HTP regulation of endothelial cell serotonylation in vitro. In vitro TNF-α treatment of endothelial cells increased endothelial cell serotonylation, and this serotonylation was blocked by treatment with 5-HTP or the TG inhibitors cystamine or dansylcadaverine (MDC) (Fig. 10, E and F); the treatments were not cytotoxic to the endothelial cells as determined by the G6PDH release assay (Fig. 14). Thus 5-HTP reduces endothelial cell serotonylation in vivo and in vitro.
Fig. 14.
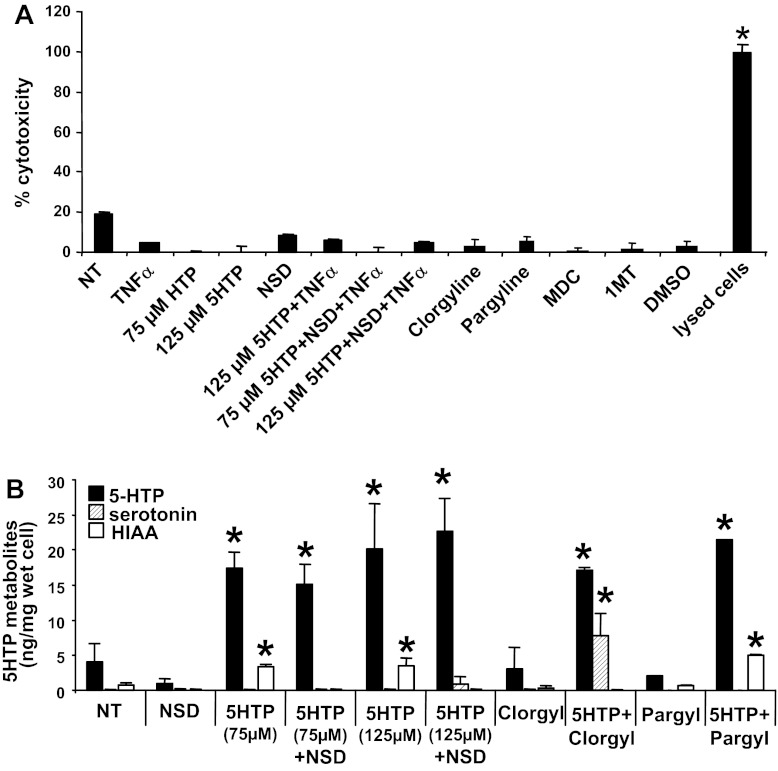
Endothelial cell metabolism of 5-HTP to serotonin or HIAA was blocked by the inhibitors without inducing endothelial cell cytotoxicity. A: inhibitors in Figs. 10, 12, and 13 did not induce cell cytotoxicity. The endothelial cell monolayers were treated for 15 min with the following inhibitors used in Figs. 10, 12, and 13: 5-HTP (75 or 125 μM), the aromatic amino acid decarboxylase inhibitor NSD1015 (NSD, 30 μM), the monoamine oxygenase inhibitors clorgyline (Clorgyl, 1 μM) or pargyline (Pargyl, 100 μM), the TG inhibitor dansylcadaverine (MDC, 100 μM), the IDO inhibitor 1-MT (100 μM 1-l-methyl-tryptophan + 100 μM 1-d-methyl-tryptophan), or the solvent control 0.01% DMSO. Then the cells were stimulated with 5 ng/ml TNF-α. After overnight culture, the endothelial cells were examined for cell cytotoxicity with the Vybrant Cytotoxicity assay. A set of cells were lysed as a positive control for cytotoxicity. B: endothelial cells were pretreated with NSD1015 (NSD, 30 μM), 5-HTP (75 or 125 μM), clorgyline (1 μM), or pargyline (Pargyl, 100 μM) where indicated and then stimulated with 5 ng/ml TNF-α. After overnight culture, the endothelial cells were examined for 5-HTP, serotonin (5-HT), and HIAA by HPLC/ECD. n = 3. *P < 0.05 indicates a significant increase compared with the nontreated (NT) group.
5-HTP supplementation in vivo does not alter leukocyte expression of chemokines or adhesion molecules or leukocyte chemotaxis, chemokinesis, or transendothelial migration.
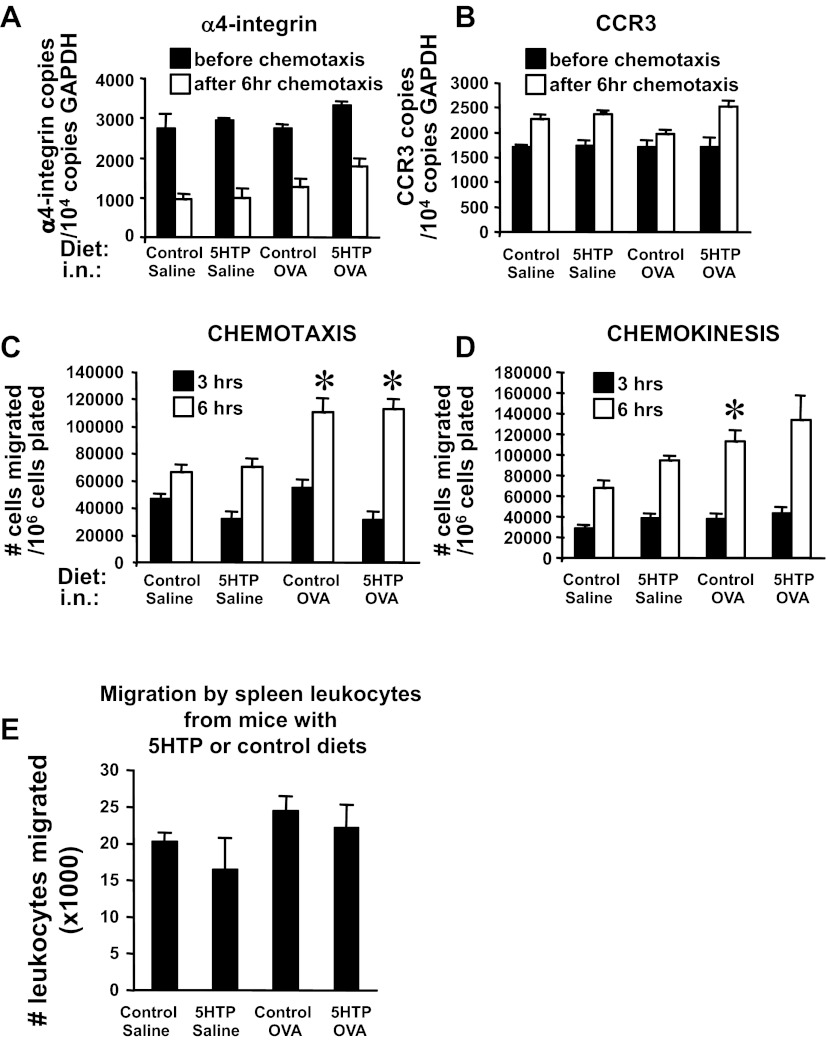
Because 5-HTP reduced leukocyte recruitment in vivo, we examined whether pretreatment of leukocytes or endothelial cells with 5-HTP regulates leukocyte transendothelial migration, which is regulated by chemokine receptors and adhesion molecules. Leukocytes from the 5-HTP-and OVA-treated mice in Fig. 3B were used to examine adhesion molecule expression, chemokine receptor expression, and transendothelial migration across a monolayer of endothelial cells in vitro. Leukocytes from 5-HTP-supplemented mice did not have altered mRNA expression of the chemokine receptor CCR3 or the VCAM-1 ligand α4-integrin (Fig. 11, A and B). As in previous reports (107), chemotaxis to eotaxin (CCL11) induced a decrease in mRNA expression of α4-integrin after 6 h (Fig. 11A). Leukocytes from 5-HTP-supplemented mice did have not altered leukocyte chemotaxis or leukocyte chemokinesis in a 6-h response to CCL11 in vitro (Fig. 11, C and D). In addition, spleen leukocytes from 5-HTP-supplemented mice did not have altered transmigration across microvascular endothelial cells in vitro (Fig. 11E). Therefore, leukocytes from 5-HTP-supplemented mice did not have altered adhesion molecule expression, chemokine receptor expression, chemotaxis, chemokinesis, or transendothelial migration in vitro.
Fig. 11.
For OVA-challenged mice, 5-HTP supplementation of leukocytes did not alter expression of leukocyte α4-integrin, the chemokine receptor CCR3, chemotaxis, chemokinesis, or transendothelial migration. A and B: spleen leukocytes from mice in Fig. 3B were placed in RNAlater before and after the chemotaxis in C. These cells were examined by quantitative PCR for α4-integrin (A) and CCR3 expression (B). C and D: chemotaxis and chemokinesis of spleen cells isolated from mice in Fig. 3B. The assays were performed with 50 ng CCL-11/ml for 3 or 6 h. E: transendothelial migration by spleen leukocytes from mice supplemented with 5-HTP as in Fig. 3B. Leukocytes were added to confluent monolayers of endothelial cells in 8-μm-pore Transwells overnight. The migrated leukocytes in the bottom chamber were counted with a hemocytometer. i.n., intranasal. n = 8–10 mice/group. *P < 0.05 compared with the saline groups. There is not a significant effect of 5-HTP supplementation of OVA-treated mice compared with control diet OVA-treated mice.
5-HTP pretreatment of endothelial cells blocks endothelial cell TG2 support of leukocyte transendothelial migration in vitro.
Endothelial cells express TG2, enzymes for synthesis of serotonin, and enzymes for metabolism of serotonin (33, 62, 78–80). Endothelial cells also regulate the migration of leukocytes into the tissue (25). To examine 5-HTP regulation of endothelial function during leukocyte migration, endothelial cells (1, 31) were treated with 5-HTP at doses reported to regulate other functions of endothelial cells and other cell types in vitro (51, 123). The endothelial cells were pretreated overnight with TNF-α (5 ng/ml) and with 5-HTP (0–125 μM) or with the TG2 inhibitors cystamine or dansylcadaverine (Fig. 12A), washed, and placed in the leukocyte transendothelial migration assay under laminar flow at 2 dynes/cm2 (1, 31). In this assay, leukocytes migrate on VCAM-1 and respond to the endothelial cell-derived chemokine MCP-1 (84, 96). Leukocyte and eosinophil transendothelial migration was blocked by pretreatment of TNF-α-stimulated endothelial cells with 5-HTP, cystamine, or dansylcadaverine (Fig. 12, B, C, and G), suggesting that endothelial cell transglutamination is important for migration. There was no effect on leukocyte or eosinophil association with endothelial cells (Fig. 12, D and H), VCAM-1 expression (Fig. 13, A and C), MCP-1 expression (Fig. 13B), endothelial cell viability (data not shown), or cell cytotoxicity as determined by the G6PDH release assay (Fig. 14A). To determine whether 5-HTP metabolism to serotonin in endothelial cells is required for leukocyte transendothelial migration, endothelial cells were pretreated with the aromatic amino acid decarboxylase inhibitor NSD1015 (Fig. 12A). 5-HTP inhibition of leukocyte and eosinophil transendothelial migration was blocked by NSD1015 (Fig. 12, E and I), whereas NSD1015 did not alter leukocyte or eosinophil association with the endothelial cells (Fig. 12, F and J). NSD1015 was not cytotoxic (Fig. 14A).
Fig. 12.
5-HTP pretreatment of endothelial cells in vitro reduced leukocyte transendothelial migration. A: diagram of 5-HTP metabolism to HIAA or serotonylation. Also indicated is the potential 5-HTP metabolism to 5-hydroxykynurenine; it is reported that 5-HTP application to intestines ex vivo generates 5-hydroxykynurenine (46). In boxes are the inhibitors. B–J: endothelial cell monolayers were pretreated with 5 ng/ml TNF-α overnight except where indicated as “no TNF”. TNF-α-stimulated endothelial cell monolayers on slide flasks were pretreated overnight as indicated with 5-HTP (75–125 μM), the TG2 inhibitors cystamine (100 μM) and dansylcadaverine (MDC, 200 μM), the aromatic amino acid decarboxylase inhibitor NSD1015 (30 μM), or the indolamine dioxygenase (IDO) inhibitor 1-MT (100 μM 1-l-methyl-tryptophan + 100 μM 1-d-methyl-tryptophan) and then washed before addition of BALB/c mouse spleen leukocytes (>90% lymphocytes) (B–F) or NJ1638 mouse eosinophils (>85% eosinophils) (G–J) for the migration and adhesion assays in a parallel plate flow chamber. The above inhibitors did not affect endothelial cell viability (data not shown) or cell cytotoxicity (Fig. 14A). Where indicated, anti-VCAM-1 antibodies (54 μg Ab/800 μl/slide flask) were added to the endothelial cells 15 min before addition of leukocytes. B–J: comparisons can only be made within an experiment because total migration can vary somewhat among experiments. In C, the “last wash” designates cells that were treated with the 5th wash from cystamine-pretreated endothelial cells, indicating that the cells were sufficiently washed to remove effective concentrations of the inhibitor. NT, nontreated. MAO, monoamine oxygenase. n = 3–5. *P < 0.05 compared with nontreated controls.
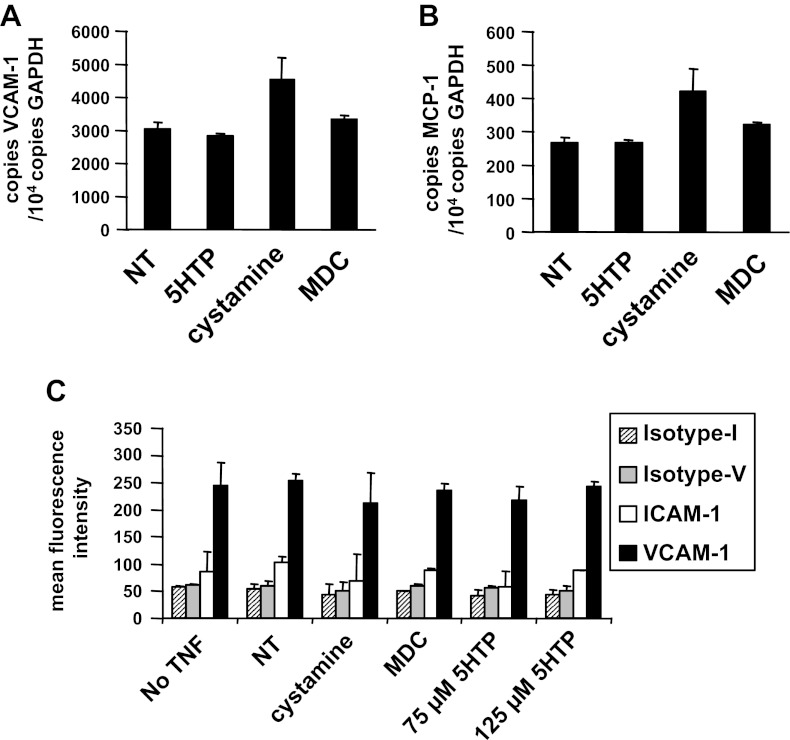
Fig. 13.
5-HTP pretreatment of endothelial cells did not alter endothelial cell expression of VCAM-1 or MCP-1. A and B: TNF-α-treated endothelial cells were incubated overnight with 125 μM 5-HTP, 100 μM cystamine, or 200 μM MDC as in Fig. 12, and the cells were placed in RNAlater; the samples were then examined by quantitative PCR for expression of the adhesion molecule VCAM-1 (A) or the chemokine MCP-1 (B). C: endothelial cells were treated as indicated, immunolabeled with anti-ICAM-1 or an isotype antibody control for the ICAM-1 antibody (isotype-I) or immunolabeled with anti-VCAM-1 or an isotype antibody control for the VCAM-1 antibody (isotype-V), and examined by flow cytometry. The endothelial cell lines expressed VCAM-1 but not ICAM-1 as previously reported (24, 25). n = 2 experiments.
There is one report that suggests that 5-HTP may be metabolized to 5-hydroxykynurenine; intestines treated ex vivo with 5-HTP generate 5-hydroxykynurenine (46) (Fig. 12A). Therefore, we determined whether 5-HTP inhibition of leukocyte transendothelial migration is blocked by treatment with the indoleamine 2,3-dioxygenase inhibitor 1-methyl-DL-tryptophan (1MT). 1MT did not alter 5-HTP-induced inhibition of leukocyte or eosinophil transendothelial migration (Fig. 12, E and I). 1MT also did not alter leukocyte or eosinophil association with the endothelial cells (Fig. 12, F and J).
The 5-HTP-treated endothelial cells were examined by HPLC with electrochemical detection as in Fig. 7A. Fresh culture medium with 20% heat-inactivated fetal calf serum did not contain detectable levels of 5-HTP, serotonin, or HIAA as determined by HPLC/ECD (data not shown). 5-HTP pretreatment of endothelial cells yielded an increase in 5-HTP and the metabolite HIAA (Fig. 14B). The l-aromatic amino acid decarboxylase inhibitor NSD1015 (6, 34, 52, 120) was used to determine whether it blocked catabolism to HIAA. The increase in HIAA in the 5-HTP-treated cells was blocked by NSD1015 (Fig. 14B) without inducing cell cytotoxicity (Fig. 14A). This suggests that the endothelial cells metabolized 5-HTP to serotonin and then HIAA. Although NSD1015 also blocks l-dihydroxyphenylalanine conversion to dopamine, we did not observe detectable levels of dopamine or its metabolite HVA in the endothelial cell lines with or without 5-HTP treatment (data not shown). Free serotonin was not detected in the 5-HTP-treated endothelial cells or in the culture supernatants from the endothelial cells as determined by HPLC/ECD (Fig. 14B), but serotonylation in the endothelial cells was detected (Fig. 10, E and F), indicating that serotonin is generated, used for serotonylation, as well as rapidly metabolized to HIAA. To detect 5-HTP catabolism to serotonin, we blocked serotonin metabolism to HIAA by using the inhibitor clorgyline to block monoamine oxygenase-A (MAO-A) and pargyline to block monoamine oxygenase-B (MAO-B) during 5-HTP treatment. MAO-A is expressed in peripheral cells including endothelial cells. In contrast, MAO-B is expressed by neurons (38) but not the endothelial cells (data not shown). Consistent with this, clorgyline but not the control pargyline increased serotonin in 5-HTP-treated endothelial cells (Fig. 14B). Together these data indicate that 5-HTP is metabolized to serotonin and then HIAA in the endothelial cells. In summary (overview in Fig. 14), 5-HTP supplementation reduced endothelial cell TG2 expression (Fig. 10B), endothelial cell serotonylation (Fig. 10, D–F) and leukocyte recruitment in vivo and in vitro (Figs. 3, 4, and 12).
DISCUSSION
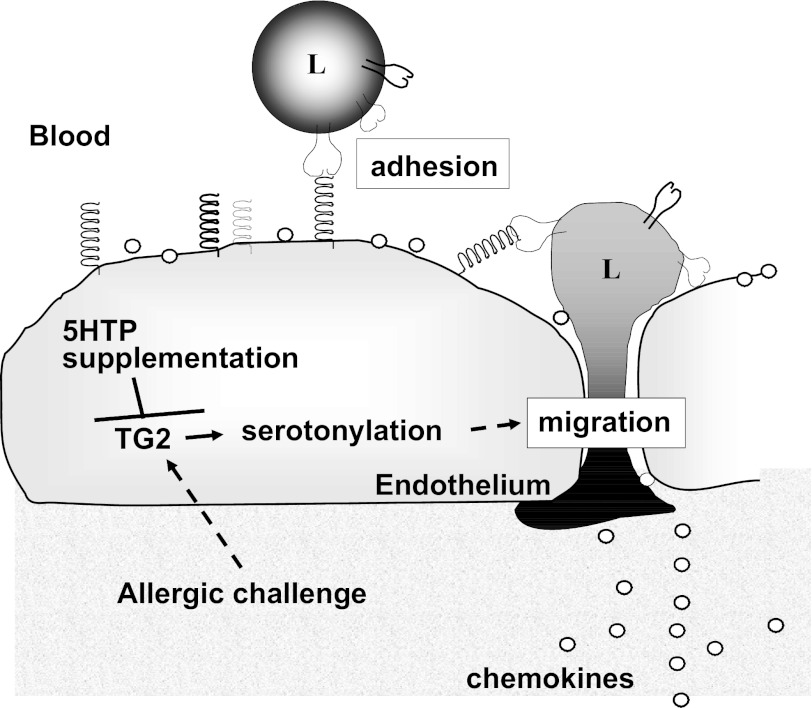
We report that 5-HTP supplementation blocks allergen-induced airway responsiveness and blocks allergic lung inflammation induced by OVA, HDM, or intratracheal administration of IL-4. This occurred without reducing lung tissue mRNA and/or protein expression of mucins, cytokines, chemokines, or adhesion molecules and without altering leukocyte adhesion molecules, chemokine receptors, chemotaxis, or chemokinesis. A mechanism for 5-HTP inhibition of inflammation was, at least in part, by inhibition of endothelial cell function during leukocyte recruitment. 5-HTP inhibited allergen-induced TG2 expression in lung endothelial cells and allergen-induced serotonylation in lung endothelial cells without altering epithelial serotonylation. Thus in vivo there were selective microenvironment changes with 5-HTP supplementation. In vitro, 5-HTP inhibited cytokine-induced serotonylation in endothelial cells and inhibited leukocyte and eosinophil transendothelial migration. Treatment of endothelial cells with TG inhibitors reduced leukocyte and eosinophil transendothelial migration. These data suggest that, during inflammation, endothelial cell serotonylation functions to support leukocyte recruitment and that this is inhibited by 5-HTP supplementation (Fig. 15).
Fig. 15.
Overview of 5-HTP inhibition of endothelial cell function during leukocyte transendothelial migration. During allergic inflammation, tissue-derived cytokines activate endothelial cells to increase expression of TG2, adhesion molecules, and chemokines. Furthermore, endothelial cell TG2-mediated serotonylation increases leukocyte transendothelial migration in response to chemokines. 5-HTP supplementation blocks allergen-induced increases in TG2 expression, TG2-mediated serotonylation, and leukocyte transendothelial migration. L, leukocyte.
It is reported that TG expression can be elevated in human asthmatic airways (56). In the mouse, daily intraperitoneal administration of a TG2 peptide inhibitor (KVLDGQDP) is reported to block OVA-induced lung inflammation (64, 65). In these studies, the cellular targets of the TG peptide inhibitor are not known. We found that, in the lung, serotonylation is present in epithelium and endothelial, but, interestingly, OVA challenge induced an increase in TG2 and serotonylation in lung endothelial cells but not in lung epithelial cells. Moreover, 5-HTP supplementation blocked the increase in TG2 expression and serotonylation in lung endothelial cells. 5-HTP did not alter total lung TG2 mRNA expression in the mixed cell population of the whole lung, but 5-HTP did reduce protein expression of TG2 in lung endothelial cells, suggesting that 5-HTP supplementation mediates a negative feedback regulation of the serotonin pathway for serotonylation in endothelial cells. There is precedence for negative feedback regulation of gene expression in the serotonin pathway. For example, treatment of mice with clorgyline to block breakdown of serotonin results in reduced brain expression of l-aromatic amino acid hydroxylase, the enzyme for 5-HTP conversion to serotonin (22).
Endothelial cells express intracellular and cell surface TG2 (7, 10, 42). TG2-mediated serotonylation is important because serotonylation regulates the function of signaling proteins (30, 65, 75, 93, 119), and it is reported that adhesion molecule signaling in endothelium is required for eosinophil recruitment in allergic lung inflammation (2, 9, 25, 63, 94). It is also reported that antibody blockade of TG on the endothelial cell surface blocks CD8+ T cell transendothelial migration in vitro, but anti-TG treatment of CD8+ cells does not have an effect (86). In our studies, pretreatment of the endothelial cells in vitro with 5-HTP blocked endothelial cell serotonylation and blocked leukocyte transendothelial migration, suggesting that 5-HTP supplementation induces a negative feedback to downregulate endothelial serotonylation and leukocyte transendothelial migration.
A TG2 peptide inhibitor (KVLDGQDP) administered by daily intraperitoneal injection is reported to block OVA-induced lung expression of TG2, VCAM-1, IL-4, IL-5, and IL-13 (64, 65). In contrast to these studies with a peptide and unknown cellular targets of the peptide, we report that supplementation with 5-HTP did not alter OVA-induced lung tissue mRNA expression of adhesion molecules, cytokines, chemokines, or chemokine receptors, including lung tissue expression for the Th2 cell cytokines IL-4 and IL-5. In addition, lung lavage cytokines were not altered. Th2 cytokines are produced by many cells in the lung. Our finding that 5-HTP inhibits allergic inflammation, OVA-induced airway responsiveness, and endothelial cell serotonylation without affecting cytokines or chemokines is consistent with previous reports, where inhibition of signals in endothelial cells blocks allergic inflammation and airway responsiveness without altering expression of cytokines and chemokines (2, 9, 25, 63, 94). This is also consistent with the active function of endothelial cells in the opening of endothelial passageways through which leukocytes migrate. Our in vitro studies demonstrate that cytokine-induced endothelial cell serotonylation functions to support leukocyte transendothelial migration and that this is blocked by treatment with 5-HTP (Fig. 12).
Very high doses of 5-HTP (1 mg/mouse, twice per day) are reported to be proinflammatory in a mouse model of colitis (49). However, these high doses of 5-HTP increase diarrhea (14, 19, 99) and thus would negatively impact colitis inflammation. It is also reported that high doses of 5-HTP (25–100 mg 5-HTP/kg body wt) increases blood or brain serotonin, whereas at 10 mg 5-HTP/kg body wt, there is no increase in 5-HTP metabolites (8, 48, 58, 95). In addition, it is reported that elevating systemic 5-HTP decreases blood pressure, whereas elevating systemic serotonin can increase vasoconstriction in humans and animal models (37, 39, 81, 105, 109, 118). Consistent with these previous reports that 10 mg 5-HTP/kg body wt did not increase 5-HTP metabolites, in our studies with lower doses of 5-HTP (0.1 mg 5-HTP/mouse/day = 5 mg 5-HTP/kg body wt/day), there was no change in systemic serotonin in plasma, lung, intestine, or brain. Nevertheless, we demonstrated that this level of 5-HTP supplementation decreased endothelial cell TG2 expression, endothelial cell serotonylation, and allergic inflammation.
Allergic inflammation is also reported to be regulated by the precursor to 5-HTP, tryptophan. Tryptophan can be converted to kynurenines by indolamine 2,3-dioxygenase and kynurenines are produced by the intestine and by dendritic cells. It is reported that kynurenines reduce OVA-induced allergic inflammation (121). In contrast to the studies with kynurenines, in our studies, 5-HTP did not alter tissue or BAL cytokine or chemokine production in vivo. In addition, when we used intratracheal IL-4 administration to bypass antigen presentation and kynurenine regulation of antigen presentation, 5-HTP blocked the IL-4-induced eosinophil inflammation in the lung. In vitro, 5-HTP inhibition of migration was blocked by pretreatment of endothelial cells with inhibitors of aromatic amino acid decarboxylase, which converts 5-HTP to serotonin in endothelial cells (33, 62, 78–80).
In summary, 5-HTP administration reduced allergic inflammation in vivo and leukocyte transendothelial migration in vitro, at least in part, by endothelial cell metabolism of 5-HTP for inhibition of TG2 (Fig. 15). Thus 5-HTP, which is reported to reduce hypertension (37) and decrease anxiety/depression (18, 53, 57, 61, 74, 82, 97, 98, 102, 111), has profound anti-inflammatory effects on allergic inflammation. This occurs without systemic increases in serotonin, which is an advantage because increases in systemic serotonin can cause vasoconstriction. These data identify 5-HTP as a potential target for intervention in allergy/asthma and the commonly asthma-associated clinical symptoms of anxiety/depression (21, 27, 36, 100, 106).
GRANTS
These studies were supported by National Institutes of Health Grant R01 AT004837 (J. Cook-Mills) and by American Heart Association 0855583G (J. Cook-Mills).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.A.-V., S.B., C.A.M., D.U., R.M., M.E.M., K.S., L.W., and J.M.C.-M. performed experiments; H.A.-V., S.B., C.A.M., D.U., R.M., M.E.M., K.S., L.W., and J.M.C.-M. analyzed data; H.A.-V., S.B., C.A.M., M.E.M., G.M.M., and J.M.C.-M. interpreted results of experiments; H.A.-V., S.B., D.U., G.M.M., and J.M.C.-M. edited and revised manuscript; H.A.-V., S.B., C.A.M., D.U., R.M., M.E.M., K.S., L.W., G.M.M., and J.M.C.-M. approved final version of manuscript; R.M., G.M.M., and J.M.C.-M. conception and design of research; J.M.C.-M. prepared figures; J.M.C.-M. drafted manuscript.
ACKNOWLEDGMENTS
We thank James J. Lee (Mayo Clinic, AZ) for helpful advice and providing the anti-MBP antibodies and the NJ.1638 mice.
REFERENCES
- 1. Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase cα via oxidation. J Immunol 177: 6379–6387, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol 292: L1111–L1125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdouh M, Albert PR, Drobetsky E, Filep JG, Kouassi E. 5-HT1A-mediated promotion of mitogen-activated T and B cell survival and proliferation is associated with increased translocation of NF-kappaB to the nucleus. Brain Behav Immun 18: 24–34, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Abdouh M, Storring JM, Riad M, Paquette Y, Albert PR, Drobetsky E, Kouassi E. Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J Biol Chem 276: 4382–4388, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Alberghina D, Amorini AM, Lazzarino G. Modulation of peripheral markers of the serotoninergic system in healthy horses. Res Vet Sci 90: 392–395, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Arita J, Kimura F. In vitro dopamine biosynthesis in the median eminence of rat hypothalamic slices: involvement of voltage-dependent Ca2+ channels. Brain Res 347: 299–305, 1985 [DOI] [PubMed] [Google Scholar]
- 7. Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res 45: 271–278, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Baumann MH, Williams Z, Zolkowska D, Rothman RB. Serotonin (5-HT) precursor loading with 5-hydroxy-l-tryptophan (5-HTP) reduces locomotor activation produced by (+)-amphetamine in the rat. Drug Alcohol Depend 114: 147–152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of Vitamin E have opposing immunoregulatory funcitons during inflammation by regulating leukocyte recruitment. J Immunol 182: 4395–4405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergamini CM, Griffin M, Pansini FS. Transglutaminase and vascular biology: physiopathologic implications and perspectives for therapeutic interventions. Curr Med Chem 12: 2357–2372, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Berry RB, Hayward LF. Selective augmentation of genioglossus electromyographic activity by l-5-hydroxytryptophan in the rat. Pharmacol Biochem Behav 74: 877–882, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev 3: 271–280, 1998 [PubMed] [Google Scholar]
- 13. Boehme SA, Lio FM, Sikora L, Pandit TS, Lavrador K, Rao SP, Sriramarao P. Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol 173: 3599–3603, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 112: 1666–1677, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown ES, Vornik LA, Khan DA, Rush AJ. Bupropion in the treatment of outpatients with asthma and major depressive disorder. Int J Psychiatry Med 37: 23–28, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci USA 106: 2047–2052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cazzola I, Matera MG. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol Sci 21: 13–16, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Chen GL, Vallender EJ, Miller GM. Functional characterization of the human TPH2 5' regulatory region: untranslated region and polymorphisms modulate gene expression in vitro. Hum Genet 122: 645–657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci 21: 6348–6361, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen YK, Richter HM, 3rd, Go VL, Tyce GM. Free and conjugated catecholamines and serotonin in canine thoracic duct lymph: effects of feeding. Am J Physiol Endocrinol Metab 265: E184–E189, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Cheung TK, Lam B, Lam KF, Ip M, Ng C, Kung R, Wong BC. Gastroesophageal reflux disease is associated with poor asthma control, quality of life, and psychological status in Chinese asthma patients. Chest 135: 1181–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Cho S, Duchemin AM, Neff NH, Hadjiconstantinou M. Modulation of tyrosine hydroxylase and aromatic l-amino acid decarboxylase after inhibiting monoamine oxidase-A. Eur J Pharmacol 314: 51–59, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol 81: 599–606, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Cook-Mills JM, Gallagher JS, Feldbush TL. Isolation and characterization of high endothelial cell lines derived from mouse lymph nodes in vitro. Cell Develop Biol 32: 167–177, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Cook-Mills JM, Marchese M, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15: 1607–1638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook-Mills JM, McCary CA. Isoforms of Vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets 10: 348–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cordina M, Fenech AG, Vassallo J, Cacciottolo JM. Anxiety and the management of asthma in an adult outpatient population. Ther Adv Respir Dis 3: 227–233, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics 40: 491–496, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol 182: 7430–7439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dai Y, Dudek NL, Patel TB, Muma NA. Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J Pharmacol Exp Ther 326: 153–162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deem TL, Abdala-Valencia H, Cook-Mills JM. VCAM-1 activation of PTP1B in endothelial cells. J Immunol 178: 3865–3873, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delaney C, Gien J, Grover TR, Roe G, Abman SH. Pulmonary vascular effects of serotonin and selective serotonin reuptake inhibitors in the late-gestation ovine fetus. Am J Physiol Lung Cell Mol Physiol 301: L937–L944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol 155: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DePietro FR, Fernstrom JD. The effect of phenylalanine on DOPA synthesis in PC12 cells. Neurochem Res 23: 1011–1020, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Di Giovanni G, Esposito E, Di Matteo V. Role of serotonin in central dopamine dysfunction. CNS Neurosci Ther 16: 179–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Marco F, Verga M, Santus P, Giovannelli F, Busatto P, Neri M, Girbino G, Bonini S, Centanni S. Close correlation between anxiety, depression, and asthma control. Respir Med 104: 22–28, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Ding XR, Stier CT, Jr, Itskovitz HD. Serotonin and 5-hydroxytryptophan on blood pressure and renal blood flow in anesthetized rats. Am J Med Sci 297: 290–293, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Domino EF. Monoamine oxidase substrates and substrate affinity. Schizophr Bull 6: 292–297, 1980 [DOI] [PubMed] [Google Scholar]
- 39. Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113: 1857–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 196: 1645–1651, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fattaccini CM, Bolanos-Jimenez F, Gozlan H, Hamon M. Tianeptine stimulates uptake of 5-hydroxytryptamine in vivo in the rat brain. Neuropharmacology 29: 1–8, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Faye C, Inforzato A, Bignon M, Hartmann DJ, Muller L, Ballut L, Olsen BR, Day AJ, Ricard-Blum S. Transglutaminase-2: a new endostatin partner in the extracellular matrix of endothelial cells. Biochem J 427: 467–475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fisher A, Starr MS. Opposite effects of glutamate antagonists and antiparkinsonian drugs on the activities of DOPA decarboxylase and 5-HTP decarboxylase in the rat brain. Brain Res 868: 268–274, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem 68: 355–381, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Freedman RR. Treatment of menopausal hot flashes with 5-hydroxytryptophan. Maturitas 65: 383–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujiwara M, Shibata M, Nomiyama Y, Sugimoto T, Hirata F, Tokuyama T, Senoh S, Hayaishi O. Formation of 5-hydroxykynurenine and 5-hydroxykynurenamine from 5-hydroxytryptophan in rabbit small intestine. Proc Natl Acad Sci USA 76: 1145–1149, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gardier AM. Mutant mouse models and antidepressant drug research: focus on serotonin and brain-derived neurotrophic factor. Behav Pharmacol 20: 18–32, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Gartside SE, Cowen PJ, Sharp T. Effect of 5-hydroxy-l-tryptophan on the release of 5-HT in rat hypothalamus in vivo as measured by microdialysis. Neuropharmacology 31: 9–14, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Cote F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Gijsman HJ, van Gerven JM, de Kam ML, Schoemaker RC, Pieters MS, Weemaes M, de Rijk R, van der Post J, Cohen AF. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol 22: 183–189, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Gomes P, Soares-da-Silva P. l-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res 829: 143–150, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Goshima Y, Nakamura S, Misu Y. l-dopa facilitates the release of endogenous norepinephrine and dopamine via presynaptic beta 1- and beta 2-adrenoceptors under essentially complete inhibition of l-aromatic amino acid decarboxylase in rat hypothalamic slices. Jpn J Pharmacol 53: 47–56, 1990 [DOI] [PubMed] [Google Scholar]
- 53. Grigoroiu-Serbanescu M, Diaconu CC, Herms S, Bleotu C, Vollmer J, Muhleisen TW, Prelipceanu D, Priebe L, Mihailescu R, Georgescu MJ, Sima D, Grimberg M, Nothen MM, Cichon S. Investigation of the tryptophan hydroxylase 2 gene in bipolar I disorder in the Romanian population. Psychiatr Genet 18: 240–247, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Gruetter CA, Lemke SM, Anestis DK, Szarek JL, Valentovic MA. Potentiation of 5-hydroxytryptamine-induced contraction in rat aorta by chlorpheniramine, citalopram and fluoxetine. Eur J Pharmacol 217: 109–118, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Haleem DJ. Exaggerated feedback control decreases brain serotonin concentration and elicits hyperactivity in a rat model of diet-restriction-induced anorexia nervosa. Appetite 52: 44–50, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS ONE 5: e8583, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hennig J, Reuter M, Netter P, Burk C, Landt O. Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behav Neurosci 119: 16–25, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Hranilovic D, Blazevic S, Ivica N, Cicin-Sain L, Oreskovic D. The effects of the perinatal treatment with 5-hydroxytryptophan or tranylcypromine on the peripheral and central serotonin homeostasis in adult rats. Neurochem Int 59: 202–207, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, Saurini F, Eddahibi S, Hamon M, Adnot S. Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 293: L1045–L1052, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Jacobs GE, Kamerling IM, de Kam ML, Derijk RH, van Pelt J, Zitman FG, van Gerven JM. Enhanced tolerability of the 5-hydroxytryptophane challenge test combined with granisetron. J Psychopharmacol 24: 65–72, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Jernej B, Stefulj J, Hranilovic D, Balija M, Skavic J, Kubat M. Intronic polymorphism of tryptophan hydroxylase and serotonin transporter: indication for combined effect in predisposition to suicide. J Neural Transm 111: 733–738, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Kawahara K, Hashiguchi T, Kikuchi K, Tancharoen S, Miura N, Ito T, Oyama Y, Nawa Y, Biswas KK, Meng X, Morimoto Y, Shrestha B, Sameshima H, Maruyama I. Induction of high mobility group box 1 release from serotonin-stimulated human umbilical vein endothelial cells. Int J Mol Med 22: 639–644, 2008 [PubMed] [Google Scholar]
- 63. Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol 174: 3709–3718, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY, Ro JY. Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br J Pharmacol 162: 210–225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim Y, Eom S, Kim K, Lee YS, Choe J, Hahn JH, Lee H, Kim YM, Ha KS, Ro JY, Jeoung D. Transglutaminase II interacts with rac1, regulates production of reactive oxygen species, expression of snail, secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation. Mol Immunol 47: 1010–1022, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol 177: 6422–6432, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Laengle UW, Markstein R, Pralet D, Seewald W, Roman D. Effect of GLC756, a novel mixed dopamine D1 receptor antagonist and dopamine D2 receptor agonist, on TNF-alpha release in vitro from activated rat mast cells. Exp Eye Res 83: 1335–1339, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Laengle UW, Markstein R, Pralet D, Seewald W, Roman D. Effects of latanoprost, timolol and GLC756, a novel dopamine D(2) agonist and D(1) antagonist on LTC(4) release after rat mast cell activation. Clin Experiment Ophthalmol 35: 645–650, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Lechin F, van der Dijs B, Orozco B, Jara H, Rada I, Lechin ME, Lechin AE. Neuropharmacologic treatment of bronchial asthma with the antidepressant tianeptine: a double-blind, crossover placebo-controlled study. Clin Pharmacol Ther 64: 223–232, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Lechin F, van der Dijs B, Orozco B, Jara H, Rada I, Lechin ME, Lechin AE. The serotonin uptake-enhancing drug tianeptine suppresses asthmatic symptoms in children: a double-blind, crossover, placebo-controlled study. J Clin Pharmacol 38: 918–925, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Lechin F, van der Dijs B, Orozco B, Lechin M, Lechin AE. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann Allergy Asthma Immunol 77: 245–253, 1996 [DOI] [PubMed] [Google Scholar]
- 72. Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109: 3139–3146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol 8: 460–471, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Lin YM, Chao SC, Chen TM, Lai TJ, Chen JS, Sun HS. Association of functional polymorphisms of the human tryptophan hydroxylase 2 gene with risk for bipolar disorder in Han Chinese. Arch Gen Psychiatry 64: 1015–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Liu Y, Wei L, Laskin DL, Fanburg BL. Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 44: 548–555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lowe SL, Yeo KP, Teng L, Soon DK, Pan A, Wise SD, Peck RW. l-5-Hydroxytryptophan augments the neuroendocrine response to a SSRI. Psychoneuroendocrinology 31: 473–484, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Lynn-Bullock CP, Welshhans K, Pallas SL, Katz PS. The effect of oral 5-HTP administration on 5-HTP and 5-HT immunoreactivity in monoaminergic brain regions of rats. J Chem Neuroanat 27: 129–138, 2004 [DOI] [PubMed] [Google Scholar]
- 78. MaassenVanDenBrink A, Centurion D, Villalon CM. Crosstalk of vascular 5-HT1 receptors with other receptors: clinical implications. Neuropharmacology 55: 986–993, 2008 [DOI] [PubMed] [Google Scholar]
- 79. MacLean MR. Pulmonary hypertension and the serotonin hypothesis: where are we now? Int J Clin Pract Suppl 156: 27–31, 2007 [DOI] [PubMed] [Google Scholar]
- 80. MacLean MR, Dempsie Y. Serotonin and pulmonary hypertension—from bench to bedside? Curr Opin Pharmacol 9: 281–286, 2009 [DOI] [PubMed] [Google Scholar]
- 81. Maclean MR, Dempsie Y. The serotonin hypothesis of pulmonary hypertension revisited. Adv Exp Med Biol 661: 309–322, 2010 [DOI] [PubMed] [Google Scholar]
- 82. Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry 45: 603–614, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, Wray SP, Gerhardt B, Lanthorn TH, Savelieva KV. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur J Pharmacol 602: 306–315, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol 164: 6550–6559, 2000 [DOI] [PubMed] [Google Scholar]
- 85. Menard G, Turmel V, Bissonnette EY. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol 150: 340–348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mohan K, Pinto D, Issekutz TB. Identification of tissue transglutaminase as a novel molecule involved in human CD8+ T cell transendothelial migration. J Immunol 171: 3179–3186, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, Nilsen M, MacLean MR. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension 49: 232–236, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Muller T, Durk T, Blumenthal B, Grimm M, Cicko S, Panther E, Sorichter S, Herouy Y, Di Virgilio F, Ferrari D, Norgauer J, Idzko M. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS ONE 4: e6453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nakagome K, Imamura M, Okada H, Kawahata K, Inoue T, Hashimoto K, Harada H, Higashi T, Takagi R, Nakano K, Hagiwara K, Kanazawa M, Dohi M, Nagata M, Matsushita S. Dopamine D1-like receptor antagonist attenuates Th17-mediated immune response and ovalbumin antigen-induced neutrophilic airway inflammation. J Immunol 186: 5975–5982, 2011 [DOI] [PubMed] [Google Scholar]
- 90. Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 587: 567–586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ortiz J, Mariscot C, Alvarez E, Artigas F. Effects of the antidepressant drug tianeptine on plasma and platelet serotonin of depressive patients and healthy controls. J Affect Disord 29: 227–234, 1993 [DOI] [PubMed] [Google Scholar]
- 92. Ortiz J, Mocaer E, Artigas F. Effects of the antidepressant drug tianeptine on plasma and platelet serotonin concentrations in the rat. Eur J Pharmacol 199: 335–339, 1991 [DOI] [PubMed] [Google Scholar]
- 93. Park D, Choi SS, Ha KS. Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids 39: 619–631, 2010 [DOI] [PubMed] [Google Scholar]
- 94. Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O'Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci USA 104: 4371–4376, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Perry KW, Fuller RW. Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus l-5-hydroxytryptophan. J Pharm Pharmacol 45: 759–761, 1993 [DOI] [PubMed] [Google Scholar]
- 96. Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA. TNF-alpha-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol 171: 4700–4707, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Rotondo A, Schuebel K, Bergen A, Aragon R, Virkkunen M, Linnoila M, Goldman D, Nielsen D. Identification of four variants in the tryptophan hydroxylase promoter and association to behavior. Mol Psychiatry 4: 360–368, 1999 [DOI] [PubMed] [Google Scholar]
- 98. Rujescu D, Giegling I, Bondy B, Gietl A, Zill P, Moller HJ. Association of anger-related traits with SNPs in the TPH gene. Mol Psychiatry 7: 1023–1029, 2002 [DOI] [PubMed] [Google Scholar]
- 99. Sanger GJ, Banner SE, Smith MI, Wardle KA. SB-207266: 5-HT4 receptor antagonism in human isolated gut and prevention of 5-HT-evoked sensitization of peristalsis and increased defaecation in animal models. Neurogastroenterol Motil 10: 271–279, 1998 [DOI] [PubMed] [Google Scholar]
- 100. Sansone RA, Sansone LA. Asthma: wheezing, woes, and worries. Psychiatry 5: 48–52, 2008 [PMC free article] [PubMed] [Google Scholar]
- 101. Schruers K, van Diest R, Overbeek T, Griez E. Acute l-5-hydroxytryptophan administration inhibits carbon dioxide-induced panic in panic disorder patients. Psychiatry Res 113: 237–243, 2002 [DOI] [PubMed] [Google Scholar]
- 102. Serretti A, Artioli P. From molecular biology to pharmacogenetics: a review of the literature on antidepressant treatment and suggestions of possible candidate genes. Psychopharmacology (Berl) 174: 490–503, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Smarius LJ, Jacobs GE, Hoeberechts-Lefrandt DH, de Kam ML, van der Post JP, de Rijk R, van Pelt J, Schoemaker RC, Zitman FG, van Gerven JM, Gijsman HJ. Pharmacology of rising oral doses of 5-hydroxytryptophan with carbidopa. J Psychopharmacol 22: 426–433, 2008 [DOI] [PubMed] [Google Scholar]
- 104. Solberg LC, Valdar W, Gauguier D, Nunez G, Taylor A, Burnett S, Arboledas-Hita C, Hernandez-Pliego P, Davidson S, Burns P, Bhattacharya S, Hough T, Higgs D, Klenerman P, Cookson WO, Zhang Y, Deacon RM, Rawlins JN, Mott R, Flint J. A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm Genome 17: 129–146, 2006 [DOI] [PubMed] [Google Scholar]
- 105. Stone RA, Worsdell YM, Fuller RW, Barnes PJ. Effects of 5-hydroxytryptamine and 5-hydroxytryptophan infusion on the human cough reflex. J Appl Physiol 74: 396–401, 1993 [DOI] [PubMed] [Google Scholar]
- 106. Strine TW, Mokdad AH, Balluz LS, Gonzalez O, Crider R, Berry JT, Kroenke K. Depression and anxiety in the United States: findings from the 2006 Behavioral Risk Factor Surveillance System. Psychiatr Serv 59: 1383–1390, 2008 [DOI] [PubMed] [Google Scholar]
- 107. Tachimoto H, Burdick MM, Hudson SA, Kikuchi M, Konstantopoulos K, Bochner BS. CCR3-active chemokines promote rapid detachment of eosinophils from VCAM-1 in vitro. J Immunol 165: 2748–2754, 2000 [DOI] [PubMed] [Google Scholar]
- 108. Tenner K, Qadri F, Bert B, Voigt JP, Bader M. The mTPH2 C1473G single nucleotide polymorphism is not responsible for behavioural differences between mouse strains. Neurosci Lett 431: 21–25, 2008 [DOI] [PubMed] [Google Scholar]
- 109. Thomas M. Pharmacological targets for pulmonary vascular disease: vasodilation versus anti-remodelling. Adv Exp Med Biol 661: 475–490, 2010 [DOI] [PubMed] [Google Scholar]
- 110. Tudor KSRS, Hess KL, Cook-Mills JM. Cytokines modulate endothelial cell intracellular signal transduction required for VCAM-1-dependent lymphocyte transendothelial migration. Cytokine 15: 196–211, 2001 [DOI] [PubMed] [Google Scholar]
- 111. Tzvetkov MV, Brockmoller J, Roots I, Kirchheiner J. Common genetic variations in human brain-specific tryptophan hydroxylase-2 and response to antidepressant treatment. Pharmacogenet Genomics 18: 495–506, 2008 [DOI] [PubMed] [Google Scholar]
- 112. Van Lieshout RJ, Bienenstock J, MacQueen GM. A review of candidate pathways underlying the association between asthma and major depressive disorder. Psychosom Med 71: 187–195, 2009 [DOI] [PubMed] [Google Scholar]
- 113. Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010 [DOI] [PubMed] [Google Scholar]
- 114. Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol 410: 165–181, 2000 [DOI] [PubMed] [Google Scholar]
- 115. Vega Lde L, Munoz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H, Farber L, Mueller W, Stratz T, Fiebich BL. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol 70: 369–380, 2005 [DOI] [PubMed] [Google Scholar]
- 116. Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299: 76, 2003 [DOI] [PubMed] [Google Scholar]
- 117. Wanstall JC, Fiore SA, Gambino A, Chess-Williams R. Potentiation of 5-hydroxytryptamine (5-HT) responses by a 5-HT uptake inhibitor in pulmonary and systemic vessels: effects of exposing rats to hypoxia. Naunyn Schmiedebergs Arch Pharmacol 368: 520–527, 2003 [DOI] [PubMed] [Google Scholar]
- 118. Watts SW, Davis RP. 5-Hydroxtryptamine Receptors in Systemic Hypertension: An Arterial Focus. Cardiovasc Ther 29: 54–67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS ONE 4: e5682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wrona MZ, Dryhurst G. A putative metabolite of serotonin, tryptamine-4,5-dione, is an irreversible inhibitor of tryptophan hydroxylase: possible relevance to the serotonergic neurotoxicity of methamphetamine. Chem Res Toxicol 14: 1184–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 121. Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett 121: 1–6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yin J, Albert RH, Tretiakova AP, Jameson BA. 5-HT(1B) receptors play a prominent role in the proliferation of T-lymphocytes. J Neuroimmunol 181: 68–81, 2006 [DOI] [PubMed] [Google Scholar]
- 123. Zabel M. Studies on in vitro effect of serotonin on calcitonin secretion by rat thyroid C cells. Histochemistry 83: 71–75, 1985 [DOI] [PubMed] [Google Scholar]