Abstract
Our laboratory has previously reported that bronchial epithelial cells (BEC) express a regulatory cascade of classic neurotransmitters and receptors that communicate in an almost neuronal-like manner to achieve physiological regulation. In this paper we show that the similarity between neurotransmitter signaling in neurons and BEC extends to the level of transmitter receptor allosteric modulators. Lynx1 is a member of the ly-6/three-finger superfamily of proteins, many of which modulate receptor signaling activity. Lynx1 specifically has been shown to modulate nicotinic acetylcholine receptor (nAChR) function in neurons by altering receptor sensitivity and desensitization. We now report that lynx1 forms a complex with α7 nAChR in BEC and serves to negatively regulate α7 downstream signaling events. Treatment of primary cultures of BEC with nicotine increased levels of nAChR subunits and that increase was potentiated by lynx1 knockdown. Lynx1 knockdown also potentiated the nicotine-induced increase in GABAA receptors (GABAAR) and MUC5AC mRNA expression, and that effect was blocked by α7 antagonists and α7 knockdown. In parallel with the increases in nAChR, GABAAR, and mucin mRNA levels, lynx1 knockdown also increased levels of p-Src. Consistent with this, inhibition of Src signaling blocked the ability of the lynx1 knockdown to increase basal and nicotine-stimulated GABAAR and mucin mRNA expression. Thus lynx1 appears to act as a negative modulator of α7 nAChR-induced events by inhibiting Src activation. This suggests that lynx1 agonists or mimetics are a potentially important therapeutic target to develop new therapies for smoking-related diseases characterized by increased mucin expression.
Keywords: nicotine, smoking, mucus, chronic obstructive pulmonary disease, asthma, GABA
our laboratory has previously reported that bronchial epithelial cells (BEC) express a regulatory cascade of classic neurotransmitters and neurotransmitter receptors including acetylcholine (ACh), GABA, glutamate, and glycine (14). Transmitter systems in BEC communicate in an almonst neuronal-like manner to achieve physiological regulation. For example, GABA signaling in BEC regulates mucin secretion (49), and activation of nicotinic ACh receptors (nAChR) in BEC by ACh or nicotine in turn activates GABA signaling in BEC to further increase mucin secretion (14). Consistent with this, levels of glutamate decarboxylase (GAD), the GABA biosynthetic enzyme, are increased in airways of smokers and correlate with levels of mucin in smokers (45). This network of nonneuronal transmitters in BEC provides a whole new layer of physiological regulation of lung function and potentially new ways to target lung disease.
In this study, we show that the similarity between neurotransmitter signaling in neurons and in BEC extend to the level of transmitter receptor allosteric modulators. The ly-6 family of proteins, also known as three-finger toxins or proteins, is a large family of proteins characterized by precisely spaced cysteine motifs (15, 21, 44). A number of these proteins bind to nAChR and act as positive or negative allosteric modulators. In particular lynx1, originally isolated by Miwa et al. (26) based on its homology with the nicotinic antagonist α-bungarotoxin (αBGT), acts as an endogenous negative regulator of nicotinic signaling in neurons (20, 27). The nAChR are ligand-gated ion channels, and the binding of their endogenous ligand (ACh) or the exogenous ligand nicotine allows entry of Na+ or Ca++ into the cell (25). Binding of lynx1 to the nAChR decreases nicotine and ACh-induced currents and increases desensitization. Multiple other members of the ly-6 family also modulate nAChR activity, including lynx2 (43), slurp-1(3, 6), slurp-2 (2), prostate stem cell antigen (19), and the prostate and testis expressed proteins (24). Because our laboratory has previously shown that lynx1 is highly expressed in airway epithelial cells, it would seem likely that lynx1 might also regulate nAChR responses to nicotine and ACh in lung (39).
Potential negative regulators of airway nicotinic signaling have important implications for chronic lung disease because so much of lung disease is linked to smoking and excess mucin production. Smoking is intimately related to asthma, chronic bronchitis, and chronic obstructive pulmonary disease (COPD). Greater than 40% of smokers develop chronic bronchitis (31), and 25% of smokers will develop COPD, which combines chronic mucus production with decreases in lung function. Similarly, smoking both increases risk of asthma and the severity of asthma (32). In COPD, increased mucus secretion is continuous and a major component of the disease; in asthma, mucus secretion is also increased, and mucus plugs are associated with the most severe exacerbations and fatalities (12, 16). Thus negative modulators of nicotinic activity would have the potential to decrease mucin expression.
The goal of this study was to further characterize the neurotransmitter cascade in BEC to determine whether lynx1 regulates cholinergic activation of GABAergic signaling and mucin expression and hence provides a new therapeutic target for smoking-induced lung disease.
MATERIALS AND METHODS
Epithelial cell culture.
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Utilization Committee. Bronchial epithelial cell cultures were established from lungs obtained from rhesus macaques (newborn to 2 yr old) undergoing necropsy from protocols not expected to alter lung function as described by Wu et al. (33, 48) with modifications as previously described by Fu et al. (14). All drugs used for the study were obtained from Sigma (St. Louis, MO).
Immunofluorescence for lynx1 and α7 nAChR receptors.
The primary antibodies used were rabbit polyclonal antisera raised against: anti-monkey lynx1 (antibody R4, rabbit, 1:300) (39) and anti-α7 nAChR (antibody 306, mouse, 1:1,000, Sigma). Secondary antibodies were FITC-conjugated horse anti-mouse (1:400 dilution) and Cy3- conjugated goat anti-rabbit (1:400 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA). Mouse brain sections were used for positive controls. Primary cell cultures were established then incubated in serum-free bronchial epithelium culture media (Cell Application, Carlsbad, CA) for 7–10 days and then fixed with 4% paraformaldehyde for immunofluorescence. For negative controls, the primary antisera were omitted. Samples were viewed on a confocal microscope (Leica TCS SP, Jena, Germany). Unless otherwise specified, antibodies used were against the human protein, but the high homology between rhesus and human proteins ensures in our experience almost 100% cross-reactivity.
RT-PCR and real-time PCR.
Total RNA was extracted from monkey brain, lung, and cultured BEC (5 × 106 cells) using Trizol (Invitrogen, Carlsbad, CA), and real-time PCR was performed as described previously (38). Primers and probes not previously described were as follows: lynx1: 5′ GGGTCCTCAGCAACATCGA, 3′ GCCCGTGTGGCACATCTT, probe AACTTGCCTCTGGTCAC; α7 nAChR; 5′ AATACGCTCAGGTGGGCTGAT, 3′ GCCCTGCTGACCGACAGT, probe, ATCCCCTTGGCACATC; α4 nAChR, 5′ GCCACACGTTTGCCACATTA, 3′ CCTGTCCAAGGCCGTCTTAC, probe TTCCTGTTCTGTGTCTGC; β2 nAChR, 5′ GGTCCACGAACGGAACTTCA, 3′ TTGCCGCCTGCCATCTAC,probe GCACTTCCCATTTGACCAGCAAGAACTG; β4 nAChR, 5′ GGCCGATGAGGCCTAAGTAAA, 3′ GATCCCTCCAAGGCATTCAG, probe ATCAAACCAGCCCAAGCCGTGGA. All other sequences and primers used for real-time PCR of GABAARs and nAChR were as described previously (14, 41). Primers and probes were specifically designed for rhesus or human probes were confirmed to have 100% homology with rhesus sequences. All reactions were run in duplex with primers and a probe for 18S (internal standard set from Life Technologies), which was used for standardization. Real-time conditions were 50°C × 2 min, 95°C × 10 min, 95°C × 15 s, 60°C × 1 min with the last two steps repeated 40 times.
siRNA knockdown.
ON-TARGET plus siRNAs for lynx1, α7 nAChR, α4 nAChR, and negative control siRNA were purchased from Dharmacon (Lafayette, CO). siRNAs were transfected at a concentration 100–150 nM with DharmaFECT 1 according to the manufacturer's instruction. Forty-eight hours after transfection, cells were harvested for real-time PCR or for Western blotting. The lynx1 siRNA (TCAGCAACATCGAGAACTT) was chosen to be distinct from the other transcripts of the lynx1 gene and was 100% homologous to NM_001266622.
Immunoprecipitation and Western blotting.
Western blots were performed as described previously (42). For immunoprecipitation, solubilized BEC cultured cell extracts (400–600 μg of protein) were incubated in the presence of anti-α7 (antibody 306, mouse, 1:800) or IgG (1–2 μg) for 4 h at 4°C, followed by the addition of 20 μl of protein A/G agarose for 12 h. Pellets were washed, boiled in SDS sample buffer, subjected to SDS-PAGE, transferred to a PVDF membrane (Millipore, Billerica, MA) and incubated with anti-lynx1 antibody (rabbit, 1:100) and or anti-β-actin (mouse, 1: 20,000, Sigma) overnight at 4°C. Blots were visualized using Immobilon Western Chemiluminescent horseradish peroxidase (HRP) substrate (Millipore) using HRP-anti-goat or anti-rabbit (1:800; Promega, Madison, WI) and analyzed using a FluorchemHD2 and FluorChem software (AlphaInnotech, Santa Clara, CA). All experiments were repeated at least three times. Antibodies used for Western blotting were anti-GABAAR β2/3(rabbit, 1:1,500, Millipore no. 05–474), anti- GABAAR α5 (rabbit, 1:500, Millipore no. AB9678), anti-c-Src (mouse, no. sc-130124; Santa Cruz Biotechnology, Santa Cruz, CA) and p-Src (mouse, Santa Cruz no. sc-81521).
Statistics.
All data are given as means ± SE, and statistical comparisons were made using ANOVA followed by Fisher's post hoc multiple comparisons or unpaired, two-tailed Student's t-tests.
RESULTS
Lynx1 and α7 nAChR expression in rhesus lung and BEC.
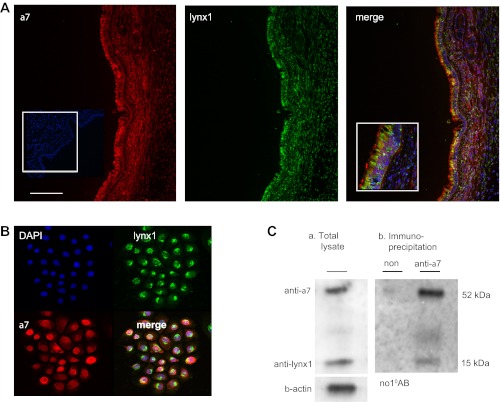
Lynx1 colocalizes with α7 nAChR. Lynx1 immunostaining in monkey lung was strongest in airway epithelium as previously reported (Fig. 1A) (39). Dual immunofluorescence for lynx1 and α7 nAChR showed colocalization in epithelium. In large airway epithelium, lynx1 and α7 staining were both primarily apical with α7 even more apical (Fig. 1A). To further prove colocalization, primary cultures of rhesus BEC were established and stained for lynx1 and α7. As shown in Fig. 1B, BEC clearly expressed both α7 and lynx1. These experiments establish that lynx1 is colocalized with α7 nAChR in monkey BEC. Next immunoprecipitation was performed to determine whether lynx1 and α7 forms a complex in BEC as has been reported for neurons and HEK cells (20, 27). As shown in Fig. 1C, Western blot analysis showed the presence of α7 and lynx1 in BEC total extracts. Immunoprecipitation of BEC with anti-α7 showed that α7 and lynx1 form a complex in BEC just as occurs in neurons.
Fig. 1.
Detection of lynx1 and α7 nicotinic acetylcholine receptor (nAChR) in monkey lung and bronchial epithelial cells. A: immunohistochemical detection of α7 nAChR (inset, nonimmune serum control), lynx1, and colocalization in monkey lung. Calibration bar represents 35 μm. Inset: box in merge shows high-power view. B: immunohistochemical detection of lynx1, α7 nAChR, and colocalization in primary cultures of rhesus bronchial epithelial cells (BEC). Calibration bar represents 60 μm. C: lynx1 and α7 nAChR form a complex in BEC. a: Western blot of total α7 nAChR and lynx1 in BEC. b: Western blot of α7 nAChR and lynx1 after immunoprecipitation with anti-α7 monoclonal antibody (mouse) or nonimmune serum showing that α7 and lynx1 form a complex in BEC. Similar results were seen if the antibody for lynx1 was used for the immunoprecipitation and the α7 antibody was used for the Western blot (data not shown). In addition, no lynx1 was seen after immunoprecipitation with an unrelated antibody (anti-GABAAR 2/3) (data not shown). The experiment was repeated 4 times.
Lynx1 is a negative regulator of nicotine-induced nAChR expression.
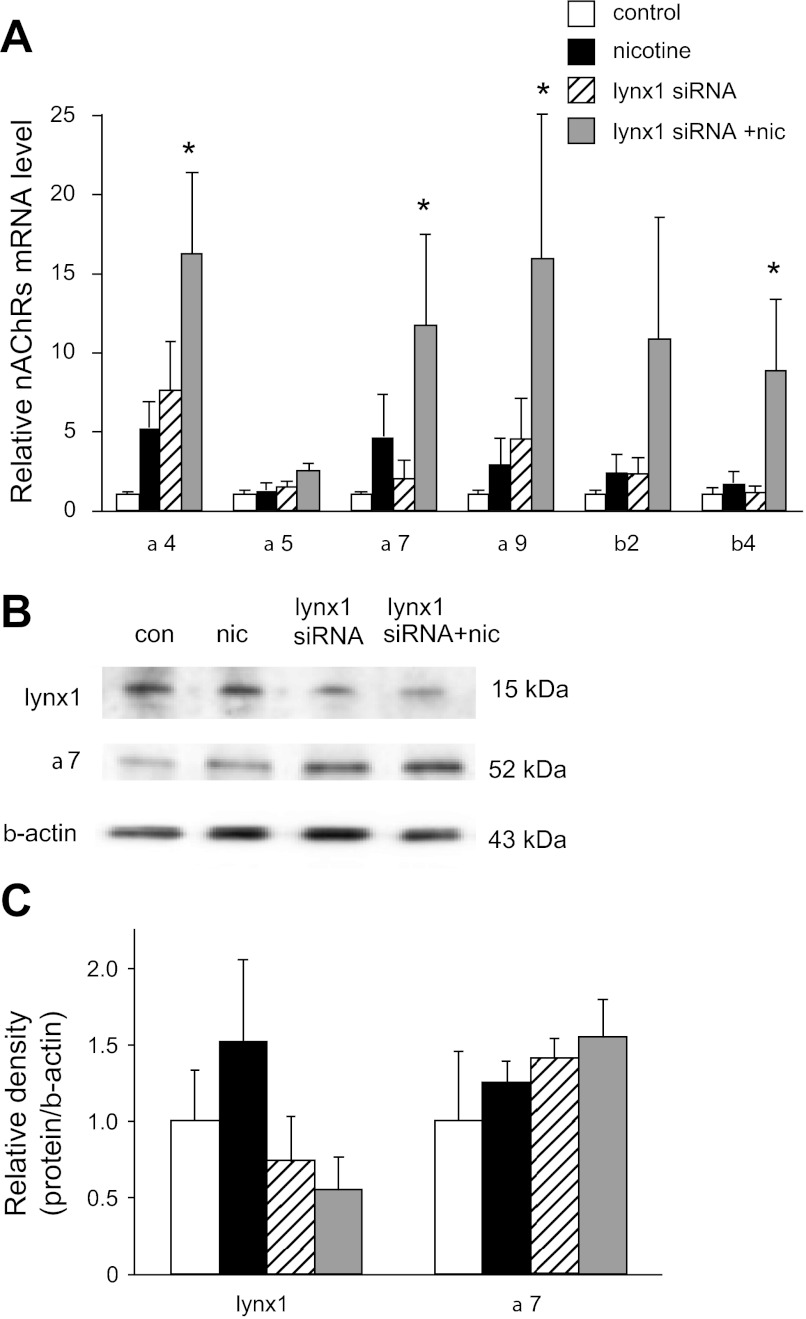
We and others have previously reported that chronic nicotine increases nAChR expression at the mRNA and protein level (1, 13, 35, 50). To determine whether lynx1 plays a role in the ability of nicotine to upregulate nAChR mRNA levels, cultured BEC were treated with 1 μM nicotine for 48 h in the presence of control siRNA or lynx1 siRNA, and levels of nAChR were measured by real-time PCR. The siRNA used was chosen to target only lynx1 and not either of the forms of slurp-2 that are also transcribed from the lynx1 gene (2). The lynx1 siRNA caused a 75% decrease in lynx1 siRNA levels. As shown in Fig. 2A, the combination of nicotine and lynx1 knockdown significantly increased levels of α4, α7, α9, and β4 nAChR subunit mRNAs (Fig. 2A). As shown in Fig. 2, B and C, changes in levels of lynx1 and α7 proteins followed the same trends as the changes in levels of their respective mRNAs. Thus these data show that lynx1 acts as a negative modulator of the ability of nicotine to upregulate nAChR mRNA and protein levels.
Fig. 2.
Lynx1 knockdown potentiated the increase in nAChR mRNA and protein caused by nicotine. A: effect of lynx1 siRNA knockdown on the ability of nicotine (1 μM for 48 h) to increase levels of nAChR subunits. Values are expressed as relative fold change of each condition vs. control with error bars showing SE (*P < 0.05 compared with control by Fisher's multiple-comparison tests after 1-way ANOVA). All control RNA levels were normalized to 1, and 18S RNA levels were used as an internal standard (n = 5–9 per group). B: representative Western blot analysis showing the lynx1 knockdown-induced decrease in lynx1 protein and the resulting increase in nicotine-induced α7 nAChR protein. C: quantification of protein changes shown in B (n = 4 per group).
Lynx1 negatively modulates the downstream effects of α7 nAChR signaling in BEC.
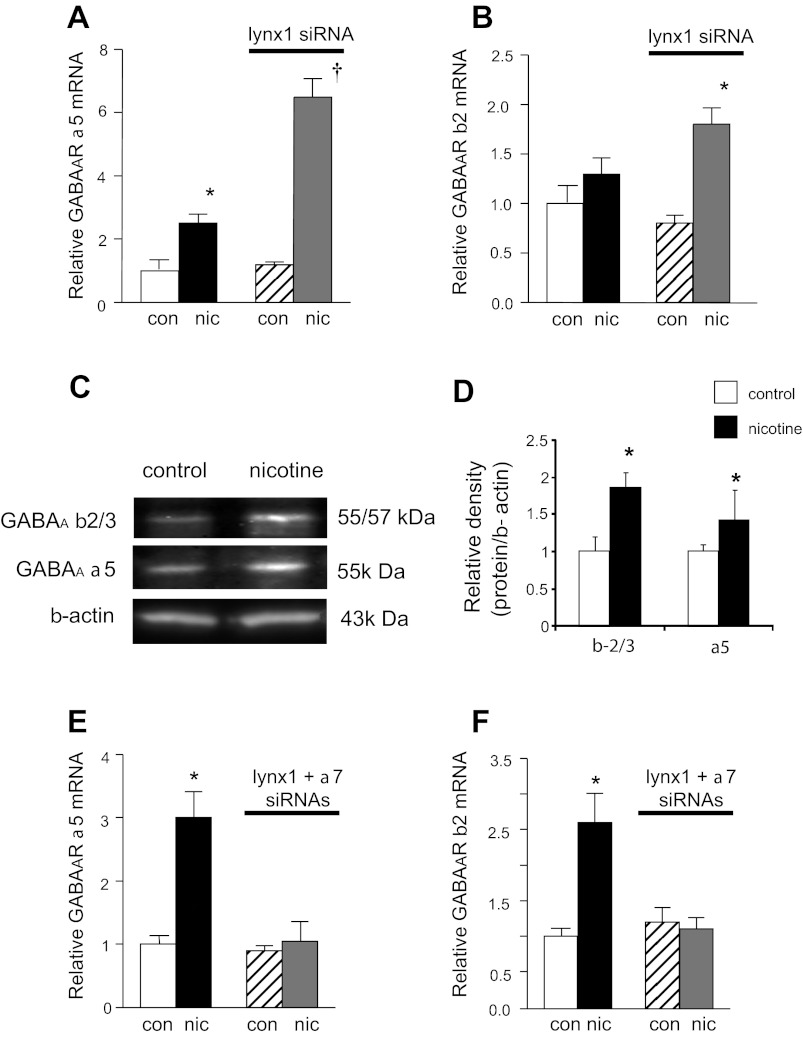
We previously reported that activation of nAChR in BEC by nicotine or ACh leads to increased levels of GAD, GABAAR, and mucin expression by BEC (14). Thus the ability of nicotine to upregulate GABA signaling in BEC provides a good readout of nAChR signaling. Consistent with our previous report (14) treatment of cultured BEC with nicotine (1 μM × 48 h) significantly increased mRNA levels for GABAAR α5 compared with control (Fig. 3A) and similar effects were seen on GABAAR protein levels (Fig. 3, C and D). Importantly, knockdown of lynx1 enhanced the ability of nicotine to increase levels of GABAAR α5 and GABAAR β2 (Fig. 3, A and B). This effect appeared to be mediated by α7 nAChR, as the effect of the lynx1 knockdown on the nicotine-induced increase in GABA was abolished by siRNA knockdown of α7 nAChR (70% knockdown of mRNA for α7 nAChR) (Fig. 3, E and F). The α7 nAChR-specific antagonist methyllycaconitine (30 nM) similarly blocked the ability of the lynx1 siRNA to increase the effects of nicotine on GABAAR expression (data not shown). These results suggest that lynx1 serves as a negative modulator of the α7 signaling as shown by the effect of lynx1 knockdown on the α7 nAChR-mediated effect of nicotine on GABAergic expression in BEC.
Fig. 3.
Lynx1 modulates the downstream effects of α7 nAChR signaling in BEC. A and B: siRNA knockdown of lynx1 increased the nicotine-induced upregulation GABAAR α5 and GABAAR β2 mRNA expression (n = 8 per group). C and D: Western blot analysis showed that changes in protein levels mirrored changes in RNA levels (n = 4 per group). E and F: siRNA knockdown of α7 nAChR blocked the effect of the lynx1 knockdown on the nicotine-induced increases in GABAAR α5 and GABAAR β2 mRNA levels (n = 5 per group). The values are expressed as relative fold change of each condition vs. control. Error bars show SE (*P < 0.05, †P < 0.01 by t-test).
Lynx1 modulation of nicotine-induced activation of GABA signaling is dependent on Src phosphorylation.
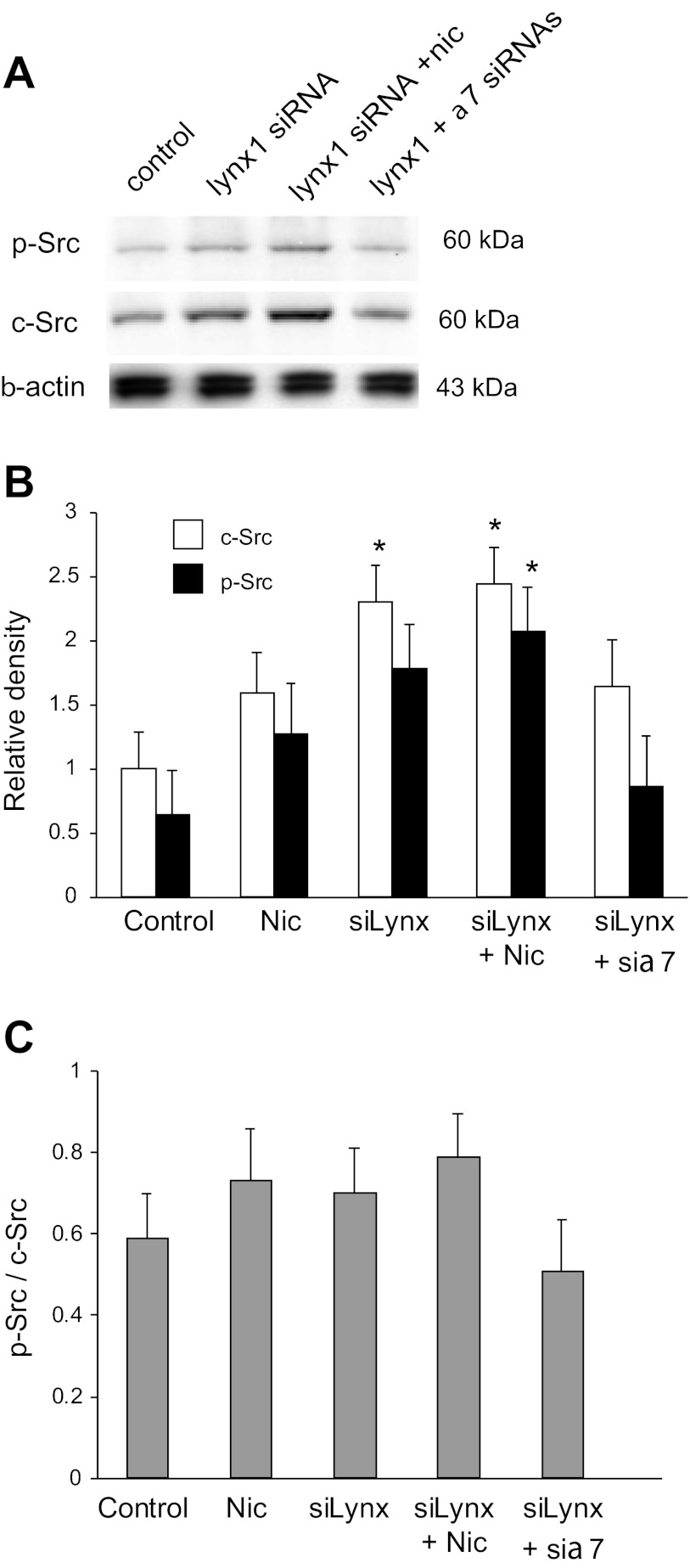
The protein tyrosine kinase Src has been shown to mediate downstream effects of nicotinic activation of α7 nAChR lung cancer cells (7, 9). We thus examined whether Src activation might also be involved in lynx1 modulation of α7 signaling in BEC. First, as shown in Fig. 4A, knockdown of lynx1 increased basal and nicotine-stimulated levels of Src and p-Src in BEC. Knockdown of also α7 prevented the increase in Src and p-Src caused by the lynx1 knockdown. These results suggest that lynx1 acts to limit activation of Src signaling by α7 nAChR and thereby modulates GABAAR levels. As shown in Fig. 5, the increases in levels of p-Src reflected both increases in total Src as well as increases in the p-Src/Src ratio.
Fig. 4.
Lynx1 modulates the ability of nicotine to activate Src signaling. A: representative Western blot showing knockdown of lynx1 increased basal and nicotine-stimulated (2 μM nicotine × 48 h) levels of Src and p-Src. Knockdown of α7 prevented the increase in Src and p-Src caused by the lynx1 knockdown. As shown in B and C, increases in p-Src primarily reflect increases in total Src, as levels of both c-Src and p-Src are increased. Images are representative from 4–5 similar experiments that were pooled for the densitometric analyses. *P < 0.05 compared with corresponding control by Fisher's multiple-comparison tests after 1-way ANOVA.
Fig. 5.
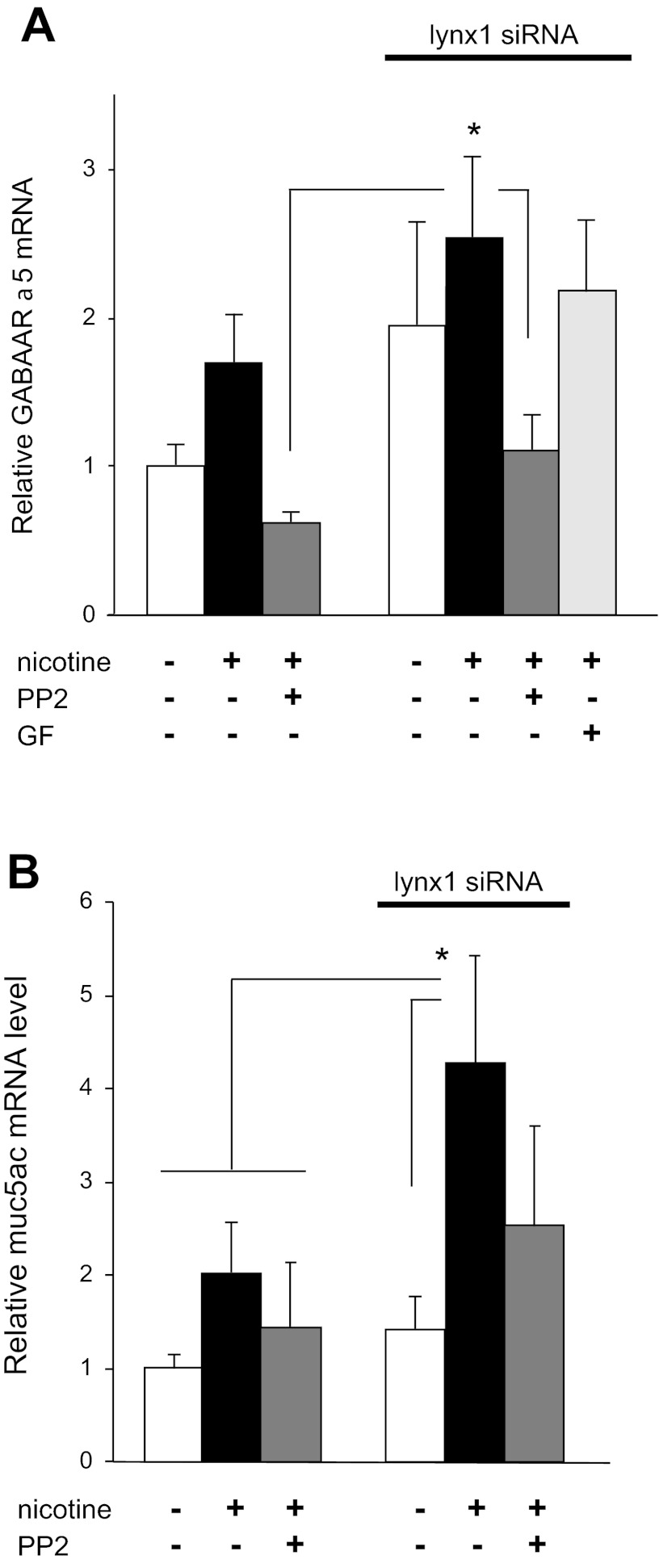
Src mediates regulation of GABAAR and mucin mRNA expression by nicotine and lynx1. A: Src inhibitor PP2 (1 μM) decreased the ability of nicotine (1 μM) and lynx1 knockdown to increase GABAAR α5 mRNA expression in cultured BEC. By comparison, the protein kinase C inhibitor GF 109203X (GF) had no effect. *P < 0.05 for nicotine + siRNA-treated group compared with groups shown by Fisher's multiple-comparison tests after 1-way ANOVA. B: nicotine-induced increase in mucin mRNA was blocked by PP2. PP2 also blocked the additional increase in mucin mRNA caused by lynx1 knockdown. *P < 0.05 for nicotine + siRNA-treated group compared with groups shown by Fisher's multiple-comparison tests after 1-way ANOVA (n = 5 per group). Drugs and siRNAs were added to cultures 48 h before harvesting of cells.
Next, the role of Src in mediating the effects of nicotine and lynx1 on GABA expression was confirmed by the use of inhibitors. As shown in Fig. 5A, 1 μM PP2, a potent inhibitor of Src family kinases, blocked the ability of nicotine and lynx1 knockdown to increase BEC GABAAR α5 mRNA expression. By contrast, the PKC inhibitor GF 109203X had no effect (Fig. 5A). This suggests that nicotine increases GABAAR expression through a Src-dependent mechanism that is inhibited by lynx1.
Lynx1 modulates MUC5AC mRNA expression.
Mucus overproduction characterizes most smoking-associated lung diseases including COPD and asthma. We have previously reported that nicotine stimulated mucin overproduction in monkey lung through activation of GABA signaling (14). This therefore suggested that, if lynx1 regulates nicotine-induced GABA signaling, then lynx1 likely also affects nicotinic regulation of mucin expression. This is the case as shown in Fig. 5B, in which lynx1 knockdown significantly increases the ability of nicotine to increase MUC5AC mRNA levels.
DISCUSSION
The present study shows that lynx1 colocalizes and forms a complex with α7 nAChR in BEC and serves as a negative regulator of α7 nAChR signaling. Knockdown of lynx1 increased the ability of nicotine to sequentially activate nicotinic and GABAergic signaling by BEC, leading to increased nicotine-stimulated MUC5AC RNA expression. This finding has implications both for the general physiology of transmitter signaling cascades in airway epithelium and specifically for potential new ways to target airway diseases characterized by the overproduction of mucin.
Airway epithelial cells express multiple classical neurotransmitters and their receptors including ACh, serotonin, GABA, glycine, and glutamate (14). We have recently reported that these systems communicate within BEC similarly to neuronal networks to modulate physiological function, as evidenced by the ability of nicotinic signaling to upregulate GABA signaling (14). In this study, we extend the parallels of BEC signaling to neuronal networks by showing that BECs similarly use transmitter receptor-negative allosteric modulators to regulate signaling. In 1999, Miwa et al. (20, 27) isolated lynx1 based on its homology to the α7 nAChR antagonist αBGT and showed that lynx1 functioned as a negative allosteric modulator of nAChR function to play critical roles in neuronal survival and plasticity (29). We have previously reported that high levels of lynx1 are expressed in BEC and that levels of lynx1 are affected by chronic nicotine exposure (39), thus suggesting that lynx1 likely affects nicotinic signaling in lung as in brain. Consistent with this, as shown in Fig. 1, lynx1 specifically colocalizes with α7 nAChR in BEC both in vivo and in primary cultures of BEC. Furthermore, lynx1 forms a complex with α7 nAChR in BEC as shown by immunoprecipitation (Fig. 2). This suggests that lynx1 serves to modify α7 signaling in BEC. Although immunohistochemistry for nicotinic receptors can be nonspecific (30), specificity of the staining reported here is supported by our previous validation of α7 expression in lung by DNA sequencing and ligand binding (37) and lynx1 expression by DNA sequencing and in situ hybridization (39).
Reflecting the high expression of nAChR in airways, multiple reports of nicotinic functions in lung have been reported, including regulation of chloride secretion (18), chemosensation (22), differentiation (23), and cell growth (34, 47). Our laboratory and others have also reported direct results of nicotinic activation in lung, including upregulation of nAChR expression (1, 13, 35, 50) and upregulation of GABA signaling and mucin expression (14). This would suggest that, if lynx1 is a negative regulator of nicotinic signaling in BEC, then these specific results of nicotinic signaling in BEC should be modified by lynx1. This is indeed the case. First, as shown in Fig. 2, siRNA knockdown of lynx1 significantly increased the ability of nicotine to increase nAChR mRNA levels. No effect was seen with control siRNAs, whereas siRNA knockdown of lynx1 significantly increased the ability of nicotine to increase levels of α4, α7, α9, and β4 nAChR mRNAs. Similarly, knockdown of lynx1 increased levels of GABAAR (Fig. 3) in an α7 nAChR-dependent manner. Thus lynx1 serves as a negative regulator of nicotinic signaling and also modulates the ability of nicotinic signaling to activate GABA signaling. These effects of lynx1 appear to be mediated by α7 nAChR because both α7 knockdown and α7 antagonists block the ability of lynx1 knockdown to increase levels of both nAChR and GABAAR mRNAs (Figs. 2 and 3). The role of α7 is further supported by its coimmunoprecipitation with lynx1 as shown in Fig. 1.
Dasgupta et al. (7) have shown that the Src signaling pathway mediates the effects of α7 activation on lung cancer proliferation. In A549 lung adenocarcinoma cells, nicotine induces the formation of an oligomeric complex, involving arrestin-β1 (ARRB1), Src, and α7 nAChR that results in the activation of Src (7–9). This in turn leads to activation of Ras, Raf, and Erk and increased cell proliferation (7, 8). Our study shows that Src similarly appears to mediate the ability of nicotine and lynx1 knockdown to increase levels of nAChR and GABAAR in normal BEC. As shown in Fig. 4, nicotine increases Src and Src phosphorylation, and this increase is further enhanced by lynx1 knockdown. The Src activation caused by nicotine and lynx1 knockdown is dependent on α7, as α7 knockdown prevents both increased Src activation and increased GABAAR expression. This suggests that one way in which lynx1 modulates nicotinic signaling is through inhibition of Src activation. This is supported by the ability of PP2, an inhibitor of Src kinase to block the nicotine and lynx1 knockdown-induced increase in and GABAAR mRNA levels (Fig. 5A). The mechanism of lynx1 regulation of activation of α7 signaling by lynx1 remains to be determined but may involve displacement of lynx1 from α7 and a resulting conformational shift in α7.
Dasgupta et al. (8) have demonstrated that nicotinic activation of α7 nAChR leads to transcriptional activation through ARRB1 translocating into the nucleus and forming a complex with E2F. Thus a similar mechanism may underlie the mechanism by which nicotinic activation of α7 nAChR in BEC increases levels of nAChR and GABAAR mRNAs although this clearly requires further investigation. Transcriptional activation may also explain the increase in total levels of Src protein (Fig. 4) although this too requires further investigation. The increases in levels of total Src caused by nicotine thus have potential to create a positive feedback loop.
Xiang at al. (49) have demonstrated that increased GABA signaling in BEC leads to increased mucus production. We recently reported that one mechanism by which smoking increases mucus production is nicotine activating α7 nAChR signaling, which in turn leads to increased GABA signaling (14). Thus, if lynx1 acts as a negative modulator of α7 signaling, then lynx1 should also modulate the ability of nicotine to cause increased mucin expression. As shown in Fig. 5B, this is indeed the case; knockdown of lynx1 significantly increases the ability of nicotine to upregulate Muc5ac mRNA levels and, like the effects on GABAAR mRNA levels, is Src dependent.
Although this study is focused on the role of lynx1 in normal lung, this also has important implications for lung cancer. Given the increased expression of nicotinic receptors in lung cancer and the ability of nicotinic activation to stimulate lung cancer growth (7, 36, 41, 46), this suggests that levels of lynx1 expression might also modulate lung cancer growth. This is indeed the case, as we have recently reported in abstract form (40) and have further studies in progress.
The finding that lynx1 can modulate the nicotine-induced increase in mucin mRNA expression is potentially important given the role that mucus hypersecretion plays in COPD and asthma. COPD is almost always associated with smoking and is characterized by increased mucus production, and the degree of mucin expression correlates with the degree of airflow obstruction (5, 5, 11, 31). In asthma, mucus plugs occlude small airways (4, 10, 11, 17, 28, 32), and overproduction of mucus is associated with mortality in severe asthma attacks (4). However, although mucin is a key component of most common respiratory diseases, there are few effective and specific treatments for excess mucin production. Thus lynx1 presents a new therapeutic target for asthma and COPD, and lynx1 agonists may serve to limit both basal and smoking-induced mucus production. Whether lynx1 levels are changed in diseases characterized by excess mucus production remains to be determined.
GRANTS
This work was supported by NIH grants OD011092, CA151601, and HL087710.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.W.F. and E.R.S. conception and design of research; X.W.F. and S.S.R. performed experiments; X.W.F., S.S.R., and E.R.S. analyzed data; X.W.F., S.S.R., and E.R.S. interpreted results of experiments; X.W.F. prepared figures; X.W.F. drafted manuscript; X.W.F. and E.R.S. edited and revised manuscript; X.W.F., S.S.R., and E.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anda Corna and the OD-011092-supported imaging core for assistance with confocal microscopy.
REFERENCES
- 1. Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: acceleration of sequential expression of α5 and α7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J 22: 1356–1368, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Arredondo J, Chernyavsky AI, Jolkovsky DL, Webber RJ, Grando SA. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol 208: 238–245, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol 125: 1236–1241, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 8: 183–192, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Caramori G, Casolari P, Di GC, Saetta M, Baraldo S, Boschetto P, Ito K, Fabbri LM, Barnes PJ, Adcock IM, Cavallesco G, Chung KF, Papi A. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology 55: 321–331, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet 12: 3017–3024, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest 116: 2208–2217, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dasgupta P, Rizwani W, Pillai S, Davis R, Banerjee S, Hug K, Lloyd M, Coppola D, Haura E, Chellappan SP. ARRB1-Mediated Regulation of E2F Target Genes in Nicotine-Induced Growth of Lung Tumors. J Natl Cancer Inst 103: 317–333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci 29: 151–158, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 15: 4–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther 121: 332–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu XW, Lindstrom J, Spindel ER. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am J Respir Cell Mol Biol 41: 93–99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am J Respir Cell Mol Biol 44: 222–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galat A, Gross G, Drevet P, Sato A, Menez A. Conserved structural determinants in three-fingered protein domains. FEBS J 275: 3207–3225, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Hays SR, Fahy JV. The role of mucus in fatal asthma. Am J Med 115: 68–69, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364: 709–721, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hollenhorst MI, Lips KS, Weitz A, Krasteva G, Kummer W, Fronius M. Evidence for functional atypical nicotinic receptors that activate K+-dependent Cl- secretion in mouse tracheal epithelium. Am J Respir Cell Mol Biol 46: 106–114, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Hruska M, Keefe J, Wert D, Tekinay AB, Hulce JJ, Ibanez-Tallon I, Nishi R. Prostate stem cell antigen is an endogenous lynx1-like prototoxin that antagonizes alpha7-containing nicotinic receptors and prevents programmed cell death of parasympathetic neurons. J Neurosci 29: 14847–14854, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron 33: 893–903, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kini RM, Doley R. Structure, function and evolution of three-finger toxins - mini proteins with multiple targets. Toxicon 56: 855–867, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schutz B, Kotlikoff M, Ibanez-Tallon I, Kummer W. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA 108: 9478–9483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res 36: 390–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levitin F, Weiss M, Hahn Y, Stern O, Papke RL, Matusik R, Nandana SR, Ziv R, Pichinuk E, Salame S, Bera T, Vincent J, Lee B, Pastan I, Wreschner DH. PATE gene clusters code for multiple, secreted TFP/Ly-6/uPAR proteins that are expressed in reproductive and neuron-rich tissues and possess neuromodulatory activity. J Biol Chem 283: 16928–16939, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56: 237–246, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N. Lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron 23: 105–114, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C, Fitzsimonds RM, Pavlides C, Lester HA, Picciotto MR, Heintz N. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron 51: 587–600, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med 12: 1–6, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330: 1238–1240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moser N, Mechawar N, Jones I, Gochberg-Sarver A, Orr-Urtreger A, Plomann M, Salas R, Molles B, Marubio L, Roth U, Maskos U, Winzer-Serhan U, Bourgeois JP, Le Sourd AM, De BM, Schroder H, Lindstrom J, Maelicke A, Changeux JP, Wevers A. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J Neurochem 102: 479–492, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Pelkonen M. Smoking: relationship to chronic bronchitis, chronic obstructive pulmonary disease and mortality. Curr Opin Pulm Med 14: 105–109, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Pietinalho A, Pelkonen A, Rytila P. Linkage between smoking and asthma. Allergy 64: 1722–1727, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Robinson CB, Wu R. Culture of conducting airway epithelial cells in serum-free medium. J Tiss Cult Meth 13: 95–102, 1991 [Google Scholar]
- 34. Roman J, Koval M. Control of lung epithelial growth by a nicotinic acetylcholine receptor. The other side of the coin. Am J Pathol 175: 1799–1801, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakurai R, Cerny LM, Torday JS, Rehan VK. Mechanism for nicotine-induced up-regulation of Wnt signaling in human alveolar interstitial fibroblasts. Exp Lung Res 37: 144–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer 9: 195–205, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 103: 637–647, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 26: 31–41, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Sekhon HS, Song P, Jia Y, Lindstrom J, Spindel ER. Expression of lynx1 in developing lung and its modulation by prenatal nicotine exposure. Cell Tissue Res 320: 287–297, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Song P, Rekow SS,., Jia Y, Singleton TC, Sekhon HS, Spindel ER. Lynx1, an endogenous negative allosteric modulator of nicotinic acetylcholine receptors, provides a new target for non-small cell lung carcinoma. Annual Meeting of the American Thoracic Society 2012 (Abstract) [Google Scholar]
- 41. Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, Saha S, Spindel ER. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res 68: 4693–4700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 63: 214–221, 2003 [PubMed] [Google Scholar]
- 43. Tekinay AB, Nong Y, Miwa JM, Lieberam I, Ibanez-Tallon I, Greengard P, Heintz N. A role for LYNX2 in anxiety-related behavior. Proc Natl Acad Sci USA 106: 4477–4482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsetlin V. Snake venom alpha-neurotoxins and other ‘three-finger’ proteins. Eur J Biochem 264: 281–286, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Wang G, Wang R, Ferris B, Salit J, Strulovici-Barel Y, Hackett NR, Crystal RG. Smoking-mediated up-regulation of GAD67 expression in the human airway epithelium. Respir Res 11: 150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81–90, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wongtrakool C, Wang N, Hyde DM, Roman J, Spindel ER. Prenatal nicotine exposure alters lung function and airway geometry through alpha7 nicotinic receptors. Am J Respir Cell Mol Biol 46: 695–702, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu R, Martin WR, Robinson CB, St George JA, Plopper CG, Kurland G, Last JA, Cross CE, McDonald RJ, Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol 3: 467–478, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O'Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med 13: 862–867, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol 37: 681–690, 2007 [DOI] [PubMed] [Google Scholar]