Abstract
Myofibroblasts are implicated in pathological stromal responses associated with lung fibrosis. One prominent phenotypic marker of fully differentiated myofibroblasts is the polymerized, thick cytoplasmic filaments containing newly synthesized α-smooth muscle actin (α-SMA). These α-SMA-containing cytoplasmic filaments are important for myofibroblast contractility during tissue remodeling. However, the molecular mechanisms regulating the formation and maturation of α-SMA-containing filaments have not been defined. This study demonstrates a critical role for neuronal Wiskott-Aldrich syndrome protein (N-WASP) in regulating the formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and in myofibroblast contractility. Focal adhesion kinase (FAK) is activated by transforming growth factor-β1 (TGF-β1) and is required for phosphorylation of tyrosine residue 256 (Y256) of N-WASP. Phosphorylation of Y256 of N-WASP is essential for TGF-β1-induced formation of α-SMA-containing cytoplasmic filaments in primary human lung fibroblasts. In addition, we demonstrate that actin-related protein (Arp) 2/3 complex is downstream of N-WASP and mediates the maturation of α-SMA-containing cytoplasmic filaments. Together, this study supports a critical role of N-WASP in integrating FAK and Arp2/3 signaling to mediate formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation.
Keywords: fibrosis, fibroblast, transforming growth factor, lung fibrosis, focal adhesion kinase
myofibroblasts are differentiated or “activated” fibroblasts; they are present in abundance within fibrotic lesions in the lung, liver, and kidney (8, 35, 47). Myofibroblast differentiation is one critical step during the development of fibrotic disorders. Persistency of myofibroblasts leads to aberrant wound healing, often resulting in excessive accumulation of extracellular matrix (ECM) proteins in affected tissues. Myofibroblasts have enhanced ability to contract and have increased ability to secrete profibrotic cytokines and to produce ECM proteins (such as collagen); therefore, they are believed to be the major cell type responsible for the excessive ECM accumulation observed in fibrotic disorders, such as idiopathic pulmonary fibrosis (IPF) (8, 23, 35). In addition, myofibroblasts are implicated in the stromal reaction associated with cancer progression and invasion (23).
Recent evidence indicates that the sources of myofibroblasts are variable among different organs (23). Myofibroblast differentiation is a complex process. Transforming growth factor-β1 (TGF-β1) is a potent cytokine that promotes myofibroblast differentiation, and TGF-β1-mediated signaling is central to fibrotic responses in lung, liver, and kidney (9, 27, 31, 41). Myofibroblast differentiation requires sequential and synergized events, including activation of TGF-β1, matrix rigidity, integrin signaling, and expression of EDA fibronectin fragment (an alternative fibronectin fragment) (18, 23, 24, 26, 32, 33, 49).
De novo expression of α-smooth muscle actin (α-SMA) and formation of α-SMA-containing cytoplasmic filaments are hallmarks of myofibroblast differentiation (8, 23, 35). During myofibroblast differentiation, the cytoplasmic filament network is reorganized, and newly synthesized α-SMA monomers are incorporated into the reorganized cytoplasmic filament network (8, 23, 35). These α-SMA-containing cytoplasmic filaments directly connect to focal adhesions and are essential for enhancing the contractile phenotype of myofibroblasts (20, 21, 23). These newly formed, thickened α-SMA-containing cytoplasmic filaments are a unique characteristic of mature myofibroblasts (20, 21, 23). The mechanisms regulating the incorporation of α-SMA monomer into cytoplasmic filaments during myofibroblast differentiation and maturation are largely unknown.
It has been demonstrated that neuronal Wiskott-Aldrich syndrome protein (N-WASP) plays an important role in filopodia formation and cell spreading and regulates cell migration (15, 36, 44, 51). The initially identified WASP is expressed specifically in hematopoietic cells, and the later identified N-WASP is ubiquitously expressed (15, 36, 44). We have previously demonstrated that focal adhesion kinase (FAK) is activated by TGF-β1, and FAK activation is required for formation α-SMA-containing filaments in TGF-β1-treated fibroblasts (10, 46). FAK plays a critical role in mediating cell migration and focal adhesion turnaround and reorganization during cell migration (15, 19, 34, 36, 39, 42). Although FAK and N-WASP mediate cell migration, their physiological interaction and roles in formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation remain to be defined.
Here, we demonstrate that N-WASP is required for formation of α-SMA-containing cytoplasmic filaments; N-WASP overexpression enhances the formation of α-SMA-containing cytoplasmic filaments in TGF-β1-treated lung fibroblasts. FAK is an upstream regulator of N-WASP, and FAK-mediated N-WASP phosphorylation is required for the formation of α-SMA-containing cytoplasmic filaments. Furthermore, actin-related protein (Arp) 2/3 complex is essential for the formation of α-SMA-containing cytoplasmic filaments via FAK and N-WASP. Together, our data suggest that N-WASP is a key intermediate in the regulation of myofibroblast differentiation and maturation by integrating TGF-β1-induced FAK and Arp2/3 signaling.
MATERIALS AND METHODS
Reagents.
TGF-β1 was obtained from R&D Systems (Minneapolis, MN). The following purified polyclonal antibodies were purchased: anti-N-WASP, anti-FAK, anti-phosphotyrosine, and anti-phosphotyrosine 256 of N-WASP (Millipore, Billerica, MA), and anti-green fluorescent protein (GFP) (Santa Cruz Biotechnology, Santa Cruz, CA). The following purified monoclonal antibodies were purchased: anti-Arp3 and anti-myc tag (Santa Cruz Biotechnology), Cy3-conjugated anti-α-SMA antibody (clone 1A4; Sigma-Aldrich, St. Louis, MO), α-SMA (American Research Products, Belmont, MA), procollagen-α1 type 1 (1A1) (Santa Cruz Biotechnology), and anti-GAPDH (Research Diagnostics, Flanders, NJ). Chemicals were purchased from Sigma-Aldrich and Thermo Fisher Scientific (Waltham, MA).
Cells and cell culture.
Primary murine lung fibroblasts were derived from 7–10-wk-old C57BL/6 mice and FAK-floxed mice (2) and maintained and propagated as described by us previously (10). IACUC approval was obtained from the University of Alabama at Birmingham for harvesting cells from mice. Briefly, primary cultures of lung fibroblasts were established by mechanical disaggregation and enzymatic digestion (collagenase and DNase) of sterilely removed lungs and by cell culture. Fibroblasts were maintained in DMEM supplemented with 10% FBS, 20 mM HEPES, and 100 U/ml penicillin/streptomycin/gentamycin. Normal adult human lung fibroblasts were purchased from the Cambrex (Walkersville, MD) and American Type Culture Collection (Manassas, VA). Human lung fibroblasts were propagated and maintained in DMEM supplemented with 10% FBS, 2 mM l-glutamine, and 100 U/ml penicillin/streptomycin as described by us previously (4).
Lentiviral vectors and stable clones.
The replication incompetent lentiviral vectors expressing short hairpin RNA (shRNA) for silencing N-WASP or Arp3 and lentiviral vectors expressing control nontargeting shRNA were from Thermo Fisher Scientific. Lung fibroblasts were transfected with lentiviral vectors according to manufacturers' instructions. Stable lung fibroblast clones expressing the above shRNAs were selected and maintained in medium containing 8 μg/ml puromycin according to the manufacturer's instructions and screened by Western blot analysis (for protein level) and quantitative RT-PCR (for mRNA level) as described by us previously (13).
Western blotting.
Western blotting was conducted as described by us previously (11). Briefly, cells were lysed in 1% NP-40 lysis buffer containing the following inhibitors, 100 μM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 100 μM sodium vanadate. Protein concentration of whole cell lysate was determined by BCA kit (Pierce, Rockford, IL). Equivalent micrograms of whole cell lysates were electrophoresed on a disulfide-reduced 7.5% SDS PAGE, transferred to Immobilon-P membrane (Millipore, Bedford, MA), probed with indicated antibodies, and developed with ECL system (Pharmacia Biotech, Piscataway, NJ). The expression of GAPDH protein was used as a loading control. For densitometric analysis of band intensity, a specific band on the ECL-developed film was subjected to densitometric analysis. The densitometric readings were pooled and averaged from three independent experiments. The background of densitometric reading on the ECL-developed film was subtracted.
Immunofluorescence analysis.
Immunofluorescence (IF) was performed as described previously (14). Briefly, cells cultured on glass coverslips were fixed in 4% buffered paraformaldehyde and permeabilized. To study the α-SMA-incorporated cytoplasmic filaments, cells were reacted with Cy3-conjugated anti-α-SMA monoclonal antibody (1:50) for overnight at 4°C. Cell nuclei were stained with blue Hoechst fluorescence dye to count the total cells per field. The α-SMA-containing cytoplasmic filaments are the prominent feature of myofibroblasts. They are long and thick and generally aligned in parallel with the long axis of the cells. These highly organized filaments are not seen in undifferentiated fibroblasts. α-SMA-positive filaments exist in a small percentage of undifferentiated fibroblasts; however, they are much thinner and shorter, and they are not aligned in a specific way or pattern. All cells containing the visible α-SMA-containing filaments in each group were counted, and the percentage is calculated over total cells per field. Digital fluorescence images were obtained by using a Nikon microscope and software (Nikon). The results were pooled from eight randomly selected, nonoverlapping fields from a total of three experiments (each performed in duplicates).
F/G-actin content.
Relative proportions of F-actin and G-actin were determined and quantified by using a kit from Cytoskeleton (Denver, CO) and Western blot analysis as described by us previously (25).
Quantitative real-time RT-PCR analysis.
Quantitative RT-PCR was performed as described by us previously (52). Briefly, total RNA is extracted from lung fibroblasts using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instruction. The following primers were used: N-WASP, sense 5′-CCCCCAAATGGTCCTAATCT-3′ and antisense 5′- ACATGTCCAATGTGCTGGAA-3; GAPDH, sense 5′-GAGTCAACGGATTTGGTCGT-3′ and antisense 5′-TTGATTTTGGAGGGATCTCG-3′. One to three micrograms of total RNA were reverse transcribed to cDNA with Maloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Quantitative RT-PCR analysis was carried out with the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) using the Roche Light Cycler 480. Samples were assayed in triplicate, and the values were normalized to the relative amounts of GAPDH.
Collagen gel contraction assay.
Collagen gel contraction assays were performed according to the instructions of commercial vendors and described by others (5, 29). Collagen gels were cast in six-well plates from type I collagen/DMEM solution composed of bovine skin collagen type I (Invitrogen, Palo Alto, CA), DMEM containing Hepes and gentamicin, NaOH (0.142 M), and PBS. The gels were in liquid form at 4°C and solidified at 37°C. Lung fibroblasts were seeded into the collagen gel (100,000 cells/well) and incubated at 37°C, CO2 level of 5% for indicated time. Fibroblast gel complex contraction was monitored by standardized photography at time 0 and at indicated time points. The area of fibroblast gel complex in digitized photographs was measured, and the ratio of collagen gel area against the culture well area was calculated. The data were pooled from three experiments (each with duplicates) and were presented as the percentage of contraction relative to vehicle-treated group.
In vitro fluorescent pyrene actin polymerization assay.
Assays were performed using commercially available kits (Cytoskeleton, Denver, CO) and as described previously by us (50). The fluorescent signal of monomer pyrene actin is enhanced during its polymerization into filaments, making it an ideal tool for monitoring α-SMA filament formation in vitro. For α-SMA actin polymerization assays, myc-tagged N-WASP (0.6 μg) or myc-tagged N-WASP mutant (N-WASP-Y256F, 0.6 μg) was incubated with recombinant FAK (1.2 μg) in actin polymerization buffer containing with or without 50 nM Arp 2/3 complex and 100 μl of pyrene α-SMA monomer stock in 0.2 mM ATP-containing buffer (final α-SMA concentration, 2.8 μM). Fluorescence changes were monitored by using a fluorometer with filters for excitation at 360 nm and emission at 410 nm according to the manufacturer's instructions.
Generation and utilization of adenoviral vectors.
The adenoviral vectors containing the myc-tagged N-WASP cDNA (Ad-NWASP), the myc-tagged N-WASP mutant (Y256F) cDNA (Ad-NWASP-Y256F), Cre recombinase (Ad-Cre), and GFP cDNA (Ad-GFP) were generated, amplified, and used as previously described (4, 10). Briefly, the replication-deficient adenoviral vectors were generated by using the Adeno-X expression system 2 according to the manufacturer's manual (Clontech, Mountain View, CA). The adenoviral vectors were rescued and amplified in 293 cells and purified by CsCl gradient centrifugation.
Statistical analysis.
Data were analyzed using the Student's t-test analysis (Sigma Plot, SPSS) for differences between two groups and expressed as means ± SE. All experiments were repeated at least three times. A P value <0.05 was considered to be statistically significant.
RESULTS
N-WASP is required for formation of α-SMA-containing cytoplasmic filaments induced by TGF-β1 in lung fibroblasts.
The appearance of thickened α-SMA-containing cytoplasmic filaments is one key cellular marker for a successful phenotypic change from fibroblasts to myofibroblasts (23). TGF-β1 is known as a potent profibrotic cytokine that induces myofibroblast differentiation in lung fibroblasts (10, 23). However, the involved molecular mechanisms, as well as the key signaling proteins, are largely under explored. It has been shown that N-WASP is involved in G-actin polymerization during cell migration and spreading (28, 43, 51). Whether N-WASP plays a role in formation of α-SMA-containing filaments through α-SMA polymerization during myofibroblast differentiation and maturation remains unknown. To understand the role of N-WASP in formation of α-SMA-containing filaments during myofibroblast differentiation and maturation, we examined the effect of loss or gain of function of N-WASP on TGF-β1-induced formation of α-SMA-containing filaments in fibroblasts.
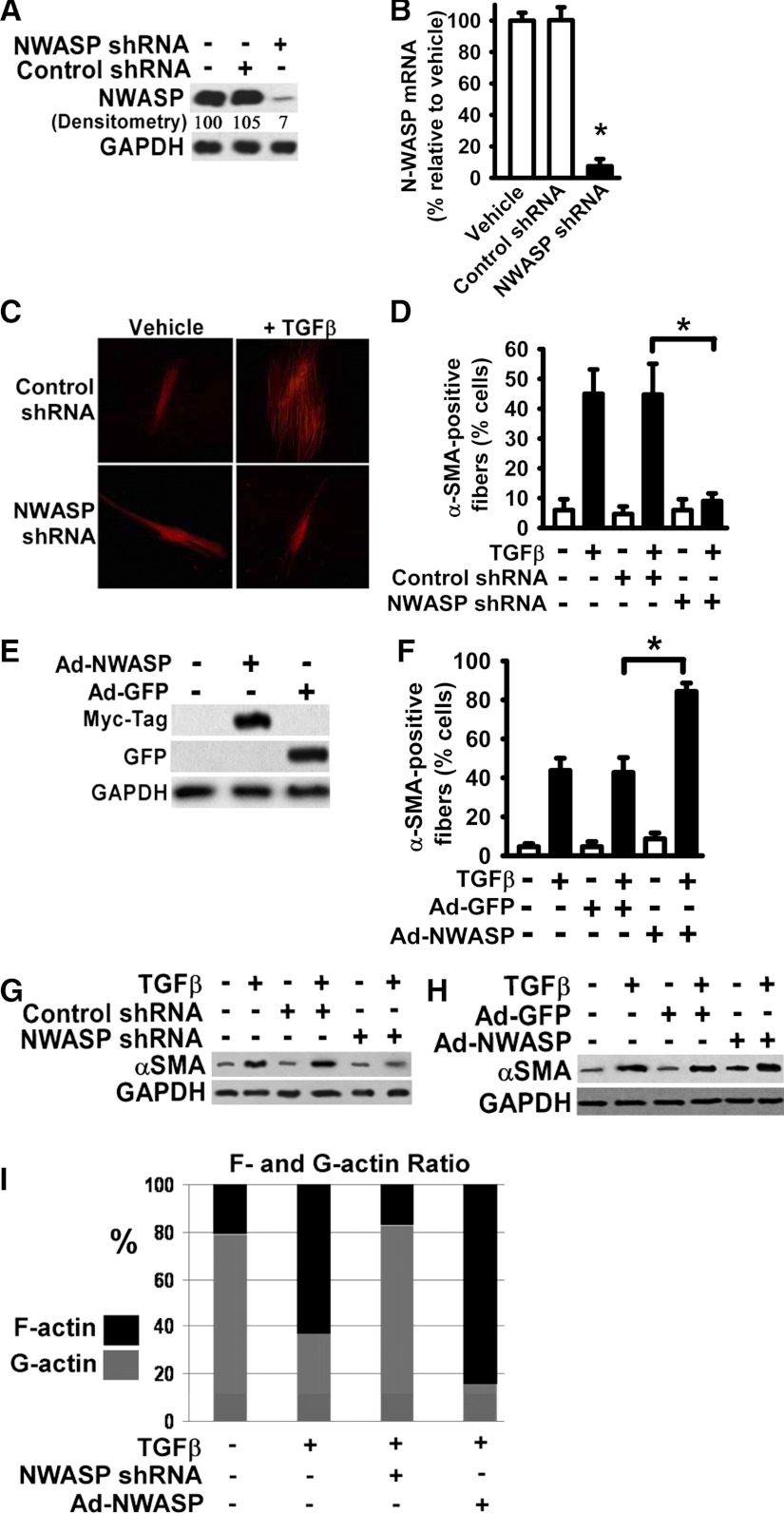
To downregulate N-WASP in normal human lung fibroblasts, lentiviral vectors expressing the shRNA specifically directed toward N-WASP mRNA were utilized. Stable clones expressing N-WASP shRNA or nontargeting control shRNA were selected by puromycin resistance as described in materials and methods. Lung fibroblasts stably expressing N-WASP shRNA or nontargeting shRNA were lysed and subjected for analysis of N-WASP protein expression. Western blot analysis demonstrated that N-WASP protein level was significantly reduced (∼93% reduction) in lung fibroblasts expressing N-WASP shRNA, compared with that in lung fibroblasts treated with vehicle only or expressing nontargeting shRNA (Fig. 1A). In addition, quantitative RT-PCR confirmed that N-WASP mRNA level had been significantly reduced by N-WASP shRNA (∼91% reduction relative to vehicle or nontargeting shRNA controls) (Fig. 1B). These data demonstrate that N-WASP shRNA effectively decreases N-WASP expression in lung fibroblasts.
Fig. 1.
Neuronal Wiskott-Aldrich syndrome protein (N-WASP) downregulation abrogated the transforming growth factor (TGF)-β1-induced α-smooth muscle actin (α-SMA)-containing cytoplasmic filaments in human lung fibroblasts. A: normal human lung fibroblasts were infected with lentiviral vectors containing N-WASP shRNA or control nontargeting shRNA, and stable clones were selected according to puromycin resistance as described in materials and methods. Fibroblasts were harvested and lysed. Equivalent amount of whole cell detergent lysates were Western blotted with the indicated antibodies. GAPDH is a loading control. Densitometry of band intensity relative to GAPDH is shown (vehicle-treated cells in lane 1 is as 100%). B: lung fibroblasts stably expressing N-WASP shRNA or nontargeting control shRNA or vehicle-treated lung fibroblasts were subjected to total RNA extraction and followed by quantitative RT-PCR to determine the level of N-WASP mRNA. Data are represented as means ± SE. *P < 0.01 for cells with N-WASP shRNA compared with cells with vehicle only. C: lung fibroblasts stably expressing N-WASP shRNA or control shRNA were cultured in serum-free media (SFM) with 1% BSA, and treated without or with TGF-β1 (10 ng/ml). Cells were fixed after 48 h and immunofluorescently stained with Cy-3-labeled monoclonal antibody toward α-SMA, and fluorescent microscopic digital images were taken (×200). Representative pictures were shown. D: quantification of the percentage of cells with α-SMA-containing filaments. The data are presented as the means ± SE. Open bars are vehicle-treated cells. Solid bars are TGF-β1-treated cells. *P < 0.01. E: lung fibroblasts were infected with adenoviral vectors expressing either myc-tagged N-WASP cDNA (Ad-NWASP) or green fluorescent protein (GFP) cDNA (Ad-GFP), lysed, and Western blotted with indicated antibodies. F: lung fibroblasts infected with Ad-NWASP or control Ad-GFP were treated with or without TGF-β1 (10 ng/ml), and stained with Cy-3-labeled antibody toward α-SMA as in C. The percentages of cells with α-SMA-containing filaments were quantified and presented as in D. * P < 0.01. G–H: lung fibroblasts stably expressing N-WASP shRNA (as in C) or infected with Ad-NWASP (as in F) were treated with or without TGF-β1 (10 ng/ml) for 48 h, lysed, and Western blotted with indicated antibodies. I: effect of N-WASP downregulation (by shRNA) or overexpression (by Ad-NWASP) on actin dynamics were determined (in cells as described in G–H) by measurements of F-/G-actin content as described in materials and methods.
To examine the effect of loss of N-WASP on formation of α-SMA-containing filaments during myofibroblast differentiation and maturation, lung fibroblasts expressing N-WASP shRNA or nontargeting control shRNA were treated with TGF-β1 (10 ng/ml) for 48 h to induce the myofibroblastic phenotype. TGF-β1 induced well-organized, thickened α-SMA-containing cytoplasmic filaments in control lung fibroblasts (Fig. 1C, top, right). Most undifferentiated fibroblasts do not show α-SMA-positive filaments. α-SMA-positive filaments exist in only about 5% of undifferentiated fibroblasts (without TGF-β1), and they are very thin, short, not aligned in a specific pattern. The percentage of cells with α-SMA-containing filaments is significantly increased in fibroblasts treated with TGF-β1. These α-SMA-containing filaments are much thicker and longer and generally aligned in parallel with the long axis of the myofibroblasts. The pattern of these filaments is not seen in undifferentiated fibroblasts. They are the prominent feature of myofibroblasts and are the most widely used molecular marker for myofibroblasts. The results indicate a phenotypic transition from fibroblasts to myofibroblasts or myofibroblast differentiation.
In contrast, N-WASP downregulation (by shRNA) abrogated the TGF-β1-induced formation of well-organized α-SMA-containing filaments in lung fibroblasts (Fig. 1C, bottom, right). Nontargeting shRNA had no effect on the TGF-β1-induced formation of α-SMA-containing filaments in these cells (Fig. 1C, top, right). The percentage of cells with α-SMA-containing filaments was quantified, and the results included all cells with visible α-SMA-containing filaments in each group. The results confirmed that N-WASP downregulation inhibited the formation of α-SMA-containing filaments in TGF-β1-treated lung fibroblasts (Fig. 1D). The findings support that N-WASP is required for the formation of α-SMA-containing filaments during myofibroblast differentiation.
Next, we examined the effects of gain of N-WASP on formation of α-SMA-containing filaments in lung fibroblasts. N-WASP overexpression was achieved by using adenoviral vector containing the myc-tagged N-WASP (Ad-NWASP) and confirmed by Western blot analysis (Fig. 1E). N-WASP overexpression significantly increased the percentage of cells with α-SMA-containing cytoplasmic filaments in lung fibroblasts in response to TGF-β1 (Fig. 1F, bar 6 vs. 4). Furthermore, these newly formed α-SMA-containing filaments are well organized and thickened. In contrast, overexpression of control GFP (mediated by adenoviral vector, Ad-GFP) had no effect in lung fibroblasts compared with the parental fibroblasts in response to TGF-β1 (Fig. 1F, bar 4 vs. 2). N-WASP also regulates TGF-β1-induced α-SMA expression. N-WASP downregulation decreased TGF-β1-induced α-SMA expression (Fig. 1G). In contrast, N-WASP overexpression increased TGF-β1-induced α-SMA expression (Fig. 1H), again supporting the important role of N-WASP in myofibroblast differentiation. To further understand the role of N-WASP in mediating actin polymerization, the G/F-actin ratio was determined. F-actin represents the polymerized actin form. TGF-β1 treatment significantly induced actin polymerization (Fig. 1I). In response to TGF-β1, the F-actin content is increased (from 21% to 63%, Fig. 1I, bars 1 and 2), whereas the G-actin content is decreased (from 79% to 37%) (Fig. 1I, bars 1 and 2). N-WASP downregulation (by shRNA) decreases F-actin content, indicating that TGF-β1-induced actin polymerization was inhibited (Fig. 1I, bars 2 and 3). In contrast, N-WASP overexpression increases F-actin content, supporting that TGF-β1-induced actin polymerization was promoted (Fig. 1I, bars 4 and 2). These data clearly indicate that N-WASP plays an important role in promoting the α-SMA incorporation into newly formed cytoplasmic filaments and promoting polymerization during myofibroblast differentiation and maturation.
N-WASP mediates TGF-β1-induced collagen gel contraction in lung fibroblasts.
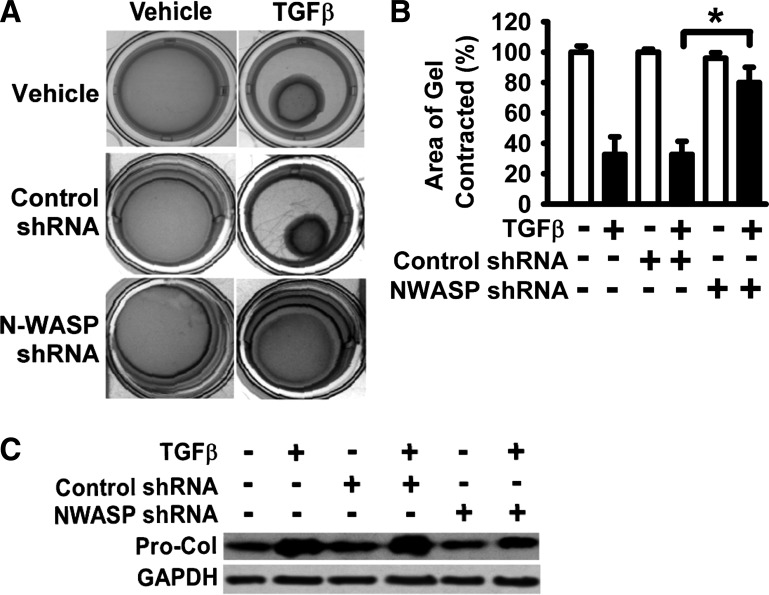
The formation of α-SMA-containing cytoplasmic filaments is, not only a hallmark of fully differentiated myofibroblasts, but also important for myofibroblast contractility (23). As N-WASP downregulation significantly reduced the formation of α-SMA-containing filaments (Fig. 1), we next examined the effect of N-WASP downregulation on myofibroblast contractility. Lung fibroblasts expressing the N-WASP shRNA or control nontargeting shRNA were treated with TGF-β1 (10 ng/ml) or vehicle and subjected to collagen gel contraction assay at 37°C, 5% CO2, for 60 h. The presence of control nontargeting shRNA had no effect on TGF-β1-induced collagen gel contraction (Fig. 2A, middle). TGF-β1 significantly induced collagen gel contraction in fibroblasts expressing the nontargeting control shRNA (Fig. 2A, middle). The collagen gels were contracted down to about 32% of the original gel area (equivalent to 68% reduction of gel size) in response to TGF-β1 (Fig. 2B, bar 4 vs. 3). In contrast, fibroblasts expressing N-WASP shRNA showed impaired ability to contract collagen gels, evidenced by that these fibroblasts only slightly contracted collagen gels in the presence of TGF-β1 (down to about 85% of the original gel area, or equivalent to 15% reduction of gel size) (Fig. 2). To further study the role of N-WASP in myofibroblast function, the collagen expression in response to TGF-β1 was determined. N-WASP downregulation (by shRNA) decreased TGF-β1-induced procollagen (Pro-Col) expression (Fig. 2C). These data support that N-WASP is required for TGF-β1-induced collagen-gel contraction, as well as collagen expression.
Fig. 2.
N-WASP downregulation blocked the ability of myofibroblasts to contract the collagen gels. A: lung fibroblasts stably expressing N-WASP shRNA or control shRNA were cultured in SFM with 1% BSA, treated with or without TGF-β1 (10 ng/ml), and followed by the collagen gel contraction assays for 60 h (at 37°C, 5% CO2) as described in materials and methods. Representative digital images were shown. B: areas of collagen gels from digitized images were measured. The ratio of collagen gel area after contraction against the original collagen gel area (before contraction and equivalent to the culture-well area) was calculated. Data are presented as the percentage of gel area relative to control (vehicle only, set as 100%). *P < 0.01. C: lung fibroblasts stably expressing N-WASP shRNA or control shRNA were cultured in SFM with 1% BSA and treated without or with TGF-β1 (10 ng/ml). Procollagen 1A1 expression was examined at 48 h by Western blot analysis.
FAK is essential for TGF-β1-induced tyrosine phosphorylation of N-WASP and formation of α-SMA-containing filaments.
We have previously shown that TGF-β1 induces FAK activation, and FAK activation is required for the formation of α-SMA-containing filaments in lung fibroblasts (10, 46). The above data demonstrate that N-WASP is required for the formation of α-SMA-containing filaments in TGF-β1-treated lung fibroblasts (Fig. 1). However, it remains to be defined whether N-WASP mediates the formation of α-SMA-containing cytoplasmic filaments through a FAK-dependent or FAK-independent pathway.
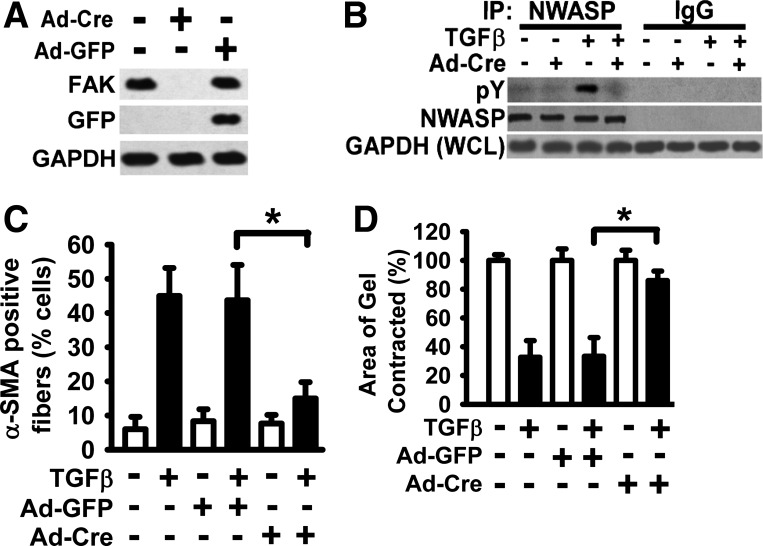
To study the interaction between FAK and N-WASP during myofibroblast differentiation and maturation, the effect of loss of FAK on N-WASP phosphorylation induced by TGF-β1 was examined first. The Cre-lox recombination system was utilized to decrease FAK expression in murine lung fibroblasts. Primary murine lung fibroblasts were derived from FAK-floxed mice and infected with adenoviral vectors encoding the Cre recombinase (Ad-Cre). FAK protein expression was effectively downregulated in fibroblasts infected with Ad-Cre according to Western blot analysis (Fig. 3A). In contrast, FAK expression was not affected in fibroblasts infected with control adenoviral vectors (Ad-GFP) (Fig. 3A).
Fig. 3.
Focal adhesion kinase (FAK) expression is essential for TGF-β1-induced tyrosine phosphorylation of N-WASP and the formation of α-SMA-containing filaments. A: primary murine lung fibroblasts were derived from FAK-floxed mice and treated with or without Ad-Cre (adenoviral vector expressing Cre recombinase) in SFM with 1% BSA. Fibroblasts were lysed, and equivalent amount of whole cell detergent lysates were Western blotted with the indicated antibodies. GAPDH is a loading control. B: cells in A were treated with or without TGF-β1 (10 ng/ml) in SFM with 1% BSA for 6 h (37°C, 5% CO2) and detergent lysed, and equivalent amount of whole cell lysates were immunoprecipitated with an anti-N-WASP antibody (NWASP IP) or control rabbit IgG (IgG IP) as indicated. The immunoprecipitates (IP) were analyzed by Western Blot with anti-phospho-tyrosine (top, pY) or anti-N-WASP (middle, NWASP). For loading controls, equivalent amounts of whole cell detergent lysates (WCL) were also analyzed with anti-GAPDH (bottom). C: lung fibroblasts as described in A were treated with or without TGF-β1 (10 ng/ml) in SFM with 1% BSA for 48 h, fixed, and stained with Cy-3-labeled antibody to visualize α-SMA-containing filaments. The percentage of cells with α-SMA-containing filaments was quantified as in Fig. 1D. Ad-GFP infection was a control for adenoviral vector. Data are presented as the means ± SE. *P < 0.01. D: lung fibroblasts were treated as in C and subjected to collagen gel contraction assay for 60 h (at 37°C, 5% CO2). Collagen gel contraction was determined, and data are presented as shown in Fig. 2. *P < 0.01.
FAK-floxed lung fibroblasts were infected with Ad-Cre or Ad-GFP, treated with TGF-β1 (10 ng/ml) or vehicle for 6 h, and detergent lysed. Whole cell lysates were immunoprecipitated by antibody specifically directed toward N-WASP or control nonimmune IgG (Fig. 3B). Western blot analysis of the immunoprecipitates showed that tyrosine phosphorylation N-WASP was significantly reduced in fibroblasts infected with Ad-Cre compared with control fibroblasts in response to TGF-β1 (Fig. 3B, top, lane 3 vs. lane 4). The findings suggest that TGF-β1-induced tyrosine phosphorylation of N-WASP is FAK dependent.
Expression of Cre (by Ad-Cre) significantly blocked the formation of α-SMA-containing filaments induced by TGF-β1 in FAK-floxed murine lung fibroblasts (Fig. 3C, bar 2 vs. 6). Control GFP expression had no effect on the formation of α-SMA-containing filaments induced by TGF-β1 (Fig. 3C, bar 2 vs. 4). These data support that FAK has an essential role in formation of α-SMA-containing filaments during myofibroblast differentiation and maturation. As α-SMA-containing filaments are associated with myofibroblast contraction, we next examined the effect of loss of FAK expression on TGF-β1-induced collagen gel contraction. TGF-β1 induced significant collagen gel contraction in FAK-floxed murine lung fibroblasts treated with vehicle only or overexpressing GFP (by Ad-GFP) (Fig. 3D, bars 2 and 4 vs. bars 1 and 3, respectively). In contrast, TGF-β1-induced collagen gel contraction was significantly reduced in fibroblasts infected with Ad-Cre (Fig. 3D, bar 6 vs. 4), indicating that FAK is required for TGF-β1-induced contractility.
TGF-β1-induced phosphorylation of tyrosine 256 (Y256) of N-WASP is FAK dependent, and N-WASP mutant (Y256F) overexpression significantly reduces the formation of α-SMA-containing filaments and contractility in lung fibroblasts.
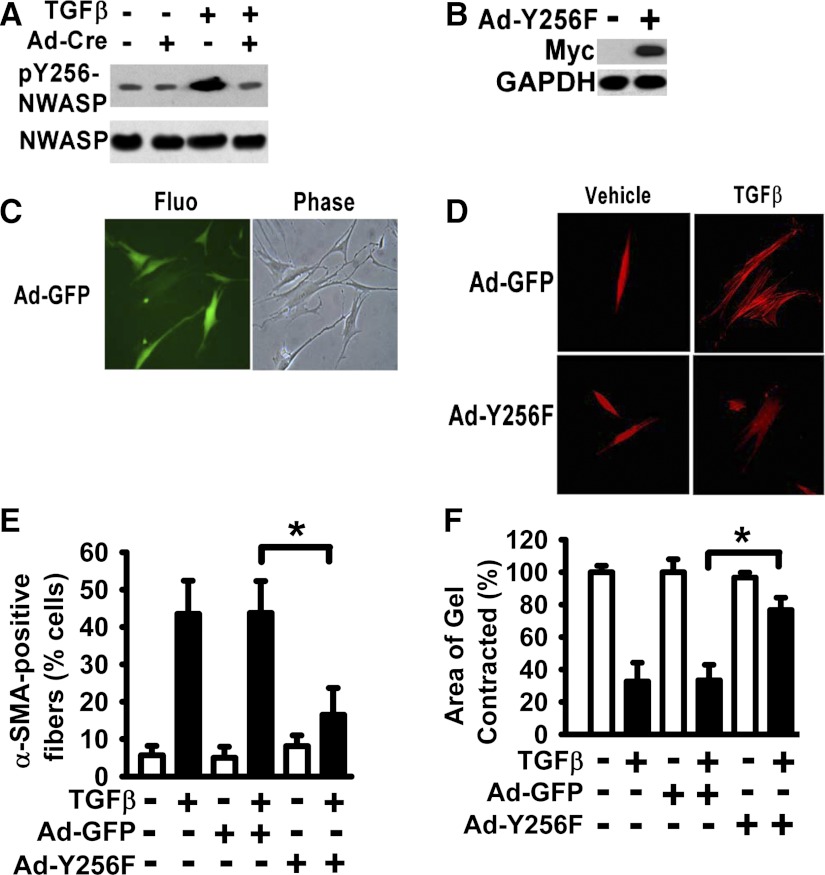
A large number of different extracellular signals can induce phosphorylation of Y256 of N-WASP (3). It takes 24–48 h for TGF-β1 to induce myofibroblast differentiation in fibroblasts. Signaling proteins leading to myofibroblast differentiation are expected to be activated earlier. To determine the role of N-WASP activation in myofibroblast differentiation, phosphorylation of Y256 of N-WASP was examined at 6 h in TGF-β1-treated cells. Our data show that TGF-β1 is able to induce phosphorylation of Y256 of N-WASP in murine lung fibroblasts (Fig. 4A, lane 3 vs. 1). To determine the effect of loss of FAK on TGF-β1-induced phosphorylation of Y256 of N-WASP, FAK-floxed murine lung fibroblasts were infected with Ad-Cre to downregulate FAK expression and then treated with or without TGF-β1 (10 ng/ml). Lung fibroblasts were detergent lysed, and whole cell lysates were Western blotted with the antibody against phosphorylation of Y256 of N-WASP. TGF-β1-induced phosphorylation of Y256 of N-WASP was significantly decreased in fibroblasts infected with Ad-Cre compared with that in control fibroblasts (Fig. 4A, top, lane 3 vs. 4), suggesting that FAK is required for phosphorylation of Y256 of N-WASP induced by TGF-β1.
Fig. 4.
Expression of N-WASP Y256F mutant significantly inhibited the formation of α-SMA-containing filaments and cell contraction in lung fibroblasts treated with TGF-β1. A: lung fibroblasts derived from FAK-floxed mice were treated with or without Ad-Cre for 24 h and treated with or without TGF-β1 (10 ng/ml) in SFM with 1% BSA for 6 h (37°C, 5% CO2) as shown in Fig. 3. Cells were lysed, and equivalent amount of whole cell lysates were subjected to Western Blot analysis with indicated antibodies. B: human lung fibroblasts were infected with adenoviral vector containing the myc-tagged Y256F N-WASP mutant (Ad-Y256F), and equivalent amount of whole cell lysates were subjected to Western Blot analysis with indicated antibodies. C: human lung fibroblasts were infected with adenoviral vector containing the GFP (Ad-GFP) for 48 h. Fluorescent image for GFP expression (left), phase contrast image (right) (×200). D: lung fibroblasts were infected with Ad-Y256F or Ad-GFP, treated with or without TGF-β1 (10 ng/ml) for 48 h, fixed, and stained with Cy-3-labeled antibody toward α-SMA as shown in Fig. 1 (×200). E: percentage of cells with α-SMA-containing filaments in D was quantified as in Fig. 1. Data are presented as the means ± SE. *P < 0.01. F: lung fibroblasts were treated as in E and subjected to collagen gel contraction assay as shown in Fig. 2. *P < 0.01.
To determine the role of Y256 of N-WASP in formation of α-SMA-containing filaments during myofibroblast differentiation and maturation, myc-tagged N-WASP mutant (Y256F, in which Y256 replaced by F256) was overexpressed in human lung fibroblasts. Expression of Y256F N-WASP mutant in lung fibroblasts was mediated by adenoviral vector (Ad-Y256F) and confirmed by Western blot with antibody directed toward the myc tag (Fig. 4B). GFP expression mediated by adenoviral vector (Ad-GFP) was used as a control for adenoviral vector and infection efficiency (Fig. 4C). Greater than 95% of fibroblasts were positive of GFP (data not shown). Human lung fibroblasts were infected with Ad-Y256F or control Ad-GFP, treated with or without TGF-β1 (10 ng/ml) for 48 h, and TGF-β1-induced α-SMA-containing filaments were immunofluorescently detected with Cy-3-labeled antibody directed toward α-SMA (Fig. 4D). The percentage of cells containing α-SMA-containing filaments was significantly decreased in Y256F N-WASP-expressing fibroblasts compared with that in control fibroblasts in response to TGF-β1 (Fig. 4, D and E). In addition, overexpression of Y256F N-WASP mutant significantly decreased TGF-β1-induced collagen gel contraction in lung fibroblasts (Fig. 4F, bar 6 vs. 4). These findings indicate that FAK is upstream of N-WASP and is required for TGF-β1-induced phosphorylation of Y256 of N-WASP. Furthermore, phosphorylation of Y256 of N-WASP is critical for the formation of α-SMA-containing filaments and contractility in response to TGF-β1 in lung fibroblasts.
Arp3 is required for TGF-β1-induced α-SMA-containing filaments and collagen gel contraction in lung fibroblasts.
Arp3 is a part of the Arp2/3 complex, and the Arp2/3 complex has been shown to bind to COOH-terminal of N-WASP and promotes the nucleation of F-actin polymerization during cell migration and spreading (3, 15, 48, 51). During myofibroblast differentiation and maturation, cytoskeleton is extensively reorganized, and de novo expressed α-SMA monomers are incorporated into the cytoplasmic filaments (20, 23, 40). To determine whether Arp2/3 complex is involved in formation of α-SMA-containing filaments during myofibroblast differentiation and maturation, Arp3 expression was downregulated by using lentiviral vectors containing Arp3-specific shRNA (∼92% protein reduction) in human lung fibroblasts (Fig. 5A). Downregulation of Arp3 significantly decreased the percentage of cells with α-SMA-containing filaments in lung fibroblasts treated with TGF-β1 (10 ng/ml, 48 h) (Fig. 5B, bar 6 vs. 2 or 4). In addition, downregulation of Arp3 significantly decreased TGF-β1-induced collagen-gel contraction in lung fibroblasts (Fig. 5C, bar 6 vs. 2). Lentiviral vectors expressing the nontargeting control shRNA had no effect on formation of α-SMA-containing filaments or collagen gel contraction in lung fibroblasts treated with TGF-β1 (Fig. 5, B and C).
Fig. 5.
Actin-related protein (Arp) 3 is required for TGF-β1-induced formation of α-SMA-containing filaments during myofibroblast differentiation and cell contraction. A: lung fibroblasts were infected with lentiviral vectors containing Arp3-specific shRNA or control nontargeting shRNA, and stable clones were selected by puromycin resistance as in Fig. 1. Cells were lysed, and equivalent amount of whole cell lysates were subjected to Western Blot analysis with indicated antibodies. B: lung fibroblasts stably expressing Arp3 shRNA or control nontargeting shRNA were treated with or without TGF-β1 (10 ng/ml) for 48 h, fixed, and stained with Cy-3-labeled antibody toward α-SMA as shown in Fig. 1. The percentage of cells with α-SMA-containing filaments was quantified and presented as the means ± SE. *P < 0.01. C: lung fibroblasts stably expressing Arp3 shRNA or control nontargeting shRNA were subjected to collagen gel contraction assays for 60 h as shown in Fig. 2. *P < 0.01. D: in vitro pyrene actin polymerization assays were performed as described in materials and methods. Polymerization of fluorescent pyrene-labeled α-SMA monomer was determined in the presence of α-SMA monomer and varying combinations of Arp2/3, FAK, N-WASP, and N-WASP mutant (N-WASP Y256F). Nonpolymerized α-SMA monomer (Actin) was used as a negative control. The VCA domain of N-WASP (VCA) served as a positive control.
To further examine the role of FAK, N-WASP, and Arp 2/3 in polymerization of newly synthesized α-SMA monomers (into the α-SMA-containing filaments), the fluorescent in vitro pyrene actin polymerization assays were performed (Fig. 5D). An increase in fluorescence indicates the actin polymerization and formation of actin filaments. The VCA domain of N-WASP (VCA) served as a positive control for actin polymerization assays (Fig. 5D, line 1). Nonpolymerized α-SMA monomer (Actin) was used as a negative control (Fig. 5D, line 7). The data show that N-WASP, with both FAK and Arp2/3 together, induced significant α-SMA polymerization (Fig. 5D, line 2). In contrast, N-WASP mutant (Y256F), with both FAK and Arp2/3 together, failed to induce α-SMA polymerization (Fig. 5D, line 4), supporting that N-WASP phosphorylation is required for Arp3 or FAK to induce α-SMA polymerization and myofibroblast differentiation. N-WASP and Arp2/3, without FAK, induced only moderate α-SMA polymerization (Fig. 5D, line 3). The data suggest that FAK is required for optimal in vitro α-SMA polymerization mediated by N-WASP and Arp2/3 (Fig. 5D, line 2) and that Arp2/3 alone, or Arp2/3 and FAK together, was not able to induce significant α-SMA polymerization (Fig. 5D, lines 5 and 6). These results demonstrate that the optimal α-SMA polymerization requires the involvement of FAK, N-WASP, and Arp2/3. Furthermore, the data indicate that phosphorylation of Y256 of N-WASP is required for the α-SMA polymerization.
DISCUSSION
The main activities of myofibroblasts are remodeling and synthesis of the ECM, generation of tensile force, and production of cytokines (16, 23). It is well accepted that myofibroblasts are responsible for granulation tissue remodeling in both normal and pathological wound healing conditions (17, 23, 38). The concept that myofibroblasts play essential roles in the development of fibrosis promotes research to better understand the mechanisms regulating myofibroblast differentiation and functions (23). During myofibroblast differentiation and maturation, fibroblasts generally become more spread and less polarized, the cytoskeleton network is being extensively reorganized, and newly synthesized α-SMA monomers are incorporated into cytoplasmic filaments (Fig. 1C) (8, 23, 35). These newly formed, thickened α-SMA-containing cytoplasmic filaments are, not only a hallmark of mature myofibroblasts, but also important for myofibroblast activities (20, 21, 23). These α-SMA-containing filaments directly connect to focal adhesions and are essential for enhancing the contractile phenotype and matrix production of myofibroblasts (20, 21, 23).
The incorporation of the newly expressed α-SMA into cytoplasmic filaments increases the contractile activity of fibroblasts (1, 20). The peptide including the NH2-terminal sequence of α-SMA blocks the formation of α-SMA-containing filaments in fibroblasts (6). Importantly, the fusion peptide including the NH2-terminal sequence of α-SMA and the antennapedia third helix sequence for cell penetration effectively decreases contraction and production of mRNA for collagen I in cultured fibroblasts and reduces granulation tissue contraction in a rat wound model (22). These findings suggest that the α-SMA-containing filaments play an important role in myofibroblast functions. Although the importance of α-SMA-containing filaments as a marker of myofibroblast differentiation and maturation is presently well accepted (7, 23), the mechanisms regulating this particular phenomenon are largely unknown.
Our data demonstrate that N-WASP plays a key role in formation of α-SMA-containing filaments during myofibroblast differentiation and maturation. This is supported by the fact that forced downregulation of N-WASP expression by shRNA abrogated the formation of α-SMA-containing filaments in TGF-β1-treated lung fibroblasts (Fig. 1). Fibroblasts with decreased N-WASP expression showed impaired ability to contract collagen gels in response to TGF-β1 (Fig. 2). Because the α-SMA-containing filaments are essential for the contractile phenotype of myofibroblasts (20, 21, 23), the decreased collagen gel contraction is likely caused by the decreased amount of α-SMA-containing filaments in TGF-β1-treated lung fibroblasts after N-WASP had been downregulated. These data are consistent with the previous findings that inhibition of α-SMA-containing filaments by α-SMA NH2-terminal peptides significantly reduces contraction (22). Furthermore, our data demonstrate that N-WASP overexpression promotes the formation of α-SMA-containing cytoplasmic filaments in TGF-β1-treated lung fibroblasts, resulting in increased percentage of cells with well-organized α-SMA-containing filaments (Fig. 1). The current studies associate the function of N-WASP protein with the formation of α-SMA-containing filaments during myofibroblast differentiation and maturation.
N-WASP protein contains 502 amino acids and has three independent small domains (also termed VCA domain) at the COOH terminus: the verprolin homology (V), central (C), and acidic (A) regions (15, 36, 44). It is known that the V domain is a G-actin-binding site and is essential for N-WASP-induced formation of microspikes or newly formed branches during F-actin polymerization (30, 45). The CA domains bind the Arp2/3 complex and stimulate it to nucleate and polymerize G-actin (15, 15, 30, 36, 37, 48). During cell migration, N-WASP brings G-actin to the branching sites and promotes Arp2/3 complex and other proteins to induce F-actin polymerization (15, 36, 44, 50). This can result in a range of dynamic structures such as lamellipodia, filopodia, and membrane ruffles at the leading edge of the migrating cell and can generate the force for cell migration (36). However, migration generally is not a prominent feature of myofibroblasts. In contrast, myofibroblasts have larger and more prominent focal adhesions than undifferentiated fibroblasts (23). Our data suggest that N-WASP functions as a key regulator to promote the α-SMA polymerization through Arp2/3 complex during myofibroblast differentiation and maturation. The role of Arp2/3 in this phenomenon is supported by the fact that Arp3 downregulation significantly blocked the formation of α-SMA-containing filaments in TGF-β1-treated lung fibroblasts (Fig. 5). However, in vitro α-SMA actin polymerization assays suggest that Arp2/3 alone is not sufficient to induce the α-SMA polymerization (Fig. 5D). N-WASP and Arp2/3 are both required for induction of α-SMA polymerization (Fig. 5D). During myofibroblast differentiation, the amount of α-SMA isoform is greatly increased (23). We speculate that N-WASP plays an important role in recruiting newly synthesized α-SMA monomer to the sites of cytoskeleton reorganization and that N-WASP and Arp2/3 complex work together to stimulate α-SMA nucleation and polymerization, resulting in formation of α-SMA-containing cytoplasmic filaments. N-WASP may play a role in other aspects and functions of myofibroblasts, at least in myofibroblast contraction and collagen expression (Fig. 2), and these possibilities will need to be investigated in the future. Our current findings and the published evidence support the fact that the main roles of the α-SMA-containing filaments are contractility and matrix protein production of myofibroblasts (22, 23).
We have previously shown that FAK is activated by TGF-β1, and FAK activation is required for TGF-β1-induced formation of SMA-containing cytoplasmic filaments (10, 46). Our current study suggests that FAK is an upstream regulator of N-WASP and mediates the formation of α-SMA-containing filaments through N-WASP and Arp2/3 during myofibroblast differentiation and maturation. FAK downregulation significantly reduced TGF-β1-induced tyrosine phosphorylation of N-WASP and inhibited the formation of α-SMA-containing filaments and collagen gel contraction in lung fibroblasts treated with TGF-β1 (Fig. 4). Our data demonstrate that phosphorylation of Y256 of N-WASP is necessary for TGF-β1-induced formation of α-SMA-containing filaments and collagen gel contraction in lung fibroblasts (Fig. 4). In addition, N-WASP mutant (Y256F) showed impaired ability to induce α-SMA polymerization in vitro (Fig. 5D). These findings suggest that TGF-β1 induces phosphorylation of Y256 of N-WASP through a FAK-dependent pathway, and FAK functions as an upstream regulator of N-WASP to promote the formation α-SMA-containing filaments through N-WASP and Arp2/3 during myofibroblast differentiation and maturation.
Myofibroblasts have been associated with wound healing and pathological fibrotic diseases, such as lung fibrosis, hypertrophic scars, and scleroderma (12, 17, 23, 38). Also, myofibroblasts may promote cancer invasion and progression through the stroma reaction in epithelial tumors (23). The current study starts to shed lights on factors contributing to the formation of α-SMA-containing filaments, which are known as a hallmark of myofibroblasts and also are important for myofibroblast functions. There are limitations of the current study. Whether N-WASP directly or indirectly regulates α-SMA and collagen expression (as shown in Figs. 1H and 2C) was not delineated by this study. One possibility is that inability to form a strong α-SMA-containing fiber network (due to N-WASP downregulation) after TGF-β treatment initiates a feedback signal, resulting in decreased α-SMA and collagen expression. Future experiments will focus on the exact mechanism(s) involved. We expect that N-WASP-mediated signaling may have an important role in fibrotic reactions in vivo due to its role in myofibroblast differentiation and function demonstrated in fibroblasts. However, the current study is not able to directly define the importance of N-WASP-mediated signaling in myofibroblast and fibrotic disease in vivo. That will have to be studied by future experiments, particularly in fibrotic tissues or cells and in animal models of fibrosis.
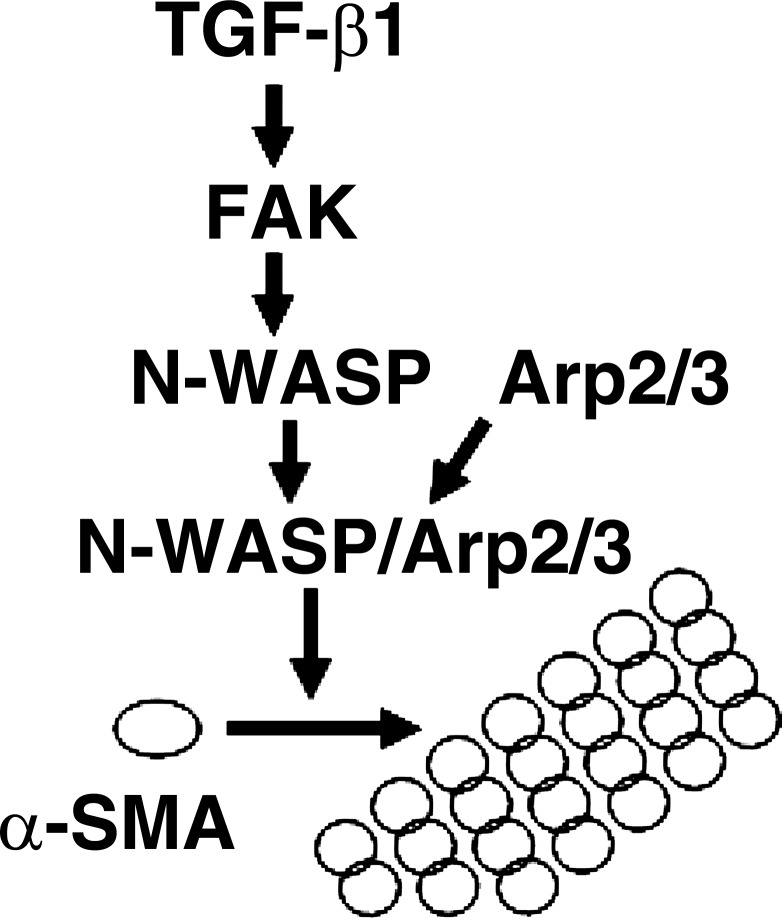
In summary, the current study shows that N-WASP plays a key role in regulating the formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation. Phosphorylation of Y256 of N-WASP is required for N-WASP to mediate the formation of α-SMA-containing filaments in lung fibroblasts in response to TGF-β1. FAK acts as an upstream regulator of N-WASP and is required for TGF-β1-induced phosphorylation of Y256 of N-WASP. In addition, we show that TGF-β1-induced α-SMA-containing cytoplasmic filaments require the involvement of Arp3 in lung fibroblasts. Together, this study supports a critical role of N-WASP in integrating FAK and Arp2/3 signaling to mediate the formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation (Fig. 6).
Fig. 6.
A working model for N-WASP in integrating FAK and Arp2/3 signaling to mediate formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation. TGF-β1 is well accepted for its ability to induce myofibroblast differentiation. The formation of α-SMA-containing filaments is the hallmark of myofibroblast differentiation and maturation and also important for myofibroblast functions. The current study shows that N-WASP plays a key role in regulating the formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation. TGF-β1 induces FAK activation, and activated FAK functions as an upstream regulator of N-WASP and mediates phosphorylation of Y256 of N-WASP. Phosphorylation of Y256 of N-WASP is required for N-WASP-mediated formation of α-SMA-containing filaments. Arp2/3 complex is recruited by N-WASP and is required for TGF-β1-induced formation of α-SMA-containing filaments. Together, this study supports a critical role of N-WASP in integrating FAK and Arp2/3 signaling to mediate formation of α-SMA-containing filaments during myofibroblast differentiation and maturation.
GRANTS
This work was supported by National Institutes of Health Grants, HL085324 and HL095451 to Q. Ding, and by Greater Southeast Affiliate, American Heart Association fellowship to G.-Q. Cai.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.-Q.C., C.-F.C., L.F.R., J.-L.G., and Q.D. conception and design of research; G.-Q.C., C.-F.C., M.H., A.Z., and H.F. performed experiments; G.-Q.C., M.H., H.F., and Q.D. analyzed data; G.-Q.C., A.Z., H.F., and Q.D. interpreted results of experiments; G.-Q.C., C.-F.C., M.H., A.Z., and H.F. prepared figures; G.-Q.C. and Q.D. drafted manuscript; T.R.L., Y.Z., V.J.T., and Q.D. edited and revised manuscript; Q.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Stuart J. Frank for generously supplying the adenoviral vector (Ad-Cre). The authors are also grateful to members of our laboratory for technical help and stimulating discussion.
REFERENCES
- 1. Arora PD, McCulloch CA. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol 159: 161–175, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40: 501–514, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bompard G, Caron E. Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol 166: 957–962, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai GQ, Zheng A, Tang Q, White ES, Chou CF, Gladson CL, Olman MA, Ding Q. Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res 316: 1600–1609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan MW, Chaudary F, Lee W, Copeland JW, McCulloch CA. Force-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia). J Biol Chem 285: 9273–9281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaponnier C, Goethals M, Janmey PA, Gabbiani F, Gabbiani G, Vandekerckhove J. The specific NH2-terminal sequence Ac-EEED of alpha-smooth muscle actin plays a role in polymerization in vitro and in vivo. J Cell Biol 130: 887–895, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63: 21–29, 1990 [PubMed] [Google Scholar]
- 8. Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13: 7–12, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biochem 122: 103–111, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding Q, Gladson CL, Wu H, Hayasaka H, Olman MA. FAK-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem 283: 26839–26849, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding Q, Grammer JR, Nelson MA, Guan JL, Stewart JE, Jr, Gladson CL. p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J Biol Chem 280: 6802–6815, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Ding Q, Luckhardt T, Hecker L, Zhou Y, Liu G, Antony VB, de Andrade J, Thannickal VJ. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis. Drugs 71: 981–1001, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding Q, Stewart J, Jr, Olman MA, Klobe MR, Gladson CL. The pattern of enhancement of Src kinase activity on platelet-derived growth factor stimulation of glioblastoma cells is affected by the integrin engaged. J Biol Chem 278: 39882–39891, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Ding Q, Stewart J, Jr, Prince CW, Chang PL, Trikha M, Han X, Grammer JR, Gladson CL. Promotion of malignant astrocytoma cell migration by osteopontin expressed in the normal brain: differences in integrin signaling during cell adhesion to osteopontin vs. vitronectin. Cancer Res 62: 5336–5343, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Dominguez R. Actin filament nucleation and elongation factors—structure-function relationships. Crit Rev Biochem Mol Biol 44: 351–366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27: 549–550, 1971 [DOI] [PubMed] [Google Scholar]
- 17. Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124: 401–404, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hagood JS, Olman MA. Muscle fatigue: MK2 signaling and myofibroblast differentiation. Am J Respir Cell Mol Biol 37: 503–506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hauck CR, Klingbeil CK, Schlaepfer DD. Focal adhesion kinase functions as a receptor-proximal signaling component required for directed cell migration. Immunol Res 21: 293–303, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12: 2730–2741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 14: 2508–2519, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biochem 157: 657–663, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19: 761–771, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. PMID: 22461426 In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116: 1606–1614, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kolodsick JE, Peters-Golden M, Larios JM, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 29: 537–544, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J Cell Sci 118: 79–87, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Lin A, Hokugo A, Choi J, Nishimura I. Small cytoskeleton-associated molecule, fibroblast growth factor receptor 1 oncogene partner 2/wound inducible transcript-3.0 (FGFR1OP2/wit30), facilitates fibroblast-driven wound closure. Am J Pathol 176: 108–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu R, Abreu-Blanco MT, Barry KC, Linardopoulou EV, Osborn GE, Parkhurst SM. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development 136: 2849–2860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int 26: 8–22, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med 177: 638–645, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Phan SH. The myofibroblast in pulmonary fibrosis. Chest 122: 286S–289S, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97: 221–231, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76: 69–125, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Schaller MD. FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J Cell Biol 166: 157–159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol 169: 941–952, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 3: 413–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2: 249–256, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Sturge J, Hamelin J, Jones GE. N-WASP activation by a beta1-integrin-dependent mechanism supports PI3K-independent chemotaxis stimulated by urokinase-type plasminogen activator. J Cell Sci 115: 699–711, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci 114: 1801–1809, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8: 37–48, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278: 12384–12389, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Welch MD. The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol 9: 423–427, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem 279: 9565–9576, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Yoo Y, Wu X, Egile C, Li R, Guan JL. Interaction of N-WASP with hnRNPK and its role in filopodia formation and cell spreading. J Biol Chem 281: 15352–15360, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Zhan S, Cai GQ, Zheng A, Wang Y, Jia J, Fang H, Yang Y, Hu M, Ding Q. Tumor necrosis factor-alpha regulates the Hypocretin system via mRNA degradation and ubiquitination. Biochim Biophys Acta 1812: 565–571, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]