Abstract
Caveolae are flask-shaped plasma membrane invaginations expressing the scaffolding caveolin proteins. Although caveolins have been found in endothelium and epithelium (where they regulate nitric oxide synthase activity), their role in smooth muscle is still under investigation. We and others have previously shown that caveolae of human airway smooth muscle (ASM), which express caveolin-1, contain Ca2+ and force regulatory proteins and are involved in mediating the effects of inflammatory cytokines such as TNF-α on intracellular Ca2+ concentration responses to agonist. Accordingly, we tested the hypothesis that in vivo, absence of caveolin-1 leads to reduced airway hyperresponsiveness, using a knockout (KO) (Cav1 KO) mouse and an ovalbumin-sensitized/challenged (OVA) model of allergic airway hyperresponsiveness. Surprisingly, airway responsiveness to methacholine, tested by use of a FlexiVent system, was increased in Cav1 KO control (CTL) as well as KO OVA mice, which could not be explained by a blunted immune response to OVA. In ASM of wild-type (WT) OVA mice, expression of caveolin-1, the caveolar adapter proteins cavins 1–3, and caveolae-associated Ca2+ and force regulatory proteins such as Orai1 and RhoA were all increased, effects absent in Cav1 KO CTL and OVA mice. However, as with WT OVA, both CTL and OVA Cav1 KO airways showed signs of enhanced remodeling, with high expression of proliferation markers and increased collagen. Separately, epithelial cells from airways of all three groups displayed lower endothelial but higher inducible nitric oxide synthase and arginase expression. Arginase activity was also increased in these three groups, and the inhibitor nor-NOHA (N-omega-nor-l-arginine) enhanced sensitivity of isolated tracheal rings to ACh, especially in Cav1 KO mice. On the basis of these data disproving our original hypothesis, we conclude that caveolin-1 has complex effects on ASM vs. epithelium, resulting in airway hyperreactivity in vivo mediated by altered airway remodeling and bronchodilation.
Keywords: bronchial smooth muscle, caveolae, calcium, airway remodeling, epithelium, arginase
increased airway contractility and impaired relaxation are major components of inflammatory airway diseases such as asthma. Enhanced intracellular calcium ([Ca2+]i) responses of airway smooth muscle (ASM) in response to bronchoconstrictors is a key component of airway contractility. In this regard, we and others have previously demonstrated in vitro that the plasma membrane of human ASM cells contain caveolae (flask-shaped invaginations) that express the constituent scaffolding proteins caveolin-1 and -2 (but not caveolin-3) (15, 17, 41). Importantly, caveolae harbor plasma membrane proteins involved in regulation of [Ca2+]i as well as force (17, 41, 48) and may thus be key regulators of ASM contractility. Indeed, we previously demonstrated that reduced caveolin-1 expression decreases ASM [Ca2+]i and contractility (46, 48), whereas proinflammatory cytokines such as TNF-α and IL-13 increase caveolin-1 expression, which in turn contributes to the enhanced [Ca2+]i response of human ASM during inflammation (46). Separately, other investigators have demonstrated that suppression of caveolin-1 is associated with a proliferative ASM cell phenotype (16, 22), another key component of asthma.
Overall, in vitro data clearly demonstrate an important role for caveolin-1 in enhanced airway contractility relevant to asthma. However, the effect of altered caveolin-1 expression or function in vivo has not been examined and was the focus of this study. Based on previous work, our hypothesis was that reduced caveolin-1 results in airway hyporesponsiveness. To this end we employed the mouse lacking caveolin-1 (Cav1 KO) as a model, with ovalbumin (OVA) sensitization and challenge as a model of allergic airway hyperresponsiveness to test our hypothesis. Cav1 KO mice are known to develop pulmonary fibrosis (9, 15, 60), and a single study showed that these mice have reduced lung compliance and increased elastance by 3 mo of age (28), with evidence of progressively greater deposition of collagen within airways and parenchyma. Whether such changes are associated with altered airway contractility and the specific role of ASM per se have not been examined. In the present study, we compared airway structure and function in wild-type (WT) vs. Cav1 KO mice that were either unsensitized [control (CTL)] or sensitized and challenged with OVA.
The above rationale and hypothesis were based on previous data suggesting a role for ASM caveolae in determining contractility. However, it is also important to consider that allergic airway inflammatory diseases such as asthma involve changes to bronchial epithelium, wherein increased epithelial thickening and the contribution of epithelium to bronchodilation may be altered (18). Although the specific role of epithelium-derived nitric oxide (NO) in bronchodilation is still under investigation (54), the role of caveolae in regulating endothelial nitric oxide synthase (eNOS) activity is well recognized (29). Furthermore, l-arginine (l-arg) serves as a common substrate for eNOS as well as arginase, the latter leading to the collagen production pathway (10), and an important area of investigation in asthma. Epithelial caveolae and caveolin-1 have been identified (1); however, the role of caveolin-1 in this cell type, especially in the setting of altered eNOS and arginine balance, is not clear and may contribute to the overall effect of caveolin-1 on airway contractility. Accordingly, the present study tested the importance of epithelium in our hypothesis that reduced caveolin-1 results in airway hyporesponsiveness.
METHODS
Mice.
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee and were in strict accordance to the guidelines of the American Physiological Society. Homozygous caveolin-1 null mice (Cav1−/−) and appropriate wild-type (WT, B6129SF2/J) mice were obtained from Jackson Laboratories (Bar Harbor, ME). As described previously (43), Cav1 KO mice lack exons 1 and 2 of the caveolin-1 gene and thus do not produce caveolin-1 protein.
OVA mouse model.
The OVA-sensitized mouse model has been previously published (25). Briefly, Cav1 KO or WT mice at 6–8 wk were sensitized with 20 μg OVA adsorbed to 1.0 mg alum or vehicle alone delivered intraperitoneally on days 0 and 14. Experimental mice were intranasally challenged with 50 μg of OVA in 50 μl of PBS under light isoflurane anesthesia on days 14, 25, 26, and 27. Control mice received intranasal PBS only. All animals were analyzed 24 h after the last challenge.
Assessment of airway function.
Under pentobarbital anesthesia and cannulation of the trachea via a tracheostomy (19Fr blunt Luer cannula), airway resistance (Rl) and compliance (Cl) were assessed at baseline and in response to increasing concentrations of methacholine (0 to 50 mg/ml in PBS) delivered via nebulization (AeroNeb). Animals were maintained at 37°C and, under muscle paralysis (pancuronium), airway function was measured by using the SciReq FlexiVent ventilator and lung mechanics system (Montreal, Quebec, Canada) (12, 34).
LCM.
Following Flexivent experiments, animals were overdosed with pentobarbital, lungs were inflated with air (25 cmH2O), the trachea was ligated, and the lungs were rapidly frozen under RNase-free conditions. Samples were then cryosectioned at 10 μm under RNase-free conditions and placed on prechilled clean slides, dehydrated with ethanol and placed on a Veritas microdissection system (Arcturus, Molecular Devices, Sunnyvale, CA). Under light microscopy (×200 magnification), small airways (300–350 μm diameter) were identified, and the ASM and epithelial layers were delineated by using software regions of interest. An infrared laser capture microdissection (LCM) laser was then used to dissect out defined areas, which were placed onto separate CapSure Macro LCM caps (Arcturus; Molecular Devices, Sunnyvale, CA) for further analyses of epithelial vs. ASM layers.
RT-PCR.
Total RNA was isolated from the CapSure LCM caps after LCM using the RNeasy micro kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized using Transcriptor reverse transcription kit (Roche, Indianapolis, IN). Total RNA from four caps per animal was isolated, and each of these RNAs served as template for two separate reverse transcription reactions. Homogeneity and relative purity of tissue source for RNA (i.e., epithelium vs. ASM) were assessed by quantifying messages for smooth muscle markers such as smooth muscle actin (sm-actin) and epithelial markers such as E-cadherin (E-cad), epithelial membrane protein 1 (EMP1), and microtubule associated protein 7 (MAP7). Following quality control analysis, samples were further analyzed for mRNA encoding specific proteins of interest (see results). In preliminary standardization experiments, neither glyceraldehyde-3-phosphate dehydrogenase (GAPDH) message nor ribosomal protein S16 varied between genotypes or by sensitization protocols (data not shown); therefore, S16 was used as the internal control for all subsequent analyses. cDNA was amplified by using an LC480 LightCycler (ABI; Carlsbad, CA) and primers listed in Table 1. Real-time PCR was performed in duplicates per cDNA template, and data for all cDNAs in a category (WT or Cav1 KO; CTL or OVA) were pooled for statistical analysis. All PCR reactions went through 60 amplification cycles. The ratio of fold change in expression of the mRNA of interest for each sample was calculated by normalization of cycle threshold (CT) values of the target gene (e.g., PTRF, Arg1, etc.) to the reference gene (S16) using the comparative CT (ΔΔCT) method. Data are reported as the ΔΔCT and the average ratio of fold change in mRNA of interest corrected for reference gene. Unsensitized (CTL) WT was used as the calibrator for quantification.
Table 1.
Primers used in PCR analysis of laser capture microdissected airway samples
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| AChR M3 | 5′-TTGCACAGTAACAGTACAACCTCG-3′ | 5′-TGTTGCCGATGATGGTCACCAATG-3′ |
| Arg1 | 5′-GGCAGTTGGAAGCATC-3′ | 5′-GGTCTACGTCTCGCAAG3-′ |
| Cav-1 | 5′-GGGCAAATACGTAGACTCCGAGG-3′ | 5′-CTTGACCACGTCGTCGTTGAGA-3′ |
| Cav-2 | 5′-GATGGGGCTGGAGACC-3′ | 5′-ACAGGATACCCGCAAT-3′ |
| E-Cad | 5′-AACAGGCCAGAGTTTACCCAGGAGG-3′ | 5′-GGTGTAGGCGATGGCAGCGTT-3′ |
| EMP1 | 5′-CACATTGCCACTGCCATTATGCTG-3′ | 5′-GGGAGATGATGGAGAAGATGATGG-3′ |
| eNOS | 5′-TCGGACTCACATTGCG-3′ | 5′-TGGTCAACCGAACGAAG-3′ |
| IL-4Rα | 5′-AGCTGTTGGTGCTGCTACTGTGGA-3′ | 5′-CTGAGAGTGCAATTTGGACTGGCTC-3′ |
| iNOS | 5′-AGCGAAATGCAATCCC-3′ | 5′-AGTATTAGAGCGGTGGC-3′ |
| MAP7 | 5′-AAACAGCTAGCTGCACGAGAAACC-3′ | 5′-TTGGCTTCTGGCTCCTTTCCATCGT-3′ |
| Orai1 | 5′-CTGGGTCAAGTTCTTACCT-3′ | 5′-GAGCGGTAGAAGTGAACA-3′ |
| PCNA | 5′-GGCTCTCAAAGACCTCATCAATGAGG-3′ | 5′-CCCGACTTCTATTACGTCTGTGGA-3′ |
| PTRF | 5′-ATGGAGGATGTCACGCTCCATATCGT-3′ | 5′-GCGCCGATGATTTTATCCAGAAGGCT-3′ |
| RhoA | 5′-CTGCCATCAGGAAGAAACTGGTGA-3′ | 5′-TAATCTTCCTGTCCAGCTGTGTCC-3′ |
| S16 | 5′-TGCAGGTCTTCGGACGCAAGAAAA-3′ | 5′-CGAATATCCACACCAGCAAATCGC-3′ |
| SDPR | 5′-AGAAGTCTCGCAAGGT-3′ | 5′-CTCATCACGGGGCAAT-3′ |
| smActin | 5′-TCCGATAGAACACGGCA-3′ | 5′-CCACATACATGGCGGG-3′ |
| SRBC | 5′-ACGACTGGAAAGAGCCCTTTTCAGGC-3′ | 5′-ACGTCCTGCTCTTCAAGGAGGAGACT-3′ |
| TNFR1 | 5′-ACAAGACCTCGGACAC-3′ | 5′-CATTTCCGGGAATAGCC-3′ |
Immunoblotting.
Mouse lung tissue homogenates were prepared by standard techniques. Briefly, lung tissue was rinsed twice with ice-cold PBS, homogenized with a Potter-Elvehjem tissue grinder in sucrose buffer (250 mM sucrose; 40 mM Tris·HCl, pH 7.2), in the presence of protease inhibitors, and the resultant supernatants assayed for total protein content using the DC protein assay kit (Bio-Rad, Hercules, CA). Approximately 30 μg total protein was loaded onto a denaturing SDS-PAGE and electrophoresed according to standard protocols. Proteins were transferred onto a polyvinylidene difluoride membrane, blocked with Li-Cor blocking buffer (Li-Cor, Lincoln, NE), and probed with antibodies of interest and IR-800 secondary antibodies (Li-Cor). Protein detection and densitometric analyses were performed on an Odyssey Infrared Imager (Li-Cor). GAPDH was used as internal control for normalizing densitometric data. Homogenates from four lung tissues per category (WT or Cav1 KO; nonsensitized or sensitized) were analyzed.
Arginase activity assay.
Arginase activity was assayed as described by Sopi et al. (53). Briefly, lung tissues snap-frozen in liquid nitrogen were thawed in storage buffer (10 mM Tris, pH 7.5, 10 mM glycine, 10 mM MnCl2, 10 mM 2-mercaptoethanol), homogenized, and centrifuged, and supernatants were collected. Supernatants were preactivated by adding 10 mM MnCl2 and heating for 5 min at 55°C. l-arg (250 mM, pH 9.7) was added to the preactivated samples, and the mixture was incubated at 37°C for 60 min. Arginase reaction was stopped by adding 1 ml of freshly prepared diacetyl monoxime/acid solution, heating in boiling water. Urea concentration was measured by using a spectrophotometer at 490 nm. Baseline urea concentration was measured without incubation with l-arg. A standard curve was obtained by including urea samples containing 10, 50, and 90 μl of 10 mM urea (0.1, 0.5, and 0.9 μM). Arginase activity was calculated by the formula [Sample (urea) − Baseline (urea)]/10 and expressed as nanomoles of urea per minute per milligram protein. One unit arginase activity is equivalent to the conversion of 1 μmol urea/min at 37°C.
RhoA activation assay.
Cytoplasmic and plasma membrane fractions were obtained from lung tissues of three animals per category by use of the FractionPREP cell fractionation kit (BioVision, Milpitas, CA), following the manufacturer's suggested protocol. Amount of RhoA present in each fraction was analyzed by immunoblotting and densitometry. RhoA activation was calculated as the ratio of RhoA expression in cytoplasmic fraction to that in membrane fraction.
BAL cell count.
Immediately after euthanasia, the lungs were lavaged three times with phosphate-buffered saline (0.5 ml per lavage) and the samples were combined. Fluid recovery was ∼95%. The bronchoalveolar lavage (BAL) samples were centrifuged at 2,000 revolution/min for 10 min at 4°C. Total leukocyte counts in BAL fluids were determined with a hemocytometer after Randolph's stain. For cell differentiation, cytospin preparations from BAL fluids were stained with Wright-Giemsa stain. The individual cell populations were counted on the basis of morphology and expressed as percent cells in BAL. BAL from at least four animals per category were collected and subjected to analysis.
Histological analyses.
Standard protocols were used for hematoxylin and eosin (H&E) and Masson trichrome (no. 250088-1, Polysciences) staining of 5-μm lung sections. Following staining procedures, slides were visualized under and images were acquired by using an Olympus Axioplan2 microscope at a magnification of ×200.
Isolated murine tracheal ring experiments.
Mice were overdosed with intraperitoneal pentobarbital, and the trachea, heart, and lungs were removed en bloc and placed in 4°C physiological saline solution (PSS). Tracheae were dissected, cleaned thoroughly of adventitia, and cut into individual rings. When necessary, epithelium denudation was performed by gentle abrasion of the lumen by using the roughened edges of a 19-gauge needle. Rings were mounted in 5-ml organ bath chambers containing PSS, under minimal tension between stainless hooks and Grass FT03 force transducers (Astromed, West Warwick, RI). Force signals were amplified (World Precision Instruments, Sarasota, FL) and collected by use of custom-built Labview-based software (National Instruments). Samples were allowed to equilibrate for 15 min and then stretched every 15 min to achieve a stable baseline tension of 0.8 g. Following the final adjustment, samples were exposed to 50 mM KCl twice and washed thrice to determine maximum force. Samples were then exposed to increasing concentrations of acetylcholine (ACh) in successive half-log increments (10−10-10−4 M), allowing 5 min for force to stabilize after each dose. All rings were then washed and allowed to return to resting tension before further experimentation (see results). Inhibitors (see results) were introduced at least 20 min prior to ACh exposure.
Statistical analysis.
Twelve animals per category (WT or Cav1 KO; nonsensitized or sensitized) were used in these analyses, with multiple protocols utilizing tissues from the same animal. Whole animal protocols were performed on at least five animals per group. All in vitro experiments were repeated at least three times with samples taken from at least five animals. For LCM, four caps containing epithelial or ASM tissue from at least four animals were collected. RNA from each cap served as a template for two RT reactions, and the resultant cDNA was used for duplicate PCR reactions. Quantification and analysis were done, based on the methods described in Ref. 33. Tissue lysates from four animals per group were used for immunoblotting and densitometric analyses, and subcellular fractions from three animals per group were used for RhoA activation assay. Comparisons were performed by two-way ANOVA with Bonferroni correction for repeated comparisons. Statistical significance was established at P < 0.05. All values are expressed as means ± SE.
Materials.
Nor-NOHA (N-omega-nor-l-arginine), a specific arginase 1 inhibitor, was purchased from Cayman Chemicals (Ann Arbor, MI). Methacholine, ACh, and OVA were obtained from Sigma Aldrich (St. Louis, MO). Antibodies against TNFR1 and IL4R and RhoA were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Primers used for LCM-PCR were obtained from Integrated DNA Technologies (Coralville, IA).
RESULTS
Effect of Cav1 KO on airway mechanics.
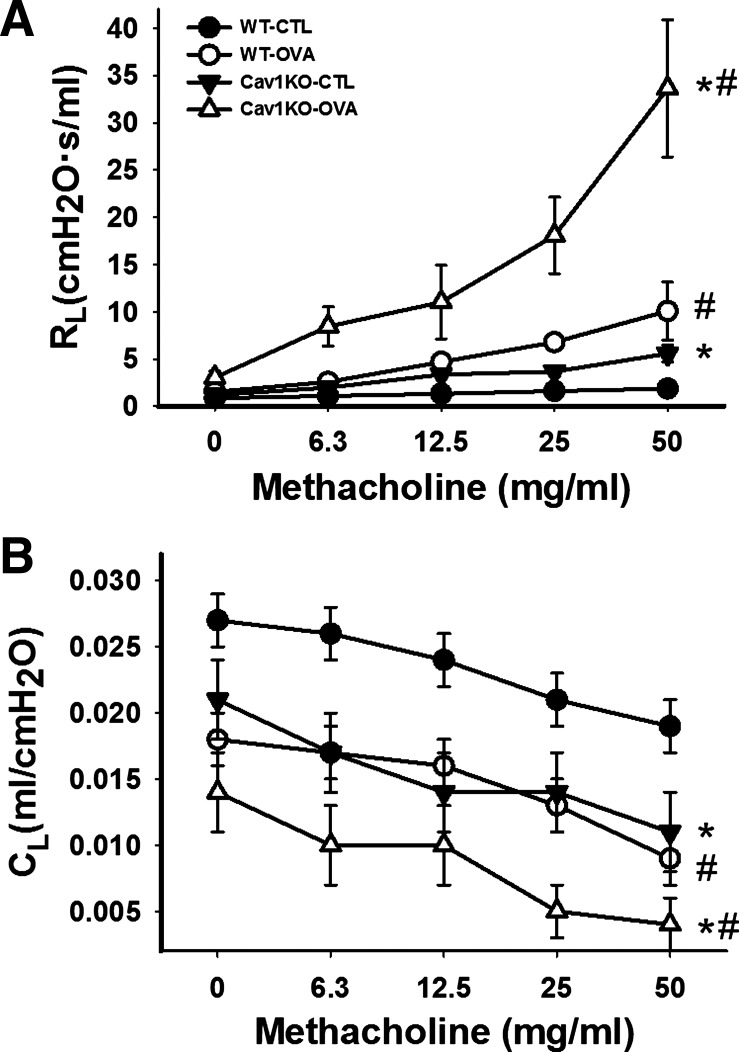
In CTL (i.e., not sensitized) WT mice that were anesthetized, paralyzed and mechanically ventilated, nebulization of methacholine resulted in a fairly linear but small increase in Rl as methacholine concentration was increased (Fig. 1A). In CTL Cav1 KO mice, baseline Rl was slightly higher but increased substantially with methacholine, such that for all drug concentrations Rl was significantly higher than for CTL WT mice (Fig. 1A; P < 0.05).
Fig. 1.
Changes in airway resistance (Rl) and compliance (Cl) in a caveolin-1 knockout (Cav1 KO) mouse model of allergic airway hyperresponsiveness induced by ovalbumin (OVA) sensitization and challenge. Wild-type (WT) and Cav1 KO animals either were unsensitized (control; CTL) or were OVA sensitized/challenged. Rl and Cl were measured under anesthesia, paralysis, and mechanical ventilation via tracheostomy (see methods for details) to increasing doses of methacholine (0–50 mg/ml). Baseline Rl was higher in both CTL and OVA Cav1 KO mice compared with WT and increased substantially with methacholine (A). In the Cav1 KO groups these values were significantly greater than in any of the other 3 groups. Cl values were significantly smaller in Cav1 KO mice (B). In the OVA Cav1 KO group, Cl dropped rapidly and significantly with increasing methacholine. Values are means ± SE (n = 5 per group). *Significant difference between WT and Cav1 KO; #significant difference between CTL and OVA (P < 0.05).
In OVA WT mice, baseline Rl was comparable to that of CTL WT, but increased to a greater extent with methacholine (Fig. 1A; P < 0.05). Importantly, baseline Rl was actually higher in OVA Cav1 KO mice, and with methacholine there was a tremendous increase in Rl such that at all concentrations these values were significantly greater than in any of the other three groups (Fig. 1A; P < 0.05 with repeated comparisons).
Cl in CTL WT mice decreased with increasing methacholine concentrations (Fig. 1B). Cl values were significantly smaller in CTL Cav1 KO mice compared with WT (P > 0.05). With OVA sensitization/challenge, compliance of OVA WT mice decreased during the methacholine challenge, but these changes were not to the same extent as the changes in Rl in the same group. Interestingly, in the OVA Cav1 KO group, Cl dropped rapidly and significantly with increasing methacholine concentrations such that the changes in this group were significantly greater than for any of the other three groups (Fig. 1B; P < 0.05 with repeated comparisons).
LCM analyses.
Given the surprising finding of enhanced airway responsiveness in the Cav1 KO mice, which disproved our initial hypothesis, we explored alternative reasons for these findings, focusing on changes in ASM vs. epithelium in terms of factors that may regulate contractility and relaxation. We used LCM-based analysis of samples from either layer. In ASM, we focused on [Ca2+]i and force-regulatory proteins that are present in caveolae, as well as proteins involved in caveolar formation and function. We examined the M3 muscarinic ACh receptor, caveolins 1 and 2, Orai1 (key component of store-operated Ca2+ entry), and RhoA (involved in Ca2+ sensitization). Recent evidence in other cell types indicates an important role for adaptor proteins called cavins, which are essential in the formation and maintenance of functional caveolar structures, in trafficking of caveolins to plasma membrane, and in membrane remodeling (5). To date, four cavins have been identified: PTRF/cavin1 (21, 58), SDPR/cavin2 (19), SRBC/cavin3 (36), and MURC/cavin4 (2, 37). There is currently limited information on cavins in ASM or airway in general (49). Furthermore, species and cell type differences may be relevant but have not been studied. In pilot studies, we found that cavins 1–3 can be detected in human ASM (not shown), and therefore, we examined these three isoforms. For epithelial samples, in addition to quality control proteins such as E-Cad (Epithelial Cadherin), EMP1 (Epithelial Membrane Protein 1), and MAP7 (Microtubule Associated Protein 7), we focused on eNOS and Arg1 with the intent of determining the potential role of airway remodeling in altered airway reactivity with Cav1 KO.
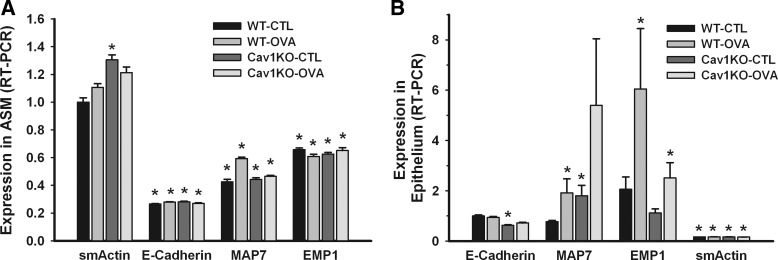
An important step in our analysis was verification of relative purity of ASM vs. epithelial layers. Although complete separation of the two layers is unlikely, we verified that the extracts were largely representative of either ASM or epithelium using the assumption that cross-contamination would result in relatively high abundance of markers of the other layer. However, our quantitative PCR analysis showed highly enriched ASM captures evidenced by high sm-actin to epithelial marker (E-Cad, MAP7, or EMP1) expression (Fig. 2A; P < 0.05), whereas epithelial captures showed high ratios of epithelial marker to sm-actin (Fig. 2B; P < 0.05).
Fig. 2.
Laser capture microdissection (LCM) and quantitative real-time PCR (RT-PCR) analyses of epithelium and airway smooth muscle (ASM) layers in mouse lung. Airways were identified under light microscopy and an infrared laser was used to dissect the layers onto LCM caps for further analysis (see methods for details). Relative purity of either the epithelial or ASM layers was verified by lack of abundance of markers of the other layer, as assayed quantitatively by RT-PCR. In the ASM layer (A), smooth muscle actin (sm-actin) expression was high whereas epithelial markers such as E-Cad, MAP7, or EMP1 were low. Conversely, in the epithelial layer (B), sm-actin expression was low whereas epithelial marker expression was high. Absence of caveolin-1 resulted in increased sm-actin expression within the ASM of Cav1 KO mice, compared with CTL WT animals. Values are means from 16 PCR reactions for each set ± SE (n = 4 animals per group). *Significant difference between the reference (WT-CTL sm-actin in ASM tissue and WT-CTL E-Cad in epithelial tissue) and the other markers (P < 0.05).
Effect of caveolin-1 KO on ASM.
Absence of caveolin-1 resulted in increased sm-actin expression within the ASM of Cav1 KO mice, compared with CTL WT animals (Fig. 2A; P < 0.05). There were no significant differences between the two Cav1 KO groups. There were some inconsistent differences in expression of epithelial markers. These were not analyzed further since the relative expression of these markers was small. In the epithelial layer, absence of caveolin-1 resulted in reduced E-Cad expression, but MAP7 expression was increased. EMP1 expression was increased by OVA, regardless of the presence of caveolin-1 (Fig. 2B; P < 0.05).
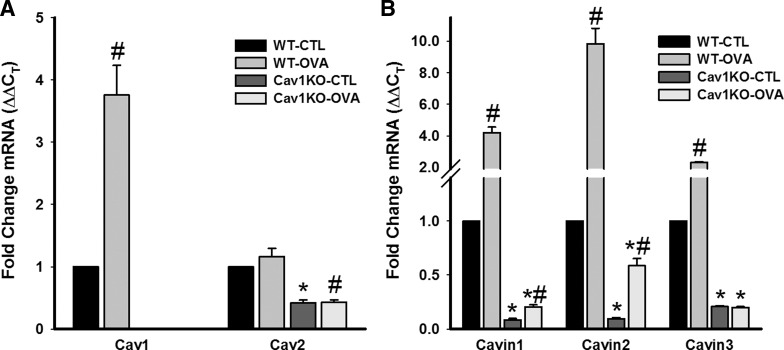
In ASM of WT animals, OVA resulted in a substantial increase in the expression of caveolin-1, whereas caveolin-2 expression was largely unchanged (Fig. 3A; P < 0.05 for caveolin-1). In Cav1 KO mice, caveolin-1 was undetectable (validating our PCR analysis), whereas caveolin-2 expression was significantly decreased (P < 0.05). Previous studies have already shown that caveolin-2 is usually associated with caveolin-1 (43), and we have shown that caveolin-3 is absent in ASM (41). However, we explored expression of caveolin-2 to determine whether a compensatory increase in this isoform could explain the enhanced contractility observed. The lack of significant difference between WT and OVA groups in the Cav1 KO animals in this regard would suggest otherwise.
Fig. 3.
Expression of caveolar regulatory proteins in ASM. mRNA expression of caveolin-1 was substantially increased in the ASM of OVA WT mice, whereas caveolin-2 was only slightly elevated (A). In Cav1 KO mice, no caveolin-1 mRNA was detected (validating our PCR analysis), whereas caveolin-2 expression was significantly decreased (A). Expression of all the 3 cavins tested (PTRF/cavin1, SDPR/cavin2, and SRBC/cavin3) was substantially increased in the WT OVA animals (B). However, in the absence of caveolin-1, their expression was highly reduced, even with OVA. All messages were measured by quantitative RT-PCR. Values are means from 16 PCR reactions for each set ± SE (n = 4 animals). *Significant difference between WT and Cav1 KO; #significant difference between CTL and OVA (P < 0.05).
In WT animals, ASM levels of cavins 1–3 (PTRF, SDPR, and SRBC) were all significantly enhanced (albeit to different extents) by OVA sensitization/challenge (Fig. 3B; P < 0.05). Absence of caveolin-1 resulted in significant decrease in the expression of all three cavins in the CTL group, which was maintained in the OVA Cav1 KO group (albeit to a lesser extent for SDPR/cavin2; P < 0.05 across groups compared with CTL or OVA WT).
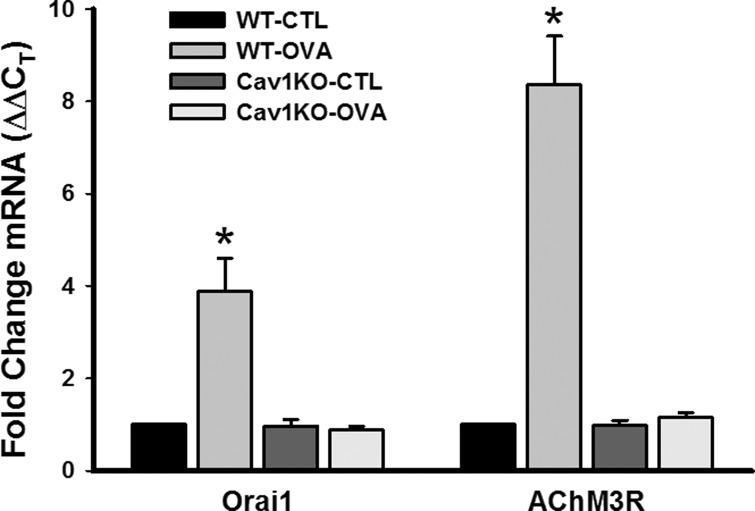
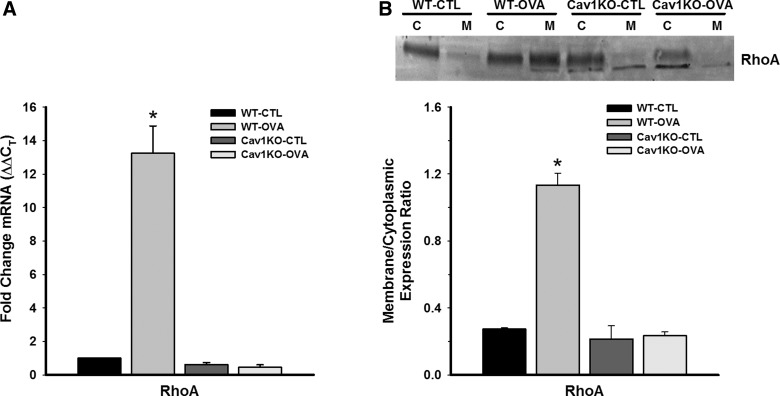
On the basis of our previous studies showing an important role for caveolin-1 in [Ca2+]i responses to agonist in ASM (41, 46), we examined changes in some [Ca2+]i and force regulatory proteins that are present in caveolae. Compared with WT CTL, expression of Orai1 and M3 ACh receptor were all substantially elevated by OVA exposure in the WT group (Fig. 4; P < 0.05). Absence of caveolin-1 resulted in expression of these proteins that was not substantially different from WT CTL (Fig. 4), potentially reflecting noncaveolar expression. There was no significant difference between CTL and OVA Cav1 KO groups in this regard. Investigation of the Ca2+ sensitization protein RhoA found increased mRNA expression in the WT OVA group, but no increase in mRNA expression in either of the Cav1 KO groups (Fig. 5A; P < 0.05). Furthermore, the ratio of plasma membrane to cytosolic expression of RhoA protein was significantly higher in WT OVA, indicating activation of RhoA in that group (Fig. 5B; P < 0.05). However, RhoA translocation was unchanged in Cav1 KO groups.
Fig. 4.
Effect of absent caveolin-1 on intracellular Ca2+ ([Ca2+]i) and force regulatory proteins in ASM. Compared with CTL WT, expression of the Ca2+ influx channel Orai1 and the M3 muscarinic receptor (AChM3R) messages were both substantially elevated by OVA (measured by quantitative RT-PCR). Absence of caveolin-1 resulted in expression of these proteins that was not substantially different from WT CTL, potentially reflecting noncaveolar expression. There was no significant difference between CTL and OVA Cav1 KO groups in this regard. Values are means ± SE (n = 4 animals). *Significant difference between CTL and OVA (P < 0.05).
Fig. 5.
Effect of Cav1 KO on the Ca2+ sensitization protein RhoA in ASM. Compared with WT CTL, RhoA mRNA expression was significantly increased in WT OVA ASM (measured by quantitative RT-PCR). However, Cav1 KO CTL and OVA mice showed no significant increase in RhoA expression (A). The ratio of plasma membrane to cytoplasmic expression of RhoA was used as an index of RhoA activation (C and M in B). RhoA expression was significantly increased in the membrane fraction of WT OVA airways but was not affected in Cav1 KO CTL or OVA groups. ΔΔCT, comparative cycle threshold method. Values are means ± SE (n = 4 animals). *Significant difference between CTL and OVA (P < 0.05).
Effect of caveolin-1 KO on epithelium.
On the basis of the above data showing that, if anything, absence of caveolin-1 results in reduced expression or activity of Ca2+ or force regulatory proteins, which would not explain the observed increase in contractility, we examined changes in the epithelium. Arginase 1 (Arg1) is the enzyme that hydrolyzes l-arg to ornithine and urea via the urea cycle and is a first step along the proline-collagen pathway (10, 30). l-arg is also a substrate for eNOS, a binding partner of caveolin-1. Although exogenous NO is a known bronchodilator, whether epithelially derived NO is involved in bronchodilation in vivo is still under investigation, although there is evidence to this point (55). Regardless, altered eNOS-arginine balance could additionally contribute to airway remodeling. Thus there was sufficient justification to determine whether altered arginase expression or activity contributed to the observed changes in the Cav1 KO mice.
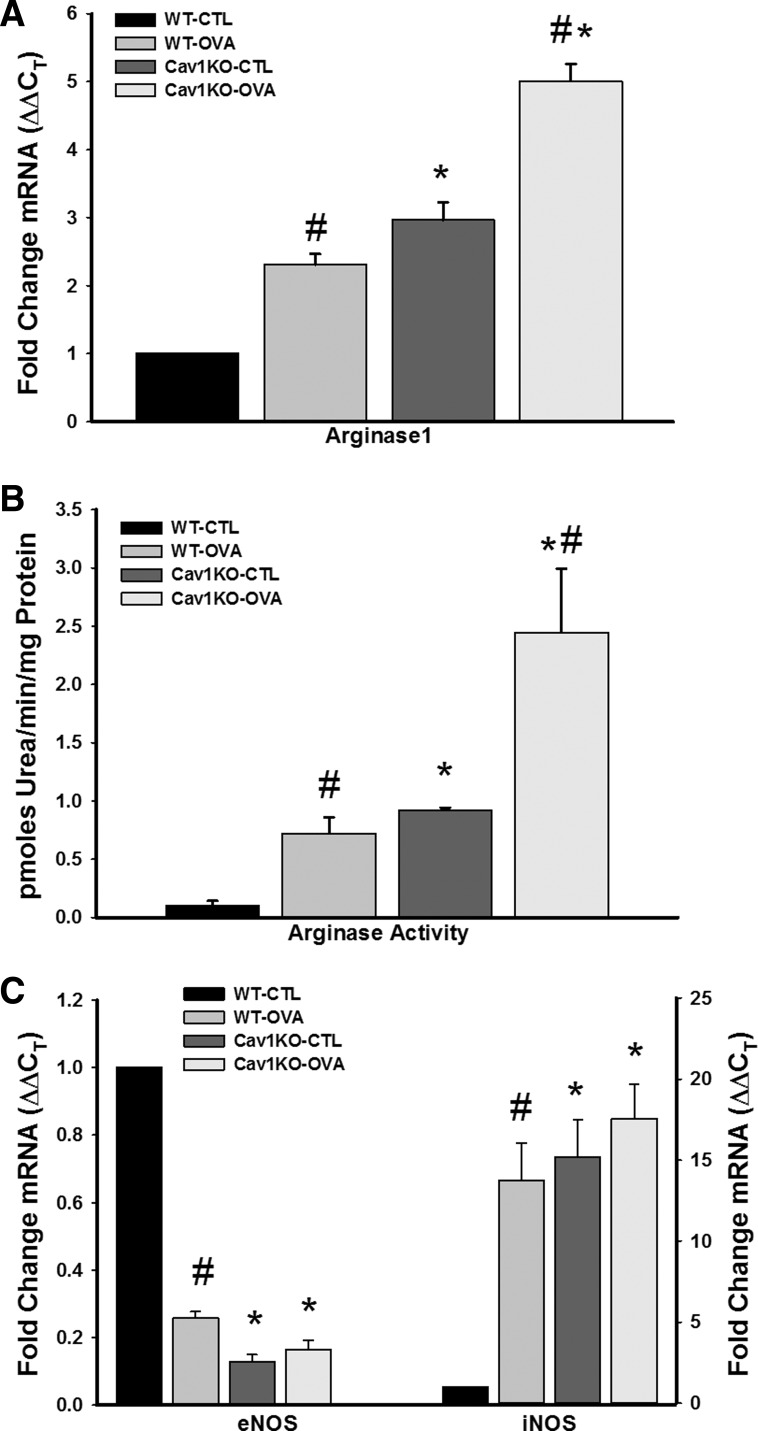
Compared with epithelium of CTL WT animals, expression of Arg1 was significantly higher in the other three groups (Fig. 6; P < 0.05). Both Cav1 KO groups showed even higher Arg1 compared with WT animals, with the Cav1 KO OVA group being the highest (Fig. 6). In addition, arginase activity was significantly increased in WT OVA and Cav1 KO CTL and OVA, with the most profound effect seen in Cav1 KO OVA mice (Fig. 6B; P < 0.05).
Fig. 6.
Effect of absence of caveolin-1 on airway epithelial expression of arginase and nitric oxide synthase. LCM-based mRNA analysis of epithelial layers showed that absence of caveolin-1, especially with OVA, resulted in the highest level of arginase 1 (Arg1) expression, as measured by quantitative RT-PCR (A, n = 4 animals for each set). The pattern of altered Arg1 expression was matched by enhanced arginase activity, especially in both Cav1 KO groups (B). Separately, in both WT and Cav1 KO airways, OVA sensitization/challenge significantly increased inducible NO synthase (iNOS) but decreased epithelial NO synthase (eNOS) expression, as measured by quantitative RT-PCR (C, n = 4 animals for each set). Values are means from 16 PCR reactions for each set ± SE. *Significant difference between WT and Cav1 KO; #significant difference between CTL and OVA (P < 0.05).
In contrast to Arg1, the pattern of eNOS expression was the converse, with absence of caveolin-1 resulting in substantially reduced eNOS (Fig. 6; P < 0.05). Given previous evidence that increased consumption of l-arg by Arg1 can result in decreased activity of inducible nitric oxide synthase (iNOS) (44), which can have potential downstream effects on airway structure and function via NO, superoxide, and peroxynitrite (23, 31), we examined epithelial iNOS in the four groups and found that its expression is indeed potentiated in epithelium of WT OVA and in both CTL and OVA Cav1 KO airways (Fig. 6).
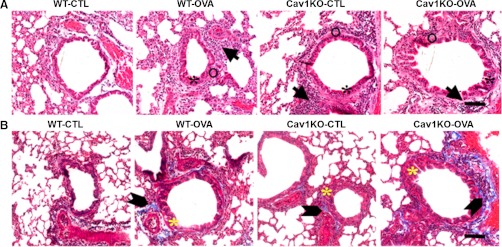
Histological analysis on lung sections from WT vs. Cav1 KO mice showed thickening of the epithelial layer in the airways of Cav1 KO mice compared with WT airways (Fig. 7A, asterisks). Sensitization and challenge with OVA further increased this thickening. Furthermore, the ASM layer of Cav1 KO airways also showed increased thickness (albeit not to the same extent as epithelium). In addition, the number of cells in both the epithelial and the ASM layers was increased in both Cav1 KO and OVA sensitized airways (Fig. 7, arrows), suggesting airway remodeling. Masson trichrome staining showed increased collagen deposition in the airways of Cav1 KO mice, especially in those sensitized and challenged with OVA (Fig. 7B, arrowheads).
Fig. 7.
Effect of absence of caveolin-1 on airway epithelium vs. ASM. In hematoxylin and eosin-stained lung sections (A), sensitization and challenge with OVA increased epithelial layer thickening (black asterisks). Interestingly, the epithelial layer was thickened in lung slices of Cav1 KO mice even without sensitization. The ASM layer (open circles) of Cav1 KO airways also showed increased thickness (albeit not to the same extent as epithelium). In addition, the number of cells was increased in the airways of Cav1 KO mice (arrows). Masson trichrome staining of lung sections (B) showed increased collagen deposition (block arrowheads) in the airways of Cav1 KO mice, especially in those sensitized and challenged with OVA. Yellow asterisks mark epithelial layer thickening in these sections. Scale bar is ∼50 μm.
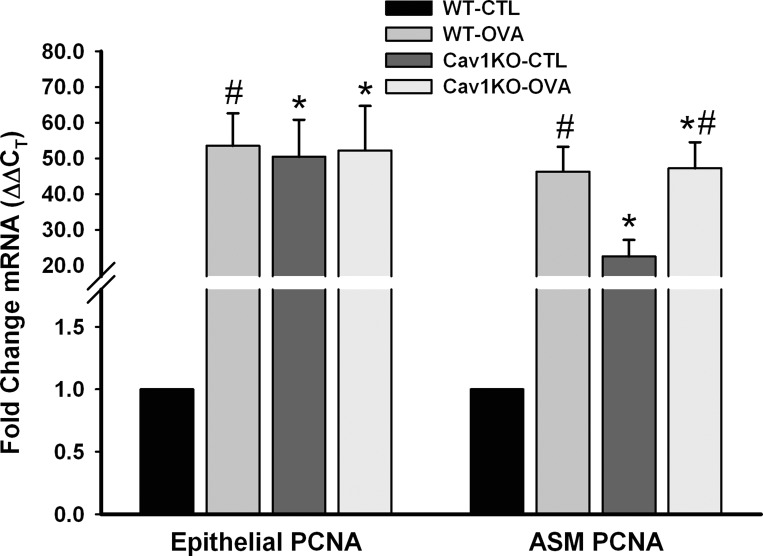
Consistent with these changes in the airway, LCM analysis for expression of proliferating cell nuclear antigen (PCNA), a specific marker for cellular proliferation in both ASM and epithelial layers showed substantial increase in the airways of OVA WT and both Cav1 KO groups (Fig. 8, P < 0.05).
Fig. 8.
Proliferative markers in the absence of caveolin-1. LCM analysis for mRNA expression of proliferating cell nuclear antigen (PCNA), a specific marker for cellular proliferation in both ASM and epithelial layers, showed substantial increase in the airways of OVA WT and both Cav1 KO groups (measured by quantitative RT-PCR). Values are means ± SE (n = 4 animals). *Significant difference between WT and Cav1 KO; #significant difference between CTL and OVA (P < 0.05).
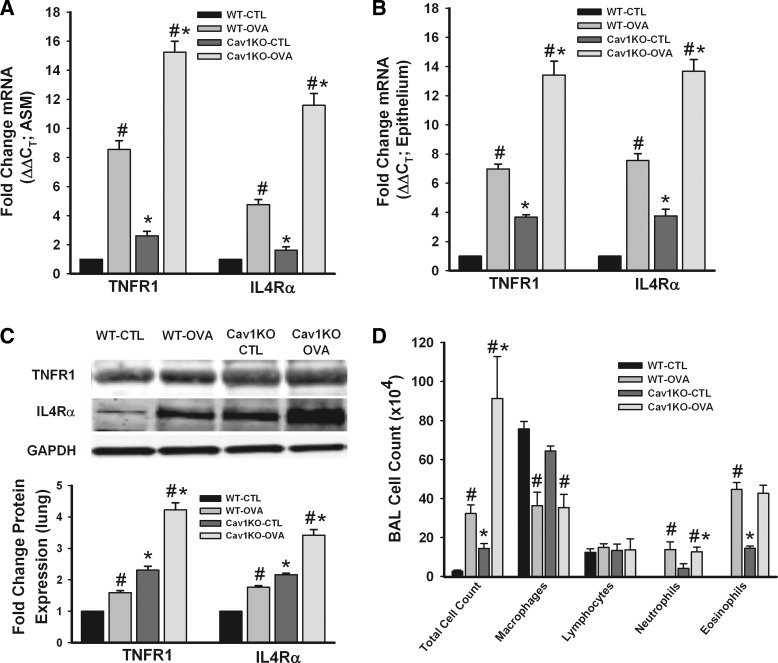
Effect of caveolin-1 absence on inflammatory response.
A potential confounder in the surprising hyperresponsiveness of Cav1 KO airways was the influence of altered caveolin-1 expression on the immune and inflammatory response to OVA. Although it was beyond the scope of this study to examine all aspects, we explored mRNA and protein expression of cytokine receptors in ASM vs. airway epithelium, and cell counts in the BAL fluid. Expression of both TNF-α receptor 1 (TNFR1) and IL4α receptor (IL4Rα; representing the IL-13 pathway) was significantly increased in WT OVA and Cav1 KO OVA in both the ASM layer and airway epithelium (Fig. 9, A and B; P < 0.05). In fact, increase in cytokine receptor expression was more pronounced in the Cav1 KO OVA group compared with WT OVA. These changes in mRNA were matched by corresponding changes in TNFR1 and IL4Rα protein expression (Fig. 9C; P < 0.05). BAL fluid analysis in these groups demonstrated a significant increase in total cell count in WT OVA, Cav1 KO CTL, and OVA groups, with the largest increases in the Cav1 KO OVA group (Fig. 9D; P < 0.05). Eosinophil counts were significantly increased in both of the Cav1 KO groups. Overall, these data suggested enhanced inflammation as a potential contributor to the observed increase in airway responsiveness.
Fig. 9.
Expression of inflammatory markers. mRNA levels for TNFR1 and IL4Rα were significantly increased in WT OVA, Cav1 KO CTL and OVA ASM (A) and airway epithelium (B) compared with WT CTL (measured by quantitative RT-PCR). This effect was most pronounced in Cav1 KO OVA group. The same pattern of effect was seen in the protein expression of these receptors (C). Separately, bronchoalveolar lavage (BAL) analysis showed significant increase in total cell count for WT OVA, Cav1 KO CTL, and OVA mice (D), with Cav1 KO OVA mice showing the maximal effect. Eosinophil count was also significantly increased in these 3 groups (n = 4 animals). Values are means ± SE (n = 4 animals). *Significant difference between WT and Cav1 KO; #significant difference between CTL and OVA (P < 0.05).
In vitro airway contractility in the absence of caveolin-1.
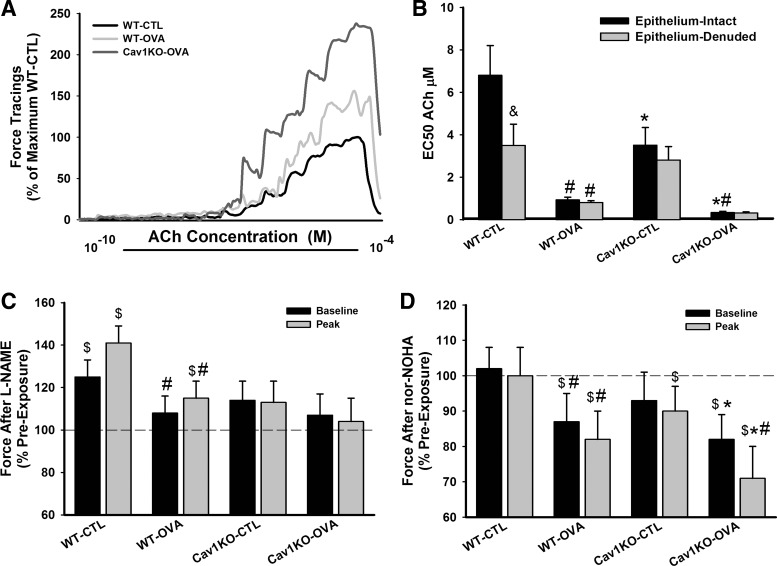
On the basis of the above data, we examined isolated tracheal rings to determine whether the epithelium plays a role in Cav1 KO airways. In epithelium-intact samples, ACh dose response curves were first performed. Following a thorough wash, samples were incubated with nor-NOHA (10 μM) for 1 h to inhibit Arg1 activity or to 100 μM NG-nitro-l-arginine methyl ester (l-NAME) to inhibit NO synthase (NOS). Samples were again subjected to ACh challenge at the same concentrations in the continued presence of nor-NOHA or l-NAME.
As shown in Fig. 10, epithelium-intact trachea from Cav1 KO mice showed significantly higher sensitivity to ACh compared with WT animals, reflected by a lower EC50 (P < 0.05). Furthermore, airways of Cav1 KO OVA mice were substantial more sensitive to ACh. Epithelial denudation enhanced airway sensitivity to methacholine but only in the WT CTL mice, with small to negligible effects in the other groups.
Fig. 10.
Physiological role of epithelium. In epithelium-intact tracheal rings, exposure to increased levels of ACh resulted in greater force production in both WT and Cav1 KO OVA mice, with much greater contractility in the absence of caveolin-1 (A). This was reflected by a significantly smaller effective concentration (EC50) of ACh in Cav1 KO mice, especially with OVA (B). Removal of epithelium reduced the EC50, but only in the WT CTL airways, suggesting dysfunctional epithelium or a lack of its role with inflammation and Cav1 KO. Accordingly, in epithelium intact rings, inhibition of NO synthase with NG-nitro-l-arginine methyl ester (l-NAME) enhanced baseline and peak force production by 1 μM ACh, but largely in WT CTL airways, and not in either Cav1 KO group (C). However, inhibition of arginase with N-omega-nor-l-arginine (nor-NOHA) substantially reduced the force responses to ACh in the Cav1 KO groups, especially with OVA (D). Values are means ± SE (n = 4 animals). *Significant difference between WT and Cav1 KO (P < 0.05). #Significant difference between CTL and OVA (P < 0.05). &Significant difference between epithelium intact and epithelium denuded rings (P < 0.05). $Significant difference between before and after inhibitor (l-NAME or nor-NOHA) treatment (P < 0.05).
To determine the role of epithelial factors, in separate experiments using epithelium-intact rings, preexposure to l-NAME significantly increased baseline and peak force in WT CTL airways but was substantially less effective in WT OVA and without effect in both Cav1 KO groups (Fig. 10 shows data at 1 μM ACh). Conversely, l-arg inhibition by nor-NOHA resulted in reduced baseline and peak forces in WT OVA and both Cav1 KO groups, with the greatest effects in Cav1 KO OVA (P < 0.05; Fig. 10). In both of these protocols, time-control experiments with no intervening l-NAME or nor-NOHA showed stable second responses to ACh that were <10% different from the first set of force responses to ACh (not shown).
DISCUSSION
Whereas in vitro studies on the role of caveolin-1 in ASM [Ca2+]i, contractility, and proliferation suggest a direct correlation between caveolin-1 and ASM contractility, the novel but surprising results of the present study in an in vivo caveolin-1 null mouse model suggest that the effect of caveolin-1 in regulation of airway contractility is much more complex and likely involves both epithelium and ASM, but in different aspects of airway hyperresponsiveness. The overall effects of caveolin-1 in the airway may be a result of several changes, importantly an increase in ASM cell proliferation (with increased expression of smooth muscle contractile protein) that contributes to increased contractility, a potential impairment of bronchodilation due an imbalance between eNOS and arginase, and the increased production of collagen that increases airway stiffness. Although the present study did not examine the role of caveolin-1 in the molecular mechanisms involved in each of these aspects, the results of the LCM analyses as well as the functional studies nonetheless suggest that caveolin-1 can regulate expression of a number of proteins involved in airway structure and function. Thus other factors, such as inflammation, may mediate airway hyperresponsiveness via caveolin-1, as shown in this study by differences in BAL cell count and LCM analyses of WT vs. Cav1 KO mice.
The flask-shaped plasma membrane invaginations called caveolae are enriched in cholesterol, sphingolipids, and integral membrane proteins including caveolins, which can regulate other caveolar proteins as well as modulate intracellular signal transduction (6, 63). In smooth muscle, including ASM, caveolae contain proteins that regulate [Ca2+]i signaling, contraction, and cellular proliferation (7, 16, 39, 40). For example, caveolae in canine ASM express binding proteins for L-type Ca2+ channels and plasma membrane Ca2+ ATPase (8), whereas human ASM caveolae express receptors for agonists such as ACh and histamine, Ca2+ influx mechanisms such as the TRPC channels and Orai1 (41), and force regulatory proteins such as RhoA (22, 41). Thus caveolae are highly important in regulating ASM structure and function.
Of the three mammalian caveolin isoforms (62), caveolin-1 and -2 are widely expressed, whereas caveolin-3 appears to be expressed mostly in striated muscle (63). In smooth muscle, caveolin isoform expression appears to be heterogeneous with some reports of all three isoforms in vascular smooth muscle (20) contrasting with other studies, including our own, showing that human ASM expresses only caveolin-1 and -2 (41, 46) (with the understanding that caveolin-2 is usually coexpressed with caveolin-1). In airway epithelium, there is currently very little data on the role of caveolins, although both caveolin-1 and -2 have been detected by immunocytochemical and imaging techniques (4, 51). These previous data formed the justification for examination of caveolin-1 in this study.
Airway inflammation and increased airway narrowing are hallmarks of diseases such as asthma (27). Here, the enhancing effect of cytokines such as TNF-α and IL-13 on ASM contractility is recognized (50). Accordingly, factors that modulate ASM structure and function in the presence of inflammation are key to understanding airway diseases. In this regard, there is limited but emerging information on the role of caveolin-1, largely derived from in vitro studies. In the lung overall, caveolin-1 has been shown to regulate NF-κB and inflammatory responses to sepsis (13). We previously showed that caveolae express the TNF-α receptor, that TNF-α increases caveolin-1 expression, and that in turn increased caveolin-1 contributes to enhancement of [Ca2+]i by cytokines (46). The role of caveolae in TNF-α enhancement of [Ca2+]i has been recently shown to involve augmented Ca2+ influx via store-operated mechanisms mediated by Orai1 (47) as well as Ca2+ sensitization via RhoA (48). Hunter and Nixon (22) demonstrated that TNFR1-mediated enhancement of RhoA involves lipid rafts. Separately, caveolin-1 regulation of p42/44 MAP kinases (16) has been demonstrated in terms of altered cellular proliferation, also a major aspect of diseases such as asthma. Thus caveolae and caveolin-1 appear to be central to regulation of airway hyperresponsiveness. In this regard, the premise of our study was based on the idea that if increased caveolin-1 contributes to enhanced [Ca2+]i and force (thus a procontractile ASM phenotype), then reduction of caveolin-1 in vivo would overall blunt ASM contractility in the presence of inflammation. Although decreased caveolin-1 would conversely allow for enhanced cellular proliferation (thus a proproliferative phenotype), the relative contribution of opposing effects of caveolin-1 is not known, and therefore we theorized that the in vivo model would provide an indication of where caveolin-1 has greater effect.
Considering the ubiquitous expression of caveolae and caveolins in different cell types, the Cav1 KO mouse, developed by Lisanti and colleagues (43) has been studied for pathology and pathophysiology of diseases other than in the airway (5). Most relevant here has been the observation that these mice develop severe pulmonary hypertension (9, 43). Defects in arterial filling and increased pulmonary vascular resistance have been observed in Cav1 KO animals (32). Considering the overall idea that pulmonary hypertension also involves enhanced vascular contractility, impaired vasodilation, and increased smooth muscle proliferation (66), the present results showing that absence of caveolin-1 leads to enhanced airway contractility is highly interesting and correlates well with previous findings in the pulmonary vasculature. Although we recognize that cell type specificity in caveolin-1 effects may limit generalization [e.g., recent studies have shown that caveolin-1 enhances pulmonary artery smooth muscle proliferation (35), in contrast to findings in ASM (16)], our findings nonetheless point to altered caveolin-1 expression being a potentially common pathological mechanism in different types of lung diseases.
Clearly, the enhanced Rl observed in both CTL and OVA Cav1 KO mice can only partially be explained by ASM contractility, and not by expression of proteins that can be expressed within caveolae. Previous in vitro data in human ASM point to enhanced caveolar expression of [Ca2+]i regulatory mechanisms (e.g., muscarinic receptors, Orai1) in inflammation (17, 18, 41). The large increases in mRNA expression of proteins such as muscarinic receptors, Orai1, sm-actin, and RhoA in OVA WT ASM, but an absence of such changes with OVA in Cav1 KO mice, are consistent with caveolin-1 regulation of these proteins that are involved in ASM and force regulation. However, the reduction of [Ca2+]i regulatory proteins and RhoA, and the diminution of RhoA activity (at least as reflected by lack of membrane translocation) in the OVA Cav1 KO mice, cannot explain the much higher Rl. Although the likely lower level of such a protein could still be all present in noncaveolar plasma membrane and thus contribute to increased ASM contractility, the lack of difference between Cav1 KO CTL vs. OVA groups would suggest otherwise in terms of airway hyperresponsiveness during inflammation. Expression of sm-actin was observed to be sustained in OVA Cav1 KO airways and may be partially responsible for the higher Rl. However, it is also clear that all of the substantial increase in force cannot be explained simply by changes at the ASM, leading us to examine other mechanisms in this model.
A single previous study that examined airway function in Cav1 KO mice (28) reported that, by 3 mo of age, lung compliance is significantly reduced whereas Rl is increased. The reduced compliance was associated with increased deposition of collagen fibrils in the airways: a finding observed in the present study as well. These authors interpreted increased Rl as being due to changes in the extracellular matrix. Thickened alveolar septa, constricted alveolar lumens, and hypercellularity have also been reported in Cav1 KO lungs (65). The findings of the present study showing increased collagen deposition in the airways of OVA-sensitized/challenged mice, and more importantly in both CTL and OVA Cav1 KO mice, are highly significant and consistent in this regard and point to the importance of caveolin-1 in airway remodeling. Furthermore, enhanced PCNA expression and a somewhat thicker smooth muscle layer in the airways of Cav1 KO mice also support the idea that enhanced cell proliferation and increased smooth muscle mass in the absence of caveolin-1 contributes, at least partly, to the observed airway hyperresponsiveness. In this regard, changes in epithelial PCNA and in the epithelial layer may also be significant, and formed a basis for further exploration of this cell type in this study.
Airway tone represents a balance between constriction and dilation. NO is recognized as an endogenous and exogenous bronchodilator (11, 14, 38). In airways, NO may be derived from epithelium (3) as well as nonadrenergic/noncholinergic innervation (26, 61). Although the epithelium is a major site for eNOS and iNOS, especially for iNOS in the asthmatic airway (24), the role of epithelially derived NO in bronchodilation (especially that mediated by eNOS) is not as clear as for endothelially derived NO in the vasculature. Regardless, a potential explanation for the higher Rl at baseline as well as with methacholine challenge in Cav1 KO mice is impaired NO production in airway epithelium, resulting in greater bronchoconstriction. This is suggested by reduced eNOS levels in epithelium (especially) of OVA Cav1 KO airways, with a concomitant increase in Arg1 expression and activity, which would only reduce substrate availability for eNOS. Although we did not specifically examine reduced NO production in this setting, our data using tracheal rings where inhibition of NOS by l-NAME showed increased baseline force and contractility in WT CTL mice, but not in either of Cav1 KO groups, highlighting lesser contribution of NO in the latter. Overall, these data suggest that caveolin-1 modulates epithelial NO levels with downstream consequences on airway tone.
Caveolin-1 is a known negative regulator of eNOS in endothelium (9, 67). Accordingly, if the same were to hold true in epithelium, eNOS expression should have been elevated in Cav1 KO animals. However, we observed downregulation of eNOS expression, highlighting the complexity of caveolin-1–eNOS interactions. Additional experiments showed a concomitant upregulation of iNOS in the Cav1 KO airway, which would then likely be a major source of NO in the knockout model. Such NO can be scavenged by superoxide free radical (O2·−) to give rise to peroxynitrite (ONOO−), a reactive nitrogen species molecule that has been shown to be proinflammatory and to induce airway hyperreactivity (56). In support, Cav1 KO animals have recently been shown to have increased levels of both peroxynitrite (64) and superoxide (42) and thus may induce a hypercontractile phenotype. However, it also important to recognize that the increase in iNOS (also observed in airway epithelium in this study) may not necessarily correlate with altered airway hyperresponsiveness, considering a recent report of lack of difference between iNOS KO and WT mice (52). Thus the specific interactions between caveolin-1 and different NOS isoforms in the inflamed airway remain to be established.
In epithelium, as with other cells, l-arg is a common substrate for both NOS and arginase. There has been much interest in arginases and in the deficiency and supplementation of l-arg in adult asthma (57, 59). Chronically elevated Arg 1 is associated with airway remodeling and hyperresponsiveness (30). Whether caveolin-1 is involved in modulation of the arginase pathway or its downstream effects is not known. However, the single previous report of increased collagen in the airways of Cav1 KO mice is highly suggestive and is supported by our findings from the LCM analysis. In this regard, our findings of increased Arg1 expression and arginase activity especially in Cav1 KO OVA mice suggest a role for caveolin-1 in the arginase regulation. This interaction may be important in regulation of airway tone, as evidenced by our novel data showing reduced baseline force and contractility when arginase is inhibited by nor-NOHA, especially in Cav1 KO OVA mice. Overall, these data highlight the importance of caveolin-1 in airway epithelium, especially in the presence of inflammation.
In addition to caveolin-1 per se, there is very recent evidence in other cell types that adaptor proteins called cavins are essential for caveolar formation and maintenance, caveolin trafficking in membrane remodeling, and caveolar interactions with intracellular structures such as endoplasmic reticulum (5). Of the four cavins identified, PTRF/cavin1 (21, 58), SDPR/cavin2 (19), and SRBC/cavin3 (36), each appear to be important in caveolar formation and shape, whereas MURC/cavin4 (2, 37) helps enhance interactions with intracellular structures. Considering the novelty of these findings, there is currently very limited information on cavins in ASM or airways (49). Nonetheless, in pilot studies, we found that cavins 1–3 are detectable in human ASM, findings confirmed in our mouse studies here. However, their role or their interactions with caveolin-1 in modulating airway contractility is not known. Furthermore, there is no information on expression of cavins in airway epithelium. The upregulation of cavins 1–3 in OVA WT airways is consistent with the known function of these proteins in enhancing caveolar structure and function (5, 19, 21, 36). The dramatic decrease in expression of cavins in Cav1 KO airways suggests not only that these proteins are transcriptionally modulated by caveolin-1 but also that cytoplasmic levels of these proteins may be crucial determinants of contractile responses in the airway. However, these issues remain to be examined.
Although our data have clearly shown the complex role of caveolin-1 in mediating airway hyperresponsiveness, a potential confounding factor is the effect of absent caveolin-1 on the inflammatory milieu. Here, if anything, our original hypothesis would have led us to the idea that Cav1 KO mice would have a less inflammatory environment. However, our data on cytokine receptor expression and the BAL analysis both suggest that in the absence of caveolin-1 the inflammatory response may be enhanced. Whether such changes contribute to the enhanced airway contractility remains to be determined.
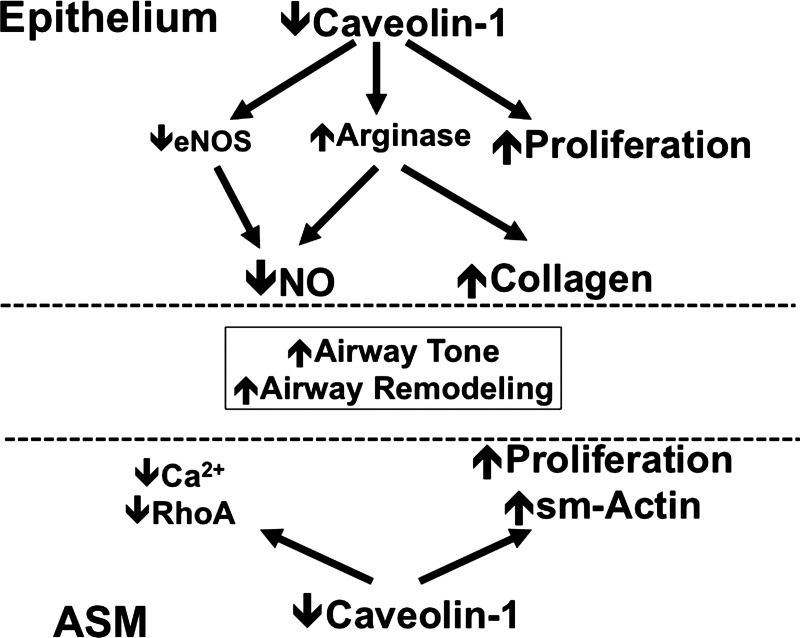
Overall, the results of the present study highlight an important but complex role for caveolin-1 in modulating expression of a number of proteins involved in airway contractility and relaxation, with a net result of enhanced airway contractility in the setting of absent caveolin-1 (schematically shown in Fig. 11). The mechanisms by which reduced caveolin-1 enhances contractility may involve the function of both epithelium and ASM. Further study is required to dissect out the relative contributions of caveolin-1 in these cell types in modulating airway structure and function. Here, reconciliation between the in vitro findings in human airways and the in vivo work of this study is necessary. A potential factor that may explain the discrepancies is that caveolin-1 effects are time and context dependent, in that short-term alterations in caveolin-1 expression, as done in many studies, including our own, may have a different effect compared with permanent absence of this protein throughout life. Addressing this issue will require a conditional and inducible caveolin-1 KO, preferably in the airway, which is currently unavailable. Furthermore, species differences in the role of caveolin-1 may also be important to consider. These issues will drive future work into this interesting but clearly complex area.
Fig. 11.
Schematic of proposed mechanisms by which caveolin-1 influences airway tone. At the level of ASM, the overall effect of caveolin-1 represents a balance between influences on [Ca2+]i, Ca2+ sensitivity mediated by RhoA, and cell proliferation and airway remodeling. A parallel effect of caveolin-1 on the balance between NO synthase and arginases further influences both NO and potentially bronchodilation, as well as airway remodeling.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL090595 (C. M. Pabelick), HL088029 (Y. S. Prakash), and HL074309 (G. C. Sieck), and by a Clinical Innovator Award from the Fight Attendant Medical Research Institute (C. M. Pabelick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.A., Y.S.P., and C.M.P. conception and design of research; B.A., S.K.V., L.W.M., P.K.V., and M.A.T. performed experiments; B.A., S.K.V., L.W.M., P.K.V., and M.A.T. analyzed data; B.A., L.W.M., P.K.V., and M.A.T. interpreted results of experiments; B.A. prepared figures; B.A. drafted manuscript; B.A., Y.S.P., and C.M.P. edited and revised manuscript; B.A., L.W.M., P.K.V., M.A.T., G.C.S., Y.S.P., and C.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Dr. Elizabeth Townsend, Ph.D. for help with the FlexiVent experiments and Thomas Keller for technical support.
REFERENCES
- 1. Barar J, Campbell L, Hollins AJ, Thomas NP, Smith MW, Morris CJ, Gumbleton M. Cell selective glucocorticoid induction of caveolin-1 and caveolae in differentiating pulmonary alveolar epithelial cell cultures. Biochem Biophys Res Commun 359: 360–366, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, Lo HP, Abankwa D, Luetterforst R, Fernandez-Rojo M, Breen MR, Gygi SP, Vinten J, Walser PJ, North KN, Hancock JF, Pilch PF, Parton RG. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol 185: 1259–1273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic Biol Med 41: 515–527, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Byrne S, Cheent A, Dimond J, Fisher G, Ockleford CD. Immunocytochemical localization of a caveolin-1 isoform in human term extra-embryonic membranes using confocal laser scanning microscopy: implications for the complexity of the materno-fetal junction. Placenta 22: 499–510, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 86: 219–225, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53: 1261–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Daniel EE, Eteraf T, Sommer B, Cho WJ, Elyazbi A. The role of caveolae and caveolin 1 in calcium handling in pacing and contraction of mouse intestine. J Cell Mol Med 13: 352–364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am J Physiol Lung Cell Mol Physiol 279: L1226–L1235, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folkerts G, Nijkamp FP. Nitric oxide in asthma therapy. Curr Pharm Des 12: 3221–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Fust A, Bates JH, Ludwig MS. Mechanical properties of mouse distal lung: in vivo versus in vitro comparison. Respir Physiol Neurobiol 143: 77–86, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol 177: 4853–4860, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Giles TD. Aspects of nitric oxide in health and disease: a focus on hypertension and cardiovascular disease. J Clin Hypertens (Greenwich) 8: 2–16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gosens R, Stelmack GL, Dueck G, Mutawe MM, Hinton M, McNeill KD, Paulson A, Dakshinamurti S, Gerthoffer WT, Thliveris JA, Unruh H, Zaagsma J, Halayko AJ. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1406–L1418, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol 11: 807–814, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardin CD, Vallejo J. Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res 69: 808–815, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132: 113–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunter I, Nixon GF. Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways. J Biol Chem 281: 34705–34715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med 162: 701–706, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Jiang J, Malavia N, Suresh V, George SC. Nitric oxide gas phase release in human small airway epithelial cells. Respir Res 10: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi T, Iijima K, Kita H. Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice. J Immunol 170: 5756–5763, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Lammers JW, Barnes PJ, Chung KF. Nonadrenergic, noncholinergic airway inhibitory nerves. Eur Respir J 5: 239–246, 1992 [PubMed] [Google Scholar]
- 27. Lazaar AL, Panettieri RA., Jr Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol 116: 488–495; quiz 496, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Le Saux O, Teeters K, Miyasato S, Choi J, Nakamatsu G, Richardson JA, Starcher B, Davis EC, Tam EK, Jourdan-Le Saux C. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol Lung Cell Mol Physiol 295: L1007–L1017, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li XA, Everson W, Smart EJ. Nitric oxide, caveolae, and vascular pathology. Cardiovasc Toxicol 6: 1–13, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Maarsingh H, Bossenga BE, Bos IS, Volders HH, Zaagsma J, Meurs H. l-arginine deficiency causes airway hyperresponsiveness after the late asthmatic reaction. Eur Respir J 34: 191–199, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Maarsingh H, Dekkers BG, Zuidhof AB, Bos IS, Menzen MH, Klein T, Flik G, Zaagsma J, Meurs H. Increased arginase activity contributes to airway remodelling in chronic allergic asthma. Eur Respir J 38: 318–328, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Maniatis NA, Shinin V, Schraufnagel DE, Okada S, Vogel SM, Malik AB, Minshall RD. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol 294: L865–L873, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol 234: 191–199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol 64: 2318–2323, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Mathew R, Huang J, Gewitz MH. Pulmonary artery hypertension: caveolin-1 and eNOS interrelationship: a new perspective. Cardiol Rev 15: 143–149, 2007 [DOI] [PubMed] [Google Scholar]
- 36. McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, Luby-Phelps K, Anderson RG. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J 28: 1001–1015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogata T, Ueyama T, Isodono K, Tagawa M, Takehara N, Kawashima T, Harada K, Takahashi T, Shioi T, Matsubara H, Oh H. MURC, a muscle-restricted coiled-coil protein that modulates the Rho/ROCK pathway, induces cardiac dysfunction and conduction disturbance. Mol Cell Biol 28: 3424–3436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park KW, Dai HB, Lowenstein E, Sellke FW. Epithelial dependence of the bronchodilatory effect of sevoflurane and desflurane in rat distal bronchi. Anesth Analg 86: 646–651, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poljakovic M, Porter DW, Millecchia L, Kepka-Lenhart D, Beighley C, Wolfarth MG, Castranova V, Morris SM., Jr Cell- and isoform-specific increases in arginase expression in acute silica-induced pulmonary inflammation. J Toxicol Environ Health A 70: 118–127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Rahman A, Sward K. The role of caveolin-1 in cardiovascular regulation. Acta Physiol (Oxf) 195: 231–245, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Santhanam L, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res 101: 692–702, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sathish V, Abcejo AJ, VanOosten SK, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 in cytokine-induced enhancement of intracellular Ca2+ in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L607–L614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 regulation of store-operated Ca2+ influx in human airway smooth muscle. Eur Respir J 40: 470–478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sathish V, Yang B, Meuchel LW, VanOosten SK, Ryu AJ, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 and force regulation in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 300: L920–L929, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharma P, Ghavami S, Stelmack GL, McNeill KD, Mutawe MM, Klonisch T, Unruh H, Halayko AJ. beta-Dystroglycan binds caveolin-1 in smooth muscle: a functional role in caveolae distribution and Ca2+ release. J Cell Sci 123: 3061–3070, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol 29: 859–866, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Silva WI, Maldonado HM, Lisanti MP, Devellis J, Chompre G, Mayol N, Ortiz M, Velazquez G, Maldonado A, Montalvo J. Identification of caveolae and caveolin in C6 glioma cells. Int J Dev Neurosci 17: 705–714, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a β2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sopi RB, Haxhiu MA, Martin RJ, Dreshaj IA, Kamath S, Zaidi SI. Disruption of NO-cGMP signaling by neonatal hyperoxia impairs relaxation of lung parenchyma. Am J Physiol Lung Cell Mol Physiol 293: L1029–L1036, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Sugiura H, Ichinose M. Nitrative stress in inflammatory lung diseases. Nitric Oxide 25: 138–144, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther 339: 815–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van der Vliet A, Eiserich JP, Shigenaga MK, Cross CE. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med 160: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Vercelli D. Arginase: marker, effector, or candidate gene for asthma? J Clin Invest 111: 1815–1817, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vinten J, Johnsen AH, Roepstorff P, Harpoth J, Tranum-Jensen J. Identification of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta 1717: 34–40, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Vonk JM, Postma DS, Maarsingh H, Bruinenberg M, Koppelman GH, Meurs H. Arginase 1 and arginase 2 variations associate with asthma, asthma severity and beta2 agonist and steroid response. Pharmacogenet Genomics 20: 179–186, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AM. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 203: 2895–2906, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ward JK, Barnes PJ, Tadjkarimi S, Yacoub MH, Belvisi MG. Evidence for the involvement of cGMP in neural bronchodilator responses in humal trachea. J Physiol 483: 525–536, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Williams TM, Lisanti MP. The caveolin proteins. Genome Biol 5: 214, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wunderlich C, Schober K, Lange SA, Drab M, Braun-Dullaeus RC, Kasper M, Schwencke C, Schmeisser A, Strasser RH. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun 340: 702–708, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Yang G, Timme TL, Naruishi K, Fujita T, Fattah el MA, Cao G, Rajagopalan K, Troung LD, Thompson TC. Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation. Exp Mol Pathol 84: 131–140, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Yildiz P. Molecular mechanisms of pulmonary hypertension. Clin Chim Acta 403: 9–16, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 99: 11375–11380, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]