Abstract
Since the discovery of tadpole collagenase in 1962, the matrix metalloproteinase (MMP) family has emerged as a significant proteinase group with recognized effects on the cardiovascular system. Over the last 40 years, many milestones have been achieved, from the identification of the first MMP, to the generation of the first MMP cDNA clone and null mouse, to the clinical approval of the first MMP inhibitor. Over the years, a few myths and misunderstandings have interwoven into the truths. In this review, we will discuss the major milestones of MMP research, as well as review the misinterpretations and misperceptions that have evolved. Clarifying the confusions and dispelling the myths will both provide a better understanding of MMP properties and functions and focus the cardiovascular field on the outstanding research questions that need to be addressed.
Keywords: review, matrix metalloproteinases, MMP, cardiovascular disease, myocardial infarction
since 1962, the matrix metalloproteinase (MMP) family has been extensively studied in a multitude of animal and tissue models, and the first review article with MMPs in the title was written by Henning Birkedal-Hansen in 1988 (12). MMPs have been evaluated using the expertise of many different disciplines, including biochemistry, cell biology, pathology, immunology, physiology, and computational biology and from many different disease viewpoints, including arthritis, cancer, periodontal disease, and cardiovascular disease.
While this is a general MMP review, we will focus on cardiovascular and inflammatory aspects, as our own investigations focus on these areas and they will be of greater appeal to this audience. In this paper, we will summarize the major milestones in the MMP research field, as well as discuss the myths and misperceptions that have arisen throughout the years. Finally, we end with discussion on where the cardiovascular MMP field is headed.
Milestones
Table 1 lists the major milestones in MMP research. Before the first MMP (collagenase/MMP-1) was identified, an initial milestone was the study by Woessner in 1962 showing that a protein enzyme in mammalian uterus could degrade collagen (165). Later that year, Jerome Gross and Charles Lapiere were the first to identify an MMP using a biochemical approach (60). They showed that the anuran tadpole had strong collagenolytic activity in the skin, gut, and gills, tissues that underwent the most radical remodeling during metamorphosis. This discovery was the first step into a field that would grow exponentially in the following years. In 1966, MMP-1 was purified from tadpole tail fin and back skin (106).
Table 1.
Major milestones in MMP research
| Milestone | Investigators |
|---|---|
| Enzyme degradation of collagen described | Woessner (165) |
| First MMP described | Gross and Lapiere (60) |
| MMP-1 purified | Nagai et al. (106) |
| MMP-2 and -3 identified, isolated, and sequenced | Chin et al. (29), Collier et al. (31), Galloway et al. (55), Okada et al. (109), and Woessner and Nagase (166) |
| MMP-3 as activator of pro-MMP-1 | Murphy et al. (105) |
| MMP term coined | Okada et al. (110) |
| TIMP-1 and RECK identified as natural MMP inhibitors | Bauer et al. (10), Oh et al (107), and Sellers et al. (132) |
| MMP zymogen and mechanism of activation | Chen et al. (27), Milla et al. (104), Rosenblum et al. (123), and Springman et al. (141), among numerous research groups |
| Crystal structure of collagenase catalytic domain solved | Lovejoy et al. (97) |
| MMP-7 is the first MMP null mouse generated | Wilson et al. (164) |
| MMP diversity and expression in human heart failure | Dixon and Spinale (42) and Spinale (138, 139) |
| MMP diversity and expression in atheromatous plaque | Libby et al. (91) |
| Plaque rupture and inflammation | Galis et al. (54) |
| MMP roles in inflammatory and fibrotic responses to myocardial infarction | Lindsey and Zamilpa (95) |
| MMP imaging in vivo | Zhang et al. (174) |
The first 10 milestones are general matrix metalloproteinase (MMP) milestones, while the last 5 relate to MMPs in cardiovascular pathophysiology. TIMP, tissue inhibitor of MMP; RECK, reversion-inducing cysteine-rich protein with kazal motifs.
When compared to MMP-1, MMP-2 was identified as a higher molecular mass species (72 kDa) with gelatinolytic activity, whereas MMP-3 was identified as a lower molecular mass species (54 kDa) with proteoglycan and casein degrading activity. MMP-2 was first described and isolated in the 1970s and initially denoted as 72-kDa type IV collagenase/gelatinase A (166). MMP-2 was sequenced by Goldberg and colleagues (31) and purified from human rheumatoid synovial fibroblasts and characterized by the Nagase laboratory (109). MMP-3 neutral proteinase activity was first described in the 1970s (166), and the enzyme was purified and described as a proteoglycanase in 1983 (55). MMP-3 was also isolated and purified from rabbit synovial fibroblasts by the Werb laboratory (29) in 1985 and subsequently named stromelysin. MMP-3 was shown in 1987 to be an activator of pro-MMP-1 (105). When these three MMPs were identified and characterized, it was noted the MMP-2 had constitutive expression that was not robustly influenced by treatment with phorbol esters, whereas MMP-1 and MMP-3 production were both greatly enhanced with stimulation.
As additional MMPs were identified, the members of the family were frequently recognized by more than one name. In the late 1980s, Ed Harris, Jr., and colleagues (110) first proposed using the name MMP for this family of enzymes. Subsequently, the International Union of Biochemistry and Molecular Biology designated the family with the unique name MMPs and assigned each family member with an enzyme number. By 1991, MMP-1, -2, -3, -7, -8, -9, and -10 as well as tissue inhibitor of metalloproteinase (TIMP)-1 and -2 had been named and characterized. The MMP family has grown to current total of 25 members, although not all of these are found in humans. The MMP family has been divided into five subgroups: collagenases, gelatinases, matrilysins, stromelysins, and membrane-type MMPs (MT-MMPs). These groups are based on sequence homology and in vitro ECM substrate characteristics (95).
To be classified as an MMP family member, the enzyme should meet the following requirements: 1) proteolysis of at least one ECM component; 2) catalysis dependent on zinc at the active site; 3) activation by proteinases or organomercurials; 4) inhibited by ethylenediaminetetraacetic acid, 1,10-phenanthroline, and one of the TIMPs; and 5) cDNA has sequence homology to MMP-1. An original qualification, that the proteinase be secreted in a proform, no longer holds, as MMP-11 and -28 are intracellularly activated by furin and are secreted in active forms and the membrane-bound MMPs are not necessarily secreted (112).
MMPs were first classified by their in vitro ECM substrate characteristics, although there was no real logic for why particular substrates were tested over others. Some of the more abundant ECM proteins, such as type I collagen, fibronectin, and laminin, were tested for many but not all MMPs. The first 10 MMPs have had broad substrate characterization, whereas for later MMPs (for example, MMP-28), only a few substrates have been identified or examined. This limited substrate classification has led to a number of misperceptions and oversimplifications in understanding the diversity of MMP functions.
Four major MMP milestones were the 1) identification of the TIMPs, 2) elucidation of MMP proenzyme forms, 3) structural determination of MMPs, and 4) creation of MMP null mice. The first TIMP, TIMP-1, was described in 1972, and four TIMPs have been identified to date (10). Additional natural MMP inhibitors that have been identified include α2-macroglobulin, tissue factor pathway inhibitor, the membrane-anchored glycoprotein reversion-inducing cysteine-rich protein with kazal motifs, and MMP prodomains (107). The recognition of MMP zymogen forms and subsequent propeptide removal as part of the MMP activation process was key to understanding MMP regulation (27, 104, 123, 141).
In 1994, the first crystal structure of human fibroblast collagenase catalytic domain was published by the laboratory of Lovejoy and colleagues (97). The crystal structure was solved at 2.4-Å resolution in the form of a collagenase-inhibitor complex. In 1997, the first MMP null mouse was generated in the Matrisian laboratory (164), which was a mouse with the targeted disruption of the MMP-7 gene. Taconic has a knockout repository website (www.taconic.com) that lists all of the available MMP null models. Current lists of null and transgenic MMP mice available with selected phenotype information are provided in Tables 2 and 3.
Table 2.
A selection of MMP and TIMP null phenotypes in mice
| MMP | Phenotypes |
|---|---|
| -1a* | ↓ Angiogenesis; ↓ tumors |
| -2 | ↑ Tumor cell apoptosis |
| -3 | ↓ Angiogenesis; ↓ tumors |
| -7 | ↓ Intestinal adenoma formation |
| -8 | ↑ Skin tumors; ↑ response in arthritis; ↓ lung fibrosis |
| -9 | ↓ MMP-2 expression, ↓ SMC migration and neovascularization |
| -10 | ↑ Inflammation to Pseudomonas aeruginosa infection |
| -11 | Accelerated neointima formation in vascular injury model |
| -12 | Early pulmonary fibrosis and ↓ airway resistance |
| -13 | ↑ Interstitial collagen; defect in growth plate cartilage |
| -14 | Arthritis; osteopenia; dwarfism; ↓ macrophage infiltration |
| -16 | ↓ Growth, ↓ mesenchymal cell viability |
| -19 | Obesity; ↑ tenascin C; ↑ Th2 inflammation |
| -20 | Decreased mineral content; deteriorating enamel organ morphology |
| -24 | Abnormal mast cell degranulation |
| -15, -17, -21, -23, -25, -27 | Mouse model available but phenotype not yet published or observed |
| -1b*, -18, -22, -26 | Mouse model not available |
| -28 | ↑ Inflammation and ECM response to cardiac aging |
| TIMP-1 | ↑ Remodeling post-myocardial infarction, ↓ adipose in high-fat diet |
| TIMP-2 | Delayed neuronal differentiation, weak muscles |
| TIMP-3 | Left ventricular dilation, cardiomyocyte hypertrophy |
| TIMP-4 | ↑ neutrophil infiltration |
Of note, most MMP null mice are viable and fertile and show phenotypes only under stressed conditions (17, 19, 34, 47, 49, 53, 68, 69, 74, 92, 99, 121, 124, 133, 164).
There are two MMP-1 genes in mice. *There are two MMP-1 genes in mice, which are MMP-1a and MMP-1b. SMC, smooth muscle cell; Th2, T-helper 2.
Table 3.
A selection of MMP and TIMP transgenic overexpression phenotypes in mice (11, 16, 18, 79, 101, 103, 126, 150, 161)
| MMP | Phenotypes |
|---|---|
| -1a | Emphysematous changes similar to human emphysema |
| -2 | Myocyte hypertrophy; systolic dysfunction |
| -3 | ↑ Squamous cell carcinoma |
| -7 | ↑ Tumor; protection from pulmonary fibrosis |
| -9 | ↑ Susceptibility of carcinogenesis; decreased fibrosis |
| -10 | Abnormality in wound epithelium organization; abnormal keratinocyte migration |
| -11 | Death during late embryogenesis in Xenopus laevis oocytes |
| -12 | ↑ Arthritic lesions; ↑ macrophage infiltration (rabbit) |
| -13 | Articular cartilage degradation, joint pathology as observed in osteoarthritis |
| -14 | Fibrosis, adenocarcinoma |
| -15 | Mouse model available but phenotype not yet published |
| -1b, -8, -16, -17, -18, -19, -20, -21, -22, -23, -24, -25, -26, -27, -28 | Mouse models not available |
| TIMP-1 | ↓ Cellular proliferation and angiogenesis during hapatocarcinogenesis |
| TIMP-2 | ↓ Tumor formation, ↓ angiogenesis, ↑ apoptosis |
| TIMP-3 | Protection from metabolic inflammation and related metabolic disorders |
| TIMP-4 | Diastolic dysfunction |
Myths and Misperceptions in the MMP Field
Over the years, several myths and misperceptions have arisen in the MMP field, in part because of a lack of understanding and in part because of unclear literature. We list here several of these misunderstandings in an attempt to clarify the facts.
Gelatin zymography.
The statement that gelatin zymography is the best way to measure MMPs is a misunderstanding. While it is true that MMPs can be measured by zymography, several myths have mutated from this idea. One myth is that a zymogram is the only way to measure MMP activity. The reason why zymograms were prominently used in the first 30 years of MMP research was that MMP antibodies were not widely available. With the current large choice in MMP antibodies that recognize both pro- and activated forms, the zymogram is for the most part now an archaic technology. Another myth was that one needed to measure active MMP in the sample in order for it to be relevant. In fact, MMPs do not need to be active to be functionally relevant. Aikawa and colleagues (37) showed that MMP-13 and Wnt compete for binding to lipoprotein receptor-related protein 5/6, without the activation of MMP-13. Nath and colleagues (32) showed that MMP-1 interacts with integrins to alter Akt phosphorylation without the activation of MMP-1. Furthermore, Bannikov and colleagues (7) demonstrated that pro-MMP-9 in the presence of substrate has enzymatic activity without the loss of the 10-kDa prodomain. Strongin (143) has reported that binding of TIMP-2 to the hemopexin domain of catalytically inactive MMP-14 induces MAPK activation and cell growth. Thus pro-MMPs can be functionally active and should not be ignored or undervalued. For MMP studies in cardiovascular research, we recommend that immunoblotting and immunohistochemistry be used to measure MMP levels, coupled with in vivo MMP imaging techniques described below.
MMP-2 and -9.
The statement that MMP-2 and MMP-9 are the most important MMPs is a myth. A literature search will clearly demonstrate that MMP-2 and MMP-9 have the most number of publications. These two MMPs, however, are not necessarily more significant than other MMPs simply because they are more frequently measured. The popularity of MMP-2 and -9 stems from earlier days when zymography was the method of choice to measure MMP levels and activity because of a lack of available antibodies for immunoblotting approaches (95). Because gelatin zymography was much more technically accessible than zymography using casein or other substrates, MMP-2 and -9 were the easiest MMPs to measure (154). As a result, the number of articles evaluating MMP-2 and -9 are a log-fold higher in number compared with other MMPs, particularly some of the newer family members.
Currently, many other MMPs are being studied in the cardiovascular system, particularly MMP-7 and MMP-14 (28, 94, 140, 172). Beyond noting expression levels, there remains a large knowledge gap in regard to roles of additional MMP family members in cardiovascular disease processes. Included in the list of MMPs that have not been fully analyzed for substrate profiles, cellular localization, and biological roles are MMP-11, MMP-20, and MMP-28 (99).
Substrates.
The statement that MMPs only process ECM proteins is a misunderstanding. MMPs degrade not only ECM but non-ECM substrates as well. The ability to cleave non-ECM proteins, such as cell surface membrane proteins, is an important mechanism to regulate cellular functions. Proteolysis can stimulate or deactivate intracellular signaling pathways, such as apoptosis and autophagy pathways (23, 142, 163). The challenge is to identify which are the most important MMP substrates. For example, in addition to gelatin (denatured collagen), MMP-2 also degrades the ECM substrates elastin, fibronectin, and aggrecan, as well as non-ECM substrates IL-1β, α1-proteinase inhibitor, pro-lysyl oxidase, and other MMPs (-1, -9, and -13) (22, 93). ECM substrates of MMP-9 include aggrecan; types I, II, III, IV, V, VII, X, and XI collagen; elastin; fibronectin; galectin-3; laminin; link protein; secreted protein acidic and rich in cysteine; and vitronectin (Table 4) (122). MMP substrate analysis is further complicated by the fact that MMPs may require cofactors for substrate cleavage. MMP-9 cleaves vascular endothelial growth factor (VEGF) in the presence but not the absence of heparin (88). An interesting website that provides information on substrates, including putative substrates, is the Center on Proteolytic Pathways (http://cpp.burnham.org/metadot/index.pl), and some of the MMP-9 putative substrates are listed in Table 5. While this website lists 375 substrates for MMP-9, it is not all inclusive since type I collagen is not listed (85).
Table 4.
A selection of ECM and non-ECM MMP-9 substrates (1, 50, 52, 115, 129, 156)
| Substrate | Effect of Cleavage on Activity |
|---|---|
| α2-Macroglobulin | ↓ |
| Plasminogen → angiostatin | ↑ |
| Endothelin-1 | ↑ |
| Fibroblast growth factor receptor 1 | Shedding |
| Growth-related oncogene-α | ↓ |
| Intercellular adhesion molecule 1 | ↓ |
| Interleukin-1β | ↑ |
| Interleukin-8 | ↑ |
| Pro-MMP-2, -9, -13 | ↑ |
| Osteopontin | ↑ |
| Platelet factor 4 | ↓ |
Table 5.
A selection of putative MMP-9 substrates from the Center on Proteolytic Pathways website (http://cpp.burnham.org/metadot/index.pl) (80, 102)
| Protein | ↓Cleavage Sequence |
|---|---|
| α1 Type-II collagen isoform 2 | GPKG ↓ANGD |
| Complement C1q | GPLG ↓ARGI |
| Interleukin-8 | LPRS ↓AKEL |
| Integrin β4 isoform | PRD ↓YST |
| Plasminogen | VAPP ↓PVVL |
| SERPINE2 (Plasminogen activator inhibitor) | LPLF ↓LLAS |
| Secreted protein acidic and rich in cysteine | GANP↓ VQVE |
| Thrombospondin 1 | PFH ↓YNP |
| Tissue factor pathway inhibitor isoform b precursor | PPLK ↓LMHS |
The MMP-14 substrate repertoire also reflects the complexity of MMP substrates. MMP-14 null mice have a more severe phenotype than MMP-2 null mice, indicating that MMP-2 is not the only relevant substrate of MMP-14 (45). MMP-14 is also a potent collagenase, and MMP-14 null mice have increased collagen deposition (45). Furthermore, MMP-2 null and collagen-resistant double-mutant mice recapitulate the MMP-14 null phenotype, indicating that both MMP-2 and collagen are critical substrates of MMP-14 (44).
Common names.
MMPs, such as collagenase or metalloelastase, were originally named based on the major substrate cleaved, but this does not mean that they only process collagen or elastin. In the early years, new MMPs were named primarily for the substrate cleaved. MMP-1 was named collagenase and MMP-2 was named gelatinase. MMP-1, however, also cleaves tenascin and aggrecan (22). MMP-7 was first called putative metalloproteinase-1 or punctuated metalloproteinase because of its truncated size, and this has led to the misstatement that MMP-7 is a putative MMP. MMP-12 was called metalloelastase but also cleaves fibronectin and tenascin (41). MMP-14 is well known as a membrane-bound MMP (MT1-MMP), but it is a highly relevant and often ignored collagenase (147).
Cell specificity.
It is a myth that MMPs have cell specificity. MMPs were often named based on the cell type from which they were first identified. MMP-8 (neutrophil collagenase) was identified as a collagen-digesting protease present in neutrophils (65, 153). This led to the incorrect assumption by some that MMP-8 was a neutrophil marker. However, further research has shown that MMP-8 is expressed in other cells, including macrophages and endothelial cells (130). Similarly, MMP-9 was first coined neutrophil gelatinase and MMP-12 was known as macrophage metalloelastase, whereas both MMPs are present in several additional cell types (61, 128). Table 6 shows the variety of cell types that express particular MMPs.
Table 6.
MMP and TIMP cell expression (known cardiovascular cell expression)
| MMP | Additional Names | Cell Expression |
|---|---|---|
| -1 | Collagenase-1; fibroblast collagenase | Endothelial, fibroblasts, macrophages |
| -2 | Gelatinase A; 72-kDa type IV collagenase | Endothelial, fibroblasts, platelets, T lymphocytes |
| -3 | Stromelysin-1 | Endothelial, fibroblasts, macrophages, vascular smooth muscle |
| -7 | Matrilysin | Macrophages |
| -8 | Collagenase-2; neutrophil collagenase | Neutrophils, endothelial, fibroblasts |
| -9 | Gelatinase B; 92-kDa type IV collagenase | Neutrophils, endothelial, eosinophils, macrophages, T lymphocytes |
| -10 | Stromelysin-2 | Fibroblasts, T lymphocytes |
| -11 | Stromelysin-3 | Fibroblasts |
| -12 | Macrophage elastase | Macrophages, stromal cells |
| -13 | Collagenase-3 | Fibroblasts |
| -14 | MT1-MMP | Fibroblasts, macrophages |
| -15 | MT2-MMP | Fibroblasts, macrophages |
| -16 | MT3-MMP | Fibroblasts, macrophages, vascular smooth muscle |
| -17 | MT4-MMP | Eosinophils, lymphocytes, monocytes |
| -18 | Xenopus laevis collagenase-4 | Xenopus expression only |
| -19 | RASI-1 | Vascular smooth muscle, endothelial, monocytes |
| -20 | Enamelysin | Endothelial |
| -23 | CA-MMP | Unknown |
| -24 | MT5-MMP | Unknown |
| -25 | MT6-MMP | Neutrophils, monocytes |
| -26 | Matrilysin-2 | B lymphocytes |
| -27 | CMMP/MMP-22 | Fibroblasts |
| -28 | Epilysin | Cardiomyocytes, macropahges, T lymphocytes |
| TIMP-1 | Collagenase inhibitor | Leukocytes, fibroblasts, mesenchymal stem cells, vascular smooth muscle |
| TIMP-2 | Fibroblasts, macrophages, vascular smooth muscle | |
| TIMP-3 | Fibroblasts, pericytes | |
| TIMP-4 | Cardiomyocytes, lymphocytes, macrophages, mast cells, vascular smooth muscle |
Activation.
The idea that MMPs are only activated extracellularly is a misunderstanding. The extracellular activation of MMPs, converting proenzymes to active forms, does occur and was first reported in 1972 (64). Tchougounova et al. (149) showed that chymase deficient mice do not have activated MMP-9, suggesting that chymase is a major in vivo activator. Plasmin, heparin, and oxidants can activate MMPs in the extracellular environment (22). Additionally, many MMPs (MMP-3, in particular) are activators of other MMPs (22). MMP-14 processes MMP-2 as well as MMP-13 to give it both direct and indirect collagenolytic activity (40). Having said this, we state that it is important to note that there are several exceptions to the extracellular activation rule. MMP-11, MT-MMPs, and MMP-28 contain furin cleavage sequences and can be activated intracellularly (45).
Intracellular functions.
The idea that MMPs only work extracellularly is a myth. MMPs can also degrade proteins in the cytoplasm, mitochondria, and nucleus. Schultz and colleagues (5, 72) have shown that MMP-2 has intracellular substrates in cardiac myocytes, including troponin. MMP-2 can proteolyze citrate synthase, a glycolytic enzyme, in the cytoplasm (20). Similarly, MMP-9 and MMP-11 can break down the cytoskeletal proteins actinin and actin (20). While these may not be typical substrates during normal homeostatic regulation, these substrates likely play important roles in the myocardial response to ischemia and reperfusion.
TIMP functions and specificity.
The concept that TIMPs only function to inhibit MMPs is a misconception. In addition to being MMP inhibitors, TIMPs have growth factor functions (67). Mann and colleagues (98) have shown that TIMPs stimulate fibroblast proliferation as well as the phenotypic differentiation into myofibroblasts. Vanhoutte and Heymans (159) have written a nice review on the MMP-independent effects of TIMPs.
The idea that TIMP-4 is the cardiac-specific TIMP is a misunderstanding. When TIMP-4 was first cloned, it was reported as being abundantly present in the heart and present at only very low levels in other tissues (59). Based on this report, TIMP-4 was coined the cardiac-specific TIMP and was even reported as the cardiac-specific inhibitor of MMPs. Subsequently, Leco et al. (87, 136) showed that TIMP-4 is robustly expressed in brain, testis, and skeletal muscle.
The idea that certain TIMPs inhibit specific MMPs is a myth. While there is some selectivity, there is a great deal of confusion about TIMP affinities for MMPs. Whereas TIMP-1 can inhibit all MMPs except MMP-14 efficiently, TIMP-1 has greater affinity for MMP-9 over MMP-2, and TIMP-2 has greater affinity for MMP-2 than MMP-9 (111). TIMP-3 can inhibit MMPs and non-MMP proteases, including A disintegrin and metalloproteinase domain-containing protein 17 and A disintegrin and metallopeptidase with thrombospondin type 1 motif-4 and -5 (14). There is some cell specificity for TIMPs. For example, TIMP-1 is the only TIMP expressed by neutrophils (118). While MMP-8, MMP-9, and TIMP-1 are all expressed by neutrophils, each is predominantly localized to a distinct granule or vesicle within the neutrophil (118). The majority of MMP-8 localizes to specific granules, MMP-9 to gelatinase granules, and TIMP-1 to secretory vesicles (73). Neutrophils can be sequentially activated, such that the specific granules are released, followed by the gelatinase granules, and finally the secretory vesicles to provide exquisite regulation (48). The Quigley laboratory (4) has shown that, because of this differential release, it is possible for neutrophils to release TIMP-free MMP-9, but this does not mean that neutrophils do not express TIMP-1.
Therapeutic potential.
The idea that all MMPs serve negative functions is a myth. The myth in the field of MMPs is that all MMPs have adverse effects and hence need to be blocked equally in all cases at all times. TIMPs and many synthetic small molecule inhibitors have been designed to target MMPs in cancer, arthritis, and cardiovascular disease (33, 43). MMP inhibitor trials have often failed for several reasons, including 1) drugs were tested in patients with advanced disease, whereas animal model studies had shown effectiveness of MMP inhibitors in early stage disease; 2) doses were not adequate; 3) combination therapies should have been applied, as MMP inhibitors are not cytotoxic; and 4) broad spectrum inhibition profiles resulted in off-target activities, inhibition of anti-target MMPs, and subsequent musculoskeletal syndrome (51, 113, 160). Peterson (116) has reviewed the need to identify more specific and selective MMP inhibitors. Along these lines, several selective inhibitors have been reported for MMP-13 (9, 15, 24, 46, 56, 58, 66, 70, 71, 84, 89, 117, 120, 125) and MMP-14 (40, 145, 171), and the Fields laboratory has developed transition state analogy, triple-helical peptide inhibitors that are selective for MMP-2/MMP-9 and collagenolytic MMPs (82, 83, 85).
There is still interest in the potential of MMP inhibitors to treat cardiovascular disease (6), but we need to understand the biology before this will be successful. Van Lint et al. (158) showed in a lethal hepatitis model that survival curves differ for MMP-2, -3, or -9 null mice, indicating different time line of responses for the different MMPs. We now know that some MMPs actually have protective roles (96). For example, MMP-1, -2, -7, -9, -14, and -17 are MMP targets (should be blocked) in cancer, but MMP-3, -8, -9, -12, -14, and -19 are antitargets (should not be blocked). Notice that both MMP-9 and MMP-14 are targets and antitargets for cancer. Angiogenesis inhibitors angiostatin, endostatin, and tumstatin can be produced by the action of MMP-9 on plasminogen, type XVIII collagen, and type IV collagen, respectively (39, 100, 113). MMP-9 inhibition may be effective in early-stage disease (when it facilitates tumor development and releases VEGF) but antagonistic in advanced disease (36, 39, 51, 76, 113). MMP-14 has been assigned as the collagenase critical for tumor cell migration and invasion (76, 127). However, inhibition of MMP-9 or MMP-14 cleavage and inactivation of CXCL12 may promote metastasis (113). TIMP-1 is a marker of fibrosis, but TIMP-1 and TIMP-3 double null mice show increased fibrosis (75, 77). In response to LPS, MMP-7 and -8 null mice are almost completely protected; MMP-2, -3, -12, and -13 null mice show some protection; and MMP-9 and -19 null mice show no difference in response (157, 158). MMP-7, -8 and -9 all process IL-1β, an initiator of the LPS response, but MMP-7 and -8 degrade, whereas MMP-9 activates IL-1β.
These myths and misunderstandings illustrate the overlapping roles of MMPs in cardiac remodeling. Because MMP functions have temporal, spatial, and cell-specific contexts, a more detailed understanding of the functional consequences of MMP actions is needed before we can fully and effectively appreciate the complexities of MMP biology.
Current Perspectives and Future Directions
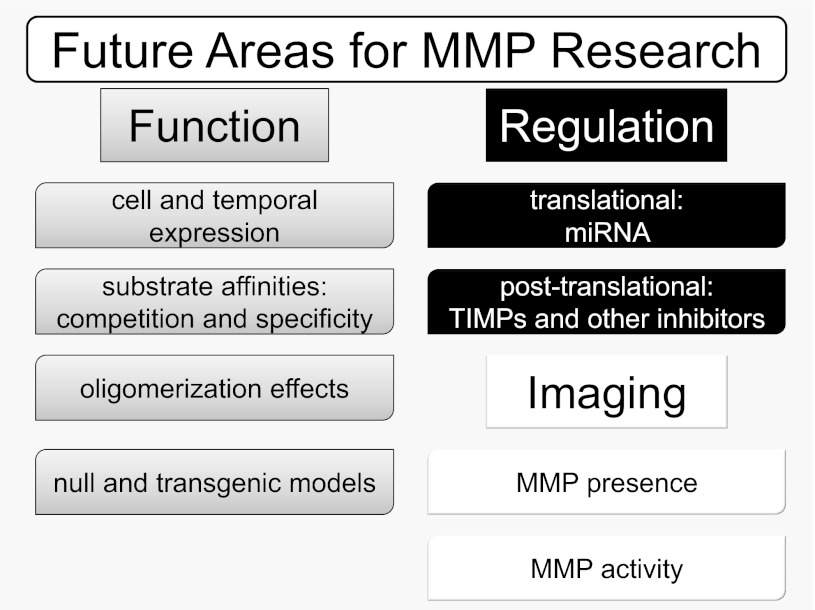
Going forward, the three major areas that need to be focused on are MMP functions during cardiovascular disease, MMP regulation, and MMP imaging in vivo (Fig. 1).
Fig. 1.
Key future areas of research for the cardiovascular matrix metalloproteinase (MMP) field. MMP function and regulation remain to be investigated from multiple perspectives, including needs for better understanding of time and expression pattern, substrate specificity patterns, and the role of MMPs, tissue inhibitors of MMPs (TIMPs), or substrates as markers of disease progression or treatment efficacy. MMP imaging techniques provide a promising avenue for future research.
MMP functions during cardiovascular disease.
More information is needed on when and where MMPs and TIMPs are expressed, which is not as simple as it sounds. MMPs are often measured at one time point, but changes over time and space need to be considered. Expression patterns are very relevant for cardiovascular diseases that are a continuum of responses, such as during the formation of atherosclerosis, after myocardial infarction (MI), or during the progression to heart failure. Not only do cell types come and go, but cells differentiate over time, and cell differentiation stages can affect MMP and TIMP expression. For example, fibroblasts stimulated with platelet-derived growth factor express MMP-1, -2, -3, -11, -14 and TIMP-1 and -2, but not MMP-9 (86). If the fibroblast is differentiated into a myofibroblast, however, MMP-9 is produced in response to platelet-derived growth factor stimulation (86). MMP-9 is expressed in macrophages, but not in circulating monocytes. The literature is very unclear on this, as many studies using isolated monocytes adhere the cells to plastic, which means that these cells are macrophages and not monocytes when examined. Little is known about how MMP and TIMP levels fluctuate with time and location.
To fully understand MMP function in physiology and pathology, the identification of the most important substrates is needed. In a complex environment of multiple MMPs and multiple substrates, we need to know which of the possible permutations provide the driving influences. For a particular MMP, we need to know which substrates it prefers; for a particular substrate, we need to know the affinity profile for all of the different MMPs that process that substrate. While we need to have more information on the substrate catalogues for the individual MMPs, we also need to know what the most critical substrates are. For MMP-9, collagen, galectin-3, and VEGF have been mentioned as critical substrates, but in the myocardium fibronectin is another very relevant substrate that is often overlooked (170). There is a need for competitive in vitro assays that better reflect the complex combinations of many MMPs and many substrates seen in biological systems. This will help to identify the substrates that propel remodeling, which can be useful predictors of outcome.
We spend a great deal of time worrying about how other MMPs, other proteases, or TIMP levels increase or decrease to compensate when a particular MMP or TIMP is deleted or overexpressed, but the presence of a net effect indicates that compensation does not really matter for that phenotype. The only concern is whether an MMP function would be masked by the appearance of the MMPs that would not be typically seen in a normal response, which could have implications when pharmacological inhibitors are applied. To confirm that a substrate is responsible for a phenotype, we need experiments where exogenously cleaved substrate is added back in to rescue the phenotype. If MMP null and substrate null show the same phenotype, this suggests the substrate is downstream of that MMP. Additionally, substrate cleavage in vitro does not matter as much as cleavage in vivo. The generation of more complex null models is required to understand MMP functions more specifically. This will help in identifying which MMPs to target, which MMPs to stimulate (antitarget), and how or if one MMP regulates the other.
Attention should be given to the functions of the different MMPs, as well as the potentially different functions for different forms of the same MMP. For example, MMP-9 has monomeric, homodimeric, and heterodimeric forms, yet we know little about the differences between these forms. The roles of MMP-9 dimerization as well as MMP-9 binding to TIMP-1 or neutrophil gelatinase-associated lipocalin have not been examined.
MMPs and TIMPs will not likely be useful biomarkers for diagnosis or prognosis when used as a single indicator. Several MMPs and TIMPs, including MMP-1, MMP-2, MMP-3, MMP-9, TIMP-1, TIMP-2, and TIMP-4, have been proposed as indicators of cardiac injury (3, 21, 90, 119, 135, 148), but these need to be evaluated in combination with other markers. All of the above studies will also help us to identify which MMPs to inhibit or promote. Once this is accomplished, more selective and specific MMP inhibitors can be designed and tested.
MMP regulation.
Several microRNAs (miR) have been shown to regulate MMPs at the translational level (35, 137). For example, miR-21 regulates MMP-2 by upregulating phosphatase and tensin homolog levels in fibroblasts (151). MMP-2 is regulated by miR-29b, MMP-9 is regulated by miR-29b and miR-491–5P, and MMP-13 is regulated by miR-27b (2, 26, 169). MMP-14 is regulated by miRNA-9, and MMP-16 is downregulated by miR-146b (167, 173). While we have some knowledge about which miRs alter which MMPs, a more detailed understanding of miR regulation of MMPs is required before we can translate its use in cardiovascular diagnostics and therapeutics.
The TIMPs and other endogenous MMP inhibitors are most often studied as output measurements, meaning that little is known beyond whether the TIMP increases or decreases. TIMP-1 and TIMP-4 are the most frequently studied TIMPs in the cardiovascular field, and more information is needed on the time and space changes in all of the TIMPs. TIMPs are also known to have roles independent of MMP inhibition, but details on how these functions regulate cardiovascular pathology are needed.
MMP imaging.
MMP imaging has greatly advanced in the last 10 years, and most imaging projects have focused on macrophage MMPs. The fluorescence resonance energy transfer reporter LaRee1, based on the MMP-12 preferential cleavage site sequence Pro-Leu-Gly-Leu-Glu-Glu-Ala, has been used to measure in vivo MMP-12 in macrophages in a mouse pulmonary inflammation model (30). The MMPSense probe (with the sequence Gly-Gly-Pro-Arg-Gln-Ile-Thr-Ala-Gly) showed MMP-2 and -9 upregulation in atherosclerotic plaques by visualizing probe accumulation via fluorescent molecular tomography (38). To distinguish between resting versus activated macrophages, Suzuki et al. (146) constructed a probe containing an MMP-9-cleavable linker with the sequence Val-Pro-Leu-Ser-Leu-Tyr-Ser-Gly. The probe binds to the scavenger receptor-AI and becomes internalized by activated macrophages upon MMP-9 cleavage and release of the trigger factor (146). rLuc technology may be a useful reporter in animal models, as the reporter does not suffer from auto fluorescent artifacts and is widely used for in vivo imaging.
Radiotracers have also been developed to monitor MMP activation in a murine model of postinfarction remodeling (144). Initial studies were performed with an 111In-labeled MMP-targeted radiotracer (111In-RP782) and a negative control enantiomeric compound (111In-RP788). The experiments were performed in control mice and in mice 1-wk postsurgically-induced MI. Subsequent in vivo imaging studies using micro-single photon emission computed tomography/computer tomography imaging studies with an analogous 99mTc-labeled MMP-targeted radiotracer (99mTc-RP805) and 201Tl demonstrated good biodistribution and clearance kinetics. Myocardial uptake in the MI region was found to be fivefold increased, and a significant twofold increase in myocardial activity in remote regions was also detected. This finding suggested activation of MMPs in regions remote from the MI. This approach holds potential clinical usefulness as a diagnostic tool for in vivo localization of MMP activation and tracking of MMP-mediated post-MI remodeling. A radiotracer to monitor the development and calcification of aortic plaques has been designed, as the MMP activity in atherosclerotic lesions is associated with plaque instability (108). The amount of uptake was also proportional to plaque size, which may make this a good method for future clinical noninvasive assessment of the extent of expression of various MMPs. With these tools in hand, imaging can be used to track the in vivo expression of MMPs, which will be useful both to monitor disease progression and therapeutic efficacy.
In conclusion, MMP research has come a long way in the last five decades. This review article has summarized the milestones, dispelled the common myths, and focused future directions to help translate MMP biology to therapeutic applications for cardiovascular disease.
GRANTS
We acknowledge support from R01-CA-098799 and the Multiple Sclerosis National Research Institute (to G. B. Fields) and from NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center and R01-HL-075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran's Administration (Merit) (to M. L. Lindsey).
DISCLOSURES
M. L. Lindsey has received grant funding from Novartis and has current grant funding from Amylin Pharmaceuticals, Inc., and Canopus Corporation. Both projects are unrelated to this paper.
AUTHOR CONTRIBUTIONS
R.P.I. and N.L.P. prepared figures; R.P.I., N.L.P., G.B.F., and M.L.L. drafted manuscript; R.P.I., N.L.P., G.B.F., and M.L.L. edited and revised manuscript; R.P.I., N.L.P., G.B.F., and M.L.L. approved final version of manuscript.
REFERENCES
- 1. Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276: 28261–28267, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum 62: 1361–1371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apple FS, Smith SW, Pearce LA, Murakami MM. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 55: 93–100, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA 104: 20262–20267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MW. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res 290: 581–591, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bannikov GA, Hinds CA, Rajala-Schultz PJ, Premanandan C, Rings DM, Lakritz J. Serum haptoglobin-matrix metalloproteinase 9 (Hp-MMP 9) complex as a biomarker of systemic inflammation in cattle. Vet Immunol Immunopathol 139: 41–49, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Bannikov GA, Karelina TV, Collier IE, Marmer BL, Goldberg GI. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J Biol Chem 277: 16022–16027, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 126: 2738–2749, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, Gallagher BM, Jr, Gege C, Hochgürtel M, Hofmann M, Jaworski A, Jin L, Kiely A, Korniski B, Kroth H, Nix D, Nolte B, Piecha D, Powers T, Richter F, Schneider M, Steeneck C, Sucholeiki I, Taveras A, Timmermann A, Van Veldhuizen J, Weik J, Wu X, Xia B. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum 60: 2008–2018, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Bauer EA, Eisen AZ, Jeffrey JJ. Regulation of vertebrate collagenase activity in vivo and in vitro. J Invest Dermatol 59: 50–55, 1972 [DOI] [PubMed] [Google Scholar]
- 11. Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 292: H1847–H1860, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Birkedal-Hansen H. From tadpole collagenase to a family of matrix metalloproteinases. J Oral Pathol 17: 445–451, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Bister VO, Salmela MT, Karjalainen-Lindsberg ML, Uria J, Lohi J, Puolakkainen P, Lopez-Otin C, Saarialho-Kere U. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-2 in normal and inflamed intestine and colon cancer. Dig Dis Sci 49: 653–661, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Black RA. Tumor necrosis factor-α converting enzyme. Int J Biochem Cell Biol 34: 1–5, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Blagg JA, Noe MC, Wolf-Gouveia LA, Reiter LA, Laird ER, Chang SP, Danley DE, Downs JT, Elliott NC, Eskra JD, Griffiths RJ, Hardink JR, Hauget AI, Jones CS, Liras JL, Lopresti-Morrow LL, Mitchell PG, Pandit J, Robinson RP, Subramanyam C, Vaughn-Bowser ML, Yocum SA. Potent pyrimidinetrione-based inhibitors of MMP-13 with enhanced selectivity over MMP-14. Bioorg Med Chem Lett 15: 1807–1810, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Blavier L, Lazaryev A, Dorey F, Shackleford GM, DeClerck YA. Matrix metalloproteinases play an active role in Wnt1-induced mammary tumorigenesis. Cancer Res 66: 2691–2699, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabrera S, Gaxiola M, Arreola JL, Ramirez R, Jara P, D'Armiento J, Richards T, Selman M, Pardo A. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int J Biochem Cell Biol 39: 2324–2338, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, Bartlett JD. Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277: 49598–49604, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol 45: 351–423, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 151: 1101.e1101–1101.e1108, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269–285, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 11: S37–S43, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen JM, Nelson FC, Levin JI, Mobilio D, Moy FJ, Nilakantan R, Zask A, Powers R. Structure-based design of a novel, potent, and selective inhibitor for MMP-13 utilizing NMR spectroscopy and computer-aided molecular design. J Am Chem Soc 122: 9648–9654, 2000 [Google Scholar]
- 25. Chen K, Li D, Zhang X, Hermonat PL, Mehta JL. Anoxia-reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J Cardiovasc Pharmacol 44: 682–687, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J 25: 1718–1728, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Chen LC, Noelken ME, Nagase H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1). Biochemistry 32: 10289–10295, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Chiao YA, Zamilpa R, Lopez EF, Dai Q, Escobar GP, Hakala KW, Weintraub ST, Lindsey ML. In vivo matrix metalloproteinase-7 substrates identified in the left ventricle post-myocardial infarction using proteomics. J Proteome Res 9: 2649–2657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem 260: 12367–12376, 1985 [PubMed] [Google Scholar]
- 30. Cobos-Correa A, Trojanek JB, Diemer S, Mall MA, Schultz C. Membrane-bound FRET probe visualizes MMP12 activity in pulmonary inflammation. Nat Chem Biol 5: 628–630, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He CS, Bauer EA, Goldberg GI. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 263: 6579–6587, 1988 [PubMed] [Google Scholar]
- 32. Conant K, St Hillaire C, Nagase H, Visse R, Gary D, Haughey N, Anderson C, Turchan J, Nath A. MMP-1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem 279: 8056–8062, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295: 2387–2392, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JFM, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 284: H364–H371, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Dangwal S, Bang C, Thum T. Novel techniques and targets in cardiovascular microRNA research. Cardiovasc Res 93: 545–554, 2012 [DOI] [PubMed] [Google Scholar]
- 36. Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med 15: 1254–1265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deguchi JO, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation 112: 2708–2715, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Deguchi Jo, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 114: 55–62, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: Contrasting, overlapping and compensatory functions. Biochim Biophys Acta 1803: 103–120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, van Gool R, Sexton DJ, Kuang G, Rank D, Hogan S, Pazmany C, Ma YL, Schoonbroodt S, Nixon AE, Ladner RC, Hoet R, Henderikx P, Tenhoor C, Rabbani SA, Valentino ML, Wood CR, Dransfield DT. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res 69: 1517–1526, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, Jahangiri M, Mayr M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics 10: M111 008128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dixon JA, Spinale FG. Myocardial remodeling: cellular and extracellular events and targets. Annu Rev Physiol 73: 47–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dorman G, Cseh S, Hajdu I, Barna L, Konya D, Kupai K, Kovacs L, Ferdinandy P. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs 70: 949–964, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Egeblad M, Shen HC, Behonick DJ, Wilmes L, Eichten A, Korets LV, Kheradmand F, Werb Z, Coussens LM. Type I collagen is a genetic modifier of matrix metalloproteinase 2 in murine skeletal development. Dev Dyn 236: 1683–1693, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 163–176, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU, Wendt KU. Structural basis for the highly selective inhibition of MMP-13. Chem Biol 12: 181–189, 2005 [DOI] [PubMed] [Google Scholar]
- 47. England KA, Price AP, Tram KV, Shapiro SD, Blazar BR, Panoskaltsis-Mortari A. Evidence for early fibrosis and increased airway resistance in bone marrow transplant recipient mice deficient in MMP12. Am J Physiol Lung Cell Mol Physiol 301: L519–L526, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5: 1317–1327, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, Mickle DA, Watson KL, Hojilla CV, Cruz W, Weisel RD, Li RK, Khokha R. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation 110: 2401–2409, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Fernandez-Patron C, Zouki C, Whittal R, Chan JS, Davidge ST, Filep JG. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1[1–32]. FASEB J 15: 2230–2240, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci 11: 479–491, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 21: 5213–5223, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Folgueras AR, Valdes-Sanchez T, Llano E, Menendez L, Baamonde A, Denlinger BL, Belmonte C, Juarez L, Lastra A, Garcia-Suarez O, Astudillo A, Kirstein M, Pendas AM, Farinas I, Lopez-Otin C. Metalloproteinase MT5-MMP is an essential modulator of neuro-immune interactions in thermal pain stimulation. Proc Natl Acad Sci USA 106: 16451–16456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94: 2493–2503, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Galloway WA, Murphy G, Sandy JD, Gavrilovic J, Crawston TE, Reynolds JJ. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J 209: 741–752, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gao DA, Xiong Z, Heim-Riether A, Amodeo L, August EM, Cao X, Ciccarelli L, Collins BK, Harrington K, Haverty K, Hill-Drzewi M, Li X, Liang S, Margarit SM, Moss N, Nagaraja N, Proudfoot J, Roman R, Schlyer S, Keenan LS, Taylor S, Wellenzohn B, Wiedenmayer D, Li J, Farrow NA. SAR studies of non-zinc-chelating MMP-13 inhibitors: Improving selectivity and metabolic stability. Bioorg Med Chem Lett 20: 5039–5043, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Gauthier MC, Racine C, Ferland C, Flamand N, Chakir J, Tremblay GM, Laviolette M. Expression of membrane type-4 matrix metalloproteinase (metalloproteinase-17) by human eosinophils. Int J Biochem Cell Biol 35: 1667–1673, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Gooljarsingh LT, Lakdawala A, Coppo F, Luo L, Fields GB, Tummino PJ, Gontarek R. Characterization of an exosite binding inhibitor of matrix metalloprotease 13. Protein Sci 17: 66–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem 271: 30375–30380, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA 48: 1014–1022, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gum RJ, Hickman D, Fagerland JA, Heindel MA, Gagne GD, Schmidt JM, Michaelides MR, Davidsen SK, Ulrich RG. Analysis of two matrix metalloproteinase inhibitors and their metabolites for induction of phospholipidosis in rat and human hepatocytes(1). Biochem Pharmacol 62: 1661–1673, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Guo C, Piacentini L. Type I collagen-induced MMP-2 activation coincides with up-regulation of membrane type 1-matrix metalloproteinase and TIMP-2 in cardiac fibroblasts. J Biol Chem 278: 46699–46708, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localized to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA 93: 9748–9753, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harper E, Bloch KJ, Gross J. The zymogen of tadpole collagenase. Biochemistry 10: 3035–3041, 1971 [DOI] [PubMed] [Google Scholar]
- 65. Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem 265: 11421–11424, 1990 [PubMed] [Google Scholar]
- 66. Heim-Riether A, Taylor SJ, Liang S, Gao DA, Xiong Z, August EM, Collins BK, Farmer BT, 2nd, Haverty K, Hill-Drzewi M, Junker HD, Margarit SM, Moss N, Neumann T, Proudfoot JR, Smith Keenan L, Sekul R, Zhang Q, Li J, Farrow NA. Improving potency and selectivity of a new class of non-Zn-chelating MMP-13 inhibitors. Bioorg Med Chem Lett 19: 5321–5324, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J Biol Chem 276: 3203–3214, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Ikonomidis JS, Barbour JR, Amani Z, Stroud RE, Herron AR, McClister DM, Jr, Camens SE, Lindsey ML, Mukherjee R, Spinale FG. Effects of deletion of the matrix metalloproteinase 9 gene on development of murine thoracic aortic aneurysms. Circulation 112: I242–I248, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol 288: H149–H158, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, Banotai CA, Mueller WT, McConnell P, Yan C, Baragi V, Lesch C, Roark WH, Wilson M, Datta K, Guzman R, Han HK, Dyer RD. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 That Reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem 282: 27781–27791, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Jüngel A, Ospelt C, Lesch M, Thiel M, Sunyer T, Schorr O, Michel BA, Gay RE, Kolling C, Flory C, Gay S, Neidhart M. Effect of the oral application of a highly selective MMP-13 inhibitor in three different animal models of rheumatoid arthritis. Ann Rheum Dis 69: 898–902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85: 413–423, 2010 [DOI] [PubMed] [Google Scholar]
- 73. Kang T, Yi J, Guo A, Wang X, Overall CM, Jiang W, Elde R, Borregaard N, Pei D. Subcellular distribution and cytokine- and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. J Biol Chem 276: 21960–21968, 2001 [DOI] [PubMed] [Google Scholar]
- 74. Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun 75: 5640–5650, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20: 1223–1235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141: 52–67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim H, Oda T, Lopez-Guisa J, Wing D, Edwards DR, Soloway PD, Eddy AA. TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 12: 736–748, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Koskivirta I, Rahkonen O, Mayranpaa M, Pakkanen S, Husheem M, Sainio A, Hakovirta H, Laine J, Jokinen E, Vuorio E, Kovanen P, Jarvelainen H. Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in inflammatory processes of human cardiovascular pathology. Histochem Cell Biol 126: 335–342, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Krampert M, Bloch W, Sasaki T, Bugnon P, Rulicke T, Wolf E, Aumailley M, Parks WC, Werner S. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell 15: 5242–5254, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kridel SJ, Chen E, Kotra LP, Howard EW, Mobashery S, Smith JW. Substrate hydrolysis by matrix metalloproteinase-9. J Biol Chem 276: 20572–20578, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol 130: 147–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lauer-Fields JL, Brew K, Whitehead JK, Li S, Hammer RP, Fields GB. Triple-helical transition-state analogs: a new class of selective matrix metalloproteinase inhibitors. J Am Chem Soc 129: 10408–10417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lauer-Fields JL, Chalmers MJ, Busby SA, Minond D, Griffin PR, Fields GB. Identification of specific hemopexin-like domain residues that facilitate matrix metalloproteinase collagenolytic activity. J Biol Chem 284: 24017–24024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lauer-Fields JL, Minond D, Chase PS, Baillargeon PE, Saldanha SA, Stawikowska R, Hodder P, Fields GB. High throughput screening of potentially selective MMP-13 exosite inhibitors utilizing a triple-helical FRET substrate. Bioorg Med Chem 17: 990–1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lauer-Fields JL, Whitehead JK, Li S, Hammer RP, Brew K, Fields GB. Selective modulation of matrix metalloproteinase 9 (MMP-9) functions via exosite inhibition. J Biol Chem 283: 20087–20095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leask A. Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106: 1675–1680, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Leco KJ, Apte SS, Taniguchi GT, Hawkes SP, Khokha R, Schultz GA, Edwards DR. Murine tissue inhibitor of metalloproteinase-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett 401: 213–217, 1997 [DOI] [PubMed] [Google Scholar]
- 88. Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169: 681–691, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li JJ, Nahra J, Johnson AR, Bunker A, O'Brien P, Yue WS, Ortwine DF, Man CF, Baragi V, Kilgore K, Dyer RD, Han HK. Quinazolinones and pyrido[3,4-d]pyrimidin-4-ones as orally active and specific matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. J Med Chem 51: 835–841, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Li MJ, Huang CX, Okello E, Yanhong T, Mohamed S. Treatment with spironolactone for 24 weeks decreases the level of matrix metalloproteinases and improves cardiac function in patients with chronic heart failure of ischemic etiology. Can J Cardiol 25: 523–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Libby P, Geng YJ, Sukhova GK, Simon DI, Lee RT. Molecular determinants of atherosclerotic plaque vulnerability. Ann NY Acad Sci 811: 134–142, 1997 [DOI] [PubMed] [Google Scholar]
- 92. Lijnen HR, Van Hoef B, Vanlinthout I, Verstreken M, Rio MC, Collen D. Accelerated neointima formation after vascular injury in mice with stromelysin-3 (MMP-11) gene inactivation. Arterioscler Thromb Vasc Biol 19: 2863–2870, 1999 [DOI] [PubMed] [Google Scholar]
- 93. Linask KK, Han M, Cai DH, Brauer PR, Maisastry SM. Cardiac morphogenesis: matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev Dyn 233: 739–753, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 113: 2919–2928, 2006 [DOI] [PubMed] [Google Scholar]
- 95. Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther 30: 31–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lopez-Otin C, Palavalli LH, Samuels Y. Protective roles of matrix metalloproteinases: from mouse models to human cancer. Cell Cycle 8: 3657–3662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lovejoy B, Cleasby A, Hassell AM, Longley K, Luther MA, Weigl D, McGeehan G, McElroy AB, Drewry D, Lambert MH, Jordan SR. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science 263: 375–377, 1994 [DOI] [PubMed] [Google Scholar]
- 98. Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol 288: H461–H468, 2005 [DOI] [PubMed] [Google Scholar]
- 99. Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc Microanal 18: 81–90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev 26: 717–724, 2007 [DOI] [PubMed] [Google Scholar]
- 101. McCawley LJ, Wright J, LaFleur BJ, Crawford HC, Matrisian LM. Keratinocyte expression of MMP3 enhances differentiation and prevents tumor establishment. Am J Pathol 173: 1528–1539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McGeehan GM, Bickett DM, Green M, Kassel D, Wiseman JS, Berman J. Characterization of the peptide substrate specificities of interstitial collagenase and 92-kDa gelatinase. Implications for substrate optimization. J Biol Chem 269: 32814–32820, 1994 [PubMed] [Google Scholar]
- 103. Menghini R, Casagrande V, Menini S, Marino A, Marzano V, Hribal ML, Gentileschi P, Lauro D, Schillaci O, Pugliese G, Sbraccia P, Urbani A, Lauro R, Federici M. TIMP3 overexpression in macrophages protects from insulin resistance, adipose inflammation, and nonalcoholic fatty liver disease in mice. Diabetes 61: 454–462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Milla ME, Gonzales PE, Leonard JD. The TACE zymogen: re-examining the role of the cysteine switch. Cell Biochem Biophys 44: 342–348, 2006 [DOI] [PubMed] [Google Scholar]
- 105. Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. Biochem J 248: 265–268, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nagai Y, Lapiere CM, Gross J. Tadpole collagenase. Preparation and purification. Biochemistry 5: 3123–3130, 1966 [DOI] [PubMed] [Google Scholar]
- 107. Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789–800, 2001 [DOI] [PubMed] [Google Scholar]
- 108. Ohshima S, Petrov A, Fujimoto S, Zhou J, Azure M, Edwards DS, Murohara T, Narula N, Tsimikas S, Narula J. Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein e or low-density-lipoprotein receptor. J Nucl Med 50: 612–617, 2009 [DOI] [PubMed] [Google Scholar]
- 109. Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, Salvesen G, Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem 194: 721–730, 1990 [DOI] [PubMed] [Google Scholar]
- 110. Okada Y, Nagase H, Harris ED., Jr Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J Rheumatol 14: 41–42, 1987 [PubMed] [Google Scholar]
- 111. Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem 272: 29975–29983, 1997 [DOI] [PubMed] [Google Scholar]
- 112. Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol 200: 2–10, 2004 [DOI] [PubMed] [Google Scholar]
- 113. Overall CM, Kleifeld O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6: 227–239, 2006 [DOI] [PubMed] [Google Scholar]
- 114. Pap T, Shigeyama Y, Kuchen S, Fernihough JK, Simmen B, Gay RE, Billingham M, Gay S. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum 43: 1226–1232, 2000 [DOI] [PubMed] [Google Scholar]
- 115. Patterson BC, Sang QX. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J Biol Chem 272: 28823–28825, 1997 [DOI] [PubMed] [Google Scholar]
- 116. Peterson JT. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc Res 69: 677–687, 2006 [DOI] [PubMed] [Google Scholar]
- 117. Piecha D, Weik J, Kheil H, Becher G, Timmermann A, Jaworski A, Burger M, Hofmann MW. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflamm Res 59: 379–389, 2010 [DOI] [PubMed] [Google Scholar]
- 118. Price B, Dennison C, Tschesche H, Elliott E. Neutrophil tissue inhibitor of matrix metalloprotetinases-1 occurs in novel vesicles that do not fuse with the phagosome. J Biol Chem 275: 28308–28315, 2000 [DOI] [PubMed] [Google Scholar]
- 119. Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, Van Veldhuisen DJ, Tavazzi L, Mann DL, Capiaumont-Vin J, Li M, Hanriot D, Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail 14: 467–474, 2008 [DOI] [PubMed] [Google Scholar]
- 120. Reiter LA, Freeman-Cook KD, Jones CS, Martinelli GJ, Antipas AS, Berliner MA, Datta K, Downs JT, Eskra JD, Forman MD, Greer EM, Guzman R, Hardink JR, Janat F, Keene NF, Laird ER, Liras JL, Lopresti-Morrow LL, Mitchell PG, Pandit J, Robertson D, Sperger D, Vaughn-Bower ML, Waller DM, Yocum SA. Potent, selective pyrimidinetrione-based inhibitors of MMP-13. Bioorg Med Chem Lett 16: 5822–5826, 2006 [DOI] [PubMed] [Google Scholar]
- 121. Rikimaru A, Komori K, Sakamoto T, Ichise H, Yoshida N, Yana I, Seiki M. Establishment of an MT4-MMP-deficient mouse strain representing an efficient tracking system for MT4-MMP/MMP-17 expression in vivo using beta-galactosidase. Genes Cells 12: 1091–1100, 2007 [DOI] [PubMed] [Google Scholar]
- 122. Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta 1803: 39–54, 2010 [DOI] [PubMed] [Google Scholar]
- 123. Rosenblum G, Meroueh S, Toth M, Fisher JF, Fridman R, Mobashery S, Sagi I. Molecular structures and dynamics of the stepwise activation mechanism of a matrix metalloproteinase zymogen: challenging the cysteine switch dogma. J Am Chem Soc 129: 13566–13574, 2007 [DOI] [PubMed] [Google Scholar]
- 124. Roten L, Nemoto S, Simsic J, Coker ML, Rao V, Baicu S, Defreyte G, Soloway PJ, Zile MR, Spinale FG. Effects of gene deletion of the tissue inhibitor of the matrix metalloproteinase-type 1 (TIMP-1) on left ventricular geometry and function in mice. J Mol Cell Cardiol 32: 109–120, 2000 [DOI] [PubMed] [Google Scholar]
- 125. Roth J, Minond D, Darout E, Liu Q, Lauer J, Hodder P, Fields GB, Roush WR. Identification of novel, exosite-binding matrix metalloproteinase-13 inhibitor scaffolds. Bioorg Med Chem Lett 21: 7180–7184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rudolph-Owen LA, Chan R, Muller WJ, Matrisian LM. The matrix metalloproteinase matrilysin influences early-stage mammary tumorigenesis. Cancer Res 58: 5500–5506, 1998 [PubMed] [Google Scholar]
- 127. Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol 167: 769–781, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Salmela MT, Karjalainen-Lindsberg ML, Puolakkainen P, Saarialho-Kere U. Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett's oesophageal adenocarcinoma. Br J Cancer 85: 383–392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 161: 3340–3346, 1998 [PubMed] [Google Scholar]
- 130. Schonherr E, Schaefer L, O'Connell BC, Kresse H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J Cell Physiol 187: 37–47, 2001 [DOI] [PubMed] [Google Scholar]
- 131. Sedlacek R, Mauch S, Kolb B, Schatzlein C, Eibel H, Peter H, Schmitt J, Krawinkel U. Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology 198: 408–423, 1998 [DOI] [PubMed] [Google Scholar]
- 132. Sellers A, Cartwright E, Murphy G, Reynolds JJ. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J 163: 303–307, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shi J, Son MY, Yamada S, Szabova L, Kahan S, Chrysovergis K, Wolf L, Surmak A, Holmbeck K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol 313: 196–209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shofuda Ki Yasumitsu H, Nishihashi A, Miki K, Miyazaki K. Expression of three membrane-type matrix metalloproteinases (MT-MMPs) in rat vascular smooth muscle cells and characterization of MT3-MMPs with and without transmembrane domain. J Biol Chem 272: 9749–9754, 1997 [DOI] [PubMed] [Google Scholar]
- 135. Shu J, Ren N, Du JB, Zhang M, Cong HL, Huang TG. Increased levels of interleukin-6 and matrix metalloproteinase-9 are of cardiac origin in acute coronary syndrome. Scand Cardiovasc J 41: 149–154, 2007 [DOI] [PubMed] [Google Scholar]
- 136. Simpson KS, Komar CM, Curry TE., Jr Localization and expression of tissue inhibitor of metalloproteinase-4 in the immature gonadotropin-stimulated and adult rat ovary. Biol Reprod 68: 214–221, 2003 [DOI] [PubMed] [Google Scholar]
- 137. Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121: 1022–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Spinale FG. Diversity of myocardial interstitial proteolytic pathways: gene deletion reveals unexpected consequences. Circulation 124: 2052–2055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 140. Spinale FG, Mukherjee R, Zavadzkas JA, Koval CN, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem 285: 30316–30327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: Evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA 87: 364–368, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sternlicht M, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta 1803: 133–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, Dione DP, Cavaliere P, Chow C, Bourke BN, Hu XY, Azure M, Yalamanchili P, Liu R, Cheesman EH, Robinson S, Edwards DS, Sinusas AJ. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation 112: 3157–3167, 2005 [DOI] [PubMed] [Google Scholar]
- 145. Suojanen J, Salo T, Koivunen E, Sorsa T, Pirilä E. A novel and selective membrane type-1 matrix metalloproteinase (MT1-MMP) inhibitor reduces cancer cell motility and tumor growth. Cancer Biol Ther 8: 2362–2370, 2009 [DOI] [PubMed] [Google Scholar]
- 146. Suzuki H, Sato M, Umezawa Y. Accurate targeting of activated macrophages based on synergistic activation of functional molecules uptake by scavenger receptor and matrix metalloproteinase. ACS Chem Biol 3: 471–479, 2008 [DOI] [PubMed] [Google Scholar]
- 147. Tam EM, Moore TR, Butler GS, Overall CM. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin C domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J Biol Chem 279: 43336–43344, 2004 [DOI] [PubMed] [Google Scholar]
- 148. Tan J, Hua Q, Gao J, Fan ZX. Clinical implications of elevated serum interleukin-6, soluble CD40 ligand, metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in patients with acute ST-segment elevation myocardial infarction. Clin Cardiol 31: 413–418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem 280: 9291–9296, 2005 [DOI] [PubMed] [Google Scholar]
- 150. Thieringer FR, Maass T, Anthon B, Meyer E, Schirmacher P, Longerich T, Galle PR, Kanzler S, Teufel A. Liver-specific overexpression of matrix metalloproteinase 9 (MMP-9) in transgenic mice accelerates development of hepatocellular carcinoma. Mol Carcinog 51: 439–448, 2012 [DOI] [PubMed] [Google Scholar]
- 151. Thum T, Fraccarollo D, Galuppo P, Tsikas D, Frantz S, Ertl G, Bauersachs J. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res 70: 50–60, 2006 [DOI] [PubMed] [Google Scholar]
- 152. Turner NA, Warburton P, O'Regan DJ, Ball SG, Porter KE. Modulatory effect of interleukin-1alpha on expression of structural matrix proteins, MMPs and TIMPs in human cardiac myofibroblasts: role of p38 MAP kinase. Matrix Biol 29: 613–620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tyagi SC. Proteinases and myocardial extracellular matrix turnover. Mol Cell Biochem 168: 1–12, 1997 [DOI] [PubMed] [Google Scholar]
- 154. Tyagi SC, Bheemanathini VS, Mandi D, Reddy HK, Voelker DJ. Role of extracellular matrix metalloproteinases in cardiac remodelling. Heart Fail Rev 1: 73–80, 1996 [Google Scholar]
- 155. Uzui H, Harpf A, Liu M, Doherty TM, Shukla A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, Shah PK, Rajavashisth TB. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation 106: 3024–3030, 2002 [DOI] [PubMed] [Google Scholar]
- 156. Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO- alpha and leaves RANTES and MCP-2 intact. Blood 96: 2673–2681, 2000 [PubMed] [Google Scholar]
- 157. Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 82: 1375–1381, 2007 [DOI] [PubMed] [Google Scholar]
- 158. Van Lint P, Wielockx B, Puimege L, Noel A, Lopez-Otin C, Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol 175: 7642–7649, 2005 [DOI] [PubMed] [Google Scholar]
- 159. Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘embracing the MMP-independent-side of the family’. J Mol Cell Cardiol 48: 445–453, 2010 [DOI] [PubMed] [Google Scholar]
- 160. Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets 5: 203–220, 2005 [DOI] [PubMed] [Google Scholar]
- 161. Wang X, Liang J, Koike T, Sun H, Ichikawa T, Kitajima S, Morimoto M, Shikama H, Watanabe T, Sasaguri Y, Fan J. Overexpression of human matrix metalloproteinase-12 enhances the development of inflammatory arthritis in transgenic rabbits. Am J Pathol 165: 1375–1383, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Welgus HG, Stricklin GP, Eisen AZ, Bauer EA, Cooney RV, Jeffrey JJ. A specific inhibitor of vertebrate collagenase produced by human skin fibroblasts. J Biol Chem 254: 1938–1943, 1979 [PubMed] [Google Scholar]
- 163. Wiesen J, Werb Z. Proteinases, cell cycle regulation, and apoptosis during mammary gland involution (minireview). Mol Reprod Dev 56: 534–540, 2000 [DOI] [PubMed] [Google Scholar]
- 164. Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 94: 1402–1407, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Woessner JF., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J 83: 304–314, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. Oxford: Oxford University Press, 2000 [Google Scholar]
- 167. Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res 1269: 158–165, 2009 [DOI] [PubMed] [Google Scholar]
- 168. Xiong W, Knispel R, MacTaggart J, Greiner TC, Weiss SJ, Baxter BT. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem 284: 1765–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yan W, Zhang W, Sun L, Liu Y, You G, Wang Y, Kang C, You Y, Jiang T. Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res 1411: 108–115, 2011 [DOI] [PubMed] [Google Scholar]
- 170. Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics 10: 2214–2223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, Hu Y, Sampson NS, Zucker S, Cao J. Inhibition of matrix metalloproteinase-14 (MMP-14)-mediated cancer cell migration. J Biol Chem 286: 33167–33177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zavadzkas JA, Mukherjee R, Rivers WT, Patel RK, Meyer EC, Black LE, McKinney RA, Oelsen JM, Stroud RE, Spinale FG. Direct regulation of membrane type-1 matrix metalloproteinase following myocardial infarction causes changes in survival, cardiac function and remodeling. Am J Physiol Heart Circ Physiol 301: H1656–H1666, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhang H, Qi M, Li S, Qi T, Mei H, Huang K, Zheng L, Tong Q. microRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Mol Cancer Ther 11: 1454–1466, 2012 [DOI] [PubMed] [Google Scholar]
- 174. Zhang J, Nie L, Razavian M, Ahmed M, Dobrucki LW, Asadi A, Edwards DS, Azure M, Sinusas AJ, Sadeghi MM. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation 118: 1953–1960, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]