Abstract
Abdominal aortic aneurysms (AAAs) are a major cause of morbidity and mortality in the United States today. We employed a model for AAA development using apolipoprotein E knock out mice fed a high-fat diet and treated with ANG II and β-aminopropionitrile (β-APN) for 4 wk. ANG II induces hypertension and atherosclerotic disease, whereas β-APN inhibits the activity of the lysyl oxidase/ lysyl oxidase-like protein (LOX/LOXL) family members. LOX/LOXL family members crosslink collagen and elastin in the extracellular matrix and therefore contribute to the integrity and stabilization of a healthy vessel wall. In this model, cotreatment with ANG II and β-APN caused a 90% AAA incidence and increased atherosclerotic lesion formation from less than 5% to greater than 25% after 4 wk. In more atheroprotected mouse strains (C57BL/6 and BalbC), cotreatment with ANG II and β-APN caused 50% and 40% AAA incidence, respectively. These data demonstrate the importance of LOX/LOXL to the stability of the vessel wall. Therapeutic strategies to overexpress LOX/LOXL enzymes or to support the crosslinking of soluble matrix proteins in a polymeric scaffold are a promising opportunity to achieve stabilization of AAAs.
Keywords: catalytic crosslinking, extracellular matrix, vascular remodeling
abdominal aortic aneurysms (AAAs) are a major cause of morbidity and mortality in the United States today. In the US, the incidence of AAA is estimated to be 4–8% in elderly men (15). Rupture occurs in 1–3% of men aged 65 and older, and the mortality rate is ∼95% (16). Furthermore, the prevalence of AAA will likely increase as the number of people in the susceptible age group continues to rise. Risk factors for AAA are not completely understood, although elderly white men with a history of smoking and/or hypertension are at the highest risk (8, 14). The current definitive therapy for AAA is surgery or percutaneous stent placement performed when the risk of rupture outweighs the risk of intervention.
Several animal models of AAA formation have been developed to better understand its pathogenesis including calcium chloride injury of the aorta, elastase infusion, and systemic ANG II infusion (4, 7, 17). Previous studies have demonstrated the importance of proteolytic degeneration of matrix components by matrix metalloproteinases (MMPs) in the pathogenesis of AAA (5, 19, 24). MMPs are a family of endopeptidases that degrade elastin and collagen fibers. MMP knockout mice 2, 3, 9, and 12 are resistant to aneurysm formation, which points directly to the critical importance of matrix stabilization in AAA pathogenesis (5, 18, 19, 24, 29).
The lysyl oxidases (LOX) are a family of enzymes that stabilize matrix components. LOX family members crosslink collagen and elastin fibers to create insoluble proteins resistant to proteolytic degeneration. This crosslinking activity stabilizes the vessel wall and imparts mechanical strength. The LOX enzymes are a family of copper-containing monoamine oxidases found in the extracellular matrix (10). These enzymes catalyze the conversion of lysine residues in collagen and elastin via a deamination reaction to allysine. The allysine residue spontaneously condenses to form highly stable crosslinks that impart additional mechanical strength to collagen and elastin.
Five different LOX enzymes are known to exist in both humans and mice: LOX and the lysyl oxidase-like proteins LOXL1, LOXL2, LOXL3, and LOXL4, collectively termed LOX/LOXL. All five LOX/LOXL family members possess catalytic crosslinking activity (21). The COOH terminus demonstrates 95% homology among the five isoforms, which is necessary and sufficient for enzyme function (3). The five isoforms have unique NH2-terminal sequences that may confer additional roles, mediate protein-protein interactions, and/or define cellular localization (3, 21).
LOX activity has been shown in vivo to be crucial for the development of elastic vessels. Rat pups weaned from their mothers and fed the general LOX/LOXL inhibitor, β-aminopropionitrile (β-APN), form aneurysms (22, 28). In addition, LOX knockout mice die soon after birth (20). The cause of lethality in the LOX knockout mice is impaired vascular development that leads to an aneurysmal dilatation of the aorta and subsequent rupture (9, 20). LOXL1 knockout mice are not lethal but have defects in elastin regeneration (17). To date there are no publications on LOXL2, LOXL3, or LOXL4 knockout mice.
Our study investigates whether the LOX/LOXL family members provide a protective and/or compensatory mechanism against AAA formation. The expression and functional contribution of LOX/LOXL to AAA formation were analyzed in a model of accelerated atherosclerosis and AAA formation consisting of apolipoprotein E knockout (ApoE−/−) mice fed an atherogenic diet and infused with ANG II (4, 32, 33).
MATERIALS AND METHODS
Animals.
ApoE−/−, C57BL/6, and BalbC mice age 6–8 wk (Jackson Laboratory, Bar Harbor, ME) were fed either a control low-fat diet (standard low-fat rodent chow Purina 5001) or a high-fat diet using modified Paigen's atherogenic diet (Research Diets, New Brunswick, NJ) and either treated with or without ANG II (0.75 mg·kg−1·day−1) via an osmotic mini-pump. Some animals were also treated with β-APN at a dose of 100 mg·kg−1·day−1 via subcutaneous osmotic mini-pumps (22). All housing, surgical procedures, and experimental protocols were approved by the Institutional Animal Care and Use Committee of Emory University. Animals had free access to chow and water during all experiments. Noninvasive systolic blood pressure measurements were obtained by using the Visitech Systems BP 2000 tail-cuff system as previously described (32). Aortic atherosclerotic lesion area was measured as previously described by pressure perfusing the vasculature at ∼100 mmHg with normal saline and subsequent fixation with 10% formalin (32). The aortic length was cleaned of periadventitial fat and connective tissue and then removed with the heart and kidneys attached. The thoracic-abdominal aorta was pinned on a wax dish and dissected en face longitudinally. Dissected aortas were digitally imaged and analyzed for lesion area and aortic circumference using ImageJ 1.61 software (National Institutes of Health, Bethesda, MD).
Immunostaining.
Pressure-fixed aortas were embedded in paraffin, and sections were obtained at 5-μm intervals. Immunostaining for the LOX/LOXL family members was carried out by using rabbit polyclonal antibodies (supplied by Drs. Fong and Csiszar from University of Hawaii At Manoa) and goat anti-rabbit biotinylated secondary antibodies. Imaging was accomplished using streptavidin tagged quantum dots (emission 605 nM) as previously reported (6).
Quantitative RT-PCR.
RNA was extracted from snap-frozen aortic tissue 7 days after treatment by using the RNeasy kit (Qiagen, Valencia, CA) according to the protocol and quantified by using a spectrophotometer. cDNA was prepared and purified from aortic RNA by using standard protocol with primers unique for each of the five lysyl oxidase isoforms, 18S and plasminogen activator inhibitor-1 (PAI-1). (Table 1).
Table 1.
LOX/LOXL and 18s primer sequences and annealing temperatures
| cDNA Targets | Primer Sequences | Annealing, °C |
|---|---|---|
| LOX | 5′ TAGCGAAGCACATAGCATTG 3′ | 60 |
| 5′ TGCAGCAATGAGTCTACAGC 3′ | ||
| LOXL1 | 5′ CTATGCCTGCACCTCTCACA 3′ | 61 |
| 5′ TGGACGATTTTGCAGTTTGT 3′ | ||
| LOXL2 | 5′ GAGTGGAGGTGCTAACAGAG 3′ | 59 |
| 5′ AGTCTGCTGGACAATCTCAG 3′ | ||
| LOXL3 | 5′ ACTGTACCCATGATGAGGAT 3′ | 57 |
| 5′ GCCATGTTGACTCTCTTTTC 3′ | ||
| LOXL4 | 5′ CTGGCGTTGCTTGTATGAACA 3′ | 64 |
| 5′ AGACAGAAGCTGGCCTTGTGT 3′ | ||
| PAI-1 | 5′ ACAGCCAACAAGAGCCAATC 3′ | 64 |
| 5′ GACACGCCATAGGGAGAGAA 3′ | ||
| 18S | 5′ Gaacgtctgccctatcaact 3′ | 64 |
| 5′ ccaagatccaactacgagct 3′ |
LOX/LOXL, lysyl oxidase/lysyl oxidase-like proteins.
Western blot analysis.
Mouse aortas were harvested and snap frozen in liquid nitrogen. After homogenization in radioimmunopreciptation assay buffer (Santa Cruz Biotechnology, Santa Cruz, CA), protein was extracted, sonicated, and quantified by using the Bradford assay. Samples were then boiled for 5 min at 95°C, and aliquots of the lysate were subjected to 8% SDS-PAGE. Proteins in the gel were transferred to a nitrocellulose membrane by electroblotting. The membranes were treated with LOX/LOXL antibodies procured from Novus (LOX) and Santa Cruz (LOXL1, LOXL2, and GAPDH) or made in-house (LOXL3). Anti-rabbit and anti-goat secondary antibodies conjugated to horseradish peroxidase were procured from Santa Cruz and Bio-Rad. Immunoreactive proteins were detected by an enhanced chemiluminescence system (GE).
LOX/LOXL activity.
Aorta tissue was isolated immediately and homogenized in 4 M urea in 0.02 M borate buffer (pH 8.0) at 4°C and centrifuged at 15,000 g for 30 min at 4°C, as previously reported (2). The supernatants were analyzed immediately for LOX assay with and without β-APN (13). Activity assays for detection of LOX were performed as previously described (23). Briefly, 500 μg of protein was added to the final reaction mixture supplied by Amplite Fluorimetric Lysyl Oxidase Assay kit (AAT Bioquest) according to manufacturer's instructions in the presence or absence of 1,000 μM β-APN. The LOX/LOXL reaction produces H2O2. Horseradish peroxidase catalyzed oxidation of N-acetyl-3,7-dihydroxyphenoxazine by H2O2 produces fluorescent resorfurin, which is measured at an excitation of 530/25 nm and emission of 580/50 nm. The fluorescence was recorded at 30 min at 37°C. A standard graph was constructed using both H2O2 (0.2–5.0 μM) and recombinant lysyl oxidase (R&D) as a measure for detectable activity. The relative fluorescence units obtained in test samples were calculated based on the difference between β-APN-treated and nontreated samples normalized to total protein.
Additional reagents.
β-Aminopropionitrile (β-APN), ANG II, and PCR primers were obtained from Sigma Chemicals, St. Louis, MO. Osmotic mini-pumps were obtained from Alzet, Cupertino, CA.
Statistical analysis.
Systolic blood pressure, cholesterol, and percent atherosclerotic lesion area are presented as means ± SD. ANOVA was performed using Graph Pad Prism Software. Post hoc analyses were performed using the Bonferroni test. P values <0.05 were considered to be significant.
RESULTS
LOXL3 mRNA expression is significantly upregulated in the aortas of ANG II-treated ApoE−/− mice fed a high-fat diet.
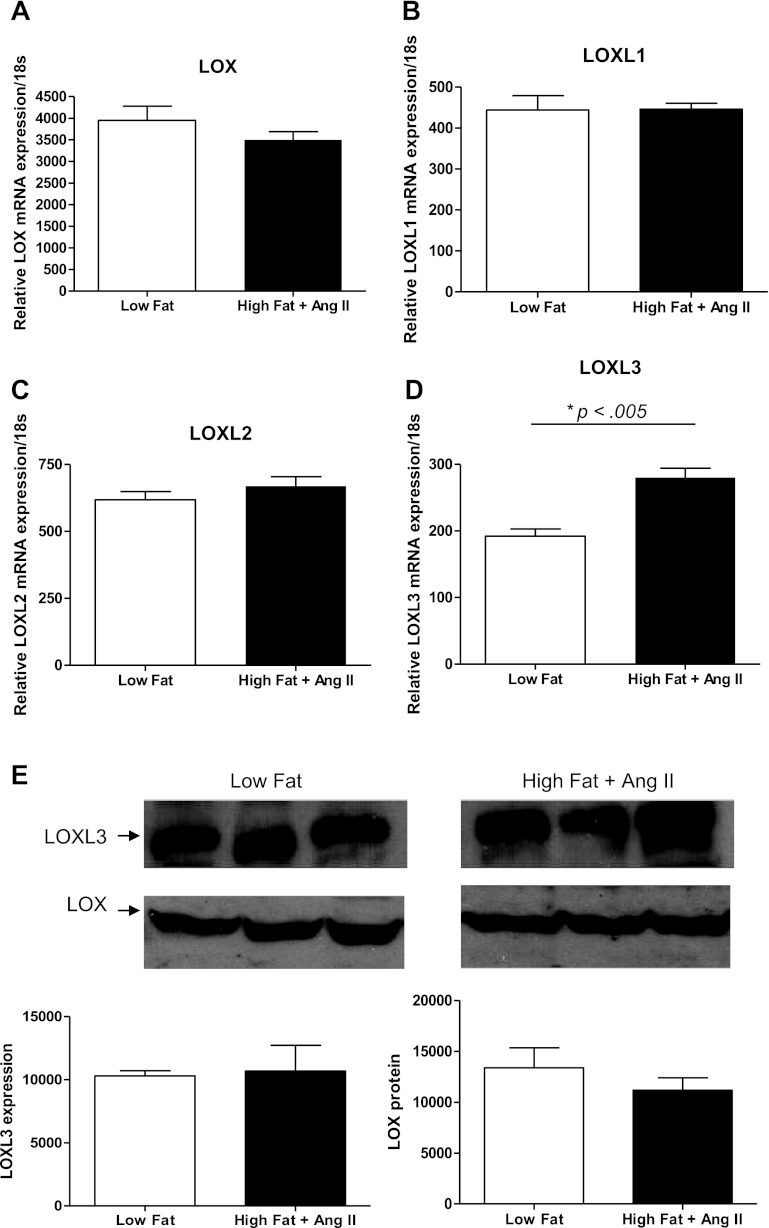
To quantitatively assess if LOX/LOXL family member expression was regulated in our model of AAA formation, LOX/LOXL mRNA levels were measured by quantitative real-time PCR using mRNA extracted from the aortas of control ApoE−/− mice fed a low-fat diet mice and compared with those fed a high-fat diet and treated with ANG II for 7 days. Mice treated with ANG II infusion and fed a high-fat diet exhibited significant increases in blood pressure (Fig. 1). At baseline LOX expression in aortic tissue was highest compared with the other family members, with expression of LOXL1, LOXL2, and LOXL3 much lower and LOXL4 expression undetectable (Fig. 2, A–D). Comparison of mRNA from our AAA mouse model to the low-fat control mice after 7 days resulted in significantly increased expression of LOXL3 mRNA. (Fig. 2D) This increase observed with regard to LOXL3 was specific to a high-fat diet in the presence of ANG II, whereas no change was observed in LOXL3 expression on a low-fat diet ± ANG II. LOXL4 expression remained undetectable after treatment. Interestingly, Western blot analysis on protein harvested from aortic tissue 7 days after treatment with ANG II on a high-fat diet failed to show any significant difference in the detected isoforms (LOX and LOXL3) over control tissue (low-fat diet) (Fig. 2E). In addition, other family members that were previously detected by quantitative RT-PCR and immunohistochemistry (LOXL1 and LOXL2) were not detected at 7 days in aortic tissue from either low-fat or high-fat + ANG II-treated mice by Western blot, suggesting temporal or spatial regulation at the protein level.
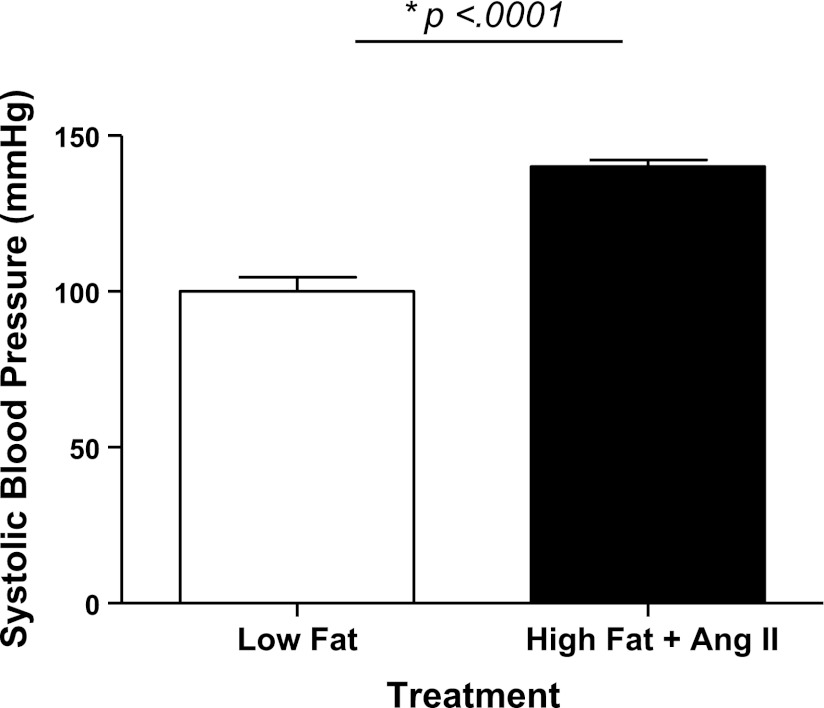
Fig. 1.
ANG II infusion increases blood pressure. Mean systolic blood pressures were taken in apolipoprotein E knockout (ApoE−/−) mice before treatment. Animals were then fed a high-fat diet and treated with ANG II for 2 wk, resulting in a significant increase in blood pressure. *P = 0.0001.
Fig. 2.
Lysyl oxidase/ lysyl oxidase-like protein (LOX/LOXL) family member expression 7 days after ANG II infusion in the presence of a high-fat diet. Quantitative RT-PCR was performed on cDNA from aortic tissue of ApoE−/− mice. A–C: LOX, LOXL1, and LOXL2 expression were not affected by ANG II in the presence of a high-fat diet. D: LOXL3 expression increased with ANG II on an atherogenic diet. *P = 0.005. E: Western blot analysis on ApoE−/− mice 7 days after treatment with low-fat diet or high-fat diet + ANG II. By Western analysis, LOX and LOXL3 were the only detectable family members in the low-fat and high-fat diet + ANG II setting 7 days post-treatment. Neither LOX nor LOXL3 showed a statistically significant change in expression with treatment (n = 3).
LOX/LOXL proteins are expressed in the aortic wall.
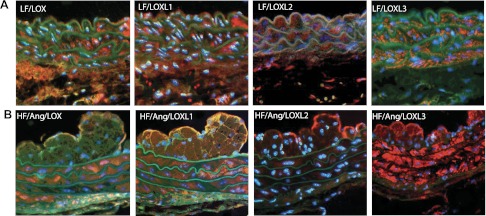
Immunostaining was performed to determine the cellular localization of individual LOX/LOXL family members within the aortic wall of ApoE−/− mice fed a high-fat diet and treated with ANG II for 8 wk. LOX, LOXL1, LOXL2, and LOXL3 expression was diffuse throughout the aortic wall (Fig. 3B). LOX, LOXL1, and LOXL3 displayed robust expression in the medial and intimal layers of the aortic wall (Fig. 3B). LOXL2 expression was most prominent in the endothelial cell layer (Fig. 3B). Interestingly, LOXL1 and LOX3 had significant expression in the neointimal layer, suggesting certain LOX/LOXL family members are expressed in migratory smooth muscle cells. LOX/LOXL family member localization was similar between low-fat and high-fat + ANG II treatment groups at 8 wk, suggesting that diet and treatment did not alter individual family member expression patterns (Fig. 3A). However, differential LOX/LOXL family member localization and expression profiles further illustrate the importance of this family of matrix crosslinking enzymes to the structural integrity of the vessel wall. Each member was clearly expressed in the vessel wall, underscoring the potential role of the LOX/LOXL family in maintaining vascular stability.
Fig. 3.
Localization of LOX/LOXL proteins in aortic tissue from ApoE−/− mice after 8 wk. A: localization of LOX/LOXL family members in aortic tissues of ApoE−/− mice after treatment with a low-fat diet for 8 wk. The spatial distribution is similar to that of the high-fat (HF) + ANG II treatment animals, with the exception of the neointimal space, which is not detected in low-fat fed animals after 8 wk. B: localization of LOX/LOXL family members in aortic tissues of ApoE−/− mice after treatment with a high-fat diet + ANG II for 8 wk. Protein expression was concentrated in the media, neointimal, and endothelial layers for LOXL1 and LOXL3. Expression of LOX was present in the medial layer and appears in the endothelial cell layer, but is absent from the neotintima. LOXL2 staining was present most abundantly within the endothelial cell layer.
LOX/LOXL activity protects against AAA formation.
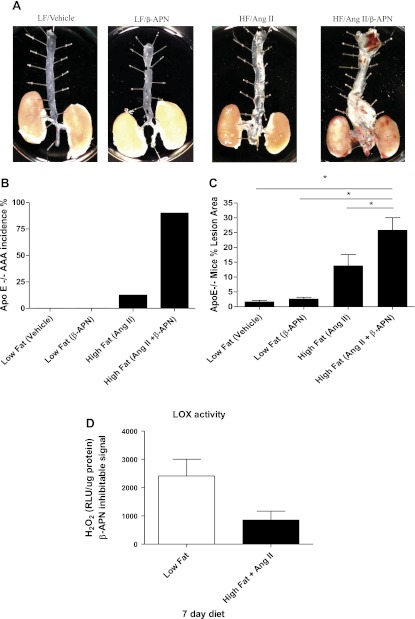
To determine the contribution of total LOX/LOXL activity to the stabilization of the aortic wall during AAA formation, we measured AAA incidence 4 wk after treatment in the ApoE−/− mice in the following settings: 1) vehicle alone, 2) β-APN alone, 3) ANG II + high-fat diet, and 4) ANG II + high-fat diet and β-APN. β-APN is a known general LOX/LOXL inhibitor and did not affect blood pressure (data not shown). Aneurysmal dilation, defined as suprarenal circumference doubling, was most pronounced in animals treated with ANG II + high-fat and β-APN (Fig. 4, A and B). Within these groups the frequency of large aneurysm formation was greater than 90%, and the high-fat/ANG II/β-APN group was associated with 60% mortality (Fig. 4B). Necropsies revealed aortic rupture as the cause of death. Mice fed a low-fat diet alone for 4 wk ± vehicle or β-APN did not demonstrate an increased frequency of aneurysm formation. Longer time points were not studied because of the high rate of mortality in the animals treated with β-APN. In addition to evaluating the contribution of LOX/LOXL family members to ANG II-induced vessel stability using β-APN inhibition, we also monitored total LOX/LOX L activity in ApoE−/− mice 7 days following treatment with either low-fat or high-fat diet + ANG II. We found a trend (P = 0.0576) toward decreased LOX/LOXL activity after treatment with ANG II and a high-fat diet, as compared with low fat controls (Fig. 4D). These data suggest that LOX/LOXL activity is critical for the crosslinking mechanisms that preserve vessel wall integrity during ANG II infusion.
Fig. 4.
Effect of β-aminopropionitrile (β-APN) on abdominal aortic aneurysms (AAA) incidence and atherosclerotic lesion area in ApoE−/− mice on a high-fat diet with ANG II infusion. A: representative examples of ApoE−/− mice treated with the lysyl oxidase inhibitor β-APN + ANG II. B: incidence of AAA in all groups. C: percent lesion area per total en face aortic area. *P < 0.001. D: total LOX/LOXL activity in aortic tissues of ApoE−/− mice after 7 days of treatment. Total LOX/LOXL activity showed a trend toward decreased LOX/LOXL activity (P = 0.0576) after treatment with a high-fat diet and ANG II. RLU, relative light units, detected as a readout for H2O2 produced. All data are normalized to 500 μg of total protein and normalized to the β-APN inhibitable signal. LF, low fat; HF, high fat.
LOX/LOXL activity protects against ANG II-induced atherosclerotic lesion formation.
To determine the functional importance of lysyl oxidase family member activity in maintaining the structural organization of the vasculature and in protection against disease, we measured the atherosclerotic lesion area in the aorta after 4 wk of control diet and after treatment with ANG II + high-fat with and without β-APN in ApoE−/− mice. ANG II + high-fat treatment induced a significant increase in atherosclerotic lesion formation above control (Fig. 4C). Conversely, mice infused with β-APN alone did not demonstrate an increase in lesion formation. However, when β-APN infusion was combined with ANG II + a high-fat diet, lesion formation increased significantly over control mice, covering nearly 35% of the aortic surface area. This dramatic increase in lesion formation demonstrates the deleterious effects of a damaged matrix on vascular disease and illustrates the protective action of LOX/LOXL against atherosclerotic lesion formation driven by an ANG II-induced mechanism.
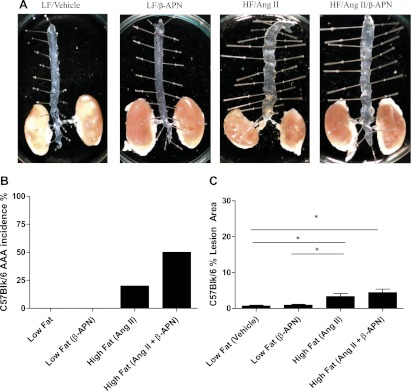
We also investigated the less atheroprone but not atheroprotected C57BL/6 mice in the same manner as the atherogenic ApoE−/− mice. The results of these experiments follow a similar trend as the ApoE−/− mice in that ANG II was sufficient to cause AAAs in some of the mice on a high-fat diet, but dual treatment with ANG II and β-APN caused further induction of AAAs, although not to the same degree as in the ApoE−/− mice (Fig. 5B). Lesion area was collectively much lower in these mice in all treatment groups, as compared with the ApoE−/− mice, and was not affected by β-APN treatment (Fig. 5, A and C).
Fig. 5.
Effect of β-APN on AAA incidence and atherosclerotic lesion area in C57BL/6 mice on a high-fat (HF) diet with ANG II. A: representative examples of C57BL/6 mice treated with the lysyl oxidase inhibitor β-APN + ANG II. B: incidence of AAA in all groups. C: percent lesion area per total en face aortic area. *P = 0.05.
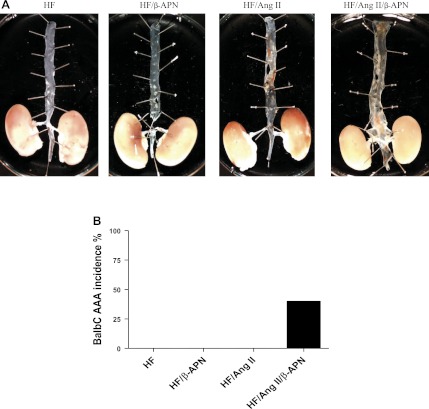
To further investigate the mechanism by which LOX/LOXL family members protect against AAA, the previously established atheroprotected BalbC mouse model (30) was treated using the same experimental strategy as described above for the atherogenic ApoE−/− mice. As expected, neither ANG II alone nor β-APN alone induced lesion formation. ANG II alone was not sufficient to produce any AAAs in the BalbC mice. However, the combination of both ANG II and β-APN induced significant aortic dilation above control (Fig. 6B), suggesting further that LOX/LOXL activity strongly counteracts ANG II-induced vascular remodeling at the matrix level and is protective against AAA formation. These data also suggest that although the presence of atherosclerotic lesion formation greatly increases the incidence of AAA formation, it is not necessary, since AAAs were induced in C57Bl/6 and BalbC mouse models, which have minimal or no atherosclerotic disease.
Fig. 6.
Effect of β-APN on AAA incidence and atherosclerotic lesion area in BalbC mice on a high-fat (HF) diet with ANG II. A: representative examples demonstrating lack of significant atherosclerotic lesion formation. However, treatment with ANG II and β-APN did cause AAA formation. B: incidence of AAA in all groups.
DISCUSSION
We have shown that LOX/LOXL family members are expressed in the aortic wall of normal and ANG II-treated mice. We have further demonstrated that although there may be spatial differences in protein expression, global LOXL3 mRNA is upregulated at the mRNA level but not at the protein level in ApoE−/− mice fed an atherogenic diet 7 days after treatment with ANG II. To elucidate the functional significance of the LOX/LOXL family members in the ApoE−/− mice fed a high-fat diet + ANG II, we inhibited LOX/LOXL activity with β-APN and observed accelerated AAA development in the setting of atherosclerosis. This suggests that the crosslinking activity of LOX/LOXL family members is a necessary compensatory mechanism for vessel stability during vascular disease. To further demonstrate that LOX/LOXL family members are crucial for maintaining matrix stability, we recapitulated this model in less atherogenic prone C57BL/6 and BalbC mice. These mice developed AAAs, although their atherosclerotic lesion area was limited. These results point to the critical significance of LOX/LOXL family members in vessel stability independent of atherosclerotic disease.
The connection between ANG II, LOX/LOXL, and AAA formation has yet to be completely elucidated. However, we can speculate on how these factors work synergistically to promote AAA formation. It is noteworthy that lesion area in the C57BL/6 and BalbC mice treated with ANG II was low (<10%) compared with the ApoE−/− mice treated with ANG II (20–30%). It is plausible that the contribution of ANG II to AAA is through the induction of atherosclerotic lesion formation, which ultimately destabilizes the vessel wall leading to dilation. This instability was more pronounced in our most atheroprone mice, which could explain why more ApoE−/− mice develop AAAs with ANG II treatment alone. The rank order of atherosclerotic potential in our mouse models is ApoE−/− > C57BL/6 > BalbC. As such, we saw AAA formation with ANG II alone only in more atheroprone models and when lesion area was more severe. Inhibition of LOX/LOXL was also capable of causing instability in the vessel wall, but not in healthy adult mice. The combination of these two factors (ANG II + high-fat diet and β-APN) promoted AAA formation regardless of the atherosclerotic potential of the mouse model. Lesion area and AAA formation reported within this manuscript are lower than previously reported numbers, which is likely attributable to differences in the ages of mice (8 wk) at the onset of treatment, as previously demonstrated by Weiss et al. (32). The use of more juvenile aged mice required the combination of the high fat + ANG II diet to detect lesion formation, similar to the method used to induce lesion formation in Low density lipoprotein receptor (LDLR−/−) mice.
It is possible that the ANG II effect on LOX/LOXL mRNA expression is a compensatory mechanism invoked to stabilize aortic structure. As determined by monitoring LOX/LOXL activity at 7 days, we detected a trend toward a decrease in LOX/LOXL activity, suggesting that a compensatory mechanism is invoked within LOXL3 to upregulate expression and counteract the decrease in LOX/LOXL activity observed at 7 days post-treatment. The catalytic function of LOX/LOXL in modifying proteins responsible for tensile and elastic strength of aortic tissue suggests a potentially important role in aneurysm formation. It is likely that inhibition of LOX/LOXL family members by β-APN induced AAA in our animal model because there was a balance that exists between extracellular matrix proteolytic activity and LOX/LOXL activity, and we altered that. We suggest that tipping the balance in favor of either can lead to expansion or stabilization of the aortic wall. It has been reported that ANG II causes the activation and/or release of MMPs that are capable of degrading collagen and elastin fibers (1, 31). β-APN treatment in combination with ANG II prevented LOX/LOXL crosslinking activity, shifting the balance in favor of aortic dilation.
To date, LOX/LOXL family members are the only known enzymes responsible for the covalent crosslinking of soluble collagen and elastin into protease-resistant fibers. LOX activity has been demonstrated to be crucial for matrix development during gestation and postnatal development (20, 28). Although synthesis of elastin into concentric laminae during development is defined, synthesis of mature elastin during adulthood is quite limited (26, 27). Furthermore, our studies show that inhibition of LOX/LOXL activity in healthy adult animals is not associated with cardiovascular disease, implying that LOX/LOXL is crucially important during times of growth and remodeling. These results were previously suggested by Kanematsu using C57BL/6 mice that were treated with ANG II and β-APN, which demonstrated that the hypertensive/hemodynamic effects of ANG II along with LOX/LOXL inhibition via β-APN contribute to the formation of AAAs (12).
Using immunohistochemistry, we have shown that LOX, LOXL1, LOXL2, and LOXL3 are expressed at the protein level and are regulated at the mRNA level by ANG II in the aortic wall. Western blot analysis did not suggest regulation in the global amount of LOX/LOXL family members after 7 days of ANG II treatment. We have further demonstrated that LOX/LOXL activity is crucial in the setting of ANG II-induced vascular disease independently of atherosclerotic lesion formation. As such, therapeutic strategies to overexpress LOX/LOXL enzymes or to support the crosslinking of soluble matrix proteins in a polymeric scaffold are a promising opportunity to achieve stabilization of AAA. It is noteworthy that attempts to overexpress LOX/LOXL activity and or expression must be done carefully since heightened levels of LOX activity have been associated with tumor progression. Losses of heterozygosity in the LOX and LOXL2 genes have been associated with colonic and esophageal neoplasms, identifying these genes as tumor suppressors (10). The by-product of LOX activity, H2O2, has been associated with cell motility and migration in breast cancer (25). Furthermore, increased LOX activity has been linked to conditions of fibrosis, such as atherosclerosis (10, 11). With this knowledge, site-specific targeting and regulated activation of the LOX/LOXL family members should be a priority of future studies.
In conclusion, these data in ApoE−/− and C57BL/6 and BalbC mice establish that the activity of the LOX/LOXL family imparts critical structural integrity to the vascular wall. Inhibition of LOX/LOXL in these models generates a novel insight to study AAA development from the standpoint of LOX/LOXL activity.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grants R01 HL-70531 and HL-090584.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.W.R., R.O., D.W., and G.J. performed experiments; E.W.R., R.O., K.M.R., and D.W. analyzed data; E.W.R., R.O., K.M.R., D.W., and G.J. interpreted results of experiments; E.W.R., R.O., K.M.R., and G.J. prepared figures; E.W.R. and K.M.R. drafted manuscript; E.W.R. and W.R.T. edited and revised manuscript; E.W.R. and W.R.T. approved final version of manuscript; R.O., D.W., K.C., S.F.F., and W.R.T. conception and design of research.
REFERENCES
- 1. Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol 286: C779–C784, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Coral K, Angayarkanni N, Madhavan J, Bharathselvi M, Ramakrishnan S, Nandi K, Rishi P, Kasinathan N, Krishnakumar S. Lysyl oxidase activity in the ocular tissues and the role of LOX in proliferative diabetic retinopathy and rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci 49: 4746–4752, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol 70: 1–32, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 24: 429–434, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 18: 1625–1633, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Ferrara DE, Weiss D, Carnell PH, Vito RP, Vega D, Gao X, Nie S, Taylor WR. Quantitative 3D fluorescence technique for the analysis of en face preparations of arterial walls using quantum dot nanocrystals and two-photon excitation laser scanning microscopy. Am J Physiol Regul Integr Comp Physiol 290: R114–R123, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Freestone T, Turner RJ, Higman DJ, Lever MJ, Powell JT. Influence of hypercholesterolemia and adventitial inflammation on the development of aortic aneurysm in rabbits. Arterioscler Thromb Vasc Biol 17: 10–17, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol 26: 2605–2613, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278: 14387–14393, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88: 660–672, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Kagan HM, Raghavan J, Hollander W. Changes in aortic lysyl oxidase activity in diet-induced atherosclerosis in the rabbit. Arteriosclerosis 1: 287–291, 1981 [DOI] [PubMed] [Google Scholar]
- 12. Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension 55: 1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuivaniemi H. Partial characterization of lysyl oxidase from several human tissues. Biochem J 230: 639–643, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuivaniemi H, Tromp G, Prockop DJ. Genetic causes of aortic aneurysms. Unlearning at least part of what the textbooks say. J Clin Invest 88: 1441–1444, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 160: 1425–1430, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 330: 750, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36: 178–182, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery 137: 457–462, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 110: 625–632, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta 1647: 220–224, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Narayanan AS, Siegel RC, Martin GR. On the inhibition of lysyl oxidase by -aminopropionitrile. Biochem Biophys Res Commun 46: 745–751, 1972 [DOI] [PubMed] [Google Scholar]
- 23. Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem 300: 245–251, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Papalambros E, Sigala F, Georgopoulos S, Menekakos C, Giatromanolaki A, Bastounis E, Sivridis E. Immunohistochemical expression of metalloproteinases MMP-2 and MMP-9 in abdominal aortic aneurysms: correlation with symptoms and aortic diameter. Int J Mol Med 12: 965–968, 2003 [PubMed] [Google Scholar]
- 25. Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SF, Csiszar K, Hendrix MJ, Kirschmann DA. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res 65: 11429–11436, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Perrin S, Foster JA. Developmental regulation of elastin gene expression. Crit Rev Eukaryot Gene Expr 7: 1–10, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87: 1828–1834, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegel RC. Lysyl oxidase. Int Rev Connect Tissue Res 8: 73–118, 1979 [DOI] [PubMed] [Google Scholar]
- 29. Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol 21: 1440–1445, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Stein O, Thiery J, Stein Y. Is there a genetic basis for resistance to atherosclerosis? Atherosclerosis 160: 1–10, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol 167: 1429–1442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 103: 448–454, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Weiss D, Sorescu D, Taylor WR. Angiotensin II and atherosclerosis. Am J Cardiol 87: 25C–32C, 2001 [DOI] [PubMed] [Google Scholar]