Abstract
We previously reported that acute inhibition of the RhoA/Rho kinase (ROCK) pathway normalized contractile function of diabetic rat hearts, but the underlying mechanism is unclear. Protein kinase C (PKC) β2 has been proposed to play a major role in diabetic cardiomyopathy at least in part by increasing oxidative stress. Further evidence suggests that PKC positively regulates RhoA expression through induction of inducible nitric oxide synthase (iNOS) in diabetes. However, in preliminary studies, we found that inhibition of ROCK itself reduced RhoA expression in diabetic hearts. We hypothesized that there is an interaction between RhoA/ROCK and PKCβ2 in the form of a positive feedback loop that sustains their activation and the production of reactive oxygen species (ROS). This was investigated in cardiomyocytes isolated from diabetic and control rat hearts, incubated with or without cytochalasin D or inhibitors of ROCK, RhoA, PKCβ2, or iNOS. Inhibition of RhoA and ROCK markedly attenuated the diabetes-induced increases in PKCβ2 activity and iNOS and RhoA expression in diabetic cardiomyocytes, while having no effect in control cells. Inhibition of PKCβ2 and iNOS also normalized RhoA expression and ROCK overactivation, whereas iNOS inhibition reversed the increase in PKCβ2 activity. Each of these treatments also normalized the diabetes-induced increase in production of ROS. Actin cytoskeleton disruption attenuated the increased expression and/or activity of all of these targets in diabetic cardiomyocytes. These data suggest that, in the diabetic heart, the RhoA/ROCK pathway contributes to contractile dysfunction at least in part by sustaining PKCβ2 activation and ROS production via a positive feedback loop that requires an intact cytoskeleton.
Keywords: diabetic cardiomyopathy, RhoA, inducible nitric oxide synthase, adult cardiomyocytes
diabetic cardiomyopathy, characterized by early diastolic and later systolic dysfunction independent of hypertension or coronary artery disease, is a common complication of diabetes mellitus (3, 51) that contributes to the development of heart failure, one of the most common causes of morbidity and mortality in this condition (3). Presently, there is no specific drug available for its prevention or treatment.
The RhoA/Rho kinase (ROCK) pathway regulates several vital cellular processes, including cell growth, morphology, contraction, and gene transcription. Elevated activity of this pathway has been implicated in a number of cardiovascular diseases, including cerebral stroke (50), hypertension (10), heart failure (29), and atherosclerosis (36). We have also shown that the RhoA/ROCK pathway is also implicated in diabetic cardiomyopathy and that acute inhibition of ROCK improved contractile function of hearts from type 1 diabetic rats both in vitro and in vivo (32). Recently, it was shown that chronic treatment with the ROCK inhibitor fasudil prevented the development of cardiac contractile dysfunction in a model of type 2 diabetes (62). However, the mechanism by which inhibition of ROCK improves the function of diabetic hearts is currently unclear.
Protein kinase Cβ2 (PKCβ2) is a member of the protein kinase C (PKC) family of serine/threonine kinases. Evidence supporting a major role for PKCβ2 in diabetic complications is compelling, and inhibition of PKCβ was successful in ameliorating diabetic nephropathy, retinopathy, neuropathy, and cardiomyopathy (reviewed in Refs. 2 and 12). Moreover, cardiac-specific overexpression of PKCβ2 led to left ventricular hypertrophy, cardiomyocyte necrosis, fibrosis, and decreased left ventricular performance without vascular lesions (55).
Increases in oxidative stress are key to the effects of PKCβ2 in diabetes. Reactive oxygen species (ROS) are believed to contribute to the activation of PKCβ2 (57), and, in turn, there is strong evidence that the adverse effects of PKCβ2 activation are mediated at least in part by increased production of ROS (30, 34, 40, 42, 44, 63) as well as by induction of inducible nitric oxide synthase (iNOS) (38), with consequent induction of oxidative and nitrosative stress. This suggests that ROS are both upstream and downstream of PKCβ2 activation.
A link between the RhoA-ROCK and PKC pathways in hyperglycemia and diabetes has been suggested by previous studies (23, 31, 41, 59). We reported that inhibition of iNOS not only decreased RhoA expression but also attenuated the increase in RhoA/ROCK pathway activity in hearts from diabetic rats, concomitant with improved cardiac function (52), implying that the diabetes-induced activation of PKCβ2 may contribute to RhoA upregulation and increased ROCK activity through induction of iNOS. At the same time, in preliminary experiments, we observed that inhibition of ROCK itself significantly decreased RhoA expression in cardiomyocytes incubated in high glucose. This suggested that the interaction between the RhoA/ROCK pathway and PKCβ2 could be in the form of a positive feedback loop that contributes to sustaining their activation and the production of ROS in diabetic rat hearts. To investigate this, we first determined if ROCK regulates PKCβ2 activity in isolated cardiomyocytes incubated in high glucose conditions as well as from diabetic rat hearts. We then investigated whether the suggested positive feedback loop occurs in diabetic rat hearts and influences the production of ROS.
MATERIALS AND METHODS
Materials.
Y-27632, H-1152, and 1400W (Calbiochem), cytochalasin D, and LY-333531 (ruboxistaurin; Enzo Life Sciences), cell permeable C3 transferase (Cytoskeleton), streptozotocin (STZ) and medium 199 (Sigma-Aldrich), collagenase II (Worthington Biochem), laminin (Roche), and pentobarbital sodium (Bimeda) were used.
Induction of diabetes mellitus in Wistar rats.
Male Wistar rats (170–200 g) were lightly anesthetized with isoflurane and given a single tail vein injection of 60 mg/kg STZ in 0.1 M citrate buffer (pH 4.5) or citrate buffer alone. STZ-treated rats with blood glucose levels >18 mmol/l, measured with a One Touch glucometer (Life Scan) 1 wk after injection, were considered diabetic. All animals were housed under identical conditions and were given free access to food and water. This investigation conforms with the Canadian Council on Animal Care Guidelines on the Care and Use of Experimental Animals and the Guide for Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH publication no. 85–23, revised 1996). All protocols were approved by the University of British Columbia Animal Care Committee.
Isolation of adult rat ventricular cardiomyocytes.
Calcium-tolerant adult ventricular cardiomyocytes were isolated as detailed previously (32). Rats were anesthetized with pentobarbital sodium (100 mg/kg ip). Once the stage of deep surgical anesthesia was reached, confirmed by loss of pedal and palpebral reflexes, hearts were rapidly excised and perfused in the Langendorff mode with calcium-free Tyrode solution (composition in mM: 100 NaCl, 10 KCl, 1.2 KH2PO4, 5 MgSO4, 50 taurine, 10 glucose, and 10 HEPES), followed by Tyrode solution containing 0.05 mM Ca2+, 0.8 mg/ml type II collagenase, and 0.1% BSA. The ventricles were removed and minced, and the resulting cell suspension was filtered using a 200-μm mesh and centrifuged briefly at 60 g. The cell pellet was washed three successive times in Tyrode solution containing 0.2, 0.5, and 1 mM Ca2+. Cardiomyocyte counts were taken using a hemocytometer, and viability was determined by assessing the percentage of cells that excluded trypan blue dye. Cell viability was >70% in all groups.
Cell culture and treatment studies.
Cardiomyocytes were resuspended in medium 199 and allowed to attach to laminin-coated plates (20 μg/ml) for 2 h before treatment. Afterwards, cells were incubated in 5.5 mM glucose (low glucose), 5.5 mM glucose plus 19.5 mM mannitol (osmotic control), or 25 mM glucose (high glucose) for 24 h to allow time for expressional changes to occur. A time course of the effect of high glucose on ROCK activity was also performed by incubating cardiomyocytes in high glucose for different time periods. Cardiomyocytes isolated from diabetic rats and their age-matched controls were incubated in low glucose medium 199 for up to 8 h. Cells were treated with the ROCK inhibitor Y-27632 (1 μM), the Rho inhibitor C3 transferase (2 μg/ml), the PKCβ inhibitor LY-333531 (20 nM), the iNOS inhibitor 1400W (10 μM), the actin depolymerizer cytochalasin D (2 μM), or left untreated.
PKCβ2 translocation inhibitor peptide synthesis and treatment.
Based on the work of Stebbins and Mochly-Rosen (53) we had a selective PKCβ2 translocation inhibitor peptide with the sequence QEVIRN synthesized (GenicBio, Shanghai, China). This sequence was conjugated to the cell penetrating HIV-1 Tat47–57 peptide by a disulfide link between cysteine residues added to the NH2-terminus of each peptide (the complete peptide sequence was RRRQRRKKRGYC-SS-CQEVIRN), which becomes reduced once the peptide enters the cell liberating the free PKCβ2 inhibitor sequence. The peptide sequence is present in the V5 region of PKCβ2 but not PKCβ1 and is responsible for the selective binding of PKCβ2 to RACK1 (53). Cardiomyocytes from control and diabetic rat hearts were cultured in medium 199 and treated with either the PKCβ2 inhibitor peptide or the Tat peptide (0.8 μM) for 30 min or 8 h to compare short- and long-term effects. The concentration of the PKCβ2 inhibitor peptide was selected based on our preliminary experiments where 0.8 μM was found to effectively inhibit PKCβ2 translocation and at the same time was devoid of cytotoxicity.
Determination of PKCβ2 translocation.
Cardiomyocytes from control and diabetic rat hearts were mechanically lysed in a detergent-free MOPS buffer. Cell lysates were then centrifuged at 2,000 g for 5 min, and the supernatant was ultracentrifuged at 100,000 g for 1 h. Afterwards, the supernatant (S1) was collected (cytosolic fraction), and the pellet was resuspended in Triton X-100 buffer and ultracentrifuged again at 100,000 g for 1 h. The supernatant (S2) was collected (membrane fraction). Both S1 and S2 were snap-frozen in liquid nitrogen and stored at −80°C.
ROCK activity assay.
The activity of ROCK was measured by determining the extent of Thr696 phosphorylation of MYPT1 in an in vitro assay as described in Liu and Liao (33). Briefly, cell lysates were added to a reaction mixture containing 50 mM Tris (pH=7.5), 0.1 mM EGTA, 10 mM magnesium acetate, 1 mM ATP, 0.1% β-mercaptoethanol, and 500 ng truncated MYPT1(654–880) and incubated at 30°C for 30 min. The level of Thr696 phosphorylation of MYPT1 was determined by Western blotting.
Western blot.
Proteins from each sample were separated by 8–12% SDS-PAGE and immunoblotted using primary antibodies against iNOS, GAPDH, Thr696p-MYPT1, Ser188p-RhoA, RhoA, PKCβ2 (Santa Cruz Biotechnology), Thr508/505pLIMK1/2 (Cell Signaling Technology), or Thr641p-PKCβ2 (Life Technologies). The intensity of the protein bands was determined by densitometry and normalized to GAPDH or its corresponding total protein in the same preparation.
RhoA activity assay.
A commercially available RhoA activation assay kit (Cytoskeleton) was used to determine the relative amount of active RhoA in 5 × 105 freshly isolated cardiomyocytes.
Adenoviral infection of adult rat ventricular cardiomyocytes.
Cardiomyocytes were isolated from nondiabetic rat hearts and plated on laminin-coated culture dishes at a cell density of 5 × 104 cells/cm2. After attachment, cells were transduced with replication incompetent (−E1/−E3) human adenovirus type 5 encoding dominant-negative RhoA mutant RhoA N19 (Ad-RhoA N19) or green fluorescent protein (Ad-GFP) as control, driven by CMV promoter at a multiplicity of infection of 40 (Applied Biological Materials), for 90 min. Afterwards, the medium was changed to medium 199 containing either 5.5 or 25 mM glucose, and cells were incubated for 24 h.
Determination of ROS levels.
The levels of ROS were measured using live cell imaging of dihydroethidium (DHE)-loaded cardiomyocytes. After treatment for the specified periods, cells were incubated in Hanks' balanced salt solution containing 5 μM DHE for 10 min at 37°C. Cells were then immediately imaged using a FV10i LIV Laser Scanning Confocal Microscope (Olympus Canada). Controls were used to correct for autofluorescence. After entering the cell, DHE is oxidized by ROS, mainly superoxide, to red fluorescent products that accumulate in the nucleus (27).
F/G-actin assay.
Freshly isolated cardiomyocytes were plated on laminin-coated culture dishes. Cells were treated with C3 exoenzyme (2 μg/ml) or cytochalasin D (1, 2, or 5 μM) for 24 h or left untreated. Myocytes were then processed for the isolation and determination of globular actin (G-actin) and filamentous actin (F-actin) content using a commercially available assay kit (Cytoskeleton).
Statistical analysis.
All values are expressed as means ± SE; n denotes the no. of animals in each group. Statistical analysis of all data was performed using one-way ANOVA followed by the Newman-Keuls test when more than two groups were compared, using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
High glucose increases RhoA/ROCK pathway activity in isolated cardiomyocytes.
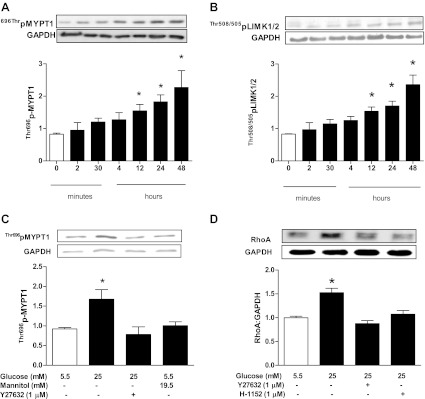
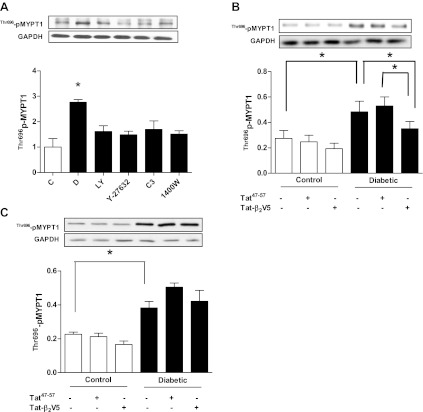
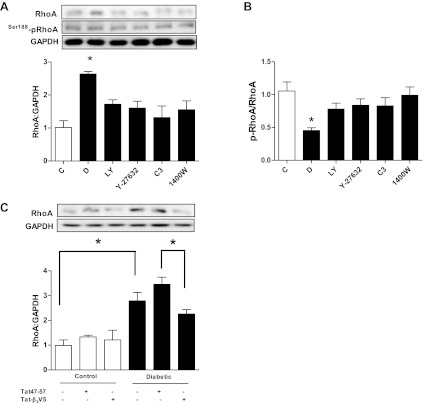
We first investigated whether the increased activation of the RhoA/ROCK pathway that we previously detected in hearts of diabetic rats (32) could be attributed to exposure of cardiomyocytes to high glucose. Incubation of adult cardiomyocytes isolated from nondiabetic rat hearts in 25 mM glucose produced a time-dependent increase in ROCK activity that reached statistical significance after 12 h or more of incubation (Fig. 1A). To further confirm the effect of high glucose on ROCK activity, we determined the level of Thr505/508 phosphorylation of LIM kinase (LIMK) 1/2, another ROCK downstream target. Indeed, we found that LIMK phosphorylation increased significantly with incubation of cardiomyocytes in high glucose for 24 h (Fig. 1B). The increase in ROCK activity was blocked by treatment with Y-27632 (Fig. 1C). An equimolar concentration of mannitol did not alter ROCK activity (Fig. 1C). Additionally, RhoA expression (Fig. 1D) and activity (Fig. 2C) were significantly elevated in cells incubated in high glucose. The elevated RhoA expression was blocked by the ROCK inhibitors Y-27632 and H-1152 (Fig. 1D).
Fig. 1.
Effect of duration of incubation of isolated cardiomyocytes in 25 mM glucose on phosphorylation of MYPT1 (A) and LIM kinase (LIMK) 1/2 (B) as indexes of Rho kinase (ROCK) activity. *p<0.05 compared with the first group (n = 4–6). C: effect of the ROCK inhibitor Y-27632 (1 μM) treatment on ROCK activity in cardiomyocytes incubated in 25 mM glucose for 24 h. Mannitol was used as an osmotic control. *P < 0.05 compared with the other groups (n = 4–6). D: effect of the ROCK inhibitors Y-27632 (1 μM) and H-1152 (1 μM) treatment for 24 h on RhoA expression in cardiomyocytes incubated in 25 mM glucose. *P < 0.05 compared with the other groups (n = 7).
Fig. 2.
A: effect of treatment with increasing concentrations of the Rho inhibitor C3 for 24 h on MYPT1 Thr696 phosphorylation as an index of ROCK activity in cardiomyocytes incubated in 25 mM glucose. #P < 0.05 compared with control. *P < 0.05 compared with untreated cardiomyocytes incubated in 25 mM glucose (n = 4). C, cells incubated in 5.5 mM glucose; HG, cells incubated in 25 mM glucose. B: effect of Y-27632 (1 μM), H-1152 (1 μM), and C3 transferase (2 μg/ml) treatment for 24 h on protein kinase C (PKC) β2 Thr641 phosphorylation in cardiomyocytes incubated in 25 mM glucose. *P < 0.05 compared with the other groups (n = 6). Effect of human adenovirus type 5 encoding dominant-negative RhoA mutant RhoA N19 (Ad-RhoA N190) on RhoA activity (C) and PKCβ2 Thr641 phosphorylation (D) in cardiomyocytes incubated in 5.5 or 25 mM glucose for 24 h. Ad-GFP, human adenovirus type 5 encoding green fluorescent protein. *P < 0.05 compared with the other groups (n = 3).
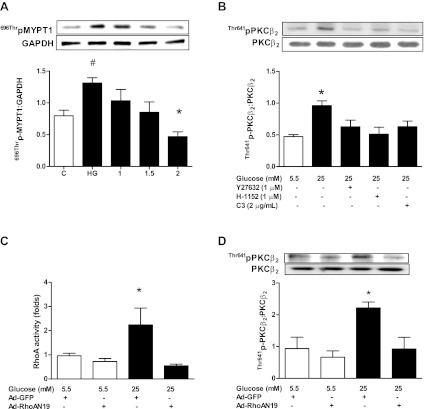
High glucose-induced PKCβ2 activation is inhibited by RhoA/ROCK inhibition.
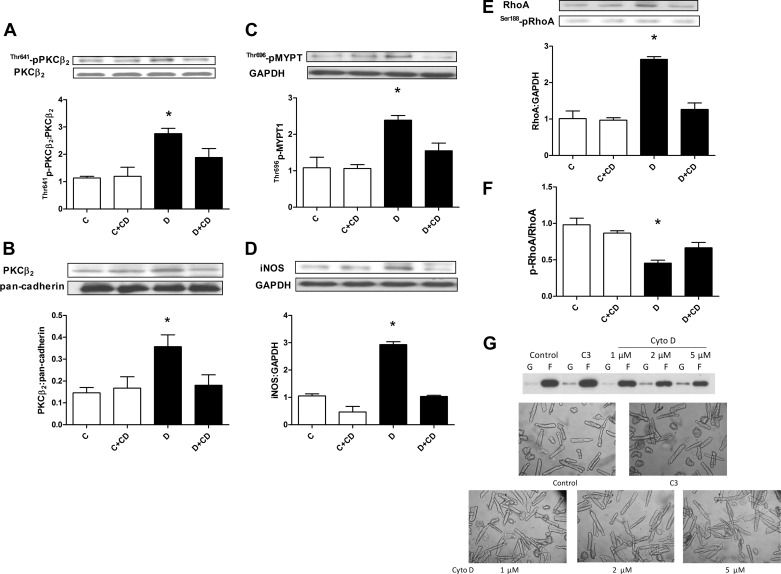
We next determined whether the RhoA/ROCK pathway regulated PKCβ2 activity under high glucose conditions. In agreement with our previous results (38), incubation in high glucose had no significant effect on total PKCβ2 levels but increased its Thr641 phosphorylation. The latter was significantly attenuated by inhibition of ROCK with Y-27632 or H-1152 (Fig. 2B). Phosphorylation of Thr641 is essential for the appropriate subcellular localization and catalytic function of PKCβ2 (16, 28) and is used as an index of PKCβ2 activation (1, 9, 35, 45). To confirm involvement of the pathway, cardiomyocytes were also treated with the Rho inhibitor C3 transferase at a concentration (2 μg/ml) that effectively inhibited ROCK activity (Fig. 2A), which significantly decreased PKCβ2 phosphorylation (Fig. 2B). Last, cardiomyocytes were infected with Ad-RhoA N19 and incubated in high or low glucose for 24 h. In preliminary experiments using Ad-GFP, we found that the cardiomyocyte transduction efficiency was 85% after 24 h (data not shown). Ad-RhoA N19 treatment completely prevented the high glucose-induced increase in RhoA activity (Fig. 2C), and this was associated with loss of the high glucose-induced increase in PKCβ2 Thr641 phosphorylation (Fig. 2D).
RhoA/ROCK inhibition attenuates PKCβ2 Thr641 phosphorylation in cardiomyocytes isolated from diabetic hearts.
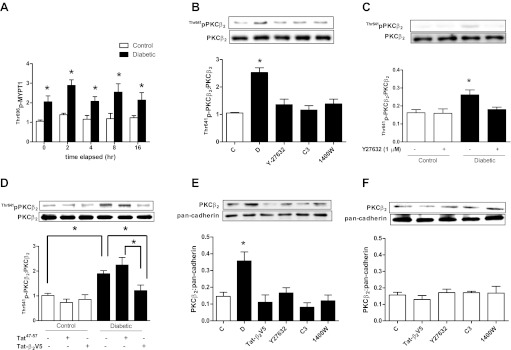
We next investigated whether RhoA/ROCK regulates PKCβ2 activity in vivo in hearts of diabetic rats. Cardiomyocytes isolated from diabetic and control rat hearts were treated with C3 transferase or Y-27632 for 8 h (longer periods of incubation caused rapid decline in the viability of cardiomyocytes from diabetic rat hearts). It is noteworthy that ROCK activity in cardiomyocytes isolated from diabetic rat hearts remained significantly elevated for up to 16 h after isolation (Fig. 3A). The diabetes-induced elevation of PKCβ2 Thr641 phosphorylation was attenuated to control levels by both Y-27632 and by C3 transferase (Fig. 3B), which had no effect on PKCβ2 phosphorylation in control cardiomyocytes (data not shown). The inhibitory effect of Y-27632 on PKCβ2 Thr641 phosphorylation in diabetic cardiomyocytes was evident even after short-term (30 min) treatment (Fig. 3C).
Fig. 3.
A: persistence of ROCK activation, assessed by MYPT1 Thr696 phosphorylation, over time after isolation of cardiomyocytes from diabetic and control rat hearts. *P < 0.05 compared with the corresponding control group (n = 4–5). Effect of Y-27632 (1 μM), C3 transferase (2 μg/ml), and 1400W (10 μM) treatment for 8 h (B) or Y-27632 (1 μM) treatment for 30 min (C) on PKCβ2 Thr641 phosphorylation in cardiomyocytes isolated from diabetic rat hearts. *P < 0.05 compared with all other groups (n = 5–7). D: effect of the PKCβ2 translocation inhibitor Tat-β2V5 (0.8 μM) treatment for 8 h on PKCβ2 Thr641 phosphorylation in cardiomyocytes isolated from control and diabetic rats. *P < 0.05 (n = 4). Effect of Tat-β2V5 (0.8 μM), Y-27632 (1 μM), C3 transferase (2 μg/ml), and 1400W (10 μM) treatment for 8 h on PKCβ2 translocation to the membrane fraction of cardiomyocytes isolated from diabetic (E) and control (F) rat hearts. C, control; D, diabetic. *P < 0.05 compared with all other groups (n = 4).
We also determined the effect of C3 transferase and Y-27632 treatment on translocation of PKCβ2 to the plasma membrane, a hallmark of PKC activation (16). There was a marked increase in the level of PKCβ2 in the membrane fraction in diabetic cardiomyocytes that was normalized by C3 transferase and Y-27632 as well as by the selective PKCβ2 translocation inhibitor peptide (Tat-β2V5; Fig. 3E), which also normalized PKCβ2 Thr641 phosphorylation in cardiomyocytes from diabetic rats (Fig. 3D). These inhibitors had no effect on PKCβ2 translocation in control cardiomyocytes (Fig. 3F).
ROCK inhibition attenuates the PKCβ2-mediated iNOS induction in cardiomyocytes isolated from diabetic rat hearts.
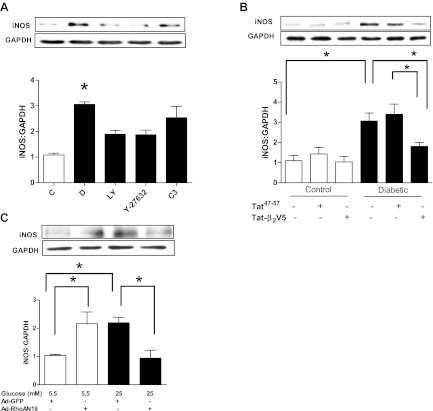
The RhoA/ROCK pathway regulation of PKCβ2 activation would also be expected to affect the expression of its downstream target, iNOS. Indeed, treatment with either Y-27632 or the PKCβ inhibitor LY-333531, at a concentration that we have previously shown to be effective in inhibiting PKCβ2 activity (38) and that is selective for PKCβ (26), significantly reduced the diabetes-induced upregulation of iNOS (Fig. 4A). Additionally, Tat-β2V5 also significantly attenuated increased iNOS expression in cardiomyocytes from diabetic rat hearts (Fig. 4B). On the other hand, C3 transferase failed to significantly alter iNOS expression in diabetic cardiomyocytes, although there was a slight downward trend (Fig. 4A). For this reason and since C3 inhibits not only RhoA, but also RhoB and -C, we determined the effect of Ad-RhoA N19 on iNOS expression in cardiomyocytes incubated in high glucose for 24 h. Ad-RhoA N19 treatment abolished the high glucose-induced increase in iNOS expression (Fig. 4C). Interestingly, Ad-RhoA N19 significantly increased iNOS expression in cardiomyocytes incubated in low glucose (Fig. 4C). We did not infect diabetic cardiomyocytes with Ad-RhoA N19 since it was not possible to maintain the cells viable for a time period sufficient for loss of RhoA activity.
Fig. 4.
A: effect of LY-333531 (LY, 20 nM), Y-27632 (1 μM), and C3 transferase (2 μg/ml) treatment for 8 h on inducible nitric oxide synthase (iNOS) expression in cardiomyocytes isolated from diabetic rat hearts. *P < 0.05 compared with the other groups except C3 (n = 5–7). B: effect of Tat-β2V5 (0.8 μM) treatment for 8 h on iNOS expression in cardiomyocytes isolated from control and diabetic rats. *P < 0.05 compared with the indicated groups (n = 4). C: effect of Ad-RhoA N19 on iNOS expression in cardiomyocytes incubated in 5.5 or 25 mM glucose for 24 h. *P < 0.05 compared with the indicated groups (n = 3).
Inhibition of PKCβ2, iNOS, RhoA, or ROCK disrupts the positive feedback loop and abrogates the sustained RhoA/ROCK pathway activation in diabetes.
We next tested whether the hypothesized feedforward loop could be detected in diabetic cardiomyocytes. We found that the diabetes-induced increase in ROCK activity was not only blocked by Y-27632 and C3 transferase but also by treatment with the iNOS inhibitor 1400W for 8 h (Fig. 5A). The latter also normalized PKCβ2 phosphorylation and translocation (Fig. 3, B and E), consistent with the proposed feedback loop. ROCK activity was also markedly attenuated by LY-333531 (Fig. 5A) and Tat-β2V5 treatment for 8 h in diabetic cardiomyocytes (Fig. 5B). In contrast, treatment with Tat-β2V5 for 30 min did not significantly alter ROCK activity (Fig. 5C).
Fig. 5.
A: effect of LY-333531 (20 nM), Y-27632 (1 μM), C3 transferase (2 μg/ml), and 1400W (10 μM) treatment for 8 h on ROCK activity in cardiomyocytes isolated from diabetic rat hearts. *P < 0.05 compared with the other groups (n = 5–7). Effect of Tat-β2V5 (0.8 μM) treatment for 8 h (B) or 30 min (C) on ROCK activity in cardiomyocytes isolated from diabetic or control rat hearts. *P < 0.05 compared with the indicated groups (n = 4–5).
To establish that ROCK positively reinforces RhoA through the PKCβ2/iNOS pathway, we determined the effect of the abovementioned inhibitors on RhoA expression and inhibitory Ser188 phosphorylation. In vehicle-treated cardiomyocytes from diabetic rats, RhoA expression but not Ser188 phosphorylation was significantly increased (Fig. 6A). The resulting diminished p-RhoA-to-RhoA ratio leads to a larger pool of readily activatable RhoA, which contributes to the elevated ROCK activity in the diabetic state. Treatment with Y-27632, 1400W, or LY-333531 failed to significantly alter RhoA Ser188 phosphorylation (Fig. 6A). On the other hand, these treatments were able to significantly decrease RhoA expression (Fig. 6A), normalizing the p-RhoA-to-RhoA ratio (Fig. 6B). The same treatments had no effect on RhoA in control cardiomyocytes (data not shown). Additionally, Tat-β2V5 significantly reduced RhoA expression in cardiomyocytes from diabetic animals compared with cardiomyocytes treated with Tat47–57 (Fig. 6C).
Fig. 6.
Effect of LY-333531 (20 nM), Y-27632 (1 μM), C3 transferase (2 μg/ml), and 1400W (10 μM) treatment for 8 h on RhoA expression (A) and Ser188 phosphorylation (B) in cardiomyocytes isolated from diabetic rat hearts. *P < 0.05 compared with other groups (n = 5–7). C: effect of Tat-β2V5 (0.8 μM) treatment for 8 h on RhoA expression in cardiomyocytes isolated from diabetic or control rat hearts. *P < 0.05 compared with the indicated groups (n = 4–5).
Actin cytoskeleton disruption mimics the effects of ROCK inhibition on the positive feedback loop.
The actin cytoskeleton is a major downstream target of the RhoA/ROCK pathway (8), and, in diabetic cardiomyocytes, actin polymerization is significantly increased (32). Therefore, we investigated whether increased actin polymerization contributes to the effects of ROCK on PKCβ2 and in turn on iNOS and RhoA. Treatment of cardiomyocytes from diabetic rats with the actin depolymerizer cytochalasin D mimicked the effects of ROCK inhibition on PKCβ2 Thr641 phosphorylation and translocation, and iNOS and RhoA expression (Fig. 7, A, B, D, and E). This was associated with loss of ROCK activity and normalization of Ser188 p-RhoA-to-total RhoA ratio (Fig. 7, C and F). The concentration of cytochalasin D used in this study did not alter cardiomyocyte morphology and produced similar extent of actin depolymerization as C3 transferase (Fig. 7G). These results suggest a role for the actin cytoskeleton in the positive feedback loop that sustains RhoA and ROCK activation.
Fig. 7.
Effect of cytochalasin D (CD, 2 μM) treatment for 8 h on Thr641 phosphorylation of PKCβ2 (A), PKCβ2 membrane translocation (B), ROCK activity (C), iNOS expression (D), RhoA expression (E), and Ser188 phosphorylation (F) in cardiomyocytes isolated from diabetic rat hearts. G: representative Western blot of the effect of C3 exoenzyme or cytochalasin D treatment for 24 h on the F-to-G-actin ratio in isolated adult rat cardiomyocytes and photomicrographs of isolated adult cardiomyocytes treated for 24 h with C3 exoenzyme or cytochalasin D. *P < 0.05 compared with the other groups (n = 3–4).
Disruption of the positive feedback loop normalizes ROS production.
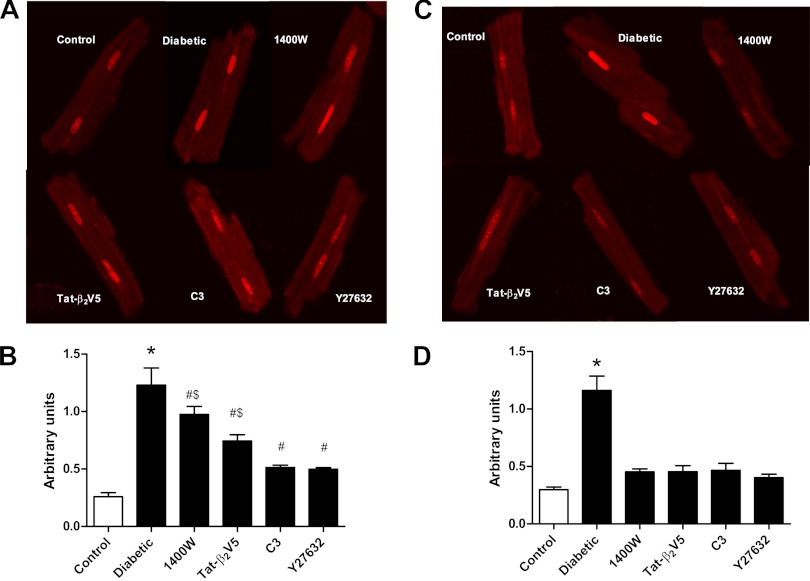
Oxidative stress plays an important role in mediating the deleterious effects of PKCβ2 in diabetes. Therefore, we determined the effect of disruption of the loop on diabetes-induced oxidative stress. Incubation of cardiomyocytes from diabetic rats with C3 or Y-26732 for 1 h produced a significantly greater reduction in ROS production than did either Tat-β2V5 or 1400W (Fig. 8B). However, following 8 h incubation, all inhibitors markedly attenuated ROS to near control levels (Fig. 8D).
Fig. 8.
Effect of Y-27632 (1 μM), C3 transferase (2 μg/ml), Tat-β2V5 (0.8 μM), and 1400W (10 μM) treatment for 1 h (A and B) or 8 h (C and D) on reactive oxygen species (ROS) levels measured by dihydroethidium staining. B and D are representative photomicrographs of dihydroethidium-stained cardiomyocytes with the various treatments. *P < 0.05 compared with all groups. #P < 0.05 compared with control. $P < 0.05 compared with Y-27632- and C3-treated cells. (n = 3–4, more than 40 cells were analyzed in each group).
DISCUSSION
The RhoA/ROCK pathway has been shown by numerous researchers to play a pivotal role in cardiovascular pathologies, and there is great interest in ROCK inhibitors as potential therapies for many of these diseases (reviewed in Ref. 15). We reported previously that the activity of the RhoA/ROCK pathway is elevated in diabetic cardiomyopathy and that acute treatment with ROCK inhibitors significantly improves the contractile function of hearts from diabetic rats (32). The novel findings of the present study are that, in the diabetic heart, 1) activation of the RhoA/ROCK pathway leads to increased PKCβ2 activity; 2) the increased activity of PKCβ2 and the RhoA/ROCK pathway is sustained by a positive feedback loop; and 3) disrupting the loop markedly attenuates diabetes-induced increases in oxidative stress. These data suggest that the mechanism by which the RhoA/ROCK pathway contributes to contractile dysfunction in the diabetic heart is at least in part through activation of PKCβ2 and promotion of the production of ROS.
There is much evidence to suggest that hyperglycemia-induced activation of PKCβ2 in the heart contributes to the development of diabetic cardiomyopathy (22, 47, 56). The results of the present study show that treatment of cardiomyocytes incubated in high glucose or isolated from diabetic rat hearts with a concentration of Y-27632 or H-1152 that has been shown to be selective for ROCK over other kinases, including PKC (13), as well as inhibition of ROCK activation with either C3 exoenzyme or by overexpression of Ad-RhoA N19, prevented the increase in PKCβ2 activity. These data strongly implicate the RhoA/ROCK pathway in the activation of PKCβ2 in the diabetic heart. Our further observation that inhibition of ROCK or PKCβ2 prevented the diabetes-induced increase in RhoA expression supports the hypothesis that the interaction between the RhoA/ROCK pathway and PKCβ2 is in the form of a positive feedback loop. The finding that inhibition of iNOS, which we have shown previously to prevent the diabetes-induced increase in RhoA expression and ROCK activity, also prevented the activation of PKCβ2 in the heart provides additional evidence in support of the loop. This could not be detected in cardiomyocytes isolated from control hearts, indicating that this feedback mechanism occurs only under pathophysiological conditions.
There is compelling evidence linking increased oxidative stress, in particular ROS production, to the development of diabetic cardiovascular complications (6, 39). As noted in the introduction, oxidative stress contributes to the activation of PKCβ2 in the heart as in other tissues (19, 57), whereas increased PKCβ2 activity has been shown to lead to increased production of ROS in diabetes (30, 34, 40, 42, 44, 63). Similarly, oxidative stress appears to positively regulate induction of iNOS (61), whereas uncoupling of both endothelial nitric oxide synthase and iNOS in diabetes leads to the generation of ROS (43, 49). Consistent with this, direct inhibition of both PKCβ2 and iNOS decreased ROS production in diabetic cardiomyocytes in the present study. In addition, however, we found that inhibition of both RhoA and ROCK also markedly attenuated ROS production. ROS production was reduced to the same extent by long-term incubation with each of the inhibitors, a finding that is consistent with the presence of the loop and that demonstrates that its disruption at any point reduces oxidative stress. It should also be noted that short-term exposure to inhibitors of the RhoA/ROCK pathway produced more profound decreases in ROS production than did direct inhibition of PKCβ2 or iNOS. This may indicate that, in addition to increasing oxidative stress by promoting the activity of the loop, RhoA/ROCK may promote production of ROS through other mechanisms.
The production of ROS has been implicated in the phenomenon known as “metabolic memory,” the persistence of diabetic complications despite normalization of glucose levels (7, 21). Interestingly, we found that ROS production remained elevated for at least 8 h after cardiomyocytes isolated from diabetic hearts were cultured in low glucose, and this was associated with continued activation of ROCK and PKCβ2. Although it is not clear how long this sustained activation continues, the possible role of the positive feedback loop in metabolic memory in diabetic hearts warrants further investigation.
The results of the present study suggest that an intact actin cytoskeleton is required to sustain the activity of the positive feedback loop and its production of ROS. The RhoA/ROCK pathway is a well-known regulator of the actin cytoskeleton (8, 54), and we have shown previously that actin polymerization is increased in cardiomyocytes from diabetic rats as a result of RhoA/ROCK activation (32). On the other hand, an intact cytoskeleton was shown to be required for RhoA activation in response to leptin in neonatal cardiomyocytes (60). An important role for the actin cytoskeleton in the activation of PKCβ2 has also been reported. For instance, a number of researchers have demonstrated that PKCβ2 associates with the actin cytoskeleton in different cell types (4, 14, 18, 25, 46). Blobe et al. (4) reported that PKCβ2, but not PKCβ1, binds to F-actin and this enhances its autophosphorylation and activation. Moreover, Pascale et al. (46) found that an intact actin cytoskeleton was essential for the translocation of PKCβ2 to the plasma membrane of astrocytes upon activation. Thus is it possible that the actin depolymerization produced by cytochalasin D in the present study interfered with the activity of the loop at multiple sites, including activation of both RhoA and PKCβ2.
A number of researchers have reported cross talk between RhoA/ROCK and PKC under high glucose conditions or in diabetes, where ROCK was reported to be either upstream (31, 41) or downstream (23, 58, 59) of PKC. Our results demonstrating that RhoA/ROCK and PKCβ2 form part of a positive feedback loop help to reconcile those reports. Interestingly, Xie et al. (59) found that, although high glucose-induced CPI-17 phosphorylation in cultured vascular smooth muscle cells was abolished by short-term RhoA or ROCK inhibition for 30 min, it was not inhibited by PKC inhibition for the same length of time. However, if the PKC inhibitor treatment was extended for 48 h, the high glucose-induced CPI-17 phosphorylation was attenuated, and this was associated with a decrease in RhoA and ROCK activity. These results are in agreement with our suggested positive feedback loop in that PKCβ2 does not activate RhoA and ROCK until iNOS and consequently RhoA expression is increased, a process that may take hours to occur. On the other hand, our data demonstrate that the effect of ROCK inhibition on PKCβ2 phosphorylation and translocation was much more rapid, occurring within 30 min.
In the present study, inhibition of ROCK with Y-27632 decreased iNOS expression in cardiomyocytes from diabetic rat hearts. Similarly, infection of cardiomyocytes incubated in high glucose with the dominant-negative RhoA mutant Ad-RhoA N19 also significantly reduced iNOS expression. On the other hand, in control cells, the use of C3 exoenzyme or RhoA N19 increased iNOS expression, and, in the case of RhoA N19, this increase was statistically significant. This is consistent with previous reports indicating that inhibition of Rho by statins or C3 exoenzyme augments cytokine-induced iNOS induction in cells incubated in low glucose media (17, 37, 48). Our data provide a possible explanation for the opposite effects of RhoA inhibition on iNOS in diabetic vs. nondiabetic conditions. While activation of RhoA appears to suppress iNOS induction under nondiabetic conditions, the diabetes-induced positive feedback loop enables RhoA to upregulate iNOS, and the latter mechanism overrides the former, resulting in a net decrease in iNOS expression on inhibition of RhoA. Interestingly, Iwasaki et al. (24) showed that ROCK inhibition decreased the transcriptional activity of nuclear factor-κB (NF-κB), the main transcription factor for iNOS, only under high glucose conditions. Additionally, we previously reported that PKCβ2 inhibition decreased Ser536 phosphorylation and activation of the p65 subunit of NF-κB only under high glucose conditions (38).
Adult ventricular cardiomyocytes were used in the present study, rather than neonatal cardiomyocytes or H9c2 cardiomyoblasts, since they have the closest resemblance to adult human cardiac cells with respect to morphology, function, and cellular signaling (20). However, a limitation of these cells, particularly those from diabetic hearts, is that, within 24 h after isolation, viability drops substantially or cells begin to dedifferentiate. This limits the use of genetic approaches that require longer times to knock down target proteins such as PKCβ2 and ROCK that have a relatively long half-life and slow turnover (5). However, we were able to use alternative approaches to support the results obtained with chemical inhibitors, including Tat-β2V5 and Ad-RhoA N19. The results obtained by these approaches are in complete agreement with those obtained with the small molecule inhibitors, providing additional support for our findings.
In conclusion, the results of this study suggest that, in the diabetic heart, the RhoA/ROCK pathway contributes to contractile dysfunction at least in part by sustaining PKCβ2 activation, iNOS induction, and ROS production via a positive feedback loop that requires an intact cytoskeleton (Fig. 9). The significance of this loop is that it links proteins that have been shown to play a pivotal role in the pathogenesis of diabetic cardiomyopathy, since inhibition of ROCK, PKCβ2, or iNOS has been shown by us or others to substantially improve cardiac function in the diabetic state (11, 32, 52, 62).
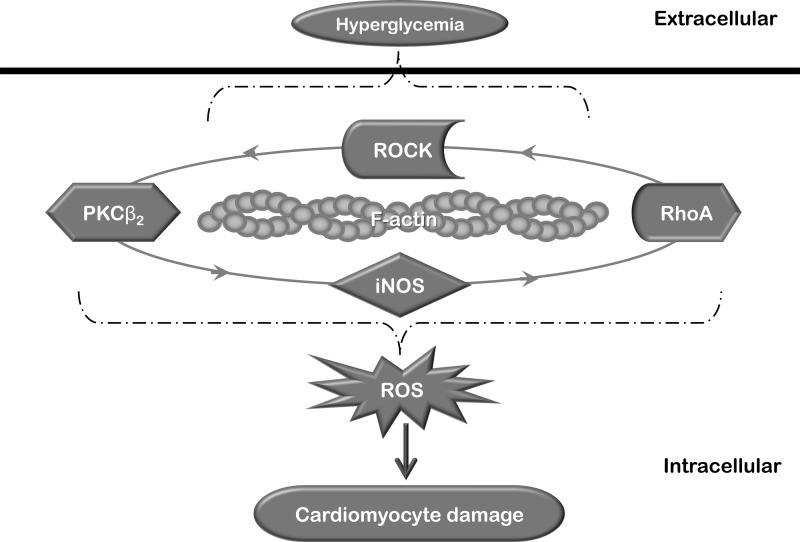
Fig. 9.
Schematic diagram summarizing the main findings of the present study. In diabetes, hyperglycemia leads to activation of a positive feedback loop that sustains the activation of RhoA/ROCK and PKCβ2 in cardiomyocytes. This loop requires an intact actin cytoskeleton for its operation and contributes to the elevation of oxidative stress, eventually resulting in cardiomyocyte damage and diabetic cardiomyopathy.
GRANTS
This study was supported by an operating grant from the Canadian Institutes of Health Research (MOP 97861). H. Soliman was supported by a doctoral student research award from the Canadian Diabetes Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.S., G.L., and K.M.M. conception and design of research; H.S., A.G., Y.-H.L., and G.B. performed experiments; H.S., A.G., and Y.-H.L. analyzed data; H.S. and K.M.M. interpreted results of experiments; H.S. prepared figures; H.S. drafted manuscript; H.S., G.L., G.B., and K.M.M. edited and revised manuscript; H.S., A.G., Y.-H.L., G.L., G.B., and K.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Timothy Kieffer and Travis Webber, Life Sciences Institute, University of British Columbia, for the expert assistance and advice they provided in the adenoviral infection work. We also thank Dr. Grant Budas, Chemical and Systems Biology, Stanford University, for advice on the use of the PKCβ2 inhibitor peptide.
REFERENCES
- 1. Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol 24: 2030–2037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avignon A, Sultan A. PKC-B inhibition: a new therapeutic approach for diabetic complications? Diabetes Metab 32: 205–213, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bell DS. Diabetic cardiomyopathy. Diabetes Care 26: 2949–2951, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem 271: 15823–15830, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Borner C, Eppenberger U, Wyss R, Fabbro D. Continuous synthesis of two protein-kinase-C-related proteins after down-regulation by phorbol esters. Proc Natl Acad Sci USA 85: 2110–2114, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Ceriello A. Hypothesis: the “metabolic memory,” the new challenge of diabetes. Diabetes Res Clin Pract 86, Suppl 1: S2–S6, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Chen X, Pavlish K, Zhang HY, Benoit JN. Effects of chronic portal hypertension on agonist-induced actin polymerization in small mesenteric arteries. Am J Physiol Heart Circ Physiol 290: H1915–H1921, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Chiasson VL, Quinn MA, Young KJ, Mitchell BM. Protein kinase CβII-mediated phosphorylation of endothelial nitric oxide synthase threonine 495 mediates the endothelial dysfunction induced by FK506 (tacrolimus). J Pharmacol Exp Ther 337: 718–723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chitaley K, Weber D, Webb RC. RhoA/Rho-kinase, vascular changes, and hypertension. Curr Hypertens Rep 3: 139–144, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Connelly KA, Kelly DJ, Zhang Y, Prior DL, Advani A, Cox AJ, Thai K, Krum H, Gilbert RE. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ Heart Fail 2: 129–137, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Danis RP, Sheetz MJ. Ruboxistaurin: PKC-beta inhibition for complications of diabetes. Expert Opin Pharmacother 10: 2913–2925, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res 210: 287–297, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Dong M, Yan BP, Liao JK, Lam YY, Yip GW, Yu CM. Rho-kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today 15: 622–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards AS, Faux MC, Scott JD, Newton AC. Carboxyl-terminal phosphorylation regulates the function and subcellular localization of protein kinase C betaII. J Biol Chem 274: 6461–6468, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Finder JD, Litz JL, Blaskovich MA, McGuire TF, Qian Y, Hamilton AD, Davies P, Sebti SM. Inhibition of protein geranylgeranylation causes a superinduction of nitric-oxide synthase-2 by interleukin-1beta in vascular smooth muscle cells. J Biol Chem 272: 13484–13488, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Goodnight JA, Mischak H, Kolch W, Mushinski JF. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem 270: 9991–10001, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med 28: 1349–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69: 1476–1486, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Ihnat MA, Thorpe JE, Ceriello A. Hypothesis: the “metabolic memory,” the new challenge of diabetes. Diabet Med 24: 582–586, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA 89: 11059–11063, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishibashi F. High glucose increases phosphocofilin via phosphorylation of LIM kinase due to Rho/Rho kinase activation in cultured pig proximal tubular epithelial cells. Diabetes Res Clin Pract 80: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Iwasaki H, Okamoto R, Kato S, Konishi K, Mizutani H, Yamada N, Isaka N, Nakano T, Ito M. High glucose induces plasminogen activator inhibitor-1 expression through Rho/Rho-kinase-mediated NF-kappaB activation in bovine aortic endothelial cells. Atherosclerosis 196: 22–28, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Jensen PV, Larsson LI. Actin microdomains on endothelial cells: association with CD44, ERM proteins, and signaling molecules during quiescence and wound healing. Histochem Cell Biol 121: 361–369, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH, 3rd, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A, Baevsky M, Ballas LM, Hall SE, Winneroski LL, Faul MM. (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-d ione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem 39: 2664–2671, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol 5: 1394–1403, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi N, Horinaka S, Mita S, Nakano S, Honda T, Yoshida K, Kobayashi T, Matsuoka H. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc Res 55: 757–767, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Korchak HM, Kilpatrick LE. Roles for beta II-protein kinase C and RACK1 in positive and negative signaling for superoxide anion generation in differentiated HL60 cells. J Biol Chem 276: 8910–8917, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Li J, O'Connor KL, Greeley GH, Jr, Blackshear PJ, Townsend CM, Jr, Evers BM. Myristoylated alanine-rich C kinase substrate-mediated neurotensin release via protein kinase C-delta downstream of the Rho/ROK pathway. J Biol Chem 280: 8351–8357, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lin G, Craig GP, Zhang L, Yuen VG, Allard M, McNeill JH, Macleod KM. Acute inhibition of Rho-kinase improves cardiac contractile function in streptozotocin-diabetic rats. Cardiovasc Res 75: 51–58, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Liu PY, Liao JK. A method for measuring Rho kinase activity in tissues and cells. Methods Enzymol 439: 181–189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Lei S, Gao X, Mao X, Wang T, Wong GT, Vanhoutte PM, Irwin MG, Xia Z. PKCbeta inhibition with ruboxistaurin reduces oxidative stress and attenuates left ventricular hypertrophy and dysfunction in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 122: 161–173, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Massip L, Garand C, Labbe A, Perreault E, Turaga RV, Bohr VA, Lebel M. Depletion of WRN protein causes RACK1 to activate several protein kinase C isoforms. Oncogene 29: 1486–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morishige K, Shimokawa H, Eto Y, Kandabashi T, Miyata K, Matsumoto Y, Hoshijima M, Kaibuchi K, Takeshita A. Adenovirus-mediated transfer of dominant-negative rho-kinase induces a regression of coronary arteriosclerosis in pigs in vivo. Arterioscler Thromb Vasc Biol 21: 548–554, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Muniyappa R, Xu R, Ram JL, Sowers JR. Inhibition of Rho protein stimulates iNOS expression in rat vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 278: H1762–H1768, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Nagareddy PR, Soliman H, Lin GR, Rajput PS, Kumar U, McNeill JH, MacLeod KM. Selective inhibition of protein kinase C beta(2) attenuates inducible nitric oxide synthase-mediated cardiovascular abnormalities in streptozotocin-induced diabetic rats. Diabetes 58: 2355–2364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Nixon JB, McPhail LC. Protein kinase C (PKC) isoforms translocate to Triton-insoluble fractions in stimulated human neutrophils: correlation of conventional PKC with activation of NADPH oxidase. J Immunol 163: 4574–4582, 1999 [PubMed] [Google Scholar]
- 41. Nobe K, Yamazaki T, Tsumita N, Hashimoto T, Honda K. Glucose-dependent enhancement of diabetic bladder contraction is associated with a rho kinase-regulated protein kinase C pathway. J Pharmacol Exp Ther 328: 940–950, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes 55: 3112–3120, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Okazaki T, Otani H, Shimazu T, Yoshioka K, Fujita M, Katano T, Ito S, Iwasaka T. Reversal of inducible nitric oxide synthase uncoupling unmasks tolerance to ischemia/reperfusion injury in the diabetic rat heart. J Mol Cell Cardiol 50: 534–544, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Omiyi D, Brue RJ, Taormina P, 2nd, Harvey M, Atkinson N, Young LH. Protein kinase C betaII peptide inhibitor exerts cardioprotective effects in rat cardiac ischemia/reperfusion injury. J Pharmacol Exp Ther 314: 542–551, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Park KH, Han DI, Rhee YH, Jeong SJ, Kim SH, Park YG. Protein kinase C betaII and delta/theta play critical roles in bone morphogenic protein-4-stimulated osteoblastic differentiation of MC3T3–E1 cells. Biochem Biophys Res Commun 403: 7–12, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Pascale A, Alkon DL, Grimaldi M. Translocation of protein kinase C-betaII in astrocytes requires organized actin cytoskeleton and is not accompanied by synchronous RACK1 relocation. Glia 46: 169–182, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Pastukh V, Wu S, Ricci C, Mozaffari M, Schaffer S. Reversal of hyperglycemic preconditioning by angiotensin II: role of calcium transport. Am J Physiol Heart Circ Physiol 288: H1965–H1975, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Rattan R, Giri S, Singh AK, Singh I. Rho A negatively regulates cytokine-mediated inducible nitric oxide synthase expression in brain-derived transformed cell lines: negative regulation of IKKalpha. Free Radic Biol Med 35: 1037–1050, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol 294: R793–R802, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36: 2251–2257, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma V, McNeill JH. Diabetic cardiomyopathy: where are we 40 years later? Can J Cardiol 22: 305–308, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soliman H, Craig GP, Nagareddy P, Yuen VG, Lin G, Kumar U, McNeill JH, Macleod KM. Role of inducible nitric oxide synthase in induction of RhoA expression in hearts from diabetic rats. Cardiovasc Res 79: 322–330, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem 276: 29644–29650, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch 453: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci USA 94: 9320–9325, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, Sandusky GE, Pechous PA, Vlahos CJ, Wakasaki H, King GL. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes 51: 2709–2718, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo Z, Guo T, Jiang J, McNeill JH. N-acetylcysteine attenuates PKCbeta2 overexpression and myocardial hypertrophy in streptozotocin-induced diabetic rats. Cardiovasc Res 73: 770–782, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Xie Z, Gong MC, Su W, Xie D, Turk J, Guo Z. Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. J Biol Chem 285: 8628–8638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xie Z, Su W, Guo Z, Pang H, Post SR, Gong MC. Up-regulation of CPI-17 phosphorylation in diabetic vasculature and high glucose cultured vascular smooth muscle cells. Cardiovasc Res 69: 491–501, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Zeidan A, Javadov S, Chakrabarti S, Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc Res 77: 64–72, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Zhen J, Lu H, Wang XQ, Vaziri ND, Zhou XJ. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am J Hypertens 21: 28–34, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Zhou H, Li YJ, Wang M, Zhang LH, Guo BY, Zhao ZS, Meng FL, Deng YG, Wang RY. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol Sin 32: 999–1008, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu LH, Wang L, Wang D, Jiang H, Tang QZ, Yan L, Bian ZY, Wang XA, Li H. Puerarin attenuates high-glucose-and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free Radic Biol Med 48: 471–482, 2010 [DOI] [PubMed] [Google Scholar]