Abstract
Forty-eight hours of water deprivation (WD) in conscious rats results in a paradoxical increase in mean arterial pressure (MAP). Previous studies suggest this may be due to increased sympathetic nerve activity (SNA). However, this remains to be investigated in conscious, freely behaving animals. The purpose of this study was to determine, in conscious rats, the role of the sympathetic nervous system (SNS) in mediating WD-induced increases in MAP and to identify which vascular beds are targeted by increased SNA. Each rat was chronically instrumented with a radiotelemetry transmitter to measure MAP and heart rate (HR) and an indwelling venous catheter for plasma sampling and/or drug delivery. MAP and HR were continuously measured during a 2-day baseline period followed by 48 h of WD and then a recovery period. By the end of the WD period, MAP increased by ∼15 mmHg in control groups, whereas HR did not change significantly. Chronic blockade of α1/β1-adrenergic receptors significantly attenuated the WD-induced increase in MAP, suggesting a role for global activation of the SNS. However, the MAP response to WD was unaffected by selective denervations of the hindlimb, renal, or splanchnic vascular beds, or by adrenal demedullation. In contrast, complete adrenalectomy (with corticosterone and aldosterone replaced) significantly attenuated the MAP response to WD in the same time frame as α1/β1-adrenergic receptor blockade. These results suggest that, in conscious water-deprived rats, the SNS contributes to the MAP response and may be linked to release of adrenocortical hormones. Finally, this sympathetically mediated response is not dependent on increased SNA to one specific vascular bed.
Keywords: osmolality, sympathetic nerve activity, water deprivation, denervation, adrenal cortex
water deprivation (WD) over long periods of time increases plasma osmolality and decreases blood volume. Despite the hypovolemia induced by WD, it has been reported that mean arterial pressure (MAP) is not only maintained but actually increases in conscious rats (4, 8, 46). The mechanisms mediating this paradoxical increase in MAP are not clear, but previous studies have shown that WD is accompanied by elevations in the renin-angiotensin-aldosterone system (4, 14, 16), plasma vasopressin and corticosterone (14, 17, 32), and sympathetic nerve activity (SNA) (10, 53, 55, 56). In this study, we investigated the sympathetic component of the MAP response to WD.
Although it is generally accepted that the sympathetic nervous system is activated during WD to support MAP, the evidence supporting this is not entirely conclusive. Studies in which plasma norepinephrine was measured as an indicator of global sympathetic activity have been inconsistent, with some showing no change (4, 20, 32) and others showing increased levels (37) during WD. Furthermore, the role of the sympathetic nervous system has not been investigated in the context of increasing MAP during WD, particularly in conscious rats. Stocker et al. (55, 56) reported that ganglionic blockade caused a greater fall in MAP in anesthetized water-deprived rats than in euhydrated controls, suggesting a greater sympathetic contribution to MAP during WD. However, this has not been confirmed in conscious rats. Moreover, because the paradoxical increase in MAP during WD is not generally present in anesthetized rats (22, 37, 55, 56), results of anesthetized experiments may not explain the mechanism of elevated MAP in conscious WD rats. To our knowledge, the contribution of global sympathetic contribution to the WD-induced increase in MAP in conscious rats has not been studied.
The question of whether WD results in sympathoexcitation is complicated by the fact that the sympathetic nervous system does not act as a single entity that is either increased or decreased. Rather, it is now clear that sympathetic activity is differentially controlled such that SNA may be increased to some vascular beds and decreased to others (39). Moreover, the precise pattern of SNA to various targets is state specific in that different stimuli (e.g., hypotension or hypoglycemia) generate different sympathetic signatures (38, 39, 43). Although the sympathetic signature associated with WD has not been specifically investigated, direct recordings of SNA to specific vascular beds during WD have been reported. Sympathetic nerve recordings in anesthetized, water-deprived rats suggest that lumbar SNA (lSNA), specifically, is increased. For example, lSNA, expressed as the percentage of maximum baroreflex curve, is elevated during WD (53), but renal SNA (rSNA) is not (54). Another study in anesthetized rats reported that inhibition of the paraventricular nucleus (PVN) caused a greater fall in lSNA than in rSNA during WD, whereas PVN blockade in euhydrated rats had no effect on either lSNA or rSNA (55). This suggests that PVN support of lSNA and rSNA is (differentially) enhanced during WD. Additionally, Brooks et al. (11) found that lSNA decreases when plasma osmolality is normalized in WD rats, suggesting that lSNA during WD is driven by osmotically sensitive brain regions (53). Finally, the mesenteric vascular bed and the adrenal gland could also be important sympathetic targets. The splanchnic sympathetic nerves are known to exert a powerful influence on both the distribution of blood volume and total vascular resistance (31); adrenal hormones could affect the MAP response to WD through central or peripheral adrenosteroid actions, for example (24, 27, 29, 50). Taken together, these studies suggest that differential sympathetic activation occurs during WD and provide rationale for selectively investigating the roles of individual sympathetic targets in mediating the WD-induced pressor response.
The objective of the present study was to determine the role of global and regional sympathetic activity in mediating the increase in MAP induced by WD in conscious rats. MAP was measured continuously in unrestrained rats under control conditions for 2 days, followed by 48 h of WD and then a recovery period. The contribution of global sympathetic activity to the MAP response during WD was established by chronic adrenoreceptor blockade. The contribution of organ/region-specific changes in SNA to WD-induced changes in MAP was determined by selective denervation of specific targets. Finally, we also investigated the effects of bilateral adrenal demedullation or complete adrenalectomies (with corticosterone and aldosterone replacement) on the control of MAP during WD.
MATERIALS AND METHODS
Animals and General Procedures
Male Sprague-Dawley rats from Charles River Laboratory (Wilmington, MA) were singly housed in a temperature-controlled animal room with a 12-h:12-h light/dark cycle (lights on at 0800 h). The rats ate normal rat chow (Lab Diet 5012) and drank deionized water. All protocols in this study were approved by the Institutional Animal Care and Use Committee.
Rats were implanted with a radiotelemetry transmitter (model TA11PA-C40; Data Sciences International, St. Paul, MN) for continuous monitoring of MAP and heart rate (HR) and an intravenous catheter for blood sampling and/or systemic drug delivery. The transmitter catheter was implanted into the descending aorta via the femoral artery. The intravenous catheter was advanced to the abdominal vena cava via the femoral vein, tunneled through a spring that was attached to the skin between the scapulae, and attached to a swivel above the cage. These procedures were described previously in greater detail (62).
General Experimental Protocol
Rats were conscious and freely moving in their home cages for the duration of the study. Each cage was placed on a receiver (model RPC1) that was connected to a computer via a Data Exchange Matrix (DSI; St. Paul, MN). MAP and HR data were collected at 500 Hz over 10 s every 4 min from the beginning of the protocol to the time of euthanasia.
All rats recovered from surgery for at least 5 days before beginning the experimental protocol; during the first 3 days of recovery, rats received amoxicillin in their drinking water (1.0 mg/ml). After the surgical recovery period, 2 days of baseline data were collected. The following day, water bottles were removed at 1200 h for 48 h. Five days after WD, rats were euthanized with isoflurane. Water intake was monitored, and rats were weighed regularly throughout the protocol.
Plasma samples were obtained on day 2 of baseline and at 24 and 48 h of WD. At 1200 h, 0.2 ml of blood was collected into heparinized syringes and stored on ice. The sampled volume was replaced with 0.2 ml isotonic saline. The blood samples were centrifuged at 4°C at 5,000 rpm for 10 min, and the plasma was collected for measurement of osmolality in triplicate using a freezing-point depression micro-osmometer (Advanced Instruments model 3320; Norwood, MA).
In the denervation experiments, tissues were harvested at the time of euthanasia for measurement of norepinephrine content to assess completeness of region/organ-specific denervation. Select organs were flash frozen in liquid nitrogen and then stored at −80°C. Upon completion of the entire study, tissues were sent to Core Assay Laboratory at Michigan State University for measurement of norepinephrine content using high-performance liquid chromatography (31).
Specific Experimental Protocols
Effect of chronic sympathetic blockade on the pressor response to WD (experiment 1).
The purpose of this protocol was to verify that the increase in MAP during WD is associated with an increase in global sympathetic activity. On the day of surgery, rats (350–400 g) were divided into two groups (vehicle and blocked) and were started on intravenous infusions of 0.9% saline vehicle or a combination of an α1-adrenergic receptor antagonist (terazosin hydrochloride; 1.2 μg/24 h; Sigma-Aldrich, St. Louis, MO) and β1-adrenergic receptor antagonist (atenolol; 24 mg/24 h; Sigma-Aldrich). Drugs and saline vehicle were continuously infused using Harvard syringe pumps with the speed adjusted to deliver ∼4 ml/day. The efficacy of α1/β1-blockade was assessed by intravenous injections of the α1-agonist phenylephrine (3.5 μg; Baxter Healthcare, Deerfield, IL) and the β1-agonist isoproterenol hydrochloride (0.7 μg; Sigma-Aldrich) on day 2 of baseline and on the day before euthanasia. Agonists were dissolved in 0.35 ml of 0.9% saline, and injections were followed by a 0.2 ml saline flush. At least an hour was allowed between the two injections.
Effect of organ/regional sympathectomy on the pressor response to WD (experiments 2–4).
These experiments were designed to establish the role of regional sympathetic activity in mediating the increase in MAP induced by WD. In these experiments, bilateral regional denervation, or a sham denervation, was performed at the time of transmitter implantation (described above).
EXPERIMENT 2: LUMBAR SYMPATHETIC DENERVATION.
A midline incision was made, and the intestines were retracted with gauze to expose the aorta below the level of the renal artery. The aorta and vena cava were gently retracted with suture to expose the lumbar sympathetic chain. For the lumbar sympathetic denervation (LDNx) group, the chain was dissected from L2 to the aortic bifurcation. Small nerve fibers on the surface of the aorta and vena cava were also removed. Sham surgery involved exposing, but not sectioning, the nerves.
EXPERIMENT 3: RENAL DENERVATION.
A midline incision was made and the intestines were retracted with gauze to expose the renal artery and vein. In the renal denervation (RDNx) group, all visible nerves from the aorta to the renal bifurcation were dissected from the artery using a microscope. The artery and vein were then brushed with a 10% phenol solution. Sham surgery involved exposing the renal vessels, but nerves were not sectioned and phenol was not applied.
EXPERIMENT 4: CELIAC GANGLIONECTOMY.
Celiac ganglionectomy (CGx) was performed via a midline abdominal incision to denervate the splanchnic vascular bed. The celiac plexus was removed, and all visible nerves were removed from the nearby aorta, celiac artery, and superior mesenteric artery (31). For the sham procedure, the celiac plexus was visualized by making a midline abdominal incision and retracting the intestines, but nerves were not sectioned.
Effect of adrenal demedullation on the pressor response to WD (experiment 5).
The aim of this experiment was to determine the contribution of adrenal medullary catecholamines to the regulation of MAP during WD. At the time of transmitter implantation (described above), the adrenals were exposed via a midline abdominal incision. A tiny incision was made on the top of each adrenal using a No. 11 scalpel blade, and forceps were used to gently extrude the adrenal medulla. For the sham procedure, the adrenal glands were visualized. Rats in this experiment were given 3 wk to recover from surgery to allow the damaged adrenal cortex to regenerate (61).
Effect of total adrenalectomy on the pressor response to WD (experiment 6).
The purpose of this protocol, in conjunction with the adrenal demedullation study above, was to determine whether hormones secreted from the adrenal cortex, such as aldosterone or corticosterone, contribute to the pressor response to WD. On the day of surgery, rats were subjected to either total adrenalectomy (ADx) or sham surgery via a midline incision. To maintain steady, near-physiological hormone levels, ADx rats received subcutaneous aldosterone (8 μg/day; Steraloids, Newport, RI) at a constant rate via an osmotic minipump (No. 2002; Alzet, Palo Alto, CA) and cholesterol pellets (Sigma-Aldrich) containing 50% corticosterone (75 mg; Roussel Uclaf, Romaineville, France). Doses were based on a previous protocol, which resulted in plasma concentrations in the physiological range (63). Sham rats received vehicle (propylene glycol; Sigma-Aldrich) in the minipump and cholesterol-only pellets.
In this study, 0.4 ml of blood was obtained during the sampling at 1200 h on day 2 of baseline and at 48 h of WD. Because the volume of blood sampled was twice that of the other experiments in this study, the 24-h plasma sample was omitted in this experiment. In addition to the osmolality measurements, some of the plasma was aliquotted and frozen for later analysis of plasma corticosterone and adrenocorticotropic hormone (ACTH) (28, 63). At the end of the experiment, body and thymus weights were also collected as physiological indicators of adequate corticosterone replacement (3).
Data Analysis and Statistics
Telemetry data were acquired and analyzed with Dataquest A.R.T. 4.0 software (DSI; St. Paul, MN). Two-hour averages of MAP and HR were plotted as means ± SE. SigmaStat software (3.5; San Jose, CA) was used to identify statistical significance within experiments. The data plotted over time were analyzed using a two-way ANOVA for repeated measures over time, followed by the Holm-Sidak post hoc test. Sham groups were used as the control between groups, and the t = 0 time point was used as the control within groups.
To summarize the effects of WD, changes in MAP and HR were grouped by lights-on/lights-off (light/dark) phases and plotted as bar graphs. Values were averaged over the last 4 h of the dark phase (t = −8 to −5) and the first 4 h of the light phase (t = −4 to −1) at the end of the control period, and these were compared with the last 4 h of the dark phase (t = 40 to 43) and first 4 h of the light phase (t = 44 to 47) on the last day of WD. These data were analyzed using a two-way ANOVA and the Holm-Sidak post hoc test, with the light phase and baseline period data set as the controls between and within groups, respectively.
One-way ANOVAs on ranks were used to detect changes in plasma osmolality during WD, and the Student's t-test was used to compare the norepinephrine data between sham and experimental groups. Results of the corticosterone and ACTH assays were analyzed using two-way ANOVAs and the Holm-Sidak post hoc test. For all comparisons, a P value of <0.05 was defined as statistically significant.
RESULTS
General Responses of All Study Groups
Baseline plasma osmolality was similar between all groups (P = 0.099) averaging 298 ± 1 mOsm/kg (Table 1). None of the experimental groups showed statistically significant differences in plasma osmolality from their sham/vehicle control groups at any time point measured. Plasma osmolality values were significantly increased by an average of 7 ± 1 mOsm/kg after 24 of WD and by 13 ± 1 mOsm/kg by the end of 48 h of WD.
Table 1.
Plasma osmolality measurements during baseline and 24- and 48-h WD
| WD |
||||
|---|---|---|---|---|
| Experiment No. and Group | n | Baseline | 24 h | 48 h |
| 1 | ||||
| Vehicle | 6 | 296 ± 3 | 300 ± 1 | 306 ± 1# |
| Block | 5 | 295 ± 1 | 302 ± 2^ | 308 ± 1^ |
| 2 | ||||
| Sham | 3 | 298 ± 2 | 306 ± 2# | 312 ± 1# |
| LDNx | 5 | 299 ± 1 | 307 ± 1^ | 314 ± 2^ |
| 3 | ||||
| Sham | 4 | 304 ± 1 | 307 ± 2 | 316 ± 2# |
| RDNx | 5 | 301 ± 2 | 311 ± 1^ | 314 ± 2^ |
| 4 | ||||
| Sham | 5 | 300 ± 1 | 308 ± 2# | 314 ± 1# |
| CGx | 5 | 299 ± 2 | 309 ± 1^ | 317 ± 2^ |
| 5 | ||||
| Sham | 2 | 296 ± 2 | 301 ± 1# | 304 ± 1# |
| ADMx | 2 | 296 ± 1 | 304 ± 1^ | 308 ± 2^ |
| 6 | ||||
| Sham | 9 | 298 ± 2 | NA | 312 ± 2# |
| ADx | 8 | 298 ± 1 | NA | 312 ± 3^ |
Values are reported as means ± SE (in mOsm/kg); n is reported because the plasma sample size in some groups was reduced due to inability to obtain blood sample from the intravenous catheter. Blood samples for plasma osmolality measurements were obtained at 1200 h on day 2 of the baseline period and after 24 and 48 h of water deprivation (WD). The 24-h sample was omitted in experiment 6 due to the larger volume of blood sampled over the duration of that protocol. ^P < 0.05 vs. light period within experimental groups; #P < 0.05 vs. light period within vehicle/sham groups.
LDNx, lumbar sympathetic denervation; RDNx, renal denervation; CGx, celiac ganglionectomy; ADMx, adrenal demedullation; ADx, total adrenalectomy; NA, not applicable.
WD for 48 h resulted in a 10–15% decrease in body weight. Sympathetically blocked rats lost the least amount of body weight (11 ± 1%), which was significantly less than the two groups with greatest weight loss (ADx, 14 ± 2%; and Sham ADx, 15 ± 1%). There were no differences between any other groups, and there were no differences in weight loss between the experimental groups and their sham/vehicle control groups. Additionally, there were no between-group differences in water intake during the 2 days of control (44 ± 1 ml/day) or during the recovery from WD (51 ± 1 ml/day).
Effect of Chronic Sympathetic Blockade on the Pressor Response to WD
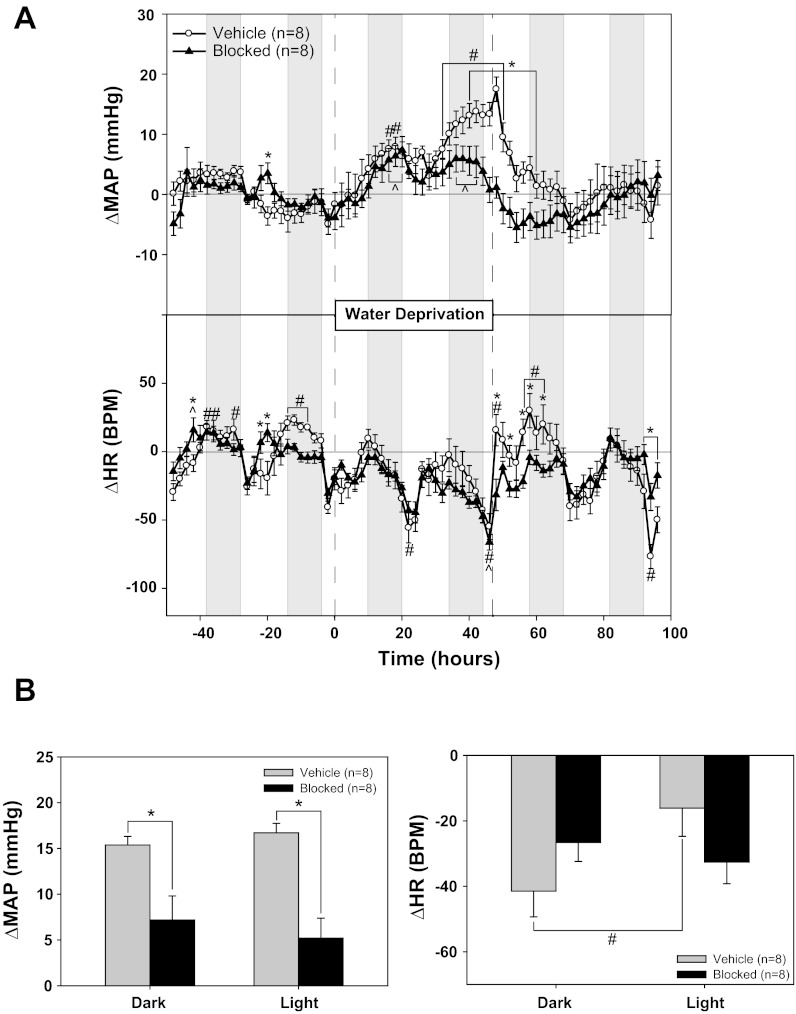
Table 2 shows 4-h averages of baseline MAP and HR during the light and dark phases (as described in materials and methods) in vehicle and α1/β1-blocked rats (experiment No. 1). Chronic α1/β1 blockade significantly lowered baseline MAP and HR during both light and dark phases. Shown in Fig. 1A are the changes from baseline for MAP and HR plotted as 2-h averages during the last 48 h of baseline, 48 h of WD, and 48 h of recovery. In vehicle rats, MAP increased ∼5 mmHg above baseline after 24 h of WD and ∼15 mmHg by the end of the 48-h WD period. In contrast, HR tended to decrease during WD but did not achieve statistical significance. More importantly, although α1/β1-blockade had no effect on the MAP response during the first 24 h of WD, by the second day of WD, the blocked rats had significantly lower MAP levels than vehicle rats. In fact, during the final light-phase hours of WD, MAP levels in α1/β1-blocked rats had returned to values similar to those of baseline (Fig. 1A). HR response to WD was unaffected by α1/β1-blockade.
Table 2.
Baseline MAP and HR values for sham and experimental groups from each experiment
| Mean Arterial Pressure |
Heart Rate |
|||
|---|---|---|---|---|
| Experiment No. and Group | Dark | Light | Dark | Light |
| 1 | ||||
| Vehicle | 99 ± 3 | 97 ± 2 | 425 ± 9# | 385 ± 8 |
| Block | 90 ± 2* | 88 ± 2* | 330 ± 4* | 318 ± 4* |
| 2 | ||||
| Sham | 103 ± 2 | 99 ± 1 | 448 ± 5# | 408 ± 5 |
| LDNx | 99 ± 2 | 97 ± 2 | 445 ± 9^ | 421 ± 7 |
| 3 | ||||
| Sham | 103 ± 5 | 101 ± 3 | 425 ± 15 | 401 ± 4 |
| RDNx | 95 ± 2 | 92 ± 2 | 425 ± 11 | 396 ± 12 |
| 4 | ||||
| Sham | 100 ± 3 | 98 ± 3 | 446 ± 12# | 403 ± 19 |
| CGx | 89 ± 4* | 85 ± 4* | 427 ± 13 | 396 ± 17 |
| 5 | ||||
| Sham | 110 ± 5 | 103 ± 2 | 396 ± 6# | 337 ± 3 |
| ADMx | 100 ± 2*^ | 93 ± 2* | 379 ± 11^ | 335 ± 10 |
| 6 | ||||
| Sham | 103 ± 1 | 100 ± 1 | 445 ± 4# | 412 ± 6 |
| ADx | 103 ± 2 | 100 ± 2 | 447 ± 5^ | 425 ± 8 |
Values are means ± SE. Four hours of baseline data from the dark and light phases were averaged as described in materials and methods.
P < 0.05 vs. vehicle/sham; ^P < 0.05 vs. light period within experimental groups; #P < 0.05 vs. light period within vehicle/sham groups.
Fig. 1.
Effect of chronic sympathetic blockade on the pressor response to water deprivation. A: mean arterial pressure (MAP) and heart rate (HR) responses to 48 h of water deprivation with continuous infusion of either saline vehicle or a combination of α1- and β1- adrenergic receptor antagonists. B: MAP and HR changes from baseline: 4-h averages from the light and dark phases at the end of water deprivation compared with the equivalent baseline averages. *P < 0.05 between groups; #P < 0.05 within vehicle; ^P < 0.05 within blocked. BPM, beats/min.
Figure 1B summarizes the responses to WD in both groups. WD increased MAP in the vehicle group by 15 ± 1 and 17 ± 1 mmHg during the dark and light phases, respectively. The MAP response to WD was significantly less in α1/β1-blocked rats, with increases of 7 ± 3 (dark phase) and 5 ± 2 (light phase) mmHg. The HR response to WD was significantly different between light and dark phases in the vehicle group, but there was no difference in HR response between the vehicle and blocked rats.
During both control and recovery periods, bolus intravenous injections of phenylephrine and isoproterenol were given to confirm successful α1/β1-blockade. The average MAP response to phenylephrine was 22 ± 3 mmHg in vehicle rats. This response was abolished in α1/β1-blocked rats (2 ± 3 mmHg). Similarly, the HR response to isoproterenol was markedly reduced in α1/β1-blocked rats (26 ± 7 beats/min) compared with vehicle rats (92 ± 12 beats/min). Because isoproterenol also caused a fall in MAP (−25 ± 6 mmHg in α1/β1-blocked rats and −23 ± 3 mmHg in vehicle rats), it is possible that the remaining HR response in α1/β1-blocked rats was due to vagal inhibition. Therefore, in several rats, we also examined the response to isoproterenol after vagal blockade. The attenuation of the HR response to isoproterenol in α1/β1-blocked rats was even greater with atropine pretreatment (14 ± 8 beats/min compared with 93 ± 11 beats/min in vehicle rats).
Effect of Organ/Regional Sympathectomy on the Pressor Response to WD
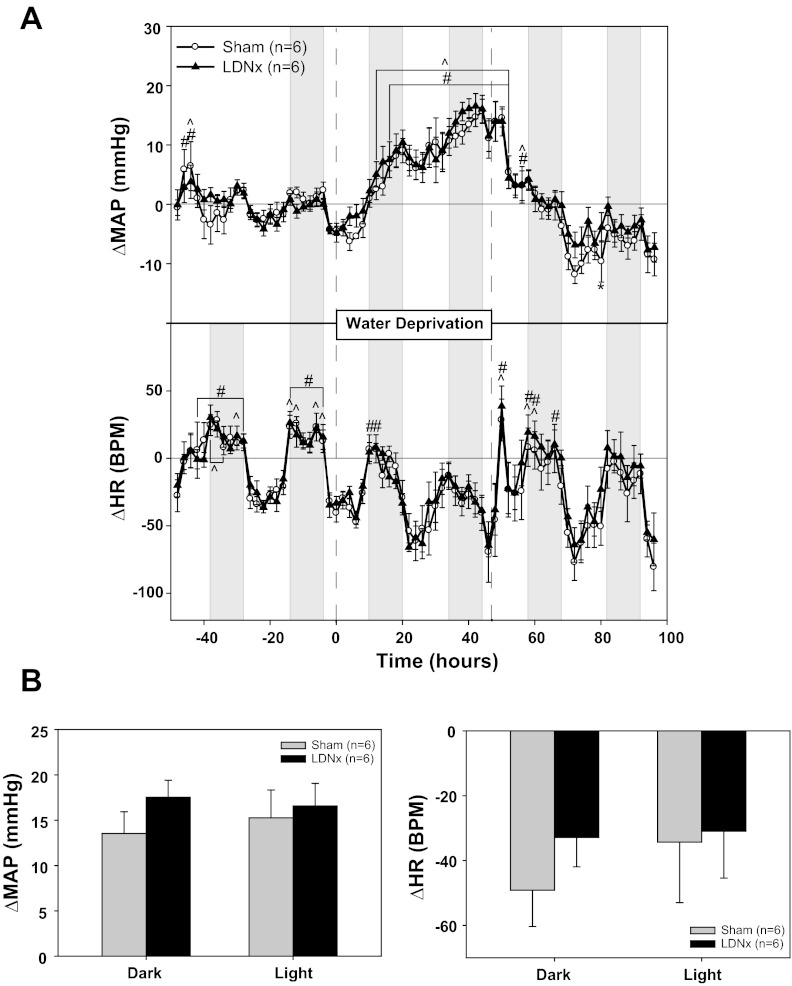
Lumbar sympathectomy (LDNx) had no effect on baseline MAP or HR (Table 2, experiment No. 2). It also had no effect on the MAP or HR response to WD (Fig. 2A). WD increased MAP in the sham group by 14 ± 2 mmHg during the dark phase and by 15 ± 3 mmHg during the light phase, whereas the respective changes in the lumbar-denervated rats were 18 ± 2 and 17 ± 2 mmHg (Fig. 2B). WD had no significant effect on HR in LDNx or sham rats (Fig. 2, A and B). Norepinephrine content was significantly decreased by 87% (left) and 68% (right) in the soleus muscles of LDNx rats compared with sham rats (Table 3, experiment No. 2). LDNx had no effect on norepinephrine levels in the kidney or spleen (data not shown).
Fig. 2.
Effect of lumbar denervation (LDNx) on the pressor response to water deprivation. A: MAP and HR responses to 48 h of water deprivation in rats with (sham) or without LDNx lumbar nerves. B: MAP and HR changes from baseline: 4-h averages from the light and dark phases at the end of water deprivation compared with the equivalent baseline averages. *P < 0.05 between groups; #P < 0.05 within sham; ^P 0.05 within LDNx.
Table 3.
Tissue norepinephrine levels in denervated organs
| Denervation, Tissue, and Group | Norepinephrine, ng/g |
|---|---|
| Experiment 2—lumbar denervation | |
| Left soleus | |
| Sham | 66 ± 13 |
| LDNx | 9 ± 2* |
| Right soleus | |
| Sham | 65 ± 12 |
| LDNx | 21 ± 8* |
| Experiment 3—renal denervation | |
| Left kidney | |
| Sham | 134 ± 12 |
| RDNx | 4 ± 1* |
| Right kidney | |
| Sham | 122 ± 14 |
| RDNx | 6 ± 1* |
| Experiment 4—celiac ganglionectomy | |
| Liver | |
| Sham | 33 ± 6 |
| CGx | 6 ± 1* |
| Spleen | |
| Sham | 189 ± 34 |
| CGx | 2 ± 0.1* |
| Duodenum | |
| Sham | 455 ± 42 |
| CGx | 6 ± 2* |
| Experiment 5—adrenal demedullation | |
| Left adrenal | |
| Sham | 156,146 ± 44,059 |
| ADMx | 868 ± 611* |
| Right adrenal | |
| Sham | 116,090 ± 24051 |
| ADMx | 359 ± 136* |
Values are means ± SE. Norepinephrine content (in ng/g tissue) was an indicator of successful denervation. Tissue was analyzed from the soleus muscles in the hindlimb to assess lumbar denervation; the kidneys to assess renal denervation; the spleen, liver, and duodenum to assess celiac ganglionectomy; and the adrenal glands to assess adrenal demedullation.
P < 0.05 vs. sham group.
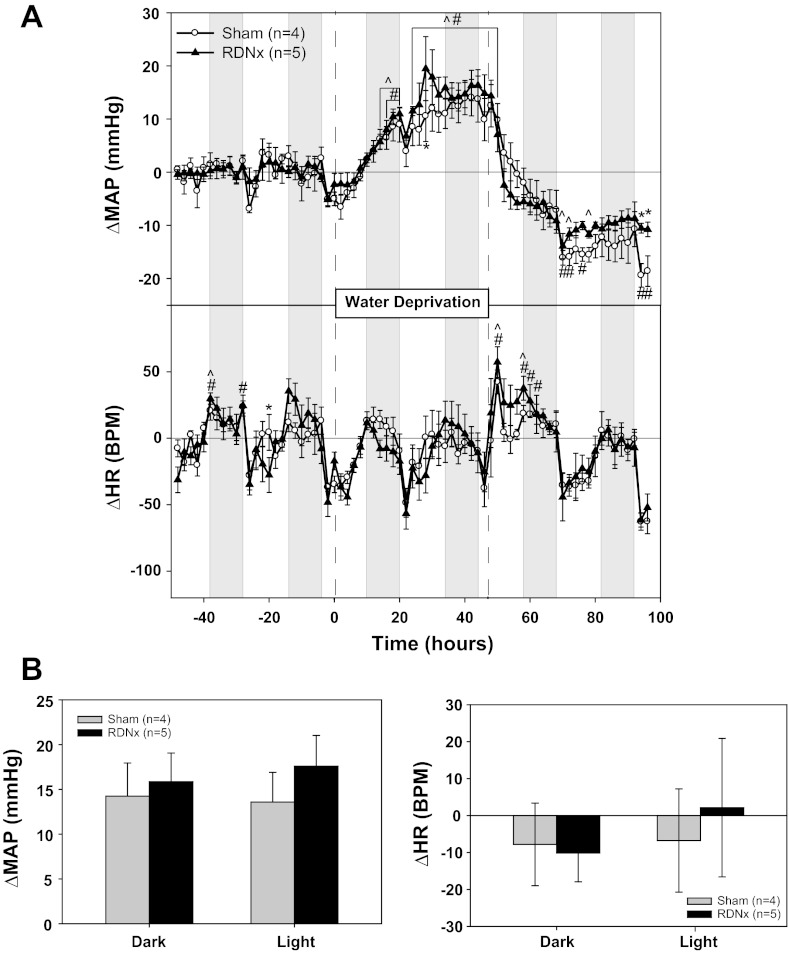
There was a statistically significant treatment effect of bilateral RDNx on baseline MAP (P = 0.0145 for sham vs. RDNx), but this effect did not achieve significance when analyzed further between the dark and light phases, as reported in Table 2 (experiment No. 3). More importantly, RDNx had no effect on the MAP or HR response to WD (Fig. 3A). WD increased MAP in the sham group by 14 ± 4 mmHg during the dark phase and by 14 ± 3 mmHg during the light phase, whereas the respective changes in the RDNx rats were 16 ± 3 and 18 ± 3 mmHg (Fig. 3B). WD had no significant effect on HR in either group. Renal norepinephrine content was decreased by 97% (left) and 95% (right) in the kidneys of RDNx rats compared with those of sham rats (Table 3, experiment No. 3).
Fig. 3.
Effect of renal denervation (RDNx) on the pressor response to water deprivation. A: MAP and HR responses to 48 h of water deprivation in rats with (sham) or without (RDNx) renal nerves. B: MAP and HR changes from baseline: 4-h averages from the light and dark phases at the end of water deprivation compared with the equivalent baseline averages. *P < 0.05 between groups; #P < 0.05 within sham; ^P 0.05 within RDNx.
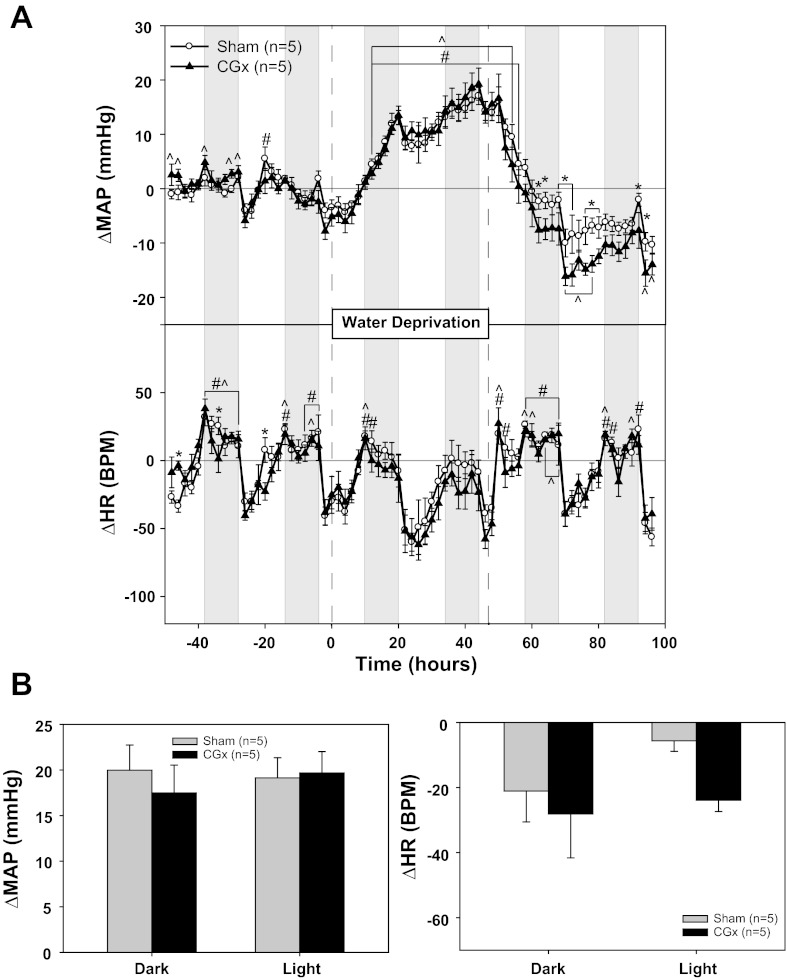
Baseline MAP was significantly lower in rats subjected to celiac ganglionectomy (CGx) compared with sham rats (Table 2, experiment No. 4). Although the CGx rats tended to have a slightly smaller increase in MAP than sham rats at some points during WD, these effects were not significant (Fig. 4A). There were, however, several points during the recovery from WD in that CGx rats had lower MAP compared with baseline than sham rats (Fig. 4A). Two days of WD caused an average increase in MAP of 20 ± 3 mmHg during the dark phase and 19 ± 2 mmHg during the light phase in the sham group, whereas the respective changes in the CGx rats were 17 ± 3 and 20 ± 2 mmHg (Fig. 4B). WD had no significant effect on HR in either group. Norepinephrine content was decreased in the liver (82%), spleen (99%), and duodenum (99%) compared with sham rats (Table 3, experiment No. 4).
Fig. 4.
Effect of celiac ganglionectomy (CGx) on the pressor response to water deprivation. A: MAP and HR responses to 48 h of water deprivation in rats with (sham) or without (CGx) splanchnic nerves. B: MAP and HR changes from baseline: 4-h averages from the light and dark phases at the end of water deprivation compared with the equivalent baseline averages. *P < 0.05 between groups; #P < 0.05 within sham; ^P 0.05 within CGx.
Effect of Adrenal Demedullation on the Pressor Response to WD
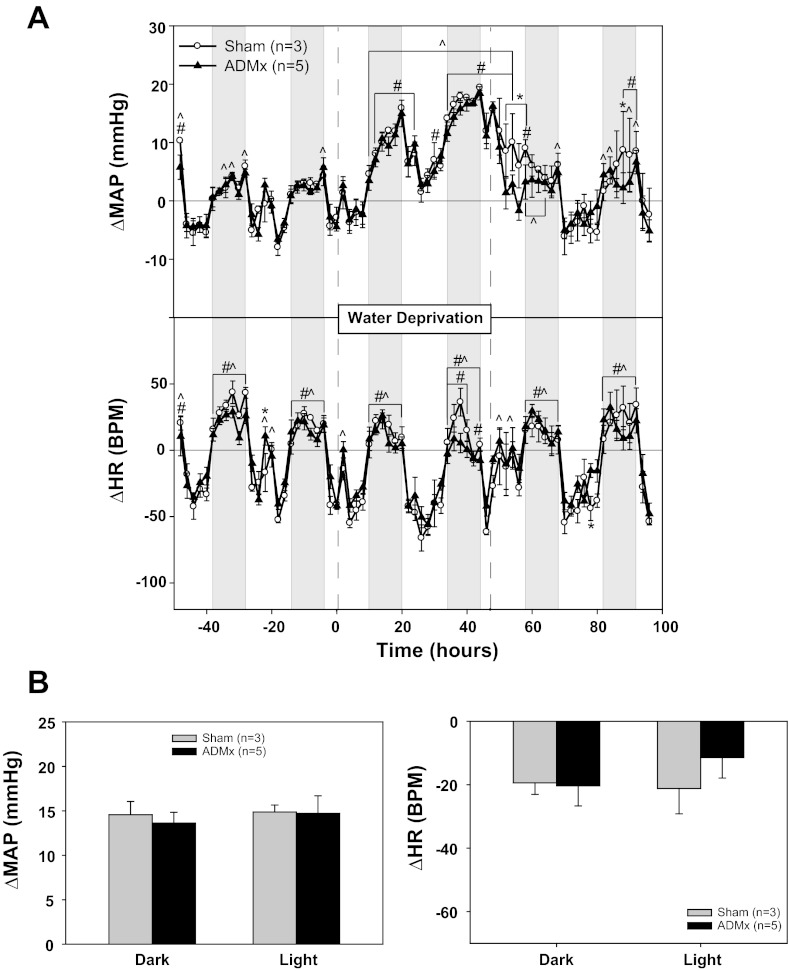
Baseline MAP was significantly lower in adrenal demedullated (ADMx) rats compared with sham controls (Table 2, experiment No. 5). However, it is important to note that the MAP values in experiment No. 5 sham rats were greater than in other experiments, at least during the dark period. ADMx had no effect on the MAP or HR response to WD, with the exception of several points during the recovery period (Fig. 5A). The average increase in MAP during 48 h of WD in the sham group was 15 ± 1 mmHg during the dark phase and 15 ± 1 mmHg during the light phase, whereas the respective changes in the ADMx rats were 14 ± 1 and 15 ± 2 mmHg (Fig. 5B). WD had no significant effect on HR in either group.
Fig. 5.
Effect of adrenal demedullation (ADMx) on the pressor response to water deprivation. A: MAP and HR responses to 48 h of water deprivation in rats with (sham) or without (ADMx) adrenal medullas. B: MAP and HR changes from baseline: 4-h averages from the light and dark phases at the end of water deprivation compared with the equivalent baseline averages. *P < 0.05 between groups; #P < 0.05 within sham; ^P 0.05 within ADMx.
Effect of Adrenalectomy on the Pressor Response to WD
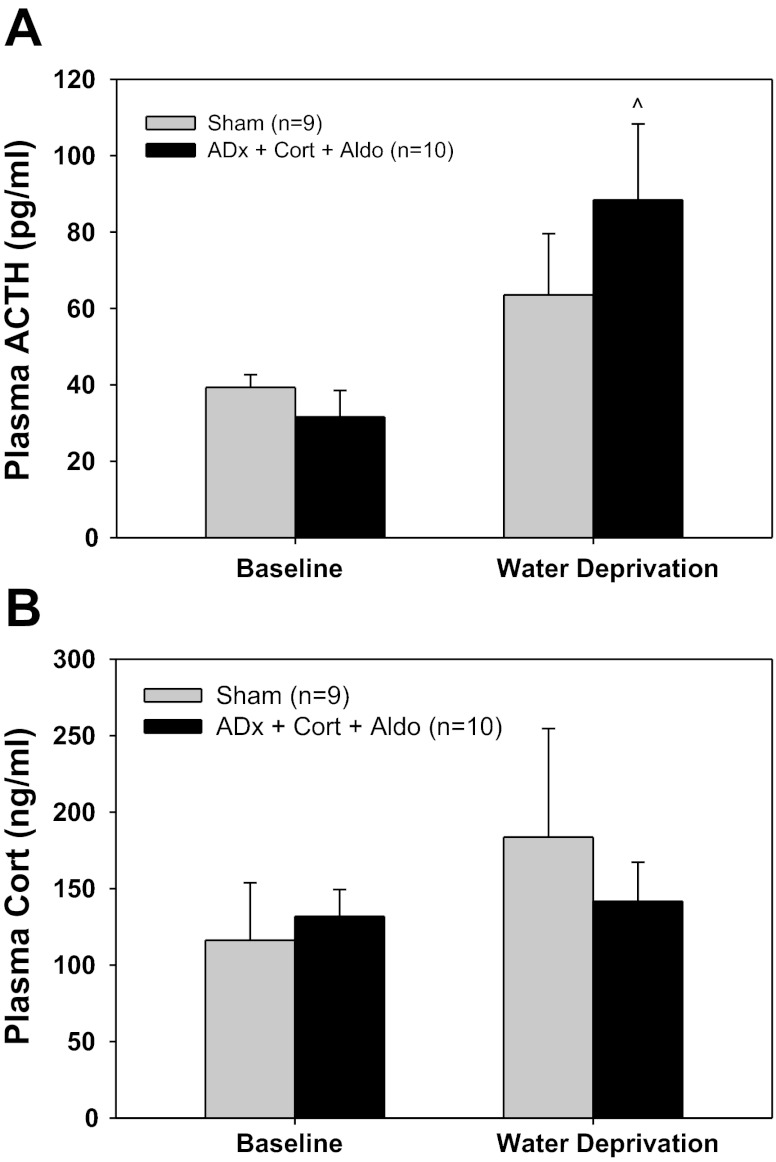
Bilateral adrenalectomy with exogenous corticosterone and aldosterone (ADx + Cort + Aldo) had no effect on baseline MAP or HR (Table 2, experiment No. 6). Similarly, there were no significant differences in baseline plasma ACTH or corticosterone concentrations between ADx + Cort + Aldo and sham rats (Fig. 6). WD increased plasma ACTH levels in both groups, but this increase was statistically significant only in ADx + Cort + Aldo rats (Fig. 6A). Plasma corticosterone tended to increase in response to WD in Sham rats, but this was not significant due to the variability of the response (Fig. 6B). As expected, plasma corticosterone remained at baseline levels during WD in ADx + Cort + Aldo rats (Fig. 6B). Finally, thymus weights, normalized to body weight, were similar between sham (1.48 ± 0.06 mg/g) and ADx + Cort + Aldo rats (1.61 ± 0.08 mg/g).
Fig. 6.
Plasma adrenocorticotropic hormone (ACTH) and corticosterone in sham rats and rats with total adrenalectomies with exogenous corticosterone and aldosterone (ADx + Cort + Aldo). A: plasma ACTH during baseline and water deprivation. B: plasma corticosterone during baseline and water deprivation. ^P 0.05 within ADx.
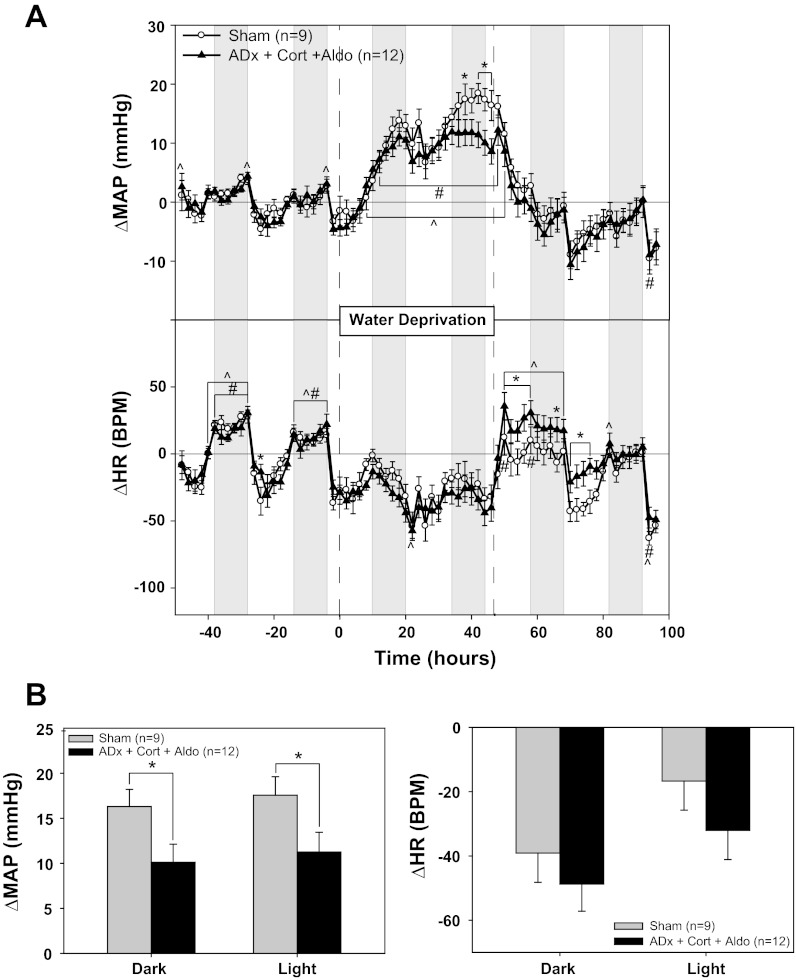
The MAP response to WD was not affected during the first 24 h of WD in the ADx + Cort + Aldo group; however, similar to the timing of the sympathetic blockade effects (Fig. 1), the pressor response was significantly attenuated beginning in the second night of WD (Fig. 7A). The average increase in MAP during WD in the sham group was 16 ± 2 mmHg during the dark phase and 18 ± 2 mmHg during the light phase, whereas the respective changes in the ADx + Cort + Aldo rats were 10 ± 2 and 11 ± 2 mmHg (Fig. 7B). HR remained unchanged from baseline in both groups.
Fig. 7.
Effect of adrenalectomy on the pressor response to water deprivation. A: MAP and HR responses to 48 h of water deprivation in rats with (sham) or without (ADx + Cort + Aldo) adrenals. Adrenalectomized rats received replacement corticosterone and aldosterone. B: MAP and HR changes from baseline: 4-h averages from the light and dark phases at the end of water deprivation compared with the equivalent baseline averages. *P < 0.05 between groups; #P < 0.05 within sham; ^P 0.05 within ADx + Cort + Aldo.
DISCUSSION
During WD, baroreceptor activation and increased plasma osmolality initiate neural and hormonal responses, which maintain perfusion of vital tissues such as the heart and brain in the face of hypovolemia. Studies in conscious rats have revealed that arterial pressure is not merely maintained during WD, but actually increases above baseline levels (4, 8, 46). We hypothesize that this paradoxical hypertensive response may be mediated by sympathoexcitation secondary to activation of osmosensitive pathways in the brain (for reviews, see Refs. 7, 9, 15, and 57–59). Indeed, it has been proposed that a detailed understanding of the mechanisms coupling plasma osmolality to sympathetic activity during WD may translate to understanding mechanisms linking dietary salt and neurogenic hypertension (11, 57).
Therefore, the objective of the present study was to investigate the role of global and regional sympathetic activity in regulating arterial pressure in conscious water-deprived rats. The approach was similar to our recent characterization of the sympathetic signature of ANG II-salt model of hypertension in the rat (43). Our results confirmed that the pressor response on the second day of 48-h WD is dependent on increased global sympathetic activity; however, the pressor response during the first 24-h period of WD appears to rely on nonsympathetic mechanisms. Additionally, unlike the ANG II-salt model in which sympathetic activity to a single vascular bed (i.e., splanchnic) was predominate, we did not find such evidence for the pressor response to WD. Finally, an unexpected finding of this study was that increased sympathetic activity during WD in conscious rats may be linked to release of adrenal cortical hormones.
Role of Global Sympathetic Activity in the Regulation of Arterial Pressure During WD
To examine global SNA, we compared the arterial pressure and HR responses to 48 h of WD between control rats and rats chronically treated with the α1- and β1- adrenergic antagonists terazosin and atenolol, respectively. In agreement with previous studies (8, 46), 48 h of WD resulted in an arterial pressure increase of ∼15 mmHg above baseline in the control group. Sympathetic blockade with α1/β1-antagonists significantly attenuated this pressor response to the extent that arterial pressure had returned to normal levels on the last day of WD. This implies that the MAP response to WD depends, in part, on sympathetic activity, particularly on the second day of WD. This finding is in agreement with a previous study employing acute ganglionic blockade in anesthetized rats (56); however, it is important to note that arterial pressure was not different between water-deprived and euhydrated rats in the presence of anesthesia in that study.
In addition to clearly summarizing the peak MAP and HR responses to WD, the data were plotted as bar graphs to assess whether the responses might be specific to one phase of the light/dark cycle. The MAP responses to WD were similar between dark and light phases for all groups in all experiments. However, the HR response to WD was significantly greater during the dark phase compared with the light phase in vehicle rats in experiment No. 1. The same trend can be seen in the sham rats in experiments Nos. 2, 4, and 6, although these data did not achieve significance. Taken together, the majority of the experiments suggest that HR decreases with WD, and this bradycardia may be greater during the dark phase compared with the light phase. In other words, the typical increase in HR during the lights-off period may be blunted in water-deprived rats; however, the importance of this observation to overall homeostasis is unclear.
Effect of Organ/Region-Specific Denervation on the Pressor Response to WD
Emerging evidence suggests that chronic activation of the sympathetic nervous system can occur in a disease-specific pattern or sympathetic signature (33). For example, in the ovine pacing-induced model of heart failure, sympathetic activity to the heart is increased, but rSNA is normal (44). This sympathetic signature appears to be similar in humans with heart failure (19). The ANG II-salt model of hypertension in the rat is characterized by increased SNA to the splanchnic vascular bed, whereas SNA to the kidneys and hindlimb is decreased or unchanged, respectively (43, 65). Additionally, the hypertension in this model is largely prevented by celiac ganglionectomy, whereas renal denervation or lumbar sympathectomy has no effect (31). Clearly, understanding disease-specific sympathetic signatures is essential for the development of targeted sympathetic-ablation therapies, which may avoid unwanted side effects associated with global sympathetic blockade. The recent success of catheter-based renal denervation to treat hypertension in humans provides convincing evidence of this approach as a novel therapeutic strategy (51).
In this study, we denervated individual vascular beds to investigate the sympathetic signature involved in the pressor response to WD. The possible peripheral targets of sympathetic outflow that could mediate the WD-induced increase in arterial pressure include the heart, kidneys, splanchnic vascular bed, skeletal muscle, and the adrenal glands. Based on our observation that HR did not increase during WD, we did not pursue selective denervation of the heart in these studies. Therefore, we focused on the effects of selective denervation of renal, splanchnic, and hindlimb vascular beds as well as adrenalectomy and adrenal demedullation on the regulation of arterial pressure during WD.
Previous studies have suggested that lSNA may increase during WD (11, 53, 55), which led to the hypothesis that LDNx would attenuate the arterial pressure response. However, there were no differences between LDNx and sham groups for either the arterial pressure or HR responses to WD. One possible explanation for this discrepancy is the method used to quantify lSNA in previous studies. Until recently, chronic recording of SNA in conscious rats over periods long enough to measure within-animal responses was unachievable. As a result, various methods have been used to normalize nerve activity to correct for differences in electrode contact with multifiber nerves and permit between-animal comparisons. For example, because lSNA during WD could not be compared with baseline, it was normalized to the plateau of the baroreflex curve (53). This method of normalization of SNA has been debated and is not resolved (12). Another possible explanation for the discrepancy between our study and those in which lSNA was measured is that previous studies were conducted within 24 h after implantation of the nerve electrode (11, 53, 55), so the lSNA may have been elevated due to postsurgical stress. A final possible explanation is that lSNA may be increased during WD as previous studies suggest, but the changes in lSNA may not quantitatively relate to arterial pressure. In other words, it is possible that the degree to which lSNA is increased during WD is not sufficient to result in increased hindlimb vascular resistance and arterial pressure.
Similar to lumbar denervation, renal denervation had no effect on the arterial pressure or HR responses to WD. This finding is in agreement with Scrogin et al. (54), who reported no elevation in rSNA, expressed as percentage of the plateau of the baroreflex curve, during WD. These findings differ from those of Blair et al. (4), who reported a decrease in the arterial pressure response to WD in renal denervated rats. In that study, rats were transferred to a recording cage and arterial pressure was recorded for a 10-min period. As a result, their protocol did not allow within-group comparisons and the acute stress induced by the method for measuring arterial pressure may have influenced the results. Stocker et al. reported that inhibition of the PVN caused a fall in both rSNA and lSNA in dehydrated rats but not in euhydrated rats (55, 56). One interpretation of these results is that both lSNA and rSNA may be elevated during WD. However, neither LDNx or RDNx affected the arterial pressure response to WD in our study.
The splanchnic sympathetic nerves have a major influence on both total vascular resistance and venous capacitance (21, 25), and mesenteric blood flow has been reported to be decreased in water-deprived rats (37). However, denervation of the splanchnic vascular bed by celiac ganglionectomy, similar to RDNx and LDNx, had no effect on the arterial pressure and HR responses to WD compared with Sham rats. To our knowledge, direct measurement of splanchnic SNA in water deprived animals has not been reported.
Role of the Adrenal Gland in the Regulation of Arterial Pressure During WD
Catecholamine-producing chromaffin cells in the adrenal medulla receive input from preganglionic nerves in segments T5-T11 of the spinal cord. These neurons travel through the greater splanchnic nerve to the suprarenal ganglion, where they either synapse onto postganglionic cells that innervate the adrenal or continue on to innervate the medulla directly (13). Removal of the celiac ganglion in the CGx experiment likely did not affect adrenal innervation, since the celiac ganglion is downstream of the suprarenal ganglion. Therefore, we examined the effect of bilateral adrenal demedullation on the arterial pressure and HR responses to WD. Similar to LDNx, RDNx, and CGx, adrenal demedullation had no effect on the cardiovascular responses to WD at any point in time during the protocol. Past studies have reported incongruent results in terms of catecholamine responses, which are in part dependent on adrenal catecholamines, during WD. Some studies found that WD increases plasma catecholamines (14, 37) while many others found no significant effect (4, 20, 32). However, as Brooks et al. (10) point out, metabolism and reuptake affect the catecholamine availability and a simple measurement of the plasma levels may be insufficient (10). Their study, which analyzed adrenal mRNA levels involved in production and reuptake of norepinephrine, suggested that WD increases adrenal activity, in part via the sympathetic nerves (10).
Although our results suggest that adrenal medullary catecholamines do not mediate the cardiovascular responses during dehydration, we also tested the hypothesis that adrenal cortical hormones play a role by examining the effect of complete bilateral adrenalectomies on the cardiovascular responses to WD. In the ADx rats, we replaced aldosterone and corticosterone at a constant rate according to past studies (63). Because the ADx rats are unable to increase production of adrenocortical hormones during WD, we reasoned that this would be an effective way to test their role in the cardiovascular response to WD. Indeed, this intervention attenuated the arterial pressure response to WD in the same time frame as chronic blockade of adrenoreceptors, suggesting a possible link between adrenocortical hormones and sympathetic activity during WD. Some evidence suggests that the adrenal cortex is innervated by sympathetic nerves that affect the release of adrenal cortical hormones (6, 18), so the effect of sympathetic blockade on the arterial pressure response to WD may be due to loss of neural control of the adrenal cortex. On the other hand, release of adrenal cortical hormones during WD could also modulate activity of the sympathetic nervous system centrally and/or peripherally. The adrenocortical hormones corticosterone (47–50, 64), aldosterone (1, 23, 24, 27, 29), and endogenous oubain (2, 5, 35, 36, 40) all have the ability to influence SNA and affect arterial pressure. Additional studies are required to determine the role of these hormones in the regulation of arterial pressure during WD.
Plasma corticosterone and ACTH were measured to assess the approximate amount of corticosterone replaced and verify that the levels were not unphysiological (aldosterone measurements were omitted due to the large blood volume required). ADx rats had plasma ACTH and corticosterone levels within physiological ranges, and thymus weights were similar to sham rats, suggesting adequate corticosterone replacement. Additionally, these measurements were an indicator of corticosterone changes in sham rats with WD. In agreement with previous studies, ACTH did not significantly increase with WD in sham rats (32, 45, 60). Although plasma corticosterone tended to increase during WD in sham rats, we did not see a statistical effect, in contrast with previous studies (32, 45, 60). This is likely due to the high variability among the plasma corticosterone measurements in our sham rats, which was likely a result of the rats being weighed 3 h before blood sampling. As expected, in ADx rats, WD did not affect corticosterone levels. However, plasma ACTH levels increased with WD in ADx rats, suggesting an attempt to increase corticosterone levels higher than the level of corticosterone replaced.
These results indicate that our corticosterone replacement levels were not abnormally high or low; nevertheless, we acknowledge that we do not have enough information to determine whether the corticosterone/aldosterone replacements affected the typical plasma renin response to WD in the present study. For example, if the dose of aldosterone was too high, basal levels of basal plasma renin activity may have been suppressed, and the response of renin to WD may have been affected. Follow-up studies are needed to examine in more detail the ways in which adrenocortical hormones affect the MAP response to WD. However, as stated previously, the similar timing of the MAP response between ADx and α1/β1-blocked rats suggests a possible link to sympathetic activation.
Effect of Organ/Region-Specific Sympathetic Blockade on Basal Levels of Arterial Pressure
Although not the goal of this study, this is the first report in which the effects of organ/region-specific blockade on arterial pressure have been systematically studied in conscious rats using continuous radiotelemetric recording. Using this approach, we have previously reported that renal denervation results in an immediate and sustained decrease in basal levels of arterial pressure of ∼10 mmHg in normotensive Sprague Dawley rats (26). That finding, which is confirmed in the present study, was surprising at the time because of the redundancy of mechanisms for regulation of arterial pressure, including sympathetic activity to other vascular beds. We extend that finding in the present study in which we observed that celiac ganglionectomy and adrenal demedullation, but not lumbar denervation, also decreased basal levels of arterial pressure on the order of ∼10 mmHg. These results suggest that basal sympathetic tone to the kidneys, splanchnic circulation, and adrenal medulla may all play an important role in the long-term control of arterial pressure under normal conditions.
Summary and Perspectives
This study demonstrates for the first time in conscious rats that increased arterial pressure during WD is dependent on increased activity of the sympathetic nervous system. Our findings suggest that this sympathetically driven pressor response is not mediated by increased activity to a specific vascular bed; however, it may be linked to adrenal cortical hormones.
It remains unclear why organ/region-selective denervation is effective in attenuating hypertension in humans (51, 52) and certain rat models of hypertension (30, 31), but no such effect was observed in water-deprived rats. One explanation might be related to the physiological concept of redundancy: that there is more than one mechanism by which a certain outcome is achieved. This is consistent with the theory of a neural set point for arterial pressure that could be altered by the changes in plasma osmolality and circulating hormones induced by WD (41, 42). This theory would predict that loss of sympathetic activity to one vascular bed would result in a compensatory increase in activity to another target to defend the arterial pressure set point. It may be that the modest increase in arterial pressure during WD relies on a more generalized increase in sympathetic activity rather than an organ-specific sympathetic signature seen in essential and experimental hypertension. Additional studies are needed to test this hypothesis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-076312 awarded to the Neurogenic Cardiovascular Diseases Consortium. B. Veitenheimer was supported by Predoctoral Neuroscience Training Grant NIH #T32 GM008471-17 and the University of Minnesota Doctoral Dissertation Fellowship. W. Engeland and M. Yoder were supported in part by funding from the National Science Foundation (IOS-1023199).
DISCLOSURES
Dr. John Osborn is a consultant for Medtronic Cardiovascular.
AUTHOR CONTRIBUTIONS
Author contributions: B.J.V. and J.W.O. conception and design of research; B.J.V. and P.A.G. performed experiments; B.J.V. analyzed data; B.J.V., W.C.E., G.D.F., and J.W.O. interpreted results of experiments; B.J.V. prepared figures; B.J.V. drafted manuscript; B.J.V., W.C.E., and J.W.O. edited and revised manuscript; B.J.V., W.C.E., G.D.F., and J.W.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marina Yoder for performing the radioimmunoassay measurements of plasma corticosterone and ACTH. We also thank Ninitha Julfiya for her instruction and advice regarding the lumbar denervation surgery, as well as Robert Burnett from the Michigan State Core Assay Laboratory for performing the norepinephrine analysis. Finally, we thank Marcos Kuroki and Jason Foss for insight and instruction on the CGx and RDNx surgeries.
REFERENCES
- 1. Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol 35: 687–694, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aileru AA, De Albuquerque A, Hamlyn JM, Manunta P, Shah JR, Hamilton MJ, Weinreich D. Synaptic plasticity in sympathetic ganglia from acquired and inherited forms of ouabain-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 281: R635–R644, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol Regul Integr Comp Physiol 249: R527–R532, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Blair ML, Woolf PD, Felten SY. Sympathetic activation cannot fully account for increased plasma renin levels during water deprivation. Am J Physiol Regul Integr Comp Physiol 272: R1197–R1203, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 302: H1031–H1049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bornstein SR, Chrousos GP. Clinical review 104: Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab 84: 1729–1736, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Brizzee BL, Harrison-Bernard L, Pretus HA, Clifton GG, Walker BR. Hemodynamic responses to vasopressinergic antagonism in water-deprived conscious rats. Am J Physiol Regul Integr Comp Physiol 255: R46–R51, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol 32: 426–432, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Brooks VL, Huhtala TA, Silliman TL, Engeland WC. Water deprivation and rat adrenal mRNAs for tyrosine hydroxylase and the norepinephrine transporter. Am J Physiol Regul Integr Comp Physiol 272: R1897–R1903, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Brooks VL, Qi Y, O′Donaughy TL. Increased osmolality of conscious water-deprived rats supports arterial pressure and sympathetic activity via a brain action. Am J Physiol Regul Integr Comp Physiol 288: R1248–R1255, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Burke SL, Head GA. Method for in vivo calibration of renal sympathetic nerve activity in rabbits. J Neurosci Methods 127: 63–74, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Celler BG, Schramm LP. Pre- and postganglionic sympathetic activity in splanchnic nerves of rats. Am J Physiol Regul Integr Comp Physiol 241: R55–R61, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Chatelain D, Montel V, Dickes-Coopman A, Chatelain A, Deloof S. Trophic and steroidogenic effects of water deprivation on the adrenal gland of the adult female rat. Regul Pept 110: 249–255, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Dampney RA, Horiuchi J, Coote JH, Yang Z, Pyner S, Deering J, Ranson RN, Motawei K, Kamel M, Brooks VL, Qi Y, O′Donaughy TL. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Di Nicolantonio R, Mendelsohn FA. Plasma renin and angiotensin in dehydrated and rehydrated rats. Am J Physiol Regul Integr Comp Physiol 250: R898–R901, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Dunn FL, Brennan TJ, Nelson AE, Robertson GL. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest 52: 3212–3219, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engeland WC. Functional innervation of the adrenal cortex by the splanchnic nerve. Horm Metab Res 30: 311–314, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol 108: 227–237, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Fejes-Toth G, Naray-Fejes-Toth A, Ratge D. Evidence against role of antidiuretic hormone in support of blood pressure during dehydration. Am J Physiol Heart Circ Physiol 249: H42–H48, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Fink GD, Osborn JW. The splanchnic circulation. In: Primer on the Autonomic Nervous System (3rd ed.), edited by David Robertson IB, Geoffrey Burnstock, Phillip A. Low, Julian F. R. Paton. Academic Press, 2010, p. 211–213 [Google Scholar]
- 22. Freeman KL, Brooks VL. AT1 and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol 292: R1675–R1682, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol 494: 515–527, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gomez-Sanchez EP, Fort CM, Gomez-Sanchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab 258: E482–E484, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Gootman PM, Cohen MI. Efferent splanchnic activity and systemic arterial pressure. Am J Physiol 219: 897–903, 1970 [DOI] [PubMed] [Google Scholar]
- 26. Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol 284: H2302–H2310, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Janiak PC, Lewis SJ, Brody MJ. Role of central mineralocorticoid binding sites in development of hypertension. Am J Physiol Regul Integr Comp Physiol 259: R1025–R1034, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol Regul Integr Comp Physiol 261: R1257–R1268, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension 11: 750–753, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol 301: H1965–H1973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kiss A, Jezova D, Aguilera G. Activity of the hypothalamic pituitary adrenal axis and sympathoadrenal system during food and water deprivation in the rat. Brain Res 663: 84–92, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Kuroki MT, Guzman PA, Fink GD, Osborn JW. Time-dependent changes in autonomic control of splanchnic vascular resistance and heart rate in ANG II-salt hypertension. Am J Physiol Heart Circ Physiol 302: H763–H769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lai FJ, Huang SS, Hsieh MC, Hsin SC, Wu CH, Hsin YC, Shin SJ. Upregulation of neuronal nitric oxide synthase mRNA and protein in adrenal medulla of water-deprived rats. J Histochem Cytochem 53: 45–53, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta 1802: 1132–1139, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Ludens JH, Clark MA, Robinson FG, DuCharme DW. Rat adrenal cortex is a source of a circulating ouabainlike compound. Hypertension 19: 721–724, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Massett MP, Johnson DG, Kregel KC. Cardiovascular and sympathoadrenal responses to heat stress following water deprivation in rats. Am J Physiol Regul Integr Comp Physiol 270: R652–R659, 1996 [DOI] [PubMed] [Google Scholar]
- 38. McAllen RM, May CN. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol Regul Integr Comp Physiol 267: R935–R944, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Nicholls MG, Lewis LK, Yandle TG, Lord G, McKinnon W, Hilton PJ. Ouabain, a circulating hormone secreted by the adrenals, is pivotal in cardiovascular disease. Fact or fantasy? J Hypertens 27: 3–8, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Osborn JW. Hypothesis: set-points and long-term control of arterial pressure. A theoretical argument for a long-term arterial pressure control system in the brain rather than the kidney. Clin Exp Pharmacol Physiol 32: 384–393, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 288: R846–R855, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Osborn JW, Kuroki MT. Sympathetic signatures of cardiovascular disease: a blueprint for development of targeted sympathetic ablation therapies. Hypertension 59: 545–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramchandra R, Hood SG, Watson AM, Allen AM, May CN. Central angiotensin type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension 59: 634–641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roberts EM, Pope GR, Newson MJ, Lolait SJ, O′Carroll AM. The vasopressin V1b receptor modulates plasma corticosterone responses to dehydration-induced stress. J Neuroendocrinol 23: 12–19, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Russ RD, Brizzee BL, Walker BR. Role of vasopressin in cardiovascular responses to acute and chronic hyperosmolality. Am J Physiol Regul Integr Comp Physiol 262: R25–R32, 1992 [DOI] [PubMed] [Google Scholar]
- 47. Saruta T. Mechanism of glucocorticoid-induced hypertension. Hypertens Res 19: 1–8, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Scheuer DA. Regulation of the stress response in rats by central actions of glucocorticoids. Exp Physiol 95: 26–31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheuer DA, Bechtold AG. Glucocorticoids potentiate central actions of angiotensin to increase arterial pressure. Am J Physiol Regul Integr Comp Physiol 280: R1719–R1726, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol 286: H458–H467, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 54: 1195–1201, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Scrogin KE, Grygielko ET, Brooks VL. Osmolality: a physiological long-term regulator of lumbar sympathetic nerve activity and arterial pressure. Am J Physiol Regul Integr Comp Physiol 276: R1579–R1586, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Scrogin KE, McKeogh DF, Brooks VL. Is osmolality a long-term regulator of renal sympathetic nerve activity in conscious water-deprived rats? Am J Physiol Regul Integr Comp Physiol 282: R560–R568, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol 286: R719–R725, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol 35: 695–700, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76: 79–92, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Ulrich-Lai YM, Engeland WC. Hyperinnervation during adrenal regeneration influences the rate of functional recovery. Neuroendocrinology 71: 107–123, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Veitenheimer B, Osborn JW. Role of spinal V1a receptors in regulation of arterial pressure during acute and chronic osmotic stress. Am J Physiol Regul Integr Comp Physiol 300: R460–R469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wotus C, Engeland WC. Differential regulation of adrenal corticosteroids after restriction-induced drinking in rats. Am J Physiol Regul Integr Comp Physiol 284: R183–R191, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Yoshimoto M, Miki K, King AJ, Fink GD, Osborn JW. Differential responses of renal and muscle sympathetic nerve activity to chronic angiotensin II administration in rats consuming a high-salt diet. Hypertension 52: e64, 2008 [Google Scholar]