Abstract
Advanced age is associated with derangements in skeletal muscle microvascular function during the transition from rest to contractions. We tested the hypothesis that, contrary to what was reported previously in young rats, selective neuronal nitric oxide (NO) synthase (nNOS) inhibition would result in attenuated or absent alterations in skeletal muscle microvascular oxygenation (Po2mv), which reflects the matching between muscle O2 delivery and utilization, following the onset of contractions in old rats. Spinotrapezius muscle blood flow (radiolabeled microspheres), Po2mv (phosphorescence quenching), O2 utilization (V̇o2; Fick calculation), and submaximal force production were measured at rest and following the onset of contractions in anesthetized old male Fischer 344 × Brown Norway rats (27 to 28 mo) pre- and postselective nNOS inhibition (2.1 μmol/kg S-methyl-l-thiocitrulline; SMTC). At rest, SMTC had no effects on muscle blood flow (P > 0.05) but reduced V̇o2 by ∼23% (P < 0.05), which elevated basal Po2mv by ∼18% (P < 0.05). During contractions, steady-state muscle blood flow, V̇o2, Po2mv, and force production were not altered after SMTC (P > 0.05 for all). The overall Po2mv dynamics following onset of contractions was also unaffected by SMTC (mean response time: pre, 19.7 ± 1.5; and post, 20.0 ± 2.0 s; P > 0.05). These results indicate that the locus of nNOS-derived NO control in skeletal muscle depends on age and metabolic rate (i.e., rest vs. contractions). Alterations in nNOS-mediated regulation of contracting skeletal muscle microvascular function with aging may contribute to poor exercise capacity in this population.
Keywords: aging, blood flow, force, oxygen uptake, S-methyl-l-thiocitrulline
nitric oxide (NO) is a ubiquitous signaling messenger synthesized primarily through the conversion of l-arginine to l-citrulline by the enzyme NO synthase (NOS). Within skeletal muscle, NO plays a critical role in the modulation of several physiological processes including vascular relaxation, oxidative metabolism, and excitation-contraction coupling (56). All three major NOS isoforms are expressed in mammalian skeletal muscle, namely, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS) (56). Several lines of evidence indicate that NO derived from nNOS participates significantly in the matching of muscle O2 delivery and utilization (Q̇o2/V̇o2) at rest and during contractions as well as submaximal force production in healthy young individuals (12; see also Refs. 13, 15, 19, 28, 33, 36, 39, 45, 52, 53, 60).
Advancing age is associated with impairments in the O2 transport pathway and exercise capacity (47). Derangements in NO-mediated function likely represent one of the main mechanisms underlying temporal Q̇o2/V̇o2 mismatch during transitions in metabolic demand in aged skeletal muscle (5, 16, 23, 25). Impairments in the ability to regulate Q̇o2 relative to V̇o2 diminish muscle microvascular O2 pressures (Po2mv) and thus the driving force for blood-myocyte O2 flux as dictated by Fick's law of diffusion. These alterations are of functional significance given that reductions in Po2mv impact negatively on mitochondrial control and could explain, at least in part, poor exercise capacity with aging (29, 57). In view of the typical deterioration of endothelial function in the elderly (54, 65), age-related decrements in NO bioavailability have been ascribed traditionally to eNOS dysfunction. Whether nNOS dysfunction is potentially involved in impaired muscle Q̇o2/V̇o2 control with aging remains unexplored.
Given that advanced aging might impact both nNOS and eNOS function possibly because of prominent oxidative stress that promotes NOS uncoupling and/or direct NO inactivation (21, 49, 54, 58), we examined whether nNOS-derived control of skeletal muscle microvascular and contractile function is altered in old rats. The hypothesis was tested that, contrary to what was observed previously in young rats (12), selective nNOS inhibition in old rats would result in attenuated or absent alterations in resting and contracting muscle blood flow, V̇o2, Po2mv, and submaximal force production, thus indicating impaired nNOS-mediated microvascular and contractile control with aging.
METHODS
A total of 21 old (27 to 28 mo; and body mass, 614 ± 11 g) male Fischer 344 × Brown Norway (F344×BN) rats were used in the present study for measurements of Po2mv and muscle blood flow (phosphorescence quenching and radiolabeled microspheres, respectively; n = 11), force production (n = 6), and time-control experiments (n = 4). Rats were obtained from Charles Rivers Laboratories and maintained on a 12-h:12-h light-dark cycle with food and water provided ad libitum. The selected age represents senescent rats according to the life span of the F344×BN rodent strain (38). The F344×BN rat has the distinct advantage of not acquiring many of the age-related pathologies that proliferate in their highly inbred counterparts (40). Upon completion of the study, rats were euthanized with intra-arterial pentobarbital sodium overdose (∼50 mg/kg). All procedures described herein were conducted under the guidelines established by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Kansas State University.
Experimental design consideration.
Comparison with young rats is facilitated using data from Copp et al. (12). The rationale for this procedure is based on the Institutional Animal Care and Use Committee stipulation that additional animals not be euthanized for replication of data. In addition, direct comparison between old and young (12) animals is facilitated by the fact that both experimental groups underwent the exact same protocols and old and young animal experiments were temporally interdigitated.
Surgical preparation.
Rats were anesthetized initially with 5% isoflurane-O2 mixture and maintained on 2 to 3% isoflurane-O2. The caudal (tail) artery was isolated surgically and cannulated (PE-50; Intra-Medic Tubing, Clay Adams Brand) for continuous monitoring of heart rate and mean arterial pressure (HR and MAP, respectively; Digi-Med BPA model 200) and infusion of the phosphorescent probe palladium meso-tetra (4-carboxyphenyl) porphyrin dendrimer (R2; 15 mg/kg; Oxygen Enterprises). Blood from the tail artery catheter was sampled at the end of each experimental condition (control and selective nNOS inhibition with S-methyl-l-thiocitrulline; SMTC) for the determination of arterial blood gases, pH, systemic hematocrit, and plasma lactate (n = 11). For blood flow measurements, an additional catheter (PE-10 connected to PE-50) was placed in the ascending aorta via the right carotid artery to allow injection of differently radiolabeled microspheres into the aortic arch. Anesthetized rats were maintained on a heating pad to maintain core temperature at ∼37 to 38°C as measured via rectal probe.
Isoflurane-O2 mixture inhalation was progressively discontinued after catheter placement procedures, and rats were then kept under anesthesia with pentobarbital sodium (administered intra-arterially to effect). The level of anesthesia was monitored frequently via the toe pinch and blink reflexes and supplemented as necessary. Overlying skin and fascia from the middorsal region of the rat were reflected surgically to expose the right spinotrapezius muscle. The muscle was moistened constantly throughout the surgery and experimental protocol via superfusion of Krebs-Henseleit bicarbonate-buffered solution, consisting of (in mM) 4.7 KCl, 2.0 CaCl2, 2.4 MgSO4, 131 NaCl, and 22 NaHCO3, equilibrated with 5% CO2-95% N2 (pH 7.4, warmed to 37–38°C), and the surrounding tissue was covered with Saran wrap (Dow Brands). Stainless steel electrodes were sutured to the rostral (cathode) and caudal (anode) regions of the spinotrapezius muscle for electrically induced contractions. Our laboratory has demonstrated previously that these surgical procedures do not alter the microvascular integrity and responsiveness of the spinotrapezius muscle (3).
Experimental protocol.
Two separate contraction bouts were performed under control (1.2 ml heparinized saline) and selective nNOS inhibition (2.1 μmol/kg SMTC dissolved in 1.2 ml heparinized saline) conditions. This dose of SMTC was selected based on previous studies designed to inhibit selectively nNOS in both humans and rodents (18, 30, 53, 64) and our analysis of the highest possible SMTC dose that could be administered without affecting the hypotensive response to ACh, indicative of nonspecific eNOS inhibition (12, 13). Each solution was infused at a rate of 0.2 ml/min into the tail artery catheter for a total time of 6 min, after which a ∼2-min period was allowed for resting muscle Po2mv to stabilize. Subsequently, 1-Hz twitch contractions (∼7 V, 2-ms pulse duration) were evoked via a stimulator (model s48; Grass Technologies) for 3 min. The muscle was then allowed to recover for a minimum of 25 min before the next condition was initiated (stimulation parameters held constant). Due to its relatively long half-life (∼40 min; refs. 18, 64), SMTC was always the last condition to prevent residual effects on vascular and skeletal muscle function. Importantly, there is no ordering (priming) effect on the Po2mv response to muscle contractions when a minimum of 20 min of recovery is allowed between stimulations (7, 16).
Effects of SMTC and NG-nitro-l-arginine methyl ester on the hypotensive responses to ACh.
In a subset of animals (n = 8 of 21 total rats), rapid ACh infusions (5 μg/kg in 0.2 ml of heparinized saline) were performed under control and SMTC conditions as well as after nonselective NOS inhibition with NG-nitro-l-arginine methyl ester (l-NAME; 10 mg/kg) administered into the caudal artery. The hypotensive responses to these infusions were recorded via the carotid artery catheter to confirm the efficacy of selective nNOS inhibition with SMTC (12, 13).
Measurement of Po2mv.
Po2mv was measured by phosphorescence quenching using a frequency domain phosphorometer (PMOD 5000; Oxygen Enterprises). As described in detail previously (6), this method applies the Stern-Volmer relationship (51) that describes quantitatively the O2 dependence of the phosphorescent probe (i.e., R2) via the following equation:
where kQ is the quenching constant and τ and τ° are the phosphorescence lifetimes in the absence of O2 and at the ambient O2 pressure, respectively. The τ of phosphorescence decay was determined using 10 scans (100 ms) in the single-frequency mode. The phosphorescent probe R2 (τ° = 601 μs and kQ = 409 mmHg−1·s−1 at pH 7.4 and temperature 38°C) (51, 63) was infused ∼15 min before initiation of muscle contractions. R2 is bound to albumin and is distributed uniformly in the plasma, thus providing a signal corresponding to the volume-weighted O2 pressure within the microvascular compartment (i.e., mainly the Po2 within the capillaries, which volumetrically constitutes the major intramuscular space) (48). The negative charge of the R2 probe also facilitates its restriction to the intravascular space (46).
The common end of the light guide was placed ∼2–4 mm superficial to the dorsal surface of the exposed right spinotrapezius muscle. The phosphorometer modulates sinusoidal excitation frequencies between 100 Hz and 20 kHz and allows phosphorescence lifetime measurements from 10 μs to ∼2.5 ms. The excitation light (524 nm) was focused on a randomly selected area of ∼2 mm diameter within the central region of the exposed muscle and has a penetration depth of ∼500 μm. Po2mv was measured continuously and recorded at 2-s intervals throughout the duration of the experimental protocols.
Analysis of Po2mv kinetics.
The kinetics of Po2mv were described by nonlinear regression analysis using the Marquardt-Levenberg algorithm (SigmaPlot 11.2; Systat software) from the onset of contractions. Transient Po2mv responses were fit with either a one- or two-component model:
One component:
Two-component:
where Po2mv(t) is the Po2mv at a given time t; Po2mv(BL) corresponds to the precontracting resting Po2mv; Δ1 and Δ2 are the amplitudes for the first and second components, respectively; TD1 and TD2 are the independent time delays for each component; and τ1 and τ2 are the time constants (i.e., time to reach 63% of the response) for each component. Goodness of fit was determined using three criteria: 1) the coefficient of determination, 2) the sum of squared residuals, and 3) visual inspection.
The amplitude of the first component was normalized to its time constant (Δ1Po2mv/τ1) to provide an index of the relative rate of Po2mv fall. The overall time necessary to attain 63% of the final amplitude of the Po2mv response following contractions onset was determined independent of modeling procedures (T63) (34) to ensure appropriateness of the model fits.
The mean response time (MRT) (43) was employed to describe the overall dynamics of the Po2mv fall
where TD1 and τ1 are defined above. The MRT analysis was constrained to the first phase of the Po2mv response since inclusion of the emergent second phase underestimates the actual rate of Po2mv fall following initiation of contractions (25).
The area under the Po2mv curve plotted as function of time (Po2Area) (23) was calculated during the 3-min contraction protocol to provide an index of the overall muscle microvascular oxygenation (i.e., incorporating from time zero to contracting steady-state Po2mv, time delays, amplitudes, and time constants of the response to yield a value, expressed in mmHg·s).
Measurement of blood flow.
Spinotrapezius muscle blood flow was measured using the radiolabeled microsphere technique, as described in detail previously (44). In each condition (control and SMTC), the stimulated right and nonstimulated left spinotrapezius muscles represented the contracting and resting blood flow measurements, respectively. Briefly, the tail artery catheter was connected to a 1-ml syringe, and blood withdrawal at a constant rate of 0.25 ml/min was performed via a Harvard pump (model 907). Differently radiolabeled microspheres (46Sc or 85Sr; 15 μm diameter; Perkin Elmer Life and Analytical Sciences) were injected in random order into the aortic arch via the carotid artery catheter during the contracting steady state (i.e., ∼3 min after onset of stimulation). Upon completion of the experiment, the right and left spinotrapezius muscles, right and left kidneys, and organs of the splanchnic region (stomach, adrenals, spleen, pancreas, small intestine, large intestine, and liver) were carefully dissected, removed, and weighed immediately after euthanasia. The thorax was opened and placement of the carotid artery catheter into the aortic arch was confirmed by anatomic dissection. Tissue radioactivity was determined on a gamma scintillation counter (Auto Gamma Spectrometer, Cobra model 5003; Hewlett-Packard), and blood flow was determined by the reference sample method (31) and expressed as milliliters per minute per 100 g of tissue. Adequate mixing of the microspheres was verified for each injection by demonstrating a <15% difference in blood flow between the right and left kidneys. Blood flow data were also normalized to MAP and expressed as vascular conductance (VC; ml·min−1·100 g−1·mmHg−1).
Calculation of muscle V̇o2.
Muscle V̇o2 was calculated from Po2mv and blood flow measurements as described previously (12, 27). Briefly, arterial O2 concentration (CaO2) was calculated directly from arterial blood samples, whereas venous O2 concentration (CvO2) was estimated from either the baseline (rest) or the contracting steady-state (contractions) Po2mv using the rat O2 dissociation curve (Hill coefficient of 2.6), the measured Hb concentration, a P50 of 38 mmHg, and an O2 carrying capacity of 1.34 ml O2/g Hb (1). The measures of the resting and contracting spinotrapezius blood flow (Q̇m) were then used to determine V̇o2 via the Fick equation, i.e., V̇o2 = Q̇m(CaO2 − CvO2).
Measurement of submaximal muscle force production.
The caudal end of the spinotrapezius muscle was exteriorized and sutured to a swivel apparatus and a nondistensible light weight (0.4 g) cable, which linked the muscle to a force transducer (model FTO3; Grass Technologies). The preload tension was set at ∼0.04 N to elicit the optimal length of the muscle for twitch force production (12, 27). Muscle force production was measured throughout control and SMTC contraction bouts which were identical to the contraction protocols described above for the measurement of Po2mv and blood flow. Force production was expressed as Newtons per gram of muscle.
Time-control experiments.
The stability and reproducibility of the spinotrapezius muscle preparation has been addressed previously (12, 27) and was reconfirmed in the current study via time-control experiments (i.e., 2 control contraction bouts performed as described above). The average coefficient of variation for Po2mv kinetics and muscle force production was 9 ± 5% with no ordering effects (P > 0.05). These data provide confidence that the significant effects (or lack thereof) detected herein were the direct result of selective nNOS inhibition with SMTC.
Statistical analyses.
Data comparison was performed using paired Student's t-tests, one-way repeated-measures ANOVA or two-way repeated-measures ANOVA where appropriate. Post hoc analyses were performed with the Student-Newman-Keuls test when a significant F-ratio was detected. The z-statistic was calculated to determine differences from zero. The significance level was set at P < 0.05. Results are reported as means ± SE.
RESULTS
Blood sampling, hemodynamic variables, and ACh injections.
There were no differences in arterial blood O2 saturation (control, 95.3 ± 0.2; and SMTC, 94.4 ± 0.7%), pH (control, 7.41 ± 0.01; and SMTC, 7.40 ± 0.01), lactate concentration (control, 1.0 ± 0.1; SMTC, 1.1 ± 0.1 mM) and systemic hematocrit (control, 35.6 ± 0.6; and SMTC, 34.8 ± 0.6%) between conditions (P > 0.05 for all).
The HR and MAP responses during control and SMTC conditions are displayed in Table 1. HR and MAP did not change during the control saline infusion, whereas MAP increased and HR decreased during the infusion of SMTC. Within each condition, there were no significant differences between rest (postinfusion) and contractions (steady state).
Table 1.
HR and MAP at rest (pre- and postinfusion) and during the steady state of muscle contractions before (control) and after selective nNOS inhibition with SMTC
| Control | SMTC | |
|---|---|---|
| At rest (preinfusion) | ||
| HR, beats/min | 297 ± 9 | 307 ± 4 |
| MAP, mmHg | 109 ± 3 | 112 ± 3 |
| At rest (postinfusion) | ||
| HR, beats/min | 305 ± 6 | 294 ± 4*† |
| MAP, mmHg | 111 ± 4 | 126 ± 3*† |
| Contractions (steady state) | ||
| HR, beats/min | 309 ± 7 | 292 ± 6*† |
| MAP, mmHg | 107 ± 3 | 129 ± 3*† |
Values are means ± SE. HR, heart rate; MAP, mean arterial pressure; nNOS, neuronal nitric oxide synthase; SMTC, S-methyl-l-thiocitrulline.
P < 0.05 vs. control;
P < 0.05 vs. rest (preinfusion). Within each condition, there were no significant differences between rest (postinfusion) and contractions (steady state).
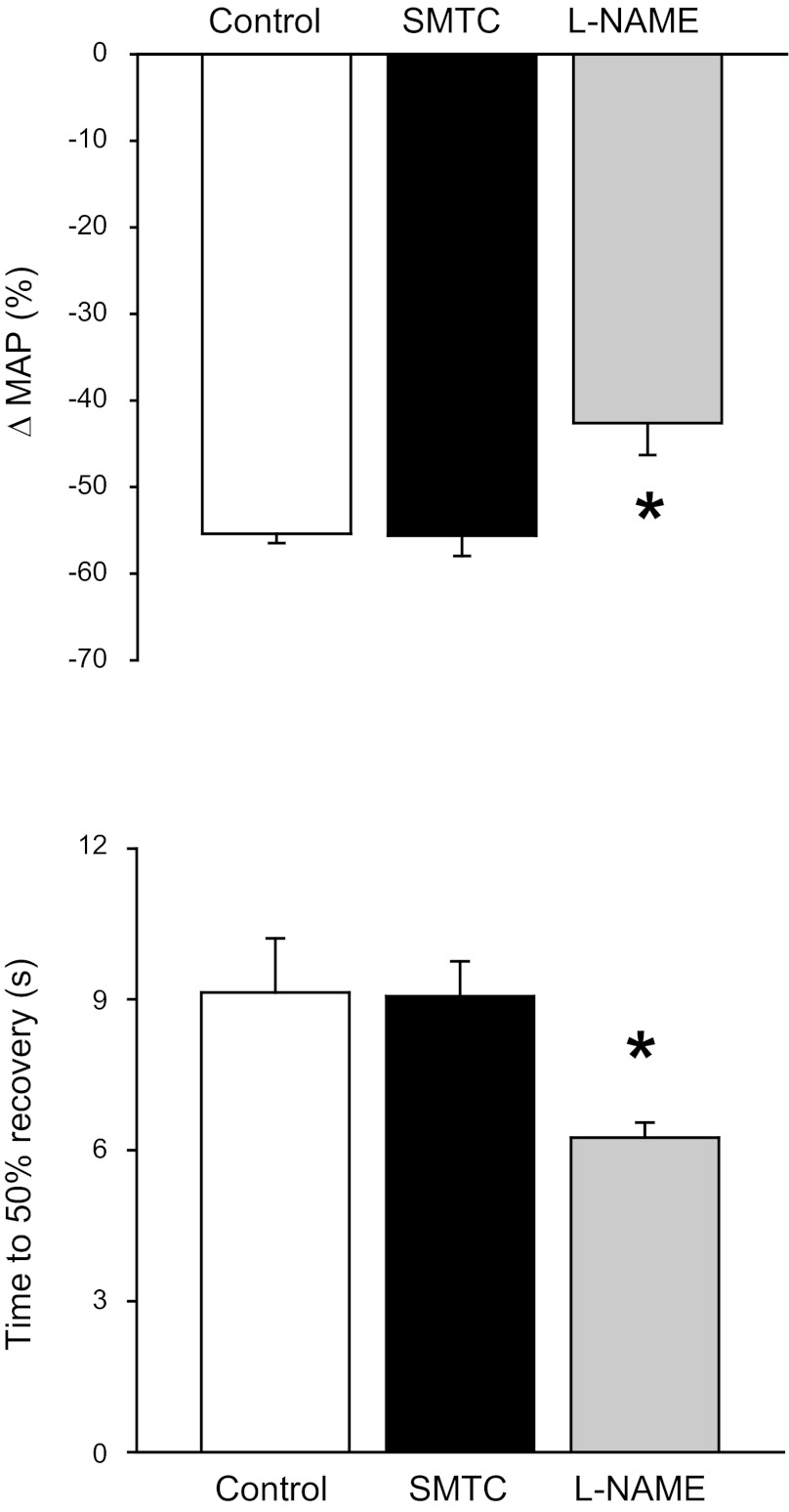
The hypotensive responses to ACh infusion during control, SMTC, and l-NAME conditions are depicted in Fig. 1. The relative change in MAP with ACh was not different between control and SMTC but decreased significantly with l-NAME. Similarly, the time to 50% recovery from the drop in MAP with ACh was not different between control and SMTC but speeded significantly with l-NAME. These data are consistent with the notion that SMTC did not impair eNOS function in the present study.
Fig. 1.
Effects of selective neuronal nitric oxide synthase (nNOS) inhibition (S-methyl-l-thiocitrulline; SMTC) and nonselective NOS inhibition (NG-nitro-l-arginine methyl ester; l-NAME) on the hypotensive responses to acetylcholine. Top and bottom: relative drop in mean arterial pressure (MAP) and time to 50% recovery from the hypotensive response to acetylcholine, respectively. *P < 0.05 vs. control and SMTC.
Spinotrapezius Po2mv.
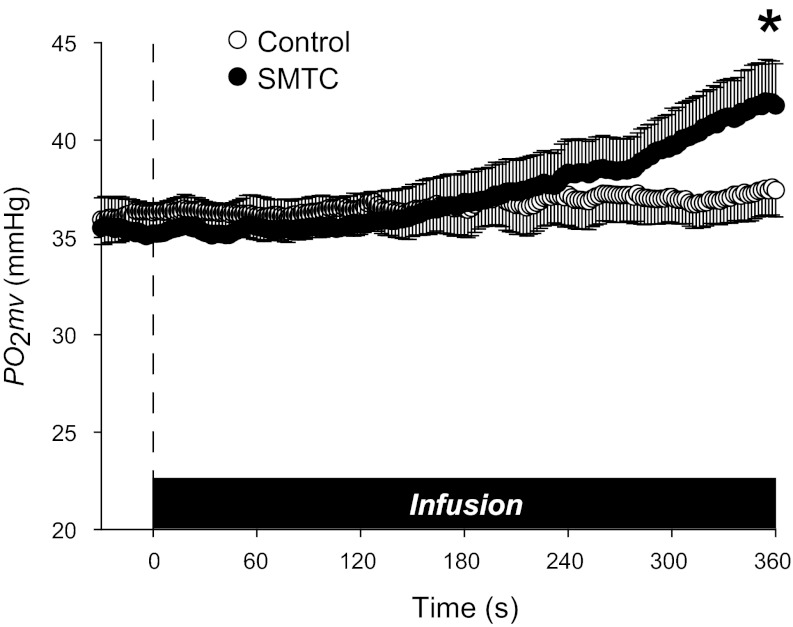
Mean spinotrapezius muscle Po2mv during control and SMTC infusions are shown in Fig. 2. The control infusion did not change Po2mv (preinfusion, 36.1 ± 1.2; and postinfusion, 37.4 ± 1.3 mmHg; P > 0.05), whereas SMTC infusion increased Po2mv from 35.3 ± 1.6 to 41.9 ± 2.2 mmHg (P < 0.05). Po2mv was not different between control and SMTC at the start or during the first 5 min of infusion (P > 0.05) but was increased significantly by the end of SMTC infusion.
Fig. 2.
Mean resting spinotrapezius muscle microvascular oxygenation (Po2mv) during infusion of saline (control) or SMTC (selective nNOS inhibition). Time zero denotes start of infusion. *P < 0.05 vs. control for end-infusion Po2mv (last 10-s average).
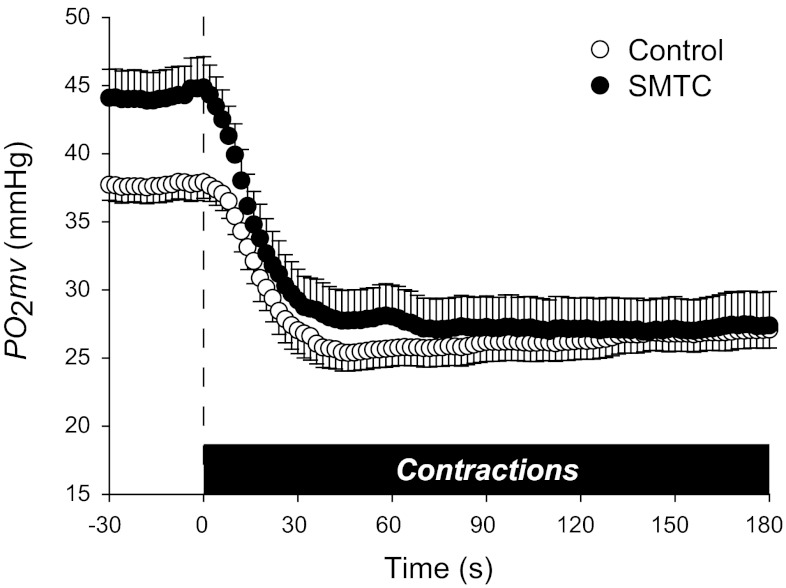
Mean spinotrapezius muscle Po2mv values following the onset of contractions during control and SMTC conditions are shown in Fig. 3 and average kinetics parameters displayed in Table 2. Although the time delay of the Po2mv fall following the onset of contractions (TD1; P < 0.05) was reduced, SMTC did not change the Po2mv time constant (τ1; P > 0.05). Furthermore, no differences in the overall dynamics of Po2mv as represented by the MRT (model dependent), T63 (model independent), and Δ1Po2mv/τ1 (relative rate of Po2mv fall) were observed between conditions (P > 0.05 for all). SMTC did, however, increase the amplitude of the Po2mv fall during contractions (Δ1Po2mv; P < 0.05), such that the contracting steady-state Po2mv [Po2mv(SS); P > 0.05] was not different between conditions. Although SMTC elevated resting Po2mv, the overall muscle microvascular oxygenation during contractions as represented by the Po2Area was not significantly different between conditions.
Fig. 3.
Mean spinotrapezius muscle Po2mv at rest and following the onset of contractions under control and selective nNOS inhibition (SMTC) conditions. Time zero denotes the onset of muscle contractions. Average kinetics parameters are displayed in Table 2. See text for further details.
Table 2.
Muscle Po2mv at rest and following the onset of contractions before (control) and after selective nNOS inhibition with SMTC
| Control | SMTC | |
|---|---|---|
| Po2mv(BL), mmHg | 37.7 ± 1.2 | 44.2 ± 2.1* |
| Δ1Po2mv, mmHg | 12.4 ± 0.9 | 17.6 ± 2.1* |
| Δ2Po2mv, mmHg | 2.3 ± 0.2 | 2.6 ± 0.5 |
| ΔTotalPo2mv, mmHg | 11.0 ± 0.7 | 17.2 ± 2.1* |
| Po2mv(SS), mmHg | 26.9 ± 1.3 | 27.3 ± 2.4 |
| TD1, s | 10.1 ± 1.5 | 6.3 ± 0.7* |
| TD2, s | 73.1 ± 11.7 | 52.8 ± 14.5 |
| τ1, s | 9.6 ± 0.9 | 13.7 ± 1.8 |
| τ2, s | 59.5 ± 15.1 | 55.7 ± 4.7 |
| MRT, s | 19.7 ± 1.5 | 20.0 ± 2.0 |
| T63, s | 20.9 ± 1.9 | 19.6 ± 1.4 |
| Δ1Po2mv/τ1, mmHg/s | 1.3 ± 0.1 | 1.4 ± 0.2 |
| Po2Area, mmHg·s | 4,920 ± 244 | 5,195 ± 402 |
Values are means ± SE.
Po2mv, microvascular oxygenation; Po2mv(BL), precontracting Po2mv; Δ1Po2mv, amplitude of the first component; Δ2Po2mv, amplitude of the second component; ΔTotalPo2mv, overall amplitude regardless of one- or two-component model fit; Po2mv(SS), contracting steady-state Po2mv; TD1, time delay for the first component; TD2, time delay for the second component; τ1, time constant for the first component; τ2, time constant for the second component; MRT, mean response time; T63, time to reach 63% of the overall amplitude as determined independent of modeling procedures; Δ1Po2mv/τ1, relative rate of Po2mv fall; Po2Area, area under the Po2mv curve over the 3-min contraction period.
The two-component exponential model was used to analyze the Po2mv kinetics in the majority of instances (7 out of 11) in the control condition, whereas the one-component model was required to fit the SMTC response in most rats (9 out of 11).
P < 0.05 vs. control.
Spinotrapezius blood flow and V̇o2.
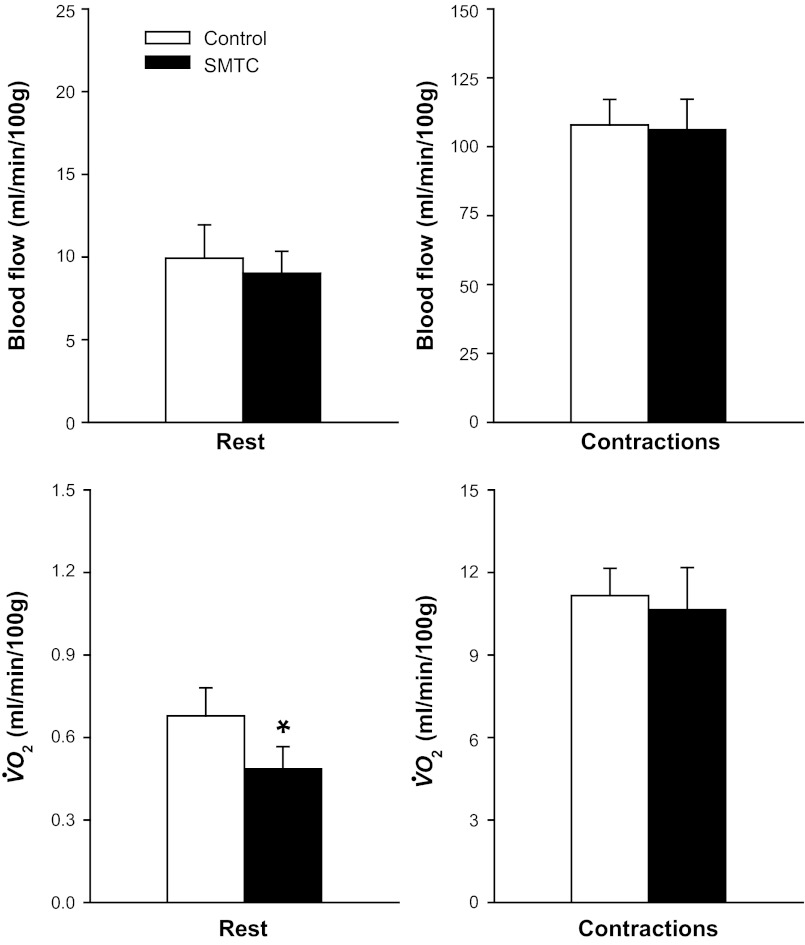
At rest, blood flow (Fig. 4, top left) and VC (control, 0.09 ± 0.02; and SMTC, 0.07 ± 0.01 ml·min−1·100 g−1·mmHg−1; P > 0.05) were not different between control and SMTC. During contractions, blood flow (Fig. 4, top right) was not different, whereas VC (control, 1.01 ± 0.09; and SMTC, 0.82 ± 0.08 ml·min−1·100 g−1·mmHg−1; P < 0.05) was reduced with SMTC compared with control. Accordingly, SMTC did not alter the change in blood flow from rest to contractions (Δblood flow: control, 98.0 ± 9.1; and SMTC, 97.1 ± 10.8 ml·min−1·100 g−1; P > 0.05) but attenuated the change in VC during the rest-contraction transient (ΔVC: control, 0.92 ± 0.09; and SMTC: 0.75 ± 0.07 ml·min−1·100 g−1·mmHg−1; P < 0.05).
Fig. 4.
Mean spinotrapezius muscle blood flow (top) and O2 utilization (V̇o2; bottom) at rest and during contractions under control and selective nNOS inhibition (SMTC) conditions. Note different scales on vertical axes.*P < 0.05 vs. control.
Relative to the control condition, SMTC reduced significantly resting but not contracting spinotrapezius muscle V̇o2 (Fig. 4, bottom). However, from rest to contractions, the change in muscle V̇o2 was not different between conditions (ΔV̇o2: control, 10.5 ± 1.0; and SMTC, 10.2 ± 1.5 ml·min−1·100 g−1; P > 0.05).
Spinotrapezius muscle force production.
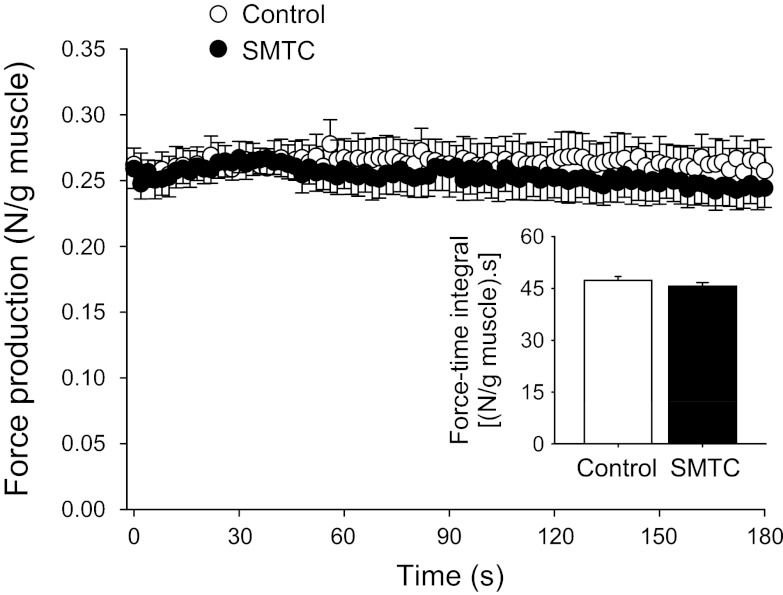
As illustrated in Fig. 5, mean spinotrapezius muscle force production throughout the contraction protocol and the force-time integral were not significantly different between control and SMTC conditions. Similarly, the average steady-state force production-to-V̇o2 ratio was also unaffected by SMTC (force/V̇o2: control, 0.024 ± 0.001; and SMTC: 0.023 ± 0.001 N·ml O2−1·min−1; P > 0.05).
Fig. 5.
Mean spinotrapezius muscle force production under control and selective nNOS inhibition (SMTC) conditions. Note that muscle force production was not significantly different throughout the contraction period between control and SMTC. Inset : force-time integral values were also not significantly different between conditions.
Abdominal organ blood flow and VC.
The effects of SMTC on resting blood flow and VC in the kidneys and organs of the splanchnic region are displayed in Table 3. Relative to control, SMTC decreased blood flow in the kidneys, stomach, adrenals, spleen, and large intestine (P < 0.05 for all). SMTC decreased VC in the kidneys, stomach, adrenals, spleen, pancreas, small intestine, and large intestine (P < 0.05 for all).
Table 3.
Resting blood flow and vascular conductance in the kidneys and organs of the splanchnic region before (control) and after selective nNOS inhibition with SMTC
| Blood Flow, ml·min−1·100 g−1 |
Vascular Conductance, ml·min−1·100 g−1·mmHg−1 |
|||
|---|---|---|---|---|
| Control | SMTC | Control | SMTC | |
| Kidneys | 515 ± 52 | 319 ± 25* | 4.88 ± 0.57 | 2.49 ± 0.19* |
| Stomach | 55 ± 7 | 34 ± 2* | 0.52 ± 0.08 | 0.27 ± 0.02* |
| Adrenals | 798 ± 75 | 542 ± 55* | 7.47 ± 0.72 | 4.20 ± 0.39* |
| Spleen | 256 ± 28 | 217 ± 26* | 2.43 ± 0.29 | 1.72 ± 0.21* |
| Pancreas | 105 ± 19 | 101 ± 26 | 0.98 ± 0.16 | 0.76 ± 0.17* |
| Small intestine | 342 ± 37 | 290 ± 18 | 3.24 ± 0.40 | 2.27 ± 0.17* |
| Large intestine | 116 ± 8 | 95 ± 5* | 1.09 ± 0.08 | 0.75 ± 0.05* |
| Liver† | 23 ± 3 | 25 ± 5 | 0.21 ± 0.03 | 0.19 ± 0.04 |
Values are means ± SE.
Arterial, not portal, blood flow and vascular conductance.
P < 0.05 vs. control.
DISCUSSION
The present investigation determined the effects of selective nNOS inhibition on skeletal muscle function at rest and during contractions in old rats. The principal novel finding was that the changes in contracting muscle blood flow, V̇o2, Po2mv kinetics, and submaximal force production produced by nNOS inhibition in healthy young rats (12) were absent in old rats (see Fig. 6). Specifically, selective nNOS inhibition in old rats resulted in 1) alterations in resting muscle V̇o2 (↓23%) and Po2mv (↑18%) but not blood flow; 2) no changes in steady-state blood flow, V̇o2, or Po2mv during contractions; 3) no changes in the overall dynamics of Po2mv following the onset of contractions; and 4) no changes in muscle force production. These data suggest that nNOS-mediated control of contracting skeletal muscle microvascular and contractile function is altered in old rats.
Fig. 6.
Effects of selective nNOS inhibition (SMTC) on contracting spinotrapezius muscle blood flow, V̇o2, overall Po2mv kinetics (MRT, mean response time), and submaximal force production in young Sprague-Dawley (data from Ref. 12; n = 10) and old Fischer 344 × Brown Norway (present study; n = 11) rats. Note that SMTC evoked significant changes in these variables in young but not old rats. *P < 0.05 vs. zero. See text for discussion.
Effects of selective nNOS inhibition on skeletal muscle function in old rats.
Skeletal muscle Po2mv is dictated by the dynamic Q̇o2/V̇o2 matching within the microvascular space (6). We reported recently that selective nNOS inhibition with SMTC in healthy young rats increased resting spinotrapezius Po2mv via reductions in V̇o2 concomitant with no alterations in muscle blood flow (12). In the present study, similar effects were observed following SMTC infusion in old rats (Figs. 2 and 4, Table 2), thus suggesting that nNOS-mediated function is preserved within aged skeletal muscle at least at rest. The lack of an effect of SMTC on resting spinotrapezius muscle blood flow in both young (12) and old (Fig. 4, top left) anesthetized rats contrasts with that seen previously in the human forearm (53) and awake rat hindlimb (13) circulations. The reasons for this discrepancy are not entirely clear but could relate partially to the effects of anesthesia. However, the reductions in blood flow to the kidneys and organs of the abdominal region following SMTC infusion in old rats at rest (Table 3) are consistent with the role of nNOS-derived NO in regulating renal and splanchnic blood flow as reported previously in the awake young rat at rest (13, see also Refs. 30, 32). These findings also suggest preserved nNOS-mediated function in the renal and splanchnic circulations with aging at rest.
The SMTC-induced reductions in resting spinotrapezius V̇o2 in both young (12) and old (Fig. 4, bottom left) rats are surprising considering the well-known inhibitory effects of NO on mitochondrial respiration (56) that may actually act to increase resting muscle V̇o2 following nonselective NOS inhibition (e.g., dogs, Ref. 20; and humans, Ref. 22). Although our data could be interpreted as reflecting a stimulatory role of NO from nNOS on mitochondrial respiration (66; see also Refs. 4, 37) and/or relate to possible differences in NOS isoform compartmentalization across species, evidence from isolated cardiac muscle suggests rather that nNOS-derived NO does not control directly tissue V̇o2 (35, 41). In the latter scenario, reduced resting V̇o2 with SMTC in old rats could result from blockade of uncoupled nNOS which alleviates the inactivation of NO from eNOS and restores partially mitochondrial respiratory inhibition (cf. 35). These intriguing possibilities remain to be tested empirically in aged skeletal muscle.
Despite the apparent preserved contribution of nNOS-derived NO to resting skeletal muscle function with aging (as discussed above), the effects of SMTC on contracting muscle blood flow, V̇o2, Po2mv kinetics, and force production observed presently in old rats differ markedly from those reported previously in young rats (12). Specifically, selective nNOS inhibition in young rats 1) reduced contracting steady-state muscle blood flow and V̇o2, 2) speeded the fall in Po2mv following the onset of contractions (i.e., shorter MRT, T63 and Δ1Po2mv /τ1), and 3) increased submaximal force production (12). In old rats, however, SMTC had no significant effects on any of these variables during muscle contractions (Table 2, Figs. 4 and 5). Figure 6 summarizes these results and illustrates the contrasting effects of SMTC on skeletal muscle hemodynamic, metabolic, and contractile function in young (12) compared with old (present study) rats during contractions. These data thus suggest that nNOS-mediated regulation of skeletal muscle microvascular and contractile function during transitions in metabolic demand is altered with advanced aging.
Alterations in nNOS-mediated regulation of skeletal muscle during contractions, but not at rest, with aging could emanate from age-related disruptions in myocyte redox state. Enhanced reactive oxygen and nitrogen species accumulation during muscle contractions (2, 8) coupled with impaired antioxidant mechanisms in old individuals (65) may exacerbate the underlying oxidative stress characteristic of aging (17) and promote nNOS uncoupling and/or direct NO inactivation (21, 49, 58). This leads to reduced nNOS-derived NO bioavailability that likely impacts contracting skeletal muscle microvascular and contractile function via multiple mechanisms. Specifically, considerable evidence in young subjects suggests critical regulatory roles for NO derived from nNOS on contracting muscle blood flow (via cGMP formation and/or functional sympatholysis; Refs. 15, 39, 60), V̇o2 (contribution to oxidative enzyme inertia at contractions onset; e.g., ref. 34), and, therefore, Po2mv kinetics (12). In addition, nNOS-derived NO bioavailability likely modulates submaximal force production via inhibitory influences on myofibrillar contractile elements (36).
Clinical implications.
Understanding how aging impacts the functional role of nNOS-derived NO on skeletal muscle represents the initial step toward the development of potential therapeutic strategies targeting this isoform. The use of isoform-selective NOS inhibitors is therefore crucial in such investigations. As discussed in detail elsewhere (12, 13) and demonstrated by previous pharmacological studies (18, 64) and the current hypotensive responses to ACh (Fig. 1), SMTC represents a viable tool for this purpose based on its selectivity for nNOS over both eNOS and iNOS inhibition. Acute selective pharmacological inhibition as used herein also minimizes the potential for chronic compensation of genetically modified nNOS models by other isoforms (33).
It is noteworthy that alterations in skeletal muscle nNOS expression and/or activity with aging (9, 11, 50, 55) may not predict nNOS-mediated function particularly in conditions associated with significant oxidative stress (e.g., aging, chronic heart failure, and diabetes), which promotes nNOS uncoupling and/or direct NO inactivation (21, 49, 58). Our current results therefore suggest that nNOS-mediated regulation of skeletal muscle microvascular and contractile function is altered in old individuals. Important clinical implications arise from these findings when considering that endurance exercise training could improve nNOS-mediated function via upregulation of nNOS expression and/or activity (55) in conjunction with augmented antioxidant capacity (62).
Besides its effects on nNOS-mediated function, it is important to acknowledge that advanced age results in downregulation of eNOS alongside upregulation of iNOS, which is consistent with a greater role for NO in inflammatory processes and a reduced participation in contractile function in aged skeletal muscle (55). This shift in the muscle NOS isoform profile with aging is hallmarked by oxidative stress and endothelial dysfunction (14, 24, 26, 54, 59, 61, 65), which likely contribute to exercise intolerance in this population (47).
Experimental considerations.
In view of the greater relative expression and activity of nNOS in fast-twitch compared with slow-twitch muscles (36, 39), potential alterations in skeletal muscle fiber-type composition with aging could partly underlie the responses seen herein with SMTC. However, it must be noted that potential age-related shifts in muscle phenotype may not only favor an increased abundance of slow-twitch fibers as traditionally considered but also promote alterations in the opposite direction (i.e., increased abundance of fast-twitch fibers) in both humans and animals (see Ref. 10 for discussion). Despite these possibilities, F344×BN rats do not appear to experience significant changes in fiber-type composition within representative skeletal muscles across the age range used herein (42).
In accordance with the latter and the fact that F344×BN rats do not develop many of the age-related pathologies seen in their highly inbred counterparts (which is essential to discern healthy aging from pathological decay; ref. 40), the National Institutes of Health support currently the use of the F344×BN rat as a model of aging. In the absence of evidence to the contrary, we therefore consider that these key facets outweigh any likely interspecies differences in nNOS-mediated control and thus facilitate comparison with young Sprague-Dawley rats (12) and suggest that differences in strain are unlikely to play a major role in the responses seen herein following selective nNOS inhibition with SMTC (Fig. 6).
Conclusions.
Pharmacological isoform-specific NOS inhibition revealed that nNOS-mediated control of contracting skeletal muscle function is altered with advanced aging. In marked contrast to the responses seen previously in young rats (12), nNOS inhibition with SMTC evoked no alterations in contracting muscle blood flow, V̇o2, Po2mv kinetics, and submaximal force production in old rats (as illustrated in Fig. 6). These novel findings suggest that, in addition to the documented deterioration in eNOS function (e.g., refs. 54, 65), alterations in nNOS-mediated regulation of contracting skeletal muscle microvascular function with aging may contribute to reduced exercise capacity in this population.
GRANTS
This research was supported in part by a Fellowship from the Brazilian Ministry of Education/CAPES-Fulbright and a Doctoral Student Research grant from the American College of Sports Medicine Foundation (to D. M. Hirai); American Heart Association Heartland Affiliate Grant 0750090Z (to T. I. Musch); Kansas State University SMILE award, American Heart Association Midwest Affiliate Grant 10GRNT4350011, and National Institutes of Health Grant HL-108328 (to D. C. Poole).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M.H., S.W.C., T.I.M., and D.C.P. conception and design of research; D.M.H., S.W.C., C.T.H., S.K.F., and T.I.M. performed experiments; D.M.H. analyzed data; D.M.H. interpreted results of experiments; D.M.H. prepared figures; D.M.H. drafted manuscript; D.M.H., S.W.C., C.T.H., S.K.F., T.I.M., and D.C.P. edited and revised manuscript; D.M.H., S.W.C., C.T.H., S.K.F., T.I.M., and D.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. Sue Hageman and Peter J. Schwagerl for invaluable technical assistance.
REFERENCES
- 1. Altman PL, Dittmer DS. Biology Data Book. Bethesda, MD: FASEB, 1974 [Google Scholar]
- 2. Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol 109: 449–456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol 279: H3131–H3137, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Baker DJ, Krause DJ, Howlett RA, Hepple RT. Nitric oxide synthase inhibition reduces O2 cost of force development and spares high-energy phosphates following contractions in pump-perfused rat hindlimb muscles. Exp Physiol 91: 581–589, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146: 259–268, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Behnke BJ, Kindig CA, Musch TI, Sexton WL, Poole DC. Effects of prior contractions on muscle microvascular oxygen pressure at onset of subsequent contractions. J Physiol 539: 927–934, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87: 465–470, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Capanni C, Squarzoni S, Petrini S, Villanova M, Muscari C, Maraldi NM, Guarnieri C, Caldarera CM. Increase of neuronal nitric oxide synthase in rat skeletal muscle during ageing. Biochem Biophys Res Commun 245: 216–219, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Carter EE, Thomas MM, Murynka T, Rowan SL, Wright KJ, Huba E, Hepple RT. Slow twitch soleus muscle is not protected from sarcopenia in senescent rats. Exp Gerontol 45: 662–670, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA 93: 9142–9147, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Copp SW, Hirai DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirculation 18: 501–511, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol 588: 1321–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol 549: 243–253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol (Oxf) 186: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Furfine ES, Harmon MF, Paith JE, Knowles RG, Salter M, Kiff RJ, Duffy C, Hazelwood R, Oplinger JA, Garvey EP. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-l-thiocitrulline and S-ethyl-l-thiocitrulline. J Biol Chem 269: 26677–26683, 1994 [PubMed] [Google Scholar]
- 19. Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics 5: 35–44, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Grassi B, Hogan MC, Kelley KM, Howlett RA, Gladden LB. Effects of nitric oxide synthase inhibition by l-NAME on oxygen uptake kinetics in isolated canine muscle in situ. J Physiol 568: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol 300: H1510–H1517, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Hirai DM, Copp SW, Ferreira LF, Musch TI, Poole DC. Nitric oxide bioavailability modulates the dynamics of microvascular oxygen exchange during recovery from contractions. Acta Physiol (Oxf) 200: 159–169, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Hirai DM, Copp SW, Hageman KS, Poole DC, Musch TI. Aging alters the contribution of nitric oxide to regional muscle hemodynamic control at rest and during exercise in rats. J Appl Physiol 111: 989–998, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Hirai DM, Copp SW, Herspring KF, Ferreira LF, Poole DC, Musch TI. Aging impacts microvascular oxygen pressures during recovery from contractions in rat skeletal muscle. Respir Physiol Neurobiol 169: 315–322, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Hirai DM, Copp SW, Schwagerl PJ, Haub MD, Poole DC, Musch TI. Acute antioxidant supplementation and skeletal muscle vascular conductance in aged rats: role of exercise and fiber type. Am J Physiol Heart Circ Physiol 300: H1536–H1544, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Hirai DM, Copp SW, Schwagerl PJ, Musch TI, Poole DC. Acute effects of hydrogen peroxide on skeletal muscle microvascular oxygenation from rest to contractions. J Appl Physiol 110: 1290–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Hirschfield W, Moody MR, O'Brien WE, Gregg AR, Bryan RM, Jr, Reid MB. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol Regul Integr Comp Physiol 278: R95–R100, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD. Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73: 728–736, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Ichihara A, Inscho EW, Imig JD, Navar LG. Neuronal nitric oxide synthase modulates rat renal microvascular function. Am J Physiol Renal Physiol 274: F516–F524, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol Heart Circ Physiol 239: H443–H449, 1980 [DOI] [PubMed] [Google Scholar]
- 32. Jansson L, Carlsson PO, Bodin B, Andersson A, Kallskog O. Neuronal nitric oxide synthase and splanchnic blood flow in anaesthetized rats. Acta Physiol Scand 183: 257–262, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol 97: 293–301, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of l-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol 91: 891–896, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res 96: 355–362, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Krause DJ, Hagen JL, Kindig CA, Hepple RT. Nitric oxide synthase inhibition reduces the O2 cost of force development in rat hindlimb muscles pump perfused at matched convective O2 delivery. Exp Physiol 90: 889–900, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Larkin LM, Halter JB, Supiano MA. Effect of aging on rat skeletal muscle β-AR function in male Fischer 344 × brown Norway rats. Am J Physiol Regul Integr Comp Physiol 270: R462–R468, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci 51: B54–B59, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loke KE, McConnell PI, Tuzman JM, Shesely EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS, Hintze TH. Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res 84: 840–845, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci 63: 921–927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macdonald M, Pedersen PK, Hughson RL. Acceleration of V̇o2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol 83: 1318–1325, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest 120: 816–826, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poole DC, Behnke BJ, McDonough P, McAllister RM, Wilson DF. Measurement of muscle microvascular oxygen pressures: compartmentalization of phosphorescent probe. Microcirculation 11: 317–326, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Poole DC, Ferreira LF. Oxygen exchange in muscle of young and old rats: muscle-vascular-pulmonary coupling. Exp Physiol 92: 341–346, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Poole DC, Wagner PD, Wilson DF. Diaphragm microvascular plasma Po2 measured in vivo. J Appl Physiol 79: 2050–2057, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 267: 24173–24176, 1992 [PubMed] [Google Scholar]
- 50. Richmonds CR, Boonyapisit K, Kusner LL, Kaminski HJ. Nitric oxide synthase in aging rat skeletal muscle. Mech Ageing Dev 109: 177–189, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241: 1649–1651, 1988 [DOI] [PubMed] [Google Scholar]
- 52. Schild L, Jaroscakova I, Lendeckel U, Wolf G, Keilhoff G. Neuronal nitric oxide synthase controls enzyme activity pattern of mitochondria and lipid metabolism. FASEB J 20: 145–147, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117: 1991–1996, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Song W, Kwak HB, Kim JH, Lawler JM. Exercise training modulates the nitric oxide synthase profile in skeletal muscle from old rats. J Gerontol A Biol Sci Med Sci 64: 540–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Stary CM, Hogan MC. Effect of varied extracellular Po2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys 471: 126–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 60. Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tian J, Yan Z, Wu Y, Zhang SL, Wang K, Ma XR, Guo L, Wang J, Zuo L, Liu JY, Quan L, Liu HR. Inhibition of iNOS protects endothelial-dependent vasodilation in aged rats. Acta Pharmacol Sin 31: 1324–1328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vincent HK, Powers SK, Stewart DJ, Demirel HA, Shanely RA, Naito H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur J Appl Physiol 81: 67–74, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Vinogradov SA, Fernandez-Searra MA, Dugan BW, Wilson DF. Frequency domain instrument for measuring phosphorescence lifetime distributions in heterogeneous samples. Rev Sci Instrum 72: 3396–3406, 2001 [Google Scholar]
- 64. Wakefield ID, March JE, Kemp PA, Valentin JP, Bennett T, Gardiner SM. Comparative regional haemodynamic effects of the nitric oxide synthase inhibitors, S-methyl-l-thiocitrulline and l-NAME, in conscious rats. Br J Pharmacol 139: 1235–1243, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Young ME, Leighton B. Fuel oxidation in skeletal muscle is increased by nitric oxide/cGMP–evidence for involvement of cGMP-dependent protein kinase. FEBS Lett 424: 79–83, 1998 [DOI] [PubMed] [Google Scholar]