Abstract
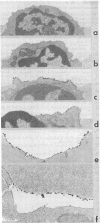
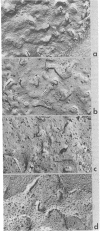
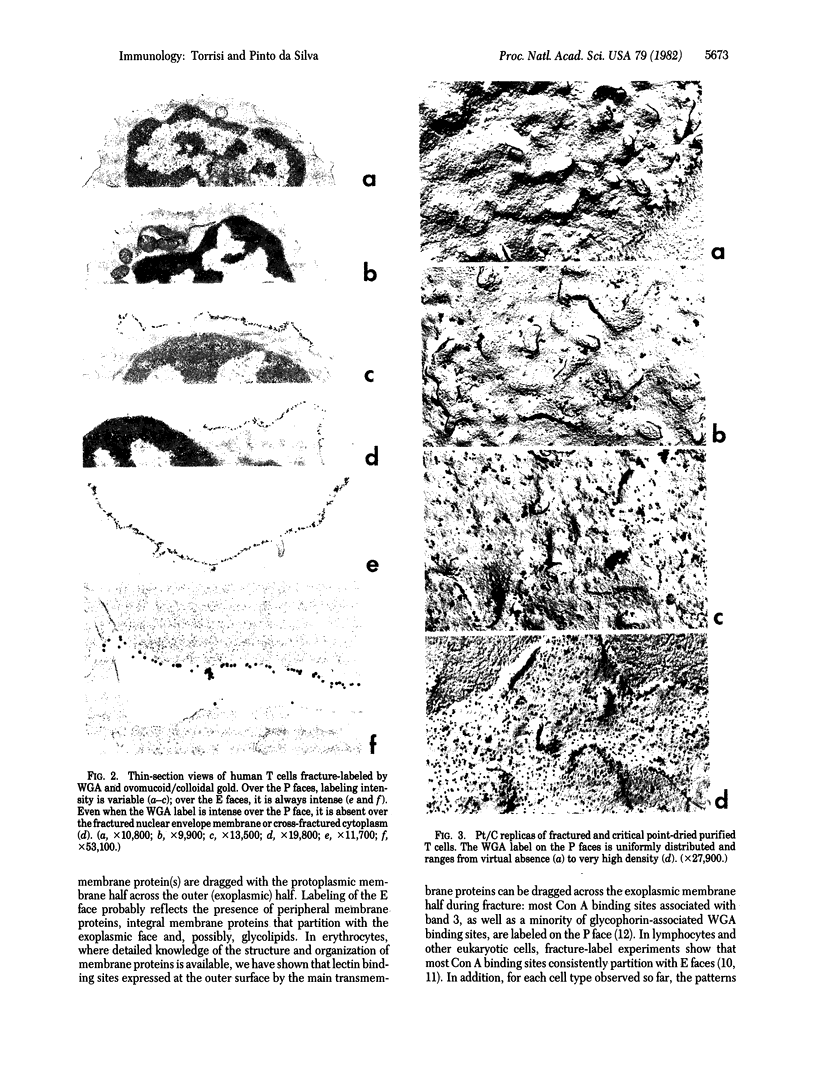
We have recently described "fracture-label" techniques that permit direct cytochemical labeling of freeze-fractured cells. We report here the use of fracture-labeling to investigate the distribution and partition of wheat germ agglutinin (WGA) receptor sites over the protoplasmic and exoplasmic plasma membrane faces of freeze-fractured human thymus-derived (T) lymphocytes. All exoplasmic faces are strongly labeled by WGA. In contrast, the protoplasmic faces exhibit remarkable variation, ranging from virtual absence of label in some faces to very high densities in other faces. We interpret the presence of WGA receptor sites over the protoplasmic faces to reflect the presence of transmembrane WGA-binding sialoglycoproteins that, during freeze-fracture, partition with the inner half of the plasma membrane. Our results, therefore, indicate heterogeneous expression of integral membrane proteins within populations of human T cells. Fracture-label techniques thus represent an additional tool in the definition of lymphocyte subpopulations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batten T. F., Hopkins C. R. Use of protein A-coated colloidal gold particles for immunoelectronmicroscopic localization of ACTH on ultrathin sections. Histochemistry. 1979 Apr 12;60(3):317–320. doi: 10.1007/BF00500659. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Boldt D. H. Interaction of wheat germ agglutinin with human peripheral blood mononuclear cells. Binding kinetics and flow microfluorometric analysis. Mol Immunol. 1980 Jan;17(1):47–55. doi: 10.1016/0161-5890(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Boldt D. H., Lyons R. D. Fractionation of human lymphocytes with plant lectins. II. Lens culinaris lectin and wheat germ agglutinin identify distinct lymphocyte subclasses. J Immunol. 1979 Aug;123(2):808–816. [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B. J., Waxdal M. J., Sharrow S. O., Thomas C. A., 3rd, Asofsky R., Mathieson B. J. Differential binding of fluorescein-labeled lectins to mouse thymocytes: subsets revealed by flow microfluorometry. J Immunol. 1980 Aug;125(2):623–630. [PubMed] [Google Scholar]
- Geoghegan W. D., Ackerman G. A. Adsorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat anti-human immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem. 1977 Nov;25(11):1187–1200. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- Gordon L. K., Hamill B., Parker C. W. The activation of blast transformation and DNA synthesis in human peripheral blood lymphocytes by wheat germ agglutinin. J Immunol. 1980 Aug;125(2):814–819. [PubMed] [Google Scholar]
- Greene W. C., Waldmann T. A. Inhibition of human lymphocyte proliferation by the nonmitogenic lectin wheat germ agglutinin. J Immunol. 1980 Jun;124(6):2979–2987. [PubMed] [Google Scholar]
- Hellström U., Dillner M. L., Hammarström S., Perlmann P. Fractionation of human T lymphocytes on wheat germ agglutinin-sepharose. J Exp Med. 1976 Nov 2;144(5):1381–1385. doi: 10.1084/jem.144.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- Pinto da Silva P., Parkison C., Dwyer N. Fracture-label:O cytochemistry of freeze-fracture faces in the erythrocyte membrane. Proc Natl Acad Sci U S A. 1981 Jan;78(1):343–347. doi: 10.1073/pnas.78.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Torrisi M. R., Kachar B. Freeze-fracture cytochemistry: localization of wheat-germ agglutinin and concanavalin A binding sites on freeze-fractured pancreatic cells. J Cell Biol. 1981 Nov;91(2 Pt 1):361–372. doi: 10.1083/jcb.91.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Con A-inducible suppression of MLC: evidence for mediation by the TH2 + T cell subset in man. J Immunol. 1979 Apr;122(4):1335–1341. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- da Silva P. P., Kachar B., Torrisi M. R., Brown C., Parkison C. Freeze-fracture cytochemistry: replicas of critical point-dried cells and tissues after fracture-label. Science. 1981 Jul 10;213(4504):230–233. doi: 10.1126/science.7244630. [DOI] [PubMed] [Google Scholar]
- da Silva P. P., Parkison C., Dwyer N. Freeze-fracture cytochemistry: thin sections of cells and tissues after labeling of fractures faces. J Histochem Cytochem. 1981 Aug;29(8):917–928. doi: 10.1177/29.8.7276536. [DOI] [PubMed] [Google Scholar]
- da Silva P. P., Torrisi M. R. Freeze-fracture cytochemistry: partition of glycophorin in freeze-fractured human erythrocyte membranes. J Cell Biol. 1982 May;93(2):463–469. doi: 10.1083/jcb.93.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]