Abstract
Various molecular forms of CCK reduce food intake in rats. Although CCK-8 is the most studied form, we reported that CCK-58 is the only detectable endocrine peptide form in rats. We investigated the dark-phase rat chow intake pattern following injection of CCK-8 and CCK-58. Ad libitum-fed male Sprague-Dawley rats were intraperitoneally injected with CCK-8, CCK-58 (0.6, 1.8, and 5.2 nmol/kg), or vehicle. Food intake pattern was assessed during the dark phase using an automated weighing system that allowed continuous undisturbed monitoring of physiological eating behavior. Both CCK-8 and CCK-58 dose dependently reduced 1-h, dark-phase food intake, with an equimolar dose of 1.8 nmol being similarly effective (−49% and −44%). CCK-58 increased the latency to the first meal, whereas CCK-8 did not. The intermeal interval was reduced after CCK-8 (1.8 nmol/kg, −41%) but not after CCK-58. At this dose, CCK-8 increased the satiety ratio by 80% and CCK-58 by 160%, respectively, compared with vehicle. When behavior was assessed manually, CCK-8 reduced locomotor activity (−31%), whereas grooming behavior was increased (+59%). CCK-58 affected neither grooming nor locomotor activity. In conclusion, reduction of food intake by CCK-8 and CCK-58 is achieved by differential modulation of food intake microstructure and behavior. These data highlight the importance of studying the molecular forms of peptides that exist in vivo in tissue and circulation of the animal being studied.
Keywords: dark phase, meal microstructure, satiation, satiety, satiety ratio, solid food
bayliss and starling first reported in 1902 that intestinal extracts stimulated pancreatic secretion (3). Ivy and Oldenberg (27) showed in 1928 that a different preparation of intestinal extracts caused contraction of the gallbladder and termed the principle “cholecystokinin”. In 1943, Harper and Raper (24) demonstrated that pancreatic secretion could be stimulated by intestinal extracts and named the principle “pancreozymin”. During the purification of CCK, Mutt and Jorpes (29) observed that a biological activity causing pancreatic enzyme secretion copurified with the CCK activity and suggested that these two activities were exerted by one molecule, then termed cholecystokinin-pancreozymin, later shortened to cholecystokinin and abbreviated CCK. CCK was originally described as a 33-amino acid peptide (38) expressed in small intestinal endocrine I-cells (40). Since then, several forms of CCK have been characterized chemically from intestinal extracts in multiple species, namely CCK-58 (14), CCK-39 (28), CCK-33 (38), CCK-22 (11), and CCK-8 (14). In rats, endocrine forms suggested from chromatography of plasma samples and assay of fractions collected have varied from laboratory to laboratory. The major forms reported were CCK-8 (28), CCK-22 (16, 34), CCK-33/39 (35, 47), and CCK-58 (42, 46). An important observation is that endocrine forms smaller than CCK-58 can be produced ex vivo during the processing of plasma samples. This could explain the disparity of results obtained when blood was not collected under conditions that prevented degradation of large molecular forms, because there are variable extents of degradation during processing of the blood. We recently established a novel blood-processing method, the RAPID method, employing reduced temperatures, acidification, protease inhibition, isotopic exogenous controls, and dilution that prevents degradation of endocrine cholecystokinin as blood is processed (54). Using this method, we found that the recovery of exogenous radiolabeled CCK-58 added to rat blood was improved from 20% to 88% compared with standard blood processing (EDTA blood on ice and plasma formation) (54). Moreover, RAPID processing resulted in detection of the added exogenous 125I-labeled CCK-58, whereas after standard blood processing, 100% of the labeled CCK-58 was degraded into smaller forms. This has led us to suggest that small forms, such as CCK-8, CCK-22, and CCK-33 observed by others are produced ex vivo during formation and processing of plasma, and we demonstrated that there is no production of smaller forms during the RAPID method (54).
If rat blood is processed in a manner that prevents degradation of 125I-labeled CCK-58, the only endocrine form detected is CCK-58 (42). Thus, endogenous endocrine CCK-8 detected by others (23, 35) is most likely a product of peptide degradation during plasma formation. Importantly, CCK-58 is the major intestinal and endocrine form in dogs (8, 13), rats (42), and humans (8, 9, 14), suggesting its biological action should be evaluated and the influence of CCK-58 on feeding patterns should be studied. The importance of this suggestion is indicated by the fact that CCK-8 and CCK-58 show different actions on various physiological processes. However, most reports have used CCK-8 to study gastrointestinal functions. The difference between the most studied form (CCK-8) and the most abundant form (CCK-58) of cholecystokinin is demonstrated by patterns of pancreatic secretion elicited by the two peptides. Intravenous infusion of CCK-58 but not CCK-8 ranging from 62.5 to 1,000 pmol·kg−1·h−1 over a 3-h period strongly stimulated pancreatic fluid secretion in a dose-dependent fashion in an in vivo rat model (46, 62). CCK-58 induces a more sustained pancreatic protein secretion than CCK-8, as shown by the return to basal values at the end of the 3-h infusion period with CCK-8, whereas protein output remained significantly elevated compared with basal in CCK-58-treated rats (46, 62). In addition, intravenous infusion of CCK-58 at 2 or 4 nmol·kg−1·h−1 for 6 h in a conscious rat model did not induce pancreatitis at a dose at which CCK-8 induced several parameters indicative of pancreatitis, including interstitial edema, inflammatory cell infiltration, intracellular vacuolization, increased pancreatic myeloperoxidase activity, and elevated serum amylase levels (61).
In 1973, Smith et al. originally reported that synthetic CCK-8 and natural CCK-33 reduce food intake (19), opening the path to a multitude of investigations on the importance of this hormone in feeding regulation. Most of these studies were performed using CCK-8 because of its availability, and the concept that all forms of CCK that contain a sulfated tyrosine would have the same biological activity (26). However, as described above, we have reported that with proper blood processing, CCK-58 is the only detectable circulating form of rat CCK (42). Similar to the observations with the pancreas, CCK-8 and CCK-58 exert differential effects on food intake, although this has not been characterized in detail. One report suggested that although CCK-8 and CCK-58 at the doses of 3.5 and 7 nmol/kg equally reduced food intake 30 min after intraperitoneal injection at the onset of the dark phase in rats that were fasted for 4 h, only the reduction of food intake induced by CCK-58 at a dose of 7 nmol/kg ip was preserved over a period of 210 min (20). However, in their study, Glatzle et al. (20) used ground powdered rat chow that does not resemble a normal rat diet and did not evaluate changes in feeding behavior to assess whether it was related to changes in intermeal interval (IMI) or other effects on the pattern of feeding behavior. In other studies, duodenal fat infusions reduced meal size and prolonged IMI, which could be attenuated by the specific CCK1 antagonist devazepide (5, 59). However, exogenous CCK-58 has not been evaluated for its influence on IMI.
In the present study, we first compared the effects of CCK-8 and CCK-58 injected intraperitoneally on dark-phase food intake, the photoperiod when rats normally eat (48). Because the analysis of the feeding microstructure is essential to assess the mechanisms regulating feeding behavior (18), we investigated the feeding pattern using a newly developed automated episodic food intake-monitoring device, which allows the undisturbed continuous monitoring during the dark phase with minimal human interference. This has been used in rats before (15, 31, 39, 49) and was established recently for the use in mice (21, 52). To investigate the specificity of the observed effects on feeding behavior, other behavioral parameters, such as grooming and locomotor activity, were assessed as established originally by Antin et al. (1) and in our previous studies (50, 53).
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (Harlan, San Diego, CA), weighing 280–350 g, were group housed under controlled illumination (0600–1800) and temperature (21–23°C) until the start of experiments. Animals had free access to standard rodent chow (Prolab RMH 2500, LabDiet; PMI Nutrition, Brentwood, MO) and tap water. All experiments were started at the onset of the dark phase. Rats had resting periods of at least 3 days between experiments. Protocols were approved by the Institutional Animal Care and Use Committee of the Veterans Administration (no. 99059–04 and no. 11045–09).
Substances.
Sulfated CCK-8 (CCK-8S; Bachem, Torrance, CA) and rat CCK-58 (46) were dissolved in 1 ml 0.1% trifluoroacetate, and concentrations were determined by their absorbance at 280 nm, diluted 50:50 with 0.2% BSA (Sigma-Aldrich, St. Louis, MO), divided to 10-pmol aliquots, and dried using vacuum centrifugation. Peptide powder was stored at −80°C and dissolved in saline-containing 0.1% BSA for a final intaperitoneal injection volume of 0.5 ml/rat immediately before use.
Food intake microstructure.
Food intake pattern was assessed as established in our previous studies (21, 52) using an automated episodic food intake-monitoring system for regular pellet rat food (BioDAQ, Research Diets, New Brunswick, NJ). Water was provided ad libitum from regular water bottles. Rats were housed singly and habituated to the system for 1 wk. Animals adapted to the system quickly, as indicated by normal food intake and body weight gained by the second day of the habituation period. During that time, rats were also handled to mimic intraperitoneal injection, including practice of the upside-down position.
The food intake monitoring system weighs the hopper with food (±0.01 g) second by second and detects “not eating” as weight stable and “eating” as weight unstable. Feeding bouts (changes in stable weight before and after a bout) are recorded with a start time, duration, and amount consumed. Bouts are separated by an interbout interval (IBI), and meals consist of one or more bouts separated by an IMI. The minimum IMI was defined as 15 min, while the minimum meal amount was defined as 0.1 g. Therefore, food intake was considered as one meal when the feeding bouts occurred within 15 min of the previous response, and their sum was equal to or greater than 0.1 g. When bouts of feeding were longer than 15 min apart, they were considered as a new meal. The software continuously records every action related to rats touching the food hopper. Meal parameters extracted from the software (BioDAQ monitoring software 2.2.02) for these studies included latency to the first meal, duration of first meal, meal size of first and second meal, IMI between first and second meal, and rate of ingestion of first meal. Amounts of food consumed for each photoperiod and for the entire 24-h period for the day of the study were also obtained. The satiety ratio (IMI in minutes between first and second meal/first meal size in grams) was calculated.
On the day of the experiment, gates were closed to prevent pre-dark phase food intake 90 min before the onset of the dark phase and for maintenance (cleaning hoppers, refilling food, and body weight monitoring). Immediately before lights off, rats were injected with CCK-8 or CCK-58 (0.6, 1.8 and 5.2 nmol/kg ip, n = 6–12 animals/group), or vehicle (saline containing 0.1% BSA, n = 9–11 animals/group), placed back in their home cage, the gates were opened, recording started, and lights were turned off. The time needed to inject rats was ∼30 s/rat. The dose of CCK was based on previous studies showing a robust reduction of food intake following injection of 2 nmol/kg CCK-8 (17, 56). Experiments were repeated in a crossover design in the same batch of rats for each treatment.
The study design had to be modified for the assessment of the latency to eat the first meal so the gates remained open during the 90 min before the dark phase, and rats were intraperitoneally injected at 0.6, 1.8 and 5.2 nmol/kg before lights off. Food intake microstructure was assessed during the dark phase. In these studies, maintenance was performed 6 h before lights off.
We have previously established the automated system in mice and shown the same food intake, as observed manually during the light and dark phase (52). Because these data were generated in a different species, we performed an additional experiment in rats to validate automated vs. manual measurement of dark-phase food intake. Rats habituated to the automated feeding system were randomly divided in two groups (n = 7 or 8) and housed in automated cages connected to the computer (feeding from hopper) or unplugged cages (feeding from cage top). Gates were closed 90 min before lights off as done in the previous experiments and similarly, food pellets were removed from the unplugged cages. Rats were intraperitoneally injected with vehicle (saline containing 0.1% BSA) right before lights off, and either food was made available or gates were opened. Food intake of standard rodent diet was monitored for 60 min by manual assessment or in an automated fashion. The experiment was repeated in a crossover design in the same batch of rats.
Behavior.
Just prior to the dark phase, singly housed, freely fed animals were injected intraperitoneally with CCK-8 (n = 6) or CCK-58 (n = 8) at the equimolar dose of 1.8 nmol/kg or vehicle (n = 8) and placed in their home cage with a paper grid under the cage divided into six equal squares. Because CCK-8 and CCK-58 dose dependently reduced 1-h dark-phase food intake with an equimolar dose of 1.8 nmol being similarly effective, this dose was chosen for the behavioral monitoring. Immediately after injection, lights were turned off, and preweighed rat chow replaced the daily chow. Rats had free access to food and water before and during the experiment. Behavior was assessed manually and simultaneously in 4 rats/investigator, as described in our previous studies (50, 51, 53). This method requires a time-sampling technique by two investigators, who performed all experiments. Interinvestigator variability was <5%. Briefly, there was a latency of 10 min after lights off; then behaviors, including grooming (washing, licking, and scratching), locomotor activity, eating, and drinking were monitored manually for 30 min by the observers, who sat motionless in front of the cages with a dim light, allowing surveillance of the animals and a silent timer to keep track of time in seconds. Eating behavior was defined as eating, as well as food approach, which consisted of sniffing and licking food. Drinking behavior included drinking and water approach, as indicated by sniffing. Locomotor activity was defined as at least one rat paw crossing the boundary of one square. The total number of squares crossed was counted. The investigators were blinded to the animals' treatment. Each behavior was counted again when it lasted >5 s. In the behavior experiments, food intake was assessed manually at one time point (40 min) by measuring the weight difference of rat chow before and after the 40-min period.
Statistical analysis.
Data are expressed as means ± SE and analyzed by one-way ANOVA followed by Tukey post hoc test and two-way ANOVA followed by Holm-Sidak method. P < 0.05 was considered significant. Correlations were determined by univariate linear regression. Because of the differential experimental set-up, the analysis for latency to the first meal was conducted separately from analyses of the other feeding parameters.
RESULTS
Automated and manual food intake monitoring yield similar results.
No differences in amount of food intake during the first hour after lights off were observed using the two different methods of examination. Rats that were monitored by the automated system ate similar amounts, as rats in which food intake was assessed manually (4.41 ± 0.48 vs. 4.67 ± 0.35 g, P > 0.05; data not shown).
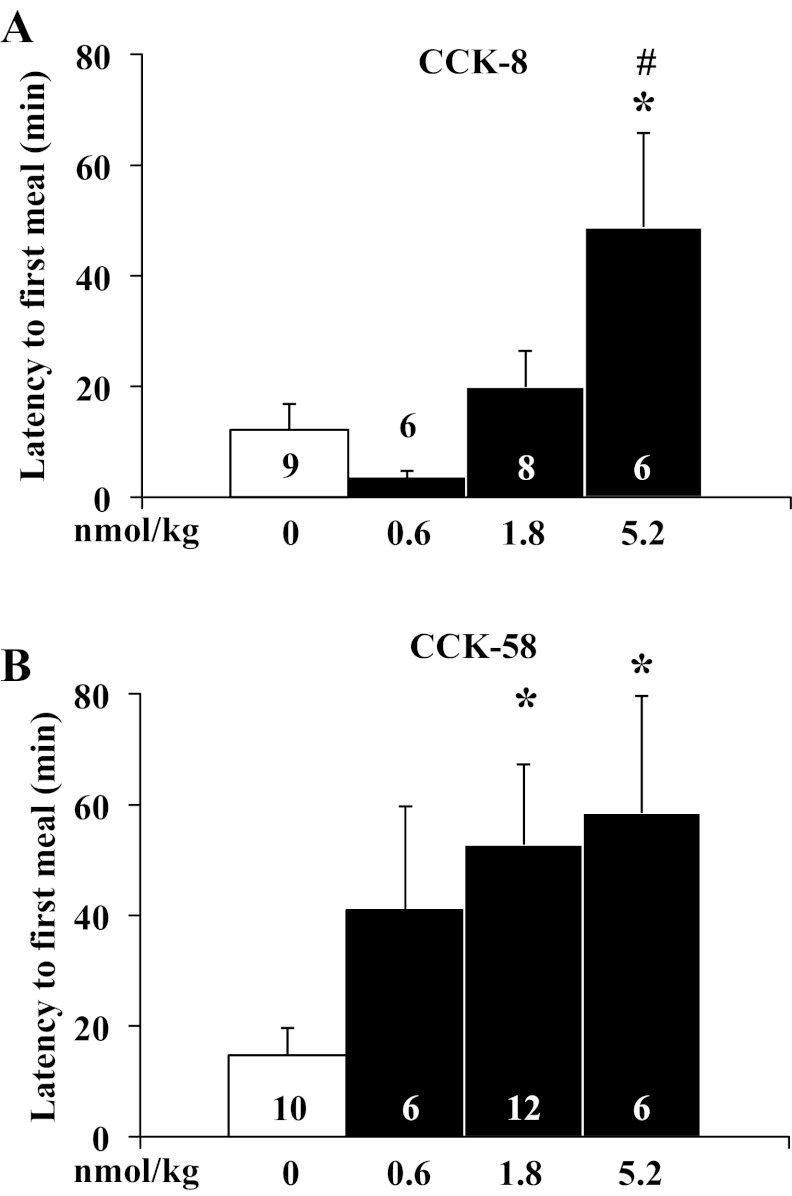
CCK-58 is more potent than CCK-8 at prolonging latency to first meal.
In the only experiments with gates open (access to food) before lights off, CCK-8 increased the latency to the first meal only at the highest dose used (5.2 nmol/kg ip) by four times compared with vehicle (F3,25 = 4.7, P < 0.05; 48.8 ± 17.0 vs. 12.2 ± 4.7 min, P < 0.05; 5.2 nmol/kg vs. vehicle) and by 15 times compared with the 0.6 nmol/kg dose (48.8 ± 17.0 vs. 3.4 ± 1.3 min, P < 0.05; 5.2 nmol/kg vs. 0.6 nmol/kg), whereas lower doses (1.8 nmol/kg or 0.6 nmol/kg) had no effect (Fig. 1A). In contrast, CCK-58 increased the time to eat almost with significance at the lowest dose (F3,30 = 3.0, P < 0.05; 40.9 ± 18.8 min vs. vehicle: 14.8 ± 4.9 min, P > 0.05), significantly by four times at 1.8 nmol/kg, and with a similar effect at 5.2 nmol/kg compared with vehicle (52.7 ± 14.5 and 58.5 ± 21.2 min, respectively, vs. 14.8 ± 4.9 min, P < 0.05; Fig. 1B). Two-way ANOVA showed a significant influence of treatment (F1,55 = 5.2, P < 0.05) and dose (F3,55 = 3.7, P < 0.05) but not treatment × dose (F3,55 = 1.0, P > 0.05).
Fig. 1.
CCK-58 at a dose of 1.8 nmol/kg increases the latency to eat, whereas CCK-8 does not. Rats housed under ad libitum feeding conditions with continuously open gates were injected intraperitoneally with CCK-8 (0.6, 1.8, and 5.2 nmol/kg; A), CCK-58 (0.6, 1.8, and 5.2 nmol/kg; B) or vehicle (saline containing 0.1% BSA), placed back in the cage, recording started and lights turned off. The latency to the first meal was extracted and shown for CCK-8 (A) and CCK-58 (B). Bars represent means ± SE of number of rats indicated at the bottom. *P < 0.05 vs. vehicle. #P < 0.05 vs. 0.6 nmol/kg.
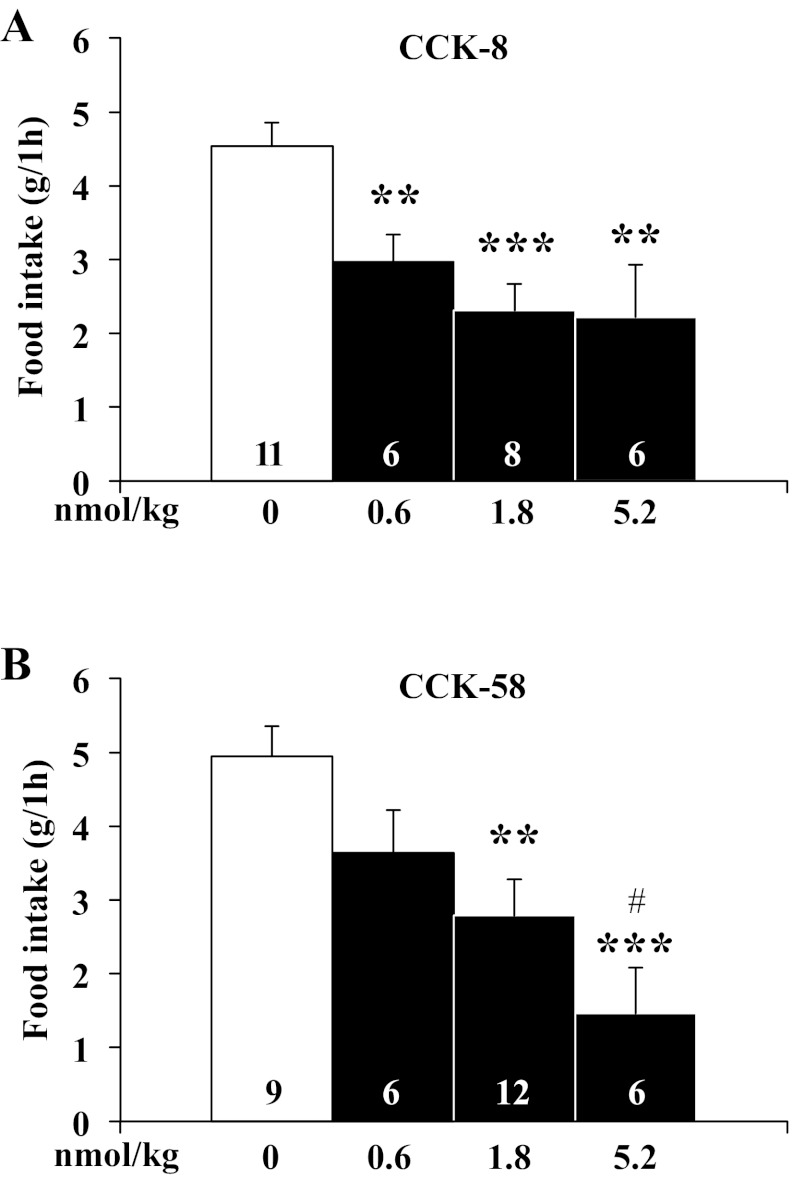
CCK-8 and CCK-58 differentially alter food intake microstructure at the onset of the dark phase.
In these experiments, gates were closed 90 min before lights off and then opened at lights off. With regard to meal consumption, the first hour dark-phase food intake, first and second meal size, as well as 12-h and 24-h food intake, were investigated. CCK-8 injected (0.6, 1.8, and 5.2 nmol/kg ip) dose dependently decreased the first 1-h dark phase food intake in ad libitum-fed rats by 35%, 49%, and 51%, respectively, compared with vehicle (F3,27 = 7.7, P < 0.001; 3.0 ± 0.4, 2.3 ± 0.4 and 2.2 ± 0.7 g, respectively, vs. 4.5 ± 0.3 g, P < 0.01; Fig. 2A). Similarly, CCK-58 (0.6, 1.8 and 5.2 nmol/kg ip) injected at the onset of the dark phase decreased the first 1-h food intake by 26%, 44%, and 70%, respectively, compared with vehicle (F3,29 = 6.4, P < 0.01; 3.6 ± 0.6, 2.8 ± 0.5, and 1.5 ± 0.6 g, respectively, vs. 4.9 ± 0.4 g, P < 0.01; CCK-58: 0.6 vs. 5.2 nmol/kg, P < 0.05; Fig. 2B). Two-way ANOVA showed a significant influence of dose (F3,54 = 13.6, P < 0.001) but not treatment (F1,54 = 0.3, P > 0.05) or treatment × dose (F3,54 = 0.7, P > 0.05).
Fig. 2.
CCK-8 and CCK-58 injected intraperitoneally dose-dependently decrease dark-phase food intake in rats. Gates were closed at 90 min before the onset of the dark phase. Directly before lights off, rats were intraperitoneally injected with CCK-8 (0.6, 1.8, and 5.2 nmol/kg; A), CCK-58 (0.6, 1.8, and 5.2 nmol/kg; B) or vehicle (saline containing 0.1% BSA), placed back in their home cage, the gates opened, recording started, and lights turned off. Food intake was extracted for the 1st h postinjection. Bars represent means ± SE of the number of rats indicated at the bottom. **P < 0.01 and ***P < 0.001 vs. vehicle and #P < 0.05 vs. 0.6 nmol/kg.
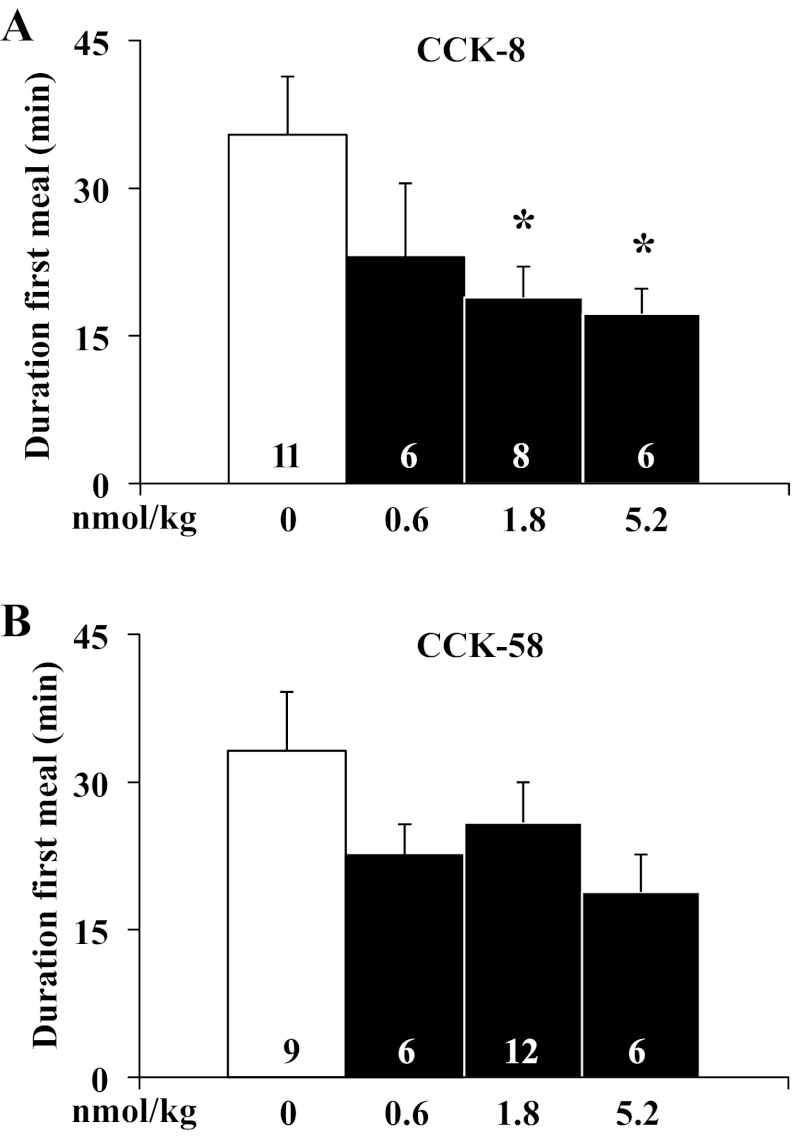
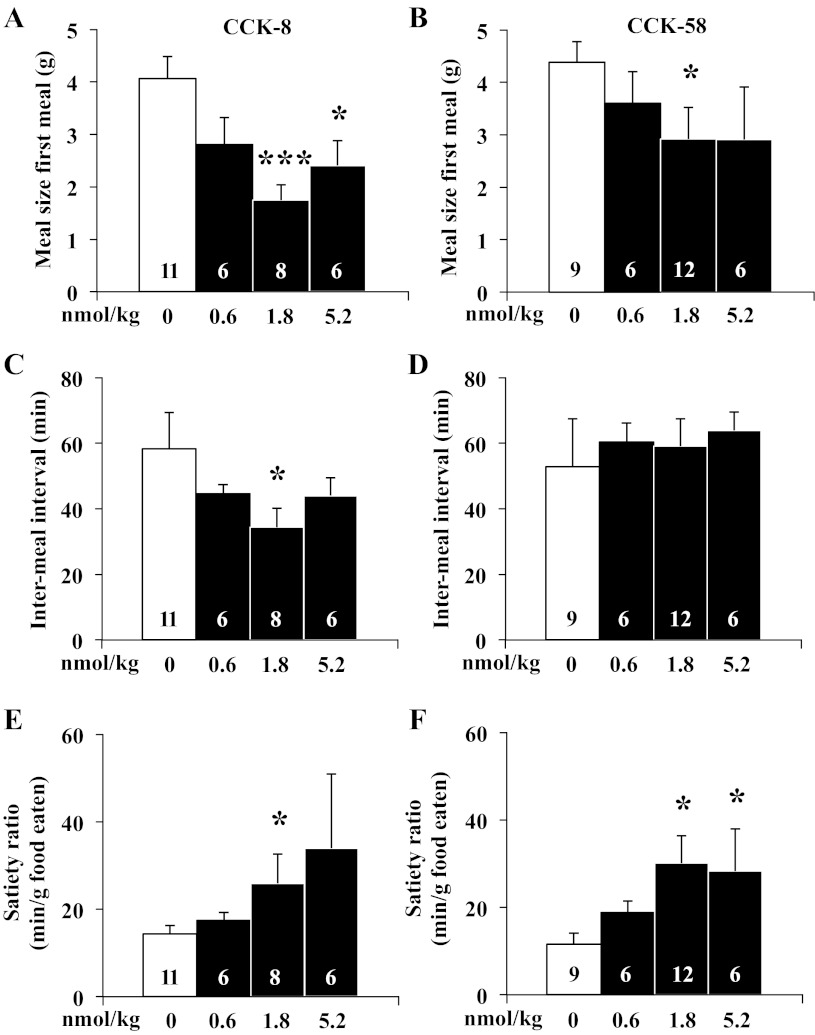
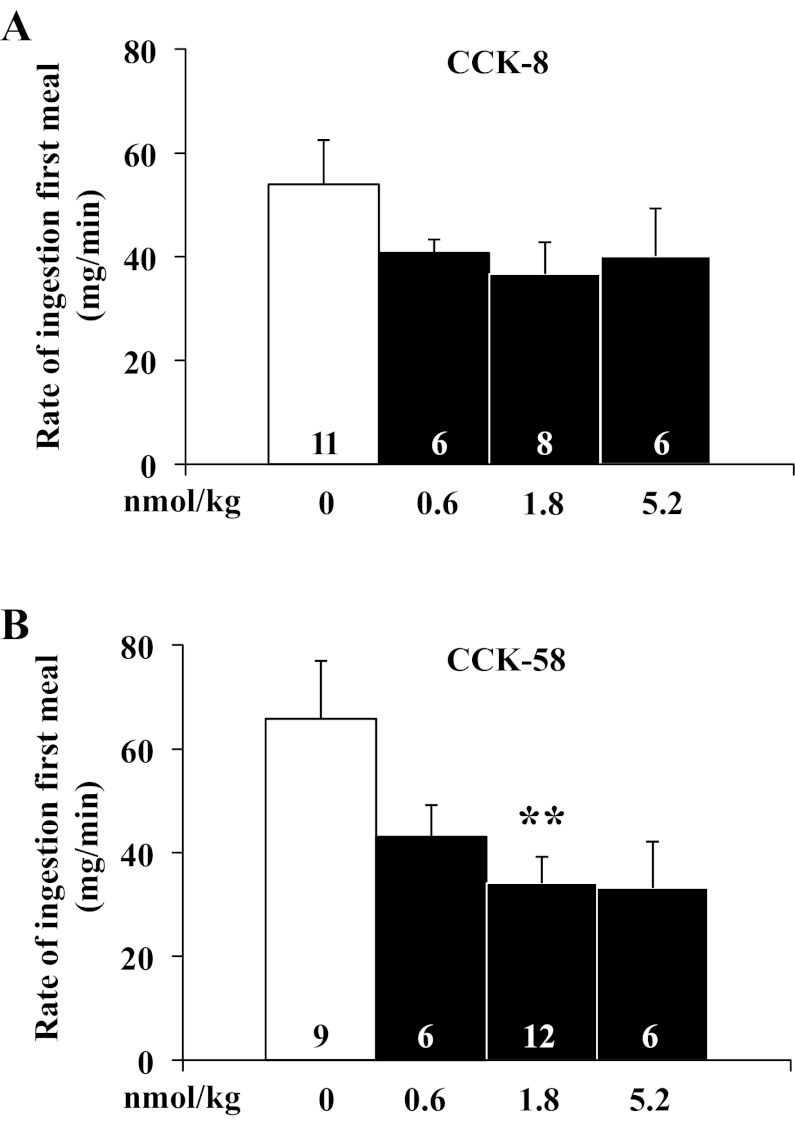
After establishing a similar potency on reduction of dark-phase food intake using equimolar doses of CCK-8 and CCK-58, the underlying food intake microstructure was investigated using automated episodic food intake monitoring. Intraperitoneal injection of CCK-8 reduced the duration of the first meal after 1.8 nmol/kg and 5.2 nmol/kg by 47% and 51%, respectively, compared with vehicle [F3,27 = 3.4, P < 0.05; 18.9 ± 3.1 and 17.2 ± 2.6 min, respectively, vs. 35.5 ± 5.9 min, P < 0.05; Fig. 3A), whereas with CCK-58, the reduction of the first meal duration did not reach significance at any dose tested (F3,29 = 1.1, P > 0.05; Fig. 3B). Two-way ANOVA showed a significant effect of dose (F3,53 = 3.5, P < 0.05), whereas treatment (F1,53 = 0.1, P > 0.05) or treatment × dose (F3,53 = 0.4, P > 0.05) had no significant effect. The meal size of the first meal of the dark phase was reduced by 1.8 nmol/kg and 5.2 nmol/kg ip CCK-8 injection by 57% and 41%, respectively, compared with vehicle (F3,27 = 6.2, P < 0.01; 1.7 ± 0.3, and 2.4 ± 0.5, respectively vs. 4.1 ± 0.4 g, P < 0.05; Fig. 4A), while for CCK-58, only 1.8 nmol/kg significantly reduced first meal size (F3,29 = 6.9, P < 0.05; −67%, 2.9 ± 0.6 vs. 4.4 ± 0.4 g, P < 0.05; Fig. 4B). Two-way ANOVA showed a significant influence of dose (F3,53 = 5.7, P < 0.01) but not treatment (F1,53 = 2.8, P > 0.05), or treatment × dose (F3,53 = 0.3, P > 0.05). The IMI between first and second meal of the dark phase was significantly reduced by intraperitoneal injection of 1.8 nmol/kg CCK-8 compared with vehicle (−41%, P < 0.05; Fig. 4C) and nonsignificantly reduced at the other doses, while there was no alteration of IMI following CCK-58 at any dose (F3,29 = 0.1, P > 0.05; 0.6 nmol/kg: 60.3 ± 5.8 min; 1.8 nmol/kg: 59.1 ± 8.3 min; 5.2 nmol/kg: 63.9 ± 5.6 min vs. vehicle: 52.8 ± 14.7 min, P > 0.05; Fig. 4D). Two-way ANOVA indicated a significant influence of treatment (F1,54 = 4.5, P < 0.05) but not dose (F3,54 = 0.3, P > 0.05) or treatment × dose (F3,54 = 0.9, P > 0.05). At 1.8 nmol/kg, these alterations resulted in a 1.8- and 2.6-times increase of the satiety ratio (IMI/meal size) induced by CCK-8 (25.9 ± 6.7 vs. 14.3 ± 1.9 min/g food eaten) and CCK-58 (30.1 ± 6.2 vs. 11.6 ± 2.5 min/g), respectively, compared with vehicle (P < 0.05; Fig. 4, E and F). Two-way ANOVA showed a significant effect of dose (F3,54 = 3.0, P < 0.05), whereas treatment (F1,54 = 0.1, P > 0.05) or treatment × dose (F3,54 = 0.2, P > 0.05) had no significant effect. The linear regression analysis of meal size and IMI following injection of CCK-8 showed a significant positive correlation (r = 0.56, P < 0.01), which was not observed following injection of CCK-58 (P > 0.05). The rate of ingestion of the first meal was not significantly altered at any dose of CCK-8 injected intraperitoneally at the onset of the dark phase (F3,27 = 1.2, P > 0.05; Fig. 5A), while CCK-58 (1.8 nmol/kg ip) decreased the rate of ingestion by 48% compared with vehicle (F3,29 = 3.6, P < 0.05; 34.1 ± 5.2 vs. 65.8 ± 11.1 mg/min, P < 0.01; Fig. 5B). Two-way ANOVA indicated a significant effect of dose (F3,53 = 4.6, P < 0.01) but not treatment (F1,53 = 0.1, P > 0.05) or treatment × dose (F3,53 = 0.5, P > 0.05).
Fig. 3.
CCK-8 reduces the duration of the first meal, whereas CCK-58 does not. Gates were closed at 90 min before the onset of the dark phase. Directly before lights off, rats were intraperitoneally injected with CCK-8 (0.6, 1.8, and 5.2 nmol/kg), CCK-58 (0.6, 1.8, and 5.2 nmol/kg) or vehicle (saline containing 0.1% BSA), placed back in their home cage, the gates opened, recording started and lights turned off. The duration of the first meal was analyzed and shown for CCK-8 (A) and CCK-58 (B). Scale bars represent mean ± SE of number of rats indicated at the bottom. *P < 0.05 vs. vehicle.
Fig. 4.
CCK-8 and CCK-58 both reduce the meal size of the first meal and increase satiety ratio, but only CCK-8 reduces the intermeal interval. Gates were closed at 90 min before the onset of the dark phase. Directly before lights off, animals were injected intraperitoneally with CCK-8 or CCK-58 (0.6, 1.8, and 5.2 nmol/kg), or vehicle (saline containing 0.1% BSA), placed back in their home cage, the gates opened, recording started, and lights turned off. The meal size of the first meal (A, B), the intermeal interval between first and second meal of the dark phase (C, D) was extracted and the satiety ratio (intermeal interval/meal size) calculated (E, F). Bars represent mean ± SE of number of rats indicated at the bottom. *P < 0.05 and ***P < 0.001 vs. vehicle.
Fig. 5.
CCK-58, but not CCK-8, decreases the rate of ingestion of the first meal. Gates were closed at 90 min before the onset of the dark phase. Directly before lights off, rats were injected with CCK-8 (0.6, 1.8 and 5.2 nmol/kg ip), CCK-58 (0.6, 1.8 and 5.2 nmol/kg ip) or vehicle (saline containing 0.1% BSA) and placed back in their home cage. Then, the gates were opened, recording started, and lights were turned off. The rate of ingestion for the first meal was analyzed and shown for CCK-8 (A) and CCK-58 (B). Bars represent means ± SE of number of rats indicated at the bottom. **P < 0.01 vs. vehicle.
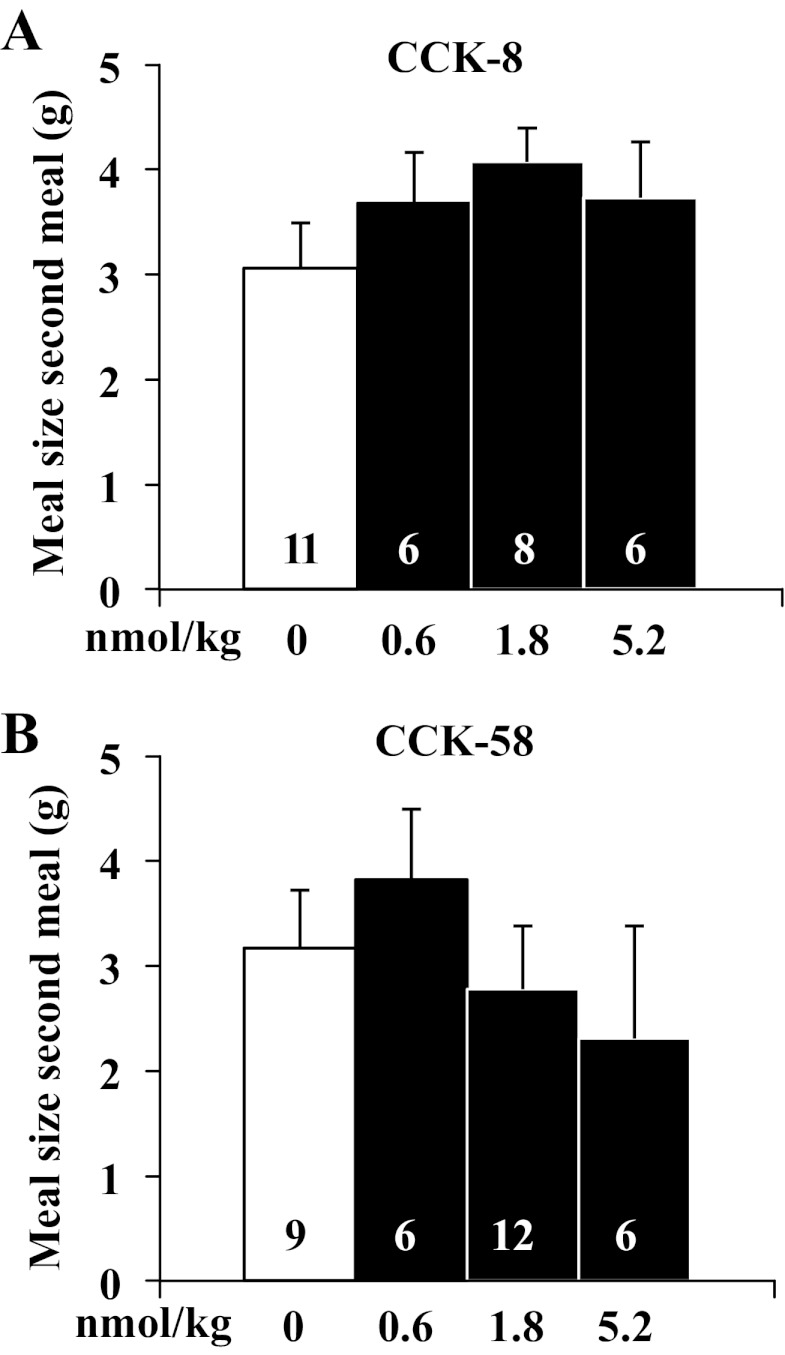
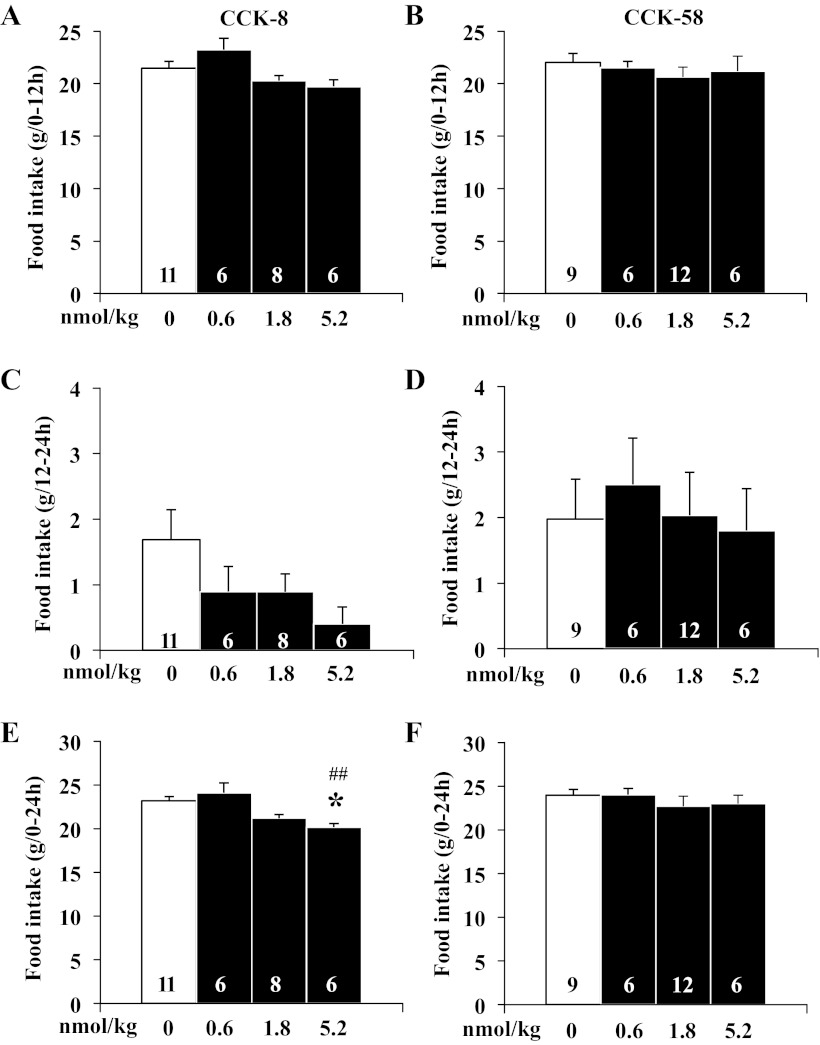
Neither CCK-8 (F3,27 = 1.1, P > 0.05) nor CCK-58 (F3,29 = 0.5, P > 0.05) had an effect on the size of the second meal (Fig. 6, A and B). Two-way ANOVA indicated no significant effect of treatment (F1,53 = 1.9, P > 0.05), dose (F3,53 = 0.5, P > 0.05), or treatment × dose (F3,53 = 1.0, P > 0.05). Similarly, there was no change in cumulative dark phase food intake (0–12 h, CCK-8: F3,27 = 3.6, P > 0.05; CCK-58: F3,29 = 0.6, P > 0.05) or consecutive light-phase food intake (12–24 h, CCK-8: F3,27 = 1.9, P > 0.05; CCK-58: F3,29 = 0.1, P > 0.05) following injection of either CCK-8 or CCK-58 (Fig. 7, A–D). Two-way ANOVA indicated no significant effect of treatment (F1,54 = 0.4, P > 0.05), dose (F3,54 = 1.8, P > 0.05), or treatment × dose (F3,54 = 1.4, P > 0.05). The cumulative 24-h food intake was not changed following injection of CCK-58 (F3,29 = 0.5, P > 0.05; Fig. 7F), whereas the highest dose of CCK-8 modestly, but significantly, reduced the 24-h food intake compared with vehicle (F3,27 = 7.4, P < 0.01; Fig. 7E). Two-way ANOVA showed a significant effect of dose (F3,54 = 3.4, P < 0.05) but not treatment (F1,54 = 3.8, P > 0.05) or treatment × dose (F3,54 = 0.7, P > 0.05).
Fig. 6.
CCK-8 and CCK-58 do not reduce the meal size of the second meal. Gates were closed at 90 min before the onset of the dark phase. Directly before lights off, animals were injected with CCK-8 or CCK-58 (0.6, 1.8, and 5.2 nmol/kg ip) or vehicle (saline containing 0.1% BSA) and placed back in their home cage. Then, the gates were opened, recording started, and lights were turned off. The meal size of the second meal was extracted. Bars represent means ± SE of number of rats indicated at the bottom.
Fig. 7.
CCK-8 and CCK-58 do not induce a compensatory increase of food intake within 24 h after peptide injection. Data were analyzed over the period of 24 h following injection. There was no change in cumulative dark-phase food intake (0–12 h, A, B) or consecutive light-phase food intake (12–24 h, C, D) following injection of either CCK-8 or CCK-58, giving no indication of a compensatory increase in food intake. The cumulative 24-h food intake was not changed following injection of CCK-58 (F), whereas the highest dose of CCK-8 (E) modestly but significantly reduced the 24-h food intake. Bars represent means ± SE of number of rats indicated at the bottom. *P < 0.05 vs. vehicle and ##P < 0.01 vs. 0.6 nmol/kg.
CCK-8 and CCK-58 differentially affect behavior.
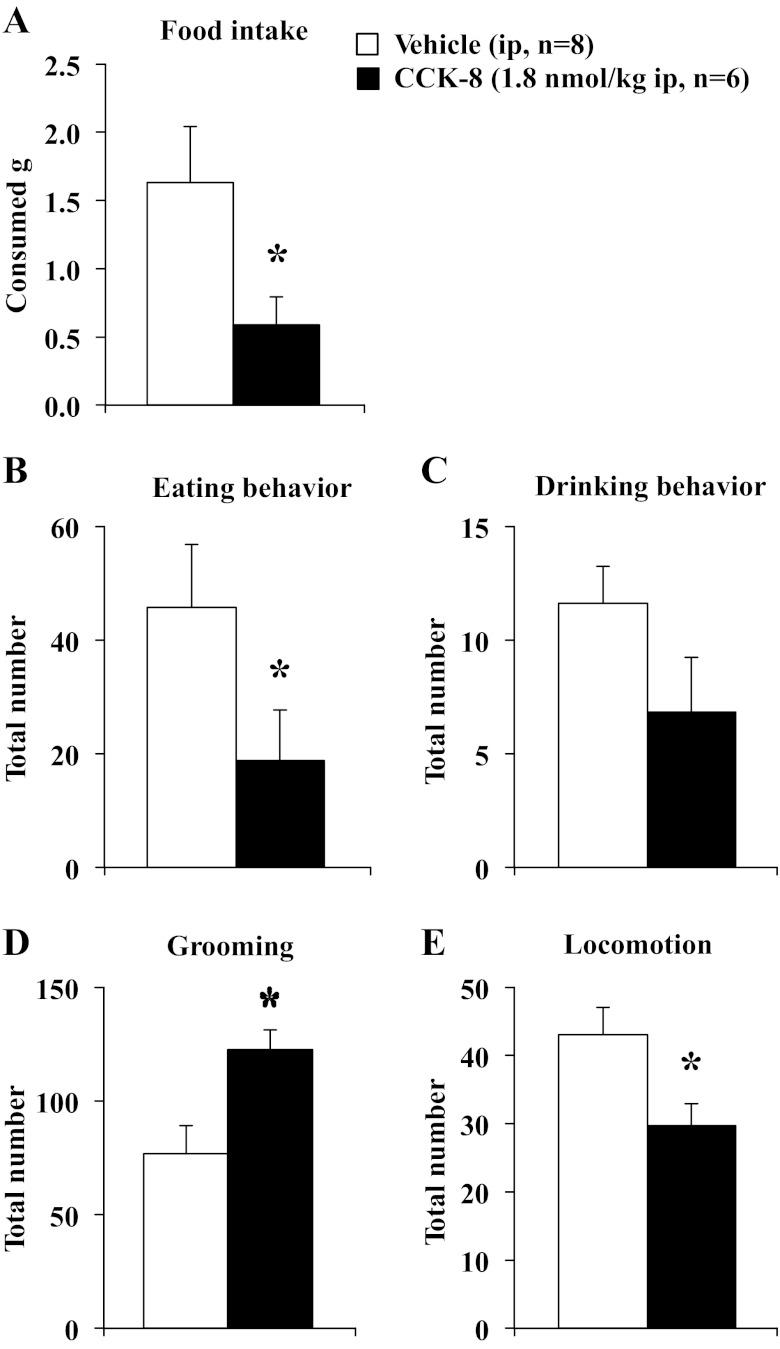
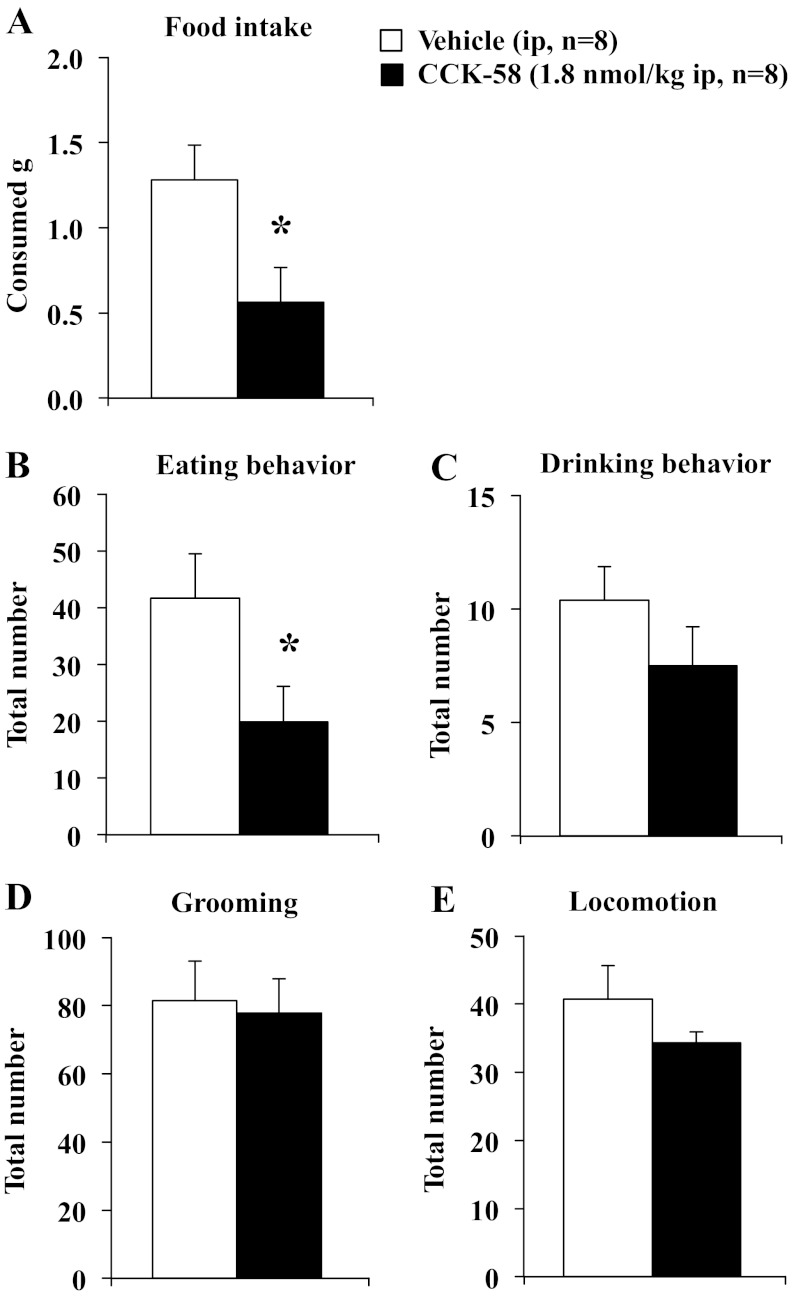
On the basis of the food intake microstructure data described above, a dose of 1.8 nmol/kg was used for the behavioral analyses following intraperitoneal injection of CCK-8 and CCK-58. To confirm the effect on feeding, food intake was assessed as well. The 40-min, dark-phase food intake was significantly reduced by 64% following CCK-8 compared with vehicle (0.6 ± 0.2 vs. 1.6 ± 0.4 g, P < 0.05) when food ingestion was monitored manually (Fig. 8A). Similarly, CCK-58 (1.8 nmol/kg ip) decreased the 40-min, dark-phase food intake by 56% compared with vehicle-injected (0.6 ± 0.2 vs. 1.3 ± 0.2 g ip, P < 0.05; Fig. 9A). Two-way ANOVA indicated a significant effect of dose (F1,26 = 9.6, P < 0.01) but not treatment (F1,26 = 0.4, P > 0.05). During that time, CCK-8 reduced the eating behavior (food intake and food approach) by 59% (18.8 ± 8.8 vs. 45.8 ± 11.1, P < 0.05; Fig. 8B), while drinking behavior (water intake and water approach) was not significantly altered compared with vehicle (P > 0.05; Fig. 8C). Following CCK-58, eating behavior was reduced compared with vehicle (−52%, 19.9 ± 6.2 vs. 41.6 ± 7.8, P < 0.05; Fig. 9B), whereas drinking behavior was not significantly altered (P > 0.05; Fig. 9C). For eating behavior, two-way ANOVA showed a significant effect of dose (F1,26 = 7.6, P < 0.05) but not treatment (F1,26 = 0.1, P > 0.05). CCK-8 increased grooming behavior by 59% (122.4 ± 8.9 vs. 76.8 ± 12.3, P < 0.05; Fig. 8D), while locomotor activity was significantly reduced compared with vehicle (−31%, 29.7 ± 3.3 vs. 43.0 ± 4.1, P < 0.05; Fig. 8E). Neither grooming behavior (Fig. 9D) nor locomotor activity (Fig. 9E) was affected by injection of CCK-58 compared with interperitoneal vehicle (P > 0.05). For grooming behavior, two-way ANOVA indicated a significant interaction of treatment × dose (F1,26 = 4.6, P < 0.05), whereas for locomotor activity, two-way ANOVA showed a significant effect of dose (F1,26 = 6.8, P < 0.05), but not treatment (F1,26 = 0.1, P > 0.05).
Fig. 8.
CCK-8 increases grooming behavior and decreases locomotor activity. Rats were injected ip with CCK-8 (1.8 nmol/kg) or vehicle (saline containing 0.1% BSA) and placed back in their home cage with paper under the cage divided into six equal squares with free access to food and water. Food intake was assessed for 40 min (A). At 10 min after injection, behaviors, including eating (including food approach, B), drinking (including water approach, C), and grooming behavior (washing, licking, and scratching; D), as well as locomotor activity (total number of squares crossed; E) were monitored manually for 30 min by an observer blinded to the animals' treatment. Each behavior was counted again when lasting >5 s. Bars indicate means ± SE of 6–8 rats/group. *P < 0.05 vs. vehicle.
Fig. 9.
CCK-58 selectively decreases eating behavior, while not altering grooming and locomotor activity. Animals were injected with CCK-58 (1.8 nmol/kg ip) or vehicle (saline containing 0.1% BSA) and placed back in their home cage with paper under the cage divided into six equal squares with free access to food and water. Food intake was assessed for 40 min (A). At 10 min after injection behaviors including eating (including food approach; B), drinking (including water approach; C) and grooming behavior (washing, licking, and scratching; D), as well as locomotor activity (total number of squares crossed; E) were monitored manually for 30 min by an observer blinded to the animals' treatment. Each behavior was counted again when lasting >5 s. Bars indicate mean ± SE of 8 rats/group. *P < 0.05 vs. vehicle.
DISCUSSION
In this study, we compared and analyzed the effects of intraperitoneal CCK-8 and CCK-58 on dark-phase meal pattern in undisturbed rats fed a solid meal. In line with previous studies, both CCK-8 and CCK-58 dose dependently reduced 1-h dark-phase food intake and first meal size at an equimolar dose of 1.8 nmol. However, microstructure analysis suggests that this was achieved by different influences on food intake parameters and behavior. At this dose, CCK-58, but not CCK-8, increased the latency to the first meal, while the IMI was reduced after CCK-8 but not after CCK-58. While CCK-8 decreased the duration of the first meal, CCK-58 decreased the rate of ingestion. In addition, CCK-8 induced fine movement and reduced locomotor activity, while CCK-58 did not elicit such changes. Importantly, both, CCK-8 and CCK-58 increased satiety ratio compared with vehicle.
Previous in vitro studies with CCK-8 and CCK-58 demonstrated that the two peptides caused similar effects on pancreatic functions, as indicated by comparable increases of intracellular Ca2+ concentrations in murine pancreatic acinar cell preparations and similar actions on cell death (7). These actions seem to be CCK1 receptor-mediated, as both peptides bind to this receptor and drive similar actions, such as Ca2+ response and receptor internalization (60). In contrast, in vivo studies showed differential effects of the two molecular forms on pancreatic physiology and pathology. CCK-58, but not CCK-8, strongly stimulates pancreatic fluid secretion in rats (46, 62), and CCK-8, but not CCK-58, induces pancreatitis (61). Sayegh and coworkers (33) have shown that effects of endogenous CCK on meal size and IMI are partly regulated by the CCK1 receptor (55). Because these findings suggest a differential action in vivo, even though both CCK-8 and CCK-58 bind to the same receptors with different affinity (45), it is important to address what accounts for these different actions. CCK-58 showed three times higher potency to bind mouse pancreatic CCK1 receptors than CCK-8, while binding to mouse brain CCK2 receptors was equipotent for the two peptides (45). Differences in tertiary structure of the carboxyl terminus of CCK-8 and CCK-58 may influence receptor binding. From pharmacokinetic studies in dogs, it is known that CCK-8 has approximately four times shorter circulating half-life than CCK-58 (25). In their study, Hoffmann et al. (25) determined that the half-life of CCK-58 was 4.4 ± 0.6 min compared with 1.3 ± 0.1 min for CCK-8 when both forms of CCK were intravenously bolus-injected in dogs. Similar experiments have not been conducted in rats, and it will be important to assess the half-life and degradation of the two CCK forms in rats injected intraperitoneally and intravenously. A possible explanation for different actions between CCK-8 and CCK-58, in addition to potential receptor binding, is the stability to enzymatic digestion of CCK-58 after intraperitoneal injection (45).
Most studies on CCK's regulation of food intake have been done with CCK-8 (2, 22, 30), after long fasting periods or during the light phase using liquid meals (32, 33, 36, 37). So far, only one report compared the feeding microstructure in response to CCK-8 and CCK-58, using an automated system measuring the intake of powdered food (20). There are several factors in our studies that help to achieve a physiological approach. The studies presented here are performed in rats with free access to solid food compared with powdered or liquid food, and they are conducted without a fasting period during the dark phase when nocturnal animals have their highest food consumption. The automated feeding system provides continuous measurement and allows for dissection of food intake parameters. Our studies suggest that both exogenous CCK-8 and CCK-58 reduce meal size. However, IMI and, therefore, satiety ratio are much less studied. In two studies, releasers of CCK, such as camostat or duodenal fat infusions, released endogenous CCK in a fashion similar to a meal, and two other studies show in rats with duodenal cannulas that these agents caused a reduction of liquid meal size and lengthening of IMI compared with vehicle infusion (5, 59). In our studies, CCK-58 tended to increase IMI, but this difference was not significant. The variation between the previous studies and our study may result from the experimental set-up, but more importantly, the use of the endogenous form of CCK-58 vs. CCK-8. In addition, at this point, we do not know whether the longer IMI produced by CCK-58 vs. CCK-8 is a paracrine or endocrine effect. This could be studied by comparing continuous intravenous and celiac artery infusions of the peptide as done by Cox et al. (6) for CCK-8.
An assumption about the IMI is that it is regulated by the size of the meal immediately preceding the IMI. Thus, the smaller the meal is, the shorter the IMI and vice versa. Therefore, to influence food consumption, either IMI or meal size can be modulated. An ideal satiety hormone would reduce meal size, but at the same time, maintain or prolong the IMI, so the total amount eaten will be decreased. Linear regression analysis of meal size and IMI following injection of CCK-8 showed a significant positive correlation, indicating that bigger meals were followed by a longer period of noneating, whereas after smaller meals, rats started to eat sooner. Whether injection of CCK-8 actively shortened the IMI or the shorter IMI reflects the anticipated concomitant shortening that follows a smaller first meal, remains to be elucidated, but most likely, this is a normal physiological phenomenon and not related to CCK-8. In contrast, injection of CCK-58 reduced the first meal size, while not altering the IMI, giving rise to a CCK-58-induced effect, preventing the compensatory decrease of the IMI. These results suggest meal size and IMI are regulated at different sites, or there is a different action of CCK-8 and CCK-58 at the same site. The small difference in half lives, which is less than 5 min for both peptides (25) suggests that the differences in IMI do not result from varying half-lives. In addition, the increased latency to start the first meal further supports that meal size and IMI result from different mechanisms. Our results with exogenous CCK-58 are in keeping with recent findings where camostat gavage (which releases endogenous CCK) reduced food intake by decreasing meal size and prolonging IMI, whereas exogenous CCK-8 reduced food intake by reducing meal size only (33).
Because the satiety ratio is calculated by IMI (min) divided by the first meal (g), an extended IMI and small meal size result in a high satiety ratio. Therefore, a negative correlation of these two parameters would be indicative of the highest satiety ratio. However, the alteration of only one factor can still significantly affect the satiety ratio. The satiety ratio was increased after CCK-58 due to the decrease in meal size and unaltered IMI. The stronger decrease of meal size observed for one dose of CCK-8 also caused an increase in satiety ratio, although the IMI was reduced. In addition, only CCK-58 significantly reduced the rate of ingestion of the first meal compared with vehicle. Although dark-phase food intake was reduced for both, CCK-8 and CCK-58 distinctly altered feeding microstructure parameters, pointing toward the hypothesis that different regulatory mechanisms control the feeding patterns induced by the two peptides.
Reduction of food intake can concur with behavioral alterations. Therefore, behavioral analyses were performed as established in our previous studies (50, 53). Both forms of CCK reduced food intake during the early dark phase due to a reduction in eating behavior (eating, as well as food approach, and sniffing the food). Whereas CCK-8 increased grooming and reduced locomotor activity compared with vehicle, the parameters were not changed after CCK-58. The changes observed after CCK-8 could be signs of postprandial fullness. Furthermore, the highest dose of CCK-8 modestly reduced the 24-h food intake, possibly due to induction of malaise, whereas cumulative 24-h food intake was not changed following injection of CCK-58. However, other studies that also evaluated different doses of CCK-8 (4–16 μg/kg, ∼3.6–16.2 nmol/kg) (10) or infused CCK-8 continuously over 8 days (57) concluded the peptide specifically reduced food intake without causing malaise (10, 57). In their study, West et al. (57) proposed a reduction in meal frequency as a determinant for malaise and taste aversion as produced by lithium chloride. In another study by West et al. (58), intermeal infusion of CCK-8 failed to prolong the IMI but initially prevented the compensatory decrease in IMI and increased feeding frequency expected after meal size was reduced. Failure of CCK-8 to affect feeding behavior similar to lithium chloride is indirect evidence that the reduction of food intake by CCK-8 is not simply a result of aversive behavior but is a specific effect (12). Thus, compared with taste-aversive agents like lithium chloride and with the lower doses used, it can be speculated that in the present study, malaise or taste aversion are unlikely to contribute to the observed CCK-8-induced reduction of food intake.
Perspectives and Significance
CCK-8 and CCK-58 injected at equimolar doses reduce food intake to a similar extent, although the underlying food intake microstructure differs. Moreover, only CCK-8 induces fine movement (grooming), while reducing locomotor activity. At equimolar doses CCK-8 significantly reduces IMI while CCK-58 does not. This suggests that meal size and IMI are regulated at different sites or they have different actions at the same site, and the common idea that all molecular forms of CCK have the same physiological actions needs reconsideration, highlighting the importance of studying the actual endogenous molecular form of peptides, especially considering the finding that CCK-58 is the only circulating form of cholecystokinin (9, 13, 42).
GRANTS
This study was supported by National Institutes of Health center grant DK-41301 (Animal Core, Peptidomic RIA Proteomic Core, to Y. Taché and J. R. Reeve, Jr.), R01 grant DK-083449 (to J. R. Reeve, Jr.), and a Veterans Administration Research Career Scientist Award and Merit Award (to Y. Taché).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.G.-S. and J.R.R.J. conception and design of research; M.G.-S., A.S., and L.W. performed experiments; M.G.-S., A.S., and J.R.R.J. analyzed data; M.G.-S., A.S., G.O., Y.F.T., and J.R.R.J. interpreted results of experiments; M.G.-S. and A.S. prepared figures; M.G.-S. and J.R.R.J. drafted manuscript; M.G.-S., A.S., L.W., G.O., Y.F.T., and J.R.R.J. edited and revised manuscript; M.G.-S., A.S., L.W., G.O., Y.F.T., and J.R.R.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sara Bassilian for her technical support.
REFERENCES
- 1. Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol 89: 784–790, 1975 [DOI] [PubMed] [Google Scholar]
- 2. Barrachina MD, Martinez V, Wang L, Wei JY, Taché Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94: 10455–10460, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 28: 325–353, 1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brand SJ, Morgan RG. The release of rat intestinal cholecystokinin after oral trypsin inhibitor measured by bio-assay. J Physiol 319: 325–343, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burton-Freeman B, Gietzen DW, Schneeman BO. Cholecystokinin and serotonin receptors in the regulation of fat-induced satiety in rats. Am J Physiol Regul Integr Comp Physiol 276: R429–R434, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cox JE, McCown SM, Bridges JM, Tyler WJ. Inhibition of sucrose intake by continuous celiac, superior mesenteric, and intravenous CCK-8 infusions. Am J Physiol Regul Integr Comp Physiol 270: R319–R325, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Criddle DN, Booth DM, Mukherjee R, McLaughlin E, Green GM, Sutton R, Petersen OH, Reeve JR., Jr Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 297: G1085–G1092, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eberlein GA, Eysselein VE, Goebell H. Cholecystokinin-58 is the major molecular form in man, dog and cat but not in pig, beef and rat intestine. Peptides 9: 993–998, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Eberlein GA, Eysselein VE, Hesse WH, Goebell H, Schaefer M, Reeve JR., Jr Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol Gastrointest Liver Physiol 253: G477–G482, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Eckel LA, Ossenkopp KP. Cholecystokinin reduces sucrose palatability in rats: evidence in support of a satiety effect. Am J Physiol Regul Integr Comp Physiol 267: R1496–R1502, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Eng J, Du BH, Pan YC, Chang M, Hulmes JD, Yalow RS. Purification and sequencing of a rat intestinal 22 amino acid C-terminal CCK fragment. Peptides 5: 1203–1206, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Ervin GN, Teeter MN. Cholecystokinin octapeptide and lithium produce different effects on feeding and taste aversion learning. Physiol Behav 36: 507–512, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR., Jr Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem 262: 214–217, 1987 [PubMed] [Google Scholar]
- 14. Eysselein VE, Eberlein GA, Schaeffer M, Grandt D, Goebell H, Niebel W, Rosenquist GL, Meyer HE, Reeve JR., Jr Characterization of the major form of cholecystokinin in human intestine: CCK-58. Am J Physiol Gastrointest Liver Physiol 258: G253–G260, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Folsch UR, Cantor P, Wilms HM, Schafmayer A, Becker HD, Creutzfeldt W. Role of cholecystokinin in the negative feedback control of pancreatic enzyme secretion in conscious rats. Gastroenterology 92: 449–458, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Garlicki J, Konturek PK, Majka J, Kwiecien N, Konturek SJ. Cholecystokinin receptors and vagal nerves in control of food intake in rats. Am J Physiol Endocrinol Metab 258: E40–E45, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Geary N. A new way of looking at eating. Am J Physiol Regul Integr Comp Physiol 288: R1444–R1446, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495, 1973 [DOI] [PubMed] [Google Scholar]
- 20. Glatzle J, Raybould HE, Kueper MA, Reeve JR, Jr, Zittel TT. Cholecystokinin-58 is more potent in inhibiting food intake than cholecystokinin-8 in rats. Nutr Neurosci 11: 69–74, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Goebel M, Stengel A, Wang L, Taché Y. Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides 32: 36–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St-Pierre DH, Taché Y. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides 27: 2811–2819, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Green GM, Taguchi S, Friestman J, Chey WY, Liddle RA. Plasma secretin, CCK, and pancreatic secretion in response to dietary fat in the rat. Am J Physiol Gastrointest Liver Physiol 256: G1016–G1021, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Harper AA, Raper HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol 102: 115–125, 1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffmann P, Eberlein GA, Reeve JR, Jr, Bunte RH, Grandt D, Goebell H, Eysselein VE. Comparison of clearance and metabolism of infused cholecystokinins 8 and 58 in dogs. Gastroenterology 105: 1732–1736, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Huttner WB. Tyrosine sulfation and the secretory pathway. Annu Rev Physiol 50: 363–376, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Ivy AC, Oldenberg E. A hormone mechanism for gall bladder contraction. Am J Physiol 86: 599–613, 1928 [Google Scholar]
- 28. Izzo RS, Brugge WR, Praissman M. Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul Pept 9: 21–34, 1984 [DOI] [PubMed] [Google Scholar]
- 29. Jorpes E, Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand 66: 196–202, 1966 [DOI] [PubMed] [Google Scholar]
- 30. Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, van der Voort IR, Veh RW, Werner CR, Klapp BF, Wiedenmann B, Wang L, Taché Y, Mönnikes H. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol 288: R751–R758, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol 497: 41–47, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Larsen CJ, Washington MC, Sayegh AI. Cholecystokinin-8 increases the satiety ratio in diabetic rats more than cholecystokinin-33. Physiol Behav 101: 649–652, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Lateef DM, Washington MC, Sayegh AI. The short-term satiety peptide cholecystokinin reduces meal size and prolongs intermeal interval. Peptides 21: 1289–1295, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Liddle RA, Goldfine ID, Williams JA. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87: 542–549, 1984 [PubMed] [Google Scholar]
- 35. Linden A, Carlquist M, Hansen S, Uvnas-Moberg K. Plasma concentrations of cholecystokinin, CCK-8, and CCK-33, 39 in rats, determined by a method based on enzyme digestion of gastrin before HPLC and RIA detection of CCK. Gut 30: 213–222, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melville LD, Smith GP, Gibbs J. Devazepide antagonizes the inhibitory effect of cholecystokinin on intake in sham-feeding rats. Pharmacol Biochem Behav 43: 975–977, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272: R1245–R1251, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem 6: 156–162, 1968 [DOI] [PubMed] [Google Scholar]
- 39. Ogilvie KM, Saladin R, Nagy TR, Urcan MS, Heyman RA, Leibowitz MD. Activation of the retinoid X receptor suppresses appetite in the rat. Endocrinology 145: 565–573, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Polak JM, Bloom SR, Rayford PL, Pearse AG, Buchan AM, Thompson JC. Identification of cholecystokinin-secreting cells. Lancet 2: 1016–1018, 1975 [DOI] [PubMed] [Google Scholar]
- 41. Reeve JR, Jr, Eysselein V, Eberlein GA, Chew P, Ho FJ, Huebner VD, Shively JE, Lee TD, Liddle RA. Characterization of canine intestinal cholecystokinin-58 lacking its carboxyl-terminal nonapeptide Evidence for similar post-translational processing in brain and gut. J Biol Chem 266: 13770–13776, 1991 [PubMed] [Google Scholar]
- 42. Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol 285: G255–G265, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Reeve JR, Jr, Keire DA, Coskun T, Green GM, Evans C, Ho FJ, Lee TD, Davis MT, Shively JE, Solomon TE. Synthesis of biologically active canine CCK-58. Regul Pept 113: 71–77, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Reeve JR, Jr, Liddle RA, McVey DC, Vigna SR, Solomon TE, Keire DA, Rosenquist G, Shively JE, Lee TD, Chew P, Green GM, Coskun T. Identification of nonsulfated cholecystokinin-58 in canine intestinal extracts and its biological properties. Am J Physiol Gastrointest Liver Physiol 287: G326–G333, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Reeve JR, Jr, McVey DC, Bunnett NW, Solomon TE, Keire DA, Ho FJ, Davis MT, Lee TD, Shively JE, Vigna SR. Differences in receptor binding and stability to enzymatic digestion between CCK-8 and CCK-58. Pancreas 25: e50–e55, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Reeve JR, Jr, Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T. Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am J Physiol Gastrointest Liver Physiol 286: G395–G402, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Rehfeld JF, Sun G, Christensen T, Hillingso JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab 86: 251–258, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Rosenwasser AM, Boulos Z, Terman M. Circadian organization of food intake and meal patterns in the rat. Physiol Behav 27: 33–39, 1981 [DOI] [PubMed] [Google Scholar]
- 49. Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, Weyer C, Parkes DG. Combination therapy with amylin and peptide YY[3–36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology 148: 6054–6061, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology 151: 4224–4235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology 150: 4911–4919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Activation of brain somatostatin 2 receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav 101: 614–622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Selective central activation of somatostatin receptor 2 increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol 61: 399–407, 2010 [PMC free article] [PubMed] [Google Scholar]
- 54. Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, Reeve JR., Jr The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology 150: 5113–5118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sullivan CN, Raboin SJ, Gulley S, Sinzobahamvya NT, Green GM, Reeve JR, Jr, Sayegh AI. Endogenous cholecystokinin reduces food intake and increases Fos-like immunoreactivity in the dorsal vagal complex but not in the myenteric plexus by CCK1 receptor in the adult rat. Am J Physiol Regul Integr Comp Physiol 292: R1071–R1080, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Weatherford SC, Laughton WB, Salabarria J, Danho W, Tilley JW, Netterville LA, Schwartz GJ, Moran TH. CCK satiety is differentially mediated by high- and low-affinity CCK receptors in mice and rats. Am J Physiol Regul Integr Comp Physiol 264: R244–R249, 1993 [DOI] [PubMed] [Google Scholar]
- 57. West DB, Greenwood MR, Marshall KA, Woods SC. Lithium chloride, cholecystokinin and meal patterns: evidence that cholecystokinin suppresses meal size in rats without causing malaise. Appetite 8: 221–227, 1987 [DOI] [PubMed] [Google Scholar]
- 58. West DB, Greenwood MR, Sullivan AC, Prescod L, Marzullo LR, Triscari J. Infusion of cholecystokinin between meals into free-feeding rats fails to prolong the intermeal interval. Physiol Behav 39: 111–115, 1987 [DOI] [PubMed] [Google Scholar]
- 59. Woltman T, Castellanos D, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of oleic acid in rats. Am J Physiol Regul Integr Comp Physiol 269: R1420–R1433, 1995 [DOI] [PubMed] [Google Scholar]
- 60. Wu SV, Harikumar KG, Burgess RJ, Reeve JR, Jr, Miller LJ. Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation. Am J Physiol Gastrointest Liver Physiol 295: G641–G647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamamoto M, Reeve JR, Jr, Green GM. Supramaximal CCK-58 does not induce pancreatitis in the rat: role of pancreatic water secretion. Am J Physiol Gastrointest Liver Physiol 292: G964–G974, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Yamamoto M, Reeve JR, Jr, Keire DA, Green GM. Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am J Physiol Gastrointest Liver Physiol 288: G866–G879, 2005 [DOI] [PubMed] [Google Scholar]