Abstract
Studies of experimental diabetes mellitus (DM) suggest that increased nitric oxide (NO) bioactivity contributes to renal hyperfiltration. However, the role of NO in mediating hyperfiltration has not been fully elucidated in humans. Our aim was to examine the effect of NO synthase inhibition on renal and peripheral vascular function in normotensive subjects with uncomplicated type 1 DM. Renal function and brachial artery flow-mediated vasodilatation (FMD) were measured before and after an intravenous infusion of the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NMMA) in 21 healthy control and 37 type 1 DM patients. Measurements in DM participants were made under clamped euglycemic conditions. The effect of l-NMMA on circulating and urinary NO metabolites (NOx) and cGMP and on urinary prostanoids was also determined. Baseline characteristics were similar in the two groups. For analysis, the DM patients were divided into those with hyperfiltration (DM-H, n = 18) and normal glomerular filtration rate (GFR) levels (DM-N, n = 19). Baseline urine NOx and cGMP were highest in DM-H. l-NMMA led to a decline in GFR in DM-H (152 ± 16 to 140 ± 11 ml·min−1·1.73 m−2) but not DM-N or healthy control participants. The decline in effective renal plasma flow in response to l-NMMA (806 ± 112 to 539 ± 80 ml·min−1·1.73 m−2) in DM-H was also exaggerated compared with the other groups (repeated measures ANOVA, P < 0.05), along with declines in urinary NOx metabolites and cGMP. Baseline FMD was lowest in DM-H compared with the other groups and did not change in response to l-NMMA. l-NMMA reduced FMD and plasma markers of NO bioactivity in the healthy control and DM-N groups. In patients with uncomplicated type 1 DM, renal hyperfiltration is associated with increased NO bioactivity in the kidney and reduced NO bioactivity in the systemic circulation, suggesting a paradoxical state of high renal and low systemic vascular NO bioactivity.
Keywords: endothelial function, hyperfiltration, nitric oxide, type 1 diabetes
glomerular hyperfiltration is an early renal hemodynamic change in animal and human studies of diabetes mellitus (DM) and may help to predict the risk for the subsequent development of diabetic nephropathy (38). The pathogenesis of hyperfiltration is complex and involves both tubuloglomerular feedback and hemodynamic abnormalities. Renal hemodynamic changes associated with hyperfiltration include afferent vasodilatation and efferent constriction. Hyperfiltration is, however, only partially corrected after selective cyclooxygenase-2 inhibition (predominant afferent constriction) or renin angiotensin system (RAS) blockade (predominant efferent vasodilatation) in humans with uncomplicated type 1 DM (8, 54). These findings suggest the presence of nonprostaglandin, non-RAS-dependent hemodynamic mechanisms that perpetuate the hyperfiltration state (60).
Functional expression studies in DM models have determined that endothelial nitric oxide (NO) synthase (eNOS) expression is consistently upregulated and localized to the afferent arteriole, renal cortex, and medulla (32). In addition to eNOS, macula densa neuronal NOS (nNOS) activation reduces afferent arteriolar tone and influences glomerular capillary pressure and tubuloglomerular feedback mechanisms that have been implicated in hyperfiltration (31, 62). Conversely, inducible NOS (iNOS) expression studies have demonstrated less consistent results (32). Therefore, it is generally accepted that eNOS and nNOS expression are upregulated in experimental models of DM leading to increased NO bioactivity, particularly in the early adaptive stages, but the functional significance of this upregulation in humans has not been fully elucidated (3).
In contrast with renal microvascular data suggesting increased NO bioactivity, studies examining systemic macrovascular function have suggested an opposite and paradoxical state of NO suppression. For example, animal models of type 1 DM have demonstrated a consistent state of NO-mediated renal hyperfiltration, despite evidence of decreased NO activity and endothelial dysfunction in large vessels (6, 37). This paradoxical state of high renal versus low macrovascular NO bioactivity may also exist in humans, since type 2 DM patients exhibit a positive association between urinary NO excretion and creatinine clearance in observational cohort studies, despite evidence of macrovascular dysfunction and low NO bioactivity (25, 26). While similar associations have been made in humans with type 1 DM (37), previous work has not, to our knowledge, used simultaneous gold-standard measures of renal microvascular and large vessel function to study this apparent renal-systemic NO paradox.
Accordingly, we hypothesized that NOS inhibition with NG-nitro-l-arginine methyl ester (l-NMMA) would result in a greater reduction in glomerular filtration rate (GFR) in hyperfiltering patients with type 1 DM (DM-H) compared with DM patients with normal GFR (DM-N) and healthy control subjects due to maximal renal NO stimulation in DM-H. Second, we hypothesized that paradoxically, impaired brachial artery FMD associated with renal hyperfiltration (9) would be associated with decreased responsiveness to l-NMMA in DM-H versus DM-N and healthy controls.
MATERIALS AND METHODS
Subjects.
Twenty-one healthy controls and thirty-seven participants with uncomplicated type 1 DM (19 DM-N and 18 with DM-H) participated in this study (Table 1). Hyperfiltration was defined using the usual definition of a GFR ≥ 135 ml·min−1·1.73 m−2 (38). Inclusion criteria were the following: duration of type 1 DM ≥5 yr, age ≥18 yr, blood pressure <140/90, no history of renal disease or macrovascular disease, and participants could not be taking any regular medications other than insulin and had to be normoalbuminuric on a 24-h urine collection. We aimed to study female subjects during the early follicular phase of the menstrual cycle, determined by cycle day and measurement of 17β-estradiol levels. None were using oral contraceptive medication. The local Research Ethics Board at the University Health Network (Toronto, Canada) approved the protocol, and all subjects gave informed consent.
Table 1.
Baseline characteristics and biochemistry
| Parameter | Healthy Control Group (n = 21) | Normofiltration Group (n = 19) | Hyperfiltration Group (n = 18) |
|---|---|---|---|
| Baseline demographic parameters | |||
| Males | 12/20 (60%) | 11/19 (58%) | 11/18 (61%) |
| Age, yr | 24 ± 4 | 23 ± 5 | 22 ± 4 |
| Diabetes duration, yr | N/A | 18 ± 7 | 17 ± 6 |
| Weight, kg | 71 ± 8 | 72 ± 12 | 73 ± 11 |
| Height, cm | 173 ± 9 | 173 ± 9 | 172 ± 9 |
| Body mass index, kg/m2 | 24 ± 1 | 24 ± 1 | 25 ± 1 |
| Baseline biochemistry | |||
| HbA1C-%, mmol/mol | N/A | 8.8 ± 0.4 (73 ± 4) | 8.8 ± 0.5 (72 ± 5) |
| Estrogen, pmol/l (in women) | 198 ± 130 | 220 ± 40 | 204 ± 135 |
| Sodium excretion, mmol/24 h | 168 ± 20 | 173 ± 20 | 180 ± 32 |
| Protein intake, g·kg−1·day−1 | 0.92 ± 0.26 | 0.90 ± 0.31 | 0.91 ± 0.24 |
| Baseline circulating RAS mediators | |||
| Aldosterone, ng/dl | 11.6 ± 9.5 | 1.73 ± 0.68* | 1.05 ± 0.43† |
| ANG II, pg/ml | 10.2 ± 6.9 | 3.1 ± 2.2* | 2.5 ± 1.5† |
| Renin, pg/ml | 10.3 ± 5.6 | 7.6 ± 5.3 | 5.5 ± 2.0† |
| Plasma renin activity, ng·ml−1·h−1 | 1.83 ± 1.0 | 1.05 ± 0.67 | 0.71 ± 0.36† |
| Angiotensinogen, ng/ml | 1,195 ± 94 | 1,290 ± 184 | 1,593 ± 366 |
Values are means ± SD; n, number of participants.
P < 0.05 in normofiltering subjects vs. healthy controls;
P < 0.05 in hyperfiltering subjects vs. healthy controls.
Experimental design.
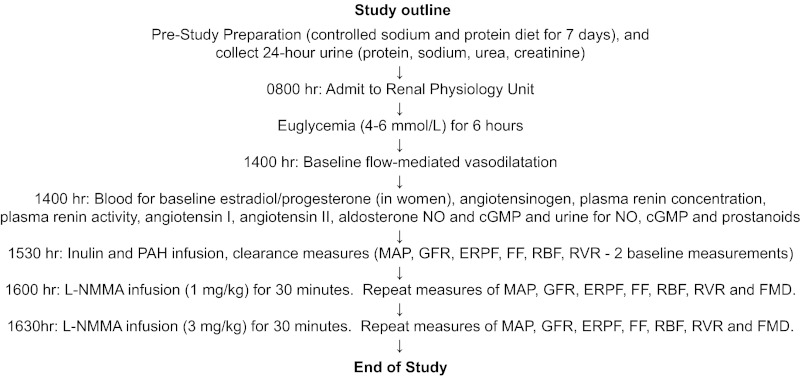
To maintain suppression of endogenous RAS activity, subjects adhered to a high-sodium (>140 mmol/day) and moderate -protein (<1.5 g·kg−1·day−1) diet during the 7-day period before each experiment, as described previously (Table 1, Fig. 1). In patients with DM, clamped euglycemic (4–6 mmol/l) conditions were maintained for ∼6 h preceding and during all investigations, a period of time previously demonstrated to be sufficient to influence vascular function (8). In all phases of the experiment, blood glucose was maintained by a modified glucose clamp technique, as described previously (8). A 16-gauge peripheral venous cannula was inserted into the left antecubital vein for infusion of glucose and insulin, and a second cannula was inserted for blood sampling more distally. Blood glucose was measured every 5–10 min, and the insulin infusion was adjusted to maintain euglycemia. In healthy control subjects, studies were performed on a single day during normoglycemic conditions. All experiments were performed in the same warm (25°C), temperature-controlled room and in a dark, quiet environment after 10 min of rest in the supine position.
Fig. 1.
Flow diagram. See text for more details and definitions of abbreviations.
After the desired level of clamped euglycemia was achieved, baseline measures of FMD were taken as described below, and baseline blood samples were collected for the following assays: inulin and p-aminohippurate (PAH) blank, baseline angiotensinogen, plasma renin concentration (PRC), plasma renin activity (PRA), angiotensin (ANG) II, and aldosterone. To assess biochemical makers of NO bioactivity, plasma NO metabolites (NO2 and NO3 or NOx) and cGMP were also measured. Baseline urine samples were also collected for NOx and cGMP excretion (corrected for creatinine concentration). Because of the important effect of NO blockade on prostanoids (58), we also measured the urinary excretion of 2,3-dinor-6-keto-PGF1-α, 6-keto-PGF1α thromboxane B2 (TXB2), PGE2, and 13,14-dihydro-15keto-PGE2 (PGEM). Next, baseline values of renal function were then obtained using inulin and PAH clearance techniques to obtain two baseline clearance periods, which were then averaged (8). l-NMMA (250 mg/vial, Clinalpha, Laüfelfingen, Switzerland) was then administered intravenously to direct drug to the renal circulation at incremental doses, first as a bolus of 1 mg/kg bolus in 5 min followed by a 1 mg/kg infusion over 25 min using established, standard dosing and infusion protocols (17, 19, 53, 61). The dose was then increased, with a 3 mg/kg bolus iv in 5 min, followed by an infusion of 3 mg/kg over 25 min. Renal function parameters (inulin and PAH clearances) were assessed at the end of each l-NMMA infusion period, at the same time that brachial artery vasodilatory responses were measured. Forearm blood flow and endothelium-dependent vasodilatation were measured starting at the midpoint of each 30-min interval after starting the l-NMMA infusion. At this 30-min time point, blood was also drawn for circulating NOx and cGMP levels. At the end of the high-dose l-NMMA infusion, we also measured urine NOx, cGMP, and prostanoid excretion. Arterial pressure and heart rate were measured by an automated sphygmomanometer over the right brachial artery (DINAMAP sphygmomanometer, Critikon) at 30-min intervals throughout the study and at 5-min intervals during the l-NMMA infusion.
Assessment of endothelial function.
Brachial artery endothelial function was determined by recording diameter changes in the brachial artery in response to increased blood flow generated during reactive hyperemia (flow-mediated dilatation) at baseline and in response to the graded infusion of l-NMMA. Briefly, the right brachial artery was scanned 2 to 5 cm above the antecubital fossa using high resolution B-mode vascular ultrasound (Vividi, 7–15 MHz linear-array transducer, GE/Vingmed). Longitudinal, ECG-gated, end-diastolic images were acquired over six cardiac cycles, and the brachial arterial diameter was determined for each image using integrated software, and the results were averaged (9). Diameter measurements were taken from the anterior to the posterior interface between the media and adventitia. After baseline images were recorded, the blood pressure cuff was inflated around the forearm distal to the elbow to >200 mmHg for 5 min. After cuff deflation, the increase in blood flow was measured (reactive hyperemia) along with the change in vessel diameter (endothelium-dependent dilatation), which was measured for a further 5 min. FMD was defined and reported as the maximal percentage changes in vessel diameter after reactive hyperemia (9). The intra-observer variability for repeated measurements of arterial diameters at flow-mediated vasodilatation was 0.01 ± 0.005 mm (absolute diameter) or 0.26 ± 0.01% (% absolute value of brachial artery at flow-mediated vasodilatation), which is similar to that previously reported (24, 30).
Assessment of renal parameters.
After the assessment of endothelial function, a third intravenous line was inserted into the right arm and was connected to a syringe infusion pump for administration of inulin and PAH. After blood was collected for inulin and PAH blank, a priming infusion containing 25% inulin (60 mg/kg) and 20% PAH (8 mg/kg) was administered. Thereafter, inulin and PAH were infused continuously at a rate calculated to maintain their respective plasma concentrations constant at 20 and 1.5 mg/dl. After a 90-min equilibration period, blood was collected for inulin, PAH, and hematocrit (Hct). Blood was further collected every 30 min for 60 min for inulin and PAH, and GFR and effective renal plasma flow (ERPF) were estimated by steady-state infusion of inulin and PAH, respectively (8).
Sample collection and analytical methods.
Blood samples collected for inulin and PAH determinations were immediately centrifuged at 3,000 rpm for 10 min at 4°C. Plasma was separated, placed on ice, and then stored at −70°C before the assay. Inulin and PAH were measured in serum by colorimetric assays using anthrone and N-(1-naphthy) ethylenediamine, respectively (14, 22, 29). The mean of two baseline clearance periods represent GFR and ERPF (expressed per 1.73 m2). Renal blood flow (RBF) was derived using ERPF/(1 − Hct), and renal vascular resistance (RVR) was derived by dividing the mean arterial pressure by the RBF. All renal hemodynamic measurements were adjusted for body surface area (14, 22).
Plasma renin concentration was measured by two-site immunoradiometric assay where two monoclonal antibodies to human active renin were used (no. 79986, Renin III Generation RIA Kit, Bio-Rad). One antibody was coupled to biotin while the second was radiolabeled for detection. The sample containing active renin was incubated simultaneously with both antibodies to form a complex. The radioactivity of this complex was directly proportional to the amount of immunoreactive renin present in the sample. PRA was measured with a radioimmunoassay kit (CA-1533, GammaCoat Plasma Renin Activity 125I RIA Kit, Diasorin, Stillwater, MN). Plasma ANG II, aldosterone, and angiotensinogen were also measured using previously described methods (7, 8).
To assess NO formation (40), plasma NOx and cGMP levels were measured at baseline and 30 min after the 1 and 3 mg/kg doses of l-NMMA. Urine NOx and cGMP levels were also measured at baseline and at the end of the 3 mg/kg l-NMMA infusion. The assay for cGMP is based on the competition between cGMP in the standards or samples and a cGMP-acetylcholinesterase (AChE) conjugate (cGMP tracer) for a limited number of cGMP-specific rabbit antibody binding sites. The rabbit antibody-cGMP complex (either free or tracer) binds to the mouse monoclonal antibody IgG that is coated to the well. The plate is washed to remove the unbound reagent, and then Ellman's Reagent (acetylthiocholine and 5,5′-dithiobis-2-nitrobenzoic acid, a substrate to AChE) is added to the well. The product of this enzymatic reaction, 5-thio-2-nitrobenzoic acid, has a distinct yellow color and absorbs strongly at 412 nm. The intensity of the color is proportional to the amount of cGMP tracer bound to the well, which is inversely proportional to the amount of free cGMP present in the standards or sample. Plasma samples are extracted with EtOH before analysis. For NOx levels (NO, Cat No. KGE001, total NO/nitrite/nitrate assay kit, R&D Systems, Minneapolis, MN) the assay determines NO concentration based on the enzymatic conversion of nitrate to nitrite by nitrate reductase. The reaction is followed by colorimetric detection of nitrite as an azo dye product of the Griess reaction. The Griess reaction is based on the two-step diazotation reaction in which acidified nitrite produces a nitrosating agent that reacts with sulfanilic acid to produce the diazonium ion. This ion is then coupled to N-(1-naphthyl)ethylenediamine to form the chromophoric azo-derivative that absorbs light at 540–570 nm.
Urinary prostanoids were also measured in all participants before and after the l-NMMA infusion, since the vascular effects of NO inhibition depend in part on prostanoid bioactivity (59). As we followed the recommended purification protocols, urinary prostaglandins (PGE2, 6-keto-PGF1α, TXB2) and their metabolites (PGEM, 2,3-dinor-6-keto-PGF1α) were measured by competitive enzyme immunoassays (Cayman Chemical). Quantification is based on a colorimetric reaction catalyzed by acetylcholinesterase. PGEM is a measure of both renal and systemic PGE2, whereas 2,3-dinor-6-keto-PGF1α corresponds to systemic prostacyclin production and subsequent urinary excretion (52). All samples were corrected for creatinine (expressed as pg/mg creatinine).
Urinary albumin excretion rate was determined from three timed overnight urine collections. Urinary albumin concentration was determined by immunoturbidimetry. Hemoglobin A1C was measured by high-performance liquid chromatography, and plasma insulin levels were measured in patients with DM using standard techniques (9).
Statistical analysis.
Descriptive statistics were used to compare baseline clinical and demographic characteristics. Between-group comparisons in baseline parameters in diabetic versus healthy control subject groups were made using analysis of variance (ANOVA). Changes in renal and systemic vascular function in response to l-NMMA were assessed using a mixed factorial two-way (group × time) repeated-measures ANOVA. All statistical analyses were performed using the statistical package SAS Version 9.2 and expressed as means ± SD. The vascular data were obtained and analyzed by a single observer (D. Z. I. Cherney) who was blinded to the renal measurements.
RESULTS
Baseline characteristics.
Healthy control, DM-N, and DM-H participants were similar with respect to gender distribution, age, body mass index, albumin excretion, and dietary parameters (Table 1). Although studies were scheduled to correspond to the early follicular phase of the menstrual cycle, plasma estrogen levels were variable and mean values were more consistent with the mid to late follicular cycle. Despite the variability, estrogen levels were similar in women in the three groups. In addition, the DM-N and DM-H groups exhibited similar plasma insulin levels (64 ± 30 vs. 74 ± 17 pmol/l, respectively), HbA1C, and diabetes duration parameters. None of the participants experienced adverse effects from the l-NMMA or other study procedures. In DM patients, mean venous blood glucose was 4.6 ± 0.6 mmol/l at the beginning of the vascular studies, indicating that the desired level of clamped euglycemia was achieved. In control subjects, mean venous blood glucose was 4.5 ± 0.2 mmol/l (range 4.2–5.4 mmol/l), again indicating normoglycemia.
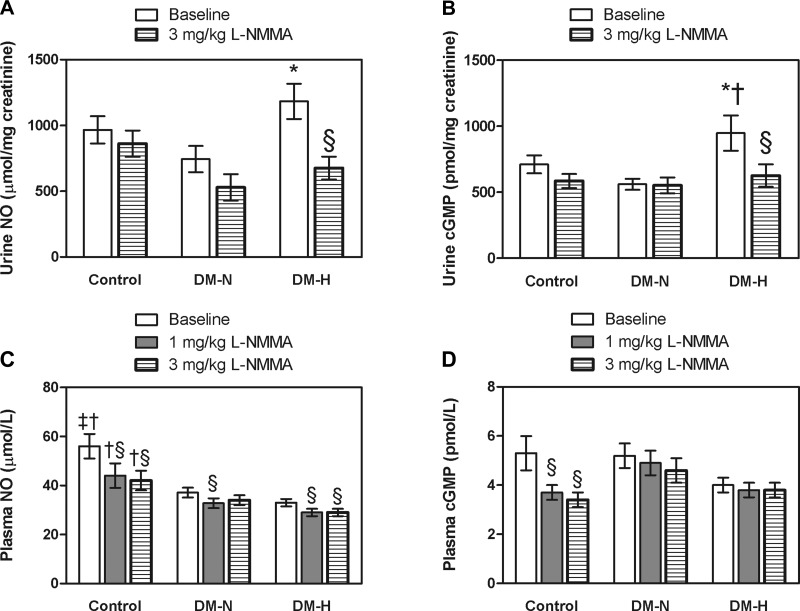
Urinary cGMP excretion was significantly higher in DM-H versus DM-N and control participants and urinary NOx excretion was higher in DM-H versus DM-N; between-group baseline differences in urinary prostanoids were not significant (Fig. 2, A and B, Table 3).
Fig. 2.
Plasma and urine nitric oxide (NO) and cGMP responses to NG-nitro-l-arginine methyl ester (l-NMMA) during clamped euglycemia in healthy controls and in type 1 diabetes patients and either normofiltration (DM-N) or hyperfiltration (DM-H) (means ± SD) *P < 0.05 for mean value in DM-H vs. DM-N groups; †P < 0.05 for mean value in healthy control vs. DM-H; ‡P < 0.05 for mean value in healthy control vs. DM-N, §P < 0.05 for within-group effect of l-NMMA.
Table 3.
Urine prostanoid responses to l-NMMA during clamped euglycemia in healthy controls and in type 1 diabetes patients and either normofiltration or hyperfiltration
| Healthy Control Subjects |
Normofiltering Type 1 DM Subjects |
Hyperfiltering Type 1 DM Subjects |
||||
|---|---|---|---|---|---|---|
| Baseline | l-NMMA (3 mg/min) | Baseline | l-NMMA (3 mg/min) | Baseline | l-NMMA (3 mg/min) | |
| Urine 2,3dinor-6-keto-PGF1α, pg/mg creatinine | 395 ± 93 | 330 ± 41 | 369 ± 69 | 350 ± 84 | 233 ± 49 | 367 ± 53 |
| Urine PGF1α, pg/mg creatinine | 344 ± 84 | 289 ± 33† | 207 ± 34 | 197 ± 23 | 272 ± 52 | 246 ± 33 |
| Urine TXB2, pg/mg creatinine | 135 ± 32 | 191 ± 20*† | 95 ± 18 | 102 ± 14 | 152 ± 34 | 119 ± 24‡ |
| Urine PGE2, pg/mg creatinine | 285 ± 70 | 219 ± 20† | 151 ± 21 | 125 ± 14 | 232 ± 44 | 195 ± 30 |
| Urine PGEM, pg/mg creatinine | 205 ± 45 | 152 ± 12 | 134 ± 18 | 112 ± 14 | 182 ± 45 | 160 ± 18 |
Values are means ± SD. See text for definition of abbreviations.
P< 0.05 for mean value in healthy control vs. DM-H;
P < 0.05 for mean value in healthy control vs. DM-N;
P < 0.05 for response to l-NMMA in DM-H vs. healthy controls.
Baseline plasma NOx levels were significantly higher in healthy control versus the DM-N and DM-H groups (Fig. 2C). Baseline plasma cGMP levels were numerically lower in DM-H compared with the DM-N and control groups, although these differences were not significant (Fig. 2C, P = 0.07); urinary excretion of 2,3-dinor-6-keto-PGF1-α was similarly lower in DM-H versus the other groups, but between-group differences were not significant (Table 3).
Circulating aldosterone, ANG II, plasma renin concentration, and PRA were higher in control versus DM-H patients; only between-group differences for aldosterone and ANG II were significant for the healthy control versus DM-N group comparison (Table 1). Numerical differences in DM-N versus DM-H patients for baseline circulating RAAS mediators did not reach significance (Table 1).
Renal function responses to l-NMMA.
Baseline values for blood pressure and heart rate were similar in the control and DM groups (Table 2). As expected from our previous work (8, 54), DM-H patients exhibited higher ERPF and GFR and lower RVR measurements compared with the healthy control participants and the DM-N group.
Table 2.
Hemodynamic responses to a graded infusion of l-NMMA during clamped euglycemia in healthy controls and in type 1 diabetes patients and either normofiltration or hyperfiltration
| Healthy Control Subjects |
Normofiltering Type 1 DM Subjects |
Hyperfiltering Type 1 DM Subjects |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | l-NMMA (1 mg/min) | l-NMMA (3 mg/min) | Baseline | l-NMMA (1 mg/min) | l-NMMA (3 mg/min) | Baseline | l-NMMA (1 mg/min) | l-NMMA (3 mg/min) | |
| Blood pressure | |||||||||
| Heart rate, beats/min | 61 ± 7 | 56 ± 8 | 55 ± 6 | 63 ± 11 | 57 ± 10 | 57 ± 9 | 68 ± 10 | 62 ± 6 | 59 ± 5 |
| SBP, mmHg | 110 ± 10 | 112 ± 10 | 119 ± 12† | 112 ± 7 | 114 ± 7 | 120 ± 10† | 112 ± 10 | 117 ± 13† | 124 ± 15† |
| DBP, mmHg | 64 ± 8 | 67 ± 8 | 74 ± 9† | 60 ± 6 | 66 ± 7† | 73 ± 8† | 62 ± 3 | 69 ± 4† | 77 ± 5† |
| Renal hemodynamic function | |||||||||
| ERPF, ml·min−1·1.73 m−2 | 586 ± 116 | 565 ± 66 | 513 ± 76† | 634 ± 107 | 564 ± 101† | 483 ± 80† | 809 ± 112* | 660 ± 95†§ | 539 ± 80†‡§ |
| GFR, ml·min−1·1.73 m−2 | 120 ± 16 | 123 ± 16 | 121 ± 14 | 113 ± 12 | 113 ± 14 | 114 ± 15 | 152 ± 16* | 149 ± 18 | 140 ± 11†‡§ |
| FF | 0.21 ± 0.05 | 0.22 ± 0.02 | 0.24 ± 0.02 | 0.18 ± 0.03 | 0.20 ± 0.03 | 0.23 ± 0.03† | 0.19 ± 0.04 | 0.22 ± 0.03 | 0.26 ± 0.04†‡§ |
| RBF, ml·min−1·1.73 m−2 | 943 ± 177 | 896 ± 95 | 813 ± 126† | 1,006 ± 174 | 891 ± 171† | 764 ± 136† | 1,281 ± 182 | 1,080 ± 196†§ | 889 ± 153†‡§ |
| RVR, mmHg·l−1·min−1 | 0.079 ± 0.039 | 0.088 ± 0.015 | 0.107 ± 0.022† | 0.080 ± 0.012 | 0.095 ± 0.015† | 0.110 ± 0.031† | 0.061 ± 0.016* | 0.079 ± 0.020† | 0.101 ± 0.032† |
Values are means ± SD. SBP, systolic blood pressure; DBP, diastolic blood pressure; ERPF, effective renal plasma flow; FF, filtration fraction; GFR, glomerular filtration rate; RBF, renal blood flow; RVR, renal vascular resistance; l-NMMA, NG-nitro-l-arginine methyl ester.
P < 0.05 for between-group baseline differences for hyperfiltering subjects vs.normofiltering and healthy controls;
P < 0.05 vs. within group baseline value;
P < 0.05 for between-group change in response to l-NMMA vs. normofiltering patients;
P < 0.05 for between group change in response to l-NMMA vs. healthy control subjects.
In response to l-NMMA, systolic (SBP) and diastolic blood pressures (DBP) increased in all three groups (Table 2). Between-group differences in blood pressure responses were not significant. For renal parameters, l-NMMA-associated declines in ERPF and RBF and increases in FF and renal vascular resistance (RVR) were observed in all groups but were exaggerated in DM-H patients (Table 2). Furthermore, l-NMMA administration was associated with a decrease in GFR in the DM-H only (Table 2, ANOVA P = 0.002). Despite a decline in GFR, the mean GFR in the DM-H group after l-NMMA remained in the hyperfiltration range.
In DM-H patients, exaggerated renal hemodynamic responses were associated with significant declines in the urinary excretion of NOx and cGMP (Fig. 2, A and B). Similar trends in healthy control and DM-N did not reach significance. After l-NMMA, urinary 6-keto-PGF1-α, PGE2, and PGEM excretion generally declined in the three groups (within-group effects P > 0.05), and after l-NMMA 6-keto-PGF1-α, TXB2, and PGE2 excretion rates were lower in DM-N versus healthy control participants. In addition, the decrease in urinary TXB2 was significant in the DM-H group versus the response in healthy control group (Table 3).
Endothelial function responses to l-NMMA.
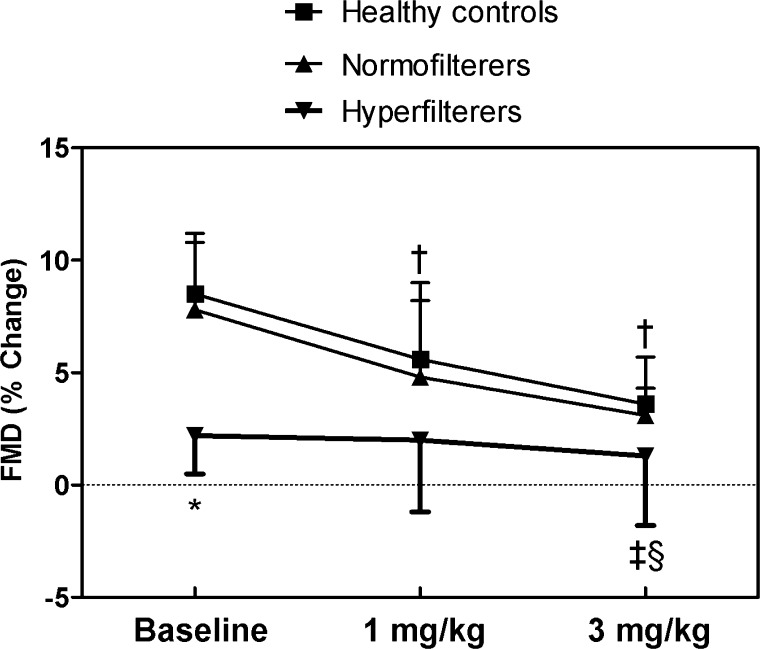
Baseline FMD was significantly higher in healthy control and DM-N compared with DM-H patients (Fig. 3, ANOVA P = 0.009). FMD responses remained significantly lower in the DM-H group throughout the rest of the study (repeated measures ANOVA P ≤ 0.01). In response to the l-NMMA infusion, FMD declined significantly in the healthy control and DM-N groups but did not change in the DM-H group. Between-group differences in the response to l-NMMA were significant at the higher dose for DM-H patients versus the healthy control and DM-N.
Fig. 3.
Flow-mediated vasodilatation (FMD) responses to l-NMMA in healthy control, DM-N and DM-H patients during clamped euglycemia (means ± SD). *P < 0.05 for between-group baseline differences for DM-H subjects vs. DM-N and healthy controls; †P < 0.05 vs. within-group baseline value in healthy control and DM-N patients; ‡P < 0.05 for between-group change in response to l-NMMA vs. DM-N patients; §P < 0.05 for between-group change in response to l-NMMA vs. healthy control subjects.
Blunted FMD responses to l-NMMA in DM-H were associated with lower plasma levels of NOx throughout the l-NMMA infusion (Fig. 2C). Declines in plasma NOx levels were greatest in healthy controls, with more modest declines in the DM groups. Plasma cGMP levels decreased significantly in response to l-NMMA in the healthy control group, but within-group changes in DM-N and DM-H were not significant (Fig. 2D). Urinary excretion of 2,3-dinor-6-keto-PGF1-α did not change in response to l-NMMA in any of the groups (Table 3).
DISCUSSION
Data from experimental models of types 1 and 2 DM suggest that increased expression and activity of NO is associated with renal hyperfiltration (27, 35). The contribution of increased NO bioactivity to early, preclinical changes in renal function in humans remains unclear. Paradoxically the hyperfiltration state has also been associated with impaired systemic endothelial function and changes in arterial stiffness, suggesting, at least in part, a state of generalized vascular dysfunction (9, 10). The simultaneous coexistence of potentially opposite levels of NO activity in humans with DM has not previously been established. Our major observations were the following: 1) declines in GFR, ERPF, and urinary excretion of renal vasodilators in response to l-NMMA during clamped euglycemia were exaggerated in DM-H versus healthy controls and DM-N patients, suggesting a state of high renal NO bioactivity in DM-H; and 2) baseline FMD and plasma vasodilators were generally suppressed in DM-H subjects. l-NMMA suppressed FMD in DM-N and healthy control subjects but not in DM-H, suggesting a state of low systemic NO bioactivity in DM-H.
Our first major observation was that similar to effects observed in diabetic animals, NOS blockade with l-NMMA during clamped euglycemia was associated with a decline in GFR in DM-H patients, suggesting a state of high renal vascular NO bioactivity (55). The 12 ml·min−1·1.73 m−2 decline in GFR was modest and GFR remained in the hyperfiltration range after l-NMMA. Moreover, l-NMMA reduced ERPF to a greater degree compared with GFR, resulting in an exaggerated 27% rise in FF, which was greater than the expected 10–13% ΔFF after l-NMMA, which is typical of healthy non-DM humans (2, 13). These effects on GFR were not observed in DM-N patients or healthy controls. The effect of l-NMMA on GFR suggests that NO bioactivity contributes to the pathogenesis of hyperfiltration in humans with uncomplicated type 1 DM. While we cannot precisely anatomically localize differences in segmental resistance in human studies, the exaggerated rise in FF with l-NMMA suggests that intraglomerular pressure increases to mitigate the renal vasoconstriction and resulting fall in ERPF associated with NOS inhibition.
Studies in normoalbuminuric patients with type 1 DM measuring urinary 15N nitrate excretion after an intravenous injection of radiolabeled l-arginine demonstrate increased whole body NO synthesis compared with healthy controls (45). Urinary 15N nitrate excretion has also been positively associated with creatinine clearance, suggesting a role for NO bioactivity in the pathogenesis of glomerular hyperfiltration (45). In the present study, urinary NOx and cGMP excretion rates were highest in DM-H participants, and l-NMMA significantly reduced urinary NOx and cGMP in this group. Combined with greater renal hemodynamic responses to l-NMMA assessed on the basis of gold-standard inulin clearances, biochemical changes in DM-H suggest a state of high-baseline NO production, which contributes to hyperfiltration in this subset of type 1 DM patients.
Functional effects of l-NMMA may also be mediated through changes in prostanoid bioactivity (11, 23, 28, 36, 39). We therefore measured urinary excretion rates of prostanoids, since these factors are important regulators of renal vascular tone and have been implicated in the pathogenesis of hyperfiltration (8, 12, 18). For example, TXB2 inhibition reduces microalbuminuria, possibly through postglomerular vasodilatory effects (20, 43). TXB2 suppression by l-NMMA has been attributed to IL-1β in in vivo animal models and in in vitro studies using human tissue, but this interaction has not, to our knowledge, been studied in in vivo human studies (11, 23, 28, 36, 39). Our results extend these previous biochemical observations by demonstrating for the first time that the l-NMMA-induced reduction in urinary TXB2 excretion was associated with a decline in GFR in DM-H. While we cannot determine causality, changes in urinary TXB2 excretion may have contributed to the renal hemodynamic effects of l-NMMA that we observed through changes in the renal microcirculation. Our results also suggest that PGE2 and prostacyclin do not contribute to l-NMMA responsiveness in humans with early type 1 DM.
Endothelial dysfunction is associated with an increased risk of diabetic nephropathy and vascular disease (44, 46, 51). The hyperfiltration state is associated with changes in macrovascular function in patients with uncomplicated type 1 DM, including higher nocturnal blood pressures, impaired FMD, and differences in arterial compliance (9, 10, 47). We have also previously reported that abnormal FMD responsiveness in DM-H depend at least in part on vasodilatory prostanoids (9). To further elucidate physiological mechanisms linking early renal and systemic vascular dysfunction in early type 1 DM, we obtained simultaneous brachial artery endothelial function measurements with renal function measurements at each time point before and during a graded infusion of l-NMMA. Consistent with our previous work in a separate younger cohort of patients with type 1 DM, baseline FMD was suppressed in the DM-H group, (9). Circulating NOx and cGMP levels were consistent with FMD responses, in that values of these vasodilators were generally lowest in the DM-H group. This finding suggests a state of systemic vasodilator suppression in DM-H, a conclusion that was further reinforced by diminished FMD responsiveness to l-NMMA and blunted changes in circulating NOx and cGMP in response to l-NMMA in the DM-H group.
The discrepancy between NO bioactivity in the renal microcirculation and systemic macrovasculature has been the subject of considerable discussion, and there are several possible explanations for this finding (48). First, although NO metabolites and NOS mRNA expression are increased in the renal microcirculation, NO activity in conduit arteries may be blunted through a variety of pathways, including superoxide anion free radical-mediated quenching of NO (48). Second, the role of NO may differ between vascular beds and other factors influencing endothelium-dependent vasodilatation, such as endothelium-derived hyperpolarizing factor and reactive oxygen species, may differ between large conduit arteries compared with the renal microcirculation (16). Third, a number of factors such as glycemic control, age, and differences in experimental design including approaches used to measure NO bioactivity may lead to inconsistent reports (31, 48). We tried to avoid some of these limitations by including a uniform, well-characterized cohort of subjects and used methods established in our laboratory that control for potential important confounders such as volume status, ambient glycemia, and diet (41, 54). Furthermore, we used a direct NO inhibitor to assess dynamic, simultaneous changes in renal and systemic vascular function in study participants who were examined at a uniform stage of the natural history of disease.
A final mechanism that may contribute to the dissociation between renal and systemic vascular function relates to the RAS. Previous work has demonstrated a consistent suppression of circulating RAS mediators in DM patients, despite evidence of RAS activation at the tissue level, referred to as the paradox of the low-renin state in DM (49). The observation that circulating RAS mediators were higher in healthy control versus DM-N patients, with even lower values in DM-H patients, is novel and may suggest that hyperfiltration is associated with the greatest disparity between circulating RAS mediators and RAS activation at the tissue level. Interestingly, the RAS does exert a suppressive effect on NOS isoforms through complex negative feedback loops in different renal compartments (1, 15). Whether upregulation of the tissue RAS in DM-H subjects was in part responsible for the low NO bioactivity observed in the systemic vasculature is an intriguing possibility and should be further investigated. Furthermore, if baseline renal RAS activity is highest in DM-H, as we have previously suggested, then, similar to observations in animals, NOS inhibition may have permitted unopposed RAS activity leading to enhanced renal vasoconstrictive and autoregulatory effects of ANG II (54, 56).
We attempted to minimize the effect of the small sample size in our study by using homogeneous study groups and by using a careful prestudy preparation phase with a focus on known factors that influence neurohormonal activation. We also scheduled studies in female participants to coincide with the early follicular phase of the menstrual cycle to avoid confounding effects of estrogen on vascular function. Although plasma estrogen levels were somewhat higher than expected and more consistent with the mid to late follicular phase, estrogen concentrations were similar in the three groups. In addition, we decreased variability by using a study design that allowed each subject to act as his/her own control. Nevertheless, the small sample size may have limited our ability to detect between-group differences in certain parameters such as urinary NOx. Next, because NO, cGMP, and prostanoids are widely expressed in different anatomical compartments of renal tissues, we were unable to determine the specific origin of these urinary factors in this intact human study (1, 5, 33, 34). In addition, tubuloglomerular feedback is likely an important factor leading to renal hyperfiltration and may have been influenced by l-NMMA. Because of the existing complexity of this set of experiments, tubular factors were not assessed and should be examined in future protocols. Next, although differences were not statistically significant, the rise in BP in response to l-NMMA was numerically greater in the DM-H group. We therefore cannot rule out the possibility that autoregulatory responses may have contributed to the greater RVR increase in the DM-H group. Next, subjects were not placed on a controlled nitrate diet, and this may have limited our ability to detect some between-group differences in plasma and urine NOx. A final limitation of this work is that changes in shear rate (area under the curve) have the potential to contribute to reductions in FMD using systemic infusions of l-NMMA (50). Future work should determine whether differences in shear rate played a role in the observed suppression of FMD in the DM-H group.
In conclusion, renal hyperfiltration is associated with simultaneously increased renal NO bioactivity and impaired endothelial function in patients with uncomplicated type 1 DM, suggesting that a state of paradoxical high renal and low systemic vascular NO activity exists in this group. Further work is required to determine the physiological basis for this differential regulation of vascular function by NO.
Perspectives and Significance
DM remains the most common cause of end-stage renal disease in developed countries, which ultimately requires dialysis or renal transplantation. Unfortunately, current therapies do not fully protect patients against renal disease progression, possibly because RAS blockade-based strategies do not abolish hyperfiltration (21, 54). While our results suggest that NO may contribute to hyperfiltration, targeted renal NOS inhibitors are not available and may be associated with risk due to systemic hypertensive effects. On the other hand, we have recently demonstrated that NO contributes to tubuloglomerular feedback pathways leading to afferent vasodilatation in patients with uncomplicated type 1 DM (42). Future work should assess the effect of increasing distal tubular sodium delivery with novel agents including sodium-glucose cotransport 2 inhibitors that reduce afferent vasodilatation and hyperfiltration in animals, in part, via effects on preglomerular NO bioactivity (4, 57).
GRANTS
This work was supported by operating grants from the Canadian Institutes of Health Research (to D. Z. I. Cherney and to R. L. Hébert) and Heart and Stroke Foundation (to D. Z. I. Cherney). D. Z. I. Cherney was also supported by a Kidney Foundation of Canada Scholarship and a Canadian Diabetes Association-KRESCENT Program Joint New Investigator Award and receives operating support from the Heart and Stroke Foundation of Canada (with H. N. Reich). H. N. Reich is a recipient of a KRESCENT Program New Investigator Award. J. W. Scholey is the CIHR/AMGEN Canada Kidney Research Chair at the University Health Network, University of Toronto. The results presented in this paper have not been published previously in whole or in part.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.Z.C. conception and design of research; D.Z.C., R.N., and V.L. performed experiments; D.Z.C., S.J., R.H., R.N., and R.L.H. analyzed data; D.Z.C. interpreted results of experiments; D.Z.C. prepared figures; D.Z.C. and J.W.S. drafted manuscript; D.Z.C., H.N.R., S.J., R.H., R.N., R.L.H., J.W.S., and E.B.S. edited and revised manuscript; D.Z.C., H.N.R., R.N., R.L.H., and J.W.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Paul Yip and Jenny Cheung-Hum for invaluable assistance with biochemical assays included in this work. Finally, the authors are grateful to the study participants whose time and effort are critical to the success of our research program.
REFERENCES
- 1. Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F885–F898, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Bech JN, Nielsen CB, Pedersen EB. Effects of systemic NO synthesis inhibition on RPF, GFR, UNa, and vasoactive hormones in healthy humans. Am J Physiol Renal Fluid Electrolyte Physiol 270: F845–F851, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Bell TD, DiBona GF, Biemiller R, Brands MW. Continuously measured renal blood flow does not increase in diabetes if nitric oxide synthesis is blocked. humans. Am J Physiol Renal Physiol 295: F1449–F1456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blantz RC, Deng A, Lortie M, Munger K, Vallon V, Gabbai FB, Thomson SC. The complex role of nitric oxide in the regulation of glomerular ultrafiltration. Kidney Int 61: 782–785, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Boone M, Kortenoeven M, Robben JH, Deen PM. Effect of the cGMP pathway on AQP2 expression and translocation: potential implications for nephrogenic diabetes insipidus. Nephrol Dial Transplant 25: 48–54, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Brooks B, Delaney-Robinson C, Molyneaux L, Yue DK. Endothelial and neural regulation of skin microvascular blood flow in patients with diabetic peripheral neuropathy: effect of treatment with the isoform-specific protein kinase C beta inhibitor, ruboxistaurin. J Diabetes Complications 22: 88–95, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Cherney DZ, Lai V, Miller JA, Scholey JW, Reich HN. The angiotensin II receptor type 2 polymorphism influences haemodynamic function and circulating RAS mediators in normotensive humans. Nephrol Dial Transplant 25: 4093–4096, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hebert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 57: 688–695, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cherney DZ, Miller JA, Scholey JW, Nasrallah R, Hebert RL, Dekker MG, Slorach C, Sochett EB, Bradley TJ. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care 33: 1344–1346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherney DZ, Sochett EB, Lai V, Dekker MG, Slorach C, Scholey JW, Bradley TJ. Renal hyperfiltration and arterial stiffness in humans with uncomplicated type 1 diabetes. Diabetes Care 33: 2068–2070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corbett JA, Kwon G, Turk J, McDaniel ML. IL-1 beta induces the coexpression of both nitric oxide synthase and cyclooxygenase by islets of Langerhans: activation of cyclooxygenase by nitric oxide. Biochemistry 32: 13767–13770, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Craven PA, Caines MA, DeRubertis FR. Sequential alterations in glomerular prostaglandin and thromboxane synthesis in diabetic rats: relationship to the hyperfiltration of early diabetes. Metabolism 36: 95–103, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Dalla Vestra M, Sacerdoti D, Bombonato G, Fioretto P, Finucci G, Saller A, Sfriso A, Bruseghin M, Sambataro M, Velussi M, Baggio B, Nosadini R, Crepaldi G. Nitric oxide modulation of renal and cardiac hemodynamics in type 2 diabetes. Eur J Endocrinol 146: 687–694, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Davidson WD, Sackner MA. Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med 62: 351–356, 1963 [PubMed] [Google Scholar]
- 15. De Nicola L, Blantz RC, Gabbai FB. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest 89: 1248–1256, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Vriese AS, Van de Voorde J, Blom HJ, Vanhoutte PM, Verbeke M, Lameire NH. The impaired renal vasodilator response attributed to endothelium-derived hyperpolarizing factor in streptozotocin–induced diabetic rats is restored by 5-methyltetrahydrofolate. Diabetologia 43: 1116–1125, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Delles C, Jacobi J, Schlaich MP, John S, Schmieder RE. Assessment of endothelial function of the renal vasculature in human subjects. Am J Hypertens 15: 3–9, 2002 [DOI] [PubMed] [Google Scholar]
- 18. DeRubertis FR, Craven PA. Eicosanoids in the pathogenesis of the functional and structural alterations of the kidney in diabetes. Am J Kidney Dis 22: 727–735, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Dijkhorst-Oei LT, Boer P, Rabelink TJ, Koomans HA. Nitric oxide synthesis inhibition does not impair water immersion-induced renal vasodilation in humans. J Am Soc Nephrol 11: 1293–1302, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Esmatjes E, Conget JI, Gaya J, Fernandez MR, Ferrer JP, Rivera F, Vilardell E. Effects of thromboxane synthesis inhibitor triflusal on renal hemodynamics in microalbuminuric diabetic patients. Diabetes Care 13: 1114–1117, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Ficociello LH, Perkins BA, Silva KH, Finkelstein DM, Ignatowska-Switalska H, Gaciong Z, Cupples LA, Aschengrau A, Warram JH, Krolewski AS. Determinants of progression from microalbuminuria to proteinuria in patients who have type 1 diabetes and are treated with angiotensin-converting enzyme inhibitors. Clin J Am Soc Nephrol 2: 461–469, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Florijn KW, Barendregt JN, Lentjes EG, van Dam W, Prodjosudjadi W, van Saase JL, van Es LA, Chang PC. Glomerular filtration rate measurement by “single-shot” injection of inulin. Kidney Int 46: 252–259, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez E, Rosello-Catafau J, Jawerbaum A, Vela J, Sinner D, Pustovrh C, White V, Xaus C, Peralta C, Gimeno MA. Involvement of inducible isoforms of COX and NOS in streptozotocin-pancreatic damage in the rat: interactions between nitridergic and prostanoid pathway. Prostaglandins Leukot Essent Fatty Acids 64: 311–316, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Henry RM, Ferreira I, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Kamp O, Bouter LM, Stehouwer CD. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not. The Hoorn Study. Atherosclerosis 174: 49–56, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hiragushi K, Sugimoto H, Shikata K, Yamashita T, Miyatake N, Shikata Y, Wada J, Kumagai I, Fukushima M, Makino H. Nitric oxide system is involved in glomerular hyperfiltration in Japanese normo- and micro-albuminuric patients with type 2 diabetes. Diabetes Res Clin Pract 53: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Ito A, Uriu K, Inada Y, Qie YL, Takagi I, Ikeda M, Hashimoto O, Suzuka K, Eto S, Tanaka Y, Kaizu K. Inhibition of neuronal nitric oxide synthase ameliorates renal hyperfiltration in streptozotocin-induced diabetic rat. J Lab Clin Med 138: 177–185, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Jawerbaum A, Gonzalez ET, Carolina P, Debora S, Christian P, Gimeno MA. Diminished levels of prostaglandin E in type I diabetic oocyte-cumulus complexes. Influence of nitric oxide and superoxide dismutase. Reprod Fertil Dev 11: 105–110, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Jung K, Klotzek S, Schulze BD. Refinements of assays for low concentrations of inulin in serum. Nephron 54: 360–361, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 34: 146–154, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Khamaisi M, Keynan S, Bursztyn M, Dahan R, Reinhartz E, Ovadia H, Raz I. Role of renal nitric oxide synthase in diabetic kidney disease during the chronic phase of diabetes. Nephron Physiol 102: p72–p80, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol 284: F1121–F1137, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest 107: 889–898, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Komhoff M, Grone HJ, Klein T, Seyberth HW, Nusing RM. Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. Am J Physiol Renal Physiol 272: F460–F468, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Levine DZ, Iacovitti M, Robertson SJ. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. II. Effects of renal mass reduction. Am J Physiol Regul Integr Comp Physiol 294: R1840–R1846, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Ling JJ, Sun YJ, Zhu DY, Chen Q, Han X. Potential role of NO in modulation of COX-2 expression and PGE2 production in pancreatic beta-cells. Acta Biochim Biophys Sin (Shanghai) 37: 139–146, 2005 [PubMed] [Google Scholar]
- 37. Lockhart CJ, Agnew CE, McCann A, Hamilton PK, Quinn CE, McCall DO, Plumb RD, McClenaghan V, McGivern CR, Harbinson MT, McVeigh G. Impaired flow mediated dilatation response in uncomplicated type 1 diabetes mellitus: influence of shear stress and microvascular reactivity. Clin Sci (Lond) 121: 129–139, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Mathy-Hartert M, Deby-Dupont GP, Reginster JY, Ayache N, Pujol JP, Henrotin YE. Regulation by reactive oxygen species of interleukin-1beta, nitric oxide and prostaglandin E(2) production by human chondrocytes. Osteoarthritis Cartilage 10: 547–555, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Metzger IF, Sertorio JT, Tanus-Santos JE. Relationship between systemic nitric oxide metabolites and cyclic GMP in healthy male volunteers. Acta Physiol (Oxf) 188: 123–127, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 10: 1778–1785, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Montanari A, Biggi A, Cabassi A, Pelloni I, Pigazzani F, Pinelli S, Pela GM, Musiari L, Cherney DZ. Renal hemodynamic response to l-arginine in uncomplicated, Type 1 diabetes mellitus: the role of buffering anions and tubuloglomerular feedback. Am J Physiol Renal Physiol (June 27, 2012).doi:10.1152/ajprenal.00149.2012 [DOI] [PubMed] [Google Scholar]
- 43. Morath R, Klein T, Seyberth HW, Nusing RM. Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J Am Soc Nephrol 10: 1851–1860, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol 7: 36–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Byrne S, Forte P, Roberts LJ, 2nd, Morrow JD, Johnston A, Anggard E, Leslie RD, Benjamin N. Nitric oxide synthesis and isoprostane production in subjects with type 1 diabetes and normal urinary albumin excretion. Diabetes 49: 857–862, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Ott C, Schneider MP, Delles C, Schlaich MP, Schmieder RE. Reduction in basal nitric oxide activity causes albuminuria. Diabetes 60: 572–576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pecis M, Azevedo MJ, Gross JL. Glomerular hyperfiltration is associated with blood pressure abnormalities in normotensive normoalbuminuric IDDM patients. Diabetes Care 20: 1329–1333, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension 31: 1047–1060, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Price DA, Porter LE, Gordon M, Fisher ND, De'Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol 10: 2382–2391, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Pyke KE, Tschakovsky Peak vs ME. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Raij L. Nitric oxide in the pathogenesis of cardiac disease. J Clin Hypertens (Greenwich) 8: 30–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schaefer WR, Seufert RJ, Casper FW, Zahradnik HP. Urinary excretion of 2,3-dinor-6-keto-PGF1 alpha and 11-dehydro-TXB2 by the gravid spontaneously hypertensive rat. Prostaglandins 52: 1–11, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Schneider MP, Klingbeil AU, Delles C, Schmidt BM, John S, Schmieder RE. Vitamin C augments the renal response to l-arginine in smokers. Nephrol Dial Transplant 18: 1512–1517, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17: 1703–1709, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Sugimoto H, Shikata K, Matsuda M, Kushiro M, Hayashi Y, Hiragushi K, Wada J, Makino H. Increased expression of endothelial cell nitric oxide synthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic nephropathy. Diabetologia 41: 1426–1434, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Takenaka T, Mitchell KD, Navar LG. Contribution of angiotensin II to renal hemodynamic and excretory responses to nitric oxide synthesis inhibition in the rat. J Am Soc Nephrol 4: 1046–1053, 1993 [DOI] [PubMed] [Google Scholar]
- 57. Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsikas D, Engeli S, Tank J, Stichtenoth DO, Jordan J. Comment on: Ott et al. Reduction in basal nitric oxide activity causes albuminuria Diabetes 2011;60:572–576. Diabetes 60: e21; author reply e22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int 70: 840–853, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Wesson DE. Moving closer to an understanding of the hyperfiltration of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 290: R973–R974, 2006 [DOI] [PubMed] [Google Scholar]
- 61. White RP, Deane C, Vallance P, Markus HS. Nitric oxide synthase inhibition in humans reduces cerebral blood flow but not the hyperemic response to hypercapnia. Stroke 29: 467–472, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993–11997, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]