Abstract
Intermittent hypoxia (IH) is a frequent occurrence in sleep and respiratory disorders. Both human and murine studies show that IH may be implicated in metabolic dysfunction. Although the effects of nocturnal low-frequency intermittent hypoxia (IHL) have not been extensively examined, it would appear that IHL and high-frequency intermittent hypoxia (IHH) may elicit distinct metabolic adaptations. To this effect, C57BL/6J mice were randomly assigned to IHH (cycles of 90 s 6.4% O2 and 90 s 21% O2 during daylight), IHL (8% O2 during daylight hours), or control (CTL) for 5 wk. At the end of exposures, some of the mice were subjected to a glucose tolerance test (GTT; after intraperitoneal injection of 2 mg glucose/g body wt), and others were subjected to an insulin tolerance test (ITT; 0.25 units Humulin/kg body wt), with plasma leptin and insulin levels being measured in fasting conditions. Skeletal muscles were harvested for GLUT4 and proliferator-activated receptor gamma coactivator 1-α (PGC1-α) expression. Both IHH and IHL displayed reduced body weight increases compared with CTL. CTL mice had higher basal glycemic levels, but GTT kinetics revealed marked differences between IHL and IHH, with IHL manifesting the lowest insulin sensitivity compared with either IHH or CTL, and such findings were further confirmed by ITT. No differences emerged in PGC1-α expression across the three experimental groups. However, while cytosolic GLUT4 protein expression remained similar in IHL, IHH, and CTL, significant decreases in GLUT4 membrane fraction occurred in hypoxia and were most pronounced in IHL-exposed mice. Thus IHH and IHL elicit differential glucose homeostatic responses despite similar cumulative hypoxic profiles.
Keywords: high-frequency intermittent hypoxia, low-frequency intermittent hypoxia, insulin resistance
sleep apnea is the most common form of sleep disordered breathing. It is highly prevalent (2–10% of the population across the age spectrum), and it is associated with multiple and important morbid consequences including metabolic disturbances (21, 50, 52, 78). One of the major perturbations of sleep apnea is the occurrence of recurrent hypoxic events, i.e., high-frequency intermittent hypoxia (IHH), resulting from periodic collapse of the upper airway during sleep. These events are often terminated by arousal, and therefore elicit fragmentation of normal sleep architecture. Epidemiological studies have shown a disruption of metabolic pathways in sleep disordered breathing, with an association between indices of insulin resistance and the degree of hypoxemia as a consequence of obstructive sleep apnea (OSA) being identified (27, 53, 55–56). However, a detailed analysis of the metabolic adaptations to long-term IH has not been extensively pursued to date.
It is now well established that low-frequency IH (IHL) as seen in multiple cardiopulmonary disorders include alteration of cellular and systemic physiology, polycythemia, pulmonary hypertension, and weight loss (25, 48–49, 79). Previous studies have also shown effects of IHL on energy metabolism via regulation of genes encoding for glucose transporters and glycolytic enzymes (29, 61, 63), primarily through modulation of hypoxia-inducible factor 1-α (HIF-1α) subunit expression and transcriptional activity. Indeed, HIF-1α was reciprocally modulated by insulin and insulin-like growth factor pathways (1, 32, 69, 72, 80).
Other studies exploring the effects of moderate IH on body weight and blood sugar in mice showed lower body weight and glycemic levels than those in normoxic mice after 40 days of IH (40). In addition, previous studies conducted at altitude or in patients with OSA have shown that glucose homeostasis is influenced by hypoxia, both acutely, and also after more prolonged exposures, suggesting that insulin resistance and glucose intolerance are positively associated with the severity of IH (27, 56, 68, 73). Liyori and colleagues (26) showed an acute decrease in whole body insulin sensitivity in animals exposed to IH mimicking the severe hypoxic stress of OSA suggesting a causal relationship between IH exposures and the development of insulin resistance in healthy animals (26). Furthermore, 2–3 days of continuous hypoxia elicited insulin resistance (7, 35), whereas more than 6 wk of continuous hypoxia reduced fasting blood glucose levels but did not affect insulin resistance or glucose tolerance (9, 15, 35).
One of the most widely physiological tests used to identify impaired glucose tolerance is the glucose tolerance test (GTT). In mouse models, GTT is carried out following a period of fasting and glucose administration, both optimized in a relevant study performed by Andrikopoulos and colleagues (3). Besides GTT, insulin tolerance test (ITT) is another widely used assay in humans and animal models, and therefore these two tests provide the opportunity to examine aspects of insulin sensitivity. However, cellular glucose uptake is facilitated through transport mediated by the hexose transporter or translocator family of membrane proteins (GLUT), specifically GLUT1 and GLUT4. GLUT4 function is highly regulated through not only changes in expression, but also by alterations in the topographic cellular distribution of GLUT4, such that increases in the GLUT4 within the plasma membrane (PM) will facilitate the reduction of plasma glucose levels (8). Overall, around 50% of intracellular GLUT4 is translocated to the cell surface upon insulin activation (44). Apart from GLUT isoforms, transcriptional coactivators can be also important targets for physiological regulation of glucose. For instance, peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) has been described as a transcriptional coactivator that operates as a key regulator of the activation a broad range of transcription factors for downstream regulation of genes encoding mitochondrial proteins and GLUT4 (10, 37, 39).
Another metabolic pathway potentially affected by hypoxia could involve leptin, the adipocyte-derived adipokine product of the ob gene. It has been shown that this hormone induces a negative energy balance by reducing appetite and increasing energy expenditure (20), which is found in circulation at proportional levels to the OSA severity (13, 28, 73). Presence of low levels of leptin or leptin resistance via its cognate receptor have been associated with high levels of insulin resistance (18, 47, 57).
Based on aforementioned considerations, we hypothesized that high- and low-frequency intermittent hypoxia would elicit differential metabolic effects on glycemic control in mice.
MATERIALS AND METHODS
Animals
Adult male C57BL/6J mice (7 wk old, 20–25 g) were 1) purchased from Jackson Laboratories (Bar Harbor, ME), 2) allowed to acclimatize to their surroundings for at least 1 wk, and 3) always housed in groups of five in standard clear polycarbonate cages. Mice were maintained in a 12-h light/dark cycle (light on 7:00 AM to 7:00 PM) at a constant temperature (26 ± 1°C) and were allowed access to food and water ad libitum. At 8 wk of age, mice were randomly assigned into three hypoxic/normoxic group exposures (26 animals per group): control, IHH, and IHL for a period of 5 wk (wk). At the end of the experimental procedures, mice were euthanized by cervical dislocation, and all testing was conducted during the light phase. Animal experiments were performed according to protocols approved by IACUC of the University of Chicago and are in close agreement with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” All efforts were made to minimize animal suffering and to reduce the number of animals used.
Exposures To High- Or Low-Frequency Intermittent Hypoxia
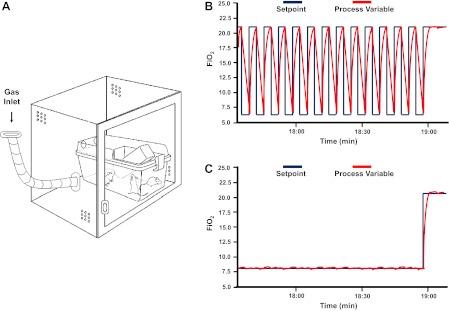
Mice were placed in identical commercially designed chambers (30 × 20 × 20 inches; Oxycycler A44XO, BioSpherix, Redfield, NY) (Fig. 1A) operated under a 12:12 h light-dark cycle (7:00 AM to 7:00 PM) for a period of 5 wk before any assay (either GTT or ITT testing or skeletal muscle harvesting). Programmed gas concentrations were circulated into each chamber, and an internal O2 analyzer measured the O2 concentration continuously. Deviations from the fixed concentrations were automatically corrected by a computerized system of solenoid valves controlling gas outlets adding either N2 or O2. Ambient CO2 in the chamber was maintained at less than 0.01%, and humidity was also maintained at 40–50% by circulating the gas through a freezer and silica gel. Ambient temperature was kept at 26°C as described previously by Gozal and colleagues (22). Two episodic hypoxia profiles were used in the study, IHH and IHL, as shown in Fig. 1, B and C, respectively. Low-frequency intermittent hypoxia-exposed mice were subjected for 12 h during daylight to a continuous FiO2 of 8% (Fig. 1C), a level that is associated with reproducible nadir of oxyhemoglobin saturations in the 75–80% range. For the rest of the 12-h lights-off period, normoxic (FiO2, 21%) conditions were applied. IHH-exposed mice were subjected for 12 h during daylight to intermittent hypoxia/normoxia cycles of 3-min duration [Hypoxia, nadir of FiO2: 6.4% for 90 s alternating with normoxia (FiO2, 21%) for 90 s; Fig. 1B]. This IH profile is associated with reproducible nadir of oxyhemoglobin saturations in the 65%-72% range. Normoxic (FiO2, 21%) conditions were used during the 12-h lights-off period. Control animals were exposed to circulating room air (FiO2, 21%) during daylight and lights-off period. The nadir of FiO2 and the cycle duration were designed to obtain similar cumulative hypoxic profiles between IHH and IHL during the 12-h daylight time period. Immediately after the 5-wk exposures, 30 animals (10 per condition) were used for GTT, 30 animals (10 per condition) were used for ITT, and 18 mice (6 per condition) were euthanized, and skeletal muscles (quadriceps) were quickly removed, frozen in liquid nitrogen, and kept at −80°C until analysis.
Fig. 1.
Schematics of the hypoxic chambers. Commercially available ventilated cages that mimic usual housing conditions are placed in computerized hypoxic chambers to achieve intermittent hypoxia (IH) exposures in mice (A). Hypoxia results from targeted nitrogen enrichment of the air, followed by reoxygenation using oxygen. The two different IH patterns are shown on right based on O2 sensor-acquired data within the experimental hypoxic chambers. IH cycles correspond to the different hypoxic patterns where the fraction of inspired oxygen (FiO2) oscillates from 21% to 6.4% during the 12-h daylight in the high-frequency IH (IHH) pattern (B) and 8% of inspired oxygen during the 12-h daylight in the low-frequency IH (IHL) pattern (C).
Body Weight
Body weight was checked weekly for a period of 5 wk always at the same time of the day (middle of the light cycle period). Body weight gain was determined by subtracting the body weight on first day of hypoxia exposure from the body weight on subsequent days.
GTT and ITT
Both tests were performed following 5 wk of either control IHH or IHL conditions. In both tests, animals were fasted for 3 h with water available ad libitum. An intraperitoneal injection (26 gauge 3/8″ needle) of sterile glucose (2 mg/g of body wt for GTT) or an intraperitoneal injection of sterile humulin (0.25 U/kg of body wt for ITT) was administered. At the beginning of both tests, the tip of the tail was nicked using a sterile surgical blade. Blood recovered from the tip of the tail at different time points (for GTT: 0, 4, 15, 30, 60, 90, 120 min after injection; ITT: 0, 4, 15, 30, 60, 75, 90, 105, 120 min after injection) was tested using an OneTouch Ultra2 glucometer (Life Scan; Milpitas, CA). At the indicated time points, venous blood samples were collected in heparin-coated capillary tubes from the tail vein. Insulin and leptin assays were carried out on selected time points using enzyme-linked immunosorbent assay (ELISA) kits (Millipore; St. Charles, MO) according to the manufacturer's protocol. The linear range of the insulin assay was 0.2–10 ng/ml, with the limit of sensitivity at 0.2 ng/ml (35 pM), and intra- and interindividual coefficients of variation up to 8.37% and 17.9% respectively at lower concentrations (0.32 ng/ml) of this analyte. Similarly for the leptin assay, the linear range was 0.2 up to 30 ng/ml with the sensitivity threshold at 0.05 ng/ml (∼3.13 pM). The intra-assay variation coefficient was up to 1.76% at high concentrations of leptin (17.60 ng/ml), and 4.59% of interindividual coefficient of variation at low concentrations (1.66 ng/ml) of this analyte.
PGC1-α and GLUT4 Protein Expression
Total cellular protein or cytosolic and membrane protein fractions were extracted from frozen skeletal muscle tissue (quadriceps) following a slightly modified protocol, as previously described by Wang and colleagues (74). Frozen tissues were homogenized using a BBX24 Bullet Blender homogenizer (Next Advance, Averill Park, NY) such that 100 mg of tissue and a volume of beads (0.5 mm zirconium oxide beads-ZSB05) equal to the mass of tissue were added to the sample. Two volumes of buffer containing 50 mM Tris·HCl (pH 7.5), 5 mM EDTA, 10 mM EGTA, 10 mM benzamidine, 50 μg/ml phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 10% glycerol (vol/vol) were also added before placing the sample into the homogenizer. At the end of the homogenization process, the homogenate was incubated on a rocking platform in the presence of 20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) at 4°C for 2 h. After centrifugation at 14,000 g for 30 min at 4°C, the supernatant was collected as total cellular protein. In another set of skeletal muscle samples, the homogenate was centrifuged without CHAPS incubation at 14,000 g for 30 min at 4°C, and the supernatant was collected as the cytosolic fraction. Next, the pellet was washed once with the previous buffer, resuspended in the sample buffer with 20 mM CHAPS, sonicated using a F60 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA), and incubated on a rocking platform at 4°C for 2 h. Finally, after a centrifugation at 14,000 g for 30 min at 4°C, the supernatant was collected as the membrane fraction. For all samples, protein concentration was determined using the modified Bradford method-DC Protein Assay (Bio-Rad Laboratories, Hercules, CA) using Protein Assay Standard II (bovine serum albumin) (Bio-Rad Laboratories) as standard.
Protein samples were electrophoresed under reducing denaturing conditions in 8% polyacrylamide-SDS gels and transferred by electroblotting onto a nitrocellulose membrane. Equal loading and transfer efficiency of total cellular protein for PGC1-α test or either cytosolic or membranous protein for GLUT4 test were carefully documented using membrane reversible Ponceau staining and anti-β-tubulin antibody (Upstate-Millipore; St. Charles, MO). After being blocked in 5% albumin solution from bovine serum (Sigma; St. Louis, MO) for 1 h, membranes were incubated with either an anti-PGC1-α monoclonal antibody (Calbiochem-EMD Millipore, St. Charles, MO) or an anti-GLUT4 polyclonal antibody (Santa Cruz; Santa Cruz, CA), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz). Signal detection was facilitated with Immun-Star WesternC Chemiluminescence Kit (Bio-Rad Laboratories). PGC1-α, GLUT4, and β-tubulin signals were quantitated using a Molecular Imager ChemiDoc XRS+ Imaging System (Bio-Rad Laboratories). PGC1-α and GLUT4 expression were normalized to the β-tubulin.
Data Analysis
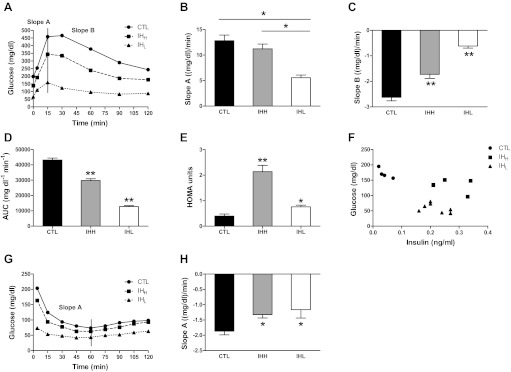
Body weight matching was not pursued among both IH conditions and CTL group, as will be discussed below. Slope A obtained from the GTT (Fig. 3B) was calculated using the glucose levels measured at times 0–15 min after glucose injection. Slope B from the same test (Fig. 3C) was computed between the peak serum glucose levels (15 min) and 120 min after glucose injection. In contrast, only slope A was calculated for the ITT (Fig. 3H), and included glucose levels measured at time 4 min till nadir of glucose levels (60 min) after humulin injection. In addition, area under the curve (AUCg) analyses were calculated for each of the three conditions during GTT. Furthermore, the homeostatic model of insulin resistance (HOMA) was calculated using baseline glucose and insulin concentrations using the following equation: fasting glucose (mg/dl) × fasting insulin (μU/ml)/405. All data are reported as means ± SE. Comparison within glucose, hormones (insulin and leptin), and protein expression levels of GLUT4 and PGC1-α between hypoxic and normoxic conditions were assessed by one-way ANOVA test followed by unpaired Student's t-test with Bonferroni posttests. A P value of <0.05 was defined as significant.
Fig. 3.
Changes in metabolic parameters during intermittent hypoxia in mice. Plasma glucose concentrations over 2 h during the intraperitoneal glucose tolerance test (GTT, 2 mg glucose/g body wt) (A) and intraperitoneal insulin tolerance test (ITT, 0.25 U/kg body wt) (G) following fasting for 3 h in C57BL/6J mice exposed to IHH, IHL, and CTL conditions. Slope A (B) was calculated using the glucose levels measured at times 0–15 min after glucose injection; slope B (C) was computed between times 15–120 min after glucose injection and slope A (H) was measured between times 4–60 min after humulin injection. The area under the curve (AUC) was calculated for glycemic levels during GTT experiments (D), and the homeostatic model for insulin resistance (HOMA) was calculated during baseline fasting conditions (E). Furthermore in F, a scatterplot of glycemic levels and corresponding plasma insulin concentrations at fasting baseline is shown for CTL (solid circles), IHH (solid squares), and IHL (solidk triangles). Slopes results are means ± SE (n = 10 per group). *Significance in both GTT and ITT have been defined as P < 0.05 (significant differences between two groups; **significant differences between all three groups).
RESULTS
Effect of Chronic IHH and IHL Exposures on Body Weight Gain
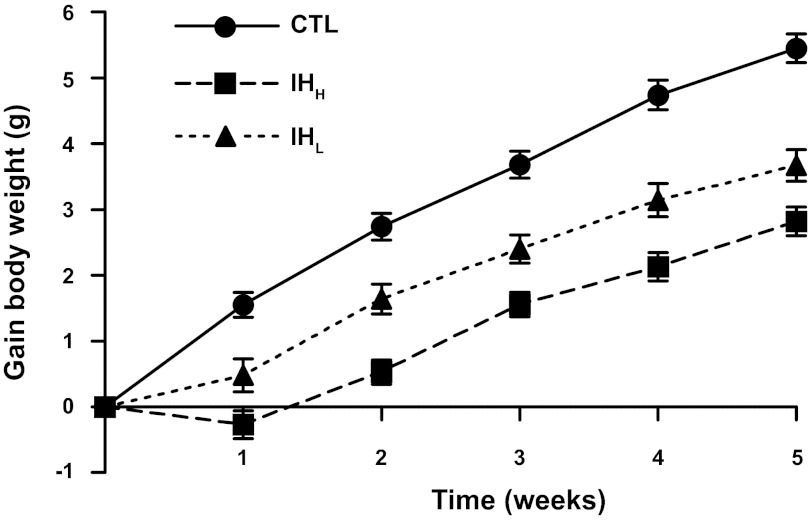
Mice exposed either to IHH or IHL gained less weight over 5 wk (2.82 ± 0.22 g and 3.67 ± 0.24 g on week 5 after exposure, respectively, n = 26 per group) compared with the normoxic controls (n = 26; 5.45 ± 0.22 g at the end of the exposure; P < 0.05) with IHH being lower than IHL. This difference reached statistical significance after the first week of exposure (Fig. 2; P < 0.05).
Fig. 2.
Changes in body weight during hypoxia. Body weight evolution during IHH, IHL, and normoxia (CTL) throughout 5 wk-exposures. Body weight alteration in mice exposed to 5 wk of intermittent hypoxia, n = 26 per group. This difference reached statistical significance after the first week of exposure (P < 0.05).
Effect of IHH and IHL on Glucose and Insulin Sensitivity
Glucose tolerance test.
As shown in Fig. 3A, IHH and IHL had lower fasting glucose levels (138.3 and 65.6 mg/dl, respectively; n = 10 per group) compared with the CTL group (198.3 mg/dl; n = 10). HOMA values were highest in IHH and were higher in IHL compared with CTL (Fig. 3E; IHH vs. IHL P < 0.05; IHH and IHL vs. CTL: P < 0.05). The complex relationships between basal levels of plasma glucose and insulin are further illustrated in Fig. 3F. Indeed, glucose levels were highest and insulin levels lowest in CTL under standard fasting conditions. AUCg analysis showed that IHH and IHL were smaller than CTL (Fig. 3D), and these findings were replicated by slope analyses. Indeed, as shown in Fig. 3, B and C, two distinct phases of blood glucose trajectory are apparent and were represented by an initial exponential increase to peak glycemic levels (mathematically represented by slope A) followed by an exponential decay (represented by the slope B). Significant differences were observed in slope A between IHL (slope A: 5.57 ± 0.52 mg·dl−1·min−1, means ± SE) and CTL (12.82 ± 1.11 mg·dl−1·min−1, means ± SE; P < 0.05) and between IHH (11.22 ± 0.94 mg·dl−1·min−1, means ± SE; n = 10) and IHL (P < 0.05), with IHL being worse than IHH and the latter worse than CTL.
Slope B represents how fast glucose levels in bloodstream decreased from minute 15 to minute 120 after glucose IP injection. The CTL group showed higher slope B values compared with both IHH and IHL, with the latter condition being the less efficient at decreasing plasma glucose levels (−2.63 ± 0.13 means ± SE, CTL; −1.73 ± 0.16 means ± SE, IHH; −0.62 ± 0.08 means ± SE, IHL; P < 0.05).
Insulin tolerance test.
As in the preceding experiments, low baseline fasting glycemic levels emerged in IHH and IHL exposed mice (133.2 and 65.1 mg/dl, respectively; n = 10 per group) compared with CTL group (169 mg/dl; n = 10). After IP humulin injection, the slope corresponding to the decreasing levels of plasma glucose over time (slope A) was considered as the indicator for insulin sensitivity. Calculations showed significantly attenuated slopes in IHH and IHL exposed mice (−1.32 ± 0.11 mg·dl−1·min−1, means ± SE, −1.16 ± 0.28 mg·dl−1·min−1, means ± SE, respectively) compared with CTL mice (−1.87 ± 0.12 mg·dl−1·min−1, means ± SE; P < 0.05).
Circulating Leptin and Insulin Levels
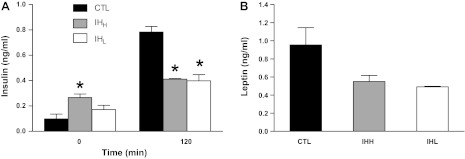
Insulin levels at time 0 min (T0) before glucose injection and insulin levels at time 120 min (T120) after exogenous glucose administration are shown in Fig. 4A. At T0, both IHH- and IHL-exposed mice were associated with increased insulin plasma concentrations, particularly among IHH-exposed animals (0.27 ± 0.03 ng/ml, means ± SE) compared with CTL group (0.10 ± 0.04 ng/ml, means ± SE; P < 0.05). In contrast, insulin levels at T120 were lower in both IH groups (0.78 ± 0.04 ng/ml, means ± SE, CTL; 0.41 ± 0.005 ng/ml, means ± SE, IHH; 0.40 ± 0.05 ng/ml, means ± SE, IHL; n = 10 per group; P < 0.05).
Fig. 4.
Changes in insulin and leptin plasma levels during IH. Insulin and leptin plasma levels in C57BL/6J mice exposed to IHH, IHL, and CTL conditions for a period of 5 wk. A: plasma insulin levels at fasting glucose baseline levels and after 120 min of glucose administration in the GTT (n = 10; *significant differences compared with control group; P < 0.05). B: leptin plasma levels measured at baseline after 3 h fasting period (n = 20; P NS). Results are means ± SE.
Leptin levels were significantly lower in animals exposed to IH (0.55 ± 0.07 ng/ml, means ± SE, IHH; 0.49 ± 0.005 ng/ml, means ± SE, IHL; P NS) compared with CTL (0.95 ± 0.19 ng/ml, means ± SE; n = 20 per group; P = 0.241; Fig. 4B).
Effect of IHH and IHL on PGC1-α and GLUT4 Protein Expression in Skeletal Muscle
PGC1-α expression.
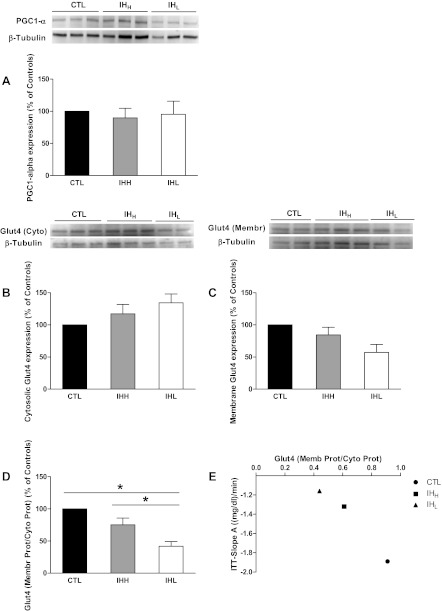
Compared with CTL mice, PGC1-α protein expression was unaltered by either IHH or IHL exposures (Fig. 5A).
Fig. 5.
Effect of IH on glucose tranporter 4 (GLUT4) and proliferator-activated receptor gamma coactivator-1α (PGC-1α) expression in skeletal muscle. Effect of 5-wk IHH, IHL, and CTL on PGC-1α and GLUT4 protein content in skeletal muscle. Representative Western blots of PGC-1α protein expression (A), GLUT4 cytosolic protein expression (B), and GLUT4 membrane protein expression (C) are shown. Samples on the autoradiographs (Western blot images panels) are expressed as percentage of the CTL group. Ratio between GLUT4 membrane protein expression and GLUT4 cytosolic protein expression were represented in D. Intensity units results are means ± SE and *P < 0.05 significantly different from the CTL group (n = 6 per group). Correlation between GLUT4 (Membr.Prot./Cyto.Prot) and slope A from ITT is shown (E).
GLUT4 expression.
Different trends in GLUT4 protein expression emerged in the cytosolic and membrane fractions (Fig. 5, B and C). GLUT4 cytosolic protein expression was increased in skeletal muscle of IHL exposed mice compared with either CTL or IHH conditions [n = 6 per group; 1.71 ± 0.54 intensity units (IU), means ± SE, IHL; 1.68 ± 0.5IU, means ± SE, IHH; 1.39 ± 0.45IU, means ± SE, CTL; P NS] (Fig. 5B). In contrast, expression of GLUT4 in the membrane fraction was significantly lower in IHH (0.62 ± 0.03 IU, means ± SE) and IHL (0.39 ± 0.06 IU, means ± SE) groups compared with CTL mice (0.74 ± 0.14 IU, means ± SE; P < 0.05), with IHL group being the lowest (Fig. 5C). Accordingly, GLUT4 membrane-to-cytosolic protein ratios, which are indicative of translocated GLUT4 protein (i.e., biologically active), were markedly lower in hypoxic mice compared with controls (Fig. 5D), and a significant correlation between GLUT4 (Membr.Prot./Cyto.Prot) and slope A from ITT was apparent (Fig. 5E).
DISCUSSION
In the present study, we aimed to assess the impact of different cycling frequencies in the application of the IH stimulus, a key clinical manifestation of OSA, on glucose regulation. Despite similar cumulative hypoxic profiles, presentation of IH using different cycling frequencies was associated with distinct glycemic homeostatic responses. From the present study, it becomes apparent that IH induces reductions in body weight gain, lowers fasting glucose levels, and also reduces the uptake of glucose after in vivo glucose administration, as well as insulin receptor sensitivity. Furthermore, lower levels of translocated GLUT4 to the plasma membrane in skeletal muscle are apparent. All of these phenomena are exacerbated in the context of IHL compared with high-frequency hypoxic oscillations, the latter differing from control conditions. Of note, although the concept that sustained and intermittent chronic hypoxic exposures imposes divergent effects on metabolic regulation is not novel and has been explored in several studies in both human and animals using exercise procedures (14, 66) or hypobaric hypoxia (46), we are unaware of any specific studies addressing the issue of hypoxia cycle duration on metabolic outcomes. In the discussion that follows, we will further explore the relationships and putative pathways linking IH frequency to insulin resistance. However, before we address these issues, several technical points appear worthy of mention. Indeed, the IH profile used herein is markedly similar to those reported by previous investigators, and as such our current findings can be potentially compared with such earlier studies (53, 58–59). Second, body weight matching was not pursued among both IH exposed mice and CTL group. Particularly, underfeeding CTL mice or overfeeding IH-exposed mice would be necessary to achieve weight matching (30, 58) between groups. Such interventions may however alter glycemic homeostasis per se and therefore mask the effects of IH explored in the present study. We reasoned that if an association between insulin resistance and IH emerged, the present findings would be all the more compelling considering the reductions in weight anticipated in the context of IH exposures. Third, GTT is routinely performed in mice following an overnight fast, with the food being removed around 4 PM, and the glucose load being administered via IP injection the following morning. However, it is well known that mice are nocturnal feeders, with ∼70% of their daily caloric intake occurring during the dark cycle (6), and that their metabolic rate is much higher than humans. Therefore, an overnight fast represents a comparatively longer fasting time compared with humans and is more akin to starvation. In addition, there is no consistency in the amount of glucose administered with studies using 1 g/kg (4, 51, 54) or 2 g/kg (5, 24, 31, 43, 81) or both doses to determine glucose tolerance (23, 62). In 2008, GTT was reevaluated in mice to determine the most appropriate fasting duration, route of glucose administration, and the ideal amount of glucose (3). Based on such considerations, we fasted mice for 3 h in both GTT and ITT and administered IP 2 mg glucose/g body wt during GTT.
Low- and High-Frequency IH and Body Weight Gain
Factors contributing to hypoxia-induced weight loss are complex and incompletely understood. Several investigators have demonstrated that body weight will continue to increase in mice submitted to chronic IH (58), whereas other laboratories have reported decreases in body weight accrual velocity in mice subjected to IH compared with controls (12, 17, 71, 76). Row et al. in 2002, measured body weights in postnatal rats and reported significantly lower body weight gain in rat pups exposed to IH than age-match controls housed in room air (60). In a recent study carried out in male lean mice exposed to different hypoxic patterns (normoxia, IH 12 times/h, IH 60 times/h or sustained hypoxia), body weights were similar among all hypoxic mice independent of the hypoxic regimen used but gained less weight than control animals (58). Consistent with such findings, we also found lower body weight gain in both hypoxic groups compared with room air-exposed adult mice independent from the IH frequency pattern for the first 7 days of exposure. However, lower body weight increases emerged in IHH compared with IHL, starting as of 14 days of hypoxic exposures. Consequently, body weight gain appears to be influenced by the IH frequency.
Low- and High-Frequency IH and Insulin Resistance
The selected IH profiles aimed to reproduce the overall cumulative hourly oxygen desaturations patterns routinely observed in moderate to severe OSA patients with oxyhemoglobin saturation levels ranging from 65 to 80% (11, 19, 22, 67). A large body of evidence derived from epidemiological cohorts and clinical populations indicates that OSA may also contribute to the development of metabolic disorders including glycemic regulation impairments (27, 55–56, 65). Furthermore, in healthy humans, acute IH induced decreased insulin sensitivity and inadequate increases in pancreatic insulin secretion (42). Iiyori and colleagues (26) used a euglycemic hyperinsulinemic clamp in mice and showed that acute IH (60 times/h over 9 h) induced insulin resistance. We now show that chronic IH will lead to lower fasting glycemic levels and relatively higher levels of insulin concentrations, specifically in IHL-exposed mice and not as much in IHH-exposed mice, yet suggesting the presence of insulin resistance state after both types of IH exposures. There were however discrepant findings between HOMA and either AUCg or slope analyses. Indeed, HOMA results suggested that IHH would be worse affected. This is not the case for the other two analytical approaches, which suggest that IHL is most severely affected. An important caveat for HOMA validity is that it imputes a dynamic β-cell function (i.e., glucose-stimulated insulin secretion) from fasting steady-state data. Therefore, based on the concordance between the two dynamic assessments of glucose and insulin relationships (i.e., AUCg and slope analyses), we would postulate that the IHL>IHH>CTL effect is most likely reflective of the true changes in glucose homeostasis. In addition, as can be seen from Fig. 3F, there were complex changes in both the baseline glycemic and insulin levels with each type of exposure, potentially reflecting underlying changes in the ”homeostat“ level of plasma glucose after prolonged hypoxic exposures, as well as changes in either basal secretion of insulin or in insulin sensitivity under those conditions. Reinke and collaborators (58) showed that even infrequent episodic hypoxic events (12 times/h) were sufficient to disturb insulin and glucose regulation in lean mice. We should note that in the present study we did not explore the presence of hypoxic injury to pancreatic β-cells during IH, and therefore the contribution of such previously reported effects of IH may have contributed to the kinetics of glycemia and insulin levels following IP glucose injection (75, 77). Another important comment pertains to the marked differences in peak glycemic levels that occurred after IP glucose injection. Notwithstanding the differences in body weight, this glucose dosage has been previously shown to reliably reflect existing differences in insulin sensitivity (3). However, it will be important in future studies to compare whether the oral glucose administration route will provide further information on the deregulation of overall glycemic homeostatic processes. Furthermore, the recent development of frequent sampling approaches in the mouse may provide improved resolution on the regulation of various glucose disposition compartments (2).
In the present study, we also show that after glucose injection, the resultant circulating glucose levels require longer times to be cleared from the circulation and to reach preinjection levels in both hypoxic groups, with the kinetics of glycemic decay in IHL-exposed mice being the most affected. Although the oral route of exogenous glucose administration is more physiological, we used an intraperitoneal administration to reduce the variability associated with inconsistent rates of gastric emptying, which can complicate data interpretation. On the other hand, the ITT was developed as a simple way to evaluate insulin action in vivo in humans. In mice, this test was modified, where a larger bolus of insulin is given after a fast of variable duration and the glucose concentration is monitored for a longer duration (∼90–120 min). For this test, the fall in blood glucose is used as a reflection of insulin action, such as a fall by ∼50% of glucose concentrations in wild-type mice on a chow diet and in normoxia (45). For both GTT and ITT, we opted to display the data as the kinetic slope from the baseline point to the highest or lowest glucose levels, respectively, thereby normalizing across potential confounders. Thus both GTT and ITT findings coincide in their findings and clearly establish a consistent perturbation of insulin sensitivity in the context of IH that is further modulated by the IH stimulus presentation frequency.
The changes in GLUT4 and PGC1-α were also examined in skeletal muscle. As mentioned, PGC1-α serves as a transcriptional coactivator that plays a key role in coordinating cellular metabolic flux in skeletal muscle, in which ∼90% of insulin-stimulated glucose uptake occurs (33). GLUT4 delivery to the cell surface is coordinated by insulin signaling and requires GLUT4 mobilization from intracellular membrane compartments, recognition of GLUT4-containing vesicles at the PM, and finally fusion of these two membranes (38). Whereas total GLUT 4 (data not shown) and PGC1-α remained unaltered by hypoxic exposures, we found that expression of GLUT4 in the membrane was significantly lower in IHL than IHH or controls, with no changes in GLUT4 expression in the cytosol. A potential explanation for such findings resides in the known activity of insulin. Indeed, insulin stimulates constitutive exocytosis (8) and particularly that of GLUT4 (38). Although the molecular mechanisms underlying the specificity of insulin action on GLUT4 vesicles remain to be established, insulin promotes tethering and fusion of GLUT4 vesicles to the plasma membrane (41), within the characteristic time frames of appearance of GLUT4 in the PM (16, 70). Interestingly, using a different IH profile, improved glucose tolerance in the absence of increased GLUT4 protein expression was previously reported (14). The unbalanced distribution of GLUT4, as clearly shown in Fig. 5D, may account for the IHL-animals inability to facilitate the transport of glucose to the intracellular space, as described in our experiments using both GTT and ITT (Fig. 5E). Thus the frequency of IH is critical for the development of insulin resistance and impaired glucose tolerance, thereby confirming Polotsky et al. previous findings (53).
Low- and High-Frequency IH and Leptin
Low fasting leptin levels probably play an important role in the alterations reported on insulin and glucose responses to IH. It has been shown that leptin can act at the level of pancreas, by downregulating insulin gene transcription and insulin secretion (34, 64). Conversely, leptin deficiency plays a key role in the acceleration of insulin resistance in mice exposed to IH, yet it remains unclear whether mice with intact leptin pathways are also susceptible to metabolic dysfunction after exposure to IH (53). We found lower baseline levels of leptin expression in both hypoxic groups with no significant differences between high- and low-frequency hypoxia. Therefore, hypoxia per se, rather than the frequency of IH, appears to be the determinant factor underlying the emergence of lower leptin levels.
We should emphasize that a significant limitation of our study included the absence of any assessments of differences in the levels of stress hormones among the three experimental groups. Indeed, a recent work by O'Donnell and colleagues (77) has postulated that the initial stress response, i.e., corticosterone levels, was a major determinant to the acute metabolic changes observed after short-lasting period of IH. This group of investigators has just published a study that emphasizes the differences between acute and chronic metabolic adaptations to IH, whereby longer durations of IH exposures appear to alleviate the magnitude of metabolic dysfunction induced by IH (36).
Perspectives and Significance
The results of the present study demonstrate that intermittent hypoxia, independently of the frequency, leads to impairments in glucose metabolism in mice. Indeed, IH is associated with reduced body weight, increase in insulin resistance, and low leptin levels. Furthermore, the alterations in GLUT4 cellular distribution in skeletal muscle are further supportive of a putative mechanism for the effects of IH on insulin sensitivity. These findings may have substantial implications for the understanding of metabolic regulation and deregulation in diseases associated with hypoxia, such a sleep apnea and chronic obstructive lung disease.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-065270 and HL-086662, and P50 HL-107160. F. Kayali was the recipient of a T-32 Training Grant HL-094282 (N. R. Prabhakar, PI).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C., F.K., J.Z., and C.H. performed experiments; A.C., J.Z., and D.G. analyzed data; A.C. and D.G. interpreted results of experiments; A.C. prepared figures; A.C. and D.G. drafted manuscript; A.C., F.K., J.Z., C.H., Y.W., and D.G. approved final version of manuscript; Y.W. and D.G. conception and design of research; D.G. edited and revised manuscript.
REFERENCES
- 1. Agani F, Semenza GL. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol 54: 749–754, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Alonso LC, Watanabe Y, Stefanovski D, Lee EJ, Singamsetty S, Romano LC, Zou B, Garcia-Ocana A, Bergman RN, O'Donnell CP. Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice. Obesity (Silver Spring) 20: 1403–1412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295: E1323–E1332, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Andrikopoulos S, Verchere CB, Terauchi Y, Kadowaki T, Kahn SE. beta-cell glucokinase deficiency and hyperglycemia are associated with reduced islet amyloid deposition in a mouse model of type 2 diabetes. Diabetes 49: 2056–2062, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Aston-Mourney K, Wong N, Kebede M, Zraika S, Balmer L, McMahon JM, Fam BC, Favaloro J, Proietto J, Morahan G, Andrikopoulos S. Increased nicotinamide nucleotide transhydrogenase levels predispose to insulin hypersecretion in a mouse strain susceptible to diabetes. Diabetologia 50: 2476–2485, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Braun B, Rock PB, Zamudio S, Wolfel GE, Mazzeo RS, Muza SR, Fulco CS, Moore LG, Butterfield GE. Women at altitude: short-term exposure to hypoxia and/or α1-adrenergic blockade reduces insulin sensitivity. J Appl Physiol 91: 623–631, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3: 267–277, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Calderon R, Llerena LA, Munive L, Kruger F. Intravenous glucose tolerance test in pregnancy in women living in chronic hypoxia. Diabetes 15: 130–132, 1966 [DOI] [PubMed] [Google Scholar]
- 10. Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol 104: 1304–1312, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Chaufour X, Issa F, Sullivan C, McLachlan C, Unger G. A fully-automated environmental chamber for examination of long-term effects of intermittent hypoxia on medium-size animals. Jpn J Physiol 49: 207–211, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Chen CY, Tsai YL, Kao CL, Lee SD, Wu MC, Mallikarjuna K, Liao YH, Ivy JL, Kuo CH. Effect of mild intermittent hypoxia on glucose tolerance, muscle morphology and AMPK-PGC-1alpha signaling. Chin J Physiol 53: 62–71, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, Mishima M, Nakao K, Ohi M. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 100: 706–712, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Chiu LL, Chou SW, Cho YM, Ho HY, Ivy JL, Hunt D, Wang PS, Kuo CH. Effect of prolonged intermittent hypoxia and exercise training on glucose tolerance and muscle GLUT4 protein expression in rats. J Biomed Sci 11: 838–846, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Davidson MB, Aoki VS. Fasting glucose homeostasis in rats after chronic exposure to hypoxia. Am J Physiol 219: 378–383, 1970 [DOI] [PubMed] [Google Scholar]
- 16. Dawson K, Aviles-Hernandez A, Cushman SW, Malide D. Insulin-regulated trafficking of dual-labeled glucose transporter 4 in primary rat adipose cells. Biochem Biophys Res Commun 287: 445–454, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 177: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, Jebb SA, Lip GY, O'Rahilly S. Partial leptin deficiency and human adiposity. Nature 414: 34–35, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Fletcher EC. An animal model of the relationship between systemic hypertension and repetitive episodic hypoxia as seen in sleep apnoea. J Sleep Res 4: 71–77, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 177: 1142–1149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, Kahn CR. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122: 337–349, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–3474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hultgren HN, Grover RF. Circulatory adaptation to high altitude. Annu Rev Med 19: 119–152, 1968 [DOI] [PubMed] [Google Scholar]
- 26. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165: 670–676, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 118: 580–586, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209: 381–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kebede M, Favaloro J, Gunton JE, Laybutt DR, Shaw M, Wong N, Fam BC, Aston-Mourney K, Rantzau C, Zulli A, Proietto J, Andrikopoulos S. Fructose-1,6-bisphosphatase overexpression in pancreatic beta-cells results in reduced insulin secretion: a new mechanism for fat-induced impairment of beta-cell function. Diabetes 57: 1887–1895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res 58: 348–351, 1998 [PubMed] [Google Scholar]
- 33. Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol Endocrinol Metab 248: E353–E362, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 100: 2729–2736, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol 504: 241–249, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee EJ, Alonso LC, Stefanovski D, Strollo HC, Romano LC, Zou B, Singamsetty S, Yester KA, McGaffin KR, Garcia-Ocana A, O'Donnell CP. Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol July 17, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leick L, Fentz J, Bienso RS, Knudsen JG, Jeppesen J, Kiens B, Wojtaszewski JF, Pilegaard H. PGC-1α is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am J Physiol Endocrinol Metab 299: E456–E465, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol 13: 383–396, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Ling Q, Sailan W, Ran J, Zhi S, Cen L, Yang X, Xiaoqun Q. The effect of intermittent hypoxia on bodyweight, serum glucose and cholesterol in obesity mice. Pak J Biol Sci 11: 869–875, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol 169: 481–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol 106: 1538–1544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, Nagy A, Woodgett JR. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab 6: 329–337, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Malide D, Ramm G, Cushman SW, Slot JW. Immunoelectron microscopic evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J Cell Sci 113: 4203–4210, 2000 [DOI] [PubMed] [Google Scholar]
- 45. McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab 297: E849–E855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP. Combining hypoxic methods for peak performance. Sports Med 40: 1–25, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Moraes D, Loscalzo J. Pulmonary hypertension: newer concepts in diagnosis and management. Clin Cardiol 20: 676–682, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naeije R. Pulmonary circulation at high altitude. Respiration 64: 429–434, 1997 [DOI] [PubMed] [Google Scholar]
- 50. O'Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Rutherford J, Mehl RC, Wang M, Tuell A, Hume BC, Gozal D. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics 111: 554–563, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 132: 325–337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131: 476–491, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165: 677–682, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Ravussin E, Pratley RE, Maffei M, Wang H, Friedman JM, Bennett PH, Bogardus C. Relatively low plasma leptin concentrations precede weight gain in Pima Indians. Nat Med 3: 238–240, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol 111: 881–890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Row BW, Kheirandish L, Li RC, Guo SZ, Brittian KR, Hardy M, Bazan NG, Gozal D. Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem 89: 189–196, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, Pedersen DJ, Braun U, Cooney GJ, Leitges M, Biden TJ. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab 6: 320–328, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci USA 96: 674–679, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, Jagia P, Kumar A. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 365: 2277–2286, 2011 [DOI] [PubMed] [Google Scholar]
- 66. Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovskaya TV. Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt Med Biol 9: 43–52, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol 121: 173–184, 2000 [DOI] [PubMed] [Google Scholar]
- 68. Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, Hoffstem VS. Insulin levels, blood pressure and sleep apnea. Sleep 17: 614–618, 1994 [DOI] [PubMed] [Google Scholar]
- 69. Tapanainen PJ, Bang P, Muller HL, Wilson K, Rosenfeld RG. Hypoxia-induced changes in insulin-like growth factors and their binding proteins in pregnant rats. Horm Res 48: 227–234, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Tengholm A, Meyer T. A PI3-kinase signaling code for insulin-triggered insertion of glucose transporters into the plasma membrane. Curr Biol 12: 1871–1876, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Truog WE, Xu D, Ekekezie II, Mabry S, Rezaiekhaligh M, Svojanovsky S, Soares MJ. Chronic hypoxia and rat lung development: analysis by morphometry and directed microarray. Pediatr Res 64: 56–62, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Tucci M, Nygard K, Tanswell BV, Farber HW, Hill DJ, Han VK. Modulation of insulin-like growth factor (IGF) and IGF binding protein biosynthesis by hypoxia in cultured vascular endothelial cells. J Endocrinol 157: 13–24, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85: 1151–1158, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 34: 5–15, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med 46: 783–790, 2009 [DOI] [PubMed] [Google Scholar]
- 76. Xu NY, Chen XQ, Du JZ, Wang TY, Duan C. Intermittent hypoxia causes a suppressed pituitary growth hormone through somatostatin. Neuro Endocrinol Lett 25: 361–367, 2004 [PubMed] [Google Scholar]
- 77. Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol 586: 899–911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 79. Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103: 691–696, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17: 5085–5094, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhong L, Georgia S, Tschen SI, Nakayama K, Bhushan A. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest 117: 2869–2876, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]