Abstract
We tested whether mild and moderate dynamic exercise and muscle metaboreflex activation (MMA) affect dynamic baroreflex control of heart rate (HR) and cardiac output (CO), and the influence of stroke volume (SV) fluctuations on CO regulation in normal (N) and pacing-induced heart failure (HF) dogs by employing transfer function analyses of the relationships between spontaneous changes in left ventricular systolic pressure (LVSP) and HR, LVSP and CO, HR and CO, and SV and CO at low and high frequencies (Lo-F, 0.04–0.15 Hz; Hi-F, 0.15–0.6 Hz). In N dogs, both workloads significantly decreased the gains for LVSP-HR and LVSP-CO in Hi-F, whereas only moderate exercise also reduced the LVSP-CO gain in Lo-F. MMA during mild exercise further decreased the gains for LVSP-HR in both frequencies and for LVSP-CO in Lo-F. MMA during moderate exercise further reduced LVSP-HR gain in Lo-F. Coherence for HR-CO in Hi-F was decreased by exercise and MMA, whereas that in Lo-F was sustained at a high level (>0.8) in all settings. HF significantly decreased dynamic HR and CO regulation in all situations. In HF, the coherence for HR-CO in Lo-F decreased significantly in all settings; the coherence for SV-CO in Lo-F was significantly higher. We conclude that dynamic exercise and MMA reduces dynamic baroreflex control of HR and CO, and these are substantially impaired in HF. In N conditions, HR modulation plays a major role in CO regulation. In HF, influence of HR modulation wanes, and fluctuations of SV dominate in CO variations.
Keywords: arterial baroreflex, exercise reflexes, pressor response, impaired cardiac performance
beat-to-beat cardiac output (CO) varies considerably across successive heart beats, whereas the average level remains remarkably constant under basal conditions (21, 50). This likely involves control mechanisms operating at markedly different frequencies. Rapid baroreflex control of heart rate (HR) is thought to play a crucial role in dynamic CO regulation. Previous studies suggest that low-frequency (Lo-F: 0.04–0.15 Hz) blood pressure fluctuations are buffered by the dynamic baroreflex control of HR (25, 28). However, we and others have observed that changes in HR do not necessarily elicit proportional changes in CO because stroke volume (SV) may also vary with the changes in ventricular filling time (17, 34, 48). Furthermore, transient increases in CO will lower ventricular filling pressure (and decreases in CO will raise filling pressure), thereby providing a self-limiting response (6, 30, 40). Furthermore, baroreflex control of HR may not functionally buffer high-frequency (Hi-F: above 0.15 Hz) blood pressure fluctuations (4, 38, 44). In this case, Hi-F variations of CO could facilitate blood pressure variability via a feed-forward mechanism rather than buffer arterial pressure. In addition, it has been shown that in anesthetized rats, the blood pressure response to cardiac pacing induced high-frequency oscillation of CO is smaller than that to low-frequency oscillation of CO (41). To our knowledge, however, the dynamic relationship between blood pressure and CO across frequencies has never been examined under conscious conditions. Furthermore, the relationships among spontaneous HR, SV, and CO fluctuations in normal subjects in both the Lo-F and Hi-F ranges are unclear at rest.
Hallmark features of congestive heart failure are increased sympathetic activity to the heart and vasculature, depressed vagal outflow to the heart, a resting tachycardia with diminished heart rate variability, and a decrease in baroreflex control of HR (14, 47, 52, 53), which coupled with lower SV, due to diminished contractility, markedly depresses baroreflex control over CO (24). However, dynamic CO regulation and its relations with HR and SV fluctuations are largely uncertain.
During exercise, the baroreflex is reset to operate around the higher prevailing blood pressure and HR. As workload increases, the rapid HR responses to spontaneous changes in arterial pressure progressively decrease as workload rises (3, 13, 23, 45). A major mechanism potentially mediating the normal rise in HR and arterial pressure during exercise is the activation of the muscle metaboreflex via stimulation of chemosensitive afferents within the active muscle, which can elicit a marked pressor response (1, 7, 9, 19, 31, 49) and also reduces this spontaneous baroreflex HR sensitivity (33, 35), and this effect of exercise and metaboreflex activation is further exacerbated in heart failure (14). The attenuated baroreflex HR sensitivity in these conditions could have an important impact on dynamic CO regulation, especially in the Lo-F range, where the baroreflex exerts strong control over HR theoretically to protect against further blood pressure fluctuations (25, 28). In subjects with congestive heart failure, the low left ventricular contractility and increased afterload sensitivity coupled with depressed baroreflex sensitivity may combine to markedly attenuate functional control of CO in cardiovascular regulation.
The effects of exercise and muscle metaboreflex activation on the dynamic CO regulation over a wide frequency range have never been investigated, even in normal subjects. In heart failure, with the marked changes in baroreflex sensitivity, cardiac inotropicity, and afterload sensitivity, these relationships may be significantly altered as the effectiveness of changes in HR to elicit changes in CO becomes even more constrained, especially so at higher frequencies. The present study was designed to address these questions. We hypothesized that exercise would attenuate dynamic baroreflex control of HR and CO in the entire frequency range. Although dynamic baroreflex HR control would decrease further with metaboreflex activation, substantial increases in ventricular contractility would also occur (37), which might attenuate further decrease in dynamic CO regulation. In addition, heart failure would markedly diminish dynamic CO control even further.
MATERIALS AND METHODS
Experiments were performed on seven adult, mongrel dogs (weight ∼20–25 kg) of either sex, selected for their willingness to run on a motor-driven treadmill. The protocols conform with the U.S. National Institutes of Health guidelines and were reviewed and approved by the Wayne State University Animal Investigation Committee. All data reported are unique to this study and have not been previously published.
Surgical preparation and procedures.
All animals were accustomed to human handling before they were surgically instrumented in two different procedures (sternotomy and left flank abdominal surgery), as described in several previous studies (10, 11, 14). Briefly, using aseptic procedures, a midline sternotomy was performed, and a fully implantable telemetered blood pressure transducer (model no. PAD-70; Data Sciences International) was placed subcutaneously on the left side of the chest. The catheter was tunneled into the thoracic cavity through the 7th or 8th intercostal space and inserted into the left ventricle for measuring left ventricular pressure (LVP). A 20- or 24-mm blood flow transducer (Transonic Systems) was placed around the ascending aorta to measure CO. Three stainless-steel ventricular pacing electrodes (O-Flexon, Ethicon) were sutured to the right ventricular free wall. For studies unrelated to the present investigation, vascular occluders were placed on the superior and inferior venous cava, and two pairs of sonomicrometry crystals were placed on the left ventricular endocardium. The pericardium was reapproximated loosely, and the chest was closed in layers. After a recovery period (at least 10 days), a second surgical procedure (left abdominal retroperitoneal surgery) was performed. A 10-mm blood flow probe (Transonic Systems) was placed on the terminal aorta to measure blood flow to the hindlimbs (HLBF). A hydraulic vascular occluder (DocXS Biomedical Products) was placed on the terminal aorta just distal to the flow probe. All arteries branching from the aorta between the iliac arteries and the HLBF probe were ligated and severed, and a catheter was placed through a lumbar artery proximal to the HLBF probe and occluder to measure mean arterial pressure (MAP). All flow probe cables, pacing wires, vascular occluder tubings, and the aortic catheter were tunneled subcutaneously and exteriorized between the scapulas at the end of its corresponding surgical procedure. The animals were allowed at least 7 days for recovery prior to any experiments.
Experimental procedures.
All experiments were performed after the animals had fully recovered from instrumentation (i.e., active, alert, afebrile, and of good appetite). All data were recorded on analog-to-digital recording systems for subsequent offline analyses. For a given experimental session, data were collected at rest and then at a randomly selected workload (mild exercise: 3.2 km/h, 0% grade elevation or moderate exercise: 6.4 km/h, 10% grade elevation). Only one workload was performed on any experimental day. All animals ran freely with only positive verbal encouragement. Steady-state data were recorded at rest, while the animal was standing on the treadmill, during exercise with unrestricted blood flow to the hindlimbs, and after metaboreflex activation elicited by reductions in HLBF achieved by partial inflation of the terminal aortic occluder, as previously described (49). After completion of the control experiments, modest congestive heart failure was induced via rapid ventricular pacing, as previously described by us and others (26, 36). Briefly, the heart was paced at 240–250 beats per minute (bpm) for ∼30 days, and the experiments were repeated while in modest heart failure conditions [defined as resting tachycardia, reduced CO, SV, and left ventricular +/−dP/dt, as described in our previous studies (8, 14, 36)]. The pacemaker was disconnected ∼30 min prior to the experiment.
Data analysis.
During the experiments, beat-to-beat CO, HLBF, HR, MAP, and LVP were collected continuously. Data were recorded for 3 to 5 min of steady state. The data were averaged at each setting (at rest, during mild or moderate exercise with unrestricted blood flow to the hindlimbs, and after metaboreflex activation) across all experiments for every animal.
We used 3 min of steady-state data for spectral and cross spectral analysis. The beat-to-beat data for the HR, CO, SV, and left ventricular systolic pressure (LVSP) were interpolated and resampled, which provided 1,024 points of equidistant time interval data. The data were then divided into five equal overlapping segments of 512 data points, and for each segment, the linear trend was removed, and the Hanning window was applied. Fast Fourier transforms were implemented in each segment and then averaged to calculate the autospectrum. The spectral resolution for these estimates was ∼0.0111 Hz. Total power as well as Lo-F (0.04 ∼ 0.15 Hz) and Hi-F (0.15 ∼ 0.6 Hz) powers of all the variables were calculated from the integration of the autospectra. The spectral powers in Lo-F and Hi-F ranges were also expressed as normalized values relative to the total powers, respectively. We then employed transfer function analysis to evaluate the relationship between LVSP and HR, LVSP and CO, HR and CO, and SV and CO. The transfer function [H(ƒ)] between two signals was calculated as H(ƒ) = Sxy(ƒ)/Sxx(ƒ), where Sxx(ƒ) is the autospectrum of LVSP or HR or SV variability, and Sxy(ƒ) is the cross spectrum between LVSP and HR, LVSP and CO, HR and CO, and SV and CO. The transfer function magnitude (gain), H(ƒ), and phase spectrum [Φ(ƒ)], were obtained from the real [HR(ƒ)] and imaginary [HI(ƒ)] parts of the complex function as H(ƒ) = [HR(ƒ)2 + HI(ƒ)2]1/2 and Φ(ƒ) = tan−1[HI(ƒ)/ HR(ƒ)]. The squared coherence function [Coh(ƒ)] was estimated as Coh(ƒ) = Sxy(ƒ) 2/[ Sxx(ƒ) Syy(ƒ)], where Syy(ƒ) is the autospectrum of changes in HR or SV or CO. The squared coherence function reflects the fraction of the output power that can be linearly related to the input power at each frequency. Similar to a correlation coefficient, it varies from 0 to 1 and reflects the validity of the transfer function estimates. For this purpose, the transfer gain and phase only at frequency data points of coherence ≥0.5 were accepted as significant responses and averaged in the Lo-F and Hi-F ranges, respectively. The minimum number of the frequency data points associated with coherence ≥0.5 were 6 out of 10 points in Lo-F range and 18 out of 41 points in Hi-F range throughout all animals across all the settings and conditions. The coherence data presented reflect the average values from all data points in each frequency range for all parameters. For phase value interpretation, a negative phase suggested that changes in the input variable preceded directionally similar changes in the output response, whereas a positive phase suggested the reverse. However, from a baroreflex perspective, positive-phase values for LVSP-HR and LVSP-CO mean that changes in LVSP are followed by changes in HR and CO in the opposite direction. Therefore, these positive-phase values for LVSP-HR and LVSP-CO relationships were taken as baroreflex relationships. We used HR rather than pulse interval for transfer function analysis of dynamic CO regulation because changes in HR and SV induce directionally similar changes in CO, while changes in pulse interval cause directionally opposite changes in CO.
Statistical analysis.
Utilizing the averaged responses for each animal, we performed statistical analyses on the data with Systat software (Systat 11.0). An α-level of P < 0.05 was set to determine statistical significance. Two-way ANOVA for repeated measures was used for comparing hemodynamic data obtained at rest, during each workload before and after metaboreflex activation, and in normal and heart failure conditions. When a significant interaction term was found, a test for simple effects post hoc analysis was performed to determine significant group mean differences. We performed dependent t-tests to determine when phase values were significantly different from zero. In figures, tables, and text, data are expressed as means ± SE and reflect data from seven animals in normal state, with an n = 6 in heart failure.
RESULTS
Tables 1 and 2 show the average values of HLBF, LVSP, CO, SV, and HR before and after induction of congestive heart failure at rest, during exercise, and during exercise with muscle metaboreflex activation at each workload. The responses to exercise and muscle metaboreflex activation before and after induction of heart failure were essentially the same as we have reported in previous studies (2, 7, 36). Briefly, metaboreflex activation caused substantial increases in HR, CO, and LVSP at both workloads in normal conditions. After induction of heart failure, CO, HLBF, SV, and LVSP were significantly depressed, and the animals were tachycardic. Metaboreflex activation in heart failure still caused a tachycardia; however, the fall in SV markedly limited any reflex increase in CO.
Table 1.
Hemodynamic values at rest, during mild exercise, and metaboreflex activation at mild exercise in normal animals and in the same animals after induction of heart failure
| Condition/Setting | HLBF, l/min | LVSP, mmHg | CO, l/min | SV, ml | HR, bpm |
|---|---|---|---|---|---|
| N, rest | 0.79 ± 0.11 | 134.4 ± 5.6 | 4.40 ± 0.33 | 42.0 ± 3.6 | 106 ± 5 |
| N, mild free-flow exercise | 1.13 ± 0.13† | 135.2 ± 5.9 | 5.84 ± 0.45† | 45.1 ± 3.8† | 131 ± 4† |
| N, mild exercise + MMA | 0.61 ± 0.05‡ | 181.1 ± 5.4‡ | 7.18 ± 0.57‡ | 47.1 ± 4.0 | 154 ± 5‡ |
| HF, rest | 0.50 ± 0.08* | 103.0 ± 6.1* | 3.34 ± 0.33* | 26 ± 2.8* | 131 ± 7* |
| HF, mild free-flow exercise | 0.75 ± 0.09*† | 110.8 ± 5.4* | 4.30 ± 0.39*† | 27.8 ± 3.4* | 158 ± 8*† |
| HF, mild exercise + MMA | 0.59 ± 0.09‡ | 141.8 ± 7.8*‡ | 4.62 ± 0.55* | 25.2 ± 2.9*‡ | 184 ± 8*‡ |
Values are expressed as means ± SE.
HLBF, hindlimb blood flow, LVSP, left ventricular systolic pressure; CO, cardiac output; SV, stroke volume; HR, heart volume; bpm, beats per minute.
For HLBF, n = 6; for all other values, n = 7 in normal (N) and n = 6 in heart failure (HF).
P < 0.05 HF vs. N;
P < 0.05, free-flow exercise vs. rest;
P < 0.05. Free-flow exercise plus metaboreflex activation (MMA) vs. free flow exercise.
Table 2.
Hemodynamic values at rest, during moderate exercise, and metaboreflex activation at moderate exercise in normal animals and in the same animals after induction of heart failure
| Condition/Setting | HLBF, l/min | LVSP, mmHg | CO, l/min | SV, ml | HR, bpm |
|---|---|---|---|---|---|
| N, Rest | 0.79 ± 0.11 | 134.4 ± 5.6 | 4.40 ± 0.33 | 42.0 ± 3.6 | 106 ± 5 |
| N, moderate free flow exercise | 2.49 ± 0.17† | 151 ± 5.7† | 8.53 ± 0.53† | 46.4 ± 3.8† | 187 ± 8† |
| N, moderate exercise + MMA | 1.82 ± 0.15‡ | 189.5 ± 7.0‡ | 9.84 ± 0.63‡ | 50.4 ± 4.1‡ | 198 ± 8‡ |
| HF, rest | 0.50 ± 0.08* | 103.0 ± 6.1* | 3.34 ± 0.33* | 26 ± 2.8* | 131 ± 7* |
| HF, moderate free flow exercise | 1.89 ± 0.22*† | 132.8 ± 7.4*† | 6.82 ± 0.59*† | 35.4 ± 3.2*† | 193 ± 3*† |
| HF, moderate exercise + MMA | 1.53 ± 0.22‡ | 160.7 ± 12.2*‡ | 7.15 ± 0.78* | 34.3 ± 3.5* | 207 ± 4*‡ |
Values are expressed as means ± SE. For HLBF, n = 6; for all other values, n = 7 in N and n = 6 in HF.
P < 0.05 HF vs. N;
P < 0.05, free-flow exercise vs. rest;
P < 0.05. Abbreviations are the same as in Table 1.
Tables 3 and 4 show spectral power for LVSP, HR, CO, and SV before and after the induction of heart failure at rest, during exercise, and muscle metaboreflex activation at both workloads. Mild exercise decreased total power for SV and the Hi-F power for all variables except LVSP, whereas it increased normalized Lo-F power for HR and CO. Moderate exercise increased total, Lo-F, and Hi-F power for LVSP and Lo-F power for HR and SV, whereas it substantially diminished Hi-F power for HR, CO, and SV. Normalized Lo-F power for HR, CO, and SV increased, whereas normalized Hi-F power for these variables decreased during moderate exercise. Metaboreflex activation at mild exercise increased total and Lo-F LVSP power (vs. free flow exercise). After induction of heart failure, spectral power in absolute units for all the variables substantially diminished with the exception of no significant change in total and Hi-F LVSP power and Lo-F HR power. Normalized Lo-F power for HR and CO increased, whereas normalized Hi-F power for HR, CO, and SV decreased in heart failure. Mild exercise decreased total, Lo-F, and Hi-F HR power. During moderate exercise, total, Lo-F, and Hi-F power for LVSP increased, whereas total, Lo-F and Hi-F power for HR and Hi-F power for SV decreased. In addition, normalized Hi-F power for CO and normalized Lo-F power for SV increased, whereas normalized Hi-F SV power decreased during moderate exercise. Metaboreflex activation at mild exercise increased total LVSP power and decreased Hi-F SV power in both absolute and normalized units. Metaboreflex activation at moderate exercise increased total and Hi-F CO power and total, Lo-F and Hi-F powers for SV, whereas it decreased normalized Lo-F power for CO.
Table 3.
Spectral power for LVSP, HR, CO, and SV at rest, during mild exercise, and muscle MMA at mild exercise in normal animals and in the same animals after induction of HF
| Normal |
Heart Failure |
|||||
|---|---|---|---|---|---|---|
| Rest | Free-Flow Exercise | MMA | Rest | Free-Flow Exercise | MMA | |
| LVSP | ||||||
| Total, mmHg2 | 16.1 ± 1.5 | 15.5 ± 1.8 | 35.8 ± 6.2‡ | 11.1 ± 2.7 | 20.9 ± 8.2 | 29.6 ± 8.0‡ |
| Lo-F | 5.8 ± 0.5 | 6.4 ± 1.8 | 19.2 ± 3.8‡ | 2.8 ± 0.4* | 4.2 ± 1.3 | 5.4 ± 1.3* |
| Hi-F | 4.8 ± 1.1 | 5.3 ± 0.8 | 9.6 ± 2.5 | 2.7 ± 0.7 | 5.1 ± 1.1 | 7.6 ± 2.6 |
| Norm Lo-F, % | 36.3 ± 2.1 | 39.8 ± 6.5 | 52.7 ± 5.9 | 30.2 ± 4.9 | 27.4 ± 7.0 | 23.0 ± 6.3* |
| Norm Hi-F | 28.9 ± 4.8 | 34.6 ± 4.7 | 26.9 ± 4.1 | 25.8 ± 3.7 | 31.4 ± 4.0 | 25.9 ± 5.3 |
| HR | ||||||
| Total, bpm2 | 146.6 ± 27.4 | 112.6 ± 24.4 | 109.4 ± 18.2 | 57.2 ± 13.3* | 6.0 ± 2.3*† | 12.5 ± 5.9* |
| Lo-F | 24.1 ± 4.0 | 45.2 ± 14.0 | 37.7 ± 8.7 | 19.3 ± 4.6 | 2.3 ± 1.6*† | 4.6 ± 2.1* |
| Hi-F | 84.9 ± 19.3 | 49.0 ± 13.9† | 52.9 ± 14.0 | 17.9 ± 5.2* | 1.5 ± 0.6*† | 4.3 ± 3.1* |
| Norm Lo-F, % | 18.6 ± 3.4 | 39.4 ± 6.9† | 35.2 ± 6.2 | 37.0 ± 6.0* | 22.9 ± 8.5* | 40.8 ± 7.8 |
| Norm Hi-F | 56.1 ± 5.6 | 41.5 ± 6.5 | 44.5 ± 6.8 | 30.8 ± 5.8* | 28.8 ± 6.4 | 21.0 ± 5.7* |
| CO | ||||||
| Total, l/min2 | 0.298 ± 0.065 | 0.216 ± 0.046 | 0.238 ± 0.051 | 0.064 ± 0.005* | 0.051 ± 0.010* | 0.041 ± 0.008* |
| Lo-F | 0.044 ± 0.008 | 0.082 ± 0.021 | 0.089 ± 0.030 | 0.022 ± 0.002* | 0.015 ± 0.004* | 0.008 ± 0.001* |
| Hi-F | 0.167 ± 0.052 | 0.077 ± 0.024† | 0.069 ± 0.017 | 0.008 ± 0.001* | 0.009 ± 0.001* | 0.010 ± 0.002* |
| Norm Lo-F, % | 17.1 ± 3.5 | 37.8 ± 7.0† | 33.7 ± 4.7 | 33.9 ± 2.5* | 34.5 ± 7.0 | 22.0 ± 2.6 |
| Norm Hi-F | 50.8 ± 7.2 | 33.7 ± 5.4 | 30.4 ± 5.8 | 13.1 ± 2.1* | 20.9 ± 3.1* | 27.8 ± 5.5 |
| SV | ||||||
| Total, ml2 | 4.72 ± 0.88 | 3.03 ± 0.69† | 3.02 ± 0.25 | 2.15 ± 0.28* | 1.87 ± 0.50 | 1.46 ± 0.33* |
| Lo-F | 0.56 ± 0.10 | 0.60 ± 0.16 | 0.46 ± 0.05 | 0.36 ± 0.03* | 0.29 ± 0.07* | 0.31 ± 0.05* |
| Hi-F | 3.07 ± 0.83 | 1.56 ± 0.45† | 1.42 ± 0.19 | 0.69 ± 0.17* | 0.49 ± 0.10* | 0.30 ± 0.06*‡ |
| Norm Lo-F, % | 13.4 ± 2.2 | 19.2 ± 2.8 | 15.1 ± 1.1 | 18.2 ± 2.4 | 19.9 ± 5.5 | 24.3 ± 3.3* |
| Norm Hi-F | 58.7 ± 6.5 | 48.3 ± 4.2 | 47.1 ± 4.7 | 31.3 ± 4.5* | 31.0 ± 5.1* | 24.9 ± 4.5*‡ |
Values are expressed as means ± SE. For HLBF, n = 6; for all other values, n = 7 in N and n = 6 in HF.
P < 0.05 HF vs. N;
P < 0.05, free-flow exercise vs. rest;
P < 0.05. Total, total spectral power; Lo-F, spectral power in low frequency range; Hi-F, spectral power in high-frequency range; Norm Lo-F, normalized spectral power in low frequency range relative to total power; Norm Hi-F, normalized spectral power in high-frequency range relative to total power. Other abbreviations are the same as in Table 1.
Table 4.
Spectral power for LVSP, HR, CO, and SV at rest, during moderate exercise and muscle MMA at moderate exercise in normal animals and in the same animals after induction of heart failure
| Normal |
Heart Failure |
|||||
|---|---|---|---|---|---|---|
| Rest | Free-Flow Exercise | MMA | Rest | Free-Flow Exercise | MMA | |
| LVSP | ||||||
| Total, mmHg2 | 16.1 ± 1.5 | 41.1 ± 5.8† | 51.5 ± 5.4 | 11.1 ± 2.7 | 32.6 ± 6.1† | 44.6 ± 10.4 |
| Lo-F | 5.8 ± 0.5 | 14.0 ± 3.2† | 18.0 ± 4.0 | 2.8 ± 0.4* | 7.8 ± 2.3† | 4.6 ± 0.6* |
| Hi-F | 4.8 ± 1.1 | 14.0 ± 4.5† | 17.0 ± 2.8 | 2.7 ± 0.7 | 12.3 ± 2.8† | 18.6 ± 5.9 |
| Norm Lo-F, % | 36.3 ± 2.1 | 35.1 ± 5.3 | 33.9 ± 5.4 | 30.2 ± 4.9 | 21.9 ± 2.8* | 13.4 ± 2.1* |
| Norm Hi-F | 28.9 ± 4.8 | 31.3 ± 7.3 | 33.7 ± 4.2 | 25.8 ± 3.7 | 37.1 ± 6.5 | 39.4 ± 3.5 |
| HR | ||||||
| Total, bpm2 | 146.6 ± 27.4 | 137.1 ± 30.5 | 81.9 ± 16.5 | 57.2 ± 13.3* | 9.1 ± 1.8*† | 8.1 ± 1.8* |
| Lo-F | 24.1 ± 4.0 | 85.5 ± 26.9† | 35.3 ± 9.4 | 19.3 ± 4.6 | 3.6 ± 0.7*† | 2.7 ± 0.6* |
| Hi-F | 84.9 ± 19.3 | 35.6 ± 11.1† | 35.1 ± 8.3 | 17.9 ± 5.2* | 1.5 ± 0.4*† | 1.6 ± 0.3* |
| Norm Lo-F, % | 18.6 ± 3.4 | 58.2 ± 7.0† | 40.0 ± 7.2 | 37.0 ± 6.0* | 41.4 ± 5.3* | 34.3 ± 5.2 |
| Norm Hi-F | 56.1 ± 5.6 | 25.9 ± 4.8† | 42.9 ± 7.1 | 30.8 ± 5.8* | 18.8 ± 5.9 | 23.3 ± 4.6* |
| CO | ||||||
| Total, l/min2 | 0.298 ± 0.065 | 0.206 ± 0.035 | 0.202 ± 0.041 | 0.064 ± 0.005* | 0.075 ± 0.018* | 0.109 ± 0.013‡ |
| Lo-F | 0.044 ± 0.008 | 0.093 ± 0.024 | 0.076 ± 0.024 | 0.022 ± 0.002* | 0.029 ± 0.006* | 0.031 ± 0.007 |
| Hi-F | 0.167 ± 0.052 | 0.042 ± 0.007† | 0.056 ± 0.014 | 0.008 ± 0.001* | 0.015 ± 0.004* | 0.025 ± 0.004*‡ |
| Norm Lo-F, % | 17.1 ± 3.5 | 44.1 ± 8.6† | 31.6 ± 6.6 | 33.9 ± 2.5* | 40.5 ± 4.6 | 26.9 ± 2.9‡ |
| Norm Hi-F | 50.8 ± 7.2 | 22.5 ± 3.7† | 28.9 ± 3.3 | 13.1 ± 2.1* | 18.9 ± 2.1† | 22.1 ± 2.3 |
| SV | ||||||
| Total, ml2 | 4.72 ± 0.88 | 3.67 ± 0.56 | 3.31 ± 0.50 | 2.15 ± 0.28* | 1.53 ± 0.27* | 2.27 ± 0.21‡ |
| Lo-F | 0.56 ± 0.10 | 1.35 ± 0.29† | 0.88 ± 0.20 | 0.36 ± 0.03* | 0.56 ± 0.12* | 0.69 ± 0.11‡ |
| Hi-F | 3.07 ± 0.83 | 1.19 ± 0.27† | 1.24 ± 0.23 | 0.69 ± 0.17* | 0.37 ± 0.09*† | 0.60 ± 0.09*‡ |
| Norm Lo-F, % | 13.4 ± 2.2 | 35.8 ± 5.8† | 25.1 ± 4.4 | 18.2 ± 2.4 | 36.9 ± 5.4† | 30.0 ± 2.4 |
| Norm Hi-F | 58.7 ± 6.5 | 31.5 ± 4.0† | 36.8 ± 2.8 | 31.3 ± 4.5* | 22.5 ± 2.8*† | 25.4 ± 1.9* |
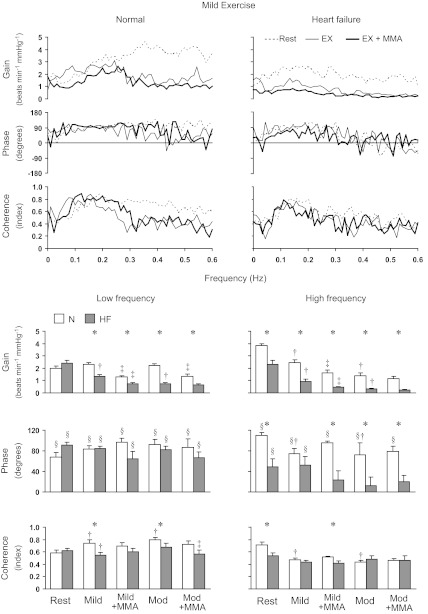
Figure 1, top, shows the group mean transfer function gain, phase, and coherence for the LVSP-HR relationship at rest, during mild exercise with and without metaboreflex activation, before and after induction of heart failure across all frequencies. Figure 1, bottom, shows the average values in the Lo-F and Hi-F ranges at each setting at both workloads. Exercise did not affect transfer function gain and phase in the Lo-F range, whereas coherence in the Lo-F range increased during exercise. In contrast, gain, phase, and coherence in the Hi-F range decreased with mild and moderate exercise. Metaboreflex activation during mild exercise decreased the gain in both frequency ranges, whereas, at moderate exercise, the metaboreflex decreased the gain only in the Lo-F range. Heart failure did not affect the gain, phase, and coherence at rest in the Lo-F range, whereas those in Hi-F range were significantly decreased. For mild and moderate exercise, the gain in both frequency ranges, the phase in Hi-F range during moderate exercise and the coherence in Lo-F range during both workloads were significantly lower compared with normal, and gain further decreased with metaboreflex activation Metaboreflex activation at moderate exercise significantly decreased the coherence in Lo-F range. Also during metaboreflex activation at both workloads, the gain in both frequency ranges and the phase in Hi-F range were significantly lower after induction of heart failure. The coherence in Hi-F range during metaboreflex activation at mild exercise was significantly lower in heart failure.
Fig. 1.
Top: group mean for the transfer function gain, phase, and coherence for the left ventricular systolic pressure-heart rate (LVSP-HR) relationship at rest, during mild exercise with and without muscle metaboreflex activation in normal and heart failure conditions. Bottom: average values in the Lo-F and Hi-F ranges at each setting at both workloads. EX, mild exercise; EX+MMA, muscle metaboreflex activation during mild exercise; N, normal; HF, heart failure; Mild, mild exercise; Mod, moderate exercise; Mild+MMA, muscle metaboreflex activation during mild exercise; Mod+MMA, muscle metaboreflex activation during moderate exercise. *Significant difference between N and HF, P < 0.05; †P < 0.05, free flow exercise vs. Rest; ‡P < 0.05, Exercise + MMA vs. free flow exercise; §P < 0.05, significant difference from zero for phase value.
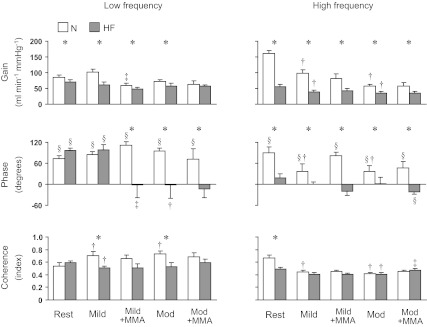
Figure 2 shows the average transfer function gain, phase, and coherence for the LVSP-CO relationship in the Lo-F and Hi-F ranges at each setting. The effect of exercise, muscle metaboreflex activation, and heart failure on the gain of the LVSP-CO relationship was similar to that seen for the LVSP-HR relationship. In contrast, the effect of heart failure on phase was much more pronounced. In heart failure, metaboreflex activation at mild exercise, as well as moderate exercise alone or with metaboreflex activation, decreased or even reversed the phase in both frequency ranges.
Fig. 2.
Group mean for the transfer function gain, phase, and coherence for the LVSP-cardiac output (CO) relationship in the low-frequency (left) and high-frequency (right) ranges at rest and during mild and moderate exercise with and without muscle metaboreflex activation in N and HF condition. Abbreviations are same as in Fig. 1. *Significant difference between N and HF, P < 0.05; †P < 0.05, free flow exercise vs. Rest; ‡P < 0.05, Exercise + MMA vs. free flow exercise; §P < 0.05, significant difference from zero for phase value.
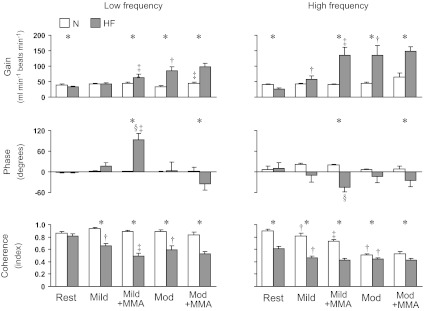
Figures 3 and 4 show the transfer function gain, phase, and coherence for the HR-CO relationship and the SV-CO relationship in the Lo-F and Hi-F ranges. In the normal animal at rest, the coherence between HR and CO was high in both frequency ranges (∼0.9), whereas, the coherence between SV and CO was relatively lower (∼0.4 in Lo-F and ∼0.7 in Hi-F). The phase for HR-CO relationship was close to 0° in both frequency ranges, whereas the phase for the SV-CO relationship was mostly negative, with the exception of low frequency at rest. The coherence for the HR-CO relationship in the Hi-F range decreased with exercise and metaboreflex activation. Metaboreflex activation during moderate exercise increased the gain for HR-CO relationship in Lo-F range, and mild exercise increased the gain for the SV-CO relationship in the Lo-F range. Moderate exercise increased the coherence in Lo-F range and the phase in Hi-F range, while it decreased the phase in Lo-F range for the SV-CO relationship. Metaboreflex activation during moderate exercise decreased the phase for SV-CO relationship in Hi-F range.
Fig. 3.
Group mean for the transfer function gain, phase, and coherence for the HR-CO relationship in the low-frequency (left) and high-frequency (right) ranges at rest and during mild and moderate exercise with and without muscle metaboreflex activation in N and HF condition. Abbreviations are same as in Fig. 1. *Significant difference between N and HF, P < 0.05; †P < 0.05, free flow exercise vs. Rest; ‡P < 0.05, Exercise + MMA vs. free flow exercise; §P < 0.05, significant difference from zero for phase value.
Fig. 4.
Group mean for the transfer function gain, phase, and coherence for the SV-CO relationship in the low-frequency (left) high-frequency (right) ranges at rest and during mild and moderate exercise with and without muscle metaboreflex activation in N and HF condition. Abbreviations are the same as in Fig. 1. *Significant difference between N and HF, P < 0.05; †P < 0.05, free flow exercise vs. Rest; ‡P < 0.05, Exercise + MMA vs. free flow exercise; §P < 0.05, significant difference from zero for phase value.
Heart failure significantly lowered the coherence between HR and CO in the Hi-F range, whereas the coherence between SV and CO in Lo-F range significantly increased. The gain for HR-CO relationship in both frequency ranges and the gain for SV-CO relationship in Hi-F range were significantly lower after induction of heart failure. Heart failure shifted the phase for SV-CO relationship in Hi-F range toward 0°. In heart failure, the coherence for HR-CO relationship in the Lo-F and Hi-F ranges decreased with exercise at both workloads and decreased further with metaboreflex activation during mild exercise. In contrast, exercise and metaboreflex activation tended to increase coherence for the SV-CO relationship in both frequency ranges. Exercise and metaboreflex activation generally increased gain for the HR-CO, as well as SV-CO relationships in both frequency ranges, although there were specific exceptions (see Figs. 3 and 4). The phase for the HR-CO relationship in Lo-F range increased with metaboreflex activation at mild exercise. The phase for the SV-CO relationship in the Lo-F range decreased modestly with mild exercise. In general, exercise and metaboreflex activation tended to decrease coherence of the HR-CO relationship. In contrast, metaboreflex activation at both workloads increased the coherence between SV and CO in both frequency ranges to substantially high values (∼0.9 at Lo-F and ∼0.95 at Hi-F ranges). The coherence between HR and CO was lower, whereas coherence between SV and CO was higher in both frequency ranges during dynamic exercise at both workloads with and without metaboreflex activation after induction of heart failure. The gain for the HR-CO relationship in both frequency ranges was significantly higher at moderate exercise and with metaboreflex activation at both workloads. The gain for SV-CO relationship in the Lo-F range at both workloads with and without metaboreflex activation was significantly lower after induction of heart failure. In addition, the gain for the SV-CO relationship in the Hi-F range at mild exercise was significantly lower. The phase for HR-CO in the Lo-F range was significantly higher, and the phase in Hi-F range was significantly lower at metaboreflex activation in mild exercise. The phase for HR-CO in both frequency ranges was significantly lower at metaboreflex activation in moderate exercise. The phase for SV-CO relationship in Hi-F range at mild exercise was significantly higher, and the phase in both frequency ranges during metaboreflex activation at both workloads was significantly higher after induction of congestive heart failure.
DISCUSSION
CO results from a complex interplay between HR and SV. This can vary under different physiological conditions, such as during exercise, as well as under pathophysiological conditions, such as in subjects with congestive heart failure in which autonomic balance to the heart changes and ventricular function is altered. To our knowledge, this is the first study to investigate dynamic CO regulation over a wide frequency range in the normal animal at rest and how this dynamic control over CO is modified by exercise workload, as well as muscle metaboreflex activation, a potentially key reflex involved in the control of CO during steady-state exercise. Furthermore, in a longitudinally designed study, we determined to what extent modest congestive heart failure affected the dynamic regulation of CO at rest, during exercise, and with metaboreflex activation. Our major findings were that dynamic baroreflex control of HR plays a major role in CO regulation and buffers blood pressure variations across a wide frequency range in normal animals at rest. In addition, the effects of dynamic exercise and muscle metaboreflex activation on dynamic control over CO were different depending on the frequency range. Furthermore, after induction of congestive heart failure, dynamic baroreflex control of HR and CO was substantially diminished, and variations in CO depend more on fluctuations in SV over a wide frequency range.
Dynamic cardiac output regulation at rest, during exercise, and metaboreflex activation in normal subjects.
Several previous studies have examined the frequency domain characteristics of baroreflex control of HR (12, 25, 28, 38). However, whether dynamic baroreflex HR regulation functionally buffers blood pressure variations has often been uncertain. To what extent the HR responses cause changes in CO can be variable, as a result of reciprocal changes in SV (17, 48), which can likely be attributed to the changes in ventricular filling time. Furthermore, even if SV is initially maintained, an increase in CO will decrease central venous pressure, thereby, decreasing right ventricular filling pressure, which would ultimately limit the ability to sustain left ventricular SV and, therefore, sustain the rise in CO (6, 30, 40). Because of the capacitance of the pulmonary circulation, the fall in the ventricular filling does take time to occur, and therefore, transient changes in HR can still cause transient changes in CO (42). This may be particularly important in compensating for rapid changes in arterial pressure, inasmuch as the parasympathetically mediated rapid HR responses and subsequent transient CO changes can occur quickly, thereby protecting pressure while the slower but sustained sympathetically mediated vascular responses develop (22, 32). In normal animals at rest, positive-phase values for both LVSP-HR and LVSP-CO relationships in both frequency ranges were consistent with baroreflex regulation of HR and CO in that a change in LVSP is followed by an opposite directional change in HR and CO. Furthermore, we observed fairly high coherence between HR and CO with relatively low coherence between SV and CO in the entire frequency range that we examined. In addition, the phase values for HR-CO and SV-CO relationships in both frequency ranges revealed that changes in HR and CO occurs simultaneously with almost no delay, whereas there are important time lags between changes in SV and changes in CO. These results suggest that at rest, dynamic baroreflex control of HR plays a major role in CO regulation and buffers blood pressure variations over a wide frequency range.
We found that the gain and phase for the LVSP-HR and LVSP-CO relationships in the Hi-F range decreased during dynamic exercise. In addition, we found that dynamic exercise reduces coherence between HR and CO and deviates phase for the HR-CO relationship from 0° in the Hi-F range, suggesting that the dominance of HR modulation on CO control diminished in this frequency range. In contrast, dynamic exercise had less impact on the HR and CO regulation in Lo-F range. The estimates of the transfer function between arterial blood pressure and HR in the Hi-F range are thought to be predominantly determined by cardiac parasympathetic activity, whereas in the Lo-F range, they might be influenced by both cardiac sympathetic and parasympathetic activity (12, 15, 25, 38). Therefore, HR and CO regulation in the Hi-F range substantially fell with vagal withdrawal during dynamic exercise, whereas those in Lo-F range showed fewer changes. However, our results appear to be inconsistent with those of earlier reports that dynamic baroreflex HR gain in the Lo-F range significantly decreased during dynamic and static exercise in humans (23, 43). These discrepancies might be attributable to differences in the balance of autonomic activity during exercise between humans and dogs (20, 23, 29). Note that as total vascular conductance rises with exercise, a given absolute change in CO has less of an effect on blood pressure. In this context, even if CO gains in Lo-F ranges are preserved during dynamic exercise, the CO contribution to blood pressure regulation could fall in these conditions.
In contrast to the small effect of dynamic exercise on Lo-F HR gain, muscle metaboreflex activation significantly decreased the HR gain in both Lo-F and Hi-F ranges. These results suggest that the mechanism(s) responsible for the decrease in dynamic HR gain by dynamic exercise vs. muscle metaboreflex activation may be different. As discussed above, the most likely cause of the decrease in HR gain in Hi-F range during dynamic exercise is the decreased parasympathetic tone. The muscle metaboreflex-induced tachycardia occurs primarily via increased sympathetic activity, which may decrease HR gain (19). Indeed, it has been previously shown that high plasma norepinephrine concentration attenuates dynamic HR regulation via the vagus nerve (18), and metaboreflex activation can elicit substantial increases in plasma norepinephrine levels, especially in subjects with heart failure (7).
We found that the muscle metaboreflex-induced attenuation of dynamic HR gain is not always associated with decrease in dynamic CO gain as in the Hi-F range at mild exercise and in the Hi-F and Lo-F ranges at moderate exercise. One explanation may be that the muscle metaboreflex-induced increase in SV via augmented ventricular performance (37) coupled with increased central blood volume mobilization (39) would work to maintain dynamic CO gain in the face of a decreased HR gain; e.g., greater SV changes partially compensate for smaller HR changes. Therefore, the dynamic baroreflex cardiac responses could be viewed as a balance between the chronotropic and inotropic responses during metaboreflex activation.
Dynamic cardiac output regulation at rest, during exercise, and metaboreflex activation in heart failure.
Hallmark features of congestive heart failure are increased sympathetic activity, depressed vagal tone, and an attenuated cardiac baroreflex strength (14, 47, 52). However, no previous studies have examined the effect of heart failure on dynamic baroreflex control of HR and CO. With the markedly reduced ventricular contractility, depressed preload sensitivity, and increased afterload sensitivity seen in heart failure, we hypothesized that dynamic CO control would be markedly attenuated. We found that at rest, the gain and phase for the LVSP-HR and LVSP-CO relationships in the Hi-F range were substantially decreased in heart failure. The phase for the LVSP-CO relationship in the Hi-F range is close to 0° in heart failure, indicating that blood pressure and CO change in similar directions with little delay. This phase relationship is inconsistent with a baroreflex relationship and suggests that the cardiac baroreflex does not functionally buffer Hi-F blood pressure fluctuations in heart failure. In contrast to the decreased HR gain in the Hi-F range, HR gain in the Lo-F range was sustained at normal levels. This difference between the effects of heart failure on Hi-F vs. Lo-F HR gain may reflect differential effects of modest heart failure on the strength of baroreflex control over sympathetic vs. parasympathetic activity. Using a similar model, Olivier and Stephenson (24) showed marked attenuation of carotid baroreflex control of heart rate but unchanged baroreflex control of total peripheral resistance. However, despite sustained HR gain in Lo-F range, CO gain in this range was significantly diminished. A substantial decrease in SV via attenuated ventricular performance (16, 36) could account for the decreased dynamic CO gain in the face of unchanged HR Lo-F gain in heart failure. In addition, a decrease in SV fluctuation could also contribute to the decrease in CO gain. Although the CO gain in the Lo-F range decreased, the phase for the LVSP-CO relationship in this frequency range remained at normal levels. Furthermore, the phase for HR-CO in the Lo-F range was almost 0° as in the normal condition, indicating the tight coupling between HR and CO changes. These suggest that cardiac baroreflex still works to buffer low-frequency blood pressure fluctuations. In addition, a given absolute change in CO has a greater effect on blood pressure in heart failure since total vascular conductance decreased ∼10% in this setting. Hence, cardiac baroreflex function in regulating blood pressure could be preserved, despite a reduced CO gain. However, the decrease in CO gain was substantially greater than the decrease in total vascular conductance. Therefore, CO contribution to blood pressure regulation would be depressed even with the lower total vascular conductance in heart failure. Our results suggest that the depressed dynamic baroreflex control of CO in heart failure is induced by both a decreased baroreflex chronotropic response (i.e., diminished HR gain) and an impaired inotropic state of the heart (i.e., reduced SV) with the relative roles of these mechanisms being frequency dependent.
The effects of exercise and metaboreflex activation on dynamic HR and CO regulation may be a consequence of the high sympathetic activity (7, 8). Indeed, our laboratory previously showed that the arterial norepinephrine levels during mild and moderate exercise in heart failure were comparable to or higher than those during metaboreflex activation at the same workload in normal animals (7). Taking into account these observations and our findings that dynamic HR gain in the Lo-F range decreased only with metaboreflex activation in normal conditions, whereas it was reduced by free-flow dynamic exercise in heart failure, an increase in sympathetic nerve activity above a certain level might be a trigger for the attenuation of HR gain in the Lo-F range.
The phase for the LVSP-HR relationship in Lo-F range was maintained at normal levels during dynamic exercise with and without metaboreflex activation, indicating that the baroreflex HR response to blood pressure fluctuations still occurs in these frequency ranges. However, the phase for the LVSP-CO relationship in both frequency ranges are close to or below 0° during mild exercise with metaboreflex activation, as well as moderate exercise with and without metaboreflex activation, indicating that a change in blood pressure was followed by a directionally similar change in CO. In addition, the phase for the SV-CO relationship in both frequency ranges is almost 0° during exercise at both workloads with and without metaboreflex activation, demonstrating that changes in SV and CO are closely coupled with little time lag. Therefore, it is possible that the cardiac baroreflex is unable to buffer blood pressure variations within the entire frequency range, and this may reflect the increased afterload sensitivity of heart in heart failure. Moreover, CO fluctuations might accentuate spontaneous variability in blood pressure in these settings.
We observed lower coherence for the HR-CO relationship in Hi-F ranges, whereas we observed higher coherence for the SV-CO relationship in the Lo-F range at rest in heart failure. Dynamic exercise and metaboreflex activation further diminished the coherence for the HR-CO relationship and increased coherence for the SV-CO relationship. Alterations in HR and SV variations could account for these changes, since CO fluctuations are determined by both HR and SV. In heart failure, CO variability declined with decreases in both HR and/or SV fluctuations in each setting. However, although variability of both HR and SV decreased, the extent of decrease in HR variability from normal was greater than that of SV variability. For example, during moderate exercise, HR and SV variability in Lo-F range decreased to 6.0 ± 2.0% and 48.0 ± 9.1% of normal, respectively. This means that SV variations relative to HR variability are greater in heart failure. This alteration of the balance between HR and SV variations would be a cause of the high coherence between SV and CO and the low coherence between HR and CO. A higher afterload sensitivity of the left ventricle in heart failure subjects (36) might be involved with the relatively greater SV fluctuations. These results suggest that in heart failure, the influence of HR modulation wanes and fluctuations of SV dominate in CO variations.
Limitations of the study.
While cross-spectral analysis provides insight into the linear interrelationship between two variables, it does not evaluate causality. Consequently, any interpretation of relationships must be made with caution. For example, respiration could simultaneously drive SV and HR and thus CO and BP in a manner that makes them appear coupled by the baroreflex. Transfer function estimates are limited by a fundamental assumption of linearity between changes in two variables and are reliable only if squared coherence values are near or above 0.5 (12, 28, 38). In the present study, the transfer gain and phase only at frequency data points of coherence ≥0.5 were accepted to confirm the validity of using this technique to evaluate those relationships. In addition, transfer function and coherence function analyses with a time frame similar to our study (i.e., several minutes) are well established and widely accepted in previous studies (15, 23, 46, 51).
Our approach that was employed to evaluate arterial baroreflex control of HR and CO (based on spontaneous fluctuations in blood pressure, HR, and CO) has several advantages and disadvantages, as discussed previously (34, 35). Briefly, this approach only examines the baroreflex gain over a relatively modest range of pressure, which, therefore, does not allow the calculation of the entire, sigmoidal baroreflex stimulus-response relationship. Therefore, we cannot exclude the possibility that the reduction in baroreflex HR gain seen with exercise and with muscle metaboreflex activation may be due to a shift of the operating point to a lower gain portion of the entire stimulus response relationship (5, 27). On the other hand, our approach does enable us to estimate the dynamic cardiac baroreflex responses during the spontaneous blood pressure fluctuations that characterize both the resting and exercise conditions, without the necessity of any pharmacological or mechanical interventions. This aspect is particularly relevant in heart failure, in which, for example, sympathostimulatory reflexes by the stretch of cardiac chambers after the phenylephrine-induced increase of afterload or a direct β-adrenergic stimulation at the sinus node level by high doses of the drug superimposed to the already heightened blood pressure of exercise may affect baroreflex responses.
Conclusions.
We conclude that at rest in normal animals, dynamic baroreflex control of HR plays a major role in CO regulation and buffers blood pressure variations within a wide frequency range. Dynamic exercise attenuates dynamic baroreflex control of HR and CO in the Hi-F range. Muscle metaboreflex activation further reduces dynamic baroreflex control of HR. However, muscle metaboreflex-induced attenuation of dynamic HR control is not always associated with attenuation of dynamic CO regulation. After induction of congestive heart failure, dynamic baroreflex control of HR and CO was substantially diminished at rest and during dynamic exercise with and without muscle metaboreflex activation. Furthermore, we found that, in normal conditions, HR modulation plays a major role in CO regulation, whereas in heart failure, influence of HR modulation wanes and fluctuations of SV dominate in CO variations.
GRANTS
Masashi Ichinose and Tomoko Ichinose were recipients of research fellowships of the Japan Society for the Promotion of Science for Young Scientists. This research was supported by National Heart, Lung, and Blood Institute Grant HL-55473.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.I., J.A.S.-M., M.C., and D.S.O. conception and design of research; M.I., J.A.S.-M., M.C., Z.L., E.J.D., and D.F. performed experiments; M.I., J.A.S.-M., T.K.I., and D.S.O. analyzed data; M.I., T.K.I., and D.S.O. interpreted results of experiments; M.I., T.K.I., and D.S.O. prepared figures; M.I., J.A.S.-M., and D.S.O. drafted manuscript; M.I. and D.S.O. edited and revised manuscript; M.I., J.A.S.-M., M.C., Z.L., T.K.I., E.J.D., D.F., and D.S.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Erin Krengel, Sue Harris, Jaime Rodriguez, and Susan Dibbley for expert technical assistance and care of the animals and also Dr. Jong-Kyung Kim for assistance with the surgeries.
Present address of ZhenHua Li: Department of Cardiology, Jinan Central Hospital, Jinan, China 250013.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ansorge EJ, Augustyniak RA, Perinot RL, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Burger HR, Chandler MP, Rodenbaugh DW, Dicarlo SE. Dynamic exercise shifts the operating point and reduces the gain of the arterial baroreflex in rats. Am J Physiol 44: R2043–R2048, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Cogliati C, Magatelli R, Montano N, Narkiewicz K, Somers VK. Detection of low- and high-frequency rhythms in the variability of skin sympathetic nerve activity. Am J Physiol Heart Circ Physiol 278: H1256–H1260, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Eiken O, Convertino VA, Doerr DF, Dudley GA, Morariu G, Mekjavic IB. Characteristics of the carotid baroreflex in man during normal and flow-restricted exercise. Acta Physiol Scand 144: 325–331, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Guyton AC, Douglas BH, Langston JB, Richardson TQ. Instantaneous increase in mean circulatory pressure and cardiac output at onset of muscular activity. Circ Res XI: 431–441, 1962 [DOI] [PubMed] [Google Scholar]
- 7. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Ichinose M, Delliaux S, Watanabe K, Fujii N, Nishiyasu T. Evaluation of muscle metaboreflex function through graded reduction in forearm blood flow during rhythmic handgrip exercise in humans. Am J Physiol Heart Circ Physiol 301: H609–H616, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Ichinose M, Sala-Mercado JA, O'Leary DS, Hammond RL, Coutsos M, Ichinose T, Pallante M, Iellamo F. Spontaneous baroreflex control of cardiac output during dynamic exercise, muscle metaboreflex activation, and heart failure. Am J Physiol Heart Circ Physiol 294: H1310–H1316, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O'Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichinose M, Koga S, Fujii N, Kondo N, Nishiyasu T. Modulation of the spontaneous beat-to-beat fluctuations in peripheral vascular resistance during activation of muscle metaboreflex. Am J Physiol Heart Circ Physiol 293: H416–H424, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Iellamo F. Neural mechanisms of cardiovascular regulation during exercise. Auton Neurosci Basic Clinical 90: 66–75, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Iellamo F, Sala-Mercado JA, Ichinose M, Hammond RL, Pallante M, Ichinose TK, Stephenson LW, O'Leary DS. Spontaneous baroreflex control of heart rate during exercise and muscle metaboreflex activation in heart failure. Am J Physiol Heart Circ Physiol 293: H1929–H1936, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Iwasaki KI, Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. Am J Physiol Regul Integr Comp Physiol 279: R2189–R2199, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Kass DA. Myocardial mechanics. In: Heart Failure: Scientific Principles and Clinical Practice, New York: Churchill Livingstone, 1997, p. 87–108 [Google Scholar]
- 17. Kumada M, Azuma T, Matsuda K. The cardiac output-heart rate relationship under different conditions. Jpn J Physiol 17: 538–555, 1967 [DOI] [PubMed] [Google Scholar]
- 18. Miyamoto T, Kawada T, Takaki H, Inagaki M, Yanagiya Y, Jin Y, Sugimachi M, Sunagawa K. High plasma norepinephrine attenuates the dynamic heart rate response to vagal stimulation. Am J Physiol Heart Circ Physiol 284: H2412–H2418, 2003 [DOI] [PubMed] [Google Scholar]
- 19. O'Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol 74: 1748–1754, 1993 [DOI] [PubMed] [Google Scholar]
- 20. O'Leary DS, Rossi NF, Churchill PC. Substantial cardiac parasympathetic activity exists during heavy dynamic exercise in dogs. Am J Physiol Heart Circ Physiol 273: H2135–H2140, 1997 [DOI] [PubMed] [Google Scholar]
- 21. O'Leary DS, Woodbury DJ. Role of cardiac output in mediating arterial blood pressure oscillations. Am J Physiol Regul Integr Comp Physiol 271: R641–R646, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol 566: 599–611, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olivier NB, Stephenson RB. Characterization of baroreflex impairment in conscious dogs with pacing-induced heart failure. Am J Physiol Regul Integr Comp Physiol 265: R1132–R1140, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Pagani M, Somers V, Furlan R, Dell'Orto S, Conway J, Baselli G, Cerutti S, Sleight P, Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension 12: 600–610, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Patel HJ, Pilla JJ, Polidori DJ, Pusca SV, Plappert TA, Sutton MS, Lankford EB, Acker MA. Ten weeks of rapid ventricular pacing creates a long-term model of left ventricular dysfunction. J Thorac Cardiovasc Surg 119: 834–841, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol 91: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 10: 538–543, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res 19: 400–411, 1966 [DOI] [PubMed] [Google Scholar]
- 30. Rothe CF, Gaddis ML. Autoregulation of cardiac output by passive elastic characteristics of the vascular capacitance system. Circulation 81: 360–368, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Rowell LB, O'Leary DS, Kellogg DL., Jr Integration of cardiovascular control systems in dynamic exercise. New York: Oxford Press, 1996, p. 770–838 [Google Scholar]
- 32. Sagawa K. Baroreflex control of systemic artrial pressure and vascular bed. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Blood Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, Vol. III, pt. 2, chapt. 14, p. 453–496 [Google Scholar]
- 33. Sala-Mercado JA, Ichinose M, Coutsos M, Li Z, Fano D, Ichinose T, Dawe EJ, O'Leary DS. Progressive muscle metaboreflex activation gradually decreases spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol 298: H594–H600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sala-Mercado JA, Ichinose M, Hammond RL, Coutsos M, Ichinose T, Pallante M, Iellamo F, O'Leary DS. Spontaneous baroreflex control of heart rate versus cardiac output: altered coupling in heart failure. Am J Physiol Heart Circ Physiol 294: H1304–H1309, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Sala-Mercado JA, Ichinose M, Hammond RL, Ichinose TK, Pallante M, Stephenson LW, O'Leary DS, Iellamo F. Muscle metaboreflex attenuates spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2867–H2873, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol Heart Circ Physiol 261: H1231–H1245, 1991 [DOI] [PubMed] [Google Scholar]
- 39. Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Sheriff DD, Zhou XP, Scher AM, Rowell LB. Dependence of cardiac filling pressure on cardiac output during rest and dynamic exercise in dogs. Am J Physiol Heart Circ Physiol 265: H316–H322, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Stauss HM, Rarick KR, Deklotz RJ, Sheriff DD. Frequency response characteristics of whole body autoregulation of blood flow in rats. Am J Physiol Heart Circ Physiol 296: H1607–H1616, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Steingrub JS, Tidswell M, Higgins TL. Hemodynamic consequences of heart-lung interactions. J Intensive Care Med 18: 92–99, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Stewart JM, Montgomery LD, Glover JL, Medow MS. Changes in regional blood volume and blood flow during static handgrip. Am J Physiol Heart Circ Physiol 292: H215–H223, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation 93: 1527–1532, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Waki H, Kasparov S, Katahira K, Shimizu T, Murphy D, Paton JF. Dynamic exercise attenuates spontaneous baroreceptor reflex sensitivity in conscious rats. Exp Physiol 88: 517–526, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Watanabe K, Ichinose M, Fujii N, Matsumoto M, Nishiyasu T. Individual differences in the heart rate response to activation of the muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol 299: H1708–H1714, 2010 [DOI] [PubMed] [Google Scholar]
- 47. White CW. Abnormalities in baroreflex control of heart rate in canine heart failure. Am J Physiol Heart Circ Physiol 240: H793–H799, 1981 [DOI] [PubMed] [Google Scholar]
- 48. White S, Patrick T, Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of altering ventricular rate on blood flow distribution in conscious dogs. Am J Physiol 221: 1402–1407, 1971 [DOI] [PubMed] [Google Scholar]
- 49. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]
- 50. Wyss CR, Bennett TD, Scher AM. Beat-by-beat control of cardiac output in awake dogs with atrioventricular block. Am J Physiol Heart Circ Physiol 242: H1118–H1121, 1982 [DOI] [PubMed] [Google Scholar]
- 51. Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Zucker IH, Wang W. Modulation of baroreflex and baroreceptor function in experimental heart failure. Basic Res Cardiol 86 Suppl 3: 133–148, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis 37: 397–414, 1995 [DOI] [PubMed] [Google Scholar]