Abstract
Ischemia-reperfusion injury (IRI) remains a significant source of early and delayed renal transplant failure. Therapeutic interventions have yet to resolve this ongoing clinical challenge although the reasons for this remain unclear. The cell surface receptor CD47 is widely expressed on vascular cells and in tissues. It has one known soluble ligand, the stress-released matricellular protein thrombospondin-1 (TSP1). The TSP1-CD47 ligand receptor axis controls a number of important cellular processes, inhibiting survival factors such as nitric oxide, cGMP, cAMP, and VEGF, while activating injurious pathways such as production of reactive oxygen species. A role of CD47 in renal IRI was recently revealed by the finding that the TSP1-CD47 axis is induced in renal tubular epithelial cells (RTEC) under hypoxia and following IRI. The absence of CD47 in knockout mice increases survival, mitigates RTEC damage, and prevents subsequent kidney failure. Conversely, therapeutic blockade of TSP1-CD47 signaling provides these same advantages to wild-type animals. Together, these findings suggest an important role for CD47 in renal IRI as a proximate promoter of injury and as a novel therapeutic target.
Keywords: thrombospondin-1, CD47, kidney injury, nitric oxide, reactive oxygen species, ischemia-reperfusion injury, transplant
ischemia-reperfusion injury (IRI) is a leading cause of acute renal injury and a major cause of both acute graft dysfunction and delayed renal transplant failure (8, 29). The role of extracellular matrix and matricellular proteins in this process remains largely unknown. The secreted matricellular protein thrombospondin-1 (TSP1) has been associated with acute renal injury and implicated in several preclinical models of chronic renal disease. TSP1 is the only known soluble high-affinity ligand for the ubiquitous cell receptor CD47 (40), although signal regulatory protein-α (SIRP-α) functions on phagocytes as a counterreceptor to CD47 (94). New findings identify CD47 as a regulator of multiple cell survival and cell death pathways (44, 46, 85), suggesting an important role for the TSP1-CD47 axis in renal injury.
TSP1
TSP1 is the first and most widely studied member of a five-member family of secreted proteins (14). It exists in vivo as a trimer of 150-kDa subunits, which with glycosylation has a mass of ∼480 kDa. This large size limits TSP1 diffusion across certain tissue barriers such as the endothelial basement membrane. TSP1 was first identified in platelet α-granules (23, 55), where it can account for >50% of the total protein. Following in utero development (16), TSP1 expression in adult animals is minimal except for the preformed pool stored in platelets and circulating at ∼100 pM levels in plasma. However, TSP1 can be induced in tissues in response to injury (2) and chronic disease (32). Recently, we reported increased plasma TSP1 in steady-state sickle cell disease (SCD) patients that correlated significantly with vasculopathic complications including acute chest syndrome (76). These observations are made prescient in light of the known increased rate of chronic kidney disease and kidney failure in SCD patients (1). As a secreted protein, TSP1 has no direct intracellular targets but alters cellular responses by interacting with cell surface receptors including several integrins (13), CD36, CD47, heparin sulfate proteoglycans (15), and LDL receptor-related protein-1/calreticulin (79). Central physiological activities of TSP1 include inhibition of angiogenesis through interaction with the vascular cell receptors CD36 (20) and CD47 (47), binding to and regulation of extracellular matrix formation and structure, activation of latent TGF-β1 (73), modulation of inflammatory cell adhesion and migration (74), and suppression of tumor growth (84).
CD47
Integrin-associated protein, or CD47, was first identified as a missing antigen in Rh-negative red blood cells (72) and as an overexpressed protein in ovarian carcinoma (11). This membrane-spanning protein also coassociates with SIRP-α and is a receptor for TSP1 (4). TSP1, binding in a high-affinity manner through its C-terminal domain (40), activates CD47 to alter numerous cellular processes. CD47 regulates inflammation, cell adhesion, and self-recognition (9). More recently, CD47 has been found to limit cell and animal survival in response to various stressors, in part through regulation of cardiovascular signaling mechanisms (5, 47, 49).
Distribution of TSP1 and CD47 in the Kidney
TSP1 is expressed in the kidney during development (39), whereas in healthy adults, TSP1 is virtually undetectable. In cell culture or with injury, TSP1 is secreted by kidney mesangial cells, a process that can be suppressed by nitric oxide (NO) pro-drugs (96). CD47 is expressed in several renal cell types including epithelial, endothelial, and mesangial cells (31) but not in podocytes (60). It is also expressed in proximal tubular HK-2 cells (7).
TSP1 and CD47 in Renal Disease
TSP1 plays a role in several preclinical models of renal injury and disease. In renal fibrosis, TSP1 expression is increased and localized to tubular epithelial cells and peritubular interstitium (92). In a rat model of deoxycorticosterone acetate/salt-induced tubulointerstitial fibrosis, TSP1 was upregulated in cortical tubular cells (52), and in mice treated with mercuric chloride subsequent tubular injury and tubulointerstitial fibrosis were associated with increased TSP1 localized in tubular epithelial cells and the peritubular interstitium (92). TSP1, in part through binding and activation of the latent form of transforming growth factor-β (TGF-β1), induces progressive renal fibrosis (19, 35). In addition to inducing collagen and other matrix proteins associated with fibrosis, TGF-β1 is a potent inducer of TSP1 gene expression in mesangial cells (56), creating a positive feedback loop. Chronic glomerulonephritis patients with marked nephrosclerosis exhibit higher TSP1 in the renal interstitium (91). A peptide that inhibits TSP1-mediated activation of latent TGF-β1 decreases renal fibrosis in rats (97). Other peptides derived from the type 1 repeats of TSP1 also inhibit renal cell proliferation in an anti-Thy1-induced glomerulonephritis rat model independent of latent TGF-β1 activation and decreased proteinuria (36).
High glucose concentrations increased TSP1 in proximal (102) and distal (99) tubular epithelial cells. In a diabetic endothelial NO synthase (eNOS) null mouse model, high glucose levels correlated with renal tubulointerstitial injury and elevated TSP1 expression, while insulin corrected the renal injury (57). Conversely, activation of the canonical NO pathway with a phosphodiesterase (PDE) 5 blocker to increase the NO second messenger cGMP inhibited anti-Thy1-induced proliferative glomerulonephritis and lowered TSP1 in rats (33). In a streptozotocin-induced model of diabetic nephropathy, a PDE inhibitor suppressed TSP1 expression and ameliorated kidney injury (95), and exogenous NO decreased TSP1 production in cultured endothelial cells (83). Also, in a mouse model of diabetic nephropathy, the LSKL sequence from the latency-associated peptide, which inhibits TSP1-mediated latent TGF-β1 activation, improved renal function (62). Finally, in kidney biopsies from human subjects with diabetic nephropathy both glomerular and cortical TSP1 expression was associated with disease (32).
TSP1 mRNA was significantly increased in a porcine model of non-heart-beating donor renal transplantation (61). Interestingly, analysis of human renal allografts with chronic nephropathy demonstrated increased TSP1 expression (34). In mice and rats, TSP1 was upregulated in the proximal renal tubules following IRI, whereas TSP1 null mice were protected from renal injury (93). Similarly, we have reported hepatic induction of TSP1 in a murine model of warm subtotal liver IRI injury and protection from tissue injury and enhanced organ reperfusion in TSP1 null animals (45).
Less is known of the role of CD47 in renal pathophysiology. In renal diseases including acute postinfectious glomerulonephritis (GN), membranoproliferative GN, and diabetic nephropathy, CD47 expression decreased in mesangial cells (31). CD47 is also markedly decreased during mesangiolysis, but increased in mesangial cells in the restoration stage (60). In mice treated with ferric nitrilotriacetate, CD47 is overexpressed in renal proximal tubular epithelial cells (RTEC) and in many of the secondary renal tumors (75). Interestingly, CD47 expression is decreased in circulating platelets in infection-mediated hemolytic uremic syndrome, and this process can be inhibited with an antibody to toll-like receptor 4 (30).
Activated CD47 Inhibits Multiple Cell Survival Pathways
Low (picomolar to nanomolar) concentrations of NO play a central role in cardiovascular physiology to promote arterial dilation and as an antithrombotic and anti-inflammatory agent (37). Work in our group has recently defined a novel regulatory role for TSP1, through binding and activating CD47, to redundantly inhibit the NO pathway (41, 43) (Fig. 1). At the level of endogenous NO production by eNOS, TSP1-CD47 signaling limits eNOS activity through suppressing calcium flux (5). Interestingly, null animals lacking endogenous TSP1 display enhanced eNOS activity under baseline conditions, as measured by eNOS phosphorylation (6). The primary intracellular target of NO is the enzyme soluble guanylyl cyclase (sGC) that, on binding NO via its prosthetic heme, increases the production of cGMP (38, 70). Activated CD47 directly inhibits NO-mediated stimulation of sGC (47), again in part by altering cellular calcium signaling (82). Activated CD47 also limits NO-independent sGC stimulation by non-heme-targeting chemical agents (71). In platelets and vascular smooth muscle cells, TSP1 inhibits downstream of sGC at the cGMP target PKG (42) (Fig. 1). Results in vascular cell culture systems translate to in vivo effects, where soluble TSP1, by binding and activating endothelial CD47, inhibits arterial vasodilation and potentiates vasoconstriction (5, 42, 46). Activated CD47 expressed on platelets can further impede blood flow and tissue perfusion by inhibiting NO to stimulate platelet aggregation (51) and thrombosis.
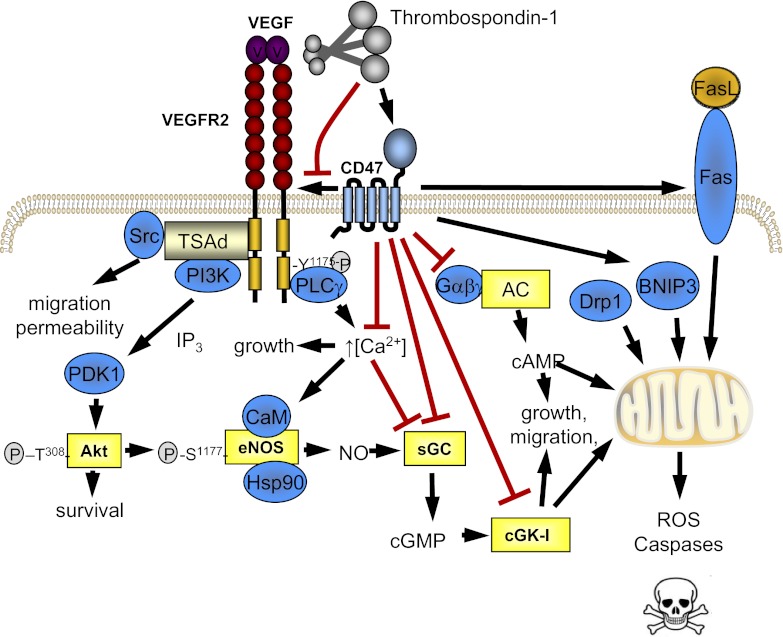
Fig. 1.
Activated CD47 is a proximate regulator of multiple cell survival and cell death pathways. CD47 is a potent and redundant inhibitor of the canonical nitric oxide (NO) pathway, limiting endothelial NO synthase (eNOS) activation (5), soluble guanylyl cyclase (sGC) stimulation (48), and the downstream cGMP target PKG (51). Thrombospondin-1 (TSP1) activation of CD47 decreases VEGF signaling by altering CD47 and VEGF receptor (VEGFR2) coassociation (54), to limit both NO-dependent and -independent effects of VEGF. TSP1, presumably via CD47, inhibits adenylyl cyclase to limit cAMP production (100). CD47 also limits mitochondrial energetics and promotes reactive oxygen species (ROS) and programmed cell death (26).
cAMP is another important cellular messenger that promotes cell survival through regulation of multiple pathways (12). In the cardiovascular system, cAMP is a potent vasodilator of arteries and critical to myocardial function (103). In the kidney, cAMP has been linked to electrolyte transport (27). Recently, we determined that TSP1 can inhibit the production of cAMP by adenylyl cyclase in vascular smooth muscle cells (100) (Fig. 1), most likely in a CD47-mediated fashion (26, 65), consistent with our pervious finding of increased basal myocardial cAMP levels in TSP1 and CD47 null mice (46).
Maintenance of a mature and healthy vascular network and adequate tissue perfusion requires the complex interaction of numerous mechanical and hormonal cues. A major role in this capacity has been ascribed to VEGF (17). Extending earlier reports from our group suggesting that TSP1 could limit VEGF-mediated effects on vascular cells (48), we recently reported that in endothelial cells CD47 constitutively associates with and binds to the VEGF receptor 2 (VEFR2) and that TSP1 binding to endothelial CD47 disrupts this association to inhibit VEGFR2 activation and VEGF signaling (54) (Fig. 1).
Mitochondria are specialized organelles that produce ATP, the primary chemical energy in eukaryotic cells. NO is a recognized salutary effector of mitochondrial health and proliferation (10). Frazier and colleagues (26) reported that TSP1 signaling through CD47 suppresses mitochondrial number and size and limits mitochondrial metabolism (Fig. 1). Mitochondria in cells from null mice that lack CD47 or TSP1 are more numerous, and mitochondria in CD47 null cells make more ATP and less reactive oxygen species (ROS) than mitochondria in wild-type cells. The in vivo implications of this are that CD47 null mice have greater endurance, are leaner, and use less oxygen than wild-type controls (26). Thus the TSP1-CD47 signaling axis inhibits multiple beneficial pathways central to the maintenance of vascular cell health, energetics, blood flow, and tissue perfusion.
Activated CD47 Stimulates Multiple Cell Injury Pathways
Activation of CD47 induces cell death in neuronal cells (88) and breast carcinoma, multiple myeloma, and leukemic cell lines (98). However, the physiological relevance of these findings is unclear as antibodies were typically used to activate CD47, or supraphysiological concentrations of the TSP1-based peptide 4N1K were employed in these experiments as a surrogate for TSP1. Cell death stimulated by CD47 ligation can be caspase dependent or independent (66). Conversely, CD47-deficient T cells are resistant to Fas-mediated apoptosis in vitro and in vivo during resolution of a delayed type hypersensitivity response (64) (Fig. 1). Consistent with these reports, we have found that TSP1 and CD47 null vascular cells and tissues do not undergo cell death in response to high-dose radiation (44). CD47-mediated cell and tissue death under radiation stress appears to occur in part by activation of apoptosis. Conversely, highly proliferative tissues such as bone marrow are protected from radiation-stimulated cell death when CD47 activation is blocked with a morpholino oligonucleotide that suppresses protein production (68). Thus activated CD47 promotes radiation-mediated cell and tissue death.
ROS potentiate many acute and chronic diseases. Under hypoxia, TSP1 and CD47 are upregulated and associated with increased vascular cell production of ROS (6). Conversely, blocking CD47 activation with a monoclonal antibody in hypoxic endothelial cells completely abrogated ROS production, suggesting a direct role for activated CD47 in promoting vascular ROS production.
CD47 in IRI
IRI is a major cause of transplant dysfunction and failure (89) and plays a critical role in morbidity and mortality resulting from myocardial infarction (101) and stroke (21). The process of organ procurement for transplantation induces acute effects that decrease tissue blood flow and perfusion and secondary late effects that activate immune inflammatory pathways (28). In a cerebral IRI model, CD47 null mice were protected with less infarct and secondary brain swelling (53). We have reported that CD47 null mice and wild-type animals treated with antibodies that block CD47 activation by TSP1 are protected from soft tissue (67) and liver IRI (45). Interestingly, CD47 blockade imparts protective effects from IRI, even if therapy is started some time after reperfusion (67).
Activated CD47 in Acute Renal Injury
It was not known whether signaling through CD47 regulates IRI responses in visceral organs other than the liver. Furthermore, the mechanism through which activated CD47 promotes IRI, and specifically renal IRI, remains unknown. To investigate this, we challenged male C57BL/6 wild-type and CD47 null mice with bilateral renal ischemia followed by 24 h of reperfusion. We found upregulation of TSP1 and its principal receptor CD47 in wild-type kidneys following IRI (86). Conversely, in CD47 null kidneys, TSP1 protein and mRNA expression was significantly reduced compared with wild-type. RTEC are a primary target of IRI (22), with the death of these cells a prominent finding in human renal transplants (58). Under hypoxia and reoxygenation (30 min of 1% O2, followed by 24 h of normoxia as a mimic of renal IRI), RTEC show persistent induction of both TSP1 and CD47. Conversely, we (6) and others (77) have shown that in the presence of elevated glucose, hypoxia upregulates TSP1 in vascular endothelial and smooth muscle cells, respectively, and as we recently reported (86), in RTEC this process is resistant to oxygen-mediated downregulation.
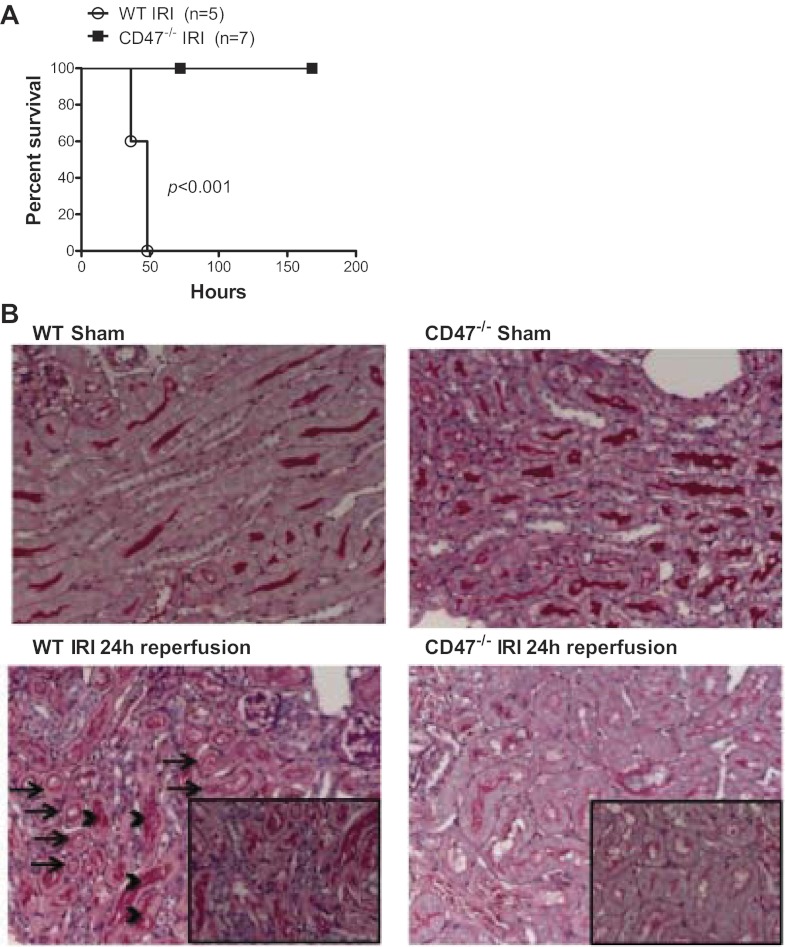
Preclinical animal models of bilateral renal IRI induce a robust stress that results in animal death. Importantly, CD47 null animals challenged with renal IRI survived indefinitely whereas all wild-type animals had expired by 50 h (Fig. 2A). Enhanced survival following IRI in CD47 null mice correlated with less evidence of infarction, tubular necrosis, and cast formation compared with wild-type controls (86) (Fig. 2B). Serum creatinine, a biomarker of renal function, was significantly lower in CD47 null animals compared with wild-type mice following IRI. Caspase 3 activation stimulates both apoptotic and necrotic cell death and is a target for therapeutic mitigation of IRI (24). Wild-type mice demonstrated a significant increase in renal caspase 3 following IRI. In contrast, CD47 null animals showed minimal to no increase in renal caspase 3 (86), suggesting CD47 promotes apoptotic cell death in renal IRI.
Fig. 2.
Absence of CD47 activation provides significant survival advantage and renal cytoprotection following ischemia-reperfusion injury (IRI) in null mice. A: wild-type (WT) C57BL/6 male mice invariably died within 50 h following renal IRI. In contrast, similarly challenged CD47−/− mice all survived as highlighted by Kaplan-Meyer analysis. B: WT mice subjected to 24-h reperfusion following bilateral renal ischemia demonstrated marked corticomedullary damage, characterized by widespread tubular epithelial cell necrosis (arrows) and cast formation (arrowheads). CD47−/− mice had minimal alterations in cytoarchitecture on histological examination. Representative images of kidneys from both mice are shown (periodic acid-Schiff-stained; original magnification ×200, with insets at ×400).
CD47 is ubiquitously expressed, and it was not clear whether CD47 in the parenchyma or in circulating cells promoted renal injury. To determine this, we created bone marrow (BM) transplant chimeras and subjected them to renal IRI. CD47 null mice receiving wild-type BM experienced minimal to no tissue injury and markedly less increase in serum creatinine following IRI (86). In contrast, wild-type mice receiving wild-type BM experienced substantial tissue injury commensurate with significantly larger increases in serum creatinine following renal IRI. CD47 null mice that received CD47 null BM displayed slightly greater protection against renal IRI than null mice that received wild-type BM, although the difference did not reach statistical significance. Thus, in renal IRI, parenchymal CD47, and not leukocyte CD47, predominantly promotes tissue injury.
Oxidative stress arising from elevated ROS production underlies the pathophysiology of numerous cardiovascular maladies (3, 63, 69, 81, 105), including IRI (18), and is a robust stimulant of renal transplant rejection (80). ROS and associated oxidative stress can induce cell death (87), activate redox-signaling pathways in the vessel wall, and induce vascular dysfunction. We analyzed oxidative stress in kidneys from animals following IRI. CD47 null tissues demonstrated significantly less ROS at 24 h following IRI compared with wild-type mice (86). Pathological ROS alters proteins, adversely impacting their function (90). Under conditions of increased ROS, tyrosine residues in proteins undergo nitration (78, 104). We found significant increases in 3-nitrotyrosine expression in wild-type kidneys following IRI. However, there was minimal evidence of this process in kidneys from CD47 null mice (86) indicative of decreased oxidative stress.
Inducible NO synthase (iNOS) is a major source of cellular ROS (59), and wild-type kidneys demonstrate robust induction of iNOS mRNA following IRI (86). In contrast in CD47 null kidneys, iNOS induction did not occur post-IRI. Inflammatory cells, particularly macrophages, are a major source of iNOS expression. CD47 null kidneys display decreased inflammatory cell invasion following IRI, which may in part explain the decrease in iNOS expression noted in null renal tissues. Alternatively, iNOS is reported to be regulated by caveolin-1 (Cav-1) (25), and we recently reported that Cav-1 is a target of CD47 in pulmonary arterial endothelial cells (6), although it is not clear whether a similar mechanism applies in the kidney.
The protective effect observed in CD47 null mice following IRI may in part be secondary to the identified role of CD47 in acute regulation of blood flow (50). Animals were challenged with 22 min of ischemia to the renal pedicle and 30 min and 24 h of reperfusion. Interestingly, renal blood flow following 30 min of reperfusion, as measured by laser Doppler, was comparable between wild-type and CD47 null mice but lagged markedly by 24 h in wild-type kidneys. In contrast, CD47 null renal perfusion approached preinjury levels at 24 h post-IRI.
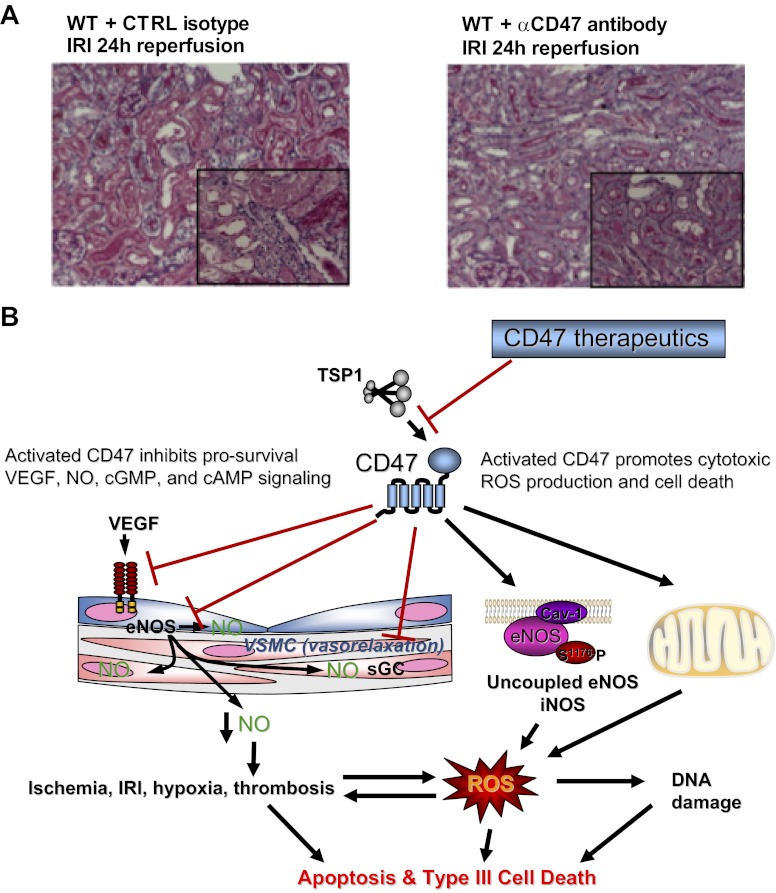
Although results in CD47 null mice and chimeras identify a significant role for parenchymal CD47 in promoting renal IRI, clinical implications were unclear. To examine this, we prevented activation of CD47 by treating wild-type mice with a monoclonal antibody previously shown to provide tissue protection in vivo (50). Consistent with increased overall survival and renal preservation in the absence of CD47, mice treated with this CD47 antibody experienced decreased renal tubular injury (Fig. 3A), lower serum creatinine, and decreased proinflammatory cytokine and chemokine transcript levels (86). At the same time, antibody-treated mice displayed decreased expression of both TSP1 and CD47. This finding is interesting in light of our report that basal and hypoxia-stimulated CD47 expressions are markedly suppressed in TSP1 null endothelial cells (6) and suggests possible cross talk at the gene level between TSP1 and its cognate receptor CD47.
Fig. 3.
Antibody blockade of CD47 activation preserves renal cytoarchitecture following IRI through targeting multiple survival and injury pathways. A: WT C57BL/6 male mice received a single ip injection of a CD47-blocking antibody (clone 301) 90 min before renal IRI. In contrast to untreated animals and animals receiving a matched isotype control antibody, all mice treated with the CD47 monoclonal antibody demonstrated preservation of near normal cytoarchitecture. Representative images of kidneys from animals treated with a CD47-blocking antibody show decreased tubular necrosis, tubular dilatation, and cast formation compared with kidney tissue sections from isotype control antibody-treated and untreated animals (original magnification ×200, insets at ×400). B: in addition to inhibiting multiple survival and growth factor pathways, in renal IRI activated CD47 can stimulate pathological ROS production including superoxide production (O2·−) though upregulation of inducible NOS (iNOS) (86) to promote programmed cell death (64, 68, 86). Therapeutic blockade of the TSP1-CD47 axis with monoclonal antibodies enhances prosurvival pathways while inhibiting cell death pathways.
Overall, these data demonstrate that the TSP1-CD47 axis is upregulated in a preclinical model of renal IRI and that activated parenchymal CD47 promotes tissue injury and renal dysfunction via multiple mechanisms including inhibiting NO signaling to limit reperfusion and blood flow following renal IRI. Activated CD47 also potentiates renal tissue injury through activation of iNOS and increased production of pathological ROS (Fig. 3B). Together, these data point to CD47 as a redundant promoter of renal IRI and unique therapeutic target.
Future Directions
The secreted protein TSP1 has been linked to kidney disease in animal models although the clinical importance of these findings remains to be determined. It is even less clear what role activated CD47, as one of the cognate receptors for TSP1, plays in kidney disease. New studies now identify activated parenchymal CD47 as a proximate promoter of renal IRI through several mechanisms. In translational experiments, an antibody that blocks TSP1-mediated activation of CD47 protects animals from renal IRI. Delivery of a humanized version of this CD47 antibody as a component of standard transplant preservation fluid could provide enhanced protection of donor organs, especially those from expanded criteria donors, from transplant IRI. Such an approach may increase the number of suitable organs for transplantation. Although preliminary studies have tested responses to IRI, it will be important to determine whether blockade of CD47 activation can provide organ protection in other types of injury and what role CD47 targeting therapies may have in chronic renal disease.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grants R01 HL-108954 (to J. S. Isenberg) and 1P01HL103455-01, the American Heart Association (11BGIA7210001 to J. S. Isenberg), the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (to J. S. Isenberg), the Intramural Research Program of the National Cancer Institute (to D. D. Roberts), and by the Australian NHMRC (APP1016276 C. J. Martin Award to N. M. Rogers).
DISCLOSURES
J. S. Isenberg is Chair of the Scientific Advisory Board of Vasculox, Inc. (St. Louis, MO) and Radiation Control Technologies, Inc. (Rockville, MD).
AUTHOR CONTRIBUTIONS
Author contributions: N.M.R. and J.S.I. provided conception and design of research; N.M.R. performed experiments; N.M.R. and J.S.I. analyzed data; N.M.R., D.D.R., and J.S.I. interpreted results of experiments; N.M.R., D.D.R., and J.S.I. prepared figures; N.M.R., M.Y., E.M.N., A.W.T., D.D.R., and J.S.I. edited and revised manuscript; N.M.R. and J.S.I. approved final version of manuscript; J.S.I. drafted manuscript.
REFERENCES
- 1. Abbott KC, Hypolite IO, Agodoa LY. Sickle cell nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 58: 9–15, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161: 831–839, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arora S, Vaishya R, Dabla PK, Singh B. NAD(P)H oxidases in coronary artery disease. Adv Clin Chem 50: 65–86, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 21: 47–52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88: 471–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 93: 682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bijuklic K, Sturn DH, Jennings P, Kountchev J, Pfaller W, Wiedermann CJ, Patsch JR, Joannidis M. Mechanisms of neutrophil transmigration across renal proximal tubular HK-2 cells. Cell Physiol Biochem 17: 233–244, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Bohmova R, Viklicky O. Renal ischemia-reperfusion injury: an inescapable event affecting kidney transplantation outcome. Folia Microbiol (Praha) 46: 267–276, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11: 130–135, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Brown GC. Nitric oxide and mitochondria. Front Biosci 12: 1024–1033, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res 52: 5416–5420, 1992 [PubMed] [Google Scholar]
- 12. Carlucci A, Lignitto L, Feliciello A. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol 18: 604–613, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem 274: 11408–11416, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol 19: 597–614, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Sottile J, Strickland DK, Mosher DF. Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related protein: localization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem J 318: 959–963, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corless CL, Mendoza A, Collins T, Lawler J. Colocalization of thrombospondin and syndecan during murine development. Dev Dyn 193: 346–358, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature 438: 937–945, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev 53: 135–159, 2001 [PubMed] [Google Scholar]
- 19. Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int 65: 459–468, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 138: 707–717, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denner L. Stroke and ischemia-reperfusion injury. IDrugs 4: 20–22, 2001 [PubMed] [Google Scholar]
- 22. Du C, Wang S, Diao H, Guan Q, Zhong R, Jevnikar AM. Increasing resistance of tubular epithelial cells to apoptosis by shRNA therapy ameliorates renal ischemia-reperfusion injury. Am J Transplant 6: 2256–2267, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol 17: 2293–2305, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metabol Disord 5: 269–287, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Felley-Bosco E, Bender F, Quest AF. Caveolin-1-mediated post-transcriptional regulation of inducible nitric oxide synthase in human colon carcinoma cells. Biol Res 35: 169–176, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol 30: 154–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fryckstedt J, Meister B, Aperia A. Control of electrolyte transport in the kidney through a dopamine- and cAMP-regulated phosphoprotein, DARPP-32. J Auton Pharmacol 12: 183–189, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Grigoryev DN, Liu M, Cheadle C, Barnes KC, Rabb H. Genomic profiling of kidney ischemia-reperfusion reveals expression of specific alloimmunity-associated genes: linking “immune” and “nonimmune” injury events. Transplant Proc 38: 3333–3336, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grinyo JM. Role of ischemia-reperfusion injury in the development of chronic renal allograft damage. Transplant Proc 33: 3741–3742, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Guo YL, Liu DQ, Bian Z, Zhang CY, Zen K. Down-regulation of platelet surface CD47 expression in Escherichia coli O157:H7 infection-induced thrombocytopenia. PLoS One 4: e7131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hafdi Z, Lesavre P, Nejjari M, Halbwachs-Mecarelli L, Droz D, Noel LH. Distribution of alphavbeta3, alphavbeta5 integrins and the integrin associated protein-IAP (CD47) in human glomerular diseases. Cell Adhes Commun 7: 441–451, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Hohenstein B, Daniel C, Hausknecht B, Boehmer K, Riess R, Amann KU, Hugo CP. Correlation of enhanced thrombospondin-1 expression, TGF-beta signalling and proteinuria in human type-2 diabetic nephropathy. Nephrol Dial Transplant 23: 3880–3887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hohenstein B, Daniel C, Wittmann S, Hugo C. PDE-5 inhibition impedes TSP-1 expression, TGF-beta activation and matrix accumulation in experimental glomerulonephritis. Nephrol Dial Transplant 23: 3427–3436, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Hotchkiss H, Chu TT, Hancock WW, Schroppel B, Kretzler M, Schmid H, Liu Y, Dikman S, Akalin E. Differential expression of profibrotic and growth factors in chronic allograft nephropathy. Transplantation 81: 342–349, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Hugo C. The thrombospondin 1-TGF-beta axis in fibrotic renal disease. Nephrol Dial Transplant 18: 1241–1245, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Hugo CP, Pichler RP, Schulze-Lohoff E, Prols F, Adler S, Krutsch HC, Murphy-Ullrich JE, Couser WG, Roberts DD, Johnson RJ. Thrombospondin peptides are potent inhibitors of mesangial and glomerular endothelial cell proliferation in vitro and in vivo. Kidney Int 55: 2236–2249, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 53: 503–514, 2002 [PubMed] [Google Scholar]
- 38. Ignarro LJ, Wood KS, Wolin MS. Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc Natl Acad Sci USA 79: 2870–2873, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn 197: 40–56, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284: 1116–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci 65: 728–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 109: 1945–1952, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer 9: 182–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, Tsokos M, Wink DA, Roberts DD. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol 173: 1100–1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 144: 752–761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 28: 110–119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 281: 26069–26080, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA 102: 13141–13146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol 28: 615–621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res 100: 712–720, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 111: 613–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iwazu Y, Muto S, Fujisawa G, Nakazawa E, Okada K, Ishibashi S, Kusano E. Spironolactone suppresses peritubular capillary loss and prevents deoxycorticosterone acetate/salt-induced tubulointerstitial fibrosis. Hypertension 51: 749–754, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Jin G, Tsuji K, Xing C, Yang YG, Wang X, Lo EH. CD47 gene knockout protects against transient focal cerebral ischemia in mice. Exp Neurol 217: 165–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem 285: 38923–38932, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almog N, Kieran MW, Folkman J. Platelets actively sequester angiogenesis regulators. Blood 113: 2835–2842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kobayashi S, Yamamoto T. The molecular biologic study of the expression of thrombospondin in vascular smooth muscle cells and mesangial cells. J Diabet Complications 5: 121–123, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Kosugi T, Heinig M, Nakayama T, Connor T, Yuzawa Y, Li Q, Hauswirth WW, Grant MB, Croker BP, Campbell-Thompson M, Zhang L, Atkinson MA, Segal MS, Nakagawa T. Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol 174: 1221–1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krol R, Chudek J, Karkoszka H, Ziaja J, Kolonko A, Pawlicki J, Kajor M, Wiecek A, Cierpka L. Apoptosis of tubular epithelial cells in preimplantation biopsies of kidney grafts with immediate, slow and delayed function. Ann Transplant 16: 17–22 [DOI] [PubMed] [Google Scholar]
- 59. Kroncke KD, Suschek CV, Kolb-Bachofen V. Implications of inducible nitric oxide synthase expression and enzyme activity. Antioxid Redox Signal 2: 585–605, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Kurihara H, Harita Y, Ichimura K, Hattori S, Sakai T. SIRP-α-CD47 system functions as an intercellular signal in the renal glomerulus. Am J Physiol Renal Physiol 299: F517–F527, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Lario S, Bescos M, Campos B, Mur C, Luque P, Alvarez R, Campistol JM. Thrombospondin-1 mRNA expression in experimental kidney transplantation with heart-beating and non-heart-beating donors. J Nephrol 20: 588–595, 2007 [PubMed] [Google Scholar]
- 62. Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol 178: 2573–2586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lu X, Guo X, Wassall CD, Kemple MD, Unthank JL, Kassab GS. Reactive oxygen species cause endothelial dysfunction in chronic flow overload. J Appl Physiol 110: 520–527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem 280: 29637–29644, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res 64: 1026–1036, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, Deist FL, Sarfati M. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood 100: 2882–2890, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg 124: 1880–1889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 1: 3ra7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H oxidase alleviates impaired NOS-dependent responses of pial arterioles in type 1 diabetes mellitus. Microcirculation 13: 567–575, 2006 [DOI] [PubMed] [Google Scholar]
- 70. McDonald LJ, Murad F. Nitric oxide and cGMP signaling. Adv Pharmacol 34: 263–275, 1995 [DOI] [PubMed] [Google Scholar]
- 71. Miller TW, Isenberg JS, Roberts DD. Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol 159: 1542–1547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Miller YE, Daniels GL, Jones C, Palmer DK. Identification of a cell-surface antigen produced by a gene on human chromosome 3 (cen-q22) and not expressed by Rhnull cells. Am J Hum Genet 41: 1061–1070, 1987 [PMC free article] [PubMed] [Google Scholar]
- 73. Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 11: 59–69, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Narizhneva NV, Razorenova OV, Podrez EA, Chen J, Chandrasekharan UM, DiCorleto PE, Plow EF, Topol EJ, Byzova TV. Thrombospondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J 19: 1158–1160, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nishiyama Y, Tanaka T, Naitoh H, Mori C, Fukumoto M, Hiai H, Toyokuni S. Overexpression of integrin-associated protein (CD47) in rat kidney treated with a renal carcinogen, ferric nitrilotriacetate. Jpn J Cancer Res 88: 120–128, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Novelli EM, Kato GJ, Ragni MV, Zhang Y, Hildesheim ME, Nouraie M, Barge S, Meyer MP, Hassett AC, Gordeuk VR, Gladwin MT, Isenberg JS. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol 87: 326–330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Osada-Oka M, Ikeda T, Akiba S, Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J Cell Biochem 104: 1918–1926, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Ostman A, Frijhoff J, Sandin A, Bohmer FD. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem 150: 345–356, 2011 [DOI] [PubMed] [Google Scholar]
- 79. Pallero MA, Elzie CA, Chen J, Mosher DF, Murphy-Ullrich JE. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J 22: 3968–3979, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pascher A, Klupp J. Biologics in the treatment of transplant rejection and ischemia/reperfusion injury: new applications for TNFalpha inhibitors? BioDrugs 19: 211–231, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Payne JA, Reckelhoff JF, Khalil RA. Role of oxidative stress in age-related reduction of NO-cGMP-mediated vascular relaxation in SHR. Am J Physiol Regul Integr Comp Physiol 285: R542–R551, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Ramanathan S, Mazzalupo S, Boitano S, Montfort WR. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry 50: 7787–7799, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 102: 13147–13152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J 10: 1183–1191, 1996 [PubMed] [Google Scholar]
- 85. Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol 31: 162–169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rogers NM, Thomson AW, Isenberg JS. Acitvated parenchymal CD47 is a proximate promoter of renal ischemia reperfusion injury. J Am Soc Nephrol [Epub before print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9: 49–89, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood 106: 658–667, 2005 [DOI] [PubMed] [Google Scholar]
- 89. Schneeberger H, Aydemir S, Illner WD, Land W. Nonspecific primary ischemia/reperfusion injury in combination with secondary specific acute rejection-mediated injury of human kidney allografts contributes mainly to development of chronic transplant failure. Transplant Proc 29: 948–949, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Shao D, Oka SI, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol 52: 550–558, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shvetsov M, Ivanov AA, Kuznetsova AV, Popova OP, Rameeva AS. [Molecular factors of angiogenesis in renal tissue of patients with chronic glomerulonephritis: association with nephrosclerosis and anemia]. Ter Arkh 81: 14–19, 2009 [PubMed] [Google Scholar]
- 92. Suzuki K, Wang R, Kubota H, Shibuya H, Saegusa J, Sato T. Kinetics of biglycan, decorin and thrombospondin-1 in mercuric chloride-induced renal tubulointerstitial fibrosis. Exp Mol Pathol 79: 68–73, 2005 [DOI] [PubMed] [Google Scholar]
- 93. Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol 175: 7781–7787, 2005 [DOI] [PubMed] [Google Scholar]
- 95. Wang X, Yan L, Chen W, Xu L, Zhang X. The renal protective effects of cilostazol on suppressing pathogenic thrombospondin-1 and transforming growth factor-beta expression in streptozotocin-induced diabetic rats. J Int Med Res 37: 145–153, 2009 [DOI] [PubMed] [Google Scholar]
- 96. Wani J, Carl M, Henger A, Nelson PJ, Rupprecht H. Nitric oxide modulates expression of extracellular matrix genes linked to fibrosis in kidney mesangial cells. Biol Chem 388: 497–506, 2007 [DOI] [PubMed] [Google Scholar]
- 97. Xie XS, Li FY, Liu HC, Deng Y, Li Z, Fan JM. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch Pharm Res 33: 275–284, 2010 [DOI] [PubMed] [Google Scholar]
- 98. Xing C, Lee S, Kim WJ, Jin G, Yang YG, Ji X, Wang X, Lo EH. Role of oxidative stress and caspase 3 in CD47-mediated neuronal cell death. J Neurochem 108: 430–436, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang YL, Chuang LY, Guh JY, Liu SF, Hung MY, Liao TN, Huang YL. Thrombospondin-1 mediates distal tubule hypertrophy induced by glycated albumin. Biochem J 379: 89–97, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res 63: 13–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 102. Yung S, Lee CY, Zhang Q, Lau SK, Tsang RC, Chan TM. Elevated glucose induction of thrombospondin-1 up-regulates fibronectin synthesis in proximal renal tubular epithelial cells through TGF-beta1 dependent and TGF-beta1 independent pathways. Nephrol Dial Transplant 21: 1504–1513, 2006 [DOI] [PubMed] [Google Scholar]
- 103. Zaccolo M. Spatial control of cAMP signalling in health and disease. Curr Opin Pharmacol 11: 649–655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca2+-ATPase. Free Radic Biol Med 27: 810–821, 1999 [DOI] [PubMed] [Google Scholar]
- 105. Zeng C, Villar VA, Yu P, Zhou L, Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clin Exp Hypertens 31: 156–178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]