Abstract
Adenosine 1 receptors (A1AR) have been shown in previous experiments to play a major role in the tubuloglomerular feedback (TGF) constrictor response of afferent arterioles (AA) to increased loop of Henle flow. Overexpression studies have pointed to a critical role of vascular A1AR, but it has remained unclear whether selective deletion of A1AR from smooth muscle cells is sufficient to abolish TGF responsiveness. To address this question, we have determined TGF response magnitude in mice in which vascular A1AR deletion was achieved using the loxP recombination approach with cre recombinase being controlled by a smooth muscle actin promoter (SmCre/A1ARff). Effective vascular deletion of A1AR was affirmed by absence of vasoconstrictor responses to adenosine or cyclohexyl adenosine (CHA) in microperfused AA. Elevation of loop of Henle flow from 0 to 30 nl/min caused a 22.1 ± 3.1% reduction of stop flow pressure in control mice and of 7.2 ± 1.5% in SmCre/A1ARff mice (P < 0.001). Maintenance of residual TGF activity despite absence of A1AR-mediated responses in AA suggests participation of extravascular A1AR in TGF. Support for this notion comes from the observation that deletion of A1ARff by nestin-driven cre causes an identical TGF response reduction (7.3 ± 2.4% in NestinCre/A1ARff vs. 20.3 ± 2.7% in controls), whereas AA responsiveness was reduced but not abolished. A1AR on AA smooth muscle cells are primarily responsible for TGF activation, but A1AR on extravascular cells, perhaps mesangial cells, appear to contribute to the TGF response.
Keywords: stop flow pressure, cre recombinase, nestin, smooth muscle actin
tubuloglomerular feedback (TGF) is defined as the change of afferent arteriolar resistance caused by alterations of luminal NaCl concentration at the tubulo-vascular contact site of the nephron, presumably the macula densa region (25). The direct relationship between the resistance of afferent arterioles and luminal NaCl concentration is, for the most part, the consequence of a gradual vasoconstriction resulting from the generation of a vasoactive mediator in the interstitium of the juxtaglomerular apparatus. Experimental evidence supports the notion that variations in the release of ATP from macula densa cells and in the subsequent formation of adenosine and its interaction with A1 adenosine receptors (A1AR) provide the critical constrictor input during increases of luminal NaCl concentration. Specifically, release of ATP from MD cells in response to increasing luminal NaCl has been demonstrated using a biosensor approach, and interference with adenosine formation and action can completely abolish TGF responses (2, 3, 29).
Nevertheless, the location of the A1AR pool contributing to TGF is not entirely clear. Expression studies as well as functional observations strongly support the presence of A1AR in afferent arterioles (14, 28, 31, 32), but expression in mesangial and juxtaglomerular granular cells has also been reported (19, 28). In the present study, we have asked the question whether the exclusive deletion of A1AR from afferent arterioles is sufficient to abolish TGF responsiveness. Site-specific deletion of arteriolar A1AR in vascular smooth muscle cells (Sm) was achieved by using the cre/loxP recombination system with a smooth muscle actin promoter expressing cre recombinase. Effective deletion of A1AR was confirmed by functional observations in isolated perfused afferent arterioles that showed nonresponsiveness to A1AR agonists. In contrast, TGF responses were not fully abolished, suggesting a TGF-supporting role for A1AR outside afferent arterioles. We have compared the results from these mice with data from animals in which A1AR were deleted from the juxtaglomerular apparatus in a less selective way by using the nestin promoter to express cre recombinase (9). TGF responses were reduced to the same extent as that seen in the SmCre animals despite the fact that the vascular responsiveness to A1AR agonists was partially maintained. Thus A1AR located on cells along the path between MD and SM cells appear to contribute to the full TGF response.
METHODS
Animals.
Experiments were performed in transgenic mice with conditional cre recombinase-mediated deletions of A1AR. Mice with the A1AR coding sequence flanked by loxP sites in a 129J/C57 mixed background were obtained from Dr. Robert Greene (University of Texas) (24). Mice were crossed in our laboratory with EIIa cre mice (FVB/C57Bl/6 background) to remove the floxed neomycin resistance (NeoR)/cytosine deaminase cassette used as selection marker. Offspring that was NeoR-negative and had loxP-flanked A1AR on both alleles (A1ARff) was crossed with transgelin promoter-cre mice (SM22-cre, mixed 129J/C57BL6 background) obtained from Jackson Laboratories to produce double-floxed and cre-positive experimental animals (SmCre/A1ARff). Similarly, A1ARff mice were crossed with transgenic nestin-cre mice (C57BL/6 background achieved by backcrossing; Jackson Laboratories) to produce NestinCre/A1ARff animals. Cre-negative mice in an A1ARff background were typically used as controls. Genotyping was done on tail DNA with standard PCR using A1AR primers that distinguish between the native (268 bp) and floxed alleles (302 bp). Primer sequences were 5′-GCTGAGTCACCACTGTCTTGT-3′ (S) and 5′-CCACCATTATCT GGCTCCCAT-3′ (AS). Presence or absence of cre DNA was determined using cre primers as described previously (6). Animals ranged in weight from 20 to 31 g and in age from 3 to 5 mo. Animals were kept on a standard diet (NaCl content 0.2%) and tap water. In some of the studies, mice received a diet containing 8% NaCl for 1 wk. Animal care and experimentation were approved and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Quantification of mRNA.
Kidneys, brains, and hearts from SmCre/A1ARff and NestinCre/A1ARff were removed from anesthetized mice. About 20 mg of each tissue were collected, immediately soaked in 600 μl of lysis buffer (Qiagen), and homogenized. The homogenates were cleaned by centrifugation and used to isolate total RNAs by using RNeasy Mini Kit (Qiagen). RNA (1 μg) was synthesized to yield 20 μl of cDNA by using SuperScript II Reverse Transcriptase (Invitrogen). cDNA (1 μl) synthesized from 50 ng of RNA was used to assess ado receptor mRNA expression by real-time PCR. Primers for adenosine receptor and hypoxanthine phosphoribosyltransferase 1 (HPRT1) cDNA amplification by real-time RT-PCR were obtained from Applied Biosystems (A1AR: Mm01308023_m1; A2bAR: Mm00839292_ml; A2aAR: Mm00802075_ml; HPRT: Mm03024075_m1). HPRT1 RNA was used as endogenous control. Differences in mRNA expression were calculated using the comparative cycle threshold (CT) method.
Animal preparation.
For micropuncture experiments, mice were anesthetized with 100 mg/kg thiobutabarbital (inactin) intraperitoneally and 100 mg/kg ketamine subcutaneously. Body temperature was maintained at 37.5°C by placing the animals on an operating table with a servo-controlled heating plate. The trachea was cannulated, and a stream of 100% oxygen was blown toward the tracheal tube throughout the experiment. The left carotid artery was catheterized with hand-drawn polyethylene tubing for continuous measurement of arterial blood pressure and blood withdrawal. A catheter connected to an infusion pump was inserted into the right jugular vein for an intravenous maintenance infusion of saline at 300 μl/h.
Micropuncture experiments.
Measurements of stop flow pressure (PSF) during perfusion of loop of Henle were done as described previously (26, 34). When PSF had stabilized, perfusion rate of the loop of Henle was increased to 30 nl/min and maximum PSF responses were determined. Perfusion rate was then reduced to 0 nl/min and maintained until steady states were achieved at each flow rate. Two such responses were determined successively in each nephron. The perfusion fluid contained 136 NaCl mM, 4 mM NaHCO3, 4 mM KCl, 2 mM CaCl2, 7.5 mM urea, and 100 mg/100 ml FD&C green (Keystone, Bellefonte, PA).
Perfusion of microdissected afferent arterioles.
To functionally assess the effectiveness of A1AR deletion in afferent arteriolar smooth muscle cells, the influence of adenosine and the A1AR agonist cyclohexyl adenosine (CHA) on vessel diameter was determined in the isolated perfused afferent arteriole preparation (12, 13). In brief, kidneys were removed and sliced along the corticomedullary axis. Microdissections were done at 4°C in albumin-enriched DMEM (0.1%) using sharpened forceps (no. 5; Dumont, Montignez, Switzerland). Afferent arterioles were identified by preparation of the arterial tree, including the interlobular artery. The afferent arteriole with attached glomerulus was then transferred into a thermoregulated chamber. Arterioles were perfused with use of a perfusion system (Vestavia Scientific, Vestavia Hills, AL), which allowed movement and adjustment of concentric holding and perfusion pipettes. The holding pipette [outer diameter (OD) of 2.13 mm, inner diameter (ID) of 1.63 mm] had an aperture of ∼26 μm at the tip and a constriction of ∼20 μm after customizing. The proximal end of the arteriole was aspirated into this pipette. The inner perfusion pipette with a tip diameter of 5 μm was advanced into the lumen of the arteriole. This pipette was connected to a reservoir containing the perfusion solution. The perfusion pressure in the afferent arteriole was 60 mmHg (21). Measurements of diameter changes were made in the distal part of the arteriole at 2 min after agonist addition.
GFR in conscious animals.
GFR was measured using single-injection elimination kientics as described by Qi et al. (8, 23). During brief isoflurane anesthesia from which the mice recovered within ∼20 s, FITC-sinistrin dissolved in saline at a concentration of 5 g% was injected at 3.74 μl/g body wt into the retroorbital plexus (FITC-sinistrin was kindly supplied by Dr. Norbert Gretz, Medical Faculty Mannheim, Mannheim, Germany). Sinistrin is a polyfructosan with greatly superior solubility and stability compared with FITC-inulin (27). At 3, 7, 10, 15, 35, 55, and 75 min, mice were placed in a restrainer, and ∼4 μl of blood were collected into heparinized 5-μl microcaps by nicking the tail vein (Drummond Scientific, Broomall, PA). Plasma (1 μl) was diluted in 9 μl of 500 mM HEPES buffer (pH 7.4), and fluorescence was determined using a NanoDrop ND-3300 fluorospectrometer. A standard curve was generated by determining fluorescence in 1 μl of 5%-FITC-sinistrin diluted 1:50, 1:100, and 1:500 in 500 mM HEPES buffer. Fluorescence was determined in 1.7 μl in a Nanodrop-ND-3300 spectrometer (Nanodrop Technologies, Wilmington, DE). GFR was calculated using a two-compartment model of two-phase exponential decay (23).
Blood pressure telemetry.
Radiotransmitters (model TA11PA-C10; Data Sciences International, St. Paul, MN) were implanted during ketamine and xylazine anesthesia (90 and 10 mg/kg, respectively) with the sensing catheter placed in the carotid artery as previously described (20). Seven to 10 days after surgery, data sampling was done for 10 s every 2 min over a period of at least 5 days. Radio signals were processed using a model RPC-1 receiver, a 20-channel data exchange matrix, APR-1 ambient pressure monitor, and a Data Quest ART Silver 2.3 acquisition system. The recording room was maintained at 21–22°C with a 12-h light/dark cycle (LD).
Statistics.
Data are expressed as means ± SE. Significance of differences between group means was tested by t-test or by ANOVA with Bonferroni post hoc test in case of multiple comparisons.
RESULTS
A1AR expression.
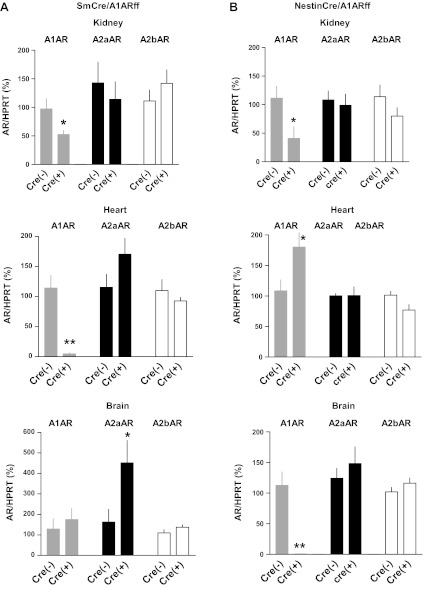
Measurements of adenosine receptor mRNA expression in wild-type, SmCre/A1ARff, and NestinCre/A1ARff mice in three organs are summarized in Fig. 1. As shown in Fig. 1A, A1AR mRNA levels in whole kidney tissue of SmCre/A1ARff mice were reduced to about half in the cre-positive animals, whereas the expression of A2 receptors was not significantly affected. An even more pronounced reduction of A1AR expression was observed in the heart, consistent with the wide SM22 alpha expression in both vascular and myocardial cells during early cardiac development (15, 17). The presence of cre recombinase did not detectably affect A1AR expression in the brain. However, there was a significant increase in the expression of A2a receptors in the brain. Figure 1B shows the effect of nestin-dependent cre on adenosine receptor mRNA expression. In the kidney, A1AR abundance in the NestinCre/A1ARff mice was significantly reduced, whereas it was elevated in the heart. A reduction of A1AR mRNA expression to virtually undetectable levels was observed in the brain. There were no differences in the expression of A2a or A2b adenosine receptors between wild-type and nestin-cre- positive mice in any of the tissues.
Fig. 1.
Relative adenosine receptor (AR) mRNA expression in SmCre/A1ARff mice (A) and NestinCre/A1ARff mice (B). Expression levels are given for kidney (top), heart (middle), and brain (bottom), and data for A1AR, A2aAR, and A2bAR are expressed relative to respective wild-type levels (cre−). Data represent means ± SE (SmCre−: n = 7, SmCre+: n = 8; NestinCre−: n = 8, NestinCre+: n = 8). Significant difference: *P < 0.05; **P < 0.01.
Arteriolar responsiveness.
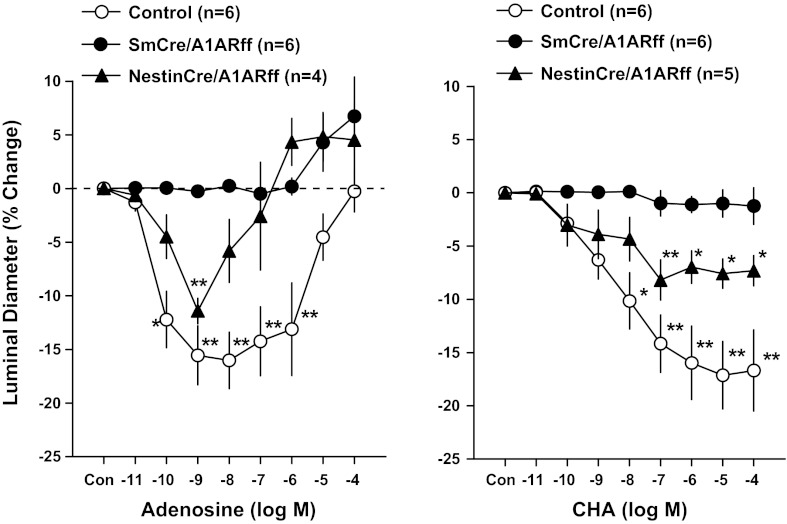
The functional impact of cre-mediated recombination on A1AR expression was examined by assessing the contractile responses of isolated perfused afferent arterioles to bath application of adenosine or of the A1AR agonist cyclohexyl adenosine (CHA). Relative changes of afferent arteriolar diameter induced by increasing concentrations of adenosine or CHA are shown in Fig. 2. Relative to untreated vessels (diameter of 10 ± 1.16 μm; n = 6), arterioles from cre-negative mice responded to application of adenosine with vessel constriction at low concentrations and an increasing diminution of this constriction at concentrations of >10−8 M without showing net vasodilatation. Observations in arterioles from NestinCre/A1ARff mice (control diameter of 9.45 ± 0/33 μm; n = 4) were qualitatively similar, although the opposing vasodilator action was more effective, leading to net vasodilatation at concentrations higher than 10−6 M. In contrast, adenosine did not elicit detectable vasoconstriction in vessels from SmCre/A1ARff mice (control diameter of 9.42 ± 0.8 μm; n = 6) with a vasodilator effect evident at higher adenosine concentrations.
Fig. 2.
Effect of bath application of adenosine (left) and cyclohexyl adenosine (CHA; right) on the diameter of perfused afferent arterioles from control (open symbols), SmCre/A1ARff (filled dots), and NestinCre/A1ARff mice (triangles). Data are expressed as percent changes of diameter from control (diameter of untreated vessels). Data represent means ± SE. Significant difference (ANOVA with Bonferroni post hoc test; shown are comparisons with control for each of the three strains): *P < 0.05; **P < 0.01.
The effects of the A1AR agonist CHA on arteriolar diameter are shown in Fig. 2, right. In cre-negative A1ARff control mice, A1AR activation by CHA caused the expected reduction of afferent arteriolar diameter by ∼15–20% in a concentration range between 10−11 and 10−5 M (control diameter of 9.06 ± 0.6 μm; n = 6). Vasoconstriction by CHA was also observed in vessels from NestinCre/A1ARff mice, but the magnitude of this effect was clearly less than in control mice (control diameter of 9.4 ± 0.32 μm; n = 5). It is likely that this diminished response is the result of reduced A1AR abundance. CHA did not cause measurable changes in the diameters of vessels from SmCre/A1ARff mice (control diameter of 8.3 ± 0.54 μm; n = 6), consistent with effective deletion of floxed A1AR by SM22-alpha-controlled cre recombinase.
TGF responsiveness.
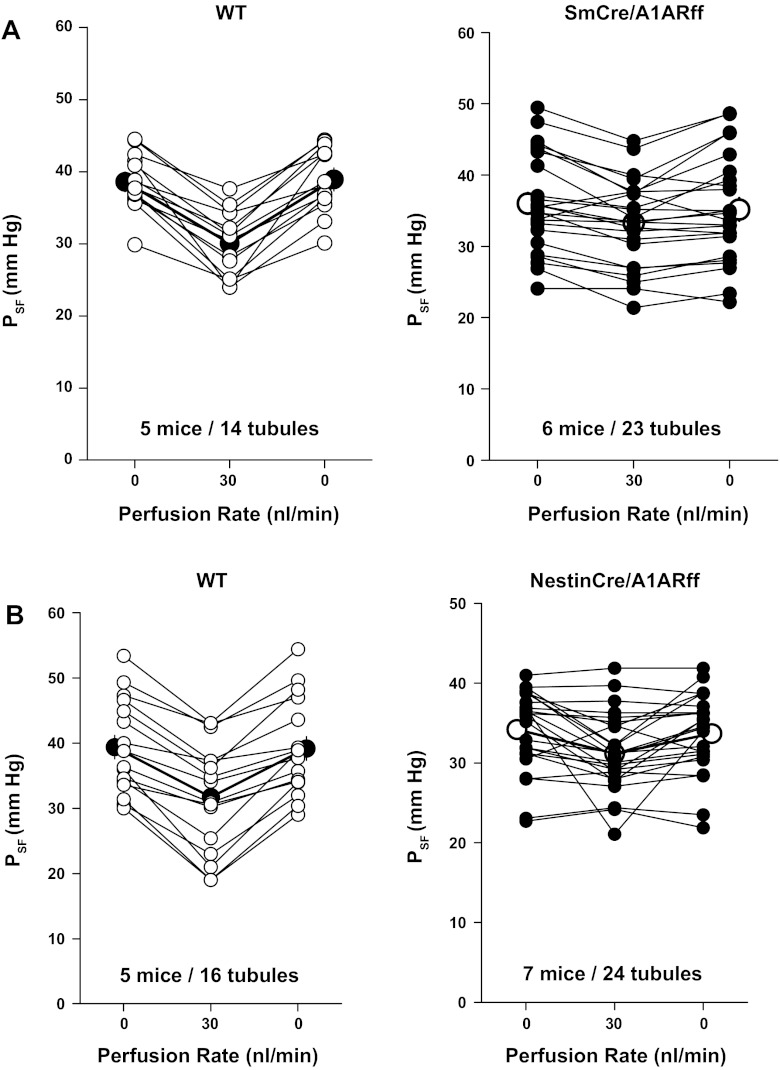
The effect of smooth muscle-specific deletion of A1AR on TGF was assessed in six male SmCre/A1ARff (23 nephrons) and in five male cre-negative A1ARff controls (14 nephrons). TGF responsiveness was quantified by measuring proximal tubular stop flow pressure (PSF) in response to changes in loop perfusion rate from 0 to 30 nl/min (on-response) and back (off-response) (Fig. 3A). Body weights and ages of the examined mice were similar, averaging 29.7 ± 1.9 g and 14.8 ± 0.7 wk in cre-positive and 31 ± 1.05 g and 16.4 ± 2.1 wk in cre-negative mice. Mean arterial pressure under micropuncture conditions was not different between SmCre/A1ARff and control mice (105.9 ± 2.9 and 97 ± 4.5 mmHg, respectively; P = 0.11). PSF at zero loop perfusion averaged 38.6 ± 1.4 mmHg in cre-negative controls and 36 ± 1.45 mmHg in cre-positive mice (P = 0.24). At a saturating perfusion rate of 30 nl/min, PSF decreased to steady-state levels of 30.1 ± 1.6 mmHg in controls and to 33.3 ± 1.3 mmHg in SmCre/A1ARff mice, corresponding to on-responses of 8.55 ± 1.3 and 2.7 ± 0.6 mmHg, respectively (P < 0.001). Subsequent reduction of perfusion rate from 30 to 0 nl/min (off-response) was associated with a PSF increase by 8.9 ± 1.34 mmHg in control mice and by 1.9 ± 0.63 mmHg in SmCre/A1ARff mice (P < 0.001). A repeat challenge with high perfusion rates caused decreases of PSF in both strains that were not significantly different from initial responses. Thus, although markedly reduced, TGF responsiveness was partially retained in the mice with smooth muscle-specific deletion of A1AR.
Fig. 3.
Tubuloglomerular feedback responses of tubular stop flow pressure (PSF) in wild-type control and SmCre/A1ARff mice (A) and in wild-type control and NestinCre/A1ARff mice (B). Lines connect data from individual nephrons at perfusion rates of 0 or 30 nl/min. Mean values are indicated by larger symbols.
Observations in regard to TGF made in seven NestinCre/A1ARff mice (mean body weight 25.3 ± 1.4 g, mean age 14.1 ± 3.7 wk) and five cre-negative control mice (mean body weight 29 ± 2.7 g, mean age 14.2 ± 2.3 wk) are graphically summarized in Fig. 3B. In response to an increase in loop perfusion rate from 0 to 30 nl/min, PSF fell from 39.4 ± 1.8 to 31.7 ± 2.1 mmHg in cre-negative control mice (P < 0.001) and from 34.2 ± 1.03 to 31.4 ± 1 mmHg in NestinCre/A1ARff mice (P < 0.01). Response magnitudes of 7.7 ± 0.9 mmHg in control mice and of 2.8 ± 0.9 mmHg in cre-positive mice were significantly different (P < 0.001). Mean arterial blood pressure during micropuncture was 102 ± 2.9 mmHg in control mice and 96 ± 1.8 mmHg in cre-positive mice (P = 0.08).
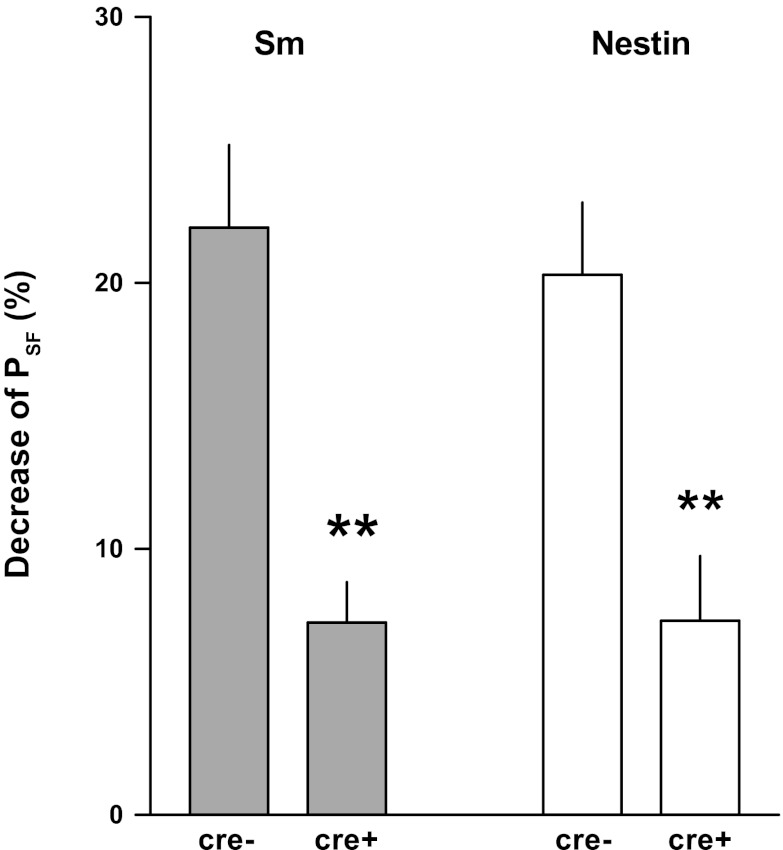
Relative reductions of PSF by a saturating flow increase are shown in Fig. 4. In SmCre/A1ARff mice, the reduction of maximum TGF responses of 7.2 ± 1.5% was significantly smaller than the 22.1 ± 3.1% decrease in control mice (P < 0.001). Similarly, response reduction in NestinCre/A1ARff mice of 7.3 ± 2.4% was significantly less than in control mice (20.3 ± 2.7%). TGF-induced reductions of PSF in the two strains of control and mutant mice were not different from each other.
Fig. 4.
Percent reduction of tubular PSF in response to a flow increase from 0 to 30 nl/min in SmCre/A1ARff (Sm) and NestinCre/A1ARff (Nestin) and their respective controls (cre−). Data are means ± SE. **Significant difference by t-test comparing cre+ and cre− (P < 0.01).
Cardiovascular characteristics and GFR.
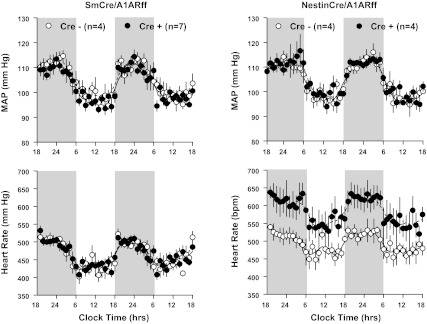
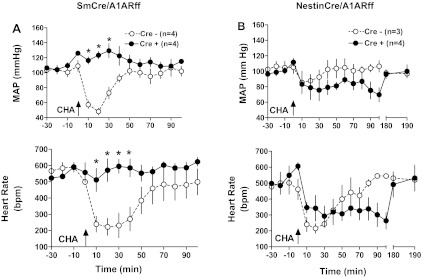
Cardiovascular variables obtained by blood pressure telemetry in control, SmCre/A1ARff, and NestinCre/A1ARff mice are summarized in Table 1. In mice on a normal diet, no significant differences in blood pressure, heart rate, and locomotor activity were observed between genotypes. There was a tendency of a higher systolic and lower diastolic pressure in control mice that translated into a noticeably larger pulse pressure in the sm cre-positive animals (P = 0.078). Furthermore, heart rates tended to be higher in nestin cre-positive than in wild-type mice (0.1 > P > 0.05). On a high NaCl diet for 1 wk before measurements, both control and SmCre/A1ARff mice showed salt sensitivity of blood pressure that reached significance for systolic and mean values in control mice and for systolic, mean, and diastolic pressures in SmCre/A1ARff mice. A similar tendency was observed in control and NestinCre/A1ARff mice, although, except for systolic pressures in the cre-positive mice, the salt effects did not reach 5% significance levels. Heart rates tended to be higher in the nestin cre-positive mice during both normal and high salt feeding (0.1 > P > 0.05). Circadian rhythms of blood pressure and heart rate were well maintained in both strains of cre-positive mice (Fig. 5). As shown in Fig. 6A, the intraperitoneal injection of the A1AR agonist cyclohexyl adenosine (1.7 μg) caused a significant reduction of mean arterial blood pressure and heart rate that was nearly completely absent in the SmCre/A1ARff mice, consistent with the marked reduction of A1AR expression in cardiac tissues of these animals reported earlier. In contrast, arterial blood pressure and heart rate fell in response to CHA in both control and NestinCre/A1ARff mice (Fig. 6B). Recovery times were prolonged in the cre-positive animals.
Table 1.
Cardiovascular variables determined by blood pressure telemetry in Cre− control, SmCre/A1ARff, and NestinCre/A1ARff mice on diets with normal or high salt contents
| Cre− |
Cre+ |
|||
|---|---|---|---|---|
| NS | HS | NS | HS | |
| SmCre/A1ARff | n = 4 | n = 4 | n = 7 | n = 7 |
| SAP, mmHg | 115.2 ± 2.8 | 121.5 ± 4.1* | 117.7 ± 1.5 | 126.4 ± 5* |
| MAP, mmHg | 105.2 ± 2.1 | 113.2 ± 7.2* | 104 ± 1.31 | 111.5 ± 1.4* |
| DAP, mmHg | 94.6 ± 3.4 | 105.1 ± 4.7 | 89.8 ± 1.4 | 96.7 ± 1.1* † |
| PP, mmHg | 20.7 ± 4.6 | 16.3 ± 4.8 | 27.8 ± 0.93 | 29.7 ± 1.4† |
| HR, beats/min | 466 ± 8 | 461 ± 16 | 411 ± 6.7 | 462 ± 13 |
| Activity, counts/min | 5.7 ± 0.9 | 4.6 ± 0.9 | 5.4 ± 1 | 4.8 ± 0.9 |
| NestinCre/A1ARff | n = 4 | n = 4 | n = 4 | n = 4 |
| SAP, mmHg | 118.5 ± 1.4 | 124.6 ± 5.2 | 119.1 ± 1.5 | 125.9 ± 2.7* |
| MAP, mmHg | 104.5 ± 1.8 | 109.9 ± 3.6 | 104.6 ± 1.1 | 108.8 ± 2.7 |
| DAP, mmHg | 90.4 ± 2.7 | 95.2 ± 3.3 | 89 ± 2.5 | 90.7 ± 4.9 |
| PP, mmHg | 29.8 ± 2.5 | 29.4 ± 4.8 | 30.1 ± 3.6 | 35.1 ± 6.1 |
| HR, beats/min | 494 ± 15.7 | 502 ± 12.6 | 573 ± 30.2‡ | 570 ± 31‡ |
| Activity, counts/min | 4.7 ± 1 | 3.8 ± 0.9 | 4.5 ± 1 | 2.1 ± 1 |
Values are means ± SE. Cre−, cre-negative control; Cre+, cre-positive mice; NS, normal salt diet; HS, high salt diet; SAP, systolic arterial pressure; MAP, mean arterial pressure; DAP, diastolic arterial pressure; PP, pulse pressure; HR, heart rate.
Significant difference between HS and NS (P < 0.05). Significant difference between genotypes for a given diet:
P < 0.05;
P < 0.1.
Fig. 5.
Telemetric recording of mean arterial blood pressure (MAP) and heart rate in cre-negative control (open symbols) and cre-positive mice (closed symbols). Measurements from the SmCre/A1ARff are on the left and from the NestinCre/A1ARff on the right. Data are shown for 2 consecutive days on 12-h light/dark cycles (dark periods from 6 PM to 6 AM indicated by shaded areas). Lines represent smoothed data using the weighted average of the nearest nine points.
Fig. 6.
Telemetric recording of the effect of an intraperitoneal injection of 1.7 μg of the A1AR agonist cyclo-hexyladenosine (CHA) on mean arterial blood pressure (top) and heart rate (bottom) in cre-negative control (open symbols) and cre-positive mice (closed symbols). A: SmCre/A1ARff mice. B: NestinCre/A1ARff mice. Note extension of time axis in the NestinCre studies.
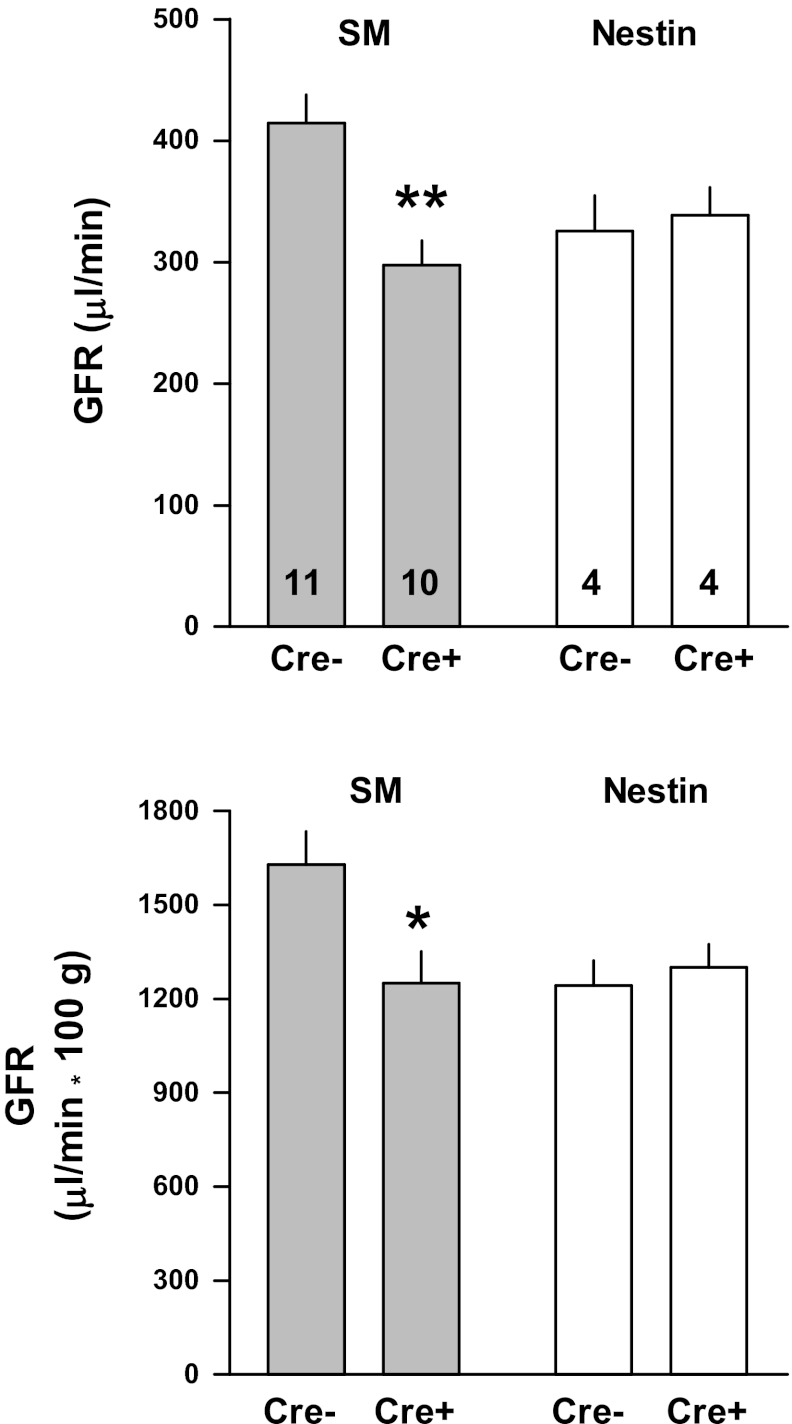
Measurements of GFR are shown in Fig. 7. GFR both in absolute and in kidney weight corrected terms was higher in control than SmCre/A1ARff mice, whereas there were no differences between control and NestinCre/A1ARff mice.
Fig. 7.
Glomerular filtration rate (GFR) measured in conscious cre-negative (Cre−) and cre-positive (Cre+) mice of the SmCre/A1ARff (shaded bars) and NestinCre/A1ARff strains (open bars). Numerals indicate numbers of animals. Significant difference between cre-negative and cre-positive mice: *P < 0.05; **P < 0.01.
DISCUSSION
Activation of adenosine 1 receptors (A1AR) has been shown by both deletion and overexpression studies to be required for the expression of tubuloglomerular feedback (TGF) responses. The goal of the present study was to further investigate the localization of A1AR involved in TGF. We used two strains of mice with conditional deletions of A1AR at overlapping but different sites to assess the impact of site-specific deletions on TGF responses. Contractile responses to CHA and adenosine in afferent arterioles, the main target site for TGF, were used as index of the efficiency of recombination in afferent arterioles. Our data show that deletion of A1AR in vascular smooth muscle cells of afferent arterioles completely abolishes the arteriolar contractions caused by A1AR agonists but that it does not abolish TGF responses. The implication that extravascular receptors contribute to TGF is supported by the finding that deletion of A1AR by nestin-driven cre recombinase reduces TGF to the same extent, although it does not abolish the vascular contractility elicited by A1AR agonists.
Global deletion of A1AR has been shown to eliminate TGF responsiveness as well as the contractile response of afferent arterioles to A1AR agonists (3, 10, 14, 29). Furthermore, overexpression of A1AR in vascular smooth muscle cells by expressing the receptors under control of a smooth muscle actin promoter caused a significant enhancement of TGF responsiveness (20). Together with additional previous evidence for a critical role of afferent arterioles in TGF, these observations strongly suggest a vascular localization of TGF-relevant A1AR. Nevertheless, this clear support of a role of vascular A1AR in TGF does not rule out a contribution of extravascular A1AR receptors to TGF responsiveness. The present results in the SmCre/A1ARff strain are in agreement with an important and dominant role for vascular receptors. Placing cre recombinase under the control of transgelin, a smooth muscle promoter element, has been shown in earlier studies to cause deletion of various target genes from smooth muscle cells (11). We have confirmed that A1AR expression in the whole kidney is decreased by about half, suggesting that half of the renal A1AR may be located in the vasculature. In the absence of reliable A1AR antibodies, studies in isolated perfused afferent arterioles were used to determine the efficiency of cre-mediated A1AR deletion in smooth muscle cells. Neither the A1AR agonist CHA nor the natural ligand adenosine caused detectable vasoconstriction in vessels of SmCre/A1ARff mice, although adenosine dilated the arterioles at concentrations of >10−6 M presumably through activation of A2AR receptors. Expression of A2AR in afferent arterioles is supported by a number of functional observations (4, 18, 30). Absence of vasoconstrictor effects indicates a virtually complete loss of functional A1AR in afferent arterioles. TGF response magnitude was reduced in SmCre/A1ARff mice by ∼80%, but it was clearly not abolished in the majority of nephrons. Because A1AR agonists do not elicit vasomotor responses in the isolated vessels, the residual TGF responsiveness is apparently not due to incomplete deletion of the receptor. This observation rather suggests that A1AR at locations not possessing SM promoter activity make a contribution of ∼20% to TGF. As discussed below, mesangial cells may play a role in TGF, and our estimate of an 80% contribution of vascular A1AR to TGF may be too high if the SM promoter driving cre recombinase is active in these cells. Although mesangial cells in culture as well as in injured kidneys clearly express smooth muscle actin, this does not typically appear to be the case in healthy kidneys in vivo, making a contribution of SmCre-driven A1AR deletion in mesangial cells somewhat unlikely (1, 7). Like in our laboratory's earlier studies in mice with global A1AR deletion (29), the marked TGF deficiency of SmCre/A1ARff mice was not associated with an increase of GFR, further indicating that measurable evidence of an operational TGF requires the imposition of preferably acute perturbations of distal NaCl delivery. The causes for the unexpected fall of GFR in the cre-positive mice are unclear but presumably unrelated to the TGF phenotype.
It is of interest that expression of A1AR in the heart of SmCre/A1ARff mice was reduced to almost undetectable levels, an observation that may be useful in future investigations of cardiac A1AR effects. Marked suppression of A1AR expression in the heart is functionally supported by absence of the blood pressure and heart rate reductions caused by activation of A1AR with cyclo-hexyladenosine. Like in our laboratory's previous studies in mice with global A1AR deletion (29), cardiac A1AR deficiency is not associated with significant changes in arterial blood pressure or heart rate, indicating that the inotropic and chronotropic effects of long-standing A1AR deficiency are fully compensated. Exposure to a high salt diet showed that mean arterial blood pressure of both control and SmCre/A1ARff mice is somewhat salt sensitive but that cardiac A1AR deficiency leads to an exaggeration of the systolic pressure increase as well as a reduction of the diastolic pressure increase. The causes and consequences for the near doubling of pulse pressure in cardiac A1AR-deficient mice on high salt diet need to be further investigated.
Nestin is an intermediate filament protein that is widely expressed during development and may be a marker for pluripotent progenitor cells (33). It has been originally considered to be specifically active in progenitors of neuronal cells (16), and the dramatic reduction of A1AR expression in brain tissue of NestinCre/A1ARff mice is consistent with strong activity of the nestin promoter in neuronal tissue. However, it is now clear that nestin is also widely expressed in stem cells of nonneuronal tissues. Although nestin expression in the mature kidney is found only in podocytes (5), it has recently been shown that sites of expression during development include intra- and extraglomerular mesangial cells, vascular smooth muscle cells of pre- and postglomerular arterioles, and juxtaglomerular renin-producing cells (9, 35). Consistent with this developmental nestin activity in the kidney is the observation of an ∼50% reduction of A1AR in the NestinCre/A1ARff animals. The constrictor response of afferent arterioles to A1AR agonists was significantly reduced, suggesting that arteriolar smooth muscle cells express nestin, presumably during development, resulting in partial deletion of A1AR in afferent arterioles. In contrast to the differential effect on vascular A1AR responsiveness, the reduction of TGF reactivity was identical between SmCre/A1ARff and NestinCre/A1ARff mice. The most likely explanation for this finding may be that A1AR on cells outside the arteriolar wall contribute to TGF. Mesangial cells have been shown to express A1AR at least in culture, and the gap-junctional connectivity of mesangial cells has been suggested to contribute to TGF by providing a pathway for Ca signal propagation (22). Compartmentalization of the A1AR pool involved in TGF may be consistent with our laboratory's recent observation that the TGF vasomotor response consists of two phases: a fast initial one and a subsequent slower one (20). It is conceivable that the fast component results from an interaction of adenosine with A1AR on mesangial cells and the elicitation of a Ca wave, whereas the slow and longer-lasting component may depend on adenosine generation, diffusion, and interaction with A1AR on vascular smooth muscle cells. The effects and causes of the upregulation of A1AR in the hearts of nestin cre-positive mice are unclear, but it could possibly contribute to the prolongation of the cardiac-depressing actions of A1AR activation. On the other hand, the strong tendency for tachycardia in the nestin cre-positive mice is vexing and unexpected in view of the increased A1AR expression. It seems conceivable that nestin-driven neuronal loss of A1AR may result in activation of cardiovascular centers in the central nervous system.
In summary, the present data confirm that the presence of A1AR in afferent arterioles is required for the expression of full TGF responses. However, maintenance of residual TGF activity despite complete abrogation of vascular A1AR responsiveness indicates that extravascular A1AR contributes to a lesser extent to TGF. Extravascular A1AR may be located on mesangial cells, but further studies are needed to verify this possibility.
GRANTS
This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases. C. S. Wilcox was supported by National Institutes of Health Grants DK-36079, DK-49870, and HL-68686.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.L., E.Y.L., Y.H., C.E., D.M., and J.S. performed experiments; L.L., E.Y.L., C.E., D.M., and J.S. analyzed data; L.L., E.Y.L., Y.H., and J.S. prepared Figs.; L.L., E.Y.L., Y.H., C.E., D.M., C.S.W., and J.S. approved final version of manuscript; E.Y.L., C.S.W., and J.S. interpreted results of experiments; C.S.W. and J.S. edited and revised manuscript; J.S. conception and design of research; J.S. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Robert Greene (University of Texas Southwestern Medical Center) for kindly donating the mice with loxP-flanked A1AR.
REFERENCES
- 1. Alpers CE, Hudkins KL, Gown AM, Johnson RJ. Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int 41: 1134–1142, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Carlstrom M, Wilcox CS, Welch WJ. Adenosine A2A receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 300: F457–F464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, Decaestecker M, Takahashi T, Breyer MD, Hao CM. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17: 1283–1291, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol 292: F27–F37, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Elger M, Drenckhahn D, Nobiling R, Mundel P, Kriz W. Cultured rat mesangial cells contain smooth muscle alpha-actin not found in vivo. Am J Pathol 142: 497–509, 1993 [PMC free article] [PubMed] [Google Scholar]
- 8. Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19: 722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanner F, von Maltzahn J, Maxeiner S, Toma I, Sipos A, Kruger O, Willecke K, Peti-Peterdi J. Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 295: R371–R380, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 99: 7142–7147, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium sensitivity in renal arterioles. Circ Res 99: 1117–1124, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Lai EY, Patzak A, Persson AE, Carlstrom M. Angiotensin II enhances the afferent arteriolar response to adenosine through increases in cytosolic calcium. Acta Physiol (Oxf) 196: 435–445, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Langlois D, Hneino M, Bouazza L, Parlakian A, Sasaki T, Bricca G, Li JY. Conditional inactivation of TGF-beta type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res 19: 1069–1082, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell 60: 585–595, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res 78: 188–195, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Nishiyama A, Inscho EW, Navar LG. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am J Physiol Renal Physiol 280: F406–F414, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Olivera A, Tomas M, Lopez-Novoa JM. Effect of adenosine A1 and A2 agonists and antagonists on cAMP and Ca2+ in cultured rat mesangial cells. Am J Physiol Cell Physiol 262: C840–C844, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Oppermann M, Qin Y, Lai EY, Eisner C, Li L, Huang Y, Mizel D, Fryc J, Wilcox CS, Briggs J, Schnermann J, Castrop H. Enhanced tubuloglomerular feedback in mice with vascular overexpression of A1 adenosine receptors. Am J Physiol Renal Physiol 297: F1256–F1264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patzak A, Lai EY, Mrowka R, Steege A, Persson PB, Persson AE. AT1 receptors mediate angiotensin II-induced release of nitric oxide in afferent arterioles. Kidney Int 66: 1949–1958, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci 23: 5762–5770, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: The Kidney. Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. San Diego, CA: Elsevier Academic, 2008, p. 589–626 [Google Scholar]
- 26. Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, Briggs JP. Absence of tubuloglomerular feedback responses in AT1A receptor- deficient mice. Am J Physiol Renal Physiol 273: F315–F320, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Schock-Kusch D, Xie Q, Shulhevich Y, Hesser J, Stsepankou D, Sadick M, Koenig S, Hoecklin F, Pill J, Gretz N. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int 79: 1254–1258, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Smith JA, Sivaprasadarao A, Munsey TS, Bowmer CJ, Yates MS. Immunolocalisation of adenosine A(1) receptors in the rat kidney. Biochem Pharmacol 61: 237–244, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang L, Parker M, Fei Q, Loutzenhiser R. Afferent arteriolar adenosine A2a receptors are coupled to KATP in in vitro perfused hydronephrotic rat kidney. Am J Physiol Renal Physiol 277: F926–F933, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Weaver DR, Reppert SM. Adenosine receptor gene expression in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 263: F991–F995, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Vasomotor effects of purinergic agonists in isolated rabbit afferent arterioles. Am J Physiol Renal Fluid Electrolyte Physiol 263: F1026–F1033, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression: a property of multi-lineage progenitor cells? Cell Mol Life Sci 61: 2510–2522, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest 53: 1695–1708, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu D, Borges GR, Davis DR, Agassandian K, Sequeira Lopez ML, Gomez RA, Cassell MD, Grobe JL, Sigmund CD. Neuron- or glial-specific ablation of secreted renin does not affect renal renin, baseline arterial pressure, or metabolism. Physiol Genomics 43: 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]