Abstract
Renal ischemia reperfusion (IR) injury causes renal tubular necrosis, apoptosis, and inflammation leading to acute and chronic kidney dysfunction. IL-11 is a multifunctional hematopoietic cytokine clinically approved to treat chemotherapy-induced thrombocytopenia. Recent studies suggest that IL-11 also has potent antiapoptotic and antinecrotic properties. In this study, we tested the hypothesis that exogenous IL-11 protects against renal IR injury and determined the mechanisms involved in renal protection. Pretreatment with human recombinant IL-11 (HR IL-11) or with long-acting site-specific polyethylene glycol (PEG)-conjugated human IL-11 analog (PEGylated IL-11) produced partial but significant protection against renal IR injury in mice. In addition, HR IL-11 or PEGylated IL-11 given 30–60 min after IR also provided renal protection in mice. Significant reductions in renal tubular necrosis and neutrophil infiltration as well as tubular apoptosis were observed in mice treated with HR IL-11 or PEGylated IL-11. Furthermore, HR IL-11 or PEGylated IL-11 decreased both necrosis and apoptosis in human proximal tubule (HK-2) cells in culture. Mechanistically, IL-11 increased nuclear translocation of hypoxia-inducible factor-1α (HIF-1α) and induced sphingosine kinase-1 (SK1) expression and activity in HK-2 cells. Moreover, selective HIF-1α inhibitors blocked IL-11-mediated induction of SK1 in HK-2 cells. Finally, HR IL-11 or PEGylated IL-11 failed to protect against renal IR injury in SK1-deficient mice. Together, our data show powerful renal protective effects of exogenous IL-11 against IR injury by reducing necrosis, inflammation, and apoptosis through induction of SK1 via HIF-1α.
Keywords: acute kidney injury, apoptosis, hypoxia inducible factor-1α, inflammation, necrosis, sphingosine kinase
renal ischemia-reperfusion (IR) injury is a frequent cause of acute kidney injury (AKI) (17). Ischemic AKI is a major clinical problem for patients subjected to major surgical procedures involving the kidney, liver, heart, or aorta, often leading to multiple organ dysfunction and systemic inflammation with an extremely high mortality (21). Currently, the incidence of renal dysfunction after major surgery in high-risk patients has been reported to be as high as 80% (15). Unfortunately, the severity and incidence of AKI has been increasing without any improvements in therapy or patient survival over the past 50 years (20). Currently, there are no proven therapies to reduce AKI in the perioperative setting.
IL-11 is a 20-kDa multifunctional member of the IL-6-type cytokine family and is a key regulator of megakaryocyte maturation (12). In addition to its hematopoietic properties, recent studies suggest cytoprotective roles for IL-11 (28). Specifically, IL-11 administration protects against intestinal, cardiomyocyte, and endothelial cell death by producing significant antinecrotic and antiapoptotic effects in these cell types (9). IL-11 also attenuates the inflammatory responses in a murine model of lipopolysaccharide -induced sepsis (53, 54). Because renal IR results in renal tubular and endothelial necrosis, apoptosis as well as inflammation (5), we tested the hypothesis that recombinant human IL-11 protects against murine renal IR injury.
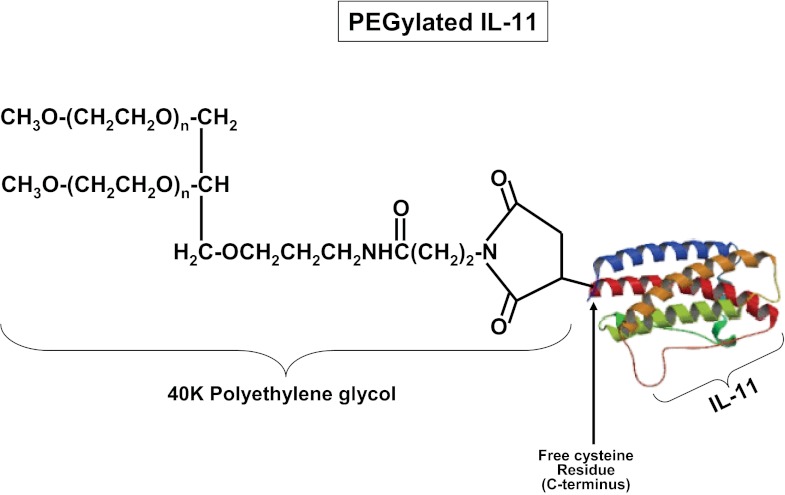
Exogenous administration of native or human recombinant (HR) IL-11 may be limited by its relatively short half-life (∼7 h) due to rapid clearance through urinary excretion, hepatic metabolism, and enzymatic degradation (9, 13). Chemical modification of IL-11 by conjugation to polyethylene glycol (PEG) reduces IL-11 glomerular filtration and hepatic uptake, therefore prolonging the half-life of IL-11 (52). In this study, we tested the renal protective effects of a site-specific cysteine residue PEG-conjugated IL-11 analog (PEGylated IL-11; Fig. 1) in mice. Finally, we examined the mechanisms of IL-11-mediated renal protection against IR. Our study suggests a critical role for induction of renal tubular sphingosine kinase-1 (SK1) via IL-11-mediated nuclear translocation of hypoxia-inducible factor-1α (HIF-1α) in IL-11-induced renal protection against ischemic AKI.
Fig. 1.
Chemical structure of site-specific polyethylene glycol-conjugated (PEGylated) IL-11. The 40-kDa branched PEG was conjugated to a carboxy-terminal cysteine residue in IL-11 (*179C).
METHODS
Interleukin-11 preparation.
Human recombinant IL-11 (HR IL-11) and cysteine residue-specific PEG-conjugated IL-11 (PEGylated IL-11) were synthesized at Bolder BioTechnology (Boulder, CO). IL-11 and IL-11 (*179C) proteins were expressed as fusion proteins in Escherichia coli strain ER2566 using the pTYB11 expression plasmid (New England Biolabs, Beverly, MA). IL-11 (*179C) is an IL-11 analog containing a cysteine residue added following the last amino acid of the native protein. The expressed fusion protein comprises an NH2-terminal chitin binding domain joined to a yeast intein sequence followed by IL-11 or IL-11 (*179C). E. coli expression of the fusion protein was induced by addition of isopropyl-β-d-thiogalactopyranoside to the cultures. After induction, the induced cells were lysed and the fusion protein captured on a chitin affinity column (New England Biolabs). The chitin column was washed with buffer containing 50 mM dithiothreitol to activate the intein domain, which cleaves IL-11 from the fusion protein. The cleaved IL-11 proteins were eluted from the column and purified by S-Sepharose column chromatography. The purified IL-11 (*179C) protein was modified with a branched 40-kDa maleimide-PEG obtained from Nippon Oil and Fat (Irvine, CA) and the PEGylated protein purified from unreacted protein and unreacted PEG by S-Sepharose column chromatography. As indicated by reverse-phase HPLC and nonreducing SDS-PAGE analyses, both proteins were >95% pure.
Murine model of renal IR injury.
After receiving Institutional Animal Care and Use Committee approval, we subjected adult male C57BL/6 (Harlan, Indianapolis, IN) as well as SK1−/− or SK2−/− mice (on a C57BL/6 background; kindly provided by Dr. R. L. Proia, National Institutes of Health, Bethesda, MD; see Refs. 2, 37) to 30 min of renal IR as described previously (22, 25). To test the renal protective effects of IL-11, we pretreated mice with saline (vehicle for HR IL-11), PEG (vehicle for PEGylated IL-11), HR IL-11 (0.1–1 mg/kg ip), or long-acting PEGylated IL-11 (0.1–1 mg/kg ip) 10 min before renal ischemia or sham operation. We also tested whether IL-11 treatment after completion of renal ischemia also provides renal protection. Separate cohorts of mice were treated with saline, PEG, HR IL-11 (1 mg/kg ip), or PEGylated IL-11 (1 mg/kg ip) 30 or 60 min after reperfusion of the ischemic kidney. We collected kidney (cortex and corticomedullary junction) and plasma 24 h after IR injury to examine the severity of renal dysfunction (plasma creatinine, renal tubular necrosis, apoptosis, and neutrophil infiltration).
Measurement of renal function.
Plasma creatinine was measured as described with an enzymatic creatinine reagent kit according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA) (50). Unlike the Jaffe method, this method of creatinine measurement largely eliminates the interference from mouse plasma chromagens.
Histological detection of necrosis, apoptosis, and neutrophil infiltration.
Morphological assessment of hematoxylin and eosin (H&E) staining was performed by an experienced renal pathologist (V.D.D.) who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (0–4, renal injury score) to the proximal tubules was used for the histopathological assessment of IR-induced damage as outlined by Jablonski et al. (18) and as described previously in our studies (32, 34). We detected apoptosis after renal IR with terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining as described elsewhere (41) by using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer. Kidney inflammation after renal IR was assessed by detection of neutrophil infiltration using immunohistochemistry 24 h after IR as described previously (41). Neutrophils infiltrating the kidney were quantified in 5–7 randomly chosen ×400 microscope image fields in the corticomedullary junction, and results are expressed as neutrophils counted per ×400 field. Apoptotic TUNEL-positive cells were quantified in 5–7 randomly chosen ×200 microscope image fields in the corticomedullary junction, and results are expressed as neutrophils counted per ×200 field.
HK-2 cell culture and induction of necrosis and apoptosis.
Necrotic injury in HK-2 cells (ATCC, Manassas, VA) was induced with exposure to 5 mM H2O2 for 3 h, and lactate dehydrogenase (LDH) released into cell culture media was measured as described using a commercial LDH assay kit (Promega, Madison, WI) (31). To induce apoptosis, HK-2 cells were exposed to tumor necrosis factor-α (TNF-α; 20 ng/ml) plus cycloheximide (10 μg/ml) for 16 h as described previously (33). Cycloheximide was added in addition to TNF-α to facilitate apoptosis. Cycloheximide has been shown to synergistically increase TNF-α cytotoxicity (46, 62). HK-2 cell apoptosis was assessed by detecting poly(adenosine diphosphate-ribose) polymerase (PARP) and caspase 3 fragmentations as described previously (33). Some HK-2 cells were pretreated with 10–1,000 ng/ml HR IL-11 or PEGylated IL-11 30 min before induction of necrosis or apoptosis. Separate cohorts of HK-2 cells were treated with HR IL-11 (100 ng/ml) or PEGylated IL-11 (100 ng/ml) for 6 h to test for induction of SK1 or SK2 mRNA and protein. To inhibit HIF-1α, some HK-2 cells were pretreated with 10 μM 2-methoxyestradiol (2ME; a posttranscriptional downregulator of HIF-1α) (36, 59) or with 25 μM 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1; an inhibitor of HIF-1α activity) (16, 63) 30 min before HR IL-11 or PEGylated IL-11 treatment.
Reverse transcription-polymerase chain reaction and immunoblotting analyses.
We measured mRNA encoding human SK1 or SK2 6 h after HR IL-11 or PEGylated IL-11 treatment in HK-2 cells as described previously (24). Table 1 lists the primer sequences utilized in this study. HK-2 cell lysates were also collected for immunoblotting analyses of SK1, SK2, and β-actin (internal protein loading control) 16 h after HR IL-11 or PEGylated IL-11 treatment as described previously (24).
Table 1.
Human RT-PCR primers used in this study
| Primers | Sequences (Sense/Antisense) | Product size, bp | Cycle Number | Annealing Temperature, °C |
|---|---|---|---|---|
| SK1 | 5′-ATCTCCTTCACGCTGATGC-3′ | 330 | 26 | 66 |
| 5′-GTGCAGAGACAGCAGGTTCA-3′ | ||||
| SK2 | 5′-GGAGGAAGCTGTGAAGATGC-3′ | 482 | 22 | 66 |
| 5′-GCAGGTCAGACACAGAACGA-3′ | ||||
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 450 | 16 | 65 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ |
Respective anticipated RT-PCR product size (bp, base pairs), PCR cycle number for linear amplification, and annealing temperatures used for each primer are provided.
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SK, sphingosine kinase.
HK-2 cell HIF-1α DNA binding assay.
Nuclear extracts from mouse kidney tissues and HK-2 cells were prepared using the TransFactor Extraction kit (Clontech, Mountain View, CA) according to the manufacturer's instructions. HIF-1α DNA-binding activity in nuclear extracts was determined using a TransFactor Family Colorimetric kit specific for HIF-1α (Clontech) according to the manufacturer's instructions.
SK activity assay.
SK activity was measured, as described previously (23), using a modified protocol according to Vessey et al. (57). To preferentially measure SK1 activity, we supplemented the assay buffer with 250 mM KCl plus 0.5% Triton X-100 (27, 42).
Statistical analysis.
The data were analyzed with Student's t-test when comparing means between two groups or with one-way ANOVA plus Tukey's post hoc multiple comparison test when comparing multiple groups. Two-way ANOVA plus Bonferroni's posttest was used to test the effects of sham operation or renal IR injury on different mouse strains or treatment groups. The ordinal values of the renal injury scores were analyzed using the Mann-Whitney nonparametric test. In all cases, a probability statistic <0.05 was taken to indicate significance. All data are means ± SE.
RESULTS
Renal protective effects of IL-11 administration.
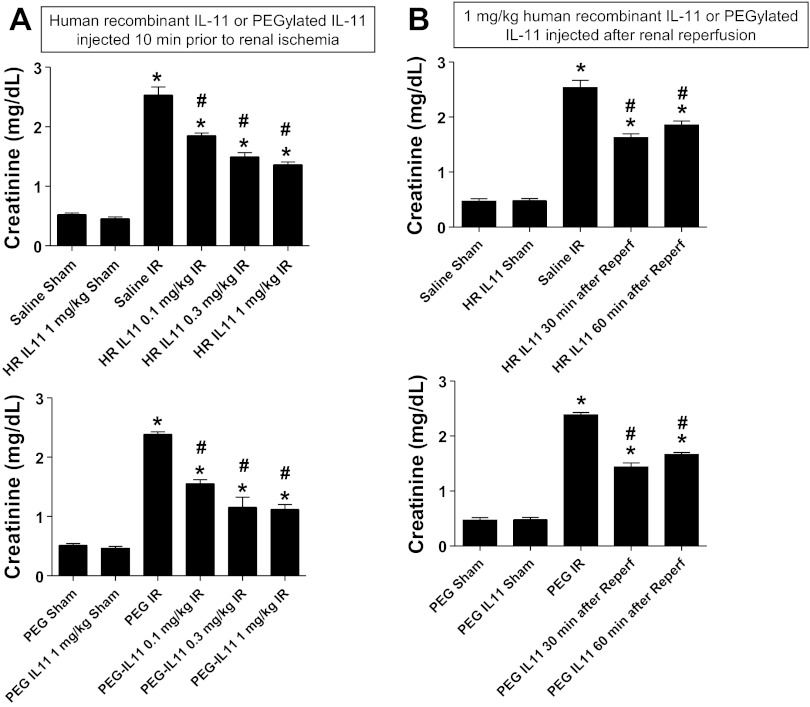
We initially tested whether HR IL-11 or PEGylated IL-11 pretreatment protects against renal IR injury in mice. Plasma creatinine values were similar between sham-operated (anesthesia, laparotomy, right nephrectomy, and recovery) saline-treated (Cr = 0.52 ± 0.03 mg/dl, n = 4), PEG-treated (Cr = 0.51 ± 0.03 mg/dl, n = 3), HR IL-11-treated (Cr = 0.45 ± 0.03 mg/dl, n = 3), or PEGylated IL-11-treated mice (Cr = 0.46 ± 0.03 mg/dl, n = 3) 24 h after surgery. Plasma creatinine significantly increased in saline- or PEG-treated mice subjected to 30 min of renal IR compared with sham-operated mice (Fig. 2). Pretreatment with HR IL-11 or PEGylated IL-11 (10 min before renal ischemia) partially but significantly attenuated the increases in plasma creatinine in mice (Fig. 2A). We also tested whether IL-11 treatment after renal reperfusion (after completion of renal ischemia) protected against renal IR injury. Figure 2B shows that HR IL-11 or PEGylated IL-11 given 30 or 60 min after reperfusion was protective against renal IR injury.
Fig. 2.
Renal protection with pre- or postischemic IL-11 treatment. Plasma creatinine levels are from wild mice subjected to sham surgery or to renal ischemia and reperfusion (IR). A: mice were pretreated with 0.1–1 mg/kg human recombinant (HR) IL-11 or PEGylated IL-11 10 min before renal ischemia (n = 5–6 per group). B: mice were treated with 1 mg/kg HR IL-11 or PEGylated IL-11 30 or 60 min after renal IR (n = 5–6 per group). Sham-operated mice were treated with either vehicle (saline or PEG) or 1 mg/kg HR IL-11 or PEGylated IL-11 (n = 4 per group). Pre-or postischemic treatment with either HR IL-11 or PEGylated IL-11 significantly attenuated the increases in plasma creatinine after renal IR. *P < 0.05 vs. vehicle-treated mice subjected to sham surgery. #P < 0.05 vs. vehicle-treated mice subjected to renal IR. Data are means ± SE.
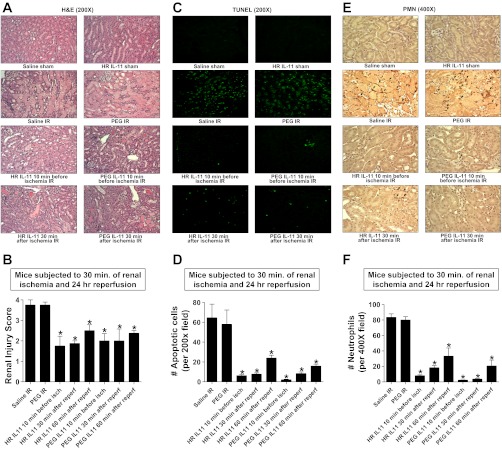
Figure 3A demonstrates severe necrotic renal injury in saline- or PEG-treated mice subjected to IR 24 h after injury. Compared with sham-operated vehicle-treated mice (not shown), the kidneys of vehicle-treated (saline or PEG) mice subjected to renal IR showed significant tubular necrosis, proteinaceous casts with increased congestion (Fig. 3A). Consistent with the plasma creatinine data, mice treated with HR IL-11 or PEGylated IL-11 10 min before or 30 min after renal ischemia had reduced renal necrosis and tubular injury (Fig. 3A). The Jablonski scale (18) renal injury score (scale 0–4) was used to grade renal tubular necrosis 24 h after renal IR (Fig. 3B). Thirty minutes of renal ischemia and 24 h of reperfusion resulted in severe acute tubular necrosis (with renal injury scores approaching 4) in saline- or PEG-treated mice. In contrast, mice treated with either HR IL-11 or PEGylated IL-11 before or after renal ischemia had partial but significantly lower renal injury scores compared with vehicle-treated mice subjected to renal IR.
Fig. 3.
IL-11 reduces renal necrosis, apoptosis, and neutrophil infiltration after IR. A, C, and E: representative photomicrographs for hematoxylin and eosin (H&E) staining (A; magnification ×200), TUNEL staining (C; representing apoptotic nuclei, magnification ×200), and immunohistochemistry for neutrophil infiltration (E; magnification ×400) of kidney sections of mice. Mice were pretreated with saline, PEG, 1 mg/kg HR IL-11, or 1 mg/kg PEGylated IL-11 and subjected to 30 min of renal ischemia and 24 h of reperfusion. Some mice were treated with HR IL-11 or PEGylated IL-11 30 min after renal IR. Images are representative of 3–5 independent experiments. B: summary of Jablonski scale renal injury scores (graded from H&E staining, scale 0–4) for mice subjected to renal IR (n = 4). D and F: quantifications of apoptotic cells per ×200 field (D) and infiltrated neutrophils per ×400 field (F) in kidneys of mice after renal IR. *P < 0.05 vs. vehicle-treated mice subjected to renal IR. Data are means ± SE. Saline- or PEG-treated mice showed severe renal tubular necrosis, apoptosis, and neutrophil infiltration after IR. Pre- or postischemic HR IL-11 or PEGylated IL-11 treatment significantly attenuated renal IR injury in mice. Reperf, reperfusion.
IL-11 treatment reduces renal apoptosis after IR.
Renal ischemia and 24 h of reperfusion resulted in severe apoptosis in the kidneys of saline- or PEG-treated mice. The TUNEL staining detected apoptotic renal cells in kidney of mice subjected to renal IR with predominant proximal tubule cell apoptosis (Fig. 3C, magnification ×200). HR IL-11 or PEG IL-11 given before or after renal ischemia significantly reduced the number of apoptotic TUNEL-positive cells in the kidney (Fig. 3D).
IL-11 treatment reduces renal neutrophil infiltration after IR.
Figure 3E shows representative images of neutrophil immunohistochemistry of kidney (magnification ×400) from mice subjected to 30 min of renal ischemia and 24 h of reperfusion. There was significant neutrophil infiltration (dark brown) in the kidneys of mice treated with saline or PEG and subjected to 24 h of renal IR. In sham-operated mice, we were unable to detect any neutrophils in the kidney (data not shown). Mice treated with HR IL-11 or PEG IL-11 before or after renal ischemia had significantly reduced neutrophil infiltration in the kidney after IR (Fig. 3F).
IL-11 reduces necrosis and apoptosis in human proximal tubule cells.
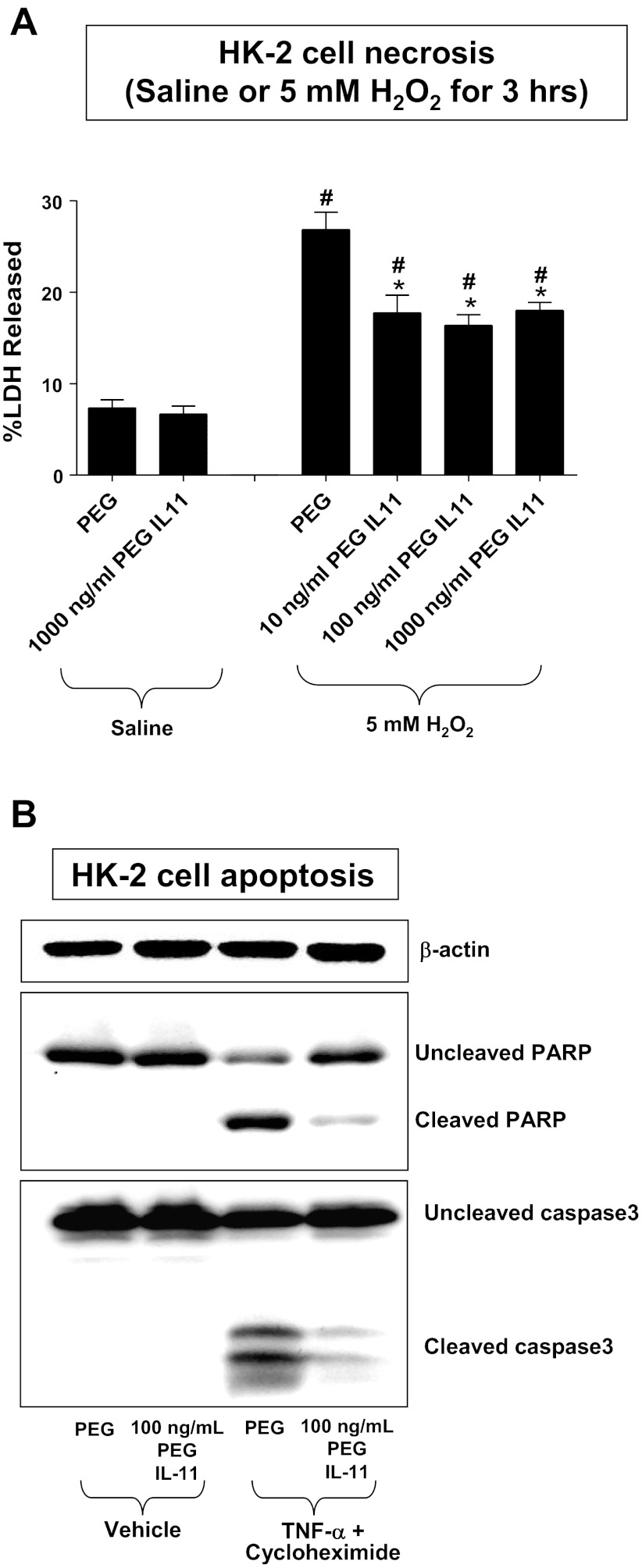
HK-2 cells pretreated with either HR IL-11 (data not shown) or PEGylated IL-11 (Fig. 4) for 6 h were protected against H2O2-induced necrosis as evidenced by reduced LDH release (Fig. 4A, 10–1,000 ng/ml PEGylated IL-11) and apoptosis as evidenced by reduced PARP and caspase 3 fragmentation (Fig. 4B, 100 ng/ml PEGylated IL-11). HR IL-11 or PEGylated IL-11 treatments themselves had no effect on basal LDH released into the cell culture media.
Fig. 4.
PEGylated IL-11 reduces necrosis and apoptosis in human renal proximal tubule (HK-2) cells. A: lactate dehydrogenase (LDH) released after saline treatment or after H2O2-induced necrosis in HK-2 cells (n = 6 for each group, expressed as a percentage of total LDH released). Vehicle (PEG) or PEGylated IL-11 (10–1,000 ng/ml) was applied 30 min before H2O2 treatment. #P < 0.05 vs. PEG-treated saline group. *P < 0.05 vs. PEG-treated H2O2 group. Data are means ± SE. Isch, ischemia. B: PEGylated IL-11 attenuates HK-2 cell apoptosis induced by TNF-α (20 ng/ml) and cycloheximide (10 mg/ml). Representative poly(adenosine diphosphate-ribose) polymerase (PARP) and caspase 3 fragmentation (n = 3–4 for each group) as indices of HK-2 cell apoptosis. PEG or PEGylated IL-11 was given 30 min before induction of apoptosis, and cells were harvested 16 h later. Similar results were obtained by treating cells with HR IL-11 (not shown here). β-actin served as internal loading controls.
IL-11 increases SK1 synthesis and induces SK activity in HK-2 cells.
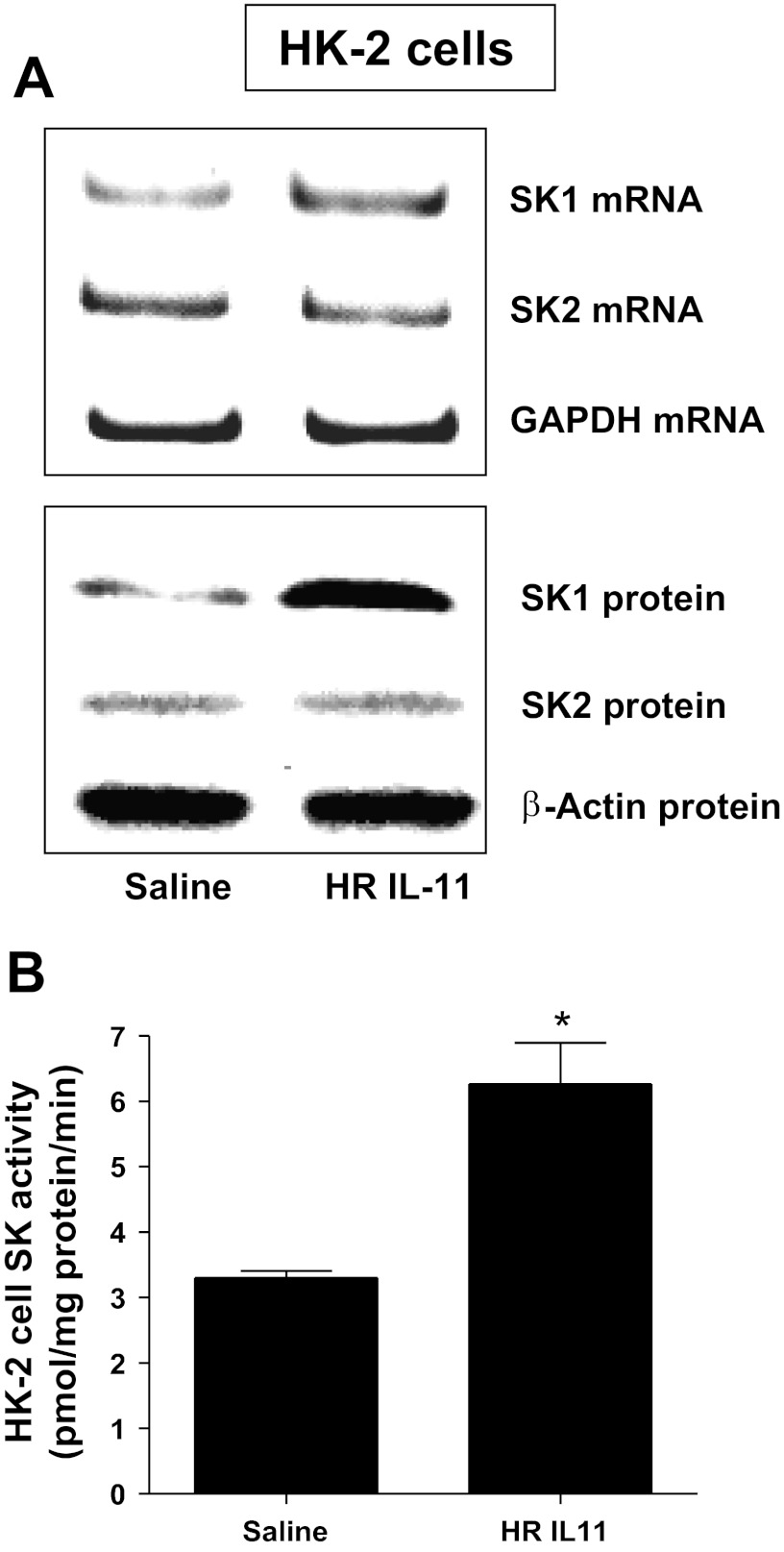
HK-2 cells were treated with HR IL-11 (100 ng/ml) or PEG IL-11 (100 ng/ml) for SK mRNA (6 h) or protein (16 h) analysis. We determined that both HR IL-11 (Fig. 5) and PEGylated IL-11 (data not shown) increased SK1 mRNA and protein expression and SK activity in HK-2 cells. SK2 mRNA or protein expression did not change. We preferentially measured SK1 activity by adding Triton X-100 in our SK activity assay.
Fig. 5.
HR IL-11 induces sphingosine kinase-1 (SK1) expression and activity in HK-2 cells. A: representative images for SK mRNA (RT-PCR) and protein (immunoblotting) expression in HK-2 cells treated with saline (vehicle for HR IL-11) or 100 ng/ml HR IL-11 (n = 4 for each group). HK-2 cells were treated for 6 h (mRNA analysis) or 16 h (protein analysis). Note that HR IL-11 increased SK1 mRNA and protein expression in HK-2 cells without changing SK2 expression. B: SK activity in HK-2 cells treated with saline or 100 ng/ml HR IL-11 (1 μM) for 16 h (n = 4 for each group). GAPDH mRNA and β-actin protein served as internal loading controls. Data are means ± SE. *P < 0.05 vs. saline treatment.
Critical role of SK1 in IL-11-mediated renal protection against IR in mice.
We next tested whether IL-11 requires induction of SK1 for renal protection in mice. We determined that SK1−/− mice were not protected against renal IR with either 1 mg/kg HR IL-11 (Cr = 2.95 ± 0.15 mg/dl, n = 4) or 1 mg/kg PEGylated IL-11 (Cr = 3.0 ± 0.18 mg/dl, n = 4) compared with saline-treated (Cr = 2.95 ± 0.15 mg/dl, n = 4) or PEG-treated (Cr = 3.0 ± 0.2 mg/dl, n = 4) SK1−/− mice subjected to renal IR. In contrast, HR IL-11 (Cr = 1.76 ± 0.12 mg/dl, n = 4) or PEGylated IL-11 (Cr = 1.52 ± 0.15 mg/dl, n = 4) protected SK2−/− mice against renal IR.
HIF-1α plays a critical role in IL-11-mediated SK1 induction.
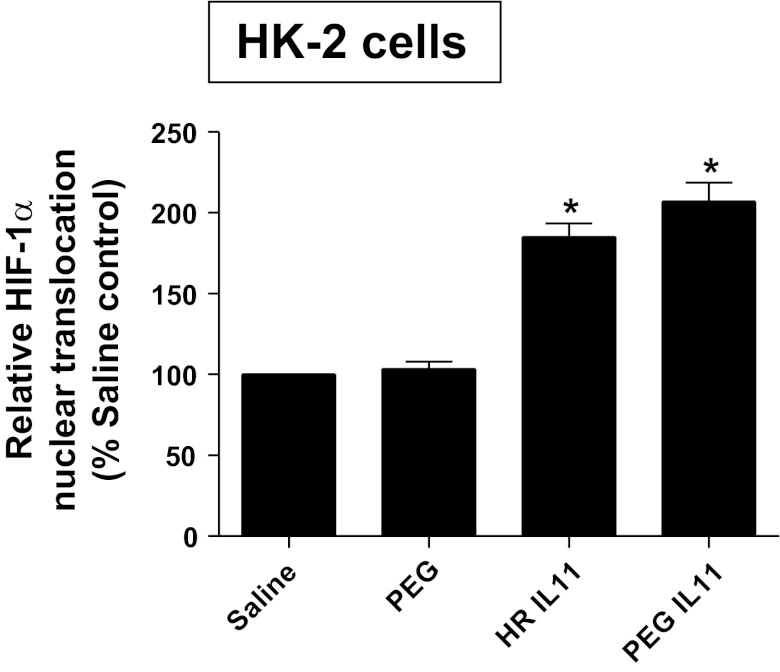
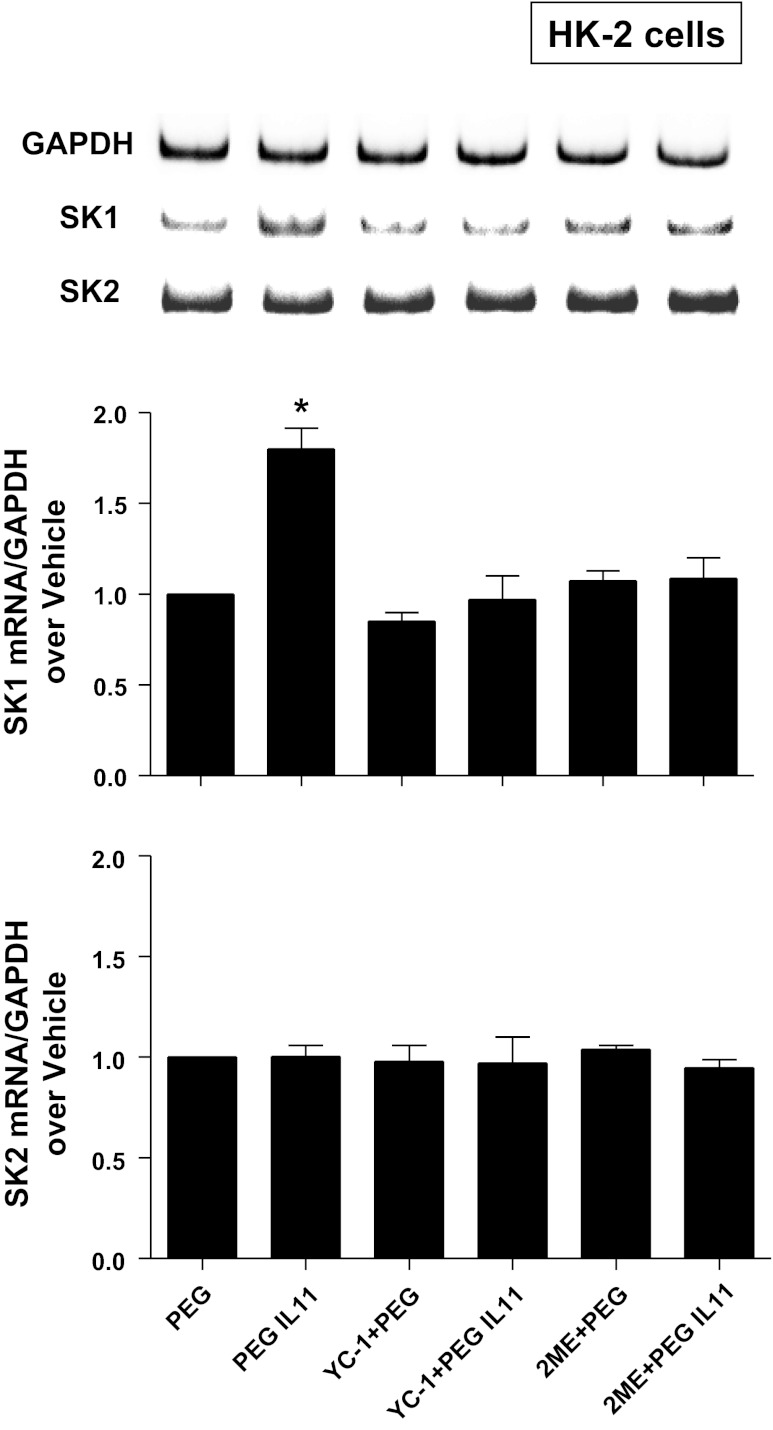
Because HIF-1α signaling can induce SK1 (48), we tested the hypothesis that IL-11 induces SK1 via a HIF-1α-dependent mechanisms in HK-2 cells. First, we tested whether HR IL-11 or PEGylated IL-11 directly increases nuclear HIF-1α translocation and subsequent DNA binding in HK-2 cells. In HK-2 cells, HR IL-11 (100 ng/ml for 6 h) or PEGylated IL-11 (100 ng/ml for 6 h) increased nuclear HIF-1α DNA binding (Fig. 6). Furthermore, when HK-2 cells were pretreated with inhibitors of HIF-1 signaling [2ME (10 μM) or YC-1 (25 μM)] 6 h before exposure to HR IL-11 (data not shown) or PEGylated IL-11 (100 ng/ml for 6 h), induction of SK1 mRNA was significantly attenuated without affecting SK2 mRNA expression (Fig. 7).
Fig. 6.
IL-11 induces nuclear translocation of hypoxia-inducible factor-1α (HIF-1α) in HK-2 cells. Shown is HIF-1α DNA-binding activity in nuclear extracts from HK-2 cells treated with saline (vehicle for HR IL-11), PEG (vehicle for PEGylated IL-11), 100 ng/ml HR IL-11, or 100 ng/ml PEGylated IL-11 for 6 h (n = 4–6 for each group). HR IL-11 or PEGylated IL-11 significantly increased the nuclear translocation of HIF-1α in HK-2 cells. *P < 0.05 vs. respective controls. Data are means ± SE.
Fig. 7.
HIF-1α plays a critical role in IL-11-mediated SK1 induction. Shown are representative bands and densitometric quantification of relative SK1 or SK2 mRNA normalized to GAPDH from RT-PCR reactions in HK-2 cells treated with PEG or 100 ng/ml PEGylated IL-11 for 6 h. PEGylated IL-11 significantly and selectively increased SK1 mRNA expression in HK-2 cells without affecting SK2 mRNA expression. PEGylated IL-11-mediated SK1 induction was blocked by selective HIF-1α inhibitors YC-1 (an inhibitor of HIF-1α activity, 25 μM) or 2-methoxyestradiol (2ME; a posttranscriptional downregulator of HIF-1α, 10 μM) given 30 min before PEGylated IL-11 treatment. *P < 0.05 vs. PEG control group. Similar results were obtained by treating cells with HR IL-11 (not shown).
DISCUSSION
AKI is a frequent and disastrous clinical complication with high mortality, morbidity, and cost (10, 21). Renal IR injury is a major cause of AKI for patients subjected to surgical procedures involving the kidney, heart, liver, or aorta. Although incompletely understood, renal tubular necrosis, apoptosis, and inflammation during and after renal IR contribute significantly to the pathogenesis of ischemic AKI (5). Our study demonstrates for the first time that HR IL-11 as well as a novel PEGylated IL-11 attenuates renal tubular cell death in vivo as well as in vitro by reducing necrosis, apoptosis, and inflammation.
IL-11, a member of the IL-6-type cytokine family, was first identified from bone marrow-derived stromal cells. It is a key regulator of hematopoiesis and promotes megakaryocyte maturation (43). Interestingly, IL-11 as well as its receptors are expressed in many tissues and cell types (9). In addition to its hematopoietic properties, recent studies suggest a cytoprotective role for IL-11 (9). In several organs, including the heart, intestine, and endothelial cells, IL-11 administration has been shown to attenuate necrotic as well as apoptotic cell death (9). In addition to its antiapoptotic and antinecrotic properties, IL-11 administration reduces inflammatory responses in lipopolysaccharide-treated mice (49, 53), macrophage inflammation (55), nephrotoxic nephritis (29), and T cell-mediated liver injury (6). Our current study also demonstrates powerful protective effects of IL-11 against renal IR injury in mice and in human HK-2 kidney proximal tubule cells. Specifically, we show that IL-11 attenuated renal tubular necrosis (Jablonski renal injury score, LDH release) as well as apoptosis (TUNEL staining, PARP/caspase 3 fragmentation). Furthermore, we demonstrate reduced influx of proinflammatory neutrophils after renal IR in IL-11-treated mouse kidneys. Therefore, we conclude that exogenous administration of IL-11 provides powerful renal protection against ischemic AKI by targeting all three pathways of cell death: necrosis, apoptosis, and inflammation.
A clinical formulation of recombinant IL-11 (oprelvekin) is already approved by the FDA to treat chemotherapy-related thrombocytopenia (43, 51). Our data suggest that HR IL-11 or PEGylated IL-11 therapy was significantly protective when given before renal ischemia as well as 30–60 min after completion of renal ischemia. This is highly exciting because IL-11 therapy may be effective for a diverse group of patients at risk for ischemic AKI. Although ischemia can be predicted in many complicated surgical procedures leading to renal injury, a significant number of patients present to the clinic after renal ischemic injury has already occurred. Postischemic therapy for AKI will increase the translational as well as clinical significance, because not all ischemic AKI can be anticipated in advance.
In this study, we used a novel, long-acting IL-11 analog that has undergone cysteine residue-specific chemical modification of the protein with PEG. Covalent modification of proteins with PEG has proven to be a very useful method to extend the circulating half-lives of proteins, and several PEGylated proteins are now approved for use in humans (8). Long-acting IL-11 analogs would not require frequent dosing and could provide significant treatment advantages in a clinical setting. In addition to improving protein half-life, PEG modification can increase protein solubility and stability and decrease protein immunogenicity (52).
Although PEGylation may increase the half-life of IL-11, it may also result in substantial decrease in biological activity or potency due to its steric hindrance (52). When amine-reactive PEGs are used, the PEG moiety can attach to the protein at any of the free amines available, resulting in a heterogeneous product mixture possessing different intrinsic biological activities. Site-specific PEGylation, by conjugating PEG to a unique, engineered cysteine residue in IL-11, overcomes this problem of product heterogeneity and loss of biological activity typical of amine-PEGylation. Site-specific PEGylation allows a protein to be selectively modified with PEG at a unique predetermined site. By targeting the PEG molecule to an optimal site in a protein, it is possible to create PEGylated proteins that are homogenously modified and have no significant loss of biological activity. Therefore, site-specific PEGylation is an innovative application to increase the potency and half-life of IL-11 (by more than 10-fold to ∼300–500 min in rats; unpublished data).
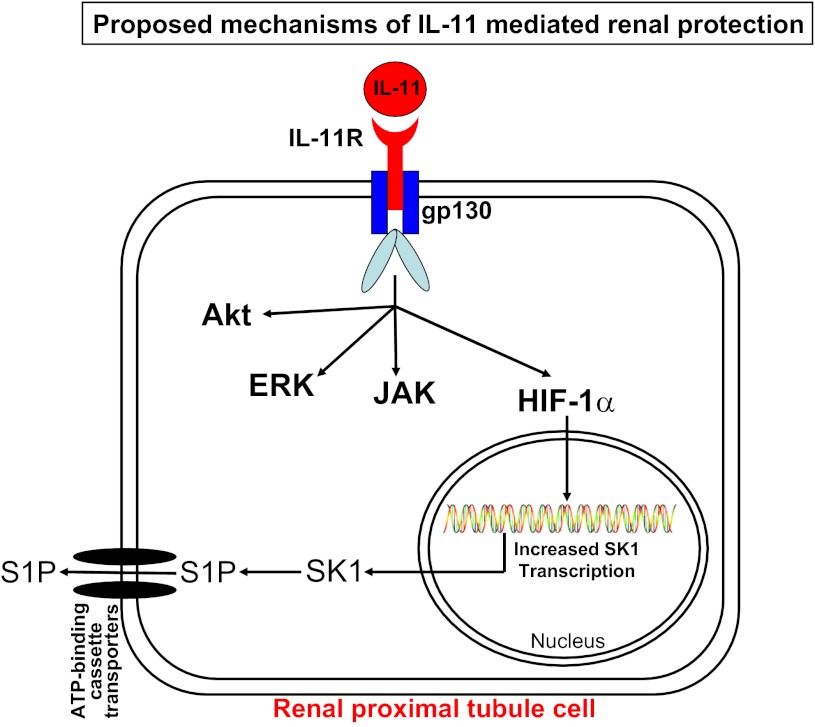
After IL-11 binds to the IL-11 receptor, the ligand-receptor complex interacts with a common receptor subunit, glycoprotein 130 (gp130), leading to gp130-associated kinase-mediated tyrosine phosphorylation (11). The cytoprotective mechanisms of IL-11 leading to reductions in necrosis, inflammation, and apoptosis have been investigated in other cell types. In cardiomyocytes, IL-11 reduces injury and fibrosis by JAK-STAT3 (Janus kinase-signal transducer and activator of transducer 3) pathway activation (11, 26, 40). In vascular endothelial and intestinal epithelial cells, IL-11 protects against oxidant-induced necrosis and apoptosis via mechanisms involving ERK MAPK, Akt (protein kinase B), and/or induction of heat shock protein 25 (39, 44, 61). Our data suggest that IL-11 produces renal protection by direct induction of SK1 via nuclear translocation of HIF-1α (Fig. 8). IL-11-mediated induction of SK1 has never been described previously and represents a novel development in the understanding of cytoprotective mechanisms of IL-11 administration. It remains to be tested whether IL-11 induces SK1 in other cell types.
Fig. 8.
Schematic of proposed cellular mechanisms of IL-11-mediated renal protection. Previous studies showed that the IL-11 and IL-11 receptor (IL-11R) complex interact with another transmembrane cytokine receptor subunit, glycoprotein 130 (gp130), to produce endothelial, cardiac, and epithelial cytoprotection involving Janus kinase signal transducer and activator of transcription (JAK STAT), ERK, and Akt (protein kinase B) (reviewed in Ref. 11). Our data also suggest that IL-11 activation results in increased HIF-1α nuclear translocation and SK1 induction. Consistent with this hypothesis, renal protective effects of IL-11 were abolished in mice deficient in SK1. We speculate that increases in sphingosine 1-phosphate (S1P) produce antinecrotic, antiapoptotic, and anti-inflammatory effects to attenuate renal IR injury. It remains to be determined whether ERK and/or Akt pathways are involved in IL-11-mediated induction of SK1.
SK is a multifunctional lipid kinase that phosphorylates sphingosine to form sphingosine 1-phosphate (S1P). Of the two forms of SK, SK1 is a cytosolic enzyme that migrates to the plasma membrane or to the nucleus upon activation (14, 30). SK1 is a well-known mediator of tissue protection (including protection against IR injury), growth, and survival (35). Overexpression of SK1 was shown to be protective in acute lung injury (60). Furthermore, in cardiac IR injury, SK1 activation protects against cardiomyocyte death, and SK1-deficient cardiomyocytes had increased injury after ischemia (58). We and others previously demonstrated a renal protective role of SK1 as well as S1P1 receptor activation (4, 19, 23, 24). Overall, activation of SK1 produces antinecrotic, anti-inflammatory, and antiapoptotic effects in several organs and cell types.
Our studies show not only that IL-11 treatment induces SK1 but also that the renal protective effects of IL-11 are dependent on SK1, because mice deficient in SK1 enzyme were not protected against renal IR injury with either HR IL-11 or PEGylated IL-11 treatment. We propose that IL-11-mediated SK1 induction enhances the synthesis of endogenous S1P in the kidney. S1P is a potent lipid signaling molecule that can activate five S1P receptors (S1PR) to regulate cell growth, cell survival, and modulation of inflammation (1, 7, 56). S1P1R activation in particular has been shown to produce tissue protection by attenuating T-lymphocyte-mediated inflammation. Activation of the S1P1Rs on endothelial cells also reduces vascular permeability, hence better preserving the integrity of the vascular endothelial cell barrier function (3). Furthermore, direct renal tubular protective effects of S1P1R activation are mediated by activation of the Akt and ERK pathways (4). Therefore, both SK1→S1P1R and IL-11→IL-11R→gp130 pathways can activate cytoprotective ERK and Akt signal transduction.
Our findings implicate an important role for HIF-1α in mediating the induction of SK1 after IL-11 treatment. HIF-1 is a heterodimeric transcription factor composed of an α- and a β-subunit (45, 47). Under normoxic conditions, prolyl hydroxylation and ubiquitination of the oxygen-dependent degradation domain of HIF-1α results in rapid HIF-1α degradation. With hypoxia or ischemia, HIF-1α stabilizes and interacts with HIF-1β, forming the HIF-1 heterodimer. Nuclear HIF-1 translocation allows binding to the hypoxia-responsive element with subsequent induction of several cytoprotective genes. Consistent with this proposed pathway, previous studies have demonstrated that HIF-1α activation protects against renal IR injury (47).
We showed that HR IL-11 or PEGylated IL-11 caused increased HIF-1α nuclear translocation and SK1 induction and enhanced SK1 activity in HK-2 cells. We propose that IL-11 receptor activation causes binding of HIF-1α to hypoxia-response elements of the SK1 promoter, leading to increases in SK1 protein synthesis and activity. Consistent with this hypothesis, we showed that selective HIF-1α blockers (2-ME or YC-1) prevented IL-11-mediated induction of SK1 in HK-2 cells. 2-ME is a natural metabolite of estrogen that is known to inhibit HIF-1α at the level of translation (38). YC-1 is a selective inhibitor of HIF-1α transcriptional activity (64).
In summary, our finding that IL-11-mediated SK1 induction leads to reduced renal injury represents a novel and a new direction in IL-11 therapy, in addition to its efficacy in attenuating chemotherapy-induced thrombocytopenia. Our studies may lead to new therapeutic approaches with a drug that can reduce all three pathways of renal cell death (necrosis, apoptosis, and inflammation) to lessen the clinical perils from AKI and have implications in organ protection strategies beyond the kidney.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 GM-06708 (to H. T. Lee) and R43 AI-088928 (to G. N. Cox).
DISCLOSURES
No conflict of interest exists for each author. G. N. Cox and L. J. Anderson are employees of Bolder BioTechnology, Inc., and have a financial interest in the company.
AUTHOR CONTRIBUTIONS
H.T.L., S.W.P., and G.N.C. conception and design of research; H.T.L., S.W.P., M.K., A.H., V.D.D., and G.N.C. analyzed data; H.T.L., S.W.P., M.K., A.H., K.M.B., and G.N.C. interpreted results of experiments; H.T.L., S.W.P., and M.K. prepared figures; H.T.L. and S.W.P. drafted manuscript; H.T.L., S.W.P., M.K., and G.N.C. edited and revised manuscript; H.T.L., S.W.P., M.K., A.H., L.J.A., K.M.B., V.D.D., and G.N.C. approved final version of manuscript; S.W.P., M.K., A.H., L.J.A., K.M.B., V.D.D., and G.N.C. performed experiments.
ACKNOWLEDGMENTS
We thank R. L. Proia for providing the SK1−/− and SK2−/− mice.
REFERENCES
- 1.Allende ML, Dreier JL, Mandela S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102: 3665–3667, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bozza M, Bliss JL, Maylor R, Erickson J, Donnelly L, Bouchard P, Dorner AJ, Trepicchio WL. Interleukin-11 reduces T-cell-dependent experimental liver injury in mice. Hepatology 30: 1441–1447, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat 73: 141–150, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cox GN, Rosendahl MS, Chlipala EA, Smith DJ, Carlson SJ, Doherty DH. A long-acting, mono-PEGylated human growth hormone analog is a potent stimulator of weight gain and bone growth in hypophysectomized rats. Endocrinology 148: 1590–1597, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood 89: 3897–3908, 1997 [PubMed] [Google Scholar]
- 10.Faubel S. Acute kidney injury and multiple organ dysfunction syndrome. Minerva Urol Nefrol 61: 171–188, 2009 [PubMed] [Google Scholar]
- 11.Fujio Y, Maeda M, Mohri T, Obana M, Iwakura T, Hayama A, Yamashita T, Nakayama H, Azuma J. Glycoprotein 130 cytokine signal as a therapeutic target against cardiovascular diseases. J Pharmacol Sci 117: 213–222, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Goldman SC, Bracho F, Davenport V, Slack R, Areman E, Shen V, Lenarsky C, Weinthal J, Hughes R, Cairo MS. Feasibility study of IL-11 and granulocyte colony-stimulating factor after myelosuppressive chemotherapy to mobilize peripheral blood stem cells from heavily pretreated patients. J Pediatr Hematol Oncol 23: 300–305, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Grosfeld JL, Du X, Williams DA. Interleukin-11: its biology and prospects for clinical use. JPEN J Parenter Enteral Nutr 23: S67–S69, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hoste EA, Kellum JA. Incidence, classification, and outcomes of acute kidney injury. Contrib Nephrol 156: 32–38, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hsu HK, Juan SH, Ho PY, Liang YC, Lin CH, Teng CM, Lee WS. YC-1 inhibits proliferation of human vascular endothelial cells through a cyclic GMP-independent pathway. Biochem Pharmacol 66: 263–271, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ikeda M, Prachasilchai W, Burne-Taney MJ, Rabb H, Yokota-Ikeda N. Ischemic acute tubular necrosis models and drug discovery: a focus on cellular inflammation. Drug Discov Today 11: 364–370, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Jablonski P, Howden BO, Rae DA, Birrel CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35: 198–204, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, Lynch KR, Okusa MD. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int 75: 167–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol 2: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol 22: 193–208, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Chen SW, Park SW, Kim M, D'Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Kim M, Kim N, D'Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol 293: F1827–F1835, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Kim M, Park SW, Pitson SM, Lee HT. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, D'Agati VD, Lee HT. Selective renal overexpression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 299: F347–F358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, Yamamoto Y, Mohri T, Kishimoto T, Kawase I, Fujio Y, Azuma J. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine 38: 107–115, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Klawitter S, Hofmann LP, Pfeilschifter J, Huwiler A. Extracellular nucleotides induce migration of renal mesangial cells by upregulating sphingosine kinase-1 expression and activity. Br J Pharmacol 150: 271–280, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuenzler KA, Pearson PY, Schwartz MZ. IL-11 pretreatment reduces cell death after intestinal ischemia-reperfusion. J Surg Res 108: 268–272, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Lai PC, Cook HT, Smith J, Keith JC, Jr, Pusey CD, Tam FW. Interleukin-11 attenuates nephrotoxic nephritis in Wistar Kyoto rats. J Am Soc Nephrol 12: 2310–2320, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life 58: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A1 and A2a adenosine receptor activation. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol 71: 493–511, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3: 363–375, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat 6: 355–361, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Naugler KM, Baer KA, Ropeleski MJ. Interleukin-11 antagonizes Fas ligand-mediated apoptosis in IEC-18 intestinal epithelial crypt cells: role of MEK and Akt-dependent signaling. Am J Physiol Gastrointest Liver Physiol 294: G728–G737, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Obana M, Maeda M, Takeda K, Hayama A, Mohri T, Yamashita T, Nakaoka Y, Komuro I, Takeda K, Matsumiya G, Azuma J, Fujio Y. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation 121: 684–691, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Park SW, Kim M, Chen SW, Brown KM, D'Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest 90: 1209–1224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitman MR, Pham DH, Pitson SM. Isoform-selective assays for sphingosine kinase activity. Methods Mol Biol 874: 21–31, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Reynolds CH. Clinical efficacy of rhIL-11. Oncology (Williston Park) 14: 32–40, 2000 [PubMed] [Google Scholar]
- 44.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology 124: 1358–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt KU, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant 23: 3472–3478, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Ruff MR, Gifford GE. Rabbit tumor necrosis factor: mechanism of action. Infect Immun 31: 380–385, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schodel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt KU, Willam C. HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury. Am J Pathol 174: 1663–1674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwalm S, Doll F, Romer I, Bubnova S, Pfeilschifter J, Huwiler A. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun 368: 1020–1025, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Sheridan BC, Dinarello CA, Meldrum DR, Fullerton DA, Selzman CH, McIntyre RC., Jr Interleukin-11 attenuates pulmonary inflammation and vasomotor dysfunction in endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 277: L861–L867, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 17: 381–387, 1965 [DOI] [PubMed] [Google Scholar]
- 51.Smith JW. Tolerability and side-effect profile of rhIL-11. Oncology (Williston Park) 14: 41–47, 2000 [PubMed] [Google Scholar]
- 52.Takagi A, Yamashita N, Yoshioka T, Takaishi Y, Sano K, Yamaguchi H, Maeda A, Saito K, Takakura Y, Hashida M. Enhanced pharmacological activity of recombinant human interleukin-11 (rhIL11) by chemical modification with polyethylene glycol. J Control Release 119: 271–278, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol 157: 3627–3634, 1996 [PubMed] [Google Scholar]
- 54.Trepicchio WL, Dorner AJ. The therapeutic utility of Interleukin-11 in the treatment of inflammatory disease. Expert Opin Investig Drugs 7: 1501–1504, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Trepicchio WL, Wang L, Bozza M, Dorner AJ. IL-11 regulates macrophage effector function through the inhibition of nuclear factor-kappaB. J Immunol 159: 5661–5670, 1997 [PubMed] [Google Scholar]
- 56.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J 397: 461–471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Anal Biochem 337: 136–142, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit 12: BR318–BR324, 2006 [PubMed] [Google Scholar]
- 59.Volpi G, Facchinetti F, Moretto N, Civelli M, Patacchini R. Cigarette smoke and alpha,beta-unsaturated aldehydes elicit VEGF release through the p38 MAPK pathway in human airway smooth muscle cells and lung fibroblasts. Br J Pharmacol 163: 649–661, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, Sammani S, Garcia JG, Natarajan V. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol 296: L603–L613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, Elias JA. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol 29: 513–522, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Wright SC, Kumar P, Tam AW, Shen N, Varma M, Larrick JW. Apoptosis and DNA fragmentation precede TNF-induced cytolysis in U937 cells. J Cell Biochem 48: 344–355, 1992 [DOI] [PubMed] [Google Scholar]
- 63.Wu Y, Dong Y, Song P, Zou MH. Activation of the AMP-activated protein kinase (AMPK) by nitrated lipids in endothelial cells. PLoS One 7: e31056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst 95: 516–525, 2003 [DOI] [PubMed] [Google Scholar]