Abstract
Vascular endothelial growth factor (VEGF)-A, a family of differentially spliced proteins produced by glomerular podocytes, maintains glomerular filtration barrier function. The expression of VEGF molecules is altered in human nephropathy. We aimed to determine the roles of the angiogenic VEGF164 isoform, and the antiangiogenic VEGF165b isoform in mature, adult glomeruli in vivo using conditional, inducible transgenic overexpression systems in mice. Podocyte-specific VEGF164 overexpression (up to 100 days) was induced by oral administration of doxycycline to adult podocin-rtTA/TetO-VEGF164 double transgenic mice. The consequences of simultaneous overexpression of VEGF164 and VEGF165b were assessed in triple-transgenic podocin-rtTA/TetO-VEGF164/nephrin-VEGF165b mice. Persistent VEGF164 overexpression did not cause proteinuria but did increase glomerular ultrafiltration coefficient between days 3 and 7. Despite persistently increased VEGF164 levels, glomerular ultrafiltration coefficient normalized by day 14 and remained normal up to 100 days. Decreased subpodocyte space (SPS) coverage of the glomerular capillary wall accompanied increased glomerular hydraulic conductivity in VEGF164-overexpressing mice. The changes in glomerular ultrafiltration coefficient and SPS coverage induced by 7 days of overexpression of VEGF164 were not present in triple transgenic VEGF164 and VEGF165b overexpressing mice. These results indicate that 1) the adult mouse glomerulus is relatively resistant to induced VEGF164 overexpression. VEGF164 overexpression altered glomerular permeability but did not cause proteinuria in these mature, adult animals; 2) the SPS is a dynamic VEGF-responsive modulator of glomerular function; and 3) the balance of VEGF isoforms plays a critical role in the regulation of glomerular permeability. VEGF165b is capable of preventing VEGF164-induced changes in glomerular permeability and ultrastructure in vivo.
Keywords: glomerulus, permeability, VEGF, proteinuria, podocyte
proteinuric kidney disease is the leading cause of end-stage renal failure in the Western world (e.g. Ref. 1). Dysfunction in any of the layers of the glomerular filtration barrier (GFB) may result in proteinuria (13), as genetic and acquired loss of a number of slit diaphragm and slit diaphragm-associated proteins in podocytes (17, 37), glomerular basement membrane components (3), and endothelial cell pathology (28) including loss of endothelial glycocalyx (15), all result in proteinuric kidney disease. Defects in individual layers of the barrier can cause proteinuria, but the multiple layers of the barrier act in concert to generate low resistance to water and high exclusion of albumin (7, 13). The sieving characteristics of the barrier are also dependent on dynamic paracrine-induced interactions between cellular components of the GFB, such as those elicited by the vascular endothelial growth factor (VEGF) axis (8).
The vegf-a gene encodes a family of proteins (termed VEGFA, or VEGF), highly expressed by podocytes, that act on cognate tyrosine kinase receptors expressed by both podocytes and endothelial cells (8). VEGF is encoded by eight exons, variably incorporated into the final molecule by alternative splicing resulting in a series of molecules of different amino acid number (from 121 to 206 in humans; Ref. 14) and functional properties (e.g., heparin binding; Ref. 16). In 2002, Bates et al. (4) described two distinct reading frames within exon 8 of the human vegf-a gene, enabling alternative splicing and resulting in different amino acid sequences in the final protein. As the encoded amino acid sequences are the same length, the mature proteins can be distinguished by composition but not by length. Proximal splice site selection in the terminal exon results in propermeability, proangiogenic isoforms of VEGF, termed VEGFxxx (e.g., human VEGF165 and the murine equivalent VEGF164). Distal splice site selection results in a second family of isoforms, termed VEGFxxxb, with contrasting functions. VEGF165b, for example, inhibits angiogenesis (14) and does not cause the chronic increase in systemic microvessel permeability typical of VEGF165 (12). These isoform families appear to be expressed in approximately equal amounts in the normal adult human kidney (5).
Genetic depletion of all VEGF isoforms from mouse glomeruli causes proteinuria (9, 10). In humans, a significant percentage (21–62%) of patients receiving anti-VEGF monoclonal antibody therapy licensed for use in cancer exhibit low-grade proteinuria, ∼2% of patients develop frank proteinuria, and the risk of developing nephrotic syndrome is increased sevenfold (38, 40).
The effect of overexpression of VEGF isoforms is more complex. Excessive glomerular VEGF levels have been reported in adult human proteinuric glomerulopathies (30), but reduced VEGF levels in the same diseases have also been reported (e.g., Ref. 2), and VEGF may play different roles in early and late nephropathy. Transgenic overexpression of VEGF164 in podocytes during glomerular development causes glomerulopathy and proteinuria, with greater degrees of overexpression resulting in more severe glomerular pathology (10). The consequences of VEGF overexpression diminish with increasing maturation of the animal: overexpression during embryonic development results in congenital nephrotic syndrome (35), overexpression at birth results in a modest minimal change disease-like phenotype (33, 35), and overexpression in more mature glomeruli (2–3 mo of age) induces proteinuria (36), which abates if VEGF164 levels return to normal.
In addition, the consequences of glomerular VEGF overexpression are isoform specific (24). VEGF165b-overexpressing animals are healthy to 18 mo of age, with a normal glomerular filtration rate, normal levels of urinary protein excretion, and normal histology by light microscopy, but with a reduction in glomerular permeability to water (normalized ultrafiltration coefficient), associated with decreased glomerular endothelial fenestral density (24). These findings raise the possibility that VEGF165b could ameliorate VEGF164 overexpression-induced proteinuria.
We therefore sought to determine whether VEGF164 overexpression in mature, adult animals causes robust glomerular disease and whether VEGF165b overexpression could ameliorate VEGF164 overexpression-induced proteinuria.
METHODS
All chemical were purchased from Sigma-Aldrich (Dorset, UK) unless otherwise stated.
Generation of Transgenic Animals
All experiments were performed in accordance with UK Home Office Legislation, with Local Ethical Committee approval. podocin-rtTA and tetO-VEGF164 mice (both mixed strain background) were supplied by Susan Quaggin with permission from Jeffrey Kopp (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) and Jeff Whitsett (Children's Hospital Medical Center, Cincinnatti, OH). Crossing these two strains resulted in double-transgenic mice (podocin-rtTA x tetO-VEGF164: pod/tet-VEGF164), as well as littermate controls bearing either none or one of these transgenes. Murine VEGF164 overexpression was induced in these mice by administration of doxycyline in light-protected drinking water at a concentration of 2 mg/ml in a solution of 5% (wt/vol) sucrose that was changed twice per week. nephrin-VEGF165b mice (neph-VEGF165b; C57Bl6 strain background) were bred in-house; these mice constitutively overexpress human VEGF165b during development and adulthood (24). Triple-transgenic podocin-rtTA x tetO-VEGF164 x nephrin-VEGF165b mice (pod/tet-VEGF164/neph-VEGF165b) were obtained by crossing pod/tet-VEGF164 and neph-VEGF165b mice. All animals in the study [26 pod/tet-VEGF164 mice; 27 littermate control mice; 6 neph-VEGF165b mice (3 neph-VEGF165b, 3 pod/tet-VEGF164/neph-VEGF165b)] were between 8 and 22 wk old.
Animal Genotyping
Genomic DNA was isolated from tail tips using Qiagen DNeasy isolation kit. The following pairs of primers were used for PCR: for the podocin-rtTA transgene, forward CGCACTTCAGTTACTTCAGGTCCTC and reverse GCTTATGCCTGATGTTGATGC; for tetO-VEGF164 transgene forward TGGATCCATGAACTTTCTGCT and reverse AATTCACCGCCTCGGCTTCTC; and for nephrin-VEGF165b forward TCAGCGCAGCTACTGCCATC and reverse GTGCTGGCCTTGGTGAGGTT.
Biochemistry
Mice were housed in metabolic cages (Tecniplast UK) for spot urine collection with food and water provided ad libitum. Urine was collected, centrifuged, and stored in 0.2-ml aliquots at −20°C. Total urinary protein was determined spectrophotometrically using pyrogallol red molybdenum complex (Thermo Fisher Scientific). Urine creatinine was measured using an enzymatic spectrophotometric assay (Konelab T-Series 981845; Thermo Fisher Scientific). Both assays were carried out by a fully automated chemistry analyzer (Konelab 30i; Thermo Fisher Scientific). Urine albumin was determined with a mouse-specific albumin ELISA (Bethyl Laboratories).
VEGF Expression
Kidney cortex was harvested immediately postmortem in euthanized mice. Fresh cortical tissue was lysed in RIPA buffer, and the levels of VEGF were determined in the supernatant by ELISA using a mouse VEGF specific kit (R&D Systems). VEGF levels were normalized on total protein concentration as determined by Bradford assay.
VEGF Receptor 2 Expression
The same kidney cortical tissue extracts used for VEGF expression ELISA assays was used for immunoblotting against VEGF receptor 2 (VEGFR2). Thirty micrograms of total protein for each sample were boiled for 5 min, separated by SDS/PAGE (7.5%) gel, and transferred to PVDF membranes. The membranes were blocked and probed overnight (4°C) using rabbit anti-VEGFR2 antibody (Cell Signaling cat. no. 55B11; dilution of 1:1,000). Further on the membranes were incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (dilution of 1:7,000), and bands were visualized using Super Signal West Femto Maximum Sensitivity Substrate (Pierce). The membranes were subsequently stripped and reprobed for α-tubulin. ImageJ was used to quantify and normalize VEGFR2 bands to α-tubulin.
Glomerular Morphology
Light microscopy.
Kidney cortex samples were flash frozen, and 5- to 10-μm sections were cut using a rotary microtome. Sections were mounted onto glass slides and stained with hematoxylin and eosin using standard techniques. Images were captured using a DCN-100 digital imaging system (Nikon Instruments, Surrey, UK).
Electron microscopy.
Small pieces (0.5- to 1-mm diameter) of kidney cortex were rapidly excised and fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (4°C), washed in 0.1 M cacodylate buffer, postfixed in 1% osmium tetroxide in cacodylate buffer, and washed in distilled water. Tissues were dehydrated with ethanol, infiltrated, and embedded in an Araldite resin mix (Agar Scientific). Survey sections (500-nm thick) were cut from each kidney and stained with toluidine blue [1% in 1% borax(aqueous)] for light microscopy. Glomeruli were identified before trimming the cut surface to include one to three glomeruli (clustered) in a smaller block face suitable for ultrathin serial sectioning. Then, 50- to 100-nm thick sections were stained with 3% (aqueous) uranyl acetate and Reynolds' lead citrate solution (25). Digital micrographs were taken on a Phillips 100CS microscope at ×1200, ×2,900, and ×6,800.
Ultrastructural analysis.
Detailed ultrastructural analysis of the glomerular filtration barrier after 7 days doxycycline exposure was undertaken along the walls of 21 glomerular capillaries (pod/tet-VEGF164 mice), 13 glomerular capillaries (control littermate mice), and 17 glomerular capillaries (pod/tet-VEGF164/neph-VEGF165b mice). Measurements were taken at random points, selected as points of intersection between a superimposed grid and the glomerular capillary wall (GCW) electron microscopy image, using Adobe Photoshop. Measurements of the subpodocyte space (SPS) were performed as previously described (20, 21). The fractional area and density of glomerular capillaries was determined according to the methods of Pagtalunan et al. (23). In brief, glomerular area (ĀG) was determined from the outline of glomeruli in toluidine blue images (n = 5 per group) traced in Adobe Photoshop, from which glomerular volume (V̄G) was calculated as
| (1) |
where β = 1.38 (the shape coefficient for spheres: the idealized shape for glomeruli) and k = 1.1 is a size distribution coefficient (23, 31).
A calibrated point-and-line lattice was superimposed on the glomerular image, and glomerular capillary surface density (SV) was calculated as
| (2) |
where ΣIPCW is the number of intersections between lattice lines and the GCW, and ΣIpolygon is the number of lattice points within the glomerular outline. Capillary surface area per glomerulus was calculated as the product of V̄G and SV.
Glomerular Water Permeability
Glomeruli were harvested from fresh cortical renal tissue pooled from three to five mice from each group using a standard sieving technique and the glomerular ultrafiltration coefficient (LPA: hydraulic conductivity * area product) measured as previously described (26). In brief, individual glomeruli that were macroscopically intact and free of Bowman's capsule were mounted on a 5-μm radius aperture holding pipette within a flow-controlled observation chamber by gentle aspiration onto <3% of the glomerular surface. The perifusate surrounding the glomerulus was rapidly exchanged from incubating solution (1 g/dl BSA in HEPES Ringer) to 8 g/dl BSA-HEPES Ringer, thereby increasing the oncotic pressure outside the GCW relative to the glomerular capillary lumen. The consequent flux of water out of the glomerular capillaries results in a rapid reduction in glomerular volume, which was recorded continuously on video-tape and analyzed off-line. Linear regression analysis was used to calculate the initial rate of reduction of glomerular volume (JV) occurring in the first 0.1 s after perifusate exchange (i.e., before longtitudinal movement of erythrocytes along capillaries; Ref. 26) in response to the applied oncotic pressure gradient (ΔΠ), from which glomerular ultrafiltration coefficient (LPA) was calculated from the relation
| (3) |
assuming that the reflection coefficient to albumin is not significantly different from 1 (26, 29).
Podocyte-specific overexpression of VEGF has previously been shown to increase glomerular volume (36). Therefore to eliminate potential confounding effects of differences in glomerular size between groups, LPA was normalized to initial glomerular volume (Vi: LPA/Vi; “normalized” ultrafiltration coefficient; Ref. 26). In addition, the hydraulic conductivity of the GCW (LP) was calculated as the quotient of LPA and the mean capillary surface area per glomerulus determined from histological sections.
Statistics
Values are expressed as means ± SE. Fold change in each parameter is calculated as the value in doxycycline-treated pod/tet-VEGF164 mice, divided by mean values in littermate controls treated with doxycycline for the same period (between 1 and 100 days, as specified) and were compared with two-tailed Wilcoxon's signed rank test vs. no fold change (i.e., 1). Unpaired Student's t-tests were used for statistical analysis of experiments involving two groups at individual time points. For three or more groups, data were analyzed with one-way ANOVA. Correlations were assessed with Pearson's correlation coefficient.
RESULTS
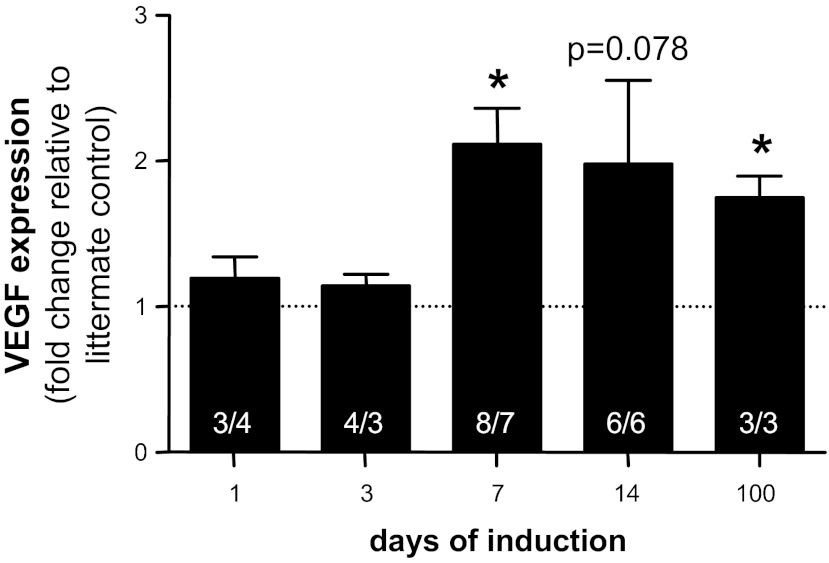
Adult double transgenic pod/tet-VEGF164 mice and control littermates received doxycycline for up to 3.5 mo. Murine VEGF levels in renal cortical tissue did not change over 100 days in doxycycline-treated control littermate mice [means ± SE; 0.79 ± 0.12 ng VEGF/mg total protein; range 1.1 ± 0.25 (7 days) to 0.53 ± 0.06 (100 days); Pearson r2 = 0.22; P > 0.3 for all time points, one-way ANOVA, Bonferroni]. In double transgenic pod/tet-VEGF164 mice treated with doxycycline, VEGF levels rose 2.1-fold (P < 0.05) compared with doxycycline-treated control littermates after 7 days (control: 1.1 ng VEGF/mg total protein; pod/tet-VEGF164: 2.3 ng VEGF/mg total protein) and remained similarly elevated after 14 days (2.0-fold; P = 0.078; control: 1.0 ng VEGF/mg total protein; pod/tet-VEGF164: 2.0 ng VEGF/mg total protein). At 100 days, VEGF levels remained 1.75-fold higher than control littermates (P < 0.05; control: 0.5 ng VEGF/mg total protein; pod/tet-VEGF164: 0.9 ng VEGF/mg total protein; Fig. 1).
Fig. 1.
Podocyte-specific, doxycycline-inducible VEGF164 overexpression increases VEGF levels in vivo. Doxycycline was added to the drinking water of pod/tet-VEGF164 mice and littermate controls for between 1 and 100 days. VEGF levels were measured by mouse VEGF-specific ELISA, and total protein by Bradford assay, in protein extracted from renal cortex. VEGF expression, determined as ng VEGF per mg total protein, was calculated relative to littermate control receiving doxycycline for the same time period. Values in bars represent numbers of mice in littermate control/pod/tet-VEGF164 groups respectively. *P < 0.05, two-tailed Wilcoxon signed rank test at each time point.
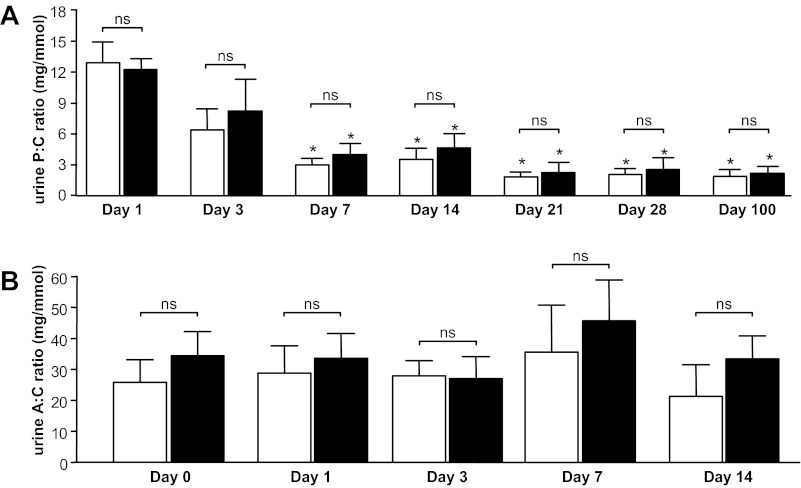
Despite this persistent VEGF164 overexpression in doxycycline-treated pod/tet-VEGF164 mice compared with control mice receiving doxycycline for the same period, there were no statistically significant differences in the urine protein-to-creatinine ratio between pod/tet-VEGF164 mice and control littermates (both doxycycline-treated) at any time point (P > 0.05, one-way ANOVA, Bonferroni, for all time point comparisons; Fig. 2A). However, a progressive reduction in the urine protein-to-creatinine ratio was noted between day 1 and day 100 of doxycycline treatment in both pod/tet-VEGF164 and control groups (both groups: day 1 vs. days 7, 14, 21, 28, and 100: all P < 0.05, one-way ANOVA, Bonferroni; test for linear trend P < 0.005; Fig. 2A). The urine albumin-to-creatinine ratio in pod/tet-VEGF164 mice and control littermates treated with doxycycline for the same period was not statistically significantly different at any of the time points examined (P > 0.05, one-way ANOVA, Bonferroni, for all time point comparisons; Fig. 2B). In addition, there were no statistically significant changes in the urine albumin-to-creatinine ratio over time (including before and after doxycycline administration) in either group of mice (both groups: day 0 vs. days 1, 3, 7, and 14: all P > 0.05, one-way ANOVA, Bonferroni; test for linear trend P > 0.75; Fig. 2B).
Fig. 2.
Podocyte-specific, doxycycline-inducible VEGF164 overexpression does not cause proteinuria or albuminuria in vivo. Protein-to-creatinine (P:C) ratio (A) was determined in spot samples of urine collected from pod/tet-VEGF164 mice (black bars) and littermate controls (open bars) treated with doxycycline for between 1 and 100 days (n = 6–18 mice per time point). albumin-to-creatinine (A:C) ratio (B) was also determined in spot urine samples but from a separate cohort of the same experimental groups (n = 4–10 mice per time point). There were no significant differences in either P:C ratio (A) or A:C ratio (B) between pod/tet-VEGF164 mice and littermate controls treated with doxycycline for the same period (ns: P > 0.05 for all time points, one-way ANOVA, Bonferroni correction for comparison between pairs). However, a significant reduction in P:C ratio with increasing duration of doxycycline treatment in both pod/tet-VEGF164 mice and littermate controls was noted {*: all P < 0.05 vs. day 1 [both wild-type (WT) and pod/tet-VEGF164 (PT) groups], one-way ANOVA, Bonferroni}. A:C ratio did not change with duration of doxycycline treatment for either WT or PT groups [all P > 0.05 vs. day 0 (both WT and PT groups), one-way ANOVA].
In the absence of statistically significant changes in overall urinary protein (and albumin) excretion in response to VEGF164 overexpression, we examined the function of individual glomeruli from VEGF164-overexpressing and control mice by measuring the glomerular ultrafiltration coefficient, normalized for glomerular volume (LPA/Vi; min−1·mmHg−1; Fig. 3). LPA/Vi was not different between pod/tet-VEGF164 and control littermate mice after 1 day of treatment with doxycycline but increased at day 3 in the double transgenic pod/tet-VEGF164 mice compared with littermate controls treated with doxycycline for the same period (P < 0.01), when renal cortical VEGF levels were only slightly increased over baseline levels. LPA/Vi was further increased at day 7 (P < 0.005 vs. doxycycline-treated littermate controls), the time at which VEGF levels were significantly increased. Despite persistent VEGF164 overexpression in doxycycline-treated pod/tet-VEGF164 animals compared with control littermates treated with doxycycline for the same period, changes in LPA/Vi resolved in doxycycline-treated pod/tet-VEGF164 at subsequent time points (14 and 100 days: both P > 0.05, unpaired t-test vs. doxycycline-treated littermate controls). Glomerular LPA/Vi in control mice did not change over the course of 100 days treatment with doxycycline throughout the study (Pearson r2 = 0.69; P > 0.05).
Fig. 3.
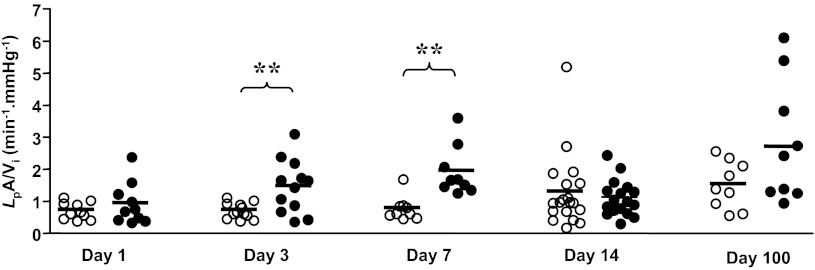
Podocyte-specific, doxycycline-inducible VEGF164 overexpression transiently increases glomerular permeability to water (normalized ultrafiltration coefficient: LpA/Vi) in vivo. Conditional overexpression of murine VEGF164 in kidneys was induced by adding doxycycline in drinking water of double-transgenic pod/tet-VEGF164 mice (●) or littermate controls (○) for 1, 3, 7 14, or 100 days. Kidneys were removed, glomeruli isolated by sieving, and glomerular normalized ultrafiltration coefficient measured by measuring the rate of fluid efflux induced by a known oncotic pressure step. Each symbol represents ultrafiltration coefficient of individual glomeruli, corrected for glomerular volume (LpA/Vi) from 3–5 mice per group. Horizontal bar: mean of group. **P < 0.01, unpaired t-test.
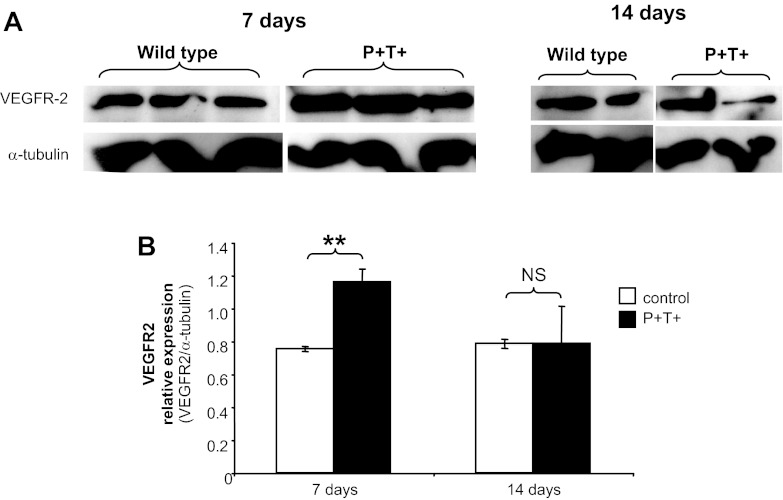
Changes in VEGFR2 expression level accompanied the loss of effect of VEGF164 overexpression after 14 days of doxycycline administration (Fig. 4). Compared with stable levels of α-tubulin in whole kidney cortex during doxycycline administration, VEGFR2 expression increased after 7 days of doxycycline-induced VEGF164 overexpression in pod/tet-VEGF164 mice (P < 0.01 vs. 7-day doxycycline-treated control littermates, unpaired t-test). After 14 days of doxycycline treatment, however, VEGFR2 expression levels in pod/tet-VEGF164 mice were no longer statistically significantly different from those observed in control littermates treated with doxycycline for the same period.
Fig. 4.
Podocyte-specific, doxycycline-inducible VEGF164 overexpression results in a transient upregulation of VEGF receprtor 2 (VEGFR2). Overexpression of VEGF164 in podocytes was induced for 7/14 days by addition of doxycycline in drinking water of double-transgenic pod/tet-VEGF164 mice (P+T+) and control littermates. A: protein extracts were prepared from kidney cortex and Western blot analysis performed with antibodies against VEGFR2 and α-tubulin (for equal loading). B: densitometry analysis shows a significant increase in VEGR2 expression in double-transgenic P+T+ mice compared with control littermates at 7 days of postinduction (P = 0.008, t-test) but not at 14 days (NS).
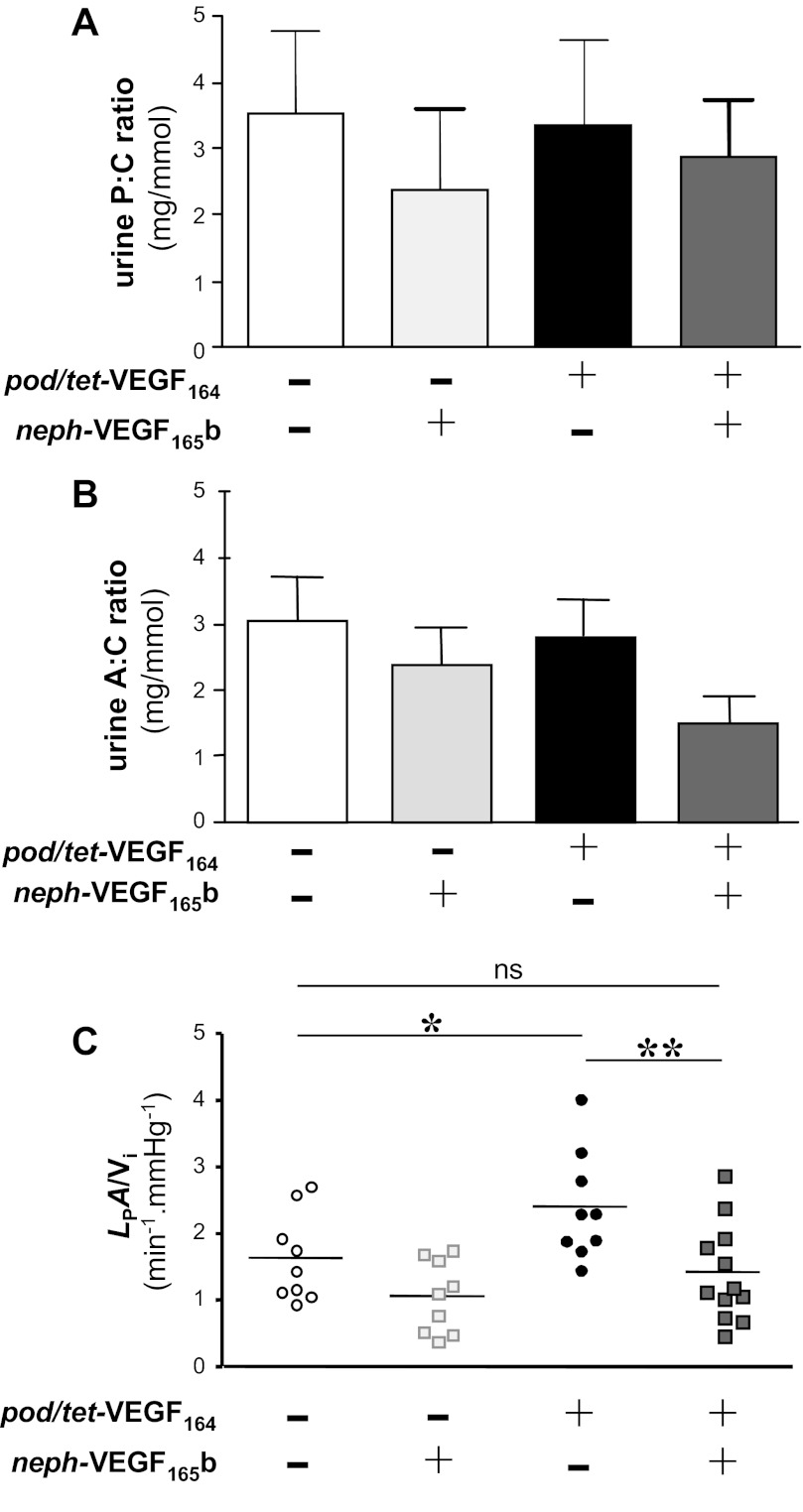
We have previously shown that VEGF165b reduces glomerular permeability to water (24). Therefore, to determine whether VEGF165b could ameliorate this VEGF164 overexpression-induced transient increase in glomerular LPA/Vi on day 7, we generated triple transgenic pod/tet-VEGF164/neph-VEGF165b mice that constitutively overexpress human VEGF165b in podocytes and overexpress murine VEGF164 upon administration of doxycycline [pod/tet-VEGF164/neph-VEGF165b (P+T+N+)], alongside appropriate control groups [constitutive VEGF165b overexpression alone: neph-VEGF165b (P−T−N+); inducible VEGF164 overexpression alone: pod/tet-VEGF164 (P+T+N−); littermate controls (P−T−N−)]. Consistent with previous results, VEGF165b-overexpressing single transgenic neph-VEGF165b mice (P−T−N+), compared with littermate controls (P−T−N−), were healthy without proteinuria (P > 0.05, one-way ANOVA; Fig. 5A) or albuminuria (P > 0.05, one-way ANOVA; Fig. 5B), with no statistically significant changes in total murine VEGF levels (P−T−N− 0.8 ± 0.2; P−T−N+ 0.6 ± 0.1; P > 0.05, one-way ANOVA) and with reduced glomerular LPA/Vi (P < 0.06, one-way ANOVA; Fig. 5C).
Fig. 5.
Human VEGF165b compensates for the increase in glomerular permeability to water observed in response to overexpression of murine VEGF164 in vivo. Conditional overexpression of murine VEGF164 in podocytes was induced for 7 days in murine VEGF164-overexpressing mice (pod/tet-VEGF164: black bar/circles) and triple-transgenic mice with conditional murine VEGF164 and constitutive human VEGF165b expression (pod/tet-VEGF164 x neph-VEGF165b: dark grey-filled bar/squares). Control littermate mice (open bar/circles) and human VEGF165b overexpressing mice (neph-VEGF165b: light grey-filled bar/squares) also received doxycycline (n = 3 mice per group). There was no significant difference in P:C ratio (A) or A:C ratio among any of the 4 groups (P > 0.05, one-way ANOVA). C: combined overexpression of murine VEGF164 and human VEGF165b in pod/tet-VEGF164 X neph-VEGF165b mice (dark grey squares) resulted in a significant decrease in LpA/Vi compared with pod/tet-VEGF164 mice overexpressing just VEGF164 (black circles; *P < 0.05, one-way ANOVA). There was a significant increase in LpA/Vi in pod/tet-VEGF164 mice (black circles) compared with controls (open circles; *P < 0.05, one-way ANOVA). Combined overexpression of murine VEGF164 and human VEGF165b (dark grey squares) restored LpA/Vi to values similar to those observed in control mice (open circles; ns: P > 0.05, one-way ANOVA).
Consistent with findings in double-transgenic mice (Fig. 2), 7 days of doxycycline administration did not cause proteinuria (Fig. 5A) or albuminuria (Fig. 5B) in any of the four groups of mice (all P > 0.05, one-way ANOVA). As with double-transgenic pod/tet-VEGF164 mice after 7 days induction of VEGF164 overexpression with doxycycline, P+T+N− mice with inducible VEGF164 overexpression had approximately twofold elevations in both murine VEGF levels and glomerular LPA/Vi in response to 7 days of treatment with doxycycline, compared with doxcycline-treated control littermate mice (P−T−N−) in which VEGF164 is not induced (P < 0.05, one-way ANOVA; Fig. 5C). Murine VEGF levels were similarly elevated 1.9-fold in doxycycline-treated triple-transgenic pod/tet-VEGF164/neph-VEGF165b mice (P+T+N+) overexpressing both VEGF164 and human VEGF165b, but the VEGF164-induced increase in LPA/Vi (P+T+N− mice) was blocked in these P+T+N+ mice overexpressing both VEGF164 and VEGF165b (P < 0.01, one-way ANOVA; Fig. 5C). LPA/Vi in these P+T+N+ mice was comparable to that in control littermates (P > 0.05, one-way ANOVA, Fig. 5C).
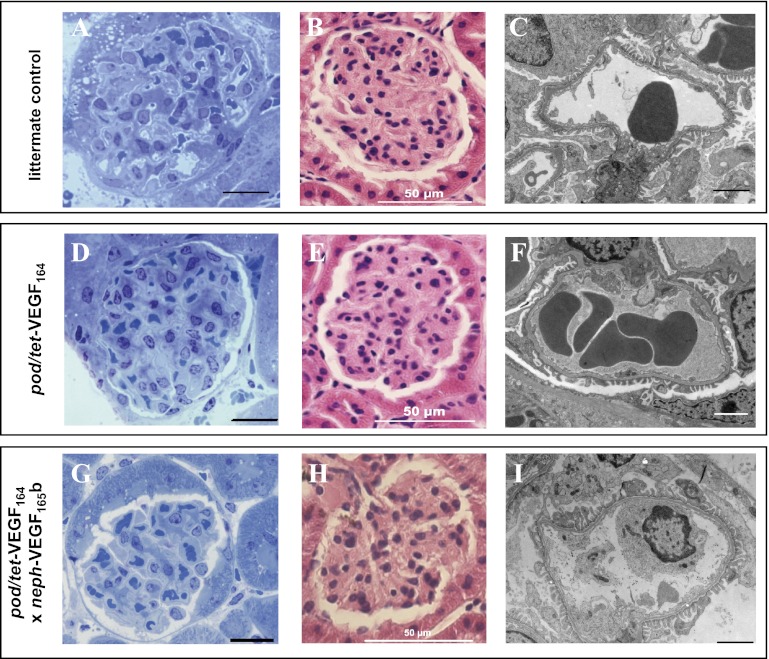
In pod/tet-VEGF164 mice treated with doxycycline for 7 days, i.e., with VEGF164 overexpression and increased glomerular LPA/Vi, as well as in triple-transgenic pod/tet-VEGF164/neph-VEGF165b mice overexpressing VEGF164 and VEGF165b, no gross abnormalities in glomerular morphology were apparent at the light or low-power electron microscopy level (Fig. 6). Of note, no differences in total glomerular volume, the fraction of glomeruli comprised of capillary, or total capillary surface area per glomerulus were discerned between VEGF164-overexpressing pod/tet-VEGF164 mice, triple-transgenic pod/tet-VEGF164/neph-VEGF165b mice, and littermate controls after 7 days of induction with doxycycline (Table 1). The absence of a significant change in glomerular volume or capillary area in VEGF164-overexpressing pod/tet-VEGF164 mice suggested that the observed increase in glomerular LPA/Vi might be attributable to a change in the hydraulic conductivity of the GCW (LP), rather than a change in surface area available for exchange. Estimates of GCW LP, derived as the quotient of LPA and the mean capillary surface area per glomerulus determined from histological sections, indicated that GCW LP was significantly elevated in VEGF164-overexpressing pod/tet-VEGF164 mice treated with doxycyline for 7 days, compared with littermate controls and triple-transgenic pod/tet-VEGF164/neph-VEGF165b mice overexpressing both VEGF164 and VEGF165b (both P < 0.05; Table 1).
Fig. 6.
Podocyte-specific overexpression of VEGF164 for 7 days and dual overexpression of VEGF164 and VEGF165b do not alter gross glomerular morphology. Control mice (A, B, and C), double-transgenic pod/tet-VEGF164 mice (D, E, and F), and triple-transgenic pod/tet-VEGF164/neph-VEGF165b mice (that constitutively overexpress VEGF165b in podocytes) (G, H, and I) received doxycycline for 7 days, to induce conditional overexpression of murine VEGF164 in podocytes. No differences in overall glomerular morphology were apparent in toluidine blue-stained kidney sections (A, D, and G; scale bar: 20 μm), hematoxylin and eosin-stained 5-μm paraffin-embedded kidney sections (B, E, and H; scale bar: 50 μm), or low-magnification electron micrographs (C, F, and I; scale bar: 2 μm).
Table 1.
Glomerular structure and function in VEGF164-overexpressing pod/tet-VEGF164 mice and control littermates after 7 days of doxycycline
| Littermate Control | pod/tet-VEGF164 | pod/tet-VEGF164 x neph-VEGF165b | |
|---|---|---|---|
| Glomerular structure | |||
| Glomerular volume, nl | 1.15 ± 0.21 (9) | 1.03 ± 0.16NS (9) | 0.74 ± 0.11NS (11) |
| Glomerular capillary surface density | 0.18 ± 0.01 (5) | 0.16 ± 0.01NS (5) | 0.21 ± 0.02NS (5) |
| Capillary surface area per glomerulus, μm2 × 10−4 | 6.55 ± 1.51 (5) | 7.63 ± 0.64NS (5) | 6.18 ± 0.83NS (5) |
| Glomerular function | |||
| LPA, nl·min−1·mmHg−1 | 0.91 ± 0.21 (9) | 1.85 ± 0.25* (9) | 0.96 ± 0.19ns,‡ (11) |
| LPA/Vi, min−1·mmHg−1 | 0.78 ± 0.13 (9) | 1.94 ± 0.26*** (9) | 1.42 ± 0.21ns,‡ (11) |
| LP, μl·min−1·mmHg−1·cm−2 | 1.39 ± 0.33 (9) | 2.43 ± 0.32* (9) | 1.25 ± 0.24ns,‡ (11) |
| Glomerular capillary wall structure | |||
| Interfenestral distance, nm | 745 ± 176 (10) | 449 ± 77ns (14) | 1084 ± 41ns,‡ (17) |
| Glomerular basement membrane depth, nm | 183 ± 14 (13) | 187 ± 10 (18) | 177 ± 7NS (17) |
| Podocyte foot process width, nm | 344 ± 32 (13) | 339 ± 19NS (18) | 343 ± 18NS (17) |
| Subpodocyte space coverage of glomerular capillary wall, % | 65 ± 4 (13) | 43 ± 6* (21) | 68 ± 4ns,‡ (17) |
| Subpodocyte space exit pore width, nm | 47 ± 9 (9) | 69 ± 21NS (8) | 66 ± 4NS (6) |
| Subpodocyte space height, nm | 163 ± 31 (12) | 193 ± 24NS (18) | 157 ± 56NS (6) |
Results are presented as means ± SE, with n numbers in parentheses denoting glomeruli (for whole glomerular measurements) and glomerular capllaries (for capillary wall measurements). LPA, glomerular ultrafiltration coefficient; Vi, initial glomerular volume; LP, hydraulic conductivity of the glomerular capillary wall.
P < 0.05,
P < 0.005 vs. littermate control, one-way ANOVA. NSno significant difference vs. any group, P > 0.05, one-way ANOVA. nsno significant difference vs. littermate control mice, P > 0.05, one-way ANOVA. ‡P < 0.05 vs. pod/tet-VEGF164 mice, one-way ANOVA.
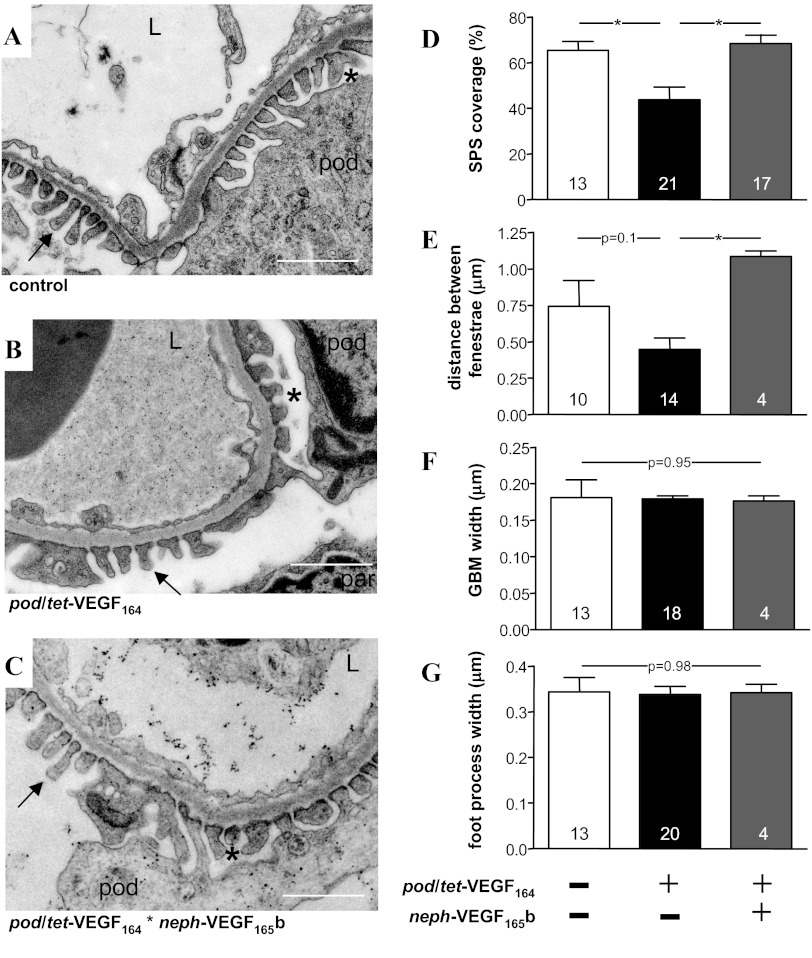
The compartment of the GCW that is modified by VEGF164 remains controversial, with evidence for and against a direct effect of VEGF164 on podocytes in vivo (33, 36). VEGF165b has previously been shown to reduce glomerular endothelial fenestral density (24). We therefore coupled the assessment of glomerular permeability with an analysis of the ultrastructural components of the GCW that determine LP (Table 1) using electron micrographs of glomeruli from control (Fig. 7A), pod/tet-VEGF164 mice (Fig. 7B), and pod/tet-VEGF164/neph-VEGF165b mice (Fig. 7C). We noted a significant reduction in the percentage of the filtering surface of the GCW covered by the subpodocyte space (SPS) in VEGF164-overexpressing pod/tet-VEGF164 mice (P < 0.05; Fig. 7D), an effect that was reversed by co-overexpression of both VEGF164 and VEGF165b (P < 0.05; Fig. 7D). No statistically significant differences in the major determinants of filtration through individual SPS (SPS height and SPS exit pore width) were apparent between the three groups of animals (Table 1). In keeping with previous reports, the distance between glomerular endothelial fenestrae was greater in pod/tet-VEGF164/neph-VEGF165b mice than pod/tet-VEGF164 mice overexpressing VEGF164 alone (P < 0.05; Fig. 7E). Other ultrastructural determinants of GCW hydraulic conductivity (podocyte filtration slit separation: Fig. 7F; glomerular basement membrane width: Fig. 7G) were not significantly different between 7-day doxycycline-treated littermate controls, VEGF164-overexpressing pod/tet-VEGF164 mice, and pod/tet-VEGF164/neph-VEGF165b mice.
Fig. 7.
Decreased subpodocyte space (SPS) coverage accompanies increased glomerular water permeability of VEGF164 overexpressing mice. A and B: High-power electron micrographs of the glomerular capillary wall from control mice (A), double-transgenic pod/tet-VEGF164 mice (B), and triple-transgenic pod/tet-VEGF164/neph-VEGF165b mice (C) treated with doxycycline-supplemented drinking water for 7 days to induce conditional overexpression of murine VEGF164 in podocytes. L, capillary lumen; pod: podocyte; arrow: podocyte foot process in SPS-free region; par: parietal epithelial cell lining Bowman's capsule. *SPS. Scale bars: 1 μm. D: significant reduction in SPS coverage of the glomerular capillary wall in VEGF164-overexpressing mice (black bar), compared with littermate control mice (open bar) and VEGF164/VEGF165b co-overexpressing mice (*P < 0.05, one-way ANOVA). E: distance between fenestrae tended to reduce (P = 0.1, one-way ANOVA) in VEGF164-overexpressing mice but was significantly increased in VEGF164/VEGF165b co-overexpressing mice (*P < 0.05, one-way ANOVA). There were no significant differences between groups in glomerular basement membrane width (F) or in podocyte foot width (G). Numbers in columns represent number of glomerular capillaries examined in each group.
DISCUSSION
We show here that persistent induction of VEGF164 overexpression in podocytes of adult mouse kidneys in vivo did not elicit proteinuria (or albuminuria) at multiple time points over the course of 100 days of VEGF164 overexpression and the consequences of glomerular VEGF164 overexpression could only be discerned by examining renal function at the single glomerular level. VEGF164 overexpression caused a significant elevation in volume-corrected glomerular ultrafiltration coefficient (LPA/Vi) with no change in the overall or capillary volume of the glomerulus, consistent with VEGF164 modifying the hydraulic conductivity (LP) of the GCW. This increase in LP appeared to be mediated by a reduction in the proportion of the GFB covered by the SPS, thereby opening up low-resistance pathways for fluid movement across the GCW. The SPS therefore appears to act as an active and dynamic regulator of glomerular permeability. Having established the consequence of VEGF164 overexpression on glomerular function, we found that VEGF165b prevents the VEGF164-induced changes in glomerular permeability and ultrastructure in vivo.
Modest Phenotype of VEGF164-Overexpressing Adult Mice
To assess whether continuous overexpression of VEGF164 can trigger the development of progressive glomerulopathy in mature, normally developed glomeruli, we investigated VEGF164 overexpression in adult mice. VEGF164 is a critical endothelial cell mitogen and survival factor (5), so overexpression of VEGF164 during glomerulogenesis can result in disordered capillary loop assembly and development of an abnormal GFB structure and has consistently been shown to result in glomerular disease in neonatal mice (33, 35).
It is the mature GFB, however, that represents the situation in adult patients with early renal disease, in whom altered glomerular function has been proposed to be a consequence of abnormal VEGF levels (41). In these mature glomeruli, however, VEGF164 overexpression appears to have more modest consequences. Sison et al. (33) and Veron et al. (36) both reported significant proteinuria in response to glomerular VEGF164 overexpression in weaned or fully-developed mice (33, 36), but the effects of VEGF164 overexpression were variable, with no albuminuria in 25–50% of mice (33, 36, respectively). Ma et al. (18) also reported no albuminuria after 3 wk of doxycyline-induced glomerular VEGF164 overexpression in adult mice (18). In this study, we also found that VEGF164 overexpression did not induce albuminuria and only resulted in modest and transient changes in glomerular water permeability.
Approximately twofold increases in VEGF164 levels (as compared with doxycycline-treated control littermate mice) were observed in response to induced VEGF164 overexpression in all of these studies, as well as the current report. However, total VEGF164 levels were higher after 7 days of doxycycline administration in this study (2.3 ng VEGF/mg total protein) than in previous reports (e.g., 0.2 and 0.128 ng total VEGF/mg total protein; Refs. 18, 36), and therefore it seems unlikely that the absence of robust renal disease in response to VEGF164 overexpression in our study is a consequence of low levels of VEGF164 overexpression. Alternatively, it is possible that differences in mouse strain modify the degree of change in glomerular function induced by supraphysiological levels of VEGF164. Mice in which VEGF164 did not elicit albuminuria [this report and Ma et al. (18)] were generated on the nephropathy-resistant C57Bl6 background, whereas those with the greatest proportion of mice displaying albuminuria were generated on an FVB background (36).
Compensation for VEGF164-Overexpression by the VEGF Axis
We find that 7 days of overexpression of VEGF164 increases VEGFR2 protein expression levels but that VEGFR2 protein levels return to baseline levels after 14 days of VEGF164 overexpression, i.e., at the time point at which glomerular LPA/Vi values are no longer statistically significantly different from doxycyline-treated control littermates. At both time points, absolute levels (2.3 and 2.0 ng VEGF/mg total protein at 7 and 14 days, respectively) and fold increases (2.1- and 2.0-fold higher than doxycycline-treated control littermates at 7 and 14 days, respectively) in VEGF164 levels were similar, although the increase in VEGF164 levels at 14 days did not reach the threshold of statistical significance (P = 0.078). These changes in VEGFR2 expression represent one biologically plausible example of how the VEGF-VEGFR axis may compensate for experimental transgenic overexpression of one component (VEGF164) of the axis. Sison et al. (33) have previously demonstrated that VEGF164 overexpression also increases VEGFR2 phosphorylation and that intact VEGFR2 signaling is required for normal glomerular function. VEGF165 also stimulates increased VEGFR2 levels (as well as phosphorylation) in other cell types (39). The observed transient changes in VEGFR2 levels may therefore also have contributed to the transient changes in glomerular LPA/Vi observed in response to VEGF164 overexpression in this report. However, the overall activity of the entire VEGF axis, which presumably mediates the transient nature of increased glomerular water permeability that follows VEGF164 overexpression, is likely to reflect expression, phosphorylation, dimerization, and downstream signals initiated by the multiple VEGF receptors that exist in multiple cell types of the glomerulus. Overall, we would argue that glomerular changes following overexpression of VEGF164 within the glomerulus reflect a combination of the age of initiating VEGF overexpression, mouse strain, and coincident changes in the levels and activity of other components of the VEGF axis. It is also possible that VEGF164 presents a more potent stimulus for glomerular dysfunction in the presence of additional insults (e.g., diabetic nephropathy; Refs. 19, 34).
Balance of VEGF164 and VEGF165b Isoforms Regulates Glomerular Water Permeability
VEGF165 increases (26), and VEGF165b reduces (24), glomerular normalized ultrafiltration coefficient in isolated intact glomeruli ex vivo; coadministration opposes the consequences of either molecule alone (24); and long-term, podocyte-specific overexpression of VEGF165b has no detectable deleterious consequences on renal function (24). We found that constitutive overexpression of VEGF165b blocked the changes in glomerular water permeability and glomerular ultrastructure that occurred in response to short-term (7 days) overexpression of VEGF164. We have not examined later time points, since we observed no changes in proteinuria, albuminuria, or single glomerular function after longer duration VEGF164 overexpression.
Appropriate total levels of VEGF isoforms are required for maintenance of healthy glomeruli (9). Our results indicate that an appropriate balance of VEGF isoforms in the adult kidney is also an important determinant of glomerular function. Early studies (6) suggested that blocking the activity of the VEGF axis might hold therapeutic potential in some forms of kidney disease, but it is becoming increasingly well illustrated that nonspecific blockade of the VEGF system has deleterious consequences. For example, a number of patients receiving the anti-VEGF antibody bevacizumab, which targets all VEGF isoforms, develop a renal thrombotic microangiopathy that is reproduced by transgenic downregulation of all VEGF isoforms in mouse models (6). Changes in VEGF isoform balance are important in Denys Drash syndrome, a cause of diffuse mesangial sclerosis and renal failure in childhood (32). Whether there are comparable changes in VEGF isoform balance in human renal disease is not yet known, but strategies that selectively return the balance of isoforms, such as modifiers of the SRPK1/ASF/SF2 splicing factor system (22), might offer a new avenue for modifying the VEGF axis for therapeutic advantage.
SPS is a Dynamic, VEGF-Responsive Regulator of Glomerular Water Permeability
The increase in glomerular ultrafiltration coefficient noted after 7 days of VEGF164 overexpression appeared to be mediated via modification of the hydraulic conductivity of the GCW. The ultrastructural correlate of this increase in hydraulic conductivity included a reduction in the coverage of glomerular capillaries by the SPS. The SPS is a restrictive urinary space, bounded on the capillary side by podocyte foot processes and on the urinary side by the plasma membrane of podocyte cell bodies or primary processes (see Fig. 7) (20). Exit foramina from the SPS lead into more peripheral urinary spaces. The narrowness of these SPS “exit pores” (47 ± 9 nm), combined with the tortuosity and length of the filtration path through the SPS, results in a high resistance to fluid (and solute) movement through the SPS. Mathematical modeling (21) and experimental (27) evidence suggest that this resistance is of similar magnitude to that imposed by the glomerular basement membrane. The GCW can therefore be considered heterogeneous: portions of the wall that are covered with SPS have high resistance (i.e., low hydraulic conductivity) and portions without an overlying SPS have lower resistance (i.e., high hydraulic conductivity). In the oncometric assay employed here, reduced SPS coverage provides a greater surface area for the oncotic effect of abluminally applied albumin solutions, eliciting greater fluid flux and therefore an increase in measured LPA. In vivo, reduced SPS coverage translates to an increased proportion of “low resistance” areas, thereby increasing the effective hydraulic conductivity of the glomerulus as a whole.
We observed a significant reduction in the proportion of the GCW covered by SPS in mice overexpressing VEGF164 and also noted that co-overexpression of VEGF164 and VEGF165b restored SPS coverage to a degree comparable to that observed in doxycycline-treated control littermate mice. We have also previously reported that transgenic overexpression of just the VEGF165b isoform alone does not alter SPS coverage (24). Different isoforms of VEGF therefore elicit isoform-specific changes in SPS coverage, as well as isoform-specific changes in glomerular water permeability. The significance of VEGF164 overexpression-induced changes in SPS to the increase in hydraulic conductivity calculated for VEGF164 overexpressing mice can be derived from the relationships given in Neal et al. (21; in particular Eq. 17). A combined increase in SPS height from 163 to 193 nm, in SEP width from 47 to 69 nm and a decrease in SPS area from 65 to 43% would increase glomerular hydraulic conductivity by 65%, accounting for 63% of the observed effect of VEGF164 overexpression on glomerular hydraulic conductivity, with the change in SPS area and exit pore width contributing most. Thus the majority of the increase in permeability can be accounted for by changes in SPS coverage and structure, although increased endothelial fenestral density in VEGF164-overexpressing mice would also be expected to increase hydraulic conductivity of the endothelial layer. In triple-transgenic VEGF164 and VEGF165b overexpressing mice, restoration of SPS height and coverage, coupled with a reduction in endothelial fenestrae, accord with the restoration of GCW hydraulic conductivity to values observed in doxycycline-treated littermate controls. In addition, SPS changes may alter the mechanical influence that the podocyte exerts on the filtration properties of other layers of the glomerular filtration barrier (11), thereby providing an additional mechanism for SPS-induced modification of glomerular permeability.
In summary, we find that long-term increased VEGF164 levels in podocyte-specific, doxycycline-inducible pod/tet-VEGF164 transgenic adult mice do not cause proteinuria but do result in a transient increase in volume-corrected glomerular ultrafiltration coefficient (LPA/Vi). Mouse factors (age and strain) and changes in other components of the VEGF axis in VEGF164-overexpressing mice (e.g., VEGFR2) may contribute to the differences in glomerular function observed at different time points and in different studies after VEGF164 overexpression. A reduction in SPS coverage of the glomerular filtration barrier, which is in keeping with the increase in LPA/Vi, was observed in VEGF164-overexpressing mice. We also show that podocyte-specific VEGF165b overexpression ameliorates the deleterious consequences of VEGF164 upregulation in vivo.
GRANTS
This work was supported by the Medical Research Council Grants GR0600920 (to D. O. Bates, S. J. Harper, and A. H. Salmon) and G0802829 (to A. H. Salmon), British Heart Foundation Grants PG08/022/21636 (to D. O. Bates, S. J. Harper, and A. H. Salmon) and BS/06/005 (to D. O. Bates), and the Richard Bright VEGF Research Trust.
DISCLOSURES
S. J. Harper, D. O. Bates, and A. H. Salmon are inventors on a patent involving VEGF165b.
AUTHOR CONTRIBUTIONS
Author contributions: S.O., C.R.N., S.J.H., D.O.B., and A.H.S. conception and design of research; S.O., C.R.N., A.M., P.P., T.A., C.A., T.L., K.S., and Y.Q. performed experiments; S.O., C.R.N., A.M., P.P., T.A., C.A., T.L., D.O.B., and A.H.S. analyzed data; S.O., C.R.N., A.M., P.P., T.A., C.A., T.L., S.J.H., D.O.B., and A.H.S. interpreted results of experiments; S.O., C.R.N., D.O.B., and A.H.S. prepared figures; S.O., S.J.H., D.O.B., and A.H.S. edited and revised manuscript; S.O., C.R.N., A.M., P.P., T.A., C.A., T.L., K.S., Y.Q., S.J.H., D.O.B., and A.H.S. approved final version of manuscript; A.H.S. drafted manuscript.
REFERENCES
- 1. Ansell D, Feehally J, Fogarty D, Ford D, Hodsman A, Tomson C, Udayaraj U, Warwick G, Williams A. UK Renal Registry 11th Annual Report. Bristol, UK: UK Renal Registry, 2008 [Google Scholar]
- 2. Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA. Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis 43: 636–650, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62: 4123–4131, 2002 [PubMed] [Google Scholar]
- 5. Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, Nishikawa A, Satchell SC, Harper SJ, Gittenberger-de Groot AC, Bates DO. The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol 110: p57–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 12: 993–1000, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–F596, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF–a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol 106: 32–37, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fissell WH, Hofmann CL, Ferrell N, Schnell L, Dubnisheva A, Zydney AL, Yurchenco PD, Roy S. Solute partitioning and filtration by extracellular matrices. Am J Physiol Renal Physiol 297: F1092–F1100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J Physiol 572: 243–257, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8: 880–887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeansson M, Bjorck K, Tenstad O, Haraldsson B. Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20: 114–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katavetin P. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 359: 205–206; author reply 206–207, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Ma J, Matsusaka T, Yang HC, Zhong J, Takagi N, Fogo AB, Kon V, Ichikawa I. Induction of podocyte-derived VEGF ameliorates podocyte injury and subsequent abnormal glomerular development caused by puromycin aminonucleoside. Pediatr Res 70: 83–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol 292: F1665–F1672, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Neal CR, Crook H, Bell E, Harper SJ, Bates DO. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol 16: 1223–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Neal CR, Muston PR, Njegovan D, Verrill R, Harper SJ, Deen WM, Bates DO. Glomerular filtration into the subpodocyte space is highly restricted under physiological perfusion conditions. Am J Physiol Renal Physiol 293: F1787–F1798, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, Bates DO. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem 285: 5532–5540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pagtalunan ME, Rasch R, Rennke HG, Meyer TW. Morphometric analysis of effects of angiotensin II on glomerular structure in rats. Am J Physiol Renal Fluid Electrolyte Physiol 268: F82–F88, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Qiu Y, Ferguson J, Oltean S, Neal CR, Kaura A, Bevan H, Wood E, Sage LM, Lanati S, Nowak DG, Salmon AH, Bates D, Harper SJ. Overexpression of VEGF165b in podocytes reduces glomerular permeability. J Am Soc Nephrol 21: 1498–1509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salmon AH, Neal CR, Bates DO, Harper SJ. Vascular endothelial growth factor increases the ultrafiltration coefficient in isolated intact Wistar rat glomeruli. J Physiol 570: 141–156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salmon AH, Toma I, Sipos A, Muston PR, Harper SJ, Bates DO, Neal CR, Peti-Peterdi J. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am J Physiol Renal Physiol 293: F1777–F1786, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia 51: 714–725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savin VJ, Terreros DA. Filtration in single isolated mammalian glomeruli. Kidney Int 20: 188–197, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Schrijvers BF, Rasch R, Tilton RG, Flyvbjerg A. High protein-induced glomerular hypertrophy is vascular endothelial growth factor-dependent. Kidney Int 61: 1600–1604, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Schumacher VA, Jeruschke S, Eitner F, Becker JU, Pitschke G, Ince Y, Miner JH, Leuschner I, Engers R, Everding AS, Bulla M, Royer-Pokora B. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J Am Soc Nephrol 18: 719–729, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 21: 1691–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, Velazquez H, Kashgarian M, Moeckel GW, Tufro A. Podocyte vascular endothelial growth factor (Vegf) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 54: 1227–1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veron D, Reidy K, Marlier A, Bertuccio C, Villegas G, Jimenez J, Kashgarian M, Tufro A. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am J Pathol 177: 2225–2233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989–999, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 21: 1381–1389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang XH, Man XY, Cai SQ, Yao YG, Bu ZY, Zheng M. Expression of VEGFR-2 on HaCaT cells is regulated by VEGF and plays an active role in mediating VEGF induced effects. Biochem Biophys Res Commun 349: 31–38, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49: 186–193, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract 82, Suppl 1: S38–41, 2008 [DOI] [PubMed] [Google Scholar]