Abstract
The present study aimed to elucidate the reciprocal interactions between oxygen (O2), nitric oxide (NO), and superoxide (O2−) and their effects on vascular and tubular function in the outer medulla. We expanded our region-based model of transport in the rat outer medulla (Edwards A, Layton AT. Am J Physiol Renal Physiol 301: F979–F996, 2011) to incorporate the effects of NO on descending vasa recta (DVR) diameter and blood flow. Our model predicts that the segregation of long DVR in the center of vascular bundles, away from tubular segments, gives rise to large radial NO concentration gradients that in turn result in differential regulation of vasoactivity in short and long DVR. The relative isolation of long DVR shields them from changes in the rate of NaCl reabsorption, and hence from changes in O2 requirements, by medullary thick ascending limbs (mTALs), thereby preserving O2 delivery to the inner medulla. The model also predicts that O2− can sufficiently decrease the bioavailability of NO in the interbundle region to affect the diameter of short DVR, suggesting that the experimentally observed effects of O2− on medullary blood flow may be at least partly mediated by NO. In addition, our results indicate that the tubulovascular cross talk of NO, that is, the diffusion of NO produced by mTAL epithelia toward adjacent DVR, helps to maintain blood flow and O2 supply to the interbundle region even under basal conditions. NO also acts to preserve local O2 availability by inhibiting the rate of active Na+ transport, thereby reducing the O2 requirements of mTALs. The dual regulation by NO of oxygen supply and demand is predicted to significantly attenuate the hypoxic effects of angiotensin II.
Keywords: mathematical model, kidney, oxygen, superoxide, tubulovascular cross talk
nitric oxide (no) and superoxide (O2−) exert opposite effects on renal vascular and tubular function. Inhibition of NO synthesis leads to a reduction in medullary blood flow (MBF), salt retention, and hypertension (27). The effects of NO on the renal medulla are mediated by several pathways: NO exerts a strong vasodilatory influence on descending vasa recta (DVR) (32), and it inhibits NaCl reabsorption in the medullary thick ascending limb (mTAL) by reducing the activity of the Na+-K+-2Cl− cotransporter (30). In contrast, O2− stimulates NaCl transport across mTALs and favors reductions in MBF (29, 11). In addition, NO and O2− diminish each other's bioavailability, as they react together to form peroxynitrite. A recent study also suggests that NO reduces flow-stimulated increases in O2− production via the cGMP/PKG pathway, independently of its scavenging effects on O2− (18). The importance of NO-O2− interactions is underscored by accumulating evidence that the balance between NO and O2− is one of the key mechanisms for the development of salt-sensitive hypertension (26).
Adding to the complexity of these interactions is the role of oxygen (O2). O2 is a substrate for the formation of each NO and O2−, yet some studies have suggested that hypoxia enhances medullary NO levels (16) and O2− production (22). Moreover, the availability of O2 depends on the balance between O2 supply, itself a function of blood flow, and O2 demand, which varies with the rate of active Na+ transport. To better understand the three-way interactions between O2, NO, and O2−, and how they impact tubular and vascular function, we recently developed a region-based model of O2, NO, and O2− transport in the outer medulla (OM) of the rat (14). Our results suggested that NaCl transport and the concentrating capacity of the OM are substantially modulated by basal and pathological levels of NO and O2−. This previous model, however, did not account for DVR vasoactivity and subsequent changes in MBF and O2 supply. It therefore lacked an important component of the feedback loops involving O2, NO, and O2−.
The model described herein incorporates the effects of NO on DVR diameter and DVR flow. It is first used to determine whether O2−-induced vasoconstriction may be in part mediated by NO. The second part of our study centers on the impact of tubular NO synthesis. We examine in particular the hypothesis that diffusion of vasoactive agents such as NO from adjacent tubules to vasa recta plays an important role in protecting the medulla from hypoxic injury, by modulating blood flow and O2 supply in response to variations in mTAL transport and metabolic requirements (28).
MODEL DESCRIPTION
Our “region-based” model accounts for the three-dimensional architecture of the rat OM by distinguishing four concentric regions, as originally proposed by Layton and Layton (21): the innermost region (R1) represents the central vascular bundle, where all the long DVR and a third of the long ascending vasa recta (AVR) are sequestered; the surrounding region (R2) represents the immediate periphery of the bundle and encompasses all short DVR (those that turn within the OM) and the remaining long AVR; the neighboring region (R3) contains most mTALs, both long and short, and some short AVR; and the region most distant from the vascular bundle (R4) includes all the collecting ducts and the remaining short AVR (see Fig. 1 in Ref. 14).
Fig. 1.
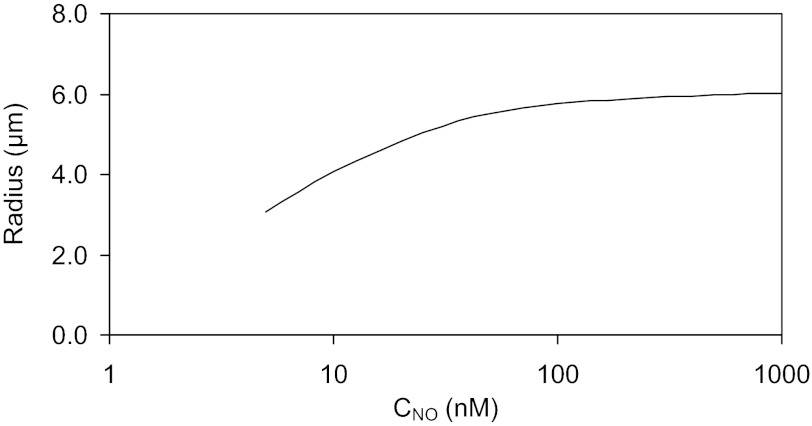
Postulated dependence of descending vasa recta (DVR) radius on nitric oxide (NO) concentration (CNO) in DVR plasma, as described by Eq. 1. The radius of the vessel cannot fall below that of a single erythrocyte (∼4 μm).
The model determines flows and solute concentrations as a function of OM depth in the interstitium, the loops of Henle, collecting ducts, vasa recta, and capillaries. Blood flow in vasa recta is divided into two compartments, plasma and red blood cells (RBCs). The solutes explicitly considered in the model are NaCl, urea, deoxy- and oxy-hemoglobin (Hb and HbO2), O2, NO, HbNO, and O2−. Detailed transport equations can be found in our previous studies (6, 14). We focus here on the new feature of our model, namely, NO-induced vasodilation of long (LDV) and short (SDV) DVR. We also briefly summarize the equations describing active Na+ transport and O2 consumption in the OM.
NO-Induced Changes in DVR Diameter
The relationship between NO levels and DVR diameter is complex. The DVR endothelium is surrounded by pericytes, vascular smooth muscle cells that impart vasocontractile properties to the vessels. The vasodilatory effects of NO appear to be mostly mediated by the cGMP/PKG pathway, which acts to reduce intracellular Ca2+ and the proportion of phosphorylated myosin light chains within pericytes (13). Moreover, vascular diameter is regulated by many other vasoactive agents, such as prostaglandins and endothelins. In light of this complexity, we opt for a simple, semiempirical approach. We assume that the local DVR radius (RDVR) depends on local NO concentration according to
| (1) |
where x denotes the position on the corticomedullary axis, CNODVR is the NO concentration in DVR plasma (more generally, Cij is the concentration of solute i in compartment j), A is a constant to be determined, and the superscript * indicates reference values. The reference radius is taken as 5.5 μm, and the reference NO concentration as 50 nM, which is the average interstitial NO concentration at the outer-inner medullary junction that we predicted in our previous base case (14).
The study of Kakoki et al. (20) relates changes in interstitial NO concentrations in the inner medulla (at a depth of 5.5 mm, that is, close to the junction between the outer and inner medulla) to changes in MBF. To relate, in turn, changes in MBF to variations in vessel radius, we use Poiseuille's law
| (2) |
where P is the luminal hydraulic pressure, FvDVR is the single-vessel blood flow, and μ is the fluid viscosity. Specifically, we assume that MBF varies in proportion to RDVR4. Kakoki et al. (20) found that different doses of NOS inhibitors respectively reduced NO levels by 32 and 66%, and MBF by 32 and 40%; with our assumptions, the latter correspond to 10 and 12% reductions in RDVR. A best fit of Eq. 1 to these data yields A = 1.0962. The postulated dependence of RDVR on CNODVR is illustrated in Fig. 1. We assume that DVR constrict along their entire OM length, since some vessels exhibit pericytes all the way to the papillary tip (33). However, it is possible that only the upper parts of OM DVR are capable of vasoconstriction. Our qualitative conclusions would nevertheless remain unaffected.
Blood flow is calculated as follows. The pressure in the efferent arteriole (denoted PE) and that in the renal vein (denoted PV) are respectively set to 20 and 3 mmHg (4, 10, 19). Each DVR is connected in series to a fixed downstream resistance (Γdown), the value of which depends on the DVR length and localization. Blood flow at the DVR inlet (i.e., at the corticomedullary junction) is computed by an iterative process, so that the sum of the pressure drop across the DVR (ΔPDVR) and that across its downstream resistance equals (PE − PV)
| (3) |
where LDVR is the vessel length, and ΔPDVR is given by
| (4) |
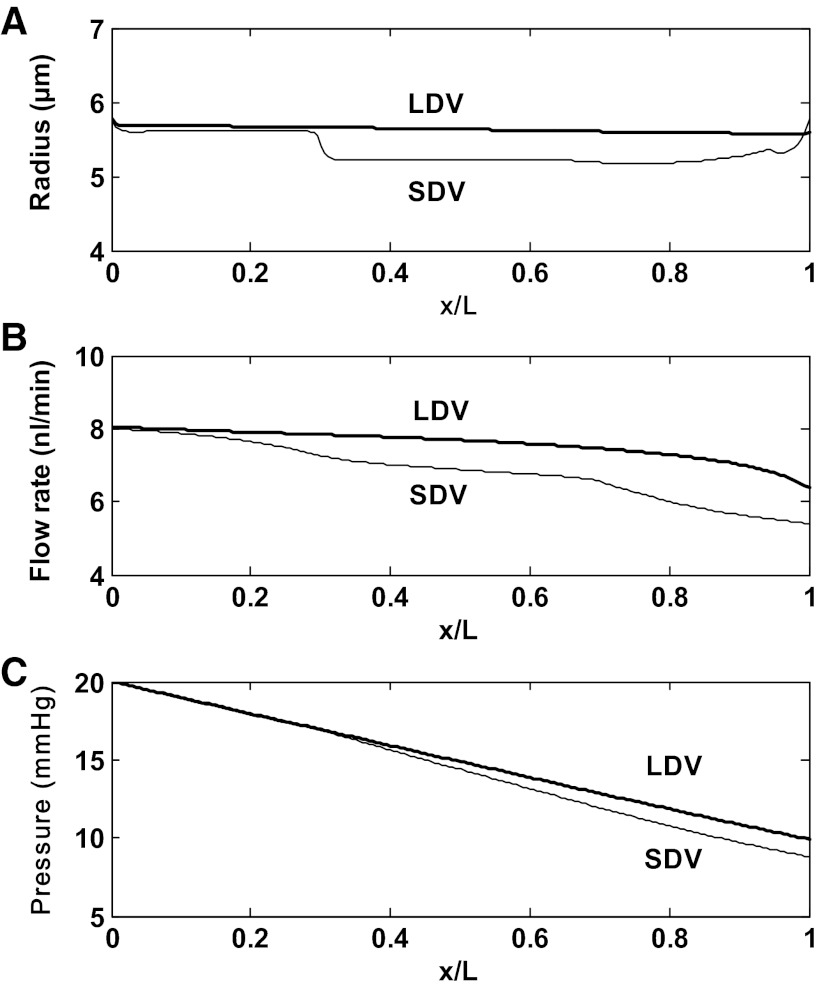
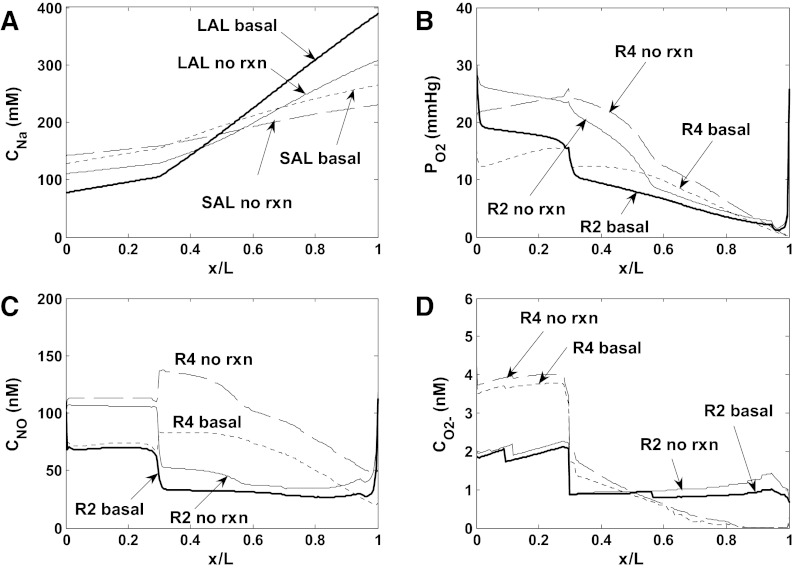
Note that blood flow variations along DVR are calculated based upon the rate of water reabsorption (see Eqs. 3 and 4 in Ref. 14). The downstream resistance Γdown of each DVR is chosen so that under baseline conditions, DVR blood flow at the inlet is 8 nl/min; Γdown then remains fixed in all other simulations. The viscosity of blood flowing through vasa recta is low, not much higher than that of plasma (34); we assume μ = 2 cp. As an illustration, the pressure drop across a DVR that extends along the entire OM length would be 11.7 mmHg if its blood flow remained constant at 8 nl/min and its radius fixed at 5.5 μm. The pressure drop across the downstream resistance would then equal (20–3) − 11.7 = 5.3 mmHg. Base-case flow and pressure profiles in long and short DVR are shown in Fig. 2.
Fig. 2.
Flow and pressure profiles in long DVR (LDV) and in the short DVR (SDV) that reach the outer-inner medullary junction, for scenario A. Results are similar for both scenarios. x: Position along the corticomedullary axis; x/L = 0 at the corticomedullary junction, and x/L = 1 at the outer-inner medullary junction. A: vessel radius. The radius is a function of luminal NO concentration (Eq. 1). B: blood flow rate. Blood flow variations along the corticomedullary axis are determined based upon the rate of water reabsorption. C: luminal pressure, calculated using the Poiseuille equation (Eq. 2). The pressure gradient is steeper in SDV than in LDV, because it is inversely proportional to the 4th power of the vessel radius.
The model represents the vasoactive effects of NO but assumes that the DVR radius is independent of the transendothelial pressure gradient. This simplifying hypothesis allows us to avoid computing local fluid pressure along the DVR and leads to substantial savings in computational cost. While elevation of luminal pressure has been shown to progressively dilate DVR ex vivo owing to the absence of a myogenic response (38), according to our calculations the effects of pressure on the radius in the scenarios simulated below are small compared with those of NO. Whereas the pressure drops from 20 mmHg in the efferent arteriole to 3 mmHg in the renal vein, pressure variations at a given medullary level are estimated to be <0.3 mmHg.
NaCl Transport Rate
The axial osmolality gradient in the OM is generated and maintained by active salt reabsorption in mTALs, which is driven by basolateral Na+-K+-ATPase pumps. As in our previous model, the rate of active Na+ transport along mTALs (ΨmTAL, Naactive) is expressed as
| (5) |
where Vmax, Na (in mol Na+·m−2·s−1) is the maximal rate of Na+ transport, KM,Na is the Michaelis-Menten constant (140 mM), and the functions f(PO2mTAL), g(CNOmTAL), and h(CO2−mTAL), respectively, represent the effects of O2, NO, and O2− on the mTAL reabsorption rate. PO2mTAL denotes the partial pressure of O2 in mTALs. The constant Vmax, Na is estimated as 57.0 nmol/(cm2·s) in the inner stripe, and 23.1 in the outer stripe (14). To simplify the notation, we omit the dependence of the variables on spatial position (x) in Eq. 5, as well as in the equations below.
We assume that when PO2mTAL falls below a critical value Pc (taken as 5 mmHg) (5), anaerobic metabolism partly takes over. We posit that in the complete absence of O2, the anaerobic pathway produces enough energy to sustain a transport rate that is a fraction λ (taken as 0.50) of the maximum rate when O2 supply is abundant, so that
| (6) |
With these assumptions, f(PO2mTAL) varies between 0.50 and 1. The function g(CNOmTAL) accounts for the inhibitory effect of NO on ΨmTAL, Naactive and is specified as (14)
| (7) |
where β is estimated as 46.9 nM. Similarly, the function h(CO2−mTAL) represents O2−-induced stimulation of mTAL NaCl transport, and is chosen as (14)
| (8) |
where the reference value CO2−* equals 20 pM in scenario A and 350 pM in scenario B (see below), so that h(CO2−mTAL) equals ∼1 under baseline conditions.
The overall rate at which Na+ is reabsorbed along mTALs, denoted TNamTAL, is calculated as the total Na+ molar flow (summed over all mTALs) at the inlet (x = L), minus that at the outlet (x = 0).
Active O2 Consumption
The volumetric rate of active O2 consumption in mTAL epithelia (γmTAL, O2active) is given by
| (9) |
where RmTAL and AmTALepi denote the mTAL radius and epithelial cross-sectional area, respectively, θ(PO2mTAL) is the proportion of the active transport rate that is supported by aerobic respiration, and 18 is the number of Na+ moles actively reabsorbed per mole of O2 consumed under maximal efficiency. We assume that (14)
| (10) |
The total consumption rate of O2 in the OM is determined by integrating γmTAL, O2active along the full length of short and long mTALs (that is, we neglect basal O2 consumption). The supply of O2 to the OM is taken as the total molar flow of O2 (in free form or bound to Hb) in LDV and SDV at the corticomedullary junction.
Effects of Hypoxia on NO and O2−
Oxygen is a substrate for NO synthesis. The volumetric rate of NO generation in compartment i (GNOi) is represented by an O2-dependent Michaelis-Menten relationship
| (11) |
where GNOi, max is the maximal NO generation rate in compartment i, PO2i is the partial pressure of O2 in i, and the constant KM, O2NO is taken as 38 mmHg. The mechanisms by which hypoxia raises renal medullary NO levels, as observed in anesthetized rats (16), remain to be elucidated. It has been suggested that hypoxia stimulates NO release via RBC nitrite (12) or SNOHb (35), but mathematical models of these pathways suggest that they deliver only picomolar amounts of NO to vascular smooth muscle, which is not sufficient to induce vasodilation (7, 8). In this study, we do not account for hypoxia-induced stimulation of NO release in the OM.
Given conflicting experimental data regarding the effects of hypoxia on O2− synthesis (9, 22), we consider two parallel scenarios throughout this study: scenario A assumes that low Po2 inhibits O2− synthesis, whereas scenario B assumes instead that low Po2 increases O2− production by 50% relative to well-oxygenated conditions. The volumetric rate of O2− generation in compartment i (GO2−i) is thus given by
| (12a) |
| (12b) |
where GO2−i, basal is fixed, and KM, O2O2− is taken as 15.4 mmHg. The boundary conditions (such as inlet O2− concentrations) are the same in both scenarios.
Parameter Values
Aside from the effects of NO on DVR radius and blood flow, the model described herein is identical to the one we recently published (14). Tables summarizing parameter values and the description of our numerical method can be found in the latter study.
MODEL RESULTS
This study focuses on the interactions between O2, NO, and O2− and the ways in which they affect tubular and vascular function in the OM. We first describe baseline concentration profiles and then simulate variations in production rates or reaction rates to probe the mechanisms by which blood flow and O2 supply can be modulated by NO to match the energetic requirements of the OM. For each set of simulations, we compare results for two cases: the “vasoactive case,” which accounts for the NO-dependence of DVR radius, and the “fixed R case,” which neglects that dependence.
Base Case
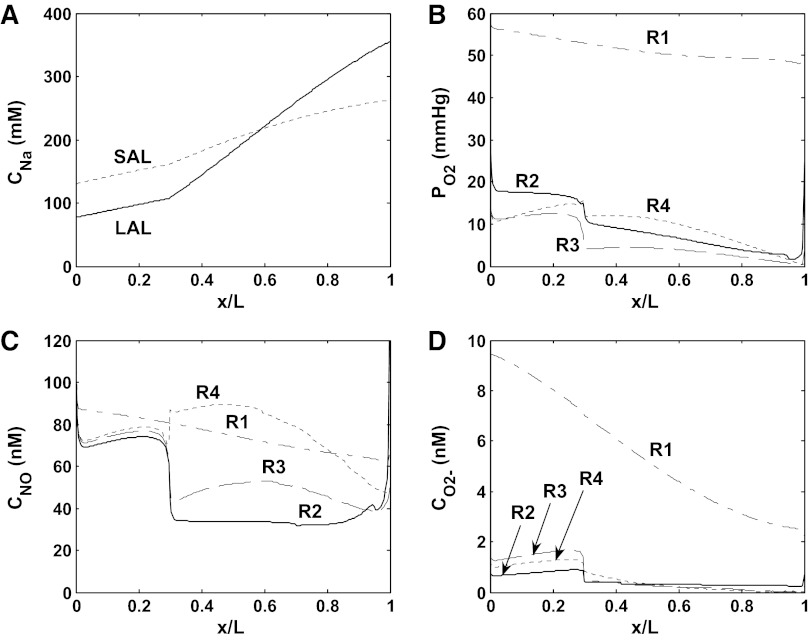
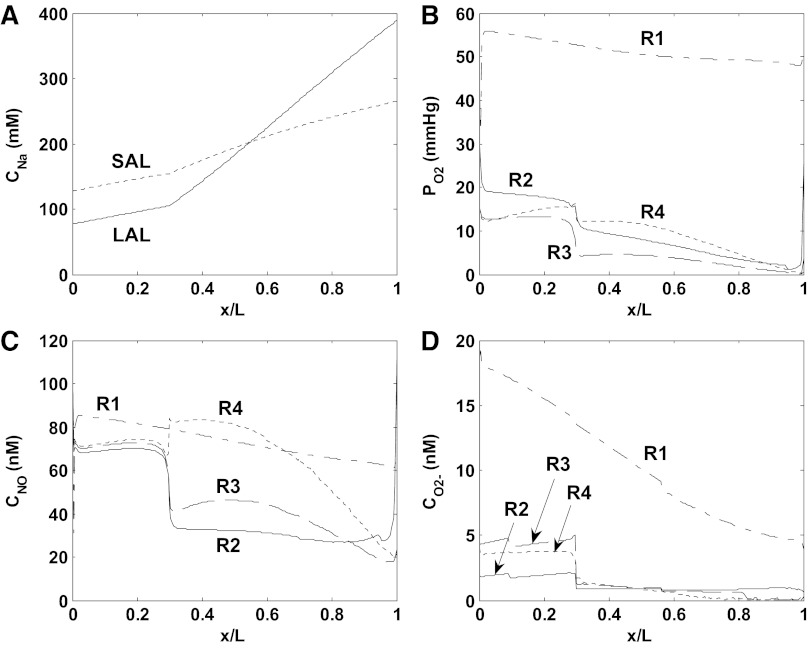
The baseline mTAL Na+ concentration profiles, and interstitial fluid O2, NO, and O2− concentration profiles are shown in Figs. 3 (scenario A) and 4 (scenario B) for the vasoactive case. Because model parameters were chosen so that baseline DVR inflow rates are analogous in the vasoactive case and the fixed R case, the profiles obtained in these two cases are similar. Both cases predict that the segregation of long DVR and long AVR within vascular bundles in the inner stripe gives rise to significant concentration differences between R1 (the center of the vascular bundles) and R2–R4 (the peripheral regions). The high metabolic requirements of mTALs in the interbundle regions substantially deplete O2 therein. NO concentrations are predicted to be higher in R1 than in R2 and R3, due to the poor availability of its substrate (i.e., O2) in the latter regions; the sharp rise in interstitial CNO in R4 in the inner stripe is caused by tubule migration (14). When the O2− generation rate (GO2−) is assumed to decrease with decreasing Po2 (scenario A), interstitial CO2− variations reflect Po2 variations (see Fig. 3, B and D); when GO2− is taken to be independent of Po2 (scenario B), interstitial CO2− varies with the fractional area occupied by vasa recta and descending limbs (i.e., the main suppliers of O2−) within each region (see Fig. 4D).
Fig. 3.
Baseline concentration profiles for scenario A (vasoactive case). A: luminal Na+ concentrations (CNa) in long (LAL) and short (SAL) ascending limbs. B: Po2 in the interstitium of the 4 concentric regions (R1–R4). R1 corresponds to the center of the vascular bundle, and R4 is the outward-most region. C: NO concentrations (CNO) in the interstitium of R1–R4. D: O2− concentrations (CO2−) in the interstitium of R1–R4.
Fig. 4.
Baseline concentration profiles for scenario B (vasoactive case). A: luminal CNa in LAL and SAL. B: interstitial Po2 in R1–R4. C: interstitial CNO in R1–R4. D: interstitial CO2− in R1–R4.
Since O2− is the predominant NO scavenger in the interbundle region, the fact that CO2− is higher in scenario B than in scenario A means that conversely, CNO is lower in R2–R4 in scenario B (compare Figs. 3C and 4C). Hence, NO inhibits NaCl reabsorption to a lesser extent in scenario B, and the concentrating ability of the OM, which is evaluated as the osmolality of the collecting duct fluid at the junction between the outer and inner medulla (denoted osmCDL), is higher in that scenario than in scenario A (867 vs. 789 mosmol/kgH2O, respectively; see Table 1). Even though mTALs actively reabsorb more NaCl in scenario B, the O2 consumption rate is slightly lower then (43 vs. 44 pmol/s per vascular bundle), because Po2 decreases more in the inner stripe, so that a greater fraction of the mTAL energetic requirements is supported by anaerobic metabolism. The predicted O2 consumption-to-supply ratio is 0.74 in scenario B and 0.75 in scenario A, in good agreement with the experimental estimate of 0.79 in the rat OM (3). As shown in Figs. 5 (scenario A) and 6 (scenario B), results for the vasoactive case and fixed R case are similar under baseline conditions.
Table 1.
Vascular and tubular function in the rat outer medulla
|
Scenario A |
Scenario B |
|||||
|---|---|---|---|---|---|---|
| SDV inflow, nl/min | O2 consumption-to-supply ratio | osmCDL, mosmol/kgH2O | SDV, inflow nl/min | O2 consumption-to-supply ratio | osmCDL, mosmol/kgH2O | |
| Base case | 8.0 (8.0) | 0.75 (0.76) | 789 (787) | 8.0 (8.0) | 0.74 (0.74) | 867 (867) |
| No NO-O2−rxn | 8.0 (8.0) | 0.75 (0.75) | 770 (771) | 8.4 (8.0) | 0.59 (0.65) | 666 (701) |
| GNOep = 0 | 7.5 (8.0) | 0.82 (0.80) | 873 (854) | 7.6 (8.0) | 0.78 (0.78) | 967 (936) |
| GNOep × 2 | 8.1 (8.0) | 0.74 (0.73) | 725 (730) | 8.1 (8.0) | 0.70 (0.71) | 800 (805) |
| GO2−ep× 2 | 7.8 (8.0) | 0.80 (0.78) | 856 (845) | 7.6 (8.0) | 0.79 (0.79) | 1,096 (1,037) |
| GO2−ep× 2 GNOep× 2 | 8.1 (8.0) | 0.76 (0.75) | 783 (788) | 7.9 (8.0) | 0.76 (0.76) | 1006 (995) |
| GO2−ep× 2 GNOep× 5 | 8.6 (8.0) | 0.47 (0.66) | 535 (694) | 8.5 (8.0) | 0.59 (0.71) | 755 (893) |
GNOep and GO2−ep: rates of NO and O2− synthesis by tubular epithelia; osmCDL, osmolality of collecting duct fluid at the outer-inner medullary junction. The short descending vasa recta (SDV) inflow is averaged over all SDV. Numbers in parenthesis correspond to the “fixed R case,” which neglects DVR vasoactivity.
Fig. 5.
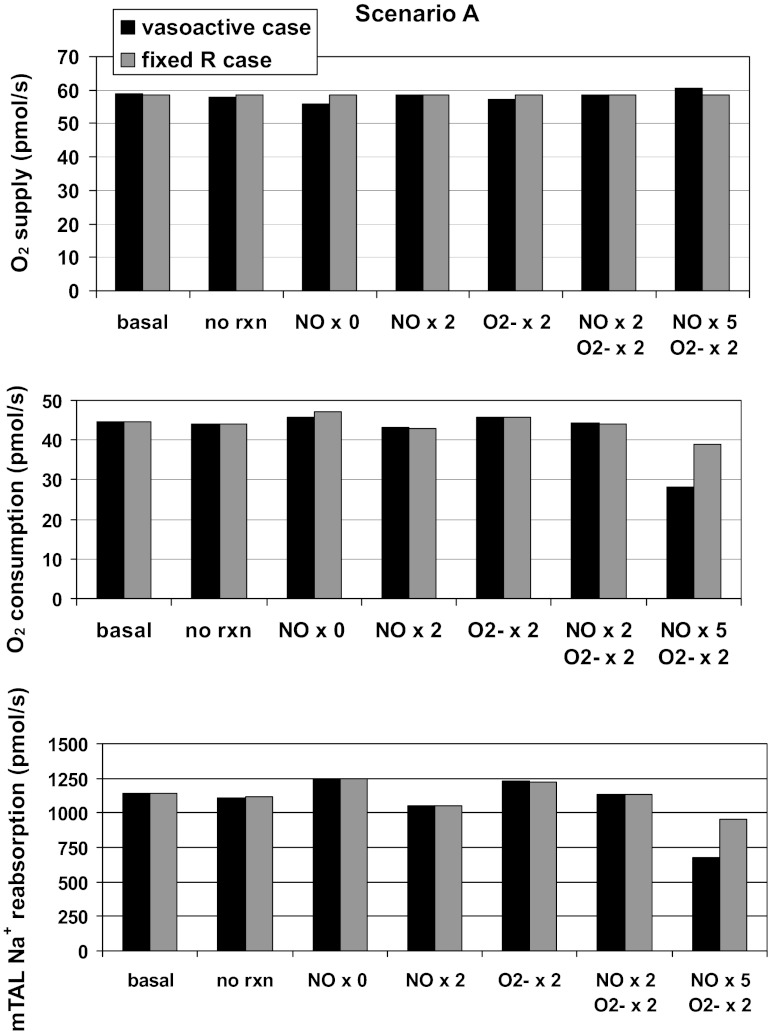
Predicted rates of O2 supply, O2 consumption, and medullary thick ascending limb of Henle's loop (mTAL) NaCl reabsorption, in pmol·s−1·vascular bundle−1, for scenario A. Results are shown for different cases: baseline conditions (“basal”), in the absence of reaction between NO and O2− (“no rxn”), when the rate of NO synthesis by tubular epithelia (GNOep) is 0, 2, or 5 times its baseline value (NO × 0, 2, or 5) , and/or when the rate of O2− synthesis by tubular epithelia (GO2−ep) is twice its baseline value (O2− × 2). Black bars, “vasoactive case”; grey bars, “fixed R case.”
Fig. 6.
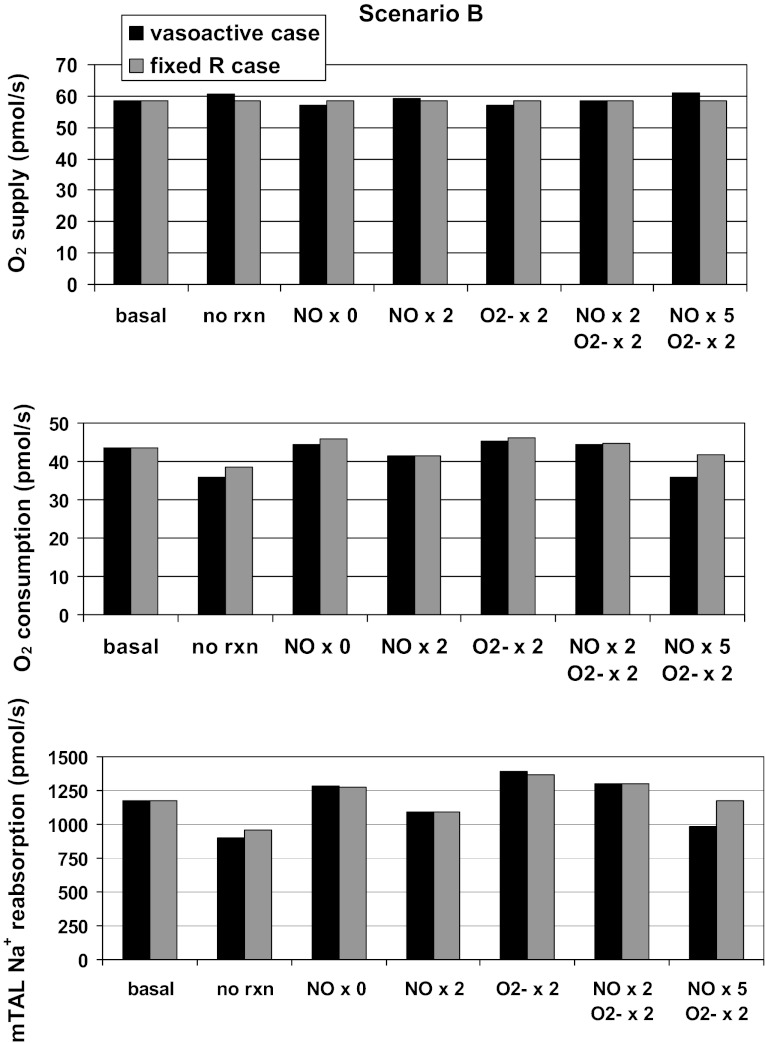
Predicted rates of O2 supply, O2 consumption, and mTAL NaCl reabsorption, in pmol·s−1·vascular bundle−1, for scenario B. Results are shown for different cases: baseline conditions (basal), in the absence of reaction between NO and O2− (no rxn), when GNOep is 0, 2, or 5 times its baseline value (NO × 0, 2, or 5) , and/or when GO2−ep is twice its baseline value (O2− × 2). Black bars, vasoactive case; grey bars, fixed R case.
Indirect Effects of O2− on MBF
The mechanisms by which O2− modulates MBF remain to be elucidated (15). Could O2−-induced vasoconstriction be mediated by NO, at least in part, given that O2− reduces NO levels via scavenging, thereby reducing the vasodilatory effects of NO? To probe this issue, we conducted simulations in which we eliminated the direct interactions between NO and O2− by setting their reaction rate to zero. We first consider the vasoactive case.
In scenario A, inhibiting the reaction raises CNO (and CO2−) by a few nanomolar in the interbundle region, not enough to raise DVR inflow (Table 1). Since NO and O2− exert counterbalancing effects on the mTAL transport rate, mNamTAL and Po2 vary only slightly. Thus, in the absence of the NO-O2− reaction, osmCDL and the O2 consumption-to-supply ratio vary by <3% (Table 1).
In scenario B, the consequences of inhibiting the NO-O2− reaction are more pronounced: because CO2− is two- to threefold higher in this scenario, O2− scavenging has a greater impact on interbundle NO levels (14). Thus, when the reaction is abolished, CNO increases more (Fig. 7), and the average SDV inflow rises from 8.0 to 8.4 nl/min (LDV inflow varies by 0.04 nl/min). The inhibitory effects of NO on mTAL transport then predominate, TNamTAL and O2 consumption both decrease (Fig. 6), and osmCDL is reduced by 23%. Altogether, the O2 consumption-to-supply ratio is predicted to fall from 0.74 to 0.59 (Table 1).
Fig. 7.
Impact of the NO-O2− reaction on concentration profiles (scenario B, vasoactive case). Results are shown for the base case (basal) and assuming that the NO-O2− reaction rate is zero (no rxn). A: luminal CNa in LAL and SAL. B: interstitial Po2 in regions R2 and R4. C: interstitial CNO in R2 and R4. D: interstitial CO2- in R2 and R4. In the interbundle region, inhibition of the NO-O2− reaction has a larger impact on CNO than on CO2−. The subsequent increase in O2 supply is accompanied by a significant decrease in the rate of NaCl reabsorption across LAL and SAL, and in the rate of O2 consumption.
A comparison with the fixed R case is instructive. If DVR vasoactivity is not taken into account, the O2 consumption-to-supply ratio in scenario B is found to decrease significantly less (from 0.74 to 0.65) when the NO-O2− reaction rate is set to zero. Indeed, the fixed R case does not consider the following positive feedback mechanism: as CNO increases, DVR dilate and carry more O2 into the medulla, which in turn raises the generation rate of NO (GNO) and therefore CNO, and so on. This cycle will stop at some point, however, because the extent to which DVR can dilate, and that to which GNO can increase, are both intrinsically limited (see the saturable expressions in Eqs. 1 and 11). Moreover, other vasoconstrictor agents, not represented in the present model, are likely to be released to limit such NO-dependent vasodilation. It is also possible that as Po2 increases, hypoxia-induced stimulation of NO release diminishes, thus putting another break on this positive feedback mechanism.
Finally, it should be noted that inhibiting the NO-O2− reaction reduces osmCDL less in the fixed R case than in the vasoactive case in scenario B (Table 1): since CNO does not rise as much, mTAL transport is less inhibited. Together, these results suggest that NO-induced vasodilation may significantly affect the O2 balance and the concentrating capacity of the OM, and that O2−-induced DVR vasoconstriction could be at least partly mediated by NO.
Impact of Epithelial NO Generation
To assess the contribution of tubular NO generation to the maintenance of medullary oxygenation, we first simulated an isolated twofold increase in the rate of NO synthesis by epithelia (GNOep). Under these conditions, calculated CNO values are 10–20 nM higher in the interbundle region (see Fig. 8 for scenario A). NO-mediated inhibition of active Na+ transport is then enhanced, and osmCDL, TNamTAL, and O2 consumption are predicted to decrease by 4–8% (Figs. 5 and 6). The impact on O2 supply is small, and the O2 consumption-to-supply ratio decreases in proportion to consumption (Table 1).
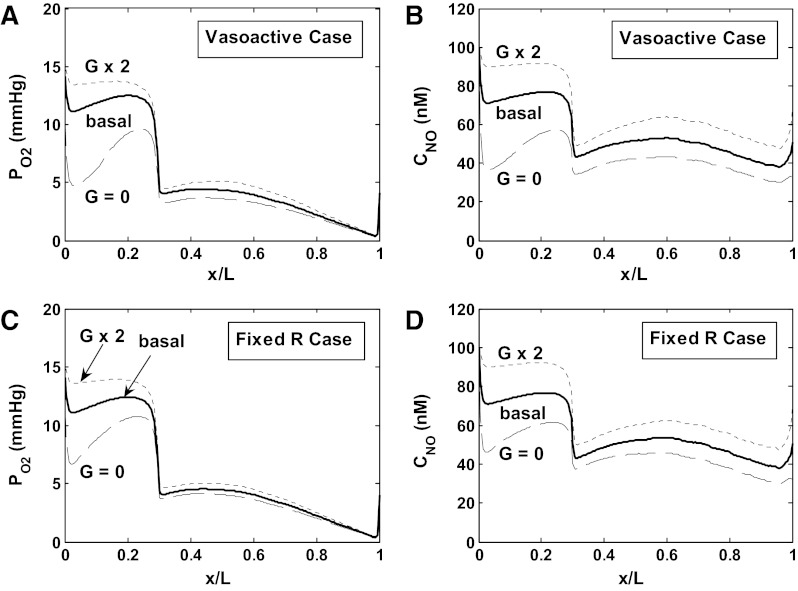
Fig. 8.
Impact of NO synthesis by tubular epithelia on Po2 and CNO in region R3, where most mTALs are located. Results are shown for scenario A. The rate of NO synthesis by tubular epithelia is either equal to its baseline value (basal), multiplied by 2 (G × 2), or 0 (G = 0). A and B: Po2 and CNO in the R3 interstitium, accounting for vasoactivity of DVR. C and D: Po2 and CNO in the R3 interstitium, assuming no vasoactivity of DVR. Results suggest that NO tubulovascular cross talk has a significant impact on O2 balance in the outer medulla.
Conversely, in the absence of NO generation by epithelia, CNO is predicted to decrease by 10–30 nM in R2–R4 (vasoactive case) relative to baseline conditions (Fig. 8). The resulting vasoconstriction of SDV reduces O2 supply to the interbundle region by ∼ 5% (Figs. 5 and 6). In parallel, the rate of active Na+ transport (which is less inhibited by NO) rises by 10%, and so does osmCDL (Table 1). Overall O2 consumption is predicted to increase only slightly (by ∼2%) for the following reason. The increase in TNamTAL requires more energy, but because O2 supply to the interbundle region decreases, in the vicinity of mTAL Po2 falls even further below the critical pressure along most of the inner stripe (see Fig. 8A), so that a smaller fraction of active Na+ transport is supported by aerobic respiration. In other words, O2 consumption does not increase in proportion to TNamTAL because a greater fraction of mTAL energy requirements is provided by anaerobic metabolism. Overall, the O2 consumption-to-supply ratio increases to 0.82 (scenario A) and 0.78 (scenario B).
In the fixed R case, the O2 supply to the interbundle region is constant. Thus, in the absence of NO generation by epithelia, Po2 does not fall as significantly in the tissue surrounding mTALs (see Fig. 8, A and C), and O2 consumption increases in proportion to the rate of active Na+ transport, that is, more than in the vasoactive case (Figs. 5 and 6). As a result, the increase in the O2 consumption-to-supply ratio is comparable in both cases (Table 1). Taken together, these results suggest that NO tubulovascular cross talk has a significant impact on OM O2 balance and concentrating capacity.
ANG II-Mediated Increases in Epithelial Generation
We then examined the impact of tubular NO synthesis specifically in response to ANG II. ANG II stimulates the production by mTAL epithelia of not only O2− but also NO (24, 25). The ANG II-induced increase in NO synthesis is thought to help abrogate tissue hypoxia (31). We simulated first an isolated increase in the tubular production of O2−, followed by an increase in that of both O2− and NO. Based on experimental measurements of O2− and NO concentrations in the presence of ANG II (24, 25, 39), epithelial GO2− (GO2−ep) was multiplied by a factor of 2, and epithelial GNO (GNOep) by a factor of 2 or 5. Note that we did not consider the NO-independent mechanisms by which ANG II may induce DVR vasoconstriction in these simulations.
As expected, an isolated twofold increase in GO2−ep raises CO2− in the vicinity of mTALs, thereby stimulating active Na+ transport, and increasing both oxygen consumption (Fig. 5 and 6) and the concentrating capacity of the medulla (Table 1). The rate of NO scavenging by O2− increases concomitantly (more so in scenario B), leading to vasoconstriction of SDV in the interbundle region and a reduction in local O2 delivery. The O2 consumption-to-supply ratio is thus predicted to increase by 5 percentage points in both scenarios.
A concomitant increase in GNOep, however, counteracts the stimulating effects of O2− on NaCl transport and O2 consumption in the OM. A twofold increase in GNOep offsets the TNamTAL increase, either fully (scenario A) or partially (scenario B) (Figs. 5 and 6). It also raises CNO sufficiently to essentially abolish SDV constriction and restore O2 supply to the interbundle region. Thus, when GO2−ep and GNOep are both doubled, the O2 consumption-to-supply ratio is predicted to remain close to its baseline value (Table 1).
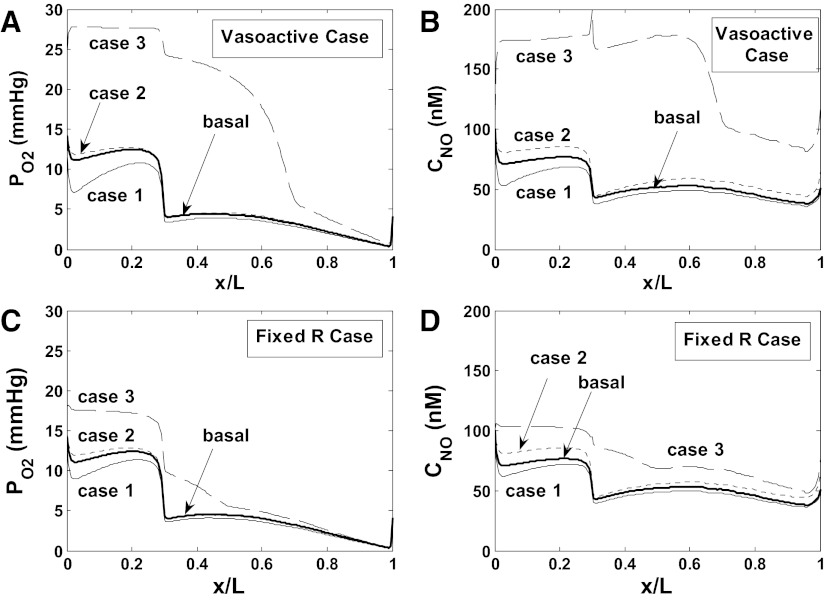
If GNOep is increased five times relative to its baseline level (while GO2−ep is doubled), O2 supply to the interbundle region is further augmented, while active Na+ transport and O2 consumption both decrease significantly below baseline levels (Figs. 5 and 6). Under these conditions, the O2 consumption-to-supply ratio is calculated as 0.47 in scenario A and 0.59 in scenario B. The corresponding predictions, displayed in Fig. 9 (case 3), conflict with experimental findings, however: in vivo, acute subpressor doses of ANG II do not significantly alter MBF and medullary Po2 (39). These results thus suggest that ANG II induces comparable increases in GO2−ep and GNOep.
Fig. 9.
Impact of epithelial production of O2− (GO2−ep) and NO (GNOep) on Po2 and CNO in region R3. Results are shown for scenario A. Basal denotes baseline profiles. In case 1, GO2−ep is multiplied by 2 (relative to its baseline value); in case 2, GO2−ep and GNOep are both multiplied by 2. In case 3, GO2−ep is multiplied by 2, and GNOep by 5. A and B: Po2 and CNO in the R3 interstitium, accounting for vasoactivity of DVR. C and D: Po2 and CNO in the R3 interstitium, assuming no vasoactivity of DVR. Results suggest that ANG II-induced stimulation of NO synthesis by mTAL counteracts the effects of oxidative stress.
As expected, the two- or fivefold GNOep increase is predicted to have a lesser impact in the fixed R case (Fig. 9). That is, in the absence of tubulovascular cross talk, TNamTAL, osmCDL and the O2 consumption-to-supply ratio are much less reduced (Table 1). Taken together, our results suggest that ANG II-mediated NO synthesis acts not only to counterbalance the effects of ANG II on vasoconstriction, as observed experimentally (39, 36), but also to reduce active Na+ transport and thereby preserve O2 availability in the OM.
DISCUSSION
The effects of NO and O2− on vascular and tubular function in the OM are difficult to untangle: not only do NO and O2− exert opposite actions on blood flow and sodium transport while reducing each other's bioavailability (15) but they also interact with O2 in ways that are not fully understood. In this study, we used a mathematical model to gain more insight into the reciprocal interactions between O2, NO, and O2−.
The most significant limitations of our model stems from the paucity of experimental data. As previously discussed (14), absolute O2− concentrations in the renal medulla have not yet been reported; the quantitative effects of NO and O2− on mTAL reabsorption rates are also uncertain. Similarly, the data we extrapolated to predict the effects of CNO on DVR diameter and blood flow (Eq. 1) are somewhat limited. In addition, the contribution of anaerobic metabolism to the energy requirements of the OM, which likely varies among species, has never been precisely determined. Further experimental studies are needed to fill those gaps, and to elucidate the effects of downstream products such as ONOO− (peroxynitrite) and H2O2 (hydrogen peroxide) on MBF (23, 26), which our current model does not consider. Finally, conflicting experimental results regarding the effects of low Po2 on medullary O2− synthesis (9, 22), as well as the uncertainty surrounding the mechanisms by which hypoxia raises medullary NO levels, also require additional investigation.
To circumvent some of these limitations, we considered two scenarios in parallel throughout the study: scenarios A and B assume that O2− synthesis respectively decreases and increases with decreasing Po2. Since medullary Po2 is low, CO2− is significantly higher in scenario B. This distinction proved useful when we examined the indirect effects of O2− on MBF. Our results suggest that O2−-induced vasoconstriction may be partly mediated by NO, provided that O2− levels are sufficiently high. When the scavenging effects of O2− on NO were eliminated, SDV inflow was predicted to remain constant in scenario A, and to increase by 5% in scenario B (from 8.0 to 8.4 nl/min).
Our model predicts differential regulation of vasoactivity in short and long DVR. The region-based approach allows us to take into account the specific architecture of the rat OM, with its sharp distinction between intra- and interbundle regions. The LDV, which are destined to the inner medulla, are positioned at the center of the vascular bundles, whereas the SDV peel off from the bundle periphery to supply blood flow to the OM capillary plexus. In all the simulations performed for this study, LDV inflow remained approximately constant (it was always comprised between 7.9 and 8.1 nl/min) even when SDV inflow varied significantly. That is, the segregation of LDV within the center of the vascular bundles is predicted to insulate these vessels from changes in tubular function in the OM, thereby preserving O2 delivery to the inner medulla.
We also found that the impact of the interactions between O2, NO, and O2− is enhanced by a positive feedback mechanism, the importance of which remains to be ascertained in vivo. Since O2 is a precursor in the synthesis of NO, an increase in Po2 should raise NO concentrations, thereby augmenting DVR diameter, blood flow, and O2 supply; this should in turn further raise CNO, and so on. This positive feedback loop should nevertheless be mitigated, if not abolished, by the combination of several factors: 1) the limited extent to which DVR can dilate, which this model accounts for; 2) other vasoconstrictor agents such as endothelins, not considered in this study; and 3) the extent to which hypoxia affects NO bioavailability. Heyman et al. (17) observed an increase in medullary NO levels when Po2 was lowered. Hypoxia-induced NO release has been reported in other tissues and is thought to be a mechanism whereby local perfusion is adjusted to match O2 requirements (1). The underlying signaling pathways are still controversial and remain to be fully elucidated (1, 7, 8, 12). The tight coupling between Po2 and CNO illustrated by the model underscores the importance of better understanding the effects of hypoxia on NO bioavailability in the renal medulla.
We used the current model to assess in particular the importance of tubulovascular cross talk in maintaining OM perfusion and O2 availability. Cowley and colleagues (36, 39) showed that ANG II-induced increases in NO production offset the vasoconstrictor effects of ANG II and maintain MBF constant. Our results suggest that even basal production of NO by tubular epithelia acts to preserve blood flow and O2 supply to the interbundle region (Figs. 5 and 6). In the absence of tubular NO synthesis and subsequent NO diffusion to neighboring DVR, O2 supply to the interbundle region is predicted to decrease by 5%, and the O2 consumption-to-supply ratio to grow by 4–7% (Table 1). These differences are not huge, but they are significant in light of the hypoxic conditions that prevail in the OM. The variations in the O2 ratio stem not only from the reduction in O2 supply but also from changes in O2 consumption, as the inhibitory effects of NO on active Na+ transport across mTALs are abrogated; in the absence of tubular NO synthesis, the rate of NaCl reabsorption and the concentrating capacity of the outer medulla are calculated to be ∼10% higher (Table 1). In other words, NO acts to preserve O2 availability in the interbundle region by modulating both oxygen supply and demand, not just by matching O2 delivery to the metabolic needs of mTALs. In the presence of ANG II, the combined regulation of O2 consumption and supply by NO is predicted to significantly attenuate the hypoxic effects of ANG II (Fig. 9), as postulated by other investigators (28, 31).
Our investigations also suggest that O2 consumption may not systematically increase in proportion to the rate of active Na+ transport, in the presence of anaerobic metabolism. In those simulations where Po2 in the vicinity of mTALs was below the critical pressure in most of the inner stripe (where the rate of NaCl reabsorption is the highest), the rate of O2 consumption did not rise as fast as TNamTAL. This prediction is contingent upon our assumption that anaerobic metabolism can be a significant source of ATP in the rat OM. The current model assumes that, when necessary, glycolysis may produce enough energy to sustain half the maximal transport rate (i.e., that at which O2 is not rate limiting; see Eq. 6). Ex vivo, the production of lactate by isolated rat mTAL segments was found to increase markedly following incubation with the oxidative metabolism inhibitor antimycin A (2). Whether, in vivo, anaerobic metabolism is sufficient to sustain an increased rate of NaCl reabsorption even as Po2 decreases remains to be ascertained. At the very least, the supply of glucose to the OM seems more than sufficient to satisfy the corresponding ATP requirements. Assuming a glucose concentration of 10 mM in DVR blood (37), the supply of glucose to the OM is 80 pmol·min−1·DVR−1, or 4.6 nmol·min−1·vascular bundle−1. The basal rate of Na+ transport across mTALs, estimated as 1,200 pmol Na+·min−1·bundle−1 (Figs. 5 and 6), requires 400 pmol ATP·min−1·bundle−1, which in turn necessitates the conversion of 200 pmol glucose·min−1·bundle−1 under anaerobic conditions, which is less than one-tenth of the glucose supply.
In conclusion, this model underlines the importance of O2 availability in regulating the balance between NO and O2− and their effects on vascular and tubular function in the rat OM. Conversely, our results suggest that basal production of NO by tubular epithelia modulates both the supply and demand of O2 in the interbundle region.
GRANTS
This work was supported by National Institutes of Health Grant DK-89066 and National Science Foundation Grant DMS-0715021 (to A. Layton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.E. and A.T.L. provided conception and design of research; A.E. and A.T.L. analyzed data; A.E. and A.T.L. interpreted results of experiments; A.E. prepared figures; A.E. drafted manuscript; A.E. and A.T.L. edited and revised manuscript; A.E. and A.T.L. approved final version of manuscript; A.T.L. performed experiments.
REFERENCES
- 1. Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide, and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15: 452–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Brezis M, Rosen S, Silva P, Epstein F. Renal ischemia: a new perspective. Kidney Int 26: 375–383, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Canton AD, Stanziale R, Corradi A, Andreucci VE, Migone VE. Effects of acute ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int 12: 403–411, 1977 [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Edwards A, Layton AT. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol 298: F1369–F1383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Layton AT, Edwards A. A mathematical model of oxygen transport in the rat outer medulla: I. Model formulation and baseline results. Am J Physiol Renal Physiol 297: F517–F536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide 18: 47–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen K, Pittman RN, Popel AS. Vascular smooth muscle NO exposure from intraerythrocytic SNOHb: a mathematical model. Antioxidants Redox Signaling 9: 1097–1110, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol 289: F749–F753, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Corradi A, Arendshorst WJ. Rat renal hemodynamics during venous compression: roles of nerves and prostaglandins. Am J Physiol Renal Fluid Electrolyte Physiol 248: F810–F820, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edwards A, Cao C, Pallone TL. Cellular mechanisms underlying nitric oxide-induced vasodilation of descending vasa recta. Am J Physiol Renal Physiol 300: F441–F456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edwards A, Layton AT. Modulation of outer medullary NaCl transport and oxygenation by nitric oxide and superoxide. Am J Physiol Renal Physiol 301: F979–F996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evans R, Fitzgerald S. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 14: 9–15, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Heyman SN, Goldfard M, Darmon D, Brezis M. Tissue oxygenation modifies nitric oxide bioavailability. Microcirculation 6: 199–203, 1999 [PubMed] [Google Scholar]
- 17. Heyman SN, Karmeli F, Rachmilewitz D, Haj-Yehia A, Brezis M. Intrarenal nitric oxide monitoring with a Clark-type electrode: potential pitfalls. Kidney Int 51: 1619–1623, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Hong NJ, Garvin JL. Nitric oxide reduces flow-induced superoxide production via cGMP-dependent protein kinase in thick ascending limbs. Am J Physiol Renal Physiol 296: F1061–F1066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ichikawa I, Brenner BM. Importance of efferent arteriolar vascular tone in regulation of proximal tubule fluid reabsorption and glomerulotubular balance in the rat. J Clin Invest 65: 1192–1201, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kakoki M, Zou A-P, Mattson DL. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am J Physiol Regul Integr Comp Physiol 281: R91–R97, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol 34: 946–952, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Mori T, O'Connor PM, Abe M, Cowley AW., Jr Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 49: 1336–1341, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Mori T, Ogawa S, Cowley AW, Jr, Ito S. Role of renal medullary oxidative and/or carbonyl stress in salt-sensitive hypertension and diabetes. Clin Exp Pharmacol Physiol 39: 125–131, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Nakanishi K, Mattson DL, Cowley AW., Jr Role of renal medullary blood flow in the development of l-NAME hypertension in rats. Am J Physiol Regul Integr Comp Physiol 268: R317–R323, 1995 [DOI] [PubMed] [Google Scholar]
- 28. O'Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol 33: 961–967, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Pallone TL, Mattson DL. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens 11: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Park F, Mattson DL, Roberts LA, Cowley AW. Evidence for the presence of smooth muscle alpha-actin within pericytes of the renal medulla. Am J Physiol Regul Integr Comp Physiol 273: R1742–R1748, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int 68: 14–22, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Szentivanyi M, Maeda CY, Cowley AW. Local renal medullary l-NAME infusion enhances the effect of long-term angiotensin II treatment. Hypertension 33: 440–445, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Thomas SR. Inner medullary lactate production and accumulation: a vasa recta model. Am J Physiol Renal Physiol 279: F468–F481, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Z, Pallone TL. Response of descending vasa recta to luminal pressure. Am J Physiol Renal Physiol 287: F535–F542, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Zou AP, Wu F, Cowley AW., Jr Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31: 271–276, 1998 [DOI] [PubMed] [Google Scholar]