Abstract
A1 receptors may participate in renal sympathetic neurotransmission by enhancing the postjunctional effects of norepinephrine. The purpose of this study was to test this concept using A1 receptor knockout (A1AR−/−) mice. In isolated kidneys from nontransgenic mice perfused with Tyrode's solution at a constant rate, renal sympathetic nerve stimulation (RSNS) increased (P < 0.0001) renal venous perfusate levels of inosine (adenosine metabolite) from 23.9 ± 3.7 to 32.7 ± 5.1, 68.2 ± 12.4, and 94.0 ± 14.3 ng/ml at 3, 5, and 7 Hz, respectively (n = 28), suggesting frequency-dependent production of adenosine. Conversely, RSNS decreased (P < 0.0001) renal venous perfusate levels of 5′-AMP (adenosine precursor) from 1.4 ± 0.3 to 1.1 ± 0.3, 0.80 ± 0.2, and 0.6 ± 0.2 ng/ml at 3, 5, and 7 Hz, respectively (n = 28), suggesting frequency-dependent increased metabolism of 5′-AMP. In kidneys from nontransgenic mice, blockade of adenosine receptors with 1,3-dipropyl-8-p-sulfophenylxanthine attenuated (P = 0.0130) vasoconstrictor responses to RSNS at 3, 5, and 7 Hz [control (n = 29): 22 ± 4, 34 ± 6, 42 ± 6 mmHg, respectively; 1,3-dipropyl-8-p-sulfophenylxanthine-treated (n = 11): 6 ± 1, 12 ± 3, 15 ± 3 mmHg, respectively]. In A1AR−/− kidneys (n = 10), vasoconstrictor responses to RSNS at 3, 5, and 7 Hz were 7 ± 3, 20 ± 5, and 36 ± 9 mmHg, respectively. In kidneys from wild-type littermates (n = 9), responses were 27 ± 9, 58 ± 14, and 59 ± 11 mmHg, respectively (effect of genotype: P = 0.0363). In kidneys from nontransgenic mice, 2-chloro-N6-cyclopentyladenosine (CCPA; highly selective A1 receptor agonist) increased renal vasoconstriction induced by norepinephrine (P = 0.0008; n = 28). In kidneys from A1AR−/− the response to norepinephrine was attenuated and the ability of CCPA to enhance responses to norepinephrine was abolished. In conclusion, adenosine formed during RSNS enhances the postjunctional effects of released norepinephrine by activating A1 receptors.
Keywords: mouse kidney, sympathetic neurotransmission, A1 receptor, adenosine, norepinephrine
a1 receptors mediate increased proximal tubular sodium reabsorption (11, 12, 23, 24). Consequently, stimulation of adenosine A1 receptors increases sodium reabsorption, and antagonism of A1 receptors reduces sodium reabsorption and promotes sodium excretion (11, 12, 24). Therefore, A1 receptor antagonists are diuretics that may be useful for the management of sodium-retaining disorders such as chronic and acute heart failure (9), liver cirrhosis (10), and hepatorenal syndrome (9). Because many sodium-retaining diseases are associated with increased renal sympathetic tone and because A1 receptor antagonists may be useful for treating such disorders, it is important to determine whether and how A1 receptors participate in renal sympathetic neurotransmission.
A recent study demonstrates that in isolated, perfused rat kidneys, pharmacological blockade of A1 receptors attenuates renal vasoconstriction induced by renal sympathetic nerve stimulation (RSNS) without altering renal spillover of norepinephrine (13). Moreover, pharmacological activation of A1 receptors increases renal vasoconstriction induced by norepinephrine, an effect that is blocked by inhibition of phospholipase C, protein kinase C, c-src, phosphatidylinositol 3-kinase, and 3-phosphoinositide-dependent protein kinase-1 (13). These results suggest that adenosine formed during RSNS enhances the postjunctional effects of released norepinephrine via coincident signaling and contributes to renal sympathetic neurotransmission (13). Likely, the coincident signaling pathway includes: phospholipase C → protein kinase C → c-src → phosphatidylinositol 3-kinase → 3-phosphoinositide-dependent protein kinase-1 (13).
The purpose of the present study was to test further the concept that A1 receptors participate in renal sympathetic neurotransmission by employing several useful tools available in our lab: 1) a technique for examining vascular responses to RSNS in the isolated, perfused mouse kidney (20), 2) a method for measuring small quantities of purines using high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) (14), and 3) the maintenance of a breeding colony of A1 receptor knockout mice (8, 16).
METHODS
Animals.
Male C57BL/6 mice were obtained from Taconic Farms (Germantown, NY). Male and female homozygous A1 receptor knockout (A1AR−/−) mice, as well as littermate heterozygous (A1AR−/+) and littermate wild-type (A1AR+/+), were bred and genotyped at the University of Pittsburgh as previously described by us (8, 16). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Drugs.
1,3-Dipropyl-8-p-sulfophenylxanthine [DPSPX; adenosine receptor antagonist that does not penetrate cell membranes (22)] and 2-chloro-N6-cyclopentyladenosine [CCPA; a highly selective A1 receptor agonist (15)] were obtained from Sigma (St. Louis, MO).
Isolated, perfused mouse kidney.
Mouse kidneys were isolated and perfused at a constant rate (1.5 ml/min) with Tyrode's solution as previously described by us (20). Kidneys were allowed to stabilize for 2 h before the protocols were initiated as described below.
RSNS.
RSNS was accomplished by placing a platinum bipolar electrode around the renal artery close to the kidney and connecting the electrode to a Grass stimulator (model SD9E; Grass Instruments, Quincy, MA) as previously described by us (20). The tissues around the electrode were kept moist with Tyrode's solution and the stimulation parameters were biphasic pulses 1-ms pulse duration; 35 V at indicated frequencies.
Analysis of norepinephrine.
Norepinephrine (NE) was quantified using the ALPCO Norepinephrine ELISA kit (catalog number 17-NORHU-E01-RES; ALPCO Diagnostics, Salem, NH).
Purines analysis.
5′-AMP, adenosine, and inosine were quantified using high-performance LC-MS/MS as previously described by us (20).
Protocol 1.
Perfused kidneys from C57BL/6 mice (n = 28) were subjected to RSNS at increasing frequencies [0 (basal), 3, 5, 7, and 9 Hz] for 5 min at each frequency and a venous perfusate sample was collected between 4 and 5 min during each stimulation period. Samples were heat inactivated to prevent enzymatic degradation of purines and stored at −80°C until assayed by LC-MS/MS. Next, after a rest period of 20 min, NE was infused at increasing concentrations (50, 100, and 150 nmol/l) for 5 min at each concentration. After another rest period of 20 min, kidneys were infused with CCPA (100 nmol/l). While maintaining the infusion of CCPA, the concentration response to NE was repeated.
Protocol 2.
In a separate group of perfused kidneys from C57BL/6 mice, DPSPX (100 μmol/l; n = 11) or vehicle (saline; n = 29) was added to the Tyrode's solution beginning 1 h into the 2-h rest period. Kidneys were subjected to RSNS at increasing frequencies [0 (basal), 3, 5, and 7 Hz] for 5 min at each frequency.
Protocol 3.
Perfused kidneys from A1AR−/− (n = 10), A1AR−/+ (n = 11), and A1AR+/+ (n = 9) mice were subjected to RSNS at increasing frequencies [0 (basal), 3, 5, and 7 Hz] for 5 min at each frequency.
Protocol 4.
Perfused kidneys from A1AR−/− (n = 7) and A1AR+/+ (n = 7) mice were subjected to increasing test concentrations of NE until a concentration was identified that increased perfusion pressure by ∼30 mmHg. Next, the concentration of NE that increased renal perfusion pressure to the desired target range was administered to the kidneys four times (5-min infusions with 10-min rest periods between responses). The first three responses were basal responses in the absence of any treatments to establish the reproducibility of the NE-induced renal vasoconstriction. CCPA was infused into the renal artery to achieve a CCPA of 1 nmol/l, and the response to NE was repeated in the presence of CCPA.
Statistics.
Data sets containing multiple groups were analyzed by repeated-measures one-factor or two-factor ANOVA or nested two-factor ANOVA with post hoc comparisons using a Fisher's least significant difference test. Data sets containing only two groups were analyzed with an unpaired Student's t-test. The criterion of significance was P < 0.05. All values in text and figures are means ± SE.
RESULTS
Protocol 1.
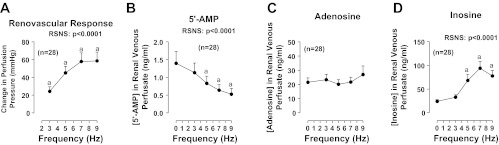
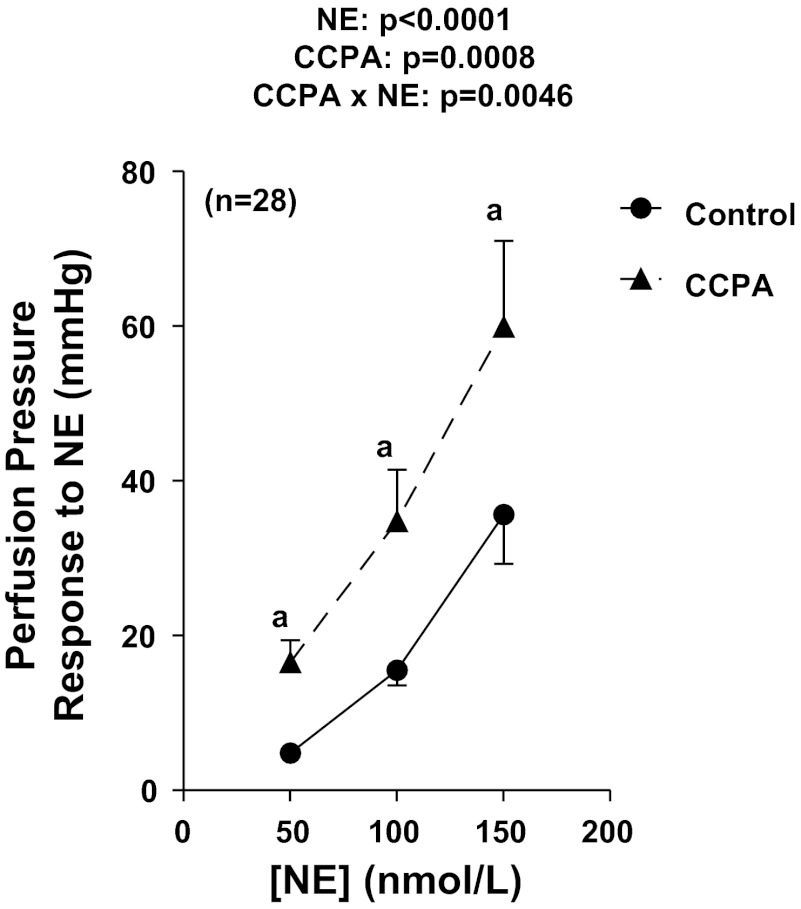
To fully characterize the response of mouse kidneys to RSNS, we first examined renovascular responses to RSNS in kidneys obtained from 28 C57BL/6 male mice. RSNS induced a frequency-dependent increase (P < 0.0001) in perfusion pressure (Fig. 1A); however, the effect reached a maximum at 7 Hz. Consistent with enhanced metabolism of adenine nucleotides, RSNS also caused a frequency-dependent reduction (P < 0.0001) in renal venous 5′-AMP levels (adenosine precursor; Fig. 1B). Although RSNS did not increase renal venous adenosine levels (Fig. 1C), RSNS provoked a frequency-dependent increase (P < 0.0001) in renal venous inosine (Fig. 1D), indicating robust formation of adenosine that was efficiently metabolized to inosine before exiting the kidney. These same 28 isolated, perfused mouse kidneys were also responsive to exogenous NE (Fig. 2). Although CCPA at a high concentration of 100 nmol/l significantly (P < 0.0001) increased basal perfusion pressure, this effect of CCPA on basal perfusion pressure was quantitatively very small (39 ± 1 mmHg before CCPA and 44 ± 2 mmHg during CCPA). More importantly, selective agonism of A1 receptors with CCPA markedly and significantly (P value for the overall effect of CCPA = 0.0008) enhanced renovascular responses to exogenous NE (Fig. 2), suggesting that activation of A1 receptors potentiates renovascular responses to NE. Moreover, the effects of CCPA on responses to exogenous NE were more pronounced at higher concentrations of NE (P value for interaction between effect of CCPA and NE = 0.0046).
Fig. 1.
Line graphs show effects of renal sympathetic nerve stimulation (RSNS) on changes in renal perfusion pressure (A) and renal venous perfusate levels of 5′-AMP (B), adenosine (C), and inosine (D) in 28 kidneys from C57BL/6 mice. P values in panels are from repeated-measures 1-factor ANOVA; aP < 0.05 compared with basal value before RSNS [Fisher's least significant difference (LSD) test]. All values represent means ± SE.
Fig. 2.
Line graph illustrates changes in renal perfusion pressure in response to exogenous norepinephrine (NE) in 28 kidneys from C57BL/6 mice both before and during treatment with 2-chloro-N6-cyclopentyladenosine (CCPA; highly selective A1 receptor agonist). P values in panels are from repeated-measures 2-factor ANOVA; aP < 0.05 compared with corresponding value in the absence of CCPA (Fisher's LSD test). All values represent means ± SE.
Protocol 2.
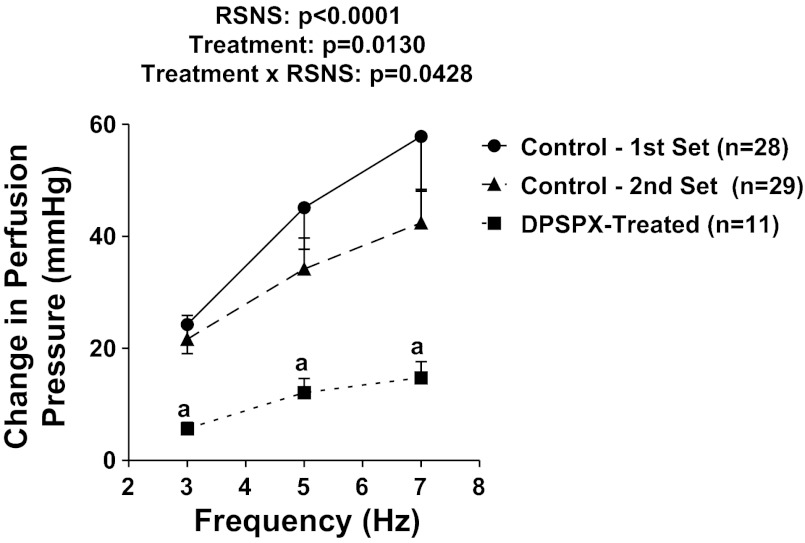
Next, we examined the effects of DPSPX (an adenosine receptor antagonist that does not penetrate cell membranes and therefore does not inhibit intracellular phosphodiesterases) on renovascular responses to RSNS. For comparison, we included two sets of control (untreated) kidneys. The first set of control kidneys (n = 28) was from the experiment performed under protocol 1 (3, 5, and 7 Hz data from Fig. 1A) and was thus not randomized with the DPSPX group. The second set of control kidneys (n = 29) was a separate group randomized with the DPSPX group (n = 11). As shown in Fig. 3, the responses to RSNS in control kidneys were similar in the first vs. second set of control kidneys, indicating the reproducibility of the responses. Importantly, DPSPX significantly suppressed vasoconstrictor responses to RSNS (P value for the overall effect of DPSPX = 0.0130 using the 2nd set of control kidneys for comparison), suggesting that blockade of adenosine receptors attenuates renovascular responses to RSNS. Moreover, the effects of DPSPX on responses to RSNS were more pronounced at higher frequencies of RSNS (P value for interaction between effect of DPSPX and RSNS = 0.0428). DPSPX did not significantly alter basal perfusion pressure (40 ± 2 vs. 44 ± 3 mmHg in control vs. DPSPX-treated kidneys).
Fig. 3.
Line graph summarizes changes in renal perfusion pressure in response to RSNS in 2 sets of control C57BL/6 kidneys and in a group of C57BL/6 kidneys treated with 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX; 100 μmol/l; adenosine receptor antagonist that does not penetrate cell membranes). P values in panels are from nested 2-factor ANOVA comparing the 2nd control set to the DPSPX-treated group; aP < 0.05 compared with corresponding value in the 2nd control set (Fisher's LSD test). All values represent means ± SE.
Protocol 3.
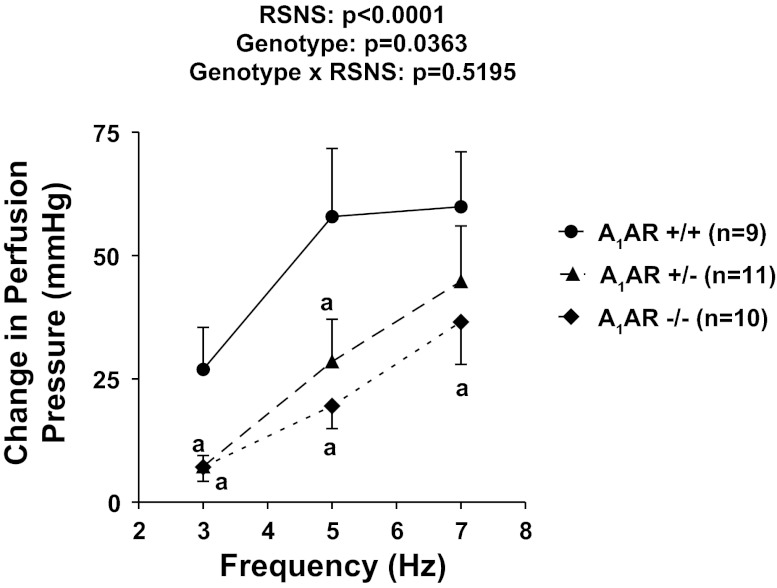
In protocol 3, kidneys were isolated from male and female A1AR−/− (n = 10), A1AR−/+ (n = 11), and A1AR+/+ (n = 9) mice and were subjected to RSNS. As shown in Fig. 4, as with C57BL/6 kidneys, kidneys from A1AR+/+ responded robustly to RSNS. However, renovascular responses to RSNS were reduced in A1AR−/+ kidneys and even more so in A1AR−/− kidneys (P value for effect of genotype = 0.0363). Basal perfusion pressures in A1AR+/+ kidneys (42 ± 2 mmHg) were similar to those observed in C57BL/6 kidneys (42 ± 1 mmHg in 28 C57BL/6 kidneys from control set 1; 40 ± 2 mmHg in 29 C57BL/6 kidneys from control set 2). Notably, basal perfusion pressures were significantly (P = 0.0081) lower in A1AR−/− kidneys (33 ± 2 mmHg) and A1AR−/+ kidneys (36 ± 2 mmHg) compared with the other three groups of kidneys with intact A1 receptors. We also measured concentrations of NE in the renal venous perfusate in a subset of A1AR−/− vs. A1AR+/+ kidneys during RSNS at 7 Hz and found no evidence that NE release was impaired in A1AR−/− kidneys [965 ± 263 (n = 4) vs. 1,064 ± 279 (n = 4) pg/ml in A1AR−/− vs. A1AR+/+].
Fig. 4.
Line graph shows changes in renal perfusion pressure in response to RSNS in homozygous A1 receptor knockout (A1AR−/−), littermate heterozygous (A1AR−/+), and littermate wild-type (A1AR+/+) kidneys. P values in panels are from nested 2-factor ANOVA comparing the 3 different genotypes; aP < 0.05 compared with corresponding value in the A1AR+/+ group (Fisher's LSD test). All values represent means ± SE.
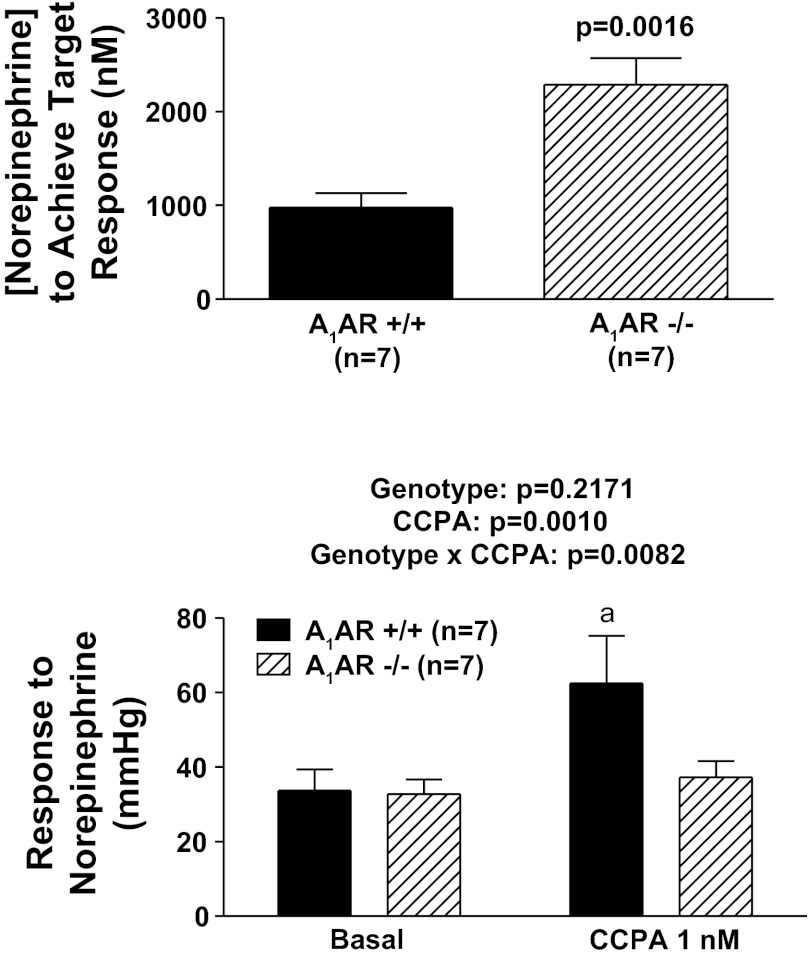
Protocol 4.
Perfused kidneys from A1AR−/− (n = 7) and A1AR+/+ (n = 7) mice were subjected to increasing test concentrations of NE until a concentration was identified that increased perfusion pressure by ∼30 mmHg. Then, three responses to this concentration of NE were induced in the absence of any treatments to establish the reproducibility of the NE-induced renal vasoconstriction. As shown in Fig. 5, a significantly higher concentration of NE (approximately twice as much) was required to induce the same renovascular response in A1AR−/− vs. A1AR+/+ kidneys (P = 0.0016). Next, the concentration of NE that increased renal perfusion pressure to the desired target range was administered to the kidneys in the presence of a very low concentration of CCPA (1 nmol/l) so as to not alter basal perfusion pressure. In A1AR+/+ kidneys, CCPA enhanced the renovascular response to NE, whereas in A1AR−/− kidneys, responses to NE were the same in the absence and presence of CCPA (P value for the interaction between genotype and CCPA = 0.0082).
Fig. 5.
Top: bar graph compares concentration of NE necessary to achieve a target response (change in perfusion pressure of ∼30 mmHg) in A1AR−/− vs. A1AR+/+ kidneys. P value is for unpaired Student's t-test. Bottom: bar graph illustrates effects of CCPA (highly selective A1 receptor agonist) on renovascular responses (changes in perfusion pressure) to exogenous NE in A1AR−/− vs. A1AR+/+ kidneys. P values in panel are from nested 2-factor ANOVA comparing the 2 different genotypes; aP < 0.05 compared with corresponding basal value (Fisher's LSD test). All values represent means ± SE.
DISCUSSION
Our previous report shows that blockade of A1 receptors with pharmacological antagonists decreases renovascular responses to RSNS in isolated, perfused rat kidneys without affecting RSNS-induced release of NE, thus suggesting that endogenous adenosine, via activation of postjunctional A1 receptors in the neuroeffector junction, contributes to renal sympathetic neurotransmission (13). Although carefully conducted pharmacological approaches can provide strong support for a physiological hypothesis, it is always desirable, when possible, to confirm inferences based on pharmacological tools with genetically modified animals. In this regard, the availability of A1AR−/− mice provides an opportunity to corroborate or refute the concept that A1 receptors participate in renal sympathetic neurotransmission.
Employing genetically modified mice for hypothesis testing, however, entails the challenge of adapting methodologies developed for larger animals to mice. Because at the time of this study we had only limited experience with isolated, perfused kidneys from mice, the first objective of the present study was to acquire more reliable information regarding how isolated, perfused kidneys from C57BL/J mice respond to RSNS. Therefore, we examined in a very large number of isolated, perfused kidneys from C57BL/J mice the effects of RSNS over a range of stimulation frequencies on perfusion pressure (an index of vasoconstriction in our constant-flow perfusion system). Importantly, RSNS caused a robust and frequency-dependent renal vasoconstriction in two separate and large cohorts of mice (n = 28 and n = 29). Although examined months apart, the renovascular responses to RSNS were similar in the two groups of mouse kidneys. These data support the reliability of the isolated, perfused mouse kidney as a model system for investigating regulation of the renal sympathetic nervous system. Moreover, as previously noted in isolated, perfused rat kidneys (13), the adenosine receptor antagonist DPSPX attenuated renovascular responses to RSNS compared with either the first or second set of control C57BL/J kidneys, indicating that the isolated, perfused mouse kidney is a useful model in which to examine the role of adenosine receptors in renal sympathetic neurotransmission.
Consistent with our previous experience in a small number of mouse kidneys (20), RSNS caused a robust and frequency-dependent release of the immediate downstream metabolite of adenosine, namely inosine, into the renal venous perfusate. Importantly, although RSNS caused a several-fold increase in the release of inosine, adenosine release was not detectably elevated and the release of 5′-AMP was clearly suppressed. These findings are reconciled by the concept developed by Westfall and co-workers (25) that adenosine is formed in the neuroeffector junction due to release of the cotransmitter ATP that is rapidly metabolized, by releasable nucleotidases (which undergo exocytosis along with ATP), to adenosine (the Westfall mechanism). Release and activation of nucleotidases by RSNS would explain the reduction in 5′-AMP reaching the renal venous effluent and the formation of adenosine in the neuroeffector junction would explain the increase in inosine, but not adenosine, because adenosine in the neuroeffector junction would likely be metabolized to inosine during the passage from its site of production in the neuroeffector junction to the renal vein. Taken together, these data indicate that in the mouse kidney, RSNS does indeed stimulate the metabolism of adenine nucleotides and induces the formation of adenosine in a sequestered compartment, findings consistent with a role for adenosine in renal sympathetic neurotransmission.
An informative result of the present study is that renovascular responses to RSNS clearly were reduced in kidneys obtained from A1AR−/+ and A1AR−/− mice vs. kidneys harvested from A1AR+/+ mice. These results indicate that life-long deletion of A1 receptors in the kidney reduces renal sympathetic neurotransmission. The fact that responses to RSNS are reduced in A1AR−/+ kidneys indicates that even a partial reduction in the expression of A1 receptors compromises renal sympathetic neurotransmission. Also, the fact that renal sympathetic neurotransmission is attenuated in mice with life-long depletion of A1 receptors indicates that with respect to renal sympathetic neurotransmission other receptor systems cannot compensate for the loss of A1 receptors. Thus, the present results corroborate our hypothesis that A1 receptors are critically involved in sympathetic neurotransmission in the kidney.
Our previous findings in isolated, perfused rat kidneys suggest that the mechanism by which endogenous adenosine via the A1 receptor facilitates renal sympathetic neurotransmission does not involve prejunctional effects (13). The present study in the mouse kidney also supports that conclusion because spillover of endogenous NE into the renal venous perfusate is not altered in A1AR−/− kidneys compared with A1AR+/+ kidneys. This conclusion is further evidenced by the fact that activation of prejunctional A1 receptors can, in some organ systems and tissues, attenuate NE release from sympathetic nerve varicosities (4), which would tend to increase, rather than attenuate, sympathetic nerve transmission in A1AR−/− kidneys.
The mechanism by which endogenous adenosine participates in renal sympathetic neurotransmission appears to involve coincident signaling at the postjunctional membrane (13). Coincident signaling is characterized by the convergence of signaling pathways such that one pathway enhances the effects of another pathway because of synergistic actions on a protein coincident detector (21). A classic example of coincident signaling is A1 receptor-induced enhancement of angiotensin II-induced renal vasoconstriction (17, 19). Since NE and angiotensin II signal via Gq-coupled receptors [α1-adrenoceptors (3) and angiotensin II AT1 receptors (18), respectively], it is not surprising that A1 receptors would enhance NE-induced renal vasoconstriction. Indeed, the present study shows that activation of A1 receptors with CCPA augments vasoconstrictor responses to exogenous NE in kidneys from both C57BL/J and A1AR+/+ mice, but not in kidneys from A1AR−/− mice. These findings are completely in line with our previous studies in rat kidneys that show that CCPA augments renovascular responses to exogenous NE via the A1 adenosine receptor (13).
In isolated, perfused mouse arterioles, A1 receptor activation causes profound vasoconstriction (6), suggesting “direct” vasoconstriction. In contrast, the present study shows that in the isolated, perfused mouse kidney, as in isolated, perfused rat kidneys (13), even high concentrations of CCPA have little effect on basal renovascular resistance (for example, 100 nmol/l of CCPA increases renal perfusion pressure, but only by ∼5 mmHg). The difference between isolated, perfused mouse arterioles and isolated, perfused mouse kidneys may relate to the availability of coincident signaling partners for the A1 receptor under basal conditions. That is to say, whether activation of A1 receptors causes renal vasoconstriction in the absence of another agonist may depend on whether a signaling pathway that converges with the A1 receptor signaling pathway is constitutively active under basal conditions.
Additional important evidence supporting a role for the A1 receptor in renal sympathetic neurotransmission is that life-long deletion of the A1 receptor beyond a doubt decreases the renovascular response to exogenous NE. For example, in the present study, the concentration of exogenous NE required to increase renal perfusion pressure by ∼30 mmHg was twice as great in A1AR−/− vs. A1AR+/+ kidneys. Thus, the postjunctional membrane in renal vascular smooth muscle cells lacking A1 receptors is less responsive to exogenous NE. This finding is in line with a previous report that renovascular responses to exogenous angiotensin II are also attenuated in A1AR−/− kidneys (7). The general attenuation of agonist-induced renal vasoconstriction in A1AR−/− kidneys probably explains the slight, but real, reduction in basal renovascular resistance in A1AR−/− and A1AR−/+ kidneys observed in the present study.
Clinical trials involving A1 receptor antagonists indicate that A1 receptor antagonists may be useful as diuretics in acute decompensated heart failure with renal impairment or diuretic resistance (1, 5), as well as in patients with chronic congestive heart failure (2), i.e., conditions associated with increased renal sympathetic tone. The results of the present study, combined with our previous findings in rat kidneys (13), provide solid evidence for a critical role of the A1 receptor in renal sympathetic neurotransmission. Thus, we conclude that a portion of the beneficial effects of A1 antagonists in disease states is due to attenuation of renal sympathetic neurotransmission, and this knowledge may be useful in guiding the selection of patients most likely to benefit from A1 receptor blocker therapy. Finally, it is conceivable that A1 antagonists could facilitate the use of NE to provide systemic support of mean arterial blood pressure in septic shock while preventing unwanted renal vasoconstriction and limiting fluid overload and thus preventing acute renal failure.
GRANTS
This work was supported by National Institutes of Health Grants DK091190, HL109002, HL069846, DK068575, and DK079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.J., J.D.V., and P.M.K. conception and design of research; E.K.J. analyzed data; E.K.J. and P.M.K. interpreted results of experiments; E.K.J. prepared figures; E.K.J. drafted manuscript; E.K.J., J.D.V., and P.M.K. edited and revised manuscript; E.K.J., D.C., Z.M., J.D.V., K.J.-F., and P.M.K. approved final version of manuscript; D.C., Z.M., and K.J.-F. performed experiments.
ACKNOWLEDGMENTS
We thank Dr. Jürgen B. Schnermann (National Institute of Diabetes and Digestive and Kidney Diseases) for contributing the breeding pairs of A1AR knockout mice that were used to establish a colony of these mice at the University of Pittsburgh.
REFERENCES
- 1. Cotter G, Dittrich HC, Weatherley BD, Bloomfield DM, O'Connor CM, Metra M, Massie BM. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail 14: 631– 640, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Dittrich HC, Gupta DK, Hack TC, Dowling T, Callahan J, Thomson S. The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. J Card Fail 13: 609– 617, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Docherty JR. Subtypes of functional α1-adrenoceptor. Cell Mol Life Sci 67: 405– 417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fredholm BB, Duner-Engstrom M, Fastbom J, Hu PS, van der Ploeg I. Role of G proteins, cyclic AMP, and ion channels in the inhibition of transmitter release by adenosine. Ann NY Acad Sci 604: 276– 288, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effects of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 50: 1551– 1560, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457– 2465, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Hansen PB, Hashimoto S, Briggs J, Schnermann J. Attenuated renovascular constrictor responses to angiotensin II in adenosine 1 receptor knockout mice. Am J Physiol Regul Integr Comp Physiol 285: R44– R49, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Haselkorn ML, Shellington D, Jackson E, Vagni VA, Janesko KL, Dubey RK, Gillespie DG, Cheng D, Bell MJ, Jenkins LW, Homanics GE, Schnermann J, Kochanek PM. Adenosine A1 receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. J Neurotrauma 27: 901– 910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hocher B. Adenosine A1 receptor antagonists in clinical research and development. Kidney Int 78: 438– 445, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Hocher B, Heiden S, von Websky K, Arafat AM, Rahnenführer J, Alter M, Kalk P, Ziegler D, Fischer Y, Pfab T. Renal effects of the novel selective adenosine A1 receptor blocker SLV329 in experimental liver cirrhosis in rats. PLos One 6: e17891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson EK. A1 receptor antagonists as diuretic/natriuretic agents. Drugs Future 27: 1057– 1069, 2002 [Google Scholar]
- 12. Jackson EK. P1 and P2 receptors in the renal system. In: Handbook of Experimental Pharmacology Volume 151/II; Purinergic and Pryimidinergic Signalling II: Cardiovascular, Respiratory, Immune, Metabolic and Gastrointestinal Tract Function, edited by Abbrachio M, Williams M. Berlin: Springer-Verlag, 2001, p. 33–71 [Google Scholar]
- 13. Jackson EK, Cheng D, Tofovic SP, Mi Z. Endogenous adenosine contributes to renal sympathetic neurotransmission via postjunctional A1 receptor-mediated coincident signaling. Am J Physiol Renal Physiol 302: F466– F476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097– 33106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobson KA, Knutsen LJS. P1 and P2 purine and pyrimidine receptor ligands. In: Purinergic and Pyrmidinergic Signalling I, edited by Abbracchio MP, Williams M. Berlin: Springer-Verlag, 2001, p. 129–175 [Google Scholar]
- 16. Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RSB, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab 26: 565– 575, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AEG. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690– 698, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82– C97, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens 12: 497– 502, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Ren J, Mi ZC, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther 325: 920– 926, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci 19: 87– 93, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther 256: 850– 860, 1991 [PubMed] [Google Scholar]
- 23. Vallon V, Miracle C, Thomson S. Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail 10: 176– 187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welch WJ. Adenosine type 1 receptor antagonists in fluid retaining disorders. Expert Opin Investig Drugs 11: 1553– 1562, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther 303: 439– 444, 2002 [DOI] [PubMed] [Google Scholar]