Abstract
Activation of the glucagon-like peptide (GLP)-1 receptor (GLP-1R) and inhibition of dipeptidyl peptidase-4 (DPP-4) are new antidiabetic strategies. The GLP-1R and DPP-4 are also expressed in the renal proximal tubular brush border, where they may regulate Na+ reabsorption. Exendin-4 (EX4) is a naturally occurring antidiabetic polypeptide (from the saliva of the lizard Heloderma suspectum) and GLP-1R agonist; however, part of its nonglucoregulatory effects are through GLP-1R-independent mechanisms. DPP-4 cleaves and inactivates GLP-1; thus the natriuretic effect of DPP-4 inhibition may be mediated by the GLP-1R. We report that parenteral application of EX4 in wild-type mice induced a diuresis and natriuresis associated with increases in glomerular filtration rate, fractional urinary fluid and Na+ excretion, and renal membrane expression of the Na+/H+ exchanger NHE3 phosphorylated at S552 and S605, established consensus sites for cAMP-dependent PKA. These effects were absent in mice lacking the GLP-1R and independent of adenylyl cyclase 6. In comparison, parenteral application of the DPP-4 inhibitor alogliptin reduced plasma DPP-4 activity by 95% and induced a diuresis and natriuresis independent of the presence of the GLP-1R or changes in phosphorylated NHE3. The inhibitory effect on renal fluid and Na+ reabsorption of EX4, but not alogliptin, was preserved in diabetic db/db mice and associated with a modest reduction in blood pressure. These results reveal mechanistic differences in how EX4 vs. DPP-4 inhibition induces diuresis and natriuresis under normal states, with preservation of GLP-1R-mediated, but not DPP-4 inhibitor-dependent, natriuretic mechanisms in a mouse model of obese type 2 diabetes.
Keywords: glucagon-like peptide-1, dipeptidyl peptidase-4, NHE3, cAMP, proximal tubule
glucagon-like peptide -1 (GLP-1), an incretin hormone secreted from enteroendocrine L cells in the intestine, stimulates glucose-dependent insulin release and may promote preservation of beta-cell function in patients with type 2 diabetes (9, 10). As a consequence, GLP-1 has been a principal focus of clinical and basic diabetes research in recent years. After secretion, active GLP-1 is rapidly cleaved by the widely expressed enzyme dipeptidyl peptidase-4 (DPP-4, CD26), such that the half-life of bioactive GLP-1 is <3 min. Therefore, therapeutic manipulation of the GLP-1 system includes strategies that inhibit the degradation of GLP-1 by DPP-4 (i.e., DPP-4 inhibitors) or degradation-resistant GLP-1 receptor (GLP-1R) agonists with a longer half-life, such as exendin-4 (EX4) or liraglutide.
In addition to its metabolic effects, GLP-1 affects kidney function. Analysis of GLP-1R expression in rats (6), pigs, and humans (34) localized the GLP-1R to the brush border microvilli of proximal tubules. Intravenous infusion of GLP-1 increased glomerular filtration rate (GFR), inhibited proximal tubular reabsorption, and increased urine flow and Na+ excretion in rats (6, 30). In healthy subjects, infusion of GLP-1 evoked a dose-dependent increase in urinary Na+ excretion without changing GFR (20, 21). Studies in intact rat and porcine renal proximal tubules indicated that GLP-1 decreases Na+/H+ exchanger (NHE3)-mediated bicarbonate reabsorption (6, 34). Moreover, GLP-1 increased expression of NHE3 phosphorylated at S552 and S605, two PKA consensus sites, without changing total NHE3 expression, in rat proximal tubular brush border microvilli (6). Similar effects on proximal tubular bicarbonate flux and NHE3 phosphorylation were observed following EX4 administration in the rat (6). Thus there is strong evidence that GLP-1 and related GLP-1R agonists not only mediate important effects on glucose homeostasis, but also stimulate renal excretion of fluid and Na+ in rats and humans.
The current studies tested whether EX4 also induces a natriuretic effect in the mouse and whether this includes effects on GFR and fractional renal Na+ excretion. It is known that GLP-1R agonists may mediate nonglucoregulatory effects, at least in part through GLP-1R-independent mechanisms (1). Moreover, EX4 has only 54% amino acid identity to GLP-1 (18). Therefore, we used a mouse model that lacks this receptor to determine whether the renal effects of EX4 are dependent on the presence of an intact GLP-1R.
DPP-4 inactivates GLP-1, is present in plasma and on the surface of capillary endothelial cells, and is one of the major proteins expressed in the apical brush border membrane of the proximal tubule (25), where it assembles with NHE3 (15). The DPP-4 inhibitors Lys [Z(NO2)]-pyrrolidide and P32/98 inhibited NHE3-mediated Na+ reabsorption in rat renal proximal tubule in vivo (6, 16). Studies in an opossum kidney proximal tubule cell line indicate that the enzymatic activity of DPP-4 in the proximal tubular brush border may locally affect the generation or breakdown of endogenous factors that regulate Na+ reabsorption via effects on NHE3 activity (17). We tested the renal effects of alogliptin (ALG), a novel, high-affinity, high-specificity DDP-4 inhibitor, in the mouse (5, 7, 13). We hypothesized that the natriuretic effect of ALG involves inhibition of the breakdown of GLP-1 and, thus, depends on an intact GLP-1R.
Studies in humans demonstrate that obese men responded to GLP-1 infusion with a natriuresis, but, in contrast to healthy subjects, this natriuresis was associated with a decrease in GFR (21), indicating that factors related to body mass may affect the renal response to EX4 and/or DPP-4 inhibition. Therefore, we also compared the effect of EX4 and ALG on renal function in obese type 2 diabetic db/db mice.
MATERIALS AND METHODS
Animal experiments were conducted according to protocols reviewed and approved by the Institutional Animal Care and Use Committee of the Veterans Affairs San Diego Healthcare System. Heterozygote breeding strategies were used to yield GLP-1R-deficient (Glp1r−/−) and littermate wild-type (WT) mice (35), as well as adenylyl cyclase (AC) type VI (AC6)-deficient and littermate WT mice (37). Homozygous C57BLKS/J db/db mice (Jackson Laboratories, Bar Harbor, ME) were used as an obese type 2 diabetic model, and littermate heterozygote db/− mice served as controls. Age- and gender-matched adult mice were used.
Metabolic cage experiments in awake mice.
Mice were randomized to application of EX4 (10 μg/kg ip; Sigma-Aldrich, St. Louis, MO), ALG (10 mg/kg ip; Takeda Pharmaceuticals, Oak Grove, IL), or vehicle (0.85% NaCl, 2 μl/g body wt ip). After their bladders were emptied, the mice were NaCl-loaded by oral gavage (0.85% NaCl, 30 μl/g body wt, ∼30% of daily NaCl intake) and placed in metabolic cages for quantitative urine collection over 3 h without access to food or water (32, 33); then blood glucose was measured by tail snip.
Two-period clearance experiments to assess GFR and absolute and fractional renal excretion.
Mice were anesthetized with thiobutabarbital (100 mg/kg ip, 2 μl/g body wt; Sigma-Aldrich, St. Louis, MO) and ketamine (100 mg/kg im, 2 μl/g body wt; Butler, Dublin, OH) and prepared for renal clearance experiments, as described elsewhere (31, 32). The jugular vein was cannulated for continuous infusion of 2.25% bovine serum albumin in 0.85% NaCl at 0.4 ml·h−1·30 g body wt−1. For assessment of two-kidney GFR by inulin clearance, [3H]inulin was added to the infusion to deliver 5 μCi·h−1·30 g body wt−1. GFR and urinary excretion of fluid, Na+, and K+ were assessed by quantitative urine collection via a bladder catheter in two 30-min periods: after completion of a basal period, EX4 (10 μg/kg), ALG (10 mg/kg), or vehicle (0.85% NaCl, 1 μl/g body wt) was given by intravenous bolus application, and 5 min later the second period was started. Blood samples (50 μl) were drawn midway in each period from an arterial catheter, which was also used to monitor blood pressure and heart rate. Concentrations of [3H]inulin in plasma and urine were measured by liquid scintillation counting. Plasma and urine were analyzed for Na+ and K+ concentrations by flame photometry (Cole-Parmer Instrument, Vernon Hills, IL). cAMP concentrations were assessed by radioimmunoassay (33). In a separate set of two-period clearance studies in WT mice, parathyroid hormone (10 μg/kg iv bolus) was administered, and 5 min later the second period was started to measure urinary cAMP excretion.
Expression of total and phosphorylated NHE3 in renal membranes.
Kidneys were harvested 1 h after application of EX4 (10 μg/kg ip), ALG (10 mg/kg ip), or vehicle (0.85% NaCl, 2 μl/g body wt). Renal membranes were prepared in the presence of protease and phosphatase inhibitors, as previously described (33, 39). Immunoblotting was performed at 4°C overnight with the primary NHE3 Ab (Millipore, Billerica, MA), phosphorylated (S552) NHE3 Ab (Novus Biologicals, Littleton, CO), and phosphorylated (S605) NHE3 Ab (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:1,000, 1:1,000, and 1:200, respectively. The latter two antibodies recognize NHE only when S552 or S605 is phosphorylated (27). Chemiluminescent detection was performed using a 1:5,000 dilution of enhanced chemiluminescent (ECL) donkey anti-rabbit IgG and anti-mouse IgG linked to horseradish peroxidase and ECL detection reagent (GE Healthcare, Buckinghamshire, UK). For verification of equal protein loading, the membrane was stripped (0.2 M NaOH for 5 min) and reprobed with monoclonal anti-β-actin Ab (Sigma-Aldrich).
DPP-4 activity in plasma.
Plasma DPP-4 activity was measured using a homogeneous luminescent assay (DPPIV-Glo Protease Assay, Promega, San Luis Obispo, CA) according to the manufacturer's instruction. For each sample, specific DPP-4 activity was determined by comparison of measurements in the presence and absence of ALG (1 μM) in the incubation.
Statistical analysis.
Values are means ± SE. Unpaired and paired t-tests were performed, as appropriate, to analyze for statistical differences between and within groups. P < 0.05 was considered statistically significant. The contribution of GLP-1R to the renal response to EX4 was determined by comparison of changes in WT vs. Glp1r−/− mice.
RESULTS
Basal kidney function in Glp1r−/− mice.
Glp1r−/− and littermate WT mice had similar body weight (27 ± 1 vs. 27 ± 1 g), food and fluid intake [determined in regular cages: 0.14 ± 0.01 vs. 0.14 ± 0.01 g·day−1·g body wt−1 and 0.15 ± 0.01 vs. 0.15 ± 0.01 ml·day−1·g body wt−1, respectively, n = 22–26, P = not significant (NS)], and plasma aldosterone concentration (879 ± 115 vs. 935 ± 65 pg/ml, n = 9, P = NS). Clearance studies under thiobutabarbital-ketamine anesthesia revealed similar blood pressure, heart rate, hematocrit, plasma Na+ and K+ concentrations, and absolute and fractional urinary excretion of H2O, Na+, and K+, while GFR was modestly greater in Glp1r−/− than WT mice (Table 1).
Table 1.
Basal parameters in WT and Glp1r−/− mice during clearance experiments
| WT (n = 12) | Glp1r−/− (n = 11) | |
|---|---|---|
| Body weight, g | 29 ± 1 | 30 ± 1 |
| Mean arterial blood pressure, mmHg | 70 ± 3 | 76 ± 2 |
| Heart rate, min−1 | 445 ± 6 | 449 ± 13 |
| Hematocrit, % | 43 ± 1 | 43 ± 1 |
| Plasma Na+, mM | 148 ± 3 | 150 ± 3 |
| Plasma K+, mM | 3.8 ± 0.2 | 3.8 ± 0.2 |
| GFR, μl/min | 373 ± 29 | 459 ± 23* |
| Urinary H2O excretion, nl· min−1 ·g body wt−1 | 45 ± 5 | 47 ± 5 |
| Urinary Na+ excretion, nmol·min−1·g body wt−1 | 7 ± 1 | 10 ± 2 |
| Urinary K+ excretion, nmol·min−1·g body wt−1 | 15 ± 2 | 18 ± 1 |
| Fractional H2O excretion, % | 0.38 ± 0.06 | 0.30 ± 0.03 |
| Fractional Na+ excretion, % | 0.39 ± 0.04 | 0.41 ± 0.05 |
| Fractional K+ excretion, % | 33 ± 3 | 31 ± 2 |
| Urinary cAMP excretion, pmol·min−1·g body wt−1 | 0.67 ± 0.16 | 1.18 ± 0.15* |
Values are means ± SE. Glp1r−/−, glucagon-like peptide-1 receptor-deficient mice; GFR, glomerular filtration rate.
P < 0.05 vs. wild-type (WT).
EX4-induced diuresis, natriuresis, and NHE3 phosphorylation in awake mice requires an intact GLP-1R.
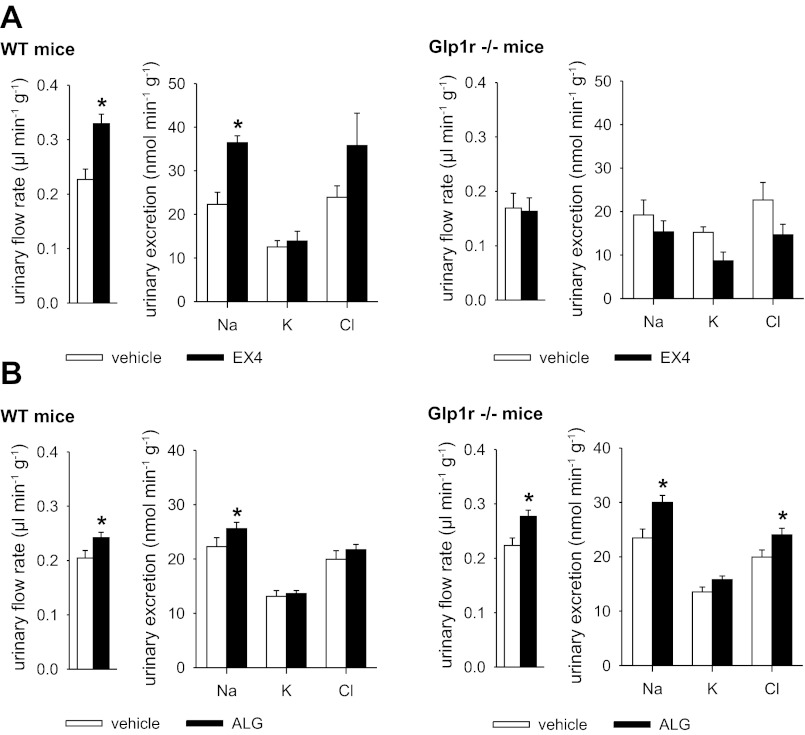
Studies in metabolic cages revealed that urinary excretion of H2O, Na+, K+, and Cl− (Fig. 1A) and blood glucose levels (154 ± 9 vs. 147 ± 8 mg/dl) was not different between WT and Glp1r−/− mice following an oral NaCl and water load with subsequent quantitative urine collection for 3 h (vehicle groups). Compared with vehicle, EX4 (10 μg/kg ip), the 39-amino acid naturally occurring GLP-1R agonist (12), which is highly resistant to the proteolytic action of DPP-4 in vivo (3, 8), lowered blood glucose levels in WT mice (104 ± 8 vs. 154 ± 9 mg/dl, P < 0.05) and increased urinary excretion of H2O and Na+, whereas K+ excretion was not significantly changed (Fig. 1A). In comparison, EX4 did not affect blood glucose levels (146 ± 6 vs. 147 ± 8 mg/dl, P = NS) or urinary H2O or Na+ excretion in Glp1r−/− mice (Fig. 1A).
Fig. 1.
Natriuretic response to exendin-4 (EX4), but not alogliptin (ALG), is blunted in awake mice lacking the glucogon-like peptide (GLP)-1 receptor (GLP-1R). GLP-1R-deficient (Glp1r−/−) and wild-type (WT) mice were randomized to application of EX4 (10 μg/kg body wt ip), ALG (10 mg/kg body wt ip), or vehicle (0.85% NaCl, 2 μl/g body wt) followed by oral gavage with isotonic saline (30 μl/g, ∼30% of daily NaCl intake), and urinary excretion in metabolic cages over 3 h was determined. A: EX4 increased urinary flow rate and Na+ excretion in WT, but not Glp1r−/−, mice. B: natriuretic effect of ALG was similar in both genotypes. Values are means ± SE; n = 5–6 per group. *P < 0.05 vs. vehicle.
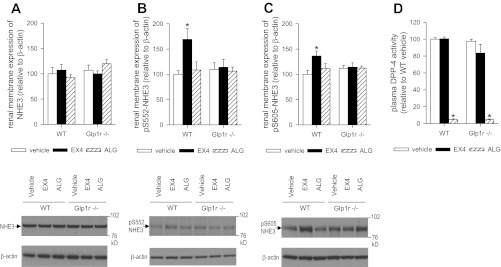
Total renal membrane expression of NHE3 in WT mice was not different from that in Glp1r−/− mice and was not altered by EX4 (Fig. 2A). EX4 increased the amount of NHE3 phosphorylated at S552 and S605 in renal membranes of WT, but not Glp1r−/−, mice (Fig. 2, B and C).
Fig. 2.
Effects of EX4 and ALG on phosphorylated Na+/H+ exchanger (NHE3) in renal membranes and plasma dipeptidyl peptidase-4 (DPP-4) activity. Kidneys and plasma were harvested 1 h after application of EX4 (10 μg/kg body wt ip), ALG (10 mg/kg body wt ip), or vehicle (0.85% NaCl, 2 μl/g body wt). A–C: natriuretic response to EX4, but not ALG, was associated with increased NHE3 phosphorylated at S552 (pS552) and S605 (pS605) in renal membranes. This effect of EX4 was absent in Glp1r−/− mice. Values are means ± SE; n = 5 per group. *P < 0.05 vs. vehicle. D: ALG inhibited DPP-4 activity in WT and Glp1r−/− mice. Data are expressed relative to the mean of the vehicle-treated WT group, which was set as 100%. EX4 was without effect. Values are means ± SE; n = 5 per group. *P < 0.05 vs. vehicle.
ALG-induced diuresis and natriuresis are independent of an intact GLP-1R and changes in NHE3 phosphorylation in awake mice.
ALG (10 mg/kg ip) administered in metabolic cage studies increased urinary excretion of fluid and Na+ in WT and Glp1r−/− mice but did not significantly change K+ excretion (Fig. 1B). In contrast to EX4, ALG did not affect the amount of NHE3 phosphorylated at S552 and S605 in renal membranes (Fig. 2, B and C), although the drug strongly inhibited plasma DPP-4 activity in WT and Glp1r−/− mice (Fig. 2D).
EX4-induced increases in GFR and fractional urinary Na+ excretion in anesthetized mice depend on an intact GLP-1R.
Two-period clearance experiments under anesthesia were performed in Glp1r−/− and WT mice with a basal period followed by EX4 administration 5 min prior to the second period. Figure 3A shows the changes in the second period compared with the basal period. Changes in blood pressure and heart rate in the second period were not different between WT and Glp1r−/− mice (9 ± 2 vs. 9 ± 4% and 5 ± 2 vs. 5 ± 2%, respectively, P = NS for both). EX4 increased GFR, urinary flow rate, and absolute and fractional urinary Na+ excretion in WT compared with Glp1r−/− mice (Fig. 3A). Thus, consistent with the metabolic cage studies in awake mice, the EX4-induced natriuresis depends on an intact GLP-1R and was the consequence of an increase in GFR and a modest decrease in fractional Na+ reabsorption.
Fig. 3.
EX4-induced increases in glomerular filtration rate (GFR) and fractional urinary Na+ excretion in anesthetized mice depend on an intact GLP-1R. A and B: 2-period clearance experiments [a basal period followed by application of EX4 (10 μg/kg body wt iv) or parathyroid hormone (PTH, 10 μg/kg body wt iv) 5 min prior to the second period] were performed under thiobutabarbital-ketamine anesthesia. Results from the basal periods of the EX4 series are summarized in Table 1. Changes in the second period vs. the basal period are as follows: EX4 increased GFR, urinary flow rate, and absolute and fractional urinary Na+ excretion in WT vs. Glp1r−/− mice but did not change urinary cAMP excretion; PTH increased urinary cAMP excretion. Values are means ± SE; n = 8–12 per group. *P < 0.05 vs. Glp1r−/−. (A) and vs. period 1 (B).
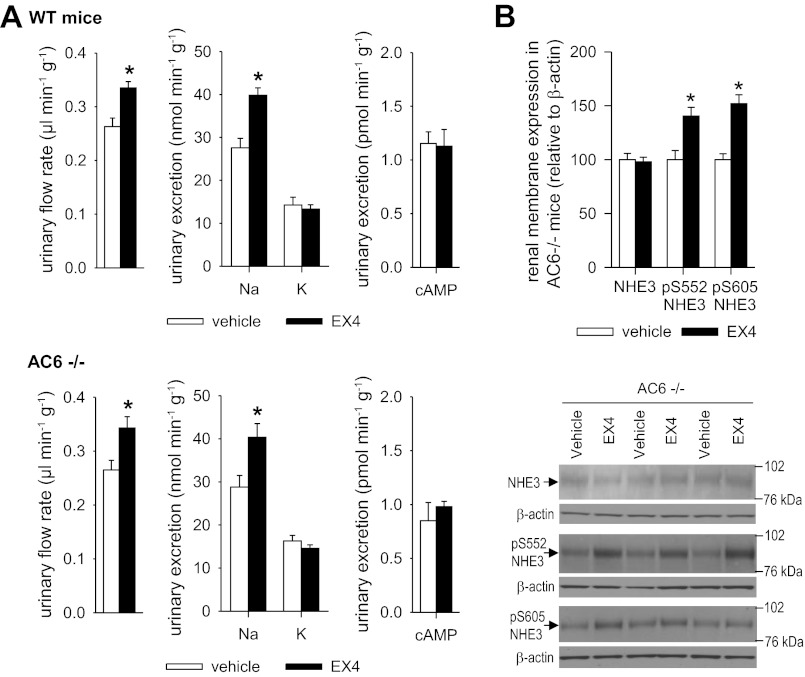
EX4-induced natriuresis and NHE3 phosphorylation occur without changes in urinary cAMP and are not affected by the absence of AC6.
Basal urinary excretion of cAMP in clearance studies under anesthesia was increased in Glp1r−/− compared with WT mice (Table 1). EX4 did not significantly change urinary excretion of cAMP in clearance studies (Fig. 3A) or in metabolic cage studies (Fig. 4A). In contrast, parathyroid hormone increased urinary cAMP excretion in WT mice in the experimental setting of the clearance studies (Fig. 3B). EX4-induced diuresis and natriuresis and phosphorylation of NHE3 were not affected by the absence of AC6 (Fig. 4).
Fig. 4.
Natriuresis and increase in NHE3 phosphorylation in response to EX4 are preserved in awake adenylyl cyclase type VI (AC6)-deficient (AC6−/−) mice. A: AC6−/− and WT mice were randomized to application of EX4 (10 μg/kg body wt ip) or vehicle (0.85% NaCl, 2 μl/g body wt) followed by oral gavage with isotonic saline (30 μl/g; ∼30% of daily NaCl intake), and urinary excretion in metabolic cages over 3 h was determined. Natriuretic effect of EX4 was similar in both genotypes. EX4 did not change urinary cAMP excretion. Values are means ± SE; n = 5–6 per group. *P < 0.05 vs. vehicle. B: kidneys were harvested from AC6−/− mice 1 h after application of EX4 (10 μg/kg body wt ip) or vehicle (0.85% NaCl, 2 μl/g body wt). Phosphorylation of renal NHE3 at S552 and S605 was preserved in AC6−/− mice. Values are means ± SE; n = 6 per group. *P < 0.05 vs. vehicle.
Inhibitory effect of EX4 on renal Na+ reabsorption, but not the stimulatory effect on GFR, is preserved in db/db mice.
Two-period clearance experiments [a basal period followed by administration of EX4 (10 μg/kg iv) or vehicle 5 min prior to the second period] under anesthesia were performed in hyperglycemic db/db and heterozygous nondiabetic db/− (control) mice. Table 2 compares data obtained in basal periods in db/db and control mice. As expected, db/db mice were heavier than control mice and hyperglycemic. Hematocrit, blood pressure, heart rate, and plasma Na+ concentration were similar between groups. Absolute GFR and renal excretion of H2O and Na+ were not different between groups but were lower in db/db mice than controls when related to body weight. Lower plasma K+ concentrations in db/db mice were associated with greater renal fractional K+ excretion and urinary K+-to-Na+ ratios.
Table 2.
Basal parameters in db/db and control mice during clearance experiments
| Control (n = 22) | db/db (n = 25) | |
|---|---|---|
| Body weight, g | 26 ± 1 | 46 ± 1* |
| Blood glucose, mg/dl | 156 ± 9 | 441 ± 20* |
| Hematocrit, % | 44 ± 1 | 45 ± 1 |
| Mean arterial blood pressure, mmHg | 89 ± 2 | 88 ± 3 |
| Heart rate, min−1 | 427 ± 12 | 441 ± 16 |
| Plasma Na+, mM | 158 ± 1 | 156 ± 2 |
| Plasma K+, mM | 4.2 ± 0.1 | 3.7 ± 0.1* |
| GFR | ||
| μl/min | 428 ± 22 | 450 ± 20 |
| μl·min−1·g body wt−1 | 16.5 ± 0.8 | 9.7 ± 0.4* |
| Urinary H2O excretion | ||
| nl/min | 1.8 ± 0.2 | 2.6 ± 0.3 |
| nl·min−1·g body wt−1 | 68 ± 6 | 57 ± 5 |
| Urinary Na+ excretion | ||
| nmol/min | 558 ± 56 | 488 ± 77 |
| nmol·min−1·g body wt−1 | 21 ± 2 | 11 ± 2* |
| Urinary K+ excretion | ||
| nmol/min | 523 ± 33 | 668 ± 43 |
| nmol·min−1·g body wt−1 | 20 ± 1 | 14 ± 1* |
| Fractional H2O excretion, % | 0.43 ± 0.04 | 0.58 ± 0.05 |
| Fractional Na+ excretion, % | 0.83 ± 0.08 | 0.69 ± 0.10 |
| Fractional K+ excretion, % | 31 ± 2 | 43 ± 3* |
| Urinary K+-to-Na+ ratio | 1.1 ± 0.1 | 1.9 ± 0.2* |
Values are means ± SE.
P < 0.05 vs. control.
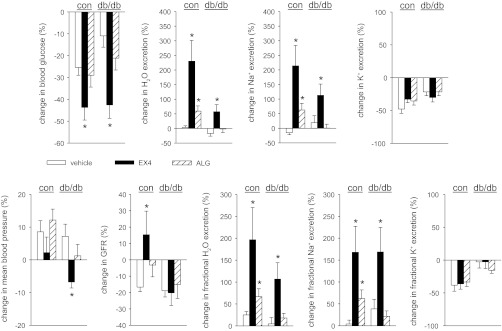
Figure 5 shows the effects of EX4 compared with vehicle in control and db/db mice. Changes in blood pressure were not different between treatments in control mice. Similar to the responses in C57BL/6 (WT) mice (see above), in control mice, EX4 lowered blood glucose levels and increased GFR, urinary flow rate, and absolute and fractional urinary Na+ excretion without altering renal K+ excretion compared with vehicle. EX4 lowered blood glucose levels in control and db/db mice (Fig. 5). EX4 modestly lowered blood pressure in db/db mice but did not alter GFR compared with vehicle; yet, and as observed in controls, EX4 increased fractional renal excretion of H2O and Na+ in db/db mice without altering renal K+ excretion.
Fig. 5.
Effects of EX4 and ALG on blood glucose, mean blood pressure, and renal function in diabetic (db/db) and nondiabetic [db/− (con)] mice. Two-period clearance experiments [a basal period followed by application of EX4 (10 μg/kg body wt iv), ALG (10 mg/kg body wt iv), or vehicle (0.85% NaCl, 1 μl/g body wt) 5 min prior to the second period] were performed under thiobutabarbital-ketamine anesthesia. Results from the basal periods are summarized in Table 2. Responses to EX4, ALG, and vehicle in the second period are as follows: in nondiabetic control mice, EX4 and ALG increased fractional H2O and Na+ excretion vs. vehicle without altering renal K+ excretion, and EX4 lowered blood glucose and increased GFR; in diabetic db/db mice, only EX4 lowered blood glucose and increased fractional H2O and Na+ excretion, and EX4 modestly lowered mean blood pressure and did not increase GFR. Values are means ± SE; n = 6–10 per group. *P < 0.05 vs. vehicle.
Inhibitory effect of ALG on renal H2O and Na+ reabsorption is blunted in db/db mice.
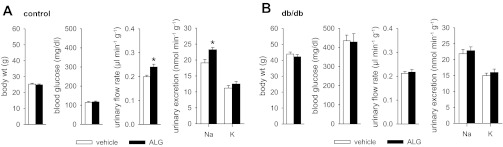
Two-period clearance experiments with application of ALG (10 mg/kg iv) prior to the second period revealed that changes in blood pressure were not different between ALG and vehicle treatment in db/db and control mice. In controls, ALG tended to increase GFR compared with vehicle (P = 0.058); this was associated with an increase in absolute and fractional urinary H2O and Na+ excretion without an alteration of renal K+ excretion (Fig. 5). As observed in controls, in db/db mice, intravenous application of ALG did not significantly lower blood glucose levels compared with vehicle (Fig. 5). In contrast to EX4, ALG did not significantly affect renal H2O or Na+ excretion in db/db mice (Fig. 5). Additional metabolic cage experiments in awake mice confirmed that ALG (10 mg/kg ip) increased urinary Na+ excretion in control, but not db/db, mice (Fig. 6). Moreover, ALG at 30 mg/kg ip did not lower blood glucose levels in control (109 ± 4 mg/dl, n = 10, P = NS) or db/db (408 ± 40 mg/dl, n = 10, P = NS) mice compared with vehicle at 3 h after application and significantly increased Na+ excretion in controls (22.6 ± 1.0 vs. 19.1 ± 1.1 nmol·min−1·g−1, P < 0.05 vs. vehicle), but not in db/db mice (23.6 ± 1.6 21.9 ± 1.4 nmol·min−1·g−1, P = NS).
Fig. 6.
ALG differentially affects urinary Na+ excretion in awake control (A) vs. db/db (B) mice. Mice were randomized to application of ALG (10 mg/kg body wt ip) or vehicle (0.85% NaCl, 2 μl/g body wt) followed by oral gavage with isotonic saline (30 μl/g body wt), and urinary excretion in metabolic cages over 3 h and then blood glucose measurements were determined. Values are means ± SE; n = 10–15 per group. *P < 0.05 vs. vehicle.
DISCUSSION
The present study shows that EX4 induces a diuresis and natriuresis due to an increase in GFR and reduction of fractional renal fluid and Na+ reabsorption. These findings in mice are in accordance with previous studies in rats (6). Gene knockout and WT mice were used to show that this response requires a functional GLP-1R.
In rats, the increase in GFR in response to GLP-1 and EX4 has been associated with an increase in renal blood flow (6), indicating a primary vascular effect. GLP-1 and GLP-1-(9–36) induced vasodilation and increased coronary flow in constant-pressure-perfused isolated hearts (1). These effects were at least in part nitric oxide/cGMP-dependent and maintained in Glp1r−/− mice, suggesting a GLP-1R-independent mechanism. Notably, EX4 did not produce vasodilatation or cGMP release in that preparation (1). In comparison, GLP-1 induced vasorelaxation in the rat aorta through classic GLP-1R-dependent AC-coupled mechanisms (19). Here we show that the effect of EX4 on GFR and, thus potentially on the renal vasculature, is dependent on an intact GLP-1R.
Natriuretic effects of GLP-1 and EX4 in the rat have been linked to the phosphorylation and inhibition of NHE3 in the proximal tubule (6). We show that the natriuretic effect of EX4 in the mouse is also associated with phosphorylation of NHE3 at S552 and S605 and that the changes in NHE3 phosphorylation are dependent on an intact GLP-1R. S552 and S605, two consensus sites for PKA on NHE3, are physiologically regulated in vitro (27) and in vivo (26, 27). However, phosphorylation of these sites does not directly alter NHE activity (26). Thus GLP-1R activation induces phosphorylation of NHE3 at S552 and S605; however, further studies are needed to understand the relevance and role of NHE3 phosphorylation in the natriuretic effects of GLP-1 and EX4.
The GLP-1R is a G protein-coupled receptor, and its activation stimulates the formation of cAMP (14). Systemic application of GLP-1 and EX4 at 1–5 μg/kg increased urinary cAMP excretion in rats by 20-fold, and the PKA inhibitor H-89 prevented the inhibitory effect of GLP-1 on bicarbonate reabsorption in renal proximal tubule (6). We found that the EX4-induced and GLP-1R-mediated natriuresis and NHE3 phosphorylation in the mouse are not associated with an increase in urinary cAMP excretion and persisted in AC6-deficient mice. AC6 is the most abundant AC isoform in rat whole kidney (36) and mouse medulla (33) on the basis of mRNA expression and the dominant AC isoform with regard to forskolin-induced cAMP formation in mouse kidney (4) and inner medulla (33). It could be that the impact of proximal tubule release on total urinary cAMP excretion is lower in mice than rats, although application of parathyroid hormone, which is thought to activate proximal tubular AC, increased urinary cAMP-to-creatinine ratios threefold in mice (43), a finding confirmed in the present studies. One may speculate that activation of proximal tubular AC by different receptors causes cAMP to enter the tubular lumen at different rates. Furthermore, an AC isoform other than AC6 may be activated by GLP-1Rs in the proximal tubule, which in the rat expresses AC2, AC3, AC6, AC7, and AC9 (2).
ALG at 10 mg/kg ip inhibited plasma DPP-4 activity by ∼95% and, in nondiabetic mice, induced a diuresis and natriuresis associated with an increase in fractional renal excretion of fluid and Na+. ALG tended to increase GFR, but, similar to a previous study of the DPP-4 inhibitor P32/98 in the rat (6), the effect did not quite reach statistical significance (P = 0.058). Overall, it appears that DPP-4 inhibition can induce a small increase in GFR [and renal plasma flow (6)] in nondiabetic rats and mice, but this effect appears to be smaller than the effect of direct GLP-1R activation (present study; 6).
Studies in an opossum kidney proximal tubule cell line showed that inhibitors of DPP-4 (diprotin A and P32/98) significantly reduced NHE3 activity (17). Treatment of rats with the DPP-4 inhibitor Lys [Z(NO2)]-pyrrolidide for 7 days decreased NHE activity in isolated proximal tubular microvillar membrane vesicles, redistributed NHE3 from the apical enriched microvillar membranes to the intermicrovillar microdomain of the brush border, and increased fractional Na+ excretion and Li+ clearance (16). Moreover, intratubular application of GLP-1 or EX4, but not the DPP-4 inhibitor P32/98, reduced the rate of bicarbonate flux during stationary in vivo microperfusion in proximal convoluted tubules, i.e., during the absence of flow and glomerular filtrate reaching the site of study (6). All these findings indicate that the enzymatic activity of DPP-4 in the proximal tubular brush border may locally affect the generation or breakdown of endogenous factors that regulate Na+ reabsorption via effects on NHE3 and that this factor may derive from the systemic circulation via glomerular filtration. The present study shows that, in contrast to EX4, the ALG-induced diuresis and natriuresis were preserved in mice lacking the GLP-1R, indicating a GLP-1R-independent mode of action. These data provide the first evidence that DPP-4 and its inhibition may regulate an endogenous substrate other than GLP-1 to affect renal reabsorption of fluid and Na+. Alternatively, GLP-1 accumulation in response to DPP-4 inhibition exerts natriuretic activity through a receptor other than the known GLP-1R.
High doses of the DPP-4 inhibitor P32/98 (50 mg/kg) increased the expression of NHE3 phosphorylated at S552 and S605 in proximal tubular microvilli of the rat, although this response was smaller than the response to EX4 (6). In the present study, ALG lowered plasma DPP-4 activity by 95% and induced a diuresis and natriuresis but did not affect NHE3 phosphorylation. The extent of the diuresis and natriuresis (and GFR effects) in response to ALG relative to that in response to EX4 in the present study was similar to the differences between P32/98 and EX4 in the previous rat study (6). Moreover, increasing the dose of ALG from 10 to 30 mg/kg in awake mice did not increase the natriuresis. Whereas higher doses of a DPP-4 inhibitor may affect additional peptides and pathways and/or induce stronger effects, the present findings indicate that the diuretic and natriuretic effect of the selective DPP-4 inhibitor ALG can occur independent of changes in NHE3 phosphorylation at S552 and S605 in mice.
Proximal tubular hyperreabsorption via the physiology of tubuloglomerular feedback has been proposed to enhance GFR in the early diabetic kidney, a risk factor for the progression to diabetic nephropathy (38). Therefore, antidiabetic drugs that lower proximal tubular hyperreabsorption may have beneficial effects on the kidney beyond blood glucose control. We observed that the acute inhibitory effect of EX4 on renal Na+ reabsorption was preserved in db/db mice. Moreover, the EX4-induced increase in GFR in nondiabetic mice was blunted in db/db mice. This may in part be due to a modest reduction in blood pressure in db/db mice by EX4. Antihypertensive effects of EX-4/GLP-1 have been reported in db/db mice (22), a rat model of the metabolic syndrome (28), and Dahl salt-sensitive rats (42). In addition, EX4 may lower GFR by inhibiting proximal reabsorption via the physiology of tubuloglomerular feedback. Along these lines, mice lacking the GLP-1R have an increased basal GFR. We speculate that knockout of the GLP-1R removes its tonic inhibitory influence on proximal tubular reabsorption, which lowers the NaCl concentration at the macula densa and increases GFR via tubuloglomerular feedback. Further studies are needed to follow up on the hypothesis that changes in proximal tubular reabsorption explain the enhanced basal GFR in the Glp1r−/− mice and the blunted GFR effect of EX4 under diabetic conditions.
In contrast to EX4, the inhibitory effect of ALG on renal fluid and Na+ reabsorption was abolished in db/db mice. This was observed in awake mice and in clearance studies under anesthesia. The results indicate that the inhibition of renal Na+ reabsorption induced by acute GLP-1R activation is preserved in diabetic db/db mice, whereas the endogenous natriuretic pathway induced by DPP-4 inhibition is rendered insensitive and/or ineffective. The reasons for the latter remain unclear. Plasma DPP-4 activity is increased in patients with type 1 and type 2 diabetes (29). Urinary DPP-4 excretion is enhanced in patients with non-insulin-dependent diabetes mellitus (24). Renal DPP-4 expression and activity (41), as well as urinary excretion of DPP-4 (23), are increased in hyperglycemic rats. Thus changes in DPP-4 expression and activity may not explain the present findings, pointing to potential changes in the availability of the DPP-4 substrate(s) or in the natriuretic signaling cascade induced by DPP-4 inhibition in db/db mice.
Whereas EX4 lowered blood glucose levels in nondiabetic control and db/db (but not Glp1r−/−) mice, intraperitoneal or intravenous application of ALG did not significantly alter blood glucose levels within the experimental time frame of 1–3 h in any of these groups compared with vehicle application. We documented a ∼95% reduction in plasma DPP-4 activity at 1 h after intraperitoneal application of ALG. It has been proposed that DPP-4 inhibition modulates glucose homeostasis through pathways distinct from those used by GLP-1R agonists in mice (11). Studies in mice proposed that the predominant mechanism through which DPP-4 inhibitors regulate blood glucose levels involves local inhibition of intestinal DPP-4 activity (40), a pathway that may not be sufficiently inhibited by the acute intravenous or intraperitoneal application of ALG in the present studies. In addition, it may be that the degree of enteral glucose loading and secretion of gut peptides necessary to unmask acute effects of intravenous DPP-4 inhibitors on blood glucose control was not established in the unchallenged control and db/db mice in the present experiments.
Summary and Perspectives
The current studies indicate that the acute natriuretic effect of intravenous and/or intraperitoneal application of EX4, but not ALG, is mediated via the GLP-1R and that only the acute natriuretic effect of EX4 is preserved in a mouse model of type 2 diabetes. Thus DPP-4 and its inhibition may regulate one or more substrates beyond GLP-1 to affect renal reabsorption of fluid and Na+. Further studies are needed to better understand the natriuretic effect of GLP-1R agonists and DPP-4 inhibitors, including the involved molecular mechanisms and their long-term effects on renal fluid and salt transport in the diabetic kidney. The physiological and pathophysiological relevance of an intestinal-renal GLP-1 system remains unclear. The present studies show that the absence of the GLP-1R increases GFR. Its absence appears not to affect the natriuresis and diuresis induced by salt loading via oral gavage, indicating a negligible contribution of the endogenous GLP-1/GLP-1R system in response to this maneuver.
GRANTS
This work was supported by National Institutes of Health Grants DK-56248, HL-094728, and P30 DK-079337 (to V. Vallon); American Heart Association Grants-in-Aid 10GRNT3440038 (to V. Vallon) and 11GRNT7610059 (to T. Tang) and Scientist Development Grant 10SDG2610034 (to T. Rieg); a Carl W. Gottschalk Research Grant of the American Society of Nephrology and a O'Brien Center Pilot Award (to T. Rieg); funding from the Heart and Stroke Foundation of Ontario, the Canada Research Chairs Program, and the Banting and Best Diabetes Centre-Novo Nordisk Chair in Incretin Biology (to D. J. Drucker); and funding from the Department of Veterans Affairs and Takeda Pharmaceuticals USA (to V. Vallon).
DISCLOSURES
V. Vallon has received within the past 12 mo research grant support for basic science studies from Takeda Pharmaceuticals USA, Inc., Bristol-Myers Squibb, and Astra-Zeneca. D. J. Drucker has served as an advisor or consultant within the past 12 mo to Amylin Pharmaceuticals, Arisaph Pharmaceuticals, Diartis Pharmaceuticals, Eli Lilly, Inc., Glaxo Smith Kline, Merck Research Laboratories, Novo Nordisk, NPS Pharmaceuticals, Pfizer, Takeda, and Transition Pharmaceuticals. Mt. Sinai Hospital receives operating grant support for basic science studies in D. J. Drucker's laboratory from Glaxo Smith Kline, Merck, and Novo Nordisk.
AUTHOR CONTRIBUTIONS
T.R., T.T., D.J.D., and V.V. are responsible for conception and design of the research; T.R., M.G., F.M., T.M., M.R., and V.V. performed the experiments; T.R., M.G., F.M., T.M., M.R., and V.V. analyzed the data; T.R., M.G., F.M., T.M., M.R., D.J.D., and V.V. interpreted the results of the experiments; T.R., M.G., and V.V. prepared the figures; T.R., M.G., F.M., T.M., T.T., M.R., D.J.D., and V.V. edited and revised the manuscript; T.R., M.G., F.M., T.M., T.T., M.R., D.J.D., and V.V. approved the final version of the manuscript; V.V. drafted the manuscript.
REFERENCES
- 1. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117: 2340–2350, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA. Differential expression of adenylyl cyclases in the rat nephron. Kidney Int 60: 890–899, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bunck MC, Corner A, Eliasson B, Heine RJ, Shaginian RM, Wu Y, Yan P, Smith U, Yki-Jarvinen H, Diamant M, Taskinen MR. One-year treatment with exenatide vs. insulin glargine: effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis 212: 223–229, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Chien CL, Wu YS, Lai HL, Chen YH, Jiang ST, Shih CM, Lin SS, Chang C, Chern Y. Impaired water reabsorption in mice deficient in the type VI adenylyl cyclase (AC6). FEBS Lett 584: 2883–2890, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER, Karim A. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther 30: 499–512, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011 [DOI] [PubMed] [Google Scholar]
- 7. DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 31: 2315–2317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 24: 2943–2952, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–1705, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Duez H, Smith AC, Xiao C, Giacca A, Szeto L, Drucker DJ, Lewis GF. Acute dipeptidyl peptidase-4 inhibition rapidly enhances insulin-mediated suppression of endogenous glucose production in mice. Endocrinology 150: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 267: 7402–7405, 1992 [PubMed] [Google Scholar]
- 13. Feng J, Zhang Z, Wallace MB, Stafford JA, Kaldor SW, Kassel DB, Navre M, Shi L, Skene RJ, Asakawa T, Takeuchi K, Xu R, Webb DR, Gwaltney SL. Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J Med Chem 50: 2297–2300, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gallwitz B, Witt M, Folsch UR, Creutzfeldt W, Schmidt WE. Binding specificity and signal transduction of receptors for glucagon-like peptide-1(7–36)amide and gastric inhibitory polypeptide on RINm5F insulinoma cells. J Mol Endocrinol 10: 259–268, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol 294: F414–F422, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268: 19650–19655, 1993 [PubMed] [Google Scholar]
- 19. Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 478: 136–142, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 73: 142–150, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hirata K, Kume S, Araki SI, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 27: 44–49, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Ishii N, Ikenaga H, Ogawa Z, Aoki Y, Saruta T, Suga T. Effects of renal sorbitol accumulation on urinary excretion of enzymes in hyperglycaemic rats. Ann Clin Biochem 38: 391–398, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Ishii N, Ogawa Z, Itoh H, Ikenaga H, Saruta T. Diagnostic significance of urinary enzymes for diabetes mellitus and hypertension. Enzyme Protein 48: 174–182, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Kenny AJ, Booth AG, George SG, Ingram J, Kershaw D, Wood EJ, Young AR. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J 157: 169–182, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kocinsky HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Laugero KD, Stonehouse AH, Guss S, Landry J, Vu C, Parkes DG. Exenatide improves hypertension in a rat model of the metabolic syndrome. Metab Syndr Relat Disord 7: 327–334, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, Cremasco F, Ognibene A, Rotella CM. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia 48: 1168–1172, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol 434: 163–167, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y2 receptor activation decreases blood pressure and increases renal Na excretion. Am J Physiol Regul Integr Comp Physiol 301: R510–R518, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther 313: 403–409, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Rieg T, Tang T, Murray F, Schroth J, Insel PA, Fenton RA, Hammond HK, Vallon V. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol 21: 2059–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept 141: 120–128, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2: 1254–1258, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Shen T, Suzuki Y, Poyard M, Miyamoto N, Defer N, Hanoune J. Expression of adenylyl cyclase mRNAs in the adult, in developing, and in the Brattleboro rat kidney. Am J Physiol Cell Physiol 273: C323–C330, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117: 61–69, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 300: R1009–R1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, Castel J, Garret C, Payros G, Maida A, Sulpice T, Holst JJ, Drucker DJ, Magnan C, Burcelin R. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology 152: 3018–3029, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci 81: 272–279, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21: 1125–1135, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Zhao N, Tenenhouse HS. Npt2 gene disruption confers resistance to the inhibitory action of parathyroid hormone on renal sodium-phosphate cotransport. Endocrinology 141: 2159–2165, 2000 [DOI] [PubMed] [Google Scholar]