Abstract
Mixed-lineage kinase 3 (MLK3) activates multiple MAPK pathways and can initiate apoptosis, proliferation, migration, or differentiation in different cell types. However, whether MLK3 signaling regulates intestinal epithelial cell sheet migration in vivo is not known. We sought to investigate whether MLK3 signaling is important in intestinal mucosal healing and epithelial cell motility in vivo and in vitro. In vivo, we compared the healing of jejunal mucosal ulcers induced in MLK3 knockout (KO) mice with healing in wild-type (WT) mice. Ulcer healing was 20.8% less at day 3 (P < 0.05) and 18.9% less at day 5 (P < 0.05) in MLK3 KO than WT mice. Within the intestinal mucosa of MLK3 KO mice, ERK and JNK signaling were reduced, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) level was increased, and p38 signaling was unchanged. Parallel in vitro studies using an MLK inhibitor assessed the role of MLK signaling in human Caco-2 intestinal epithelial migration across collagen substrates. The MLK inhibitor reduced closure of circular wounds in Caco-2 monolayers. MLK inhibition reduced ERK and JNK, but not p38, signaling in Caco-2 cells. Although PTEN is increased after MLK inhibition, it does not influence MLK-mediated cell migration. These findings indicate that disruption of MLK3 signaling impairs ulcer healing by suppressing ERK and JNK signaling in vitro and in mouse intestinal mucosa in vivo. These results reveal a novel role for MLK3 signaling in the regulation of intestinal epithelial migration in vivo and suggest that MLK3 may be an important target for the regulation of intestinal mucosal healing.
Keywords: cell migration, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), ERK, JNK, small intestine
the intestinal mucosa is repetitively subjected to injury during normal gut function and must be able to heal to maintain barrier function. In addition, intestinal mucosal ulceration occurs during processes as diverse as inflammatory bowel disease, infectious gastroenteritis, and peptic ulcer disease (45). The healing of such mucosal lesions requires epithelial sheet migration (restitution) and proliferation, each of which is regulated by various MAPKs after growth factor activation (18, 38). For instance, ERK signaling correlates with migration speed in mammary 184A1 epithelial cells (25, 54), and ERK increases in migrating epithelial cells at the edge of rat gastric mucosa and mouse jejunal ulcers (34, 46). Similarly, p38 and JNK signaling influence intestinal epithelial sheet migration in vitro in response to cyclic strain (53) and improve skin wound healing after stimulation by activin B (55). However, although MAPK signaling is important for intestinal mucosal healing, the upstream signals that influence MAPK activation during intestinal mucosal healing are poorly understood.
Mixed-lineage kinase (MLK) 3 (MLK3) is a ubiquitously expressed serine/threonine kinase capable of regulating multiple MAPK pathways in mammalian cells. In some cells, MLK3 can variably activate the JNK, p38, and MEK/ERK MAPK pathways (17) and mediate the mitogenic effects of cytokines and mitogens in vitro (6, 10). MLK3 has been proposed to upregulate phosphatase and tensin homolog deleted on chromosome 10 (PTEN) expression by activating the transcription factor ATF-2 in human endothelial cells (41). However, MLK3-null murine embryonic fibroblasts (MEF) display reduced JNK signaling without reduction in ERK or p38 activation after TNFα stimulation (6). Moreover, JNK signaling may be activated by the MEKK pathway in the absence of functional MLK3 in kidney cells (47). Thus the upstream regulation of the terminal MAPK ERK, p38, and JNK differs from cell type to cell type. Although epithelial sheet migration is qualitatively different from the movement of cancer cells, it is worth noting that MLK3 has also been implicated in the regulation of motility and invasion in epithelial cancer cells (13, 30, 43) and may induce expression of activator protein 1-regulated genes associated with invasive cancer (13). MLK3 influences motility and JNK and p38 activity in breast cancer cells (13). However, the role of MLK3 in modulating two-dimensional restitutive sheet migration in nonmalignant epithelium in vivo is unknown. MLK3 knockout (KO) mice develop pathology of their dorsal skin, suggesting that MLK3 could be involved in developmental epithelial cell migration (6), but the effects of MLK3 signaling on cell motility or the MAPK that regulates it in vivo have not been examined.
Since MLK3 might influence several of the intracellular signaling pathways relevant to intestinal epithelial cell migration and/or proliferation, we used a model of ulcer healing in engineered mice with a targeted deletion of the MLK3 gene in vivo, as well as MLK inhibition in Caco-2 and IEC-6 intestinal epithelial cells in vitro, to investigate its role in mucosal wound healing. In vivo, we measured migration, proliferation, apoptosis, and MAPK pathways to determine their involvement, if any, in ulcer healing. Using an MLK inhibitor in vitro, we confirmed that suppressed MLK3 signaling affects ERK and JNK signaling pathways that may be critical for cell migration. Our results suggest that MLK3 signaling plays an important role in enterocyte migration in vivo and in vitro via ERK and JNK, but not PTEN or p38 signaling. This is the first reported evidence delineating a key role for MLK3 signaling in the regulation of epithelial sheet migration in vivo.
MATERIALS AND METHODS
Animal and surgical procedures.
All experiments were approved by the University Laboratory Animal Resources at Michigan State University. MLK3 KO mice (6) were a gift from Pfizer (Ann Arbor, MI). The wild-type (WT) mice used as controls were of the same genetic background (C57BL/6) and age as the MLK3 KO mice. Male C57BL/6J mice were used at 8–12 wk of age to match the age of the MLK3 KO experimental mice. Mice were housed in a 12:12-h light-dark facility. Food and water were provided ad libitum. Mean body weight was not different between MLK3 KO and WT groups at baseline or at harvest. Experimental procedures were carried out simultaneously in MLK3 KO and control animals.
Mucosal ulcers.
WT and MLK3 KO animals were anesthetized with isoflurane, and a laparotomy was performed to expose the jejunum. To create a circumscribed ischemic mucosal ulcer, tissue paper disks (3.4 mm2) soaked with a 75% acetic acid solution were applied to the small bowel serosa, with care taken to avoid large vessels, for 15 s, as previously described (16, 34) using a modification of a previously published method to create gastric ulcers (44). The application area was washed three times with sterile saline. We previously demonstrated that this method yields reproducible-sized ulcers at day 1 (34). Mice were euthanized at 1, 3, and 5 days after ulcer induction. The segment of intestine in which the ulcer was created was incised along the mesenteric border, and the mucosal ulcer was photographed using a dissecting microscope equipped with a digital camera (Q-color5, Olympus, Tokyo, Japan). Images were analyzed using National Institutes of Health ImageJ software (version 1.43u, public domain). Ulcer healing was measured as percent ulcer area closure at days 3 and 5 relative to the average area of the ulcers measured at day 1.
Immunohistochemical studies of proliferation and histomorphometry.
Samples of the intestinal segments containing induced ulcers from experimental MLK3 KO and WT mice were fixed in 10% formalin for 24 h and embedded in paraffin. Step sections 4.0 μm thick were prepared from all the blocks and stained with hematoxylin-eosin. Immunohistochemical analysis for proliferation was done on unstained, formalin-fixed paraffin sections utilizing a Ki-67 kit (Zymed). Sections were counterstained with hematoxylin for histological orientation, visualized, and photographed. Proliferation was assessed as the percentage of Ki-67-positive nuclei in intestinal mucosa, as previously described (28). Morphometric parameters analyzed in small intestine, including mucosal thickness, fibromuscular stromal thickness, villus width and height, crypt depth, villus epithelial cell area, number of cells per 100 μm, and mucosa-to-stroma thickness ratio, were determined in hematoxylin-eosin-stained sections of intestine using Image Pro Plus 5.5 (Media Cybernetics, Silver Spring, MD) image analysis software and appropriate standards (×200 magnification, 5 areas per small intestine of each animal). Measurements were done in longitudinal sections of villi and crypts and expressed in micrometers. At least 30 measurements were taken for each animal, and the group average was calculated. Immunohistochemical analysis for intestinal cell signaling was performed on unstained, formalin-fixed paraffin sections. Antigen retrieval for all antibodies was done by boiling the slides in citrate buffer (Biogenex, San Ramon, CA) for 30 min. Endogenous peroxidase activity was quenched with 20 min of incubation in 3% H2O2 in methanol. Changes in intestinal epithelial cell signaling in vivo were assessed in sections of mouse intestine with antibodies to phosphorylated ERK, phosphorylated JNK, phosphorylated p38, and PTEN (Cell Signaling Technology, Beverly, MA), at 1:200 dilution in PBS, followed by Vectastain Universal ABC kit detection (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin, coverslips were applied, and sections were visualized and photographed on a Nikon Microphot-FXA (Nikon, Tokyo, Japan).
Cell and culture conditions.
For in vitro studies, we utilized colon carcinoma-derived Caco-2 cells, a common model of intestinal epithelial biology capable of differentiation in culture (12). Caco-2 intestinal epithelial cells (CRL-2102, American Type Culture Collection) were maintained at 37°C with 8% CO2 in DMEM with 25 mM d-glucose, 4 mM glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 g/ml transferrin, 10 mM HEPES (pH 7.4), and 3.7 g/l NaHCO3 supplemented with 10% heat-inactivated FBS. IEC-6 nonmalignant rat intestinal epithelial cells (CRL-1592, American Type Culture Collection) were maintained at 5% CO2 in high-glucose (4.5 g/l) DMEM supplemented with 10% FBS and 0.1 U/ml bovine insulin. Only cells below passage 25 were used for these experiments. To compare MLK3 levels in motile and static conditions, Caco-2 cells were seeded simultaneously at 31,200 and 6,370 cells/cm2 into 35 × 10 and 100 × 10 mm tissue culture plates, respectively, to create static confluent monolayers and populations of small islands of migrating epithelial cells of the same age after plating, as previously described (52). Cells in the first population reached confluence at 4 days and were used for experiments at 24, 48, and 72 h after confluence; the second group remained subconfluent and motile at all time points. To determine whether PTEN level changes with ERK inhibition, ERK signaling in Caco-2 cells was blocked by the ERK antagonist PD-98059 (20 mmol/l; Calbiochem, La Jolla, CA) for 24 h. Control cells in these studies were treated with the 0.1% DMSO vehicle.
Motility measurement.
Caco-2 or IEC-6 cells were cultured to confluence on sterile six-well dishes precoated with collagen I. Small uniform circular wounds in the cell monolayers were created as previously described (54). Monolayers of cells with created wounds were immediately treated for 24 h (Caco-2) or 6 h (IEC-6) with medium containing 0 (0.1% DMSO), 100, 200, 400, 800, or 1,600 nM CEP-11004 or CEP-1347 (n = 6), as previously described (13). At 0 and 24 h, the remaining wound area was calculated after visualization on a Kodak Image Station (Perkin Elmer, Boston, MA).
Proliferation.
Caco-2 cells were seeded at 100,000 cells/well on type I collagen-precoated six-well culture plates for 24 h. Subconfluent (30–40%) cells were serum-starved for 24 h. A single six-well plate was reserved for a time 0 measurement, and the remaining serum-starved cells were incubated in normal growth medium containing 0.1% DMSO or CEP-11004 inhibitor (400 nM) dissolved in DMSO for 24 h before trypsinization and cell counting. Cell number was determined in each of the six wells independently with an automated cell counter (Countess, Invitrogen, Carlsbad, CA) using the manufacturer's protocol. Data from each experiment were analyzed with six observations in each group.
PTEN small interfering RNA studies.
Caco-2 cells were seeded on type I collagen-coated six-well culture plates to attain 30–40% confluence 1 day before transfection with nontargeting (NT1) small interfering RNA (siRNA) or siRNA to PTEN (Dharmacon, Lafayette, CO; 50 nM final concentration) using Oligofectamine according to the manufacturer's protocol. Effectiveness of the siRNA transfection (routinely 70–90%) was verified by immunoblotting 72 h after transfection. Uniform circular wounds were created, and the cells were treated with 0.1% DMSO or 400 nM CEP-11004 for 24 h.
Protein isolation and Western blot analysis.
Mucosal scrapings from target intestinal segments or harvested Caco-2 cells were immediately immersed in ice-cold lysis buffer (50 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% dichloroacetate, glycerol, 10 mM sodium pyrophosphate, and 50 mM NaF). Tissue was homogenized using a BulletBlender (Next Advance, Averill Park, NY) and then centrifuged at 15,000 g for 10 min at 4°C. Protein concentrations were determined using bicinchoninic acid (Pierce Chemical, Rockford, IL). Equal amounts of protein were resolved by SDS-PAGE and then electrophoretically transferred to a Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blotted for phosphorylated ERK (Thr202, Tyr204), ERK, phosphorylated JNK, JNK, phosphorylated AKT, AKT, phosphorylated p38, p38, and cleaved caspase-3 (Cell Signaling Technology, Beverly, MA). Membranes were then reprobed with antibodies specific for β-actin as a loading control and the appropriate fluorophore-conjugated secondary antibody. Bands were visualized with an Odyssey imaging system (LI-COR) and analyzed with the Kodak Image Station 440CF, and phosphorylated-to-total protein ratios for each sample were adjusted to β-actin. All exposures of probed membranes used for densitometric analysis were within the linear range.
Data analysis.
Values are group means ± SE of the nontransformed data. Prior to analysis, all data were checked to ensure that they fit a normal distribution using the plot of predicted values vs. residuals, as well as the Shapiro-Wilk and Kolmogorov-Smirnov tests for normality. Two-tailed Student's t-test or ANOVA was used when appropriate. Skewed or nonnormally distributed data were logarithmically transformed prior to analysis, and the correction to a normal distribution was confirmed using tests described above. Differences between means were considered significant at P < 0.05.
RESULTS
Disruption of MLK3 signaling impairs intestinal mucosal healing in mice.
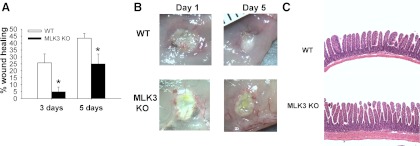
Ulcers were induced in MLK3 KO and age-matched WT mice at 8–12 wk of age and measured in animals euthanized 3 and 5 days after surgery. Ulcer size was compared with that in animals of the same genotype measured 1 day after ulcer induction. Analysis of the digital images of the mucosal ulcer surface 3 days after surgery demonstrated that ulcer healing was 20.8% slower in MLK3 KO than WT mice (n = 6, P < 0.05; Fig. 1A). Similarly, the ulcer area was 18.9% larger in the MLK3 KO than WT mice 5 days after induction (P < 0.05; Fig. 1, A and B).
Fig. 1.
Mixed-lineage kinase 3 (MLK3) disruption reduces mucosal ulcer healing in mouse small intestine. A: targeted deletion of MLK3 [MLK3 knockout (KO)] reduces ulcer healing 3 and 5 days after ulcer induction compared with wild-type (WT) mice (n = 6 per group, 10–12 wk old). *P < 0.05. B: representative images of mucosal ulcers at day 1 in WT and 5 days after surgery in WT and MLK3 KO mice. Ulcers were induced by application of filter disks saturated with 75% acetic acid to the intestinal serosa. Original magnification ×7.5. C: representative hematoxylin-eosin-stained sections of WT and MLK3 KO mice intestine. Increased mucosal and stromal thickness was observed in MLK3 KO animals. Original magnification ×100.
Deletion of MLK3 alters intestinal mucosal and intestinal epithelial cell morphology but not proliferation.
Since MLK3 has been implicated in the regulation of cell proliferation in some settings, using an antibody against Ki-67, a protein expressed during the active phases of proliferation (40), we compared the proliferation of epithelial cells within the mucosa at the ulcer edge 5 days after ulcer induction. The percentage of Ki-67-positive intestinal mucosal cells within 2 mm of the ulcer was similar in WT and MLK3 KO mice, as well as in intact mucosa distant from the induced ulcer (n = 12; Table 1). Similar proliferation rates were observed between genotypes at 3 days and 1 day after surgery (data not shown). We next examined the effects of MLK3 deletion on intestinal wall composition that might account for the migration defects in intestinal cells or the ability of the intestine to withstand ulcer damage. Thickness of the mucosa and the fibromuscular layer of the intact intestinal wall of MLK3 KO mice was significantly increased compared with that of WT mice (P < 0.05, n = 12; Fig. 1C, Table 1). Villous height, crypt depth, and villous width were also significantly greater in MLK3 KO animals than WT controls (P < 0.05, n = 12). Deletion of MLK3 significantly increased the number of cells per 100 μm of villus length compared with mucosa from WT mice (P < 0.05, n = 6; Fig. 1C, Table 1). Although the epithelial cell cross-sectional area tended to be smaller in MLK3 KO animals than WT controls, this did not reach significance. The ratio of the thickness of the mucosa to the thickness of the stroma was preserved in MLK3 KO and WT groups (data not shown). Taken together, these results suggest that the reduced rate of ulcer healing in MLK3 KO mice is not due to reduced proliferation of mucosal epithelial cells at the ulcer edge or to reduced thickness of the intestine, which might be protective against injury (42).
Table 1.
Morphometric parameters of WT and MLK3 KO mouse intact small intestine and proliferation rate in the mucosa at the ulcer edge and intact area of experimental animals
| Ki-67-Positive Cells, % |
Thickness, μm |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intact area | Ulcer edge at 5 days | Mucosa | Stroma | Total | Crypt Depth, μm | Villous Height, μm | Cells per 100 μm of Villi | Villus Width, μm | Villus Cell Area, μm2 | |
| WT | 76.4 ± 1.9 | 73.6 ± 2.1 | 441.7 ± 24.7 | 63.5 ± 3.8 | 492.5 ± 26.4 | 137.3 ± 12.5 | 330 ± 20.2 | 16.2 ± 5.5 | 83.1 ± 3.5 | 12.5 ± 1.3 |
| MLK3 KO | 74.8 ± 1.5 | 75.0 ± 1.4 | 602.3 ± 17.1* | 97.7 ± 6.0* | 690.2 ± 22.8* | 171 ± 8.3* | 405 ± 13.8* | 20.2 ± 7.5* | 113.5 ± 8.9* | 10.2 ± 0.5 |
Values are means ± SE; n = 6–12.
MLK3, mixed-lineage kinase 3; WT, wild-type; KO, knockout.
P < 0.05.
Signals crucial for cell migration are altered in the mucosa of mice deficient in MLK3.
To identify the effect of the absent MLK3 signaling on downstream targets, potential MLK3-dependent signals, ERK, JNK, PTEN, and p38, were evaluated in sections of intestinal ulcer by immunohistochemistry and in mucosal scrapings by Western blotting.
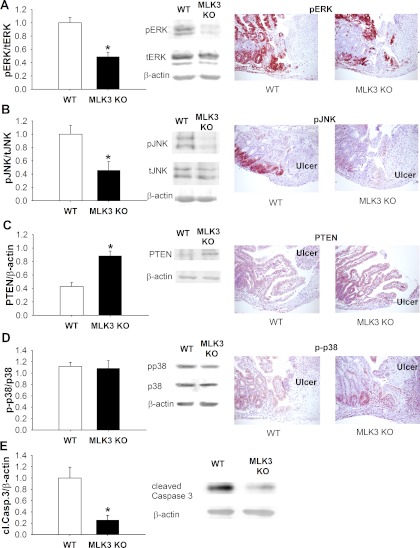
Phosphorylated ERK immunoreactivity was readily visible at the ulcer edge, as well as in intact mucosa, 5 days after ulcer induction in WT mice (Fig. 2A). Although phosphorylated ERK immunoreactivity was higher at the ulcer edge in MLK3 KO and WT animals than in more distant mucosa in these animals, the level of phosphorylated ERK immunoreactivity was significantly lower in MLK3 KO than WT mice. Western blot analysis also showed a significant difference in phosphorylated ERK levels in intact mucosa between WT and MLK3 KO animals (n = 12, P < 0.05; Fig. 2A).
Fig. 2.
Reduced mucosal wound healing in MLK3 KO mice is associated with changes in ERK, JNK, and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling. A: ERK signaling is reduced in intestinal mucosa of MLK3 KO mice (n = 12). pERK and tERK, phosphorylated and total ERK. B: JNK signaling is lower in intestinal mucosa of MLK3 KO mice (n = 12). pJNK and tJNK, phosphorylated and total JNK. C: PTEN levels are higher in intestinal mucosa of MLK3 KO mice (n = 12). D: p38 activation is not different in MLK3 KO and WT mice (n = 12). p-p38, phosphorylated p38. Original magnification ×100. E: levels of cleaved caspase-3 (cl.Casp.3) are lower in intestinal mucosa of MLK3 KO mice (n = 5). *P < 0.05.
Phosphorylated JNK has been reported to decrease after inhibition of MLK3 signaling (6). In MLK3 KO animals, phosphorylated JNK immunoreactivity appeared significantly reduced at the ulcer site and within intact mucosa compared with WT animals (Fig. 2B). The significant reduction of phosphorylated JNK in the mucosa was confirmed by Western blot analysis (n = 12, P < 0.05; Fig. 2B).
PTEN expression has been shown to regulate levels of phosphorylated ERK and JNK (21). WT animals had relatively low PTEN levels at the ulcer site and in the intact mucosa (Fig. 2C). Conversely, MLK3 KO mice exhibited significantly higher PTEN immunoreactivity in the intestinal mucosa by immunohistochemistry and Western blot analysis (n = 12, P < 0.05; Fig. 2C). In contrast, immunohistochemical and Western blot analysis of phosphorylated p38 revealed no difference at the ulcer edge or in the intact mucosa between WT and MLK3 KO intestine (Fig. 2D).
We also examined if MLK3 deletion changes levels of activated caspase-3 in mouse intestinal mucosa. Western blot analysis of intestinal mucosa of WT and MLK3 KO mice indicated that MLK3 deletion reduced caspase-3 cleavage, indicative of reduced apoptosis (Fig. 2E).
MLK3 signaling is involved in intestinal epithelial cell migration.
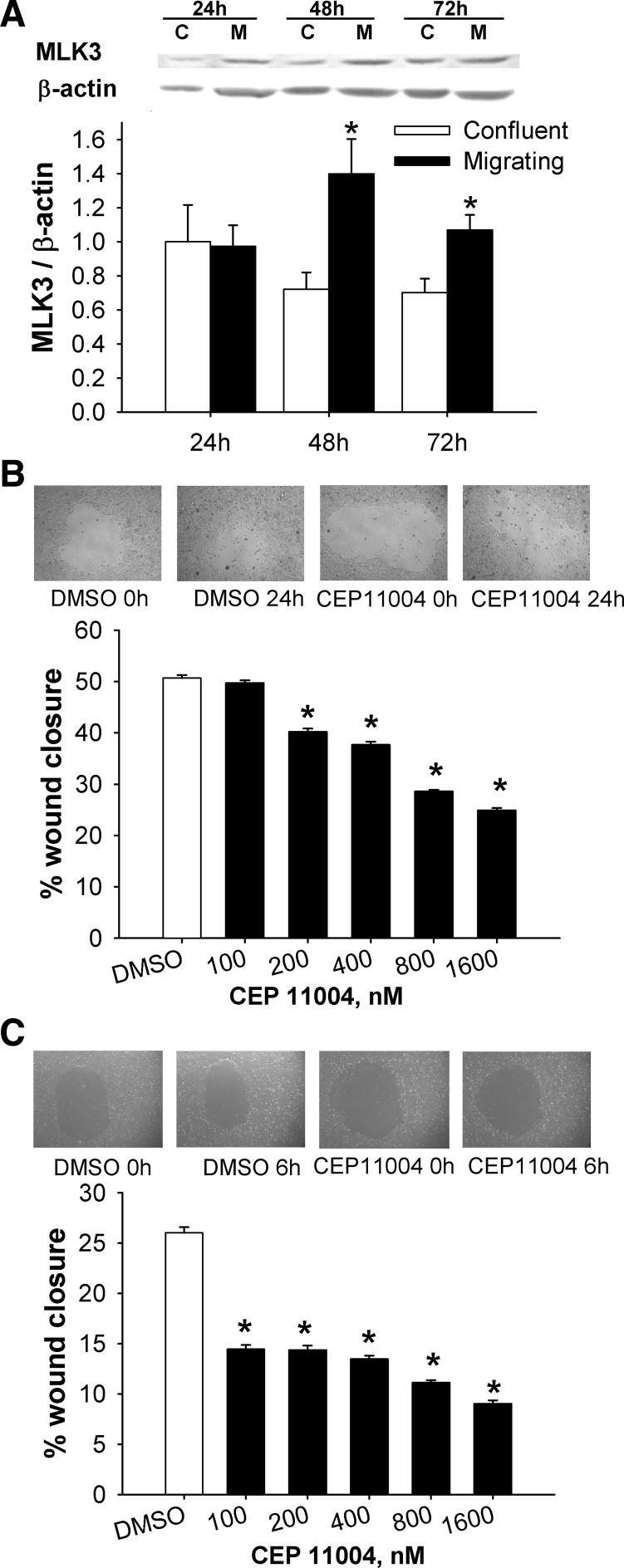
We next sought to determine whether MLK3 levels differ between migrating and static confluent Caco-2 cells. MLK3 levels were significantly increased at 48 and 72 h in migrating cells compared with confluent cells (n = 6, P < 0.05; Fig. 3A), consistent with the concept that a high level of functional MLK3 might contribute to the migration of intestinal epithelial cells.
Fig. 3.
MLK3 modulates intestinal epithelial cell migration in vitro. A: MLK3 levels are higher in migrating (M) than confluent (C) human Caco-2 cells at 48 and 72 h (n = 6 per group). B: representative 24-h circular wounds of vehicle- and CEP-11004 (400 nM)-treated Caco-2 cells (top) and dose-dependent suppression of migration of Caco-2 cells (n = 15) by the MLK inhibitor CEP-11004 after 24 h (bottom). C: representative 6-h circular wounds of vehicle- and CEP-11004 (400 nM)-treated IEC-6 cells (top) and dose-dependent decrease in migration of rat IEC-6 cells (n = 15) by CEP-11004 after 6 h (bottom). *P < 0.05.
To investigate this possibility in vitro, Caco-2 cells were treated with different doses of the MLK inhibitors CEP-11004 and CEP-1347. In Chinese hamster ovary cells, Murakata et al. (32) demonstrated that CEP-11004 and CEP-1347 have the highest specificity for MLK3 compared with their ability to inhibit MLK1 and MLK2 but are not effective against PKC or neurotrophic tyrosine kinase receptor (TrkA) activity. Moreover, expression of MLK2 is limited to brain, skeletal muscles, and testis (26), so MLK2 is not expressed in the intestine. Whether MLK1 is expressed in the intestine is not known. CEP-11004 is capable of reducing the phosphorylation of MLK3 in MCF-7 breast cancer cells as soon as 30 min after it is introduced (39).
CEP-11004, at a concentration as low as 200 nM, significantly reduced wound closure (P < 0.01; Fig. 3B), whereas 800 nM CEP-1347 inhibited wound closure (P < 0.01; data not shown). Significant reductions in wound closure in normal intestinal IEC-6 cells were also observed at CEP-11004 concentrations as low as 100 nM (P < 0.01; Fig. 3C), while 200 mM CEP-1347 significantly reduced wound closure (P < 0.05; data not shown).
MLK inhibitors alter ERK and JNK signaling in confluent and migrating Caco-2 cells.
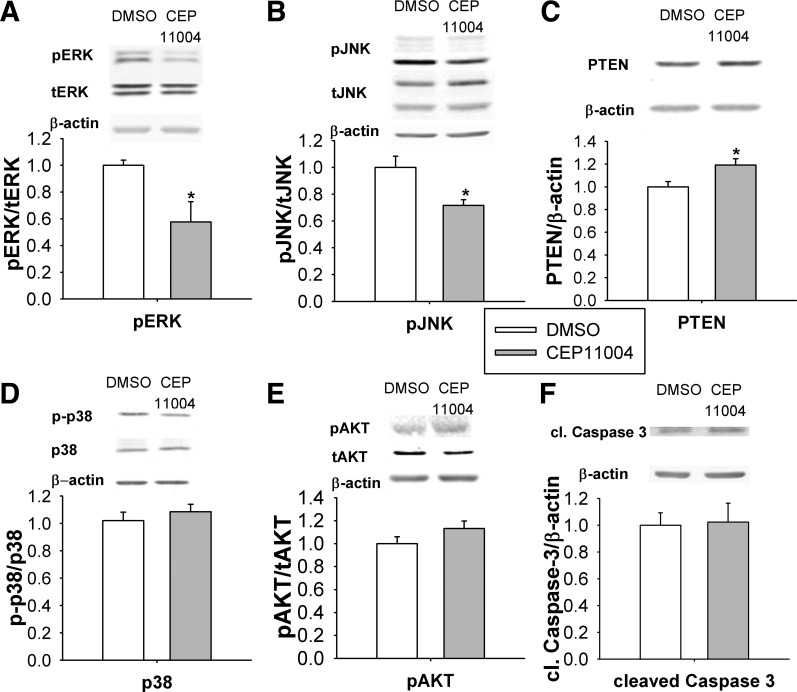
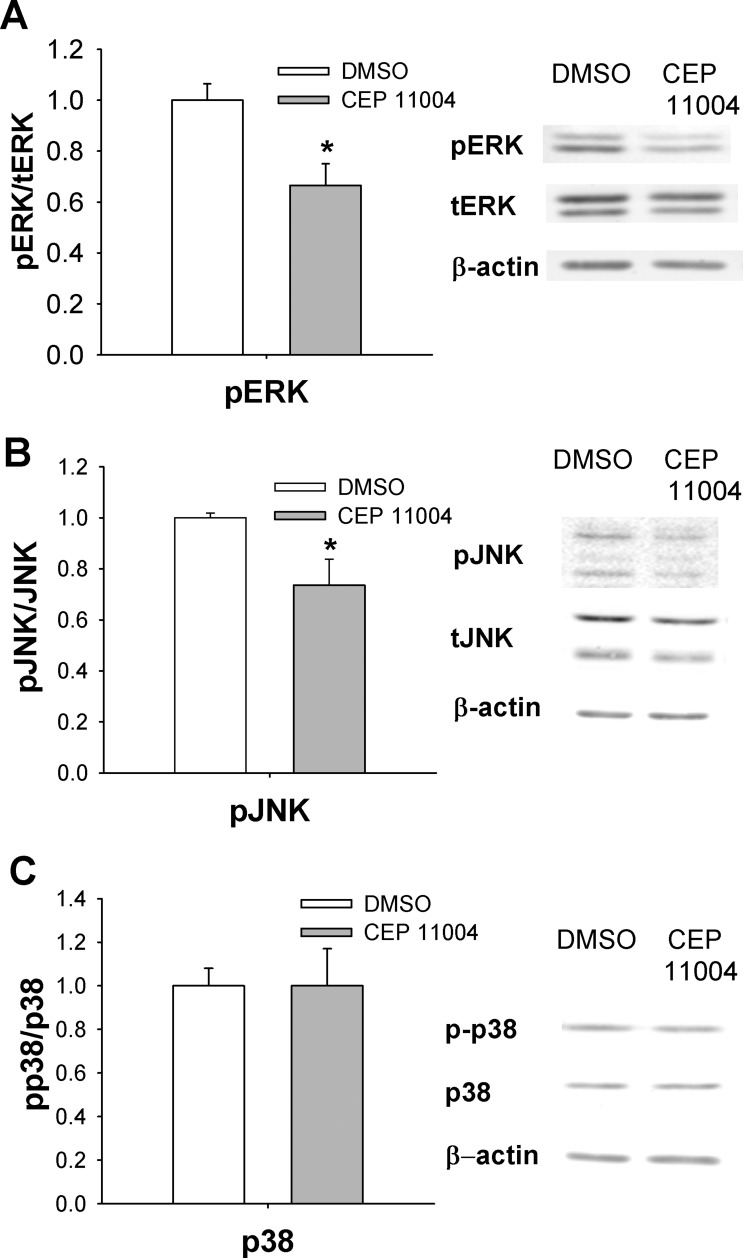
We further investigated the effect of MLK inhibitor treatment on potential downstream signals that might be involved in wound healing and to correlate the effects of short-term MLK3 inhibition in vitro with long-term MLK3 KO in vivo. Phosphorylated ERK and JNK were decreased in Caco-2 cells 24 h after MLK inhibition (P < 0.05; Fig. 4, A and B). PTEN levels were significantly increased in Caco-2 cells after treatment with CEP-11004 (P < 0.05; Fig. 4C), but this MLK inhibitor did not alter the level of phosphorylated p38 (Fig. 4D) or AKT (Fig. 4E) activation. Similar changes in intracellular signaling were observed after the use of CEP-11004 in normal intestinal IEC-6 cells (data not shown). Western blot analysis of the CEP-11004-treated subconfluent Caco-2 cells revealed no difference in the level of activated caspase-3 (Fig. 4E).
Fig. 4.
MLK signaling is required for activation of ERK and JNK signaling in vitro in confluent Caco-2 cells. A: MLK inhibitor CEP-11004 reduces ERK phosphorylation in confluent Caco-2 cells (n = 6) after 24 h. B and C: CEP-11004 reduces JNK phosphorylation (n = 6) and increases total PTEN levels (n = 6) in Caco-2 cells. D and E: CEP-11004 does not affect p38 signaling (n = 6) or phosphorylated AKT levels (n = 6). F: levels of cleaved caspase-3 are not changed after 24 h of treatment with CEP-11004. *P < 0.05.
We also investigated the effect of MLK inhibitor treatment on MAPK signals that might be affected by suppression of MLK signaling in migrating Caco-2 cells. As seen in confluent Caco-2 cells, phosphorylated ERK and JNK were decreased in migrating Caco-2 cells 24 h after MLK inhibition (P < 0.05; Fig. 5, A and B). The level of the phosphorylated p38 was not changed under these conditions (Fig. 5C).
Fig. 5.
MLK signaling is required for activation of ERK and JNK signaling in vitro in migrating Caco-2 cells. A: CEP-11004 (400 nM) reduces ERK phosphorylation in migrating (30–40% confluent) Caco-2 cells (n = 6) after 24 h. B: CEP-11004 reduces JNK phosphorylation (n = 6). C: CEP-11004 does not affect p38 signaling in migrating Caco-2 cells (n = 6). *P < 0.05.
MLK inhibition does not change cell proliferation.
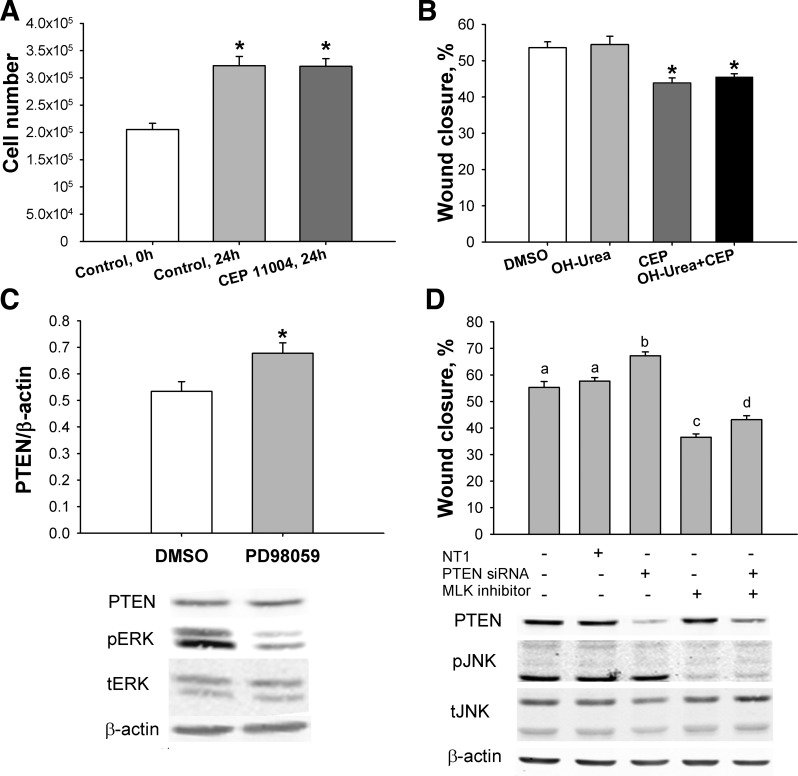
To rule out the possibility that an effect of MLK suppression on cell proliferation was contributing to the effects on Caco-2 monolayer wound closure, we measured proliferation after 24 h of treatment with CEP-11004. Cell number was not different from control after treatment with CEP-11004 (n = 6; Fig. 6A). We repeated these studies in cell monolayers simultaneously treated with hydroxyurea to prevent cell proliferation (48). Hydroxyurea did not change the antimotogenic effects of CEP-11004 (n = 6; Fig. 6B). These results suggest that intestinal epithelial cell wound closure and migration depend on MLK3 activity and that this effect is independent of cell proliferation.
Fig. 6.
Motogenic effect of MLK3 does not depend on cell proliferation or PTEN level. A: CEP-11004 does not change Caco-2 cell proliferation (n = 12). B: antimotogenic effects of CEP-11004 (CEP, 400 nM) are not reversed by hydroxyurea (OH-urea, n = 6). C: inhibition of ERK signaling increases PTEN level in Caco-2 cells (n = 6). D: loss of MLK signaling reduces cell migration in nontargeting (NT1) and PTEN small interfering RNA (siRNA)-treated Caco-2 cells (n = 15). Bars without a common letter (a, b, c, d) are significantly different at P < 0.01. *P < 0.05.
MLK mediated regulation of cell migration does not require PTEN.
PTEN levels in Caco-2 cells were significantly increased after inhibition of ERK signaling by PD-98059 for 24 h, suggesting that an increase in PTEN could contribute to the reduced intestinal cell migration after MLK inhibition-initiated ERK suppression (n = 6, P < 0.05; Fig. 6C). We tested this hypothesis by reducing PTEN level in Caco-2 cells with specific siRNA. Reduction of PTEN significantly increased wound closure in Caco-2 cells (n = 5, P < 0.01; Fig. 6D). However, wound closure in PTEN siRNA-transfected Caco-2 cells after MLK inhibition was not only significantly suppressed compared with control, but it was also significantly increased compared with MLK inhibitor treatment alone (P < 0.01) without change in JNK signaling, indicating that an increase in PTEN is not central to MLK regulation of cell migration.
DISCUSSION
Mucosal ulcer healing is a complex process requiring cell proliferation, migration, and matrix formation and angiogenesis (45). Migration of epithelial cells at the ulcer edge, critical for ulcer healing, is regulated by cytokines and growth factors (4). This study establishes a critical role for MLK3 in this process and traces its downstream effectors. MLK3 is a widely expressed mammalian serine/threonine kinase that functions as a MAPKKK and can activate multiple MAPK pathways in response to cytokines or cellular stresses (17). In this study, we show that a targeted deletion of MLK3 impaired the healing of experimental small intestinal mucosal ulcers in mice and that MLK3 inhibition delayed intestinal epithelial monolayer wound closure in vitro. Furthermore, MLK3 appears to critically mediate ERK and JNK, but not p38, activation during intestinal mucosal wound healing in vivo as well as in confluent and migrating cells in vitro. Although we observed PTEN upregulation in the MLK3 KO intestine, PTEN deletion does not affect MLK-mediated cell migration, so PTEN may be less likely than the MAPK to be a critical mediator of migration in this setting.
Although mucosal healing is stimulated by a variety of endogenous growth factors and cytokines, these stimuli require active intracellular signaling pathways to promote cell migration and proliferation (15, 33). Most growth factors are produced locally as a result of mucosal damage or inflammation (2, 15), so delays in wound healing may reflect abnormalities of the intracellular signaling response to these factors, rather than the absence of the growth factors themselves. Furthermore, cell signaling and biology in vitro may be very different from the kinome and its responsiveness to stimuli in the complex in vivo environment (8, 22). It therefore becomes essential to delineate the relevant intracellular pathways that govern intestinal epithelial sheet migration in vivo to identify targets for intervention to promote mucosal healing.
This study clearly demonstrates that MLK3 is an important regulator of intestinal epithelial sheet migration in vitro and mucosal wound healing in vivo. Previous studies demonstrated that MLK3 is involved in breast cancer cell migration (13), colon cancer cell proliferation (10), and enhancement of hepatoma cell apoptosis (27). In contrast, our results suggest that, in the nonmalignant intestinal epithelium, MLK3 is essential only for intestinal epithelial cell motility, while MLK3 seems less important or more easily compensated for in the pathways that influence intestinal epithelial proliferation. Moreover, MLK3 KO mice reveal an epidermal phenotype (6), and we found that intact intestinal mucosa of adult MLK3 KO mice also differs from mucosa in the WT animals, suggesting a wider role for MLK3 signaling in epithelial development. The difference in intestinal cell migration, but not proliferation or apoptosis, in our study supports a major role in cell migration during developmental formation of the intestinal wall, as well as during mucosal recovery after damage.
MLK3 is traditionally known to exhibit its effects via activation of JNK signaling in vivo and in neuronally derived cells in response to cytokines and LPS in vitro (14, 50). Chemokines and growth factors influence JNK signaling and modulate migration and invasion of human colorectal cancer cells (7) or rat intestinal IEC-6 cells (49). JNK signaling has been shown to regulate cell migration in breast cancer cells (13) and in gastric cancer cells after gastrin induction (30). Our observation suggests that, in intestinal cells, MLK3 suppression correlates with reduced JNK signaling in vitro and in vivo. This is consistent with a previous report of impaired JNK activation in MEF derived from MLK3 KO mice in response to TNFα (6) or free fatty acids (24). However, these cells also did not differ in their migratory or invasive properties from WT MEF (6). Suppression of MLK3 signaling also reduces cytokine-mediated JNK activation in human colonic CCD-18Co fibroblasts (10) or microglial cells (23) in a fashion similar that described here. Moreover, JNK signaling is necessary for isolated cell migration of breast cancer cells (unlike nonmalignant epithelial cells, these cells do not migrate as sheets), but not matrix metalloproteinase 9-mediated invasiveness (37). Thus, MLK3 may act as a primary regulator of JNK signaling in promoting cell sheet migration, but not proliferation, in intestinal mucosa ulcer healing.
ERK appears to be an important downstream mediator of the effect of MLK3 on wound healing. ERK influences diverse cellular activities and has important effects on intestinal epithelial cell migration (20). Chadee et al. (11) reported that MLK3, independent of its MAPK3 catalytic activity, complexes with B-Raf and Raaf-1 to promote ERK signaling. Our data suggest that MLK3-mediated activation of ERK modulates migration of intestinal epithelial cells in vitro and in vivo, similar to that observed in HT29 colon adenocarcinoma cells (9) and gastric cancer cells (30). Suppression of MLK3 signaling in metastatic breast cancer cells by CEP-11004 reduced migration twofold and prevented activation of JNK and p38, but not ERK, signaling (13), again emphasizing the differences in MLK3 downstream signaling in different cell types. In this study, we observed reduced ERK signaling within the normal small intestinal mucosa after deletion of MLK3. Such a reduction of mediators of signal transduction may reduce the responsiveness of epithelial cells to local growth factors and cytokines (35). MLK3 overexpression has been demonstrated to increase phosphorylated ERK in response to TGFβ1 in FaO rat hepatoma cells (27). In contrast, in primary MEF derived from MLK3 KO animals, ERK activation by TNFα and serum-induced chemotaxis do not differ from similar phenomena in MEF from WT mice (6). These differences may reflect differences in cell type, cell context, or variations in migration across different substrates or specific difference in MLK3 activation of downstream ERK pathways in response to different stimuli (52). We demonstrated that MLK inhibition inhibited ERK activation and migration in vitro, as it does in vivo. In neuronal cell lines, reducing ERK signaling with a MEK inhibitor attenuates the neuroprotective effects of CEP-11004 against mutant Huntington-associated neurotoxicity (1) or CEP-1347 against cell death induced by serum deprivation (36), suggesting that activation of ERK may be necessary for at least some MLK3 actions. Although the CEP-11004 and CEP-1347 inhibitors are specific for MLK1, MLK2, and MLK3, the KO mouse data were obtained in MLK3-null models. Thus the inhibitor data are chiefly important, because they confirm that the effects we saw in the MLK3 KO animals do not reflect some sort of other compensation for MLK3 reduction or inhibition that may occur after a prolonged period of MLK3 reduction or inhibition.
p38 signaling has been implicated in regulation of cell migration and spreading in response to growth factors in vitro (3, 51). MLK3 mediates TGFβ1-induced apoptosis via p38 signaling in hepatoma cells (27), and in human fibroblasts MLK3 activates p38, as well as other MAPKs, in response to EGF stimulation (9). However, our results do not support MLK3 regulation of p38 signaling in the intestinal mucosa in vivo, as we did not observe differences in phosphorylated p38 within the mucosa of the KO mice or in intestinal epithelial cells treated with MLK3 inhibitor in vitro. We previously reported that although p38 is increased during strain-induced Caco-2 cell migration, it does not modulate cell motility in this setting (52). TNFα stimulation of MEF isolated from MLK3 KO mice also did not increase p38 signaling (6). Activation of p38 by MLK3 may be tissue-specific or require growth factor stimulation, but it does not appear central to the influence of MLK3 on intestinal epithelial cell migration.
PTEN plays a major role in many cellular activities and has been shown to interact with MAPK signaling pathways regulating cell spreading and cell migration (21). PTEN has been reported to influence the migration of differentiated Caco-2 cells in vitro and dedifferentiated metastatic colon cancer HCT116 cells in vivo (29). Conversely, PTEN can be transcriptionally suppressed via activation of RAS/ERK signaling by bone morphogenic protein in SW480 colon cancer cells (5). We also observed increased PTEN within the mucosa of the MLK3 KO mice and were able to reproduce this effect in vitro with the MLK inhibitor CEP-11004. Thus it is possible that the alterations in PTEN within the MLK3 KO mucosa could contribute in some fashion to the reduced mucosal healing that we observed in vivo in these mice. However, in vitro results with siRNA suggest that PTEN may not be necessary, since loss of PTEN did not reverse MLK-mediated inhibition of wound closure. Understanding why some MLK3 inhibition or reduction affects PTEN awaits further study. In addition, inhibition or reduction of MLK3 could well invoke feedback signaling that drives other upstream kinases in an attempt to reregulate the downstream MAPK signaling.
Activation of MLK3 and its downstream target JNK signaling often contributes to cell death. In differentiated neuronal PC-12 cells, MLK3 suppression blocks NGF withdrawal-induced cell death (31) and CEP-11004 treatment suppresses neuronal cell death in vivo (19). However, the antimotogenic effects of MLK3 signaling in vitro are independent of caspase-3 activation, despite reduced level of caspase-3 in vivo.
In summary, our results demonstrate that MLK3 signaling contributes to in vivo mucosal healing in the small intestine independently of cell proliferation and to the in vitro epithelial sheet migration of human intestinal Caco-2 cells. Targeted deletion of MLK3 in vivo or inhibition of MLK3 in vitro modulates distinct signaling pathways, including ERK and JNK, but not p38. MLK3 signaling may modulate PTEN level, but this is not required for regulation of cell migration. MLK3 may prove to be an important target modulating intestinal epithelium renewal and maintenance and improving repair of gut mucosal injury.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-067257 (M. D. Basson) and a VA Merit Research Award (M. D. Basson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.L.K., L.K., K.A.G., and M.D.B. are responsible for conception and design of the research; P.L.K. and L.K. performed the experiments; P.L.K., L.K., J.C., and K.A.G. analyzed the data; P.L.K., L.K., and K.A.G. interpreted the results of the experiments; P.L.K. prepared the figures; P.L.K. drafted the manuscript; P.L.K., L.K., and M.D.B. edited and revised the manuscript; P.L.K., L.K., J.C., K.A.G., and M.D.B. approved the final version of the manuscript.
REFERENCES
- 1.Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL, Thompson LM. CEP-1347 reduces mutant Huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci 39: 8–20, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Baatar D, Jones MK, Tsugawa K, Pai R, Moon WS, Koh GY, Kim I, Kitano S, Tarnawski AS. Esophageal ulceration triggers expression of hypoxia-inducible factor-1α and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am J Pathol 161: 1449–1457, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 115: 3193–3206, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Basson MD, Modlin IM, Flynn SD, Jena BP, Madri JA. Independent modulation of enterocyte migration and proliferation by growth factors, matrix proteins, and pharmacologic agents in an in vitro model of mucosal healing. Surgery 112: 299–307, 1992 [PubMed] [Google Scholar]
- 5.Beck SE, Carethers JM. BMP suppresses PTEN expression via RAS/ERK signaling. Cancer Biol Ther 6: 1313–1317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol 25: 3670–3681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Goke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res 310: 117–130, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res 89: 718–727, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol 6: 770–776, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chadee DN, Kyriakis JM. A novel role for mixed lineage kinase 3 (MLK3) in B-Raf activation and cell proliferation. Cell Cycle 3: 1227–1229, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chadee DN, Xu D, Hung G, Andalibi A, Lim DJ, Luo Z, Gutmann DH, Kyriakis JM. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc Natl Acad Sci USA 103: 4463–4468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Miller EM, Gallo KA. MLK3 is critical for breast cancer cell migration and promotes a malignant phenotype in mammary epithelial cells. Oncogene 29: 4399–4411, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Ciallella JR, Saporito M, Lund S, Leist M, Hasseldam H, McGann N, Smith CS, Bozyczko-Coyne D, Flood DG. CEP-11004, an inhibitor of the SAPK/JNK pathway, reduces TNF-α release from lipopolysaccharide-treated cells and mice. Eur J Pharmacol 515: 179–187, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dominguez JA, Coopersmith CM. Can we protect the gut in critical illness? The role of growth factors and other novel approaches. Crit Care Clin 26: 549–565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanigan TL, Owen CR, Gayer C, Basson MD. Supraphysiologic extracellular pressure inhibits intestinal epithelial wound healing independently of luminal nutrient flow. Am J Surg 196: 683–689, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3: 663–672, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Gamell C, Susperregui AG, Bernard O, Rosa JL, Ventura F. The p38/MK2/Hsp25 pathway is required for BMP-2-induced cell migration. PLos One 6: e16477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganguly A, Oo TF, Rzhetskaya M, Pratt R, Yarygina O, Momoi T, Kholodilov N, Burke RE. CEP11004, a novel inhibitor of the mixed lineage kinases, suppresses apoptotic death in dopamine neurons of the substantia nigra induced by 6-hydroxydopamine. J Neurochem 88: 469–480, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gayer CP, Chaturvedi LS, Wang S, Alston B, Flanigan TL, Basson MD. Delineating the signals by which repetitive deformation stimulates intestinal epithelial migration across fibronectin. Am J Physiol Gastrointest Liver Physiol 296: G876–G885, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol 143: 1375–1383, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haring HU, Kellerer M, Mosthaf L. Modulation of insulin receptor signalling: significance of altered receptor isoform patterns and mechanism of hyperglycaemia-induced receptor modulation. Diabetologia 37 Suppl 2: S149–S154, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Hidding U, Mielke K, Waetzig V, Brecht S, Hanisch U, Behrens A, Wagner E, Herdegen T. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochem Pharmacol 64: 781–788, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell 27: 498–508, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joslin EJ, Opresko LK, Wells A, Wiley HS, Lauffenburger DA. EGF-receptor-mediated mammary epithelial cell migration is driven by sustained ERK signaling from autocrine stimulation. J Cell Sci 120: 3688–3699, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Katoh M, Hirai M, Sugimura T, Terada M. Cloning and characterization of MST, a novel (putative) serine/threonine kinase with SH3 domain. Oncogene 10: 1447–1451, 1995 [PubMed] [Google Scholar]
- 27.Kim KY, Kim BC, Xu Z, Kim SJ. Mixed lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates transforming growth factor-β-induced apoptosis in hepatoma cells. J Biol Chem 279: 29478–29484, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kovalenko PL, Zhang Z, Yu JG, Li Y, Clinton SK, Fleet JC. Dietary vitamin D and vitamin D receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev Res 4: 1617–1625, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langlois MJ, Bergeron S, Bernatchez G, Boudreau F, Saucier C, Perreault N, Carrier JC, Rivard N. The PTEN phosphatase controls intestinal epithelial cell polarity and barrier function: role in colorectal cancer progression. PLos One 5: e15742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra P, Senthivinayagam S, Rangasamy V, Sondarva G, Rana B. Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol Endocrinol 24: 598–607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra R, Barthwal MK, Sondarva G, Rana B, Wong L, Chatterjee M, Woodgett JR, Rana A. Glycogen synthase kinase-3β induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem 282: 30393–30405, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakata C, Kaneko M, Gessner G, Angeles TS, Ator MA, O'Kane TM, McKenna BA, Thomas BA, Mathiasen JR, Saporito MS, Bozyczko-Coyne D, Hudkins RL. Mixed lineage kinase activity of indolocarbazole analogues. Bioorg Med Chem Lett 12: 147–150, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T, Chai J, Li A, Akahoshi T, Tanigawa T, Tarnawski AS. Novel roles of local insulin-like growth factor-1 activation in gastric ulcer healing: promotes actin polymerization, cell proliferation, re-epithelialization, and induces cyclooxygenase-2 in a phosphatidylinositol 3-kinase-dependent manner. Am J Pathol 170: 1219–1228, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen CR, Yuan L, Basson MD. Smad3 knockout mice exhibit impaired intestinal mucosal healing. Lab Invest 88: 1101–1109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology 114: 706–713, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Roux PP, Dorval G, Boudreau M, Angers-Loustau A, Morris SJ, Makkerh J, Barker PA. K252a and CEP1347 are neuroprotective compounds that inhibit mixed-lineage kinase-3 and induce activation of Akt and ERK. J Biol Chem 277: 49473–49480, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Safina A, Vandette E, Bakin AV. ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene 26: 2407–2422, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Samarakoon R, Goppelt-Struebe M, Higgins PJ. Linking cell structure to gene regulation: signaling events and expression controls on the model genes PAI-1 and CTGF. Cell Signal 22: 1413–1419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schachter KA, Du Y, Lin A, Gallo KA. Dynamic positive feedback phosphorylation of mixed lineage kinase 3 by JNK reversibly regulates its distribution to Triton-soluble domains. J Biol Chem 281: 19134–19144, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem 281: 7727–7736, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Sheth SU, Lu Q, Twelker K, Sharpe SM, Qin X, Reino DC, Lee MA, Xu DZ, Deitch EA. Intestinal mucus layer preservation in female rats attenuates gut injury after trauma-hemorrhagic shock. J Trauma 68: 279–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swenson-Fields KI, Sandquist JC, Rossol-Allison J, Blat IC, Wennerberg K, Burridge K, Means AR. MLK3 limits activated Gαq signaling to Rho by binding to p63RhoGEF. Mol Cell 32: 43–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarnawski A, Hollander D, Krause WJ, Dabros W, Stachura J, Gergely H. “Healed” experimental gastric ulcers remain histologically and ultrastructurally abnormal. J Clin Gastroenterol 12 Suppl 1: S139–S147, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci 50 Suppl 1: S24–S33, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Tarnawski AS, Pai R, Wang H, Tomikawa M. Translocation of MAP (Erk-1 and -2) kinases to cell nuclei and activation of c-fos gene during healing of experimental gastric ulcers. J Physiol Pharmacol 49: 479–488, 1998 [PubMed] [Google Scholar]
- 47.Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem 271: 27225–27228, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Turowski GA, Rashid Z, Hong F, Madri JA, Basson MD. Glutamine modulates phenotype and stimulates proliferation in human colon cancer cell lines. Cancer Res 54: 5974–5980, 1994 [PubMed] [Google Scholar]
- 49.Walsh MF, Ampasala DR, Rishi AK, Basson MD. TGF-β1 modulates focal adhesion kinase expression in rat intestinal epithelial IEC-6 cells via stimulatory and inhibitory Smad binding elements. Biochim Biophys Acta 1789: 88–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol 21: 4713–4724, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaoka T, Frey MR, Dise RS, Bernard JK, Polk DB. Specific epidermal growth factor receptor autophosphorylation sites promote mouse colon epithelial cell chemotaxis and restitution. Am J Physiol Gastrointest Liver Physiol 301: G368–G376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu CF, Sanders MA, Basson MD. Human Caco-2 motility redistributes FAK and paxillin and activates p38 MAPK in a matrix-dependent manner. Am J Physiol Gastrointest Liver Physiol 278: G952–G966, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Li W, Sumpio BE, Basson MD. Fibronectin blocks p38 and JNK activation by cyclic strain in Caco-2 cells. Biochem Biophys Res Commun 306: 746–749, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology 131: 1179–1189, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Liu NY, Wang XE, Chen YH, Li QL, Lu KR, Sun L, Jia Q, Zhang L. Activin B promotes epithelial wound healing in vivo through RhoA-JNK signaling pathway. PLos One 6: e25143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]