Abstract
Changes in intestinal luminal pH affect mucosal ion transport. The aim of this study was to compare how luminal pH and specific second messengers modulate the membrane traffic of four major ion transporters (CFTR, NHE3, NKCC1, and NBCe1) in rat small intestine. Ligated duodenal, jejunal, and ileal segments were infused with acidic or alkaline saline, 8-Br-cAMP, or the calcium agonist carbachol in vivo for 20 min. Compared with untreated intestine, lumen pH was reduced after cAMP or carbachol and increased following HCO3−-saline. Following HCl-saline, lumen pH was restored to control pH levels. All four secretory stimuli resulted in brush-border membrane (BBM) recruitment of CFTR in crypts and villi. In villus enterocytes, CFTR recruitment was coincident with internalization of BBM NHE3 and basolateral membrane recruitment of the bicarbonate transporter NBCe1. Both cAMP and carbachol recruited NKCC1 to the basolateral membrane of enterocytes, while luminal acid or HCO3− retained NKCC1 in intracellular vesicles. Luminal acid resulted in robust recruitment of CFTR and NBCe1 to their respective enterocyte membrane domains in the upper third of the villi; luminal HCO3− induced similar membrane changes lower in the villi. These findings indicate that each stimulus promotes a specific transporter trafficking response along the crypt-villus axis. This is the first demonstration that physiologically relevant secretory stimuli exert their actions in villus enterocytes by membrane recruitment of CFTR and NBCe1 in tandem with NHE3 internalization.
Keywords: cystic fibrosis transmembrane conductance regulator, membrane trafficking, electrogenic sodium/bicarbonate cotransporter, sodium- and potassium-coupled chloride cotransporter, sodium/proton exchanger, goblet cell
intestinal diseases are commonly linked to impaired anion transport and changes in lumen pH. Both responses may underlie pathophysiology of diseases such as secretory diarrhea, cystic fibrosis (CF), Crohn's disease, and ulcerative colitis (13, 17, 37, 62). For example, defective trafficking of the CF transmembrane conductance regulator (CFTR) chloride channel leads to CF and reduced intestinal lumen pH (62). In the human CF intestine, this abnormally low duodenal luminal pH contributes to bacterial overgrowth, dysmotility, and malabsorption (31, 62–63). How defective intestinal ion transport, including CFTR dysfunction, contributes to the reduced luminal pH is unknown.
It is widely accepted that the balanced ratio of secreted chloride (Cl−) and bicarbonate (HCO3−) is critical to intestinal fluid secretion. This balance is primarily maintained by the following anion transporters: the sodium- and potassium-coupled chloride (Na+-K+-2Cl−) cotransporter 1 (NKCC1), the major basolateral Cl− entry gateway, the electrogenic sodium-coupled bicarbonate (Na+/HCO3−) cotransporter 1 (NBCe1), the major basolateral HCO3− entry gateway; CFTR, the major apical Cl− and HCO3− exit gateway, in association with the Na+/H+ exchanger 3 (NHE3), and the Cl−/HCO3− exchangers SLC26A6 (PAT-1) and SLC26A3 (DRA). The relative rates of stimulated secretion of HCO3− and Cl− appear to be determined by their relative conductivity through CFTR, and by cell-to-lumen concentration differences for the two anions (47). Thus, mucosal responses to an acid or alkaline load and modulation of intestinal luminal pH could involve a number of ion transporters.
Changes in the levels of intestinal ion transporters, including both up- and downregulation, can affect small bowel luminal pH and contribute to disease. The reduced pH in CF results from a loss of CFTR-mediated HCO3− secretion from duodenal villus enterocytes that involves several transporters, including CFTR, members of the SLC26 family of apical anion exchangers (SLC26A6 and SLC26A3), and NBCe1 (18, 30, 35, 50–51). In cyclic nucleotide-elicited secretory diarrhea due to cholera and Escherichia coli, fluid and HCO3− secretion are linked to upregulation of CFTR, while absence of CFTR protects against enterotoxin-induced diarrhea (19, 27, 32–33). We have reported the cellular sites of origins for CFTR mediated Cl− vs. HCO3− secretion along the crypt-villus axis in rat intestine (40). Our studies, and those from other groups, suggest that intestinal ion transporters exhibit plasticity in response to second messengers and stress (21, 30, 33, 57–58). Importantly, defects in transporters can cause a loss of these adaptive responses and disordered luminal pH.
To provide a systematic and comprehensive analysis of the trafficking of key transporters that mediate small intestinal luminal pH, their distribution within the full mucosa was examined in the basal state and with response to relevant stimuli (Ca2+, cAMP, luminal HCl and luminal HCO3−) in rodent and human intestine. The results of this study provide insights into selectivity and coordination of transport secretory responses to luminal stimuli along the crypt-villus axis. Specifically, our data reveal the following key new observations: 1) consistent with a role in bicarbonate secretion, villus enterocytes respond to Ca2+, cAMP, luminal acid, and luminal HCO3− conditions by recruiting CFTR into the brush-border membrane (BBM), internalizing NHE3, and recruiting NBCe1 to the basolateral membrane; 2) membrane recruitment of CFTR and NBCe1, and internalization of NHE3 in villus enterocytes are synchronized in response to secretory stimuli; 3) luminal acid selectively elicits robust enterocyte responses in the upper third of the villi by recruiting CFTR and NBCe1 to their respective membrane domains, consistent with acid-stimulated bicarbonate secretion; and 4) alkalinization also elicits CFTR/NBCe1 responses but selectively in the lower villi.
These observations suggest that luminal stress and/or stimuli elicit rapid and cell-specific membrane trafficking responses along the crypt-villus axis that can determine the composition of secreted fluid in the lumen and modulate luminal pH.
MATERIALS AND METHODS
Antibodies
Anti-CFTR.
AME4991 rabbit polyclonal anti-CFTR antibody was used for immunocytochemistry (4, 33, 40). The monoclonal mouse anti-CFTR antibody (cat. no. M3A7; Chemicon International, Temecula, FL) was used in some double-label studies. The use of NBCe1, NHE3, and NKCC1 antibodies were described previously (40).
Human Tissue
Rectosigmoid biopsy specimens (provided by Dr. Anthony Bauer; University of Pittsburgh, Pittsburgh, PA) were obtained from patients undergoing colonoscopy and subsequently found to have no pathology of the large intestine. The study was approved by the Institutional Review Board at the University of Pittsburgh School of Medicine. Colonic biopsies (1.5 mm × 3 mm) were maintained in DMEM and left untreated, or treated ex vivo with 0.2 ml normal saline (pH 7.4) or acidified saline (pH 6.0) that was added to the ∼1 ml culture solution for 20 min at 37°C in 5% CO2/90% air atmosphere incubator. Tissues were fixed in 2% paraformaldehyde for 1 h.
Animals
The Institutional Animal Care and Use Committee of Yale University School of Medicine approved the study. Male Sprague-Dawley rats (200–250 g wt, Charles River Laboratories, Wilmington, MA) were fasted overnight but allowed free access to drinking water and anesthetized with Inactin (120 mg/kg ip) injection. Body temperature was maintained with a heating pad.
Luminal pH Measurement and Luminal Treatment of Ligated Small Intestinal Loops
Intestinal loops (∼2.5-cm length) were created with ligatures in the duodenum, proximal jejunum, and ileum. Lumen pH was first measured in untreated segments juxtaposed to a respective treated segment. The pH of lumen samples was measured using the i-STAT blood gas analyzer (Abaxis, Union City, CA) equipped with EC8+ sample cartridges (Abbott Point of Care, Princeton, NJ). The cartridges were stored at 4°C, and allowed to equilibrate to room temperature before use. Samples (60–90 μl) were collected from the intestinal lumen by suction into airtight 25-gauge syringes. Care was taken to avoid air bubbles in the samples. Samples were immediately introduced into the well, allowed to fill by passive movement to the indicated level, and the cartridge was inserted into the analyzer. After successful completion of the calibration and analysis cycle, the pH values were recorded as measured at 37°C.
For in vivo luminal infusion, the following solutions were prepared at 37°C: normal saline (pH 7.4); 100 μM 8-Br-cAMP or 10 μM carbachol diluted in pH 7.4 saline; pH 7.0 saline and pH 2.0 saline acidified with HCl; pH 8.0 saline alkalinized with NaOH; and pH 8.0 HCO3−-saline gassed with 95% O2-5% CO2 and alkalinized with 24 mM NaHCO3−. The pH of the solutions was measured immediately prior to injection. Syringes were filled with the solutions and ligated intestinal segments were infused in vivo for 10 or 20 min. The abdomen was closed, and the animal was kept warm. Immediately following treatment luminal samples (60–90 μl) were collected, and pH was measured as described above. Each lumen pH data point was collected in at least five animals. At the end of the experiment, the animals were killed by administration of intraperitoneal injection of Inactin (200 mg/kg).
Tissue Preparation/Immunofluorescence Labeling
Preparation of intestinal tissues by fixation, embedding, sectioning, and the use of tissue arrays for immunolabeling, microscopy, and densitometric image analysis were performed as described in our previous studies (40).
Fluorescence Image Analysis and Statistics
Fluorescence intensity levels were measured over the apical membrane (for CFTR and NHE3), lateral membrane (for NBCe1 and NKCC1), and intracellular apical pole on digital images as before (40). Briefly, data from six to twelve selected areas were averaged in each image, six to eight images were analyzed for each measurement group in one animal, and data were collected from four animals. All measured values were presented as means ± SE. Statistical significance between two individual measurement groups was determined by unpaired Student's t-test. Differences among groups were determined using one-way ANOVA and the Tukey's post hoc method of multiple comparisons. The level of significance was set at P < 0.05.
RESULTS
Changes in Lumen pH in Rat Small Intestine
Since secretory stimuli can modulate the electrolyte composition in the intestinal lumen, changes in lumen pH in small intestinal segments (duodenum, proximal jejunum, ileum) were measured before and after treatment for 20 min with 1) HCO3−-saline pH 8.0, 2) HCl−-saline pH 2.0, 3) 8-Br-cAMP-saline pH 7.4, and 4) carbachol-saline pH 7.4 (Fig. 1). The mean lumen pH in untreated small intestine were proximal duodenum 7.24, proximal jejunum 6.68, and ileum 7.83. After treatment with HCl−-saline pH 2.0, lumen pH did not change significantly, suggesting that the small intestine is capable of rapidly restoring its pH after exposure to strong acidic conditions. Treatment with HCO3−-saline pH 8.0 induced small increases in lumen pH: ∼0.2 in duodenum, 0.3 in jejunum, and 0.2 in ileum. On the other hand, both cAMP and carbachol induced a small but significant decrease in lumen pH by ∼0.3 to 0.4 in each small intestinal segment.
Fig. 1.
Intestinal lumen pH of rat small intestine before and 20 min after in vivo treatments. Lumen pH was measured in adult male overnight-fasted rats before and after in vivo treatments of ligated segments with: 1) HCl-saline pH 2.0, 2) HCO3−-saline pH 8.0, 3) 8-Br-cAMP-saline pH 7.4, and 4) carbachol-saline pH 7.4 (see materials and methods for details). Mean ± SE lumen pH values were derived from at least 5 experiments for each intestinal segment (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
cAMP and Carbachol-Induced Redistribution of CFTR, NHE3, NKCC1, and NBCe1
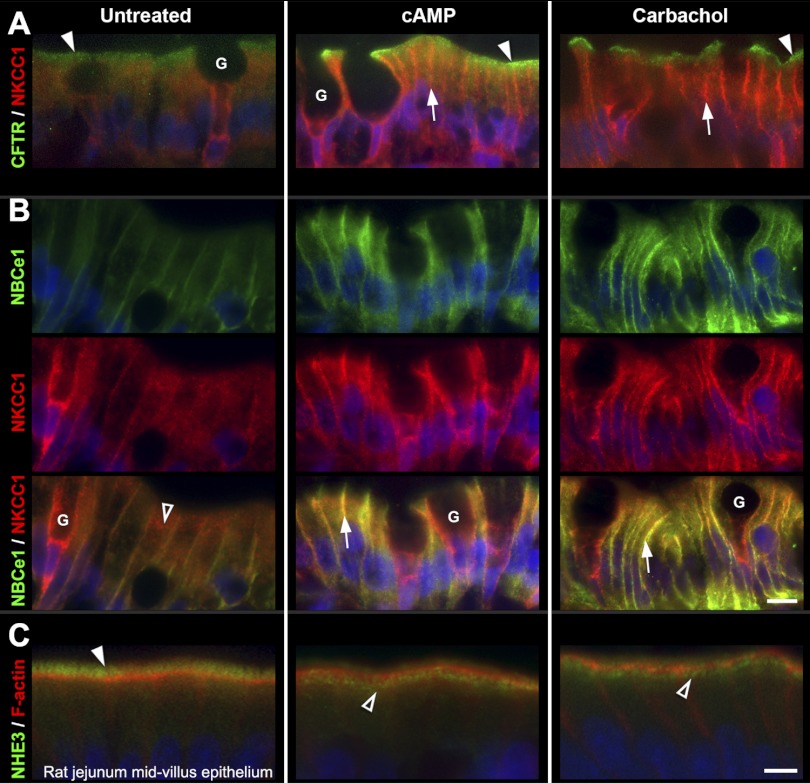
cAMP-activated fluid secretion depends on the insertion of CFTR from subapical vesicles into the BBM of villus enterocyte (7). CFTR-mediated fluid secretion in the intestine requires coordinate activities with other transporters including NHE3, NBCe1, and NKCC1 and can be stimulated by both cAMP and calcium agonists (12, 30, 50, 54). The effects of cAMP and the muscarinic receptor agonist carbachol on the distribution of these transporters was examined in the small intestine (Fig. 2). In untreated jejunum, immunolabeled CFTR and NHE3 were largely confined to the BBM and subapical vesicles, consistent with published reports (40). CFTR was present in both crypt and villus enterocytes, but NHE3 was confined to villus enterocytes. NKCC1 labeling was largely intracellular, but NBCe1 was localized both in intracellular compartments and on the basolateral membranes of villus enterocytes (40). Following stimulation with either carbachol or cAMP, CFTR redistributed to the BBM of enterocytes in the crypt and villi (Figs. 2A and 3, A–B) consistent with previous findings (6, 40). In contrast, a large proportion of NHE3 in the BBM redistributed to a subapical compartment (Fig. 2C). Both carbachol and cAMP stimulation resulted in translocation of NKCC1 and NBCe1 to the basolateral membranes of villus enterocytes (Fig. 2B) and increased NKCC1 in the basolateral membranes of goblet cells (Fig. 2, B and C). The cellular redistribution of transporters in response to both secretagogues was observed throughout the length of the villus. Figure 2 shows images of transporter redistribution patterns in representative midvillus regions that were confirmed by densitometry (Fig. 3). The movement of CFTR into and NHE3 out of the apical BBM in response to the secretory agonists cAMP and carbachol are consistent with CFTR-mediated anion secretion that is maximized by concomitant inhibition of Na+ absorption. The observed parallel movement of chloride (NKCC1) and bicarbonate (NBCe1) transporters into the basolateral membranes provide the anions for apical secretion.
Fig. 2.
cAMP and carbachol-induced redistribution of CFTR, NKCC1, NBCe1, and NHE3 in rat jejunum. Representative images from untreated (left), 8-Br-cAMP-treated (center), or carbachol-treated (right) midvillus jejunum tissue sections double-labeled to detect CFTR (green) and NKCC1 (red) (A); NBCe1 (green), NKCC1 (red), and NBCe1/ NKCC1 (B); NHE3 (green) and F-actin (red) (C). A: arrowheads, apical CFTR; arrows, basolateral NKCC1 label in enterocytes. B: open arrowhead, intracellular NKCC1 labeling in untreated enterocytes; arrows, colocalization (yellow) of NBCe1 and NKCC1 at the basolateral membranes of enterocytes in cAMP (center) or carbachol-treated tissues (right). G, goblet cells. C: arrowhead, brush-border NHE3; open arrowheads, NHE3 label in subapical compartment. The images represent the results of 4 experiments (n = 4). Scalebars: A–B, 10 μm; C, 5 μm.
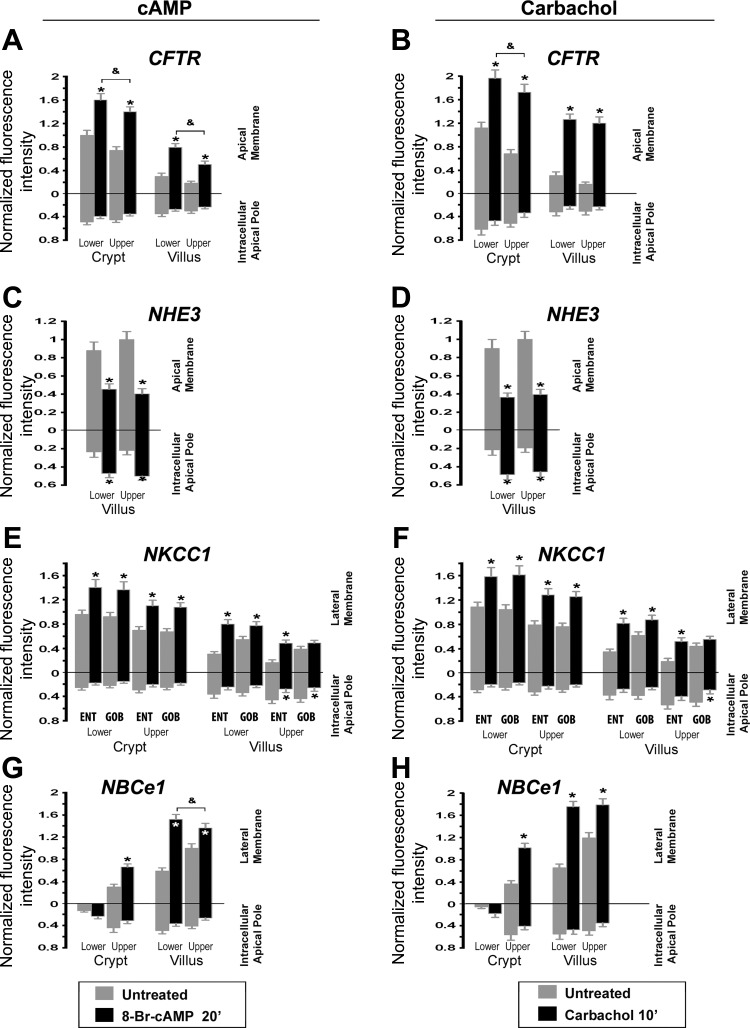
Fig. 3.
Densitometry of normalized CFTR, NHE3, NKCC1, and NBCe1 fluorescence intensity at apical or lateral membranes and subapical compartments of enterocytes and goblet cells in cAMP and carbachol-treated rat jejunum. Cells were analyzed from the lower and upper third of crypts and lower and upper third of villi. A, C, E, and G: cAMP-treated tissues. B, D, F and H: carbachol-treated tissues. cAMP treatment increased apical CFTR in lower and upper crypts 1.6- and 1.87-fold, respectively, and lower and upper villus 2.67 and 2.78-fold (A); decreased apical NHE3 in lower and upper villus to 51% and 40% (C); increased enterocyte (ENT) lateral membrane NKCC1 in lower and upper crypt 1.49- and 1.57-fold, and lower and upper villus 2.50- and 2.67-fold; increased lateral membrane NKCC1 of lower and upper crypt goblet (GOB) cells 1.51- and 1.58-fold, and lower and upper villus goblet cells 1.43- and 1.25-fold (E); and increased lateral membrane NBCe1 of upper crypt enterocytes 2.03-fold, lower and upper villus enterocytes 2.5- and 1.35-fold (G). Carbachol treatment increased apical CFTR in lower and upper crypts 1.73- and 2.57-fold, and lower and upper villus 4.07- and 6.82-fold (B); decreased apical NHE3 of lower and upper villus to 40% and 39% (D); increased enterocyte lateral membrane NKCC1 in lower and upper crypt 1.46- and 1.67-fold, and lower and upper villus 2.60- and 2.88-fold (F); increased lateral membrane NKCC1 of lower and upper crypt goblet cells 1.57- and 1.72-fold, and lower and upper villus goblet cells 1.43- and 1.30-fold; and increased lateral membrane NBCe1 of upper crypt enterocytes 2.74-fold, lower and upper villus enterocytes 2.68- and 1.51-fold (H). *Significant difference from the untreated group; &upper villus-treated group significantly different from lower villus-treated group.
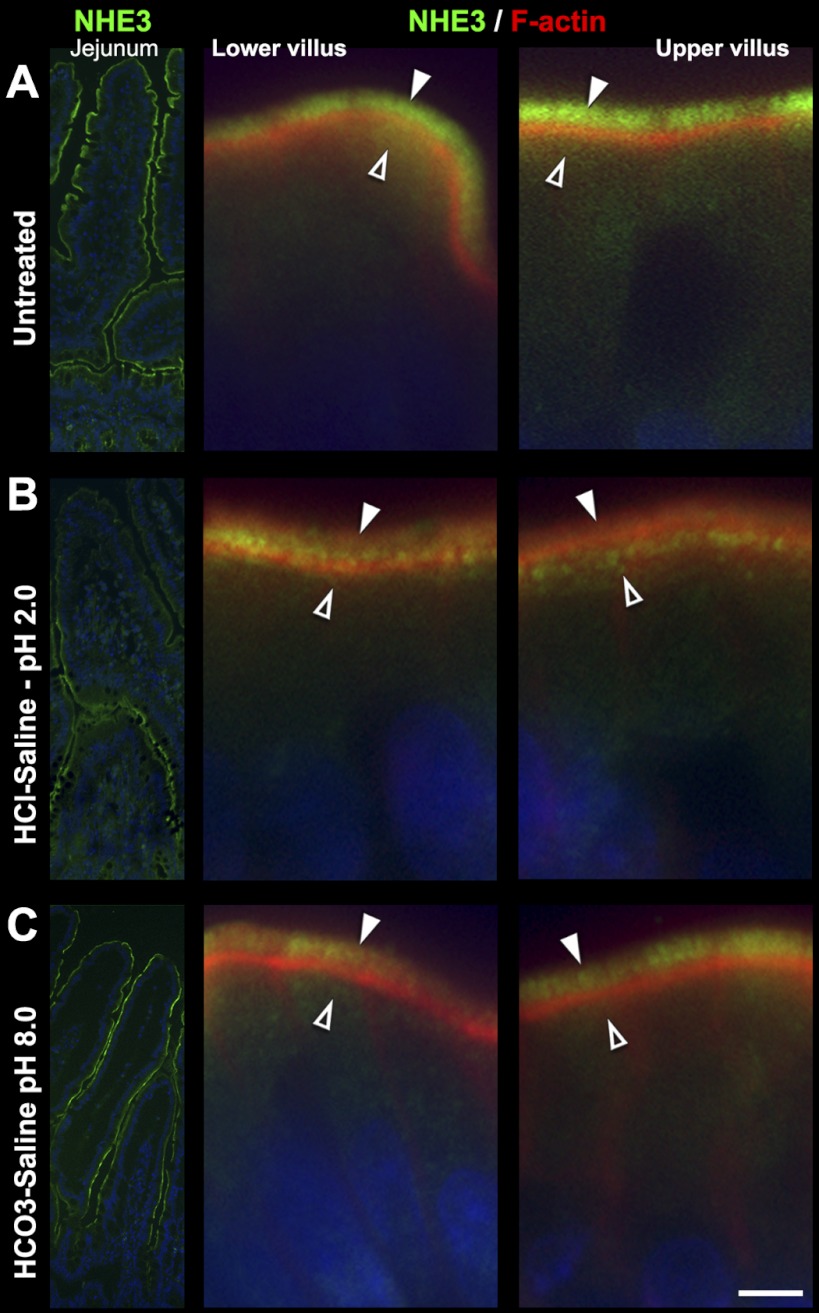
HCl-Saline Induced Redistribution of CFTR, NHE3, NKCC1, and NBCe1
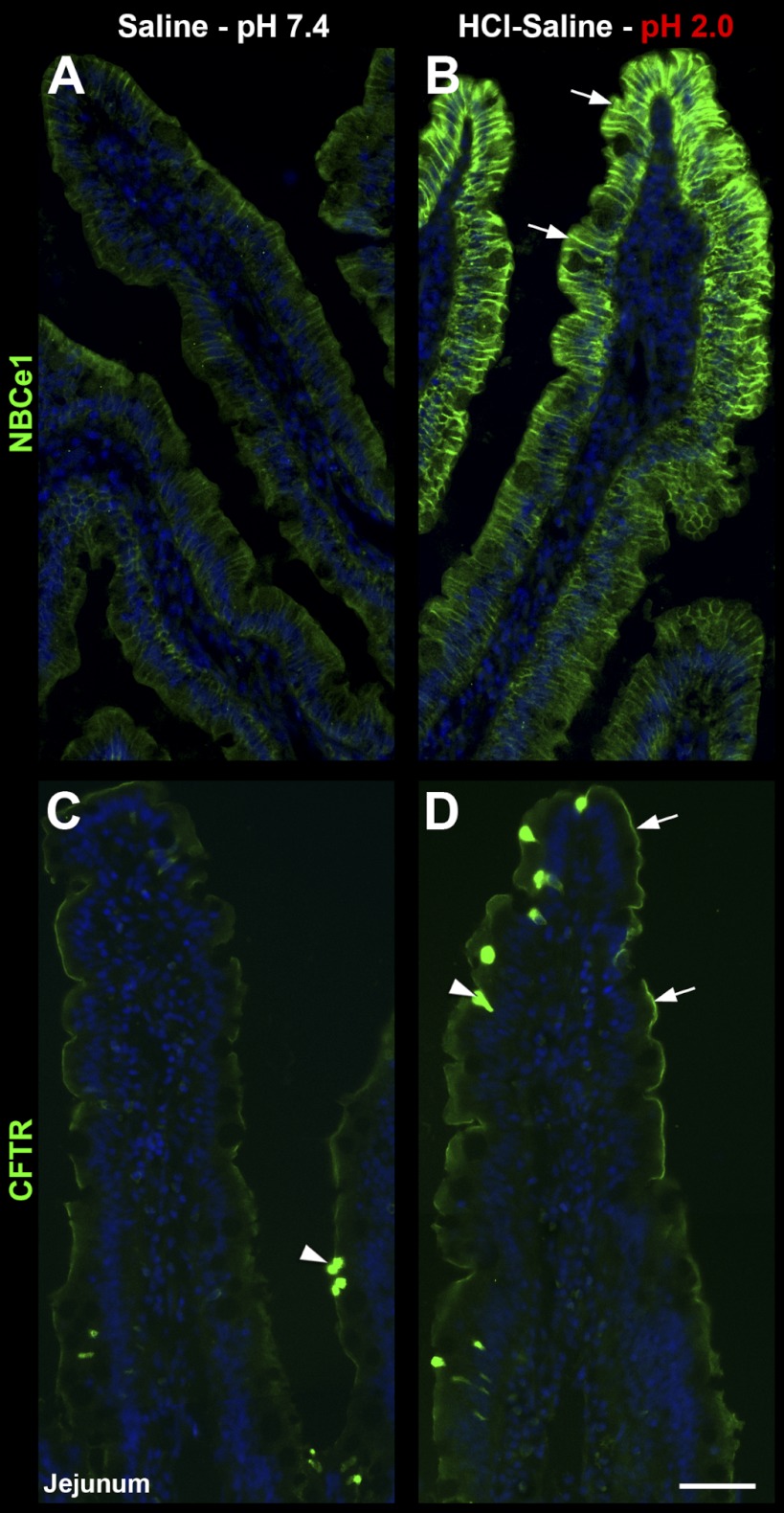
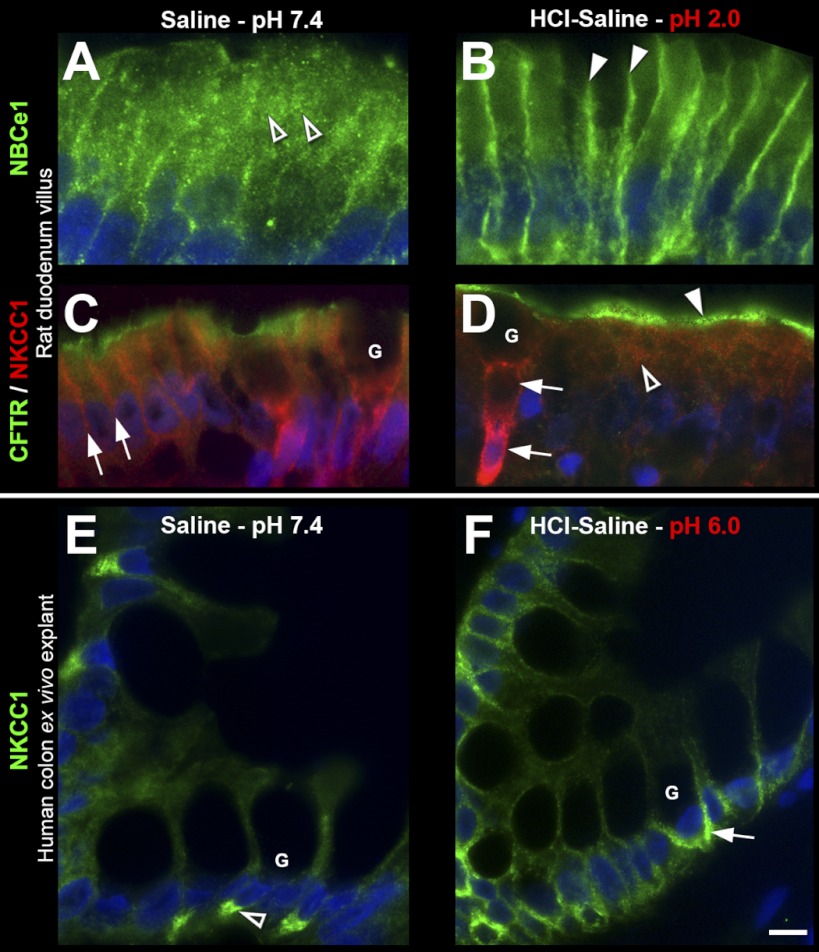
Next, we examined whether luminal exposure to an acidic saline solution alters the subcellular distribution of anion transporters in the small intestine. Treatment of ligated segments with HCl-saline pH 2.0 for 20 min resulted in robust recruitment of NBCe1 to the enterocyte basolateral membrane in jejunum (Fig. 4, A–B), duodenum (Fig. 5, A–B), and ileum (not shown). Surprisingly, NBCe1 membrane recruitment was restricted to enterocytes of the upper villus (Fig. 4, A–B, arrows) in each small intestinal segment. In a similar fashion, acid treatment selectively induced recruitment of CFTR into the BBM of enterocytes in the upper villus in duodenum (Fig. 5, C–D, arrowhead) and jejunum, including the CFTR high-expresser (CHE) cells (arrowheads, Fig. 4, C–D). Although not the focus of this study, CHE cells represent a small (∼2.5%) subpopulation of enterocytes in the small intestine that express very high levels of CFTR and undergo robust second messenger regulated traffic into and out of the BBM (5, 7–8). Acid treatment resulted in retention of NKCC1 in intracellular compartments in villus enterocytes, but its recruitment to the basolateral membrane of goblet cells (Fig. 5, C–D). Finally, acid treatment induced partial internalization of NHE3 from the BBM to a subapical compartment of enterocytes of the upper villus (Fig. 6). Densitometry analysis of HCl-saline induced changes in fluorescence intensity of transporter distribution is shown in Fig. 7, left column. These observations suggest that epithelial cells of the upper villus epithelium respond to low pH by upregulating CFTR on the BBM. In contrast to cAMP and carbachol, low pH does not lead to NKCC1 recruitment on the basolateral membranes of enterocytes, but to its retention within the cytoplasm, suggesting that under this condition, chloride secretion is limited and bicarbonate secretion predominates because of robust membrane recruitment of NBCe1 to the basolateral membranes of enterocytes.
Fig. 4.
HCl-saline induced redistribution of CFTR and NBCe1 in rat jejunum. Rat jejunum was treated in vivo with normal saline pH 7.4 (left) or HCl-saline pH 2.0 (right). A–B: distribution of NBCe1 (green) in villus epithelium. Arrows, increased basolateral NBCe1 label in the upper villus in HCl-saline-treated tissue. C–D: distribution of CFTR (green) in villus epithelium. Arrows, recruitment of apical CFTR in the upper villus in HCl-saline-treated tissue; Arrowheads, CFTR high-expresser (CHE) cells. The images represent the results of 4 experiments (n = 4). Scalebars: A–D, 100 μm.
Fig. 5.
HCl-saline induced redistribution of CFTR, NBCe1, and NKCC1 in rat duodenum in vivo and NKCC1 in human colon explant ex vivo. Representative images of immunolabeled sections from (A–D) rat duodenum treated with saline pH 7.4 (left) or HCl-saline pH 2.0 (right). A–B: NBCe1 (green) distribution in villus epithelium: open arrowheads, intracellular staining; arrowheads, basolateral staining. C–D: double-label for CFTR (green) and NKCC1 (red) in villus epithelium. Arrows, basolateral NKCC1 label; open arrowhead, intracellular NKCC1 label; arrowhead, apical CFTR label. E–F: distribution of NKCC1 (green) in human colon treated with saline pH 7.4 (left) or HCl-saline pH 6.0 (right): open arrowhead, intracellular localization; arrow, basolateral labeling. G, goblet cells. The images represent the results of 4 experiments (n = 4). Scalebars: A–F, 10 μm.
Fig. 6.
Redistribution of NHE3 in HCl- saline and HCO3−-saline-treated rat jejunum. A: untreated tissue. B: HCl-saline (pH 2.0)-treated tissue. C: HCO3−-saline (pH 8.0)-treated tissue. Left: low-power images of NHE3 (green) distribution; Center: high-power images from lower villus. Right: image from upper villus double-labeled for NHE3 (green) and F-actin (red). Arrowheads, apical brush-border localization; open arrowheads, subapical localization. Images represent the results of 4 experiments (n = 4). Scalebars: A–C, 5 μm.
Fig. 7.
Densitometry of normalized CFTR, NHE3, NKCC1, and NBCe1 and fluorescence intensity at apical or lateral membrane, and subapical compartments of enterocytes and goblet cells from HCl-saline- and HCO3−-saline-treated rat jejunum. Cells were analyzed from the lower and upper third of crypts, and lower and upper third of villi. A, C, E, and G: HCl-saline treatment. B, D, F, and H: HCO3− treatment. HCl treatment increased BBM CFTR in lower and upper crypt enterocytes 1.35- and 1.27-fold, and lower and upper villus enterocytes 1.11- and 4.07-fold (A); decreased BBM NHE3 in lower and upper villus enterocytes to 88% and 61% (C); decreased enterocyte lateral membrane NKCC1 in lower and upper crypt to 88% and 76%, and lower and upper villus to 67% and 82% (E); increased lateral membrane NKCC1 in lower and upper crypt goblet cells 1.24- and 1.63-fold, and lower and upper villus goblet cells 1.67- and 2.21-fold; increased lateral membrane NBCe1 in upper crypt enterocytes 1.91-fold, lower and upper villus enterocytes 1.33- and 1.55-fold (G). HCO3− saline induced an increase in BBM CFTR in lower and upper crypt enterocytes 1.3- and 1.6-fold, and lower and upper villus enterocytes 3.93- and 2.78-fold (B); slight decrease in BBM NHE3 in lower and upper villus enterocytes to 86% and 92% (D); decrease in enterocyte lateral membrane NKCC1 in lower and upper crypt to 73% and 64%, and lower and upper villus 61% and 53%; increased lateral membrane NKCC1 of lower and upper crypt goblet cells 1.39- and 1.52-fold, and lower and upper villus goblet cells 1.93- and 2.52-fold (F); and increased lateral membrane NBCe1 of upper crypt enterocytes 1.6-fold, lower and upper villus enterocytes 1.56- and 1.43-fold (H). *Significant difference from the untreated group; &upper villus-treated group significantly different from lower villus-treated group.
HCl-Saline pH 6.0 Redistributes NKCC1 in Human Colonic Goblet Cells
The observed acid-induced redistribution of NKCC1 to the basolateral membranes of rat goblet cells following pH 2.0 were recapitulated in human colon explants following treatment with HCl-saline pH 6.0 for 20 min ex vivo (Fig. 5, E and F). Since a pH 2.0 solution caused tissue damage to the ex vivo tissue explant, its effect on transporters was not studied.
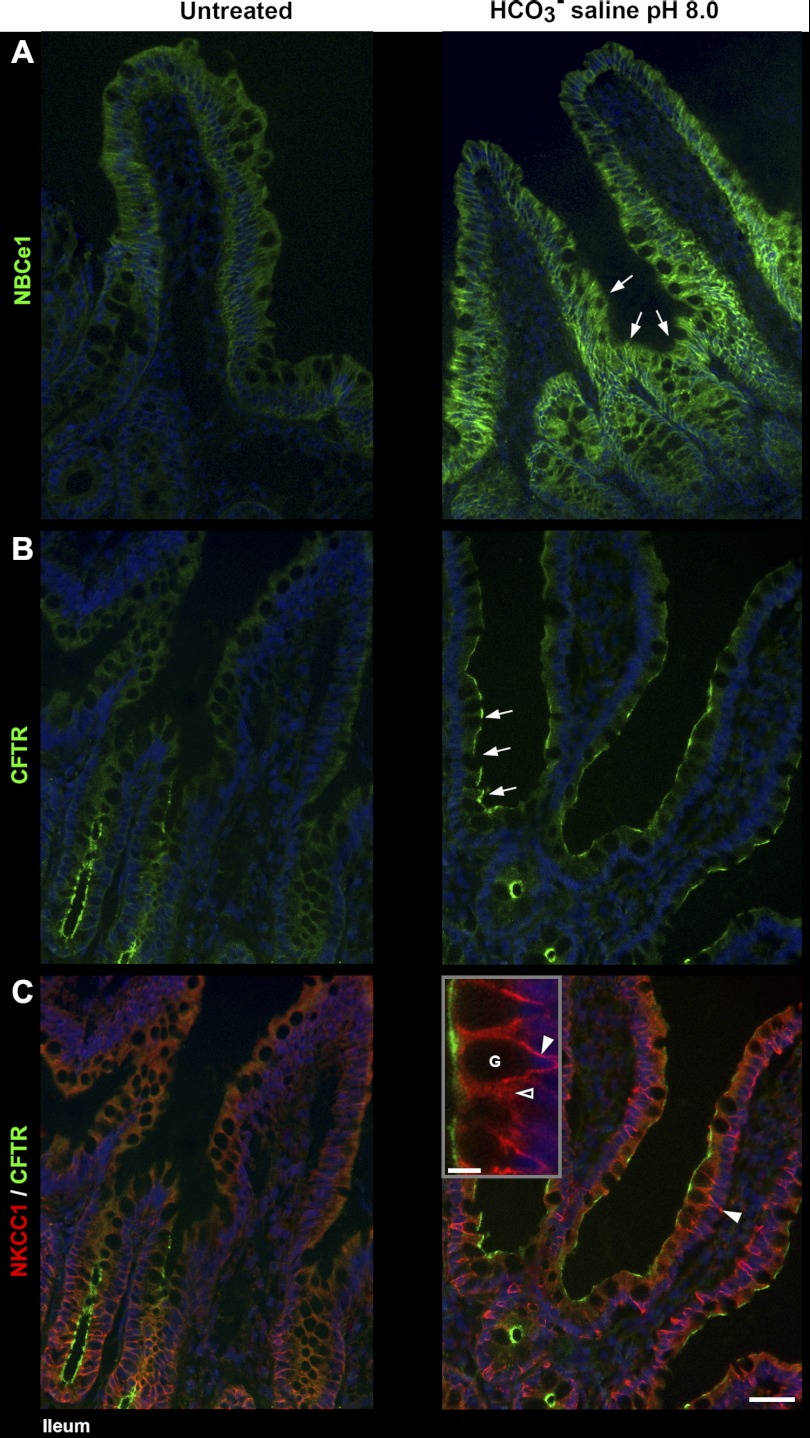
HCO3−-Saline Induced Redistribution of CFTR, NHE3, NKCC1, and NBCe1
In contrast to the observed effects of acid, luminal HCO3− in jejunum and ileum appeared to preferentially target enterocytes in the lower villus epithelium. Both basolateral NBCe1 (Fig. 8A) and apical CFTR label (Fig. 8B) were selectively increased in lower villi. HCO3− treatment also increased basolateral NKCC1 in goblet cells but decreased basolateral NKCC1 in enterocytes (Fig. 8C). After HCO3− treatment, NHE3 remained largely localized to the BBM, although at a slightly reduced intensity (Fig. 6C) as confirmed by densitometry (Fig. 7, right graphs).
Fig. 8.
Luminal bicarbonate induced redistribution of CFTR, NKCC1, and NBCe1 in rat ileum. Left: untreated tissue; right: tissue treated with HCO3−-saline pH 8.0. A: distribution of NBCe1 (green). Arrows, increased NBCe1 label in the lower villus epithelium in HCO3−-saline-treated tissue. B: distribution of CFTR (green). Arrows, increased CFTR label in the lower villus epithelium of HCO3−-saline-treated tissue (C) double-labeled for CFTR (green) and NKCC1 (red). Arrowhead, increased NKCC1 label at the basolateral membrane of goblet cells in HCO3−-saline-treated tissue; high-powered inset, intense NKCC1 label on the basolateral membrane (arrowhead) of goblet cells (G); open arrowhead, intracellular NKCC1 label in neighboring enterocyte. The images represent the results of 4 experiments (n = 4). Scalebar: A–C, 50 μm; insert, 10 μm.
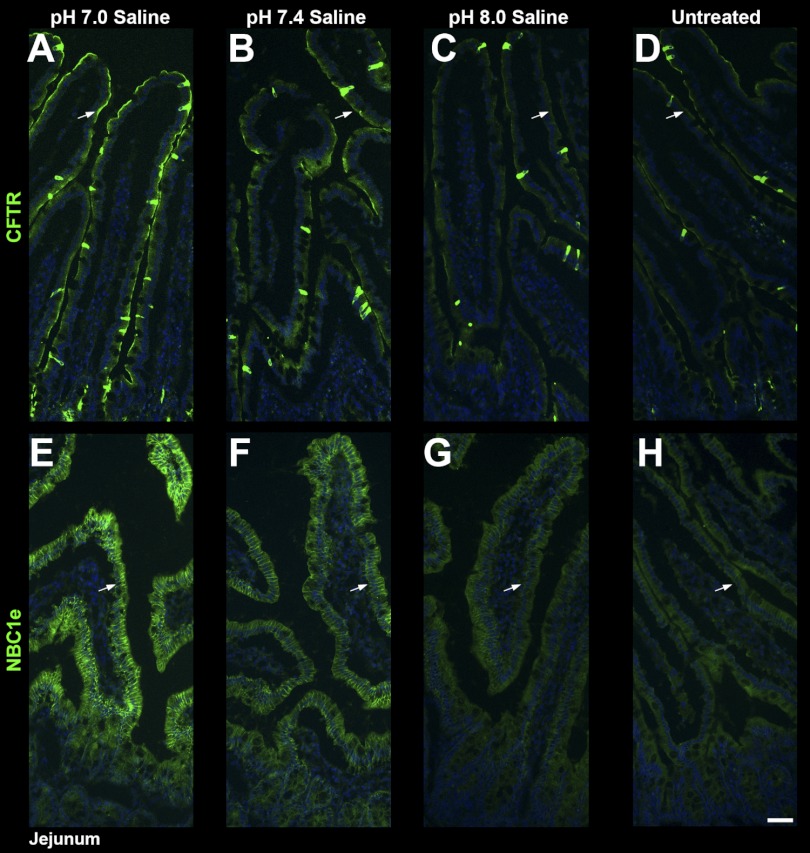
Effects of Mildly Acidic, Neutral, and Mildly Alkaline Saline on the Distribution of CFTR and NBCe1
The distribution of CFTR and NBCe1 were examined in tissue sections from jejunal loops that were left untreated or treated with mildly acidic saline (H+-saline; pH 7.0), saline (pH 7.4), or mildly alkaline saline (OH−-saline; pH 8.0). Compared with untreated tissue (Fig. 9, D and H), BBM CFTR label was highest at pH 7.0 followed by pH 7.4, and at pH 8.0 CFTR label intensity resembled that of the untreated tissues (Fig. 9, A–D). The pattern of basolateral NBCe1 distribution under the same conditions paralleled CFTR (Fig. 9, E–H).
Fig. 9.
Distribution of CFTR (green) and NBCe1 (green) in jejunum following pH 7.0, pH 7.4, or pH 8.0 saline. Ligated, neighboring proximal jejunal segments were treated with pH 7.0 (A and E), pH 7.4 (B and F), or pH 8.0 saline (C and G), or left untreated (D–H). Tissue sections were immunolabeled for NBCe1 (green), or CFTR (green). A–D, arrows: CFTR label. E–H, arrows: NBCe1 label. Compared with pH 8.0 saline-treated and untreated tissues, CFTR and basolateral NBCe1 label are more intense in the saline pH 7.0 or pH 7.4-treated tissues. The images represent the results of 3 experiments (n = 3). Scalebars: A–F, 50 μm.
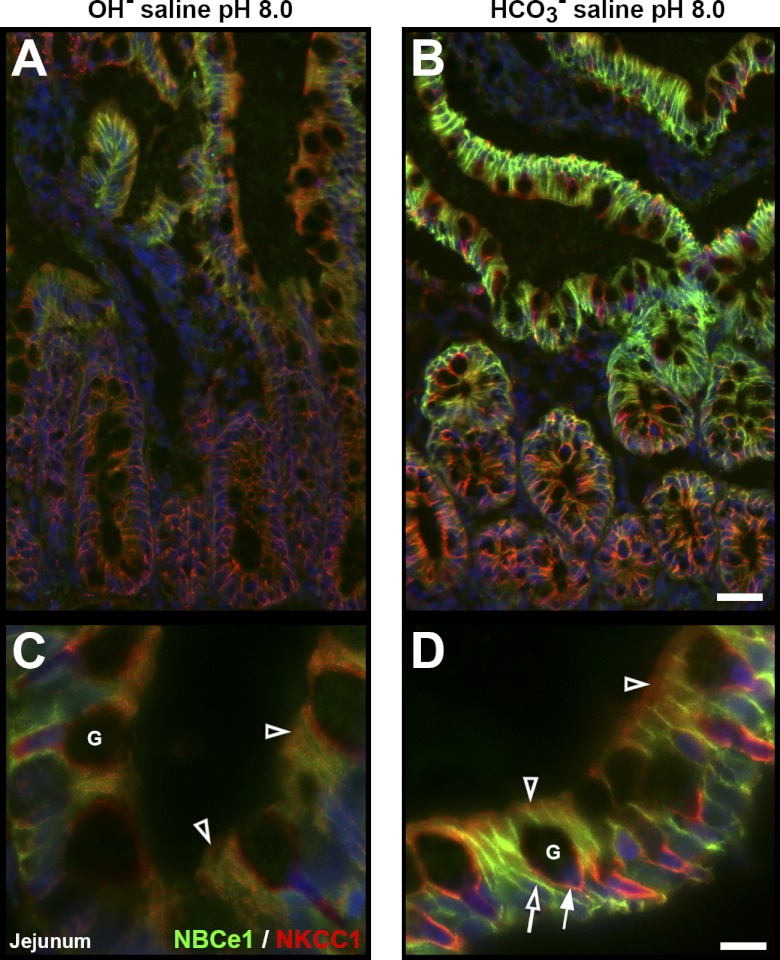
Comparison of Tissue Responses to OH−-Saline or HCO3−-Saline at the Same pH (pH 8.0)
Studies have suggested that epithelial cells directly sense Pco2 rather than pH (36). Under this scenario, CO2 generated from the interaction of luminal H+ and HCO3− at the mucosal surface stimulates bicarbonate and mucus secretion (2). We examined transporter distribution patterns in surface mucosa in the presence or absence of generated CO2. Neighboring ligated proximal jejunal loops were treated with OH− saline or HCO3−-saline at pH 8.0. In the OH−-saline-treated epithelium, membrane CFTR (not shown) and NBCe1 levels remained low in enterocytes, and membrane NKCC1 levels remained low in enterocytes and goblet cells (Fig. 10, A and C). Thus, neither the enterocytes (involved in bicarbonate secretion) nor the goblet cells (mucus secretion) responded to luminal alkalinization when CO2 was not generated. In contrast, after HCO3−-saline treatment, apical CFTR (not shown), as well as basolateral NBCe1 labeling increased strongly in enterocytes, and basolateral labeling of NKCC1 selectively increased in goblet cells (Fig. 10, B and D and graphs in Fig. 7, B, F, and H). Thus, anion transporter trafficking was robust in bicarbonate (enterocytes) and mucus secreting (goblet) cells when CO2 was generated. Following both OH− or HCO3−-saline treatments, NKCC1 was largely internalized in enterocytes (Fig. 10, B and D; open arrowheads), suggesting that stimulated basolateral Cl− transport was not activated in these conditions. The observed differences in trafficking responses in the presence and absence of CO2 support the hypothesis of epithelial CO2 sensing and suggest that enterocytes and goblet cells respond to CO2 simultaneously; CFTR and NBCe1 traffic to their respective membrane domain in enterocytes and NKCC1 in goblet cells.
Fig. 10.
Redistribution of NBCe1 and NKCC1 in OH−-saline pH 8.0 or HCO3−-saline pH 8.0-treated rat jejunum. Neighboring ligated proximal jejunal segments were treated with OH−-saline pH 8.0 (left) or HCO3−-saline pH 8.0 (right). A–B: tissue sections double-labeled for NBCe1 (green) and NKCC1 (red). C–D: high-powered micrographs of NBCe1/NKCC1 double-labeled in villus section. Open arrowheads, intracellular NKCC1 label; open arrow, basolateral NBCe1 label; arrow, basolateral NKCC1 label; G, goblet cell. Scalebars: A–B, 50 μm; C–D, 10 μm. The images represent the results of 4 experiments (n = 4).
DISCUSSION
Redistribution Patterns of Ion Transporters
The present study examined cell-specific transporter trafficking patterns in tissues from rat and human intestine under basal conditions and following luminal administration of four physiologically relevant stimuli: cAMP or Ca2+ mediated secretagogues and HCl or HCO3−-saline, conditions that can lead to anion secretion and alterations in luminal pH. CFTR abundance increased in the enterocyte BBM after all treatments, consistent with published studies of CFTR-mediated anion secretion following cAMP and Ca2+ activation (35), luminal acid, or CO2 challenge (44, 54). NHE3 dramatically redistributed to a subapical compartment of enterocytes along the entire villus axis after cAMP or carbachol. Robust NHE3 internalization was observed only in upper villus enterocytes after luminal acid. NHE3 internalization was observed following HCO3− treatment but was less pronounced compared with that observed following acid, carbachol, or cAMP. The cellular redistribution patterns of NHE3 observed in this study correspond to functional data indicating that both cAMP (30, 46) and carbachol (43) strongly inhibit NHE3 activity. The observed NHE3 trafficking responses suggest that NHE3 remains partially active under acid or CO2 challenge (25, 44) and are in agreement with functional data on NHE3 activity under these conditions.
The finding that all four conditions (luminal acid, luminal HCO3−, carbachol, and cAMP) lead to simultaneous BBM recruitment of CFTR and NHE3 internalization extends our previous observations that a single stimulus (carbachol) causes simultaneous BBM recruitment of CFTR and NHE3 internalization in the same villus enterocytes (40). These reciprocal trafficking events align closely with physiological findings that: 1) cAMP-induced inhibition of NHE3 activity and stimulation of CFTR activity occur in parallel in villus epithelium (30), 2) CFTR inhibition augments NHE3 activity during luminal high CO2 exposure in rat duodenum (44), and 3) NHE3 inhibition upregulates CFTR function during duodenal bicarbonate secretion (25). The data presented in the present and previous study (40) unambiguously demonstrate that both CFTR and NHE3 are present in villus enterocytes along the entire small intestine.
In the present study, apical CFTR recruitment is consistently accompanied by basolateral NBCe1 recruitment in the same villus epithelium after secretory stimuli elicited by cAMP, carbachol, HCl-saline, or HCO3−-saline, similar to responses elicited by the prosecretory drug lubiprostone (39). In fact, we found no condition where CFTR or NBCe1 was recruited without the other (Jakab RL, Ameen NA, unpublished observations). This suggests that the two trafficking events are functionally linked in small intestinal villus enterocytes. Membrane recruitment of CFTR or NBCe1 alone was observed in response to cAMP or carbachol (6, 10–11, 40). It is recognized that CFTR expression is a sine qua non condition for cAMP and Ca2+-stimulated duodenal HCO3− secretion (50). Although the morphological nature of this study allows limited speculations on function, the observed corecruitment of CFTR and NBCe1 suggests their coactivation and implies that CFTR function is consistently linked to HCO3− secretion in villus enterocytes. Overall, our data provide a compelling morphological argument for the concept that CFTR, NHE3, and NBCe1 are functionally linked in the same villus enterocytes and further support their roles in anion secretion in diarrheal disease (21, 23, 45, 50).
cAMP and carbachol recruited NKCC1 to the basolateral membrane, but NKCC1 was internalized after HCl-saline or HCO3−-saline treatments in enterocytes. Membrane recruitment of NKCC1 can follow low intracellular Cl− levels, hypotonic swelling, or cell shrinkage, while Cl− secretion is associated with BBM recruitment of CFTR that occurs in conjunction with basolateral membrane recruitment of NKCC1 following secretagogue (20). The observed internalization of NKCC1 in enterocytes following HCl-saline or HCO3−-saline treatment is consistent with reduced Cl− secretory activity. NKCC1 was recruited to the basolateral membrane of goblet cells after all four treatments, but was particularly robust after HCl-saline (upper villus 2.21-fold increase) and HCO3−-saline treatment (upper villus 2.51-fold increase) compared with the relatively modest (1.3- to 1.4-fold) increase after treatments with cAMP or carbachol. The approaches used in the present study cannot determine whether this trafficking event is a volume regulatory phenomenon to counterbalance cell shrinkage following exocytosis of mucus granules, or activation of Cl− secretion in association with mucus secretion by goblet cells, as suggested (41). In either case, the recruitment of NKCC1 in goblet cells is likely linked to mucus secretion. Indeed, both luminal acid or CO2 challenge potently stimulate mucus secretion (2). Unlike enterocytes, goblet cells are devoid of CFTR and NBCe1, but may contribute to anion secretion via transporters not studied here, e.g., the Ca2+-activated Cl− channel bestrophin 2 (64) or the TMEM16 anoctamin channels (42).
Lumen pH Profile Along the Small Intestine
The pH in the small intestine was relatively high in the duodenum (mean pH 7.24), lower in the proximal jejunum (mean pH 6.68), and highest in the ileum (mean pH 7.83; see Fig. 1). These data indicate that the lumen is relatively alkaline with higher HCO3− concentrations in the unstimulated duodenum and ileum. The small intestinal pH profile agrees with physiological data that the duodenum and ileum preferentially secrete HCO3−, whereas the jejunum preferentially absorbs HCO3− in the steady-state fasting condition (14–15, 26). The intestinal pH profile observed in this study corresponds well with that reported earlier for the rat (61) and humans (22, 31, 48–49). Our lumen pH findings were comparable to previous studies that employed pH electrodes in rats (61). In the referenced study, the mean luminal duodenal value was pH 6.9 in fed rats and pH 7.1 in fasted rats, and the jejunum-ileum value was pH 7.4 in fed rats and pH 8.0 in fasted rats. Human intestinal pH profiles are similar to the present study, but the pH values are ∼0.5 to 1.0 point lower (22, 31, 48–49). The most likely explanation is that all human studies were conducted under nonfasting conditions and feeding markedly lowers lumen pH (61). Our data indicate that the lumen pH changes in individual segments following a physiological stimulus (see below) but maintains the overall pH gradient along the small intestine. The untreated lumen pH data were obtained from nonligated intestine. However, the lumen pH responses to the 20-min treatments were obtained in ligated segments. Thus, the lumen pH responses represent nonphysiological conditions, since in ligated segments lumen content propulsion is blocked, whereas the intact intestine is able to alter lumen pH by propulsion of the lumen content.
Experimentally Induced Lumen pH Responses
The observation that lumen pH was restored 20 min after pH 2.0 HCl-saline was particularly notable, indicating that each intestinal segment is able to rapidly restore its pH after exposure to strong acidic conditions. Lumen pH was slightly reduced by cAMP or carbachol and slightly increased after luminal HCO3− treatment. Fluid secretion induced by the secretagogues cAMP or carbachol are associated with reduction in lumen pH. Both cAMP and carbachol induced robust recruitment of NKCC1, NBCe1, and CFTR and endocytosis of NHE3 from the apical BBM. These transporter trafficking events are consistent with collective coordinate activities that result in increased NKCC1-mediated Cl− secretion and reduced lumen pH.
After both luminal HCl and luminal HCO3− treatment, apical CFTR was recruited into the BBM but, of the basolateral transporters, only NBCe1 was recruited, and basolateral NKCC1 was reduced in crypt enterocytes and remained internalized in villus enterocytes. The internalization of apical BBM NHE3 was partial and incomplete along the villus axis, a response consistent with fluid absorption at a reduced level. These transporter trafficking behaviors are collectively consistent with CFTR/NBCe1-mediated HCO3− secretion but a diminished NKCC1-mediated Cl− secretion component. These events could lead to the observed restoration of lumen pH after acid treatment and account for the slight lumen pH increase after HCO3− treatment.
Functional Relevance of CFTR/NBCe1 Coexpression In Villus Enterocytes
We previously demonstrated (40), and confirmed here that CFTR and NBCe1, the transporters involved in stimulated HCO3− secretion are present together in villus enterocytes along the small intestine. A compelling clinical correlate of this finding is that disorders involving villus atrophy have abnormal responsiveness to luminal pH imbalances. For example, HCO3− secretion is minimal in irradiation pathology, where villus atrophy is a hallmark effect (65). Also, both CFTR and NBCe1 are expressed most robustly in the villus epithelium of the proximal duodenum (40), a region where the function of neutralizing gastric acid was found to be impaired in the human CF intestine (31).
It is a basic tenet of intestinal physiology that bicarbonate and mucus secretion occur in tandem (2). All four secretory stimuli studied here (cAMP, Ca2+, acid, or CO2 challenge) induce bicarbonate and mucus secretion simultaneously (2). In agreement with this, the present data (see graphs in Figs. 3 and 7), show a coordinated activation of CFTR/NBCe1 coexpressing villus enterocytes and NKCC1-expressing goblet cells (as measured by the respective trafficking response) following all four secretory stimuli (cAMP, Ca2+, acid, or CO2 challenge). The data provided in the present study align well with a model (see Fig. 11), whereby CFTR/NBCe1 coexpressing villus enterocytes maintain a loose mucus layer by infusion of HCO3−-rich fluid into the adherent firm mucus secreted by NKCC1-expressing goblet cells. This process can aid mucus emptying and removal if the ion transporters involved function normally (3, 16). The importance of defective bicarbonate secretion in the pathogenesis of intestinal CF mucoviscidosis is increasingly recognized as recently demonstrated (34). Using a CF mouse model, investigators examined mucus layer removability from CF and non-CF small intestinal villi, and demonstrated a critical role for bicarbonate in unpacking and transforming secreted mucins to removable loose mucus by neutralizing pH and removing Ca2+. Loss of basolateral HCO3− transport in wild-type mucus resulted in an adherent CF mucus, and apical HCO3− buffers transformed the CF mucus to that of loose mucus resembling wild-type. The findings of the present study and those of other groups (3, 16, 28, 34) provide a credible explanation for compromised mucus removal in CF disease where the HCO3− secreting capability of CFTR-deficient villus enterocytes is defective. Garcia et al. (28) found that NBCe1- and NKCC1-mediated effects are additive in removing the loose mucus, and both converge on a common CFTR pathway. These findings align with morphological observations in this study that support CFTR/NKCC1/NBCe1 coexpressing villus enterocytes in mediating such an effect. Indeed, the phenotype of corkscrew ceca was observed in CFTR null mice (56), NKCC1-null mice (24), as well as NBCe1-null mice (29), indicating that all three transporters are required for preventing mucus impactions.
Fig. 11.
Hypothetical model of proposed mechanisms underlying the effects of luminal acid or bicarbonate on membrane localization of CFTR and NBCe1 in upper vs. lower villus enterocytes. The results of this study support a hypothetical model whereby the villus axis-specific tissue responses may be a consequence of mucus layer differences. A: under steady-state condition, an H+/HCO3− equilibrium may be maintained in the intestinal lumen: the firm mucus layer is more acidic (enriched in H+), but the loose mucus layer can accumulate HCO3−, as shown recently (34). B: excess luminal H+ could react with the HCO3− content of the loose mucus layer on upper villus. The released CO2 could diffuse into and acidify upper villus enterocytes that respond to acidification with NBCe1 and CFTR upregulation and enhanced HCO3− secretion. C: excess luminal HCO3− may be absorbed by the loose mucus layer. CO2 is not generated here, hence upper villus enterocytes are not acidified, and NBCe1 and CFTR are not upregulated. However, some of the HCO3− can diffuse deeper, react with the H+ of the firm mucus layer, and the released CO2 can diffuse into and acidify lower villus enterocytes that respond to acidification by NBCe1 and CFTR upregulation and enhanced HCO3− secretion. For an easier understanding of how this model may align with data from this study, NBCe1 distribution patterns in untreated (A), pH 2.0 HCl saline-treated (B), and pH 8.0 HCO3−-saline-treated (C) jejunum villus epithelium are shown (bottom).
Differential Responses of Upper vs. Lower Villus Enterocytes and Role of Mucus Layers
This study revealed that acidic challenge (luminal HCl−-saline at pH 2.0) preferentially recruited apical BBM CFTR and basolateral NBCe1 in the upper villus. Indeed, upper villus enterocytes appear to sense acidity (the relative levels of H+ and HCO3−) through a carbonic anhydrase II-mediated mechanism (55). On the other hand, tissue responses to HCO3−-saline at pH 8.0 preferentially involved cells in the lower villus. The selectivity in responses by upper vs. lower villus epithelium to luminal acid vs. bicarbonate may also be linked to mucus layer differences (9) along the villus axis. In the absence of direct evidence, our findings complement existing (1, 9, 16, 28, 55) and emerging data (34) in support of our proposed model (Fig. 11). Under steady-state conditions, a luminal H+/HCO3− equilibrium could be maintained, in part because the loose mucus layer accumulating HCO3− and the firm layer enriched in H+ can be reversibly transformed into each other as previously suggested (34). Under conditions of excess luminal acid, luminal H+ first contacts the loose mucus layer over the upper villus (34). Here luminal H+ can react with the HCO3− content of the loose mucus layer to produce CO2 to acidify upper villus enterocytes that respond by upregulation of NBCe1 and CFTR to enhance HCO3− secretion. On the contrary, when excess luminal HCO3− contacts the loose mucus layer, CO2 is not generated. Here, upper villus enterocytes are not acidified, and NBCe1 and CFTR are not upregulated. However, the excess HCO3− could diffuse deeper and react with the H+ content of the firm mucus layer, and the released CO2 can diffuse into and acidify lower villus enterocytes that can respond by upregulating NBCe1 and CFTR to enhance HCO3− secretion.
Precedence for functional selectivity between lower and upper villus epithelium has been observed for other transporters such as DRA and PAT-1 that are functionally linked to CFTR, but further studies are required to understand in more detail how these exchangers respond to stimuli at the cellular level. The Cl-/HCO3− anion exchangers DRA and PAT-1 possess differential expression profiles along the crypt-villus axis of duodenum (38, 52–53, 59–60). Expression levels for DRA are greater in the lower villus/crypt while PAT-1 is more abundant in the upper villus. Recent studies suggest that PAT-1 Cl−out/HCO3−in exchange provides a HCO3− import pathway in upper villus epithelium to sustain intracellular pH homeostasis during acid challenge (53). Microfluorometry studies identified PAT-1 as the major contributor to total Cl−/HCO3− exchange in upper villus, and DRA as the main contributor in lower villus. But under conditions of outwardly directed HCO3−, functional activity of PAT-1 was detected in lower villus epithelium (60).
In conclusion, the results of this study reveal novel details regarding the distinct roles, coordination, and selectivity of secretory responses by epithelial cells and transporters along the crypt/villus axis. An important role for CFTR-mediated bicarbonate secretion emanating from villus enterocytes was recently recognized, and emerging evidence suggest that this activity is central to the pathogenesis of intestinal disease in CF. But whether acid-stimulated bicarbonate secretion originated from specific sites along the crypt-villus axis was unclear. The observed synchronized trafficking responses of CFTR, NHE3, and NBCe1 to low pH in upper villus enterocytes of proximal small intestine support robust acid-stimulated bicarbonate secretion in cells that first encounter low pH from stomach effluent entering the duodenum. These observations also provide more understanding of the underlying pathophysiology of abnormally low luminal pH in the human CF duodenum where CFTR is absent from the enterocyte BBM.
While electrophysiological approaches such as Ussing chamber have been central to investigating the physiological and pharmacological responses of epithelial tissues, complementary morphological approaches, such as those applied in this study, are necessary to fully grasp the complexity of the functional organization of an intact epithelium. Studies of intestinal tissues that employ a combination of in vivo treatments and morphological analytical techniques are critical to identification of new cell type-specific functions and regulation.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-077065 (to N. A. Ameen) and DK-34989 (to the Digestive Diseases Research Core at Yale University).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L.J. and N.A.A. conception and design of research; R.L.J., A.M.C., and N.A.A. performed experiments; R.L.J., A.M.C., and N.A.A. analyzed data; R.L.J., A.M.C., and N.A.A. interpreted results of experiments; R.L.J. prepared figures; R.L.J. drafted manuscript; R.L.J., A.M.C., and N.A.A. edited and revised manuscript; N.A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Fred Gorelick and Nadia Hoekstra for reviewing the manuscript.
REFERENCES
- 1. Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 279: G437–G447, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288: C1–C19, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJ, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA 109: 5645–5650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Ameen NA, Marino C, Salas PJ. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am J Physiol Cell Physiol 284: C429–C438, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+-HCO3− cotransport activity in murine colonic crypts in a M3-, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol 291: G650–G657, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol 284: G37–G45, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Bachmann O, Wuchner K, Rossmann H, Leipziger J, Osikowska B, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. Expression and regulation of the Na+-K+-2Cl− cotransporter NKCC1 in the normal and CFTR-deficient murine colon. J Physiol 549: 525–536, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bell S, Kamm MA. Antibodies to tumour necrosis factor-α as treatment for Crohn's disease. Lancet 355: 858–860, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Physiology of the Gastrointestinal Tract (3rd ed.), edited by Johnson LR. New York: Raven, 1994, p. 2133–2171 [Google Scholar]
- 15. Chang EB, Rao MC. Intestinal water and electrolyte transport: mechanisms of physiological and adaptive responses. In: Physiology of the Gastrointestinal Tract (3rd ed.), edited by Johnson LR. New York: Raven, 1994, p. 2027–2081 [Google Scholar]
- 16. Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299: L542–L549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciancio MJ, Chang EB. Epithelial secretory response to inflammation. Ann NY Acad Sci 664: 210–221, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Cuthbert AW, Hickman ME, MacVinish LJ, Evans MJ, Colledge WH, Ratcliff R, Seale PW, Humphrey PP. Chloride secretion in response to guanylin in colonic epithelial from normal and transgenic cystic fibrosis mice. Br J Pharmacol 112: 31–36, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D'Andrea L, Lytle C, Matthews JB, Hofman P, Forbush B, 3rd, Madara JL. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J Biol Chem 271: 28969–28976, 1996 [DOI] [PubMed] [Google Scholar]
- 21. de Jonge HR. The response of small intestinal villus and crypt epithelium to cholera toxin in rat. Evidence against a specific role of the crypt cells in choleragen induced secretion. Biochimica Biphysica Acta 381: 128–143, 1975 [DOI] [PubMed] [Google Scholar]
- 22. Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29: 1035–1041, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Field M, Semrad CE. Toxigenic diarrheas, congenital diarrheas and cystic fibrosis: disorders of intestinal ion transport. Ann Rev Physiol 55: 631–655, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274: 26946–26955, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Furukawa O, Bi LC, Guth PH, Engel E, Hirokawa M, Kaunitz JD. NHE3 inhibition activates duodenal bicarbonate secretion in the rat. Am J Physiol Gastrointest Liver Physiol 286: G102–G109, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Furukawa O, Hirokawa M, Zhang L, Takeuchi T, Bi LC, Guth PH, Engel E, Akiba Y, Kaunitz JD. Mechanism of augmented duodenal HCO3− secretion after elevation of luminal CO2. Am J Physiol Gastrointest Liver Physiol 288: G557–G563, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266: 107–109, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem 282: 9042–9052, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125: 1148–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 2012. May 17 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32. Goldstein JL, Sahi J, Bhuva M, Layden TJ, Rao MC. Escherichia coli heat-stable enterotoxin-mediated colonic Cl− secretion is absent in cystic fibrosis. Gastroenterology 107: 950–956, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Golin-Bisello F, Bradbury NA, Ameen NA. Heat stable enterotoxin (STa) and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. CFTR mediates cAMP and calcium activated duodenal epithelial bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 272: G872–G878, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Holm M, Johansson B, Pettersson A, Fandriks L. Carbon dioxide mediates duodenal mucosal alkaline secretion in response to luminal acidity in the anesthetized rat. Gastroenterology 115: 680–685, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Isenberg J, Hogan D, Koss MA, Selling J. Human duodenal mucosal bicarbonate secretion. Gastroenterology 91: 370–378, 1986 [PubMed] [Google Scholar]
- 38. Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci 2012. August 25 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl− secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol Cell Physiol 261: C574–C582, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Kunzelmann K, Kongsuphol P, Chootip K, Toledo C, Martins JR, Almaca J, Tian Y, Witzgall R, Ousingsawat J, Schreiber R. Role of the Ca2+-activated Cl− channels bestrophin and anoctamin in epithelial cells. Biol Chem 392: 125–134, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 556: 791–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mizumori M, Choi Y, Guth PH, Engel E, Kaunitz JD, Akiba Y. CFTR inhibition augments NHE3 activity during luminal high CO2 exposure in rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol 294: G1318–G1327, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Moritz M, Iber F, Moore E. Rabbit cholera: effects of cycloheximide on net water and ion fluxes and transmural electric potentials. Gastroenterology 63: 76–82, 1972 [PubMed] [Google Scholar]
- 46. Musch MW, Arvans DL, Wang Y, Nakagawa Y, Solomaha E, Chang EB. Cyclic AMP-mediated endocytosis of intestinal epithelial NHE3 requires binding to synaptotagmin 1. Am J Physiol Gastrointest Liver Physiol 298: G203–G211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poulsen J, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capabilty of cystic fibrosis transmembrane conductance regulator. Physiology 91: 5340–5344, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Press AG, Hauptmann IA, Hauptmann L, Fuchs B, Fuchs M, Ewe K, Ramadori G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther 12: 673–678, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Pye G, Evans DF, Ledingham S, Hardcastle JD. Gastrointestinal intraluminal pH in normal subjects and those with colorectal adenoma or carcinoma.. Gut 31: 1355–1357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossman H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP, cGMP and calcium-dependent HCO3− secretion. J Physiol 505: 411–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates basal Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl-−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Simpson JE, Walker NM, Supuran CT, Soleimani M, Clarke LL. Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in duodenal villous epithelium. Am J Physiol Gastrointest Liver Physiol 298: G683–G691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCO3− secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol 298: C1057–C1065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sjoblom M, Singh AK, Zheng W, Wang J, Tuo BG, Krabbenhoft A, Riederer B, Gros G, Seidler U. Duodenal acidity “sensing” but not epithelial HCO3− supply is critically dependent on carbonic anhydrase II expression. Proc Natl Acad Sci USA 106: 13094–13099, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992 [DOI] [PubMed] [Google Scholar]
- 57. Stewart CP, Turnberg LA. A microelectrode study of responses to secretagogues by epithelial cells in villus and crypt of rat small intestine. Am J Physiol Gastrointest Liver Physiol 257: G334–G343, 1989 [DOI] [PubMed] [Google Scholar]
- 58. Tuo B, Wen G, Zhang Y, Liu X, Wang X, Dong H. Involvement of phosphatidylinositol 3-kinase in cAMP- and cGMP-induced duodenal epithelial CFTR activation in mice. Am J Physiol Cell Physiol 297: C503–C515, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL. Functional activity of Pat-1 (Slc26a6) Cl−/HCO3− exchange in the lower villus epithelium of murine duodenum. Acta Physiol (Oxf) 201: 21–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ward FW, Coates ME. Gastrointestinal pH measurement in rats: influence of the microbial flora, diet and fasting. Lab Anim 21: 216–222, 1987 [DOI] [PubMed] [Google Scholar]
- 62. Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut 56: 1153–1163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Youngberg CA, Berardi RR, Howatt WF, Hyneck ML, Amidon GL, Meyer JH, Dressman JB. Comparison of gastrointestinal pH in cystic fibrosis and healthy subjects. Dig Dis Sci 32: 472–480, 1987 [DOI] [PubMed] [Google Scholar]
- 64. Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest 120: 1722–1735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang K, Yin L, Zhang M, Parker MD, Binder HJ, Salzman P, Zhang L, Okunieff P, Vidyasagar S. Radiation decreases murine small intestinal HCO3− secretion. Int J Radiat Biol 87: 878–888, 2011 [DOI] [PubMed] [Google Scholar]