Abstract
The pancreas, liver, and gallbladder are commonly involved in cystic fibrosis (CF), and acidic, dehydrated, and protein-rich secretions are characteristic findings. Pancreatic function studies in humans have been done by sampling the jejunal fluid. However, it has been difficult to separately study the function of pancreatic and biliary systems in humans with CF, because jejunal fluid contains a mixture of bile and pancreatic fluids. In contrast, pancreatic and biliary ducts open separately into the porcine intestine; therefore, biliary and pancreatic fluid can be individually analyzed in CF pigs. We studied newborn wild-type (WT) and CF pigs and found that CFTR was localized to the pancreatic ducts. We collected bile and pancreatic fluid and analyzed pancreatic enzymes with activity assays and immunoblot. Pancreatic enzyme expression was significantly decreased in CF compared with WT pigs. The volume and pH of pancreatic fluid were significantly lower and protein concentration was >5-fold higher in CF pigs. Secretin stimulation increased pancreatic fluid volume and pH in WT, but not CF, pigs. Baseline bile volume did not differ between WT and CF pigs, but volume did not increase in response to secretin in CF pigs. Bile pH was lower and protein concentration was twofold higher in CF pigs. These results indicate that pancreatic and biliary secretions are altered in CF pigs. Abnormal pancreatic and biliary secretion in CF may have important implications in disease pathogenesis.
Keywords: pancreas, bile, liver, porcine, gallbladder
cystic fibrosis (CF) is caused by mutations in the gene that encodes the CFTR (63). CFTR is expressed in many epithelial cells (sweat duct, airway, pancreatic duct, intestine, biliary tree, and vas deferens) and functions as an apical membrane anion channel, primarily involved in anion secretion (9, 70, 86). The pancreas is universally involved, and prior to administration of pancreatic enzymes, it caused malnutrition (5, 9, 64). Today, lung disease is the major cause of mortality in CF (23). However, pancreatic disease continues to impact nutrition, growth, and the progression of lung disease (18, 39, 49, 52).
Pancreatic disease starts in utero in CF; significant pancreatic involvement can be found in young infants (5, 64, 69), and lesions can even be observed in aborted fetuses (10, 65). Pancreatic lesions in CF vary considerably in severity but, in general, progress with increasing age (5, 64, 69) until complete loss of organ function in ∼85% of patients with CF (12, 19, 24, 40, 84). Pancreatic disease in CF is characterized by acidic, dehydrated, and protein-rich secretions (27, 37, 41, 75).
Liver involvement is also common in CF. Most patients with CF develop some evidence of liver abnormalities, including slightly elevated liver enzymes, fatty infiltration, and focal biliary cirrhosis (31, 86). Approximately 2–5% of patients develop advanced liver disease and liver failure (17). The gallbladder is also involved, and microgallbladder occurs in 20–30% of patients with CF (74, 85). Less than 10% of patients develop cholelithiasis/cholecystitis (80, 87).
It has been difficult to separately study the function of the liver and pancreas in humans with CF. Pancreatic function studies in humans have been done by sampling the fluid in the jejunum (50, 51). Because the pancreatic duct converges with the common bile duct (CBD) shortly before opening at the duodenum, the jejunal fluid invariably contains a mixture of bile and pancreatic fluids. Furthermore, pancreatic duct and cholangiocyte epithelia secrete HCO3−-rich fluid in response to secretin (43, 54, 55, 83); therefore, it is not possible to evaluate the relative contributions of biliary and pancreatic systems, unless the fluids are collected separately. Early pancreatic studies in humans with CF have shown that the jejunal fluid is low in volume, concentrated, and acidic in patients with CF (26, 34, 46, 51). As in humans with CF, the jejunal fluid is dehydrated and acidic in CF mice (36). Because pancreaticobiliary anatomy is similar in humans and mice, the contribution of biliary and pancreatic fluids has not been individually analyzed. Moreover, pancreatic disease is milder in mice than in humans (6, 28, 35, 36). Investigating pancreatic secretions in CF is important, because CFTR is proposed to facilitate HCO3− transport in pancreatic ducts (16, 41, 50, 77), and its loss in CF is thought to play a key role in the pathogenesis of pancreatic disease (75). Similarly, knowledge of hepatic secretions in CF might provide insight into CF liver disease.
Hepatic and pancreatic histopathology in CF pigs is very similar to that in humans with CF (61, 66, 72, 81). However, pigs have separate CBD and pancreatic duct openings into the intestine (42). Thus the porcine model offers a unique opportunity to examine pancreatic fluid and bile separately. The goal of this work was to study enzyme and CFTR expression in the pancreas, sample the pancreatic fluid and bile separately, and examine whether biliary and pancreatic secretory function were affected in CF. We found that the exocrine pancreatic function was abnormal in CF pigs (decreased pancreatic enzymes, acidic, dehydrated pancreatic secretions with a high protein concentration, and lack of response to secretin). Bile composition was also affected in CF pigs (normal baseline fluid secretion, relatively lower pH and higher protein concentration, and lack of response to secretin). Our results suggest that abnormal pancreatic and biliary secretion in CF may have important implications in disease pathogenesis.
METHODS
Animals.
All studies were approved by the University of Iowa Animal Care and Use Committee. Four wild-type (WT, CFTR+/+, 2 males and 2 females), 3 CFTR−/− (1 male and 2 females), and 2 CFTRΔF508/ΔF508 (1 male and 1 female) piglets were obtained from Exemplar Genetics (Sioux Center, IA) for pancreatic fluid and bile collection within 24 h after birth. At the end of the study, animals were euthanized with pentobarbital sodium-phenytoin sodium (Euthasol, Virbac, Fort Worth, TX), and the pancreas was collected. For gallbladder pH studies, separate animals were used: 8 WT (CFTR+/+, 5 males and 3 females) and 12 CF (CFTR−/−, 6 males and 6 females) piglets were studied at the time of euthanasia.
Tissues and pathology examination.
After euthanasia, pancreata were collected and immersed in fixative for 48–96 h, routinely processed, embedded, sectioned (4 μm), and stained with hematoxylin and eosin.
Immunofluorescence.
Pancreatic tissues were excised from newborn piglets, immediately placed in ice-cold 30% sucrose, and quick-frozen in optimal cutting temperature compound with liquid N2. Tissue segments were kept at −80°C. Tissues were cut into 7-μm sections, fixed in 100% methanol at −20°C for 10 min, permeabilized in 0.2% Triton X-100 (Thermo Scientific) in PBS, and blocked in SuperBlock (Thermo Scientific) with 10% normal goat serum (Jackson ImmunoResearch). Tissue sections were incubated for 2 h at 37°C in anti-CFTR-596 (21, 56) (Chemicon) and polyclonal antibody to β-catenin (Zymed), all diluted at 1:100, and then in secondary antibodies (goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 568; Molecular Probes/Invitrogen), diluted 1:1,000. Sections were mounted with VECTASHIELD HardSet containing 4′,6-diamidino-2-phenylindole (Vector Laboratories) to visualize nuclei. Images were acquired with identical parameters on an Olympus Fluoview FV1000 confocal microscope with a UPLSAPO ×60 oil lens. Images were scanned sequentially at 2 ms/pixel. Postcollection enhancements were processed using an Olympus Fluoview FV10-ASW2.0 viewer.
Collection of pancreatic and biliary fluid with the blind-loop technique.
Pancreaticobiliary anatomy in pigs is slightly different from that in humans. In humans, the pancreatic duct converges with the CBD shortly before it opens at the duodenum. In pigs, the CBD is located distal to the pylorus, and the pancreatic duct opens to the duodenum distal to the CBD (Fig. 1) (42). We collected the pancreatic fluid using a blind-loop technique similar to the method described by Freedman et al. (36) and Meyerholz et al. (60). After sedation with ketamine (10–20 mg/kg im) and acepromazine (0.1–2.2 mg/kg im), piglets were anesthetized with inhaled halothane, endotracheally intubated, and ventilated. Heart rate, pulse oximetry, and level of anesthesia were monitored continuously. After the animals were fully anesthetized, a laparotomy was done, and three sutures were placed to create the intestinal blind loops. A biliary blind loop was created by placing the first suture at the distal end of the pylorus and the second suture 1 cm caudally to the first suture. A third suture was placed 1 cm distal to the pancreatic duct, and the intestinal loop was created between the second and third suture as the pancreatic blind loop. The blind loops were entered with 22-gauge Angiocaths that were secured in place with tissue adhesive. After a basal collection for 30 min, secretin (0.2 μg/kg iv; ChiRhoClin, Burtonsville, MD; generously donated by Dr. Edward D. Purich) was administered, and bile and pancreatic fluid were collected for 30 min. Animals were euthanized upon completion of the procedure, as described above. Volume was measured with micropipettes, pH was measured with pH strips, and protein concentrations were measured in 2 μl of fluid using a spectrophotometer (NanoDrop 1000, Thermo Scientific). Because CF pancreatic secretions and bile were not homogenous and contained pieces of mucoid material, we could not measure protein concentration in each sample; therefore, our data may underestimate the effect of CF on protein concentration. The pH of bile and pancreatic secretions was measured using colorpHast pH test strips (catalog no. 9590, EMD Chemicals, Gibbstown, NJ). The pH of gallbladder bile was measured using a needle-type fiber-optic pH meter (catalog no. 502123, World Precision Instruments, Sarasota, FL).
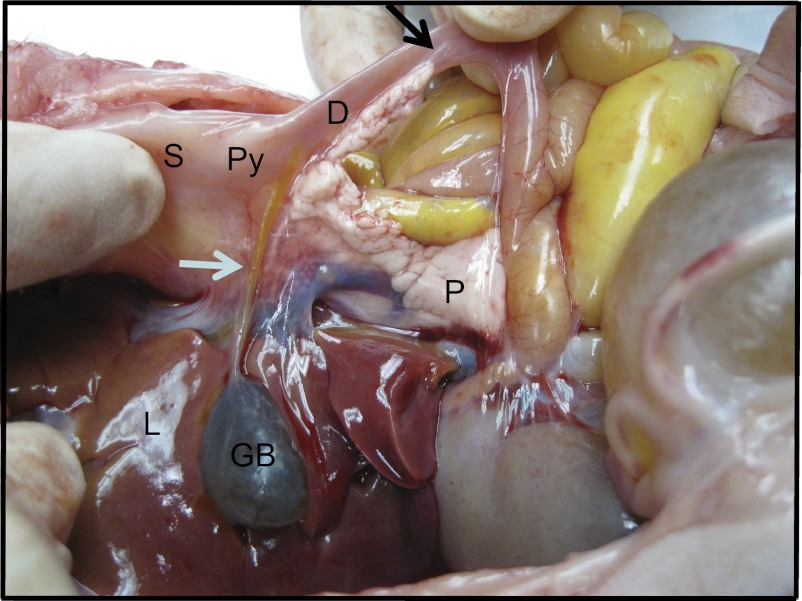
Fig. 1.
Pancreatic and biliary duct anatomy in pigs: biliary and pancreatic duct openings to the duodenum in a newborn pig. S, stomach; L, liver; GB, gallbladder; P, pancreas; Py, pylorus; D, duodenum. White arrow points to common bile duct; black arrow points to pancreatic duct.
Pancreatic enzyme expressions.
Pancreas tissue samples were homogenized in phosphate-buffered saline (20 mM Na2HPO4 and 140 mM NaCl, pH 7.4) containing 0.1% BSA and protease inhibitors. Homogenates were centrifuged at 13,000 g at 4°C for 15 min, and the supernatant was collected. Protein concentrations were estimated with a bicinchoninic acid (BCA) assay.
Amylase activity.
Pancreatic amylase activity (IU/μg total protein) was determined using blue starch as a substrate (Phadebas Amylase Kit, Magle Life Sciences, Cambridge, MA) (29).
Lipase activity.
Lipase activity in the pancreas homogenates was measured by the Clinical Biochemistry Laboratory at the University of Iowa using a commercially available chromogenic substrate (Roche).
Trypsin activity.
Pancreatic trypsin activity was determined using N-α-benzoyl-l-Arg-p-nitroanilide as the substrate in the presence of enterokinase (Sigma-Aldrich, St. Louis, MO) (53). Absorbance at 405 nm was measured every minute for 10 min at room temperature. Enzyme activity was expressed as units and defined as the amount of enzyme that hydrolyzed 1 μmol of substrate per minute.
Immunoblot.
Snap-frozen tissues from the pancreas were homogenized in lysis buffer (0.15 M NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM Tris·Cl, and 100 μM PMSF), and protein was quantified with a Micro BCA protein assay reagent kit (Pierce Biotechnology, Rockford, IL). Proteins were separated by SDS-PAGE using 12% acrylamide resolving gels. After electrophoretic transfer to a nitrocellulose membrane, blots were blocked in TTBS buffer (10 mM Tris, pH 7.4, 130 mM NaCl, 0.8 mM disodium EDTA, and 1% Tween 20) with 5% nonfat dry milk for ≥1 h and subsequently incubated for 1 h with pancreatic elastase antibody (1:2,000 dilution; Assay Designs, Ann Arbor, MI) or chymotrypsinogen antibody (diluted 1:2,000 in TTBS buffer). The protein of interest was detected using goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (1:10,000 dilution; Upstate Biotechnology, Lake Placid, NY). Blots were washed several times with TTBS buffer. Antibody-labeled bands were visualized by incubation of the blots for 1 min with ECL chemiluminescent substrate (Amersham, Arlington Heights, IL) and exposure of Kodak XAR film for 1–5 min.
Statistics.
Values are means ± SE. Differences between groups were analyzed using two-way ANOVA with Bonferroni's posttest analysis; P < 0.05 was considered significant. Results from 3 CFTR−/− and 2 CFTRΔF508/ΔF508 pigs were similar, and they were grouped together as CF pigs.
RESULTS
Loss of CFTR function reduces pancreatic enzyme levels in pigs.
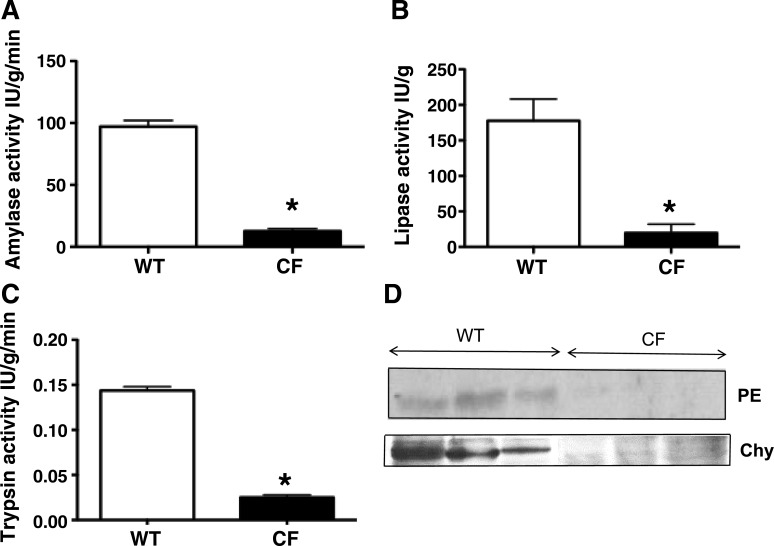
Our previous studies suggested that the CF pigs have pancreatic disease at birth, with reduced number of acini, decreased cytoplasmic zymogen granules, and ectatic and plugged ducts surrounded by degenerative exocrine tissue (2, 61, 66, 72, 81). To further test for pancreatic disease in CF pigs, we assayed enzyme levels in the pancreas. Activities of pancreatic amylase, lipase, and trypsin were significantly lower (Fig. 2, A–C) and levels of pancreatic elastase and chymotrypsinogen were reduced (Fig. 2D) in newborn CF pigs. These data are consistent with our previous observations and the degree of pancreatic damage and reduced numbers of acini and zymogen granules in CF pigs (2, 61, 66, 72, 81).
Fig. 2.
Pancreatic enzyme expression in wild-type (WT) pigs and pigs with cystic fibrosis (CF). Pancreatic amylase (A), lipase (B), and trypsin (C) activities (n = 4 each) and pancreatic elastase (PE) and chymotrypsinogen (Chy) expression (D) (n = 3 each) were severely diminished in CF compared with WT pigs. *P < 0.01 vs. WT.
CFTR is localized to the pancreatic ducts in newborn pigs.
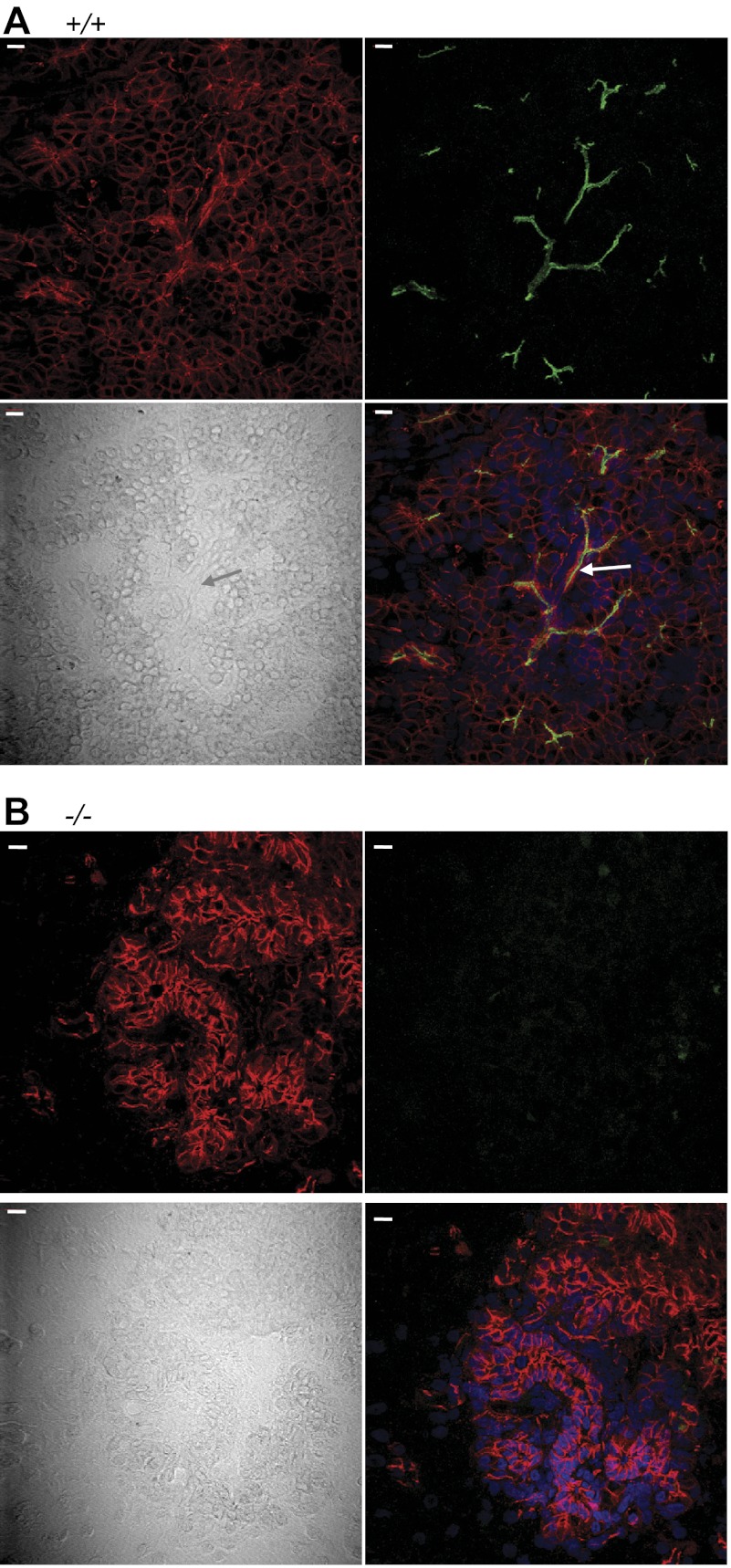
Although the CF pancreas has significant pancreatic acinar cell damage, the secretory defects are secondary to decreased CFTR function in the pancreatic ducts and ductules. Studies of human samples have shown that CFTR is expressed at high levels in the pancreas and mainly localizes to the pancreatic duct epithelia (13, 57, 82). To determine whether CFTR was also localized to the pancreatic ducts in pigs, we examined its localization with immunostaining and confocal microscopy. We used the adherens junction protein β-catenin to outline pancreatic ducts and differential interference contrast microscopy to delineate tissue architecture. We detected CFTR immunostaining in ducts and ductules from WT piglets but no CFTR immunostaining in pancreas from CFTR−/− piglets (Fig. 3). Thus, CFTR immunolocalization is similar to that reported for human pancreatic epithelia.
Fig. 3.
Immunocytochemical localization of CFTR in pancreas from newborn pigs shown as stacks of confocal and differential interference contrast microscopic images. Scale bars, 10 μm. A: WT pancreas. CFTR is green, β-catenin is red, and nuclei are blue (4′,6-diamidino-2-phenylindole). B: CFTR−/− pancreas. Arrows point to intercalated ducts.
Pancreatic secretion is defective in newborn CF pigs.
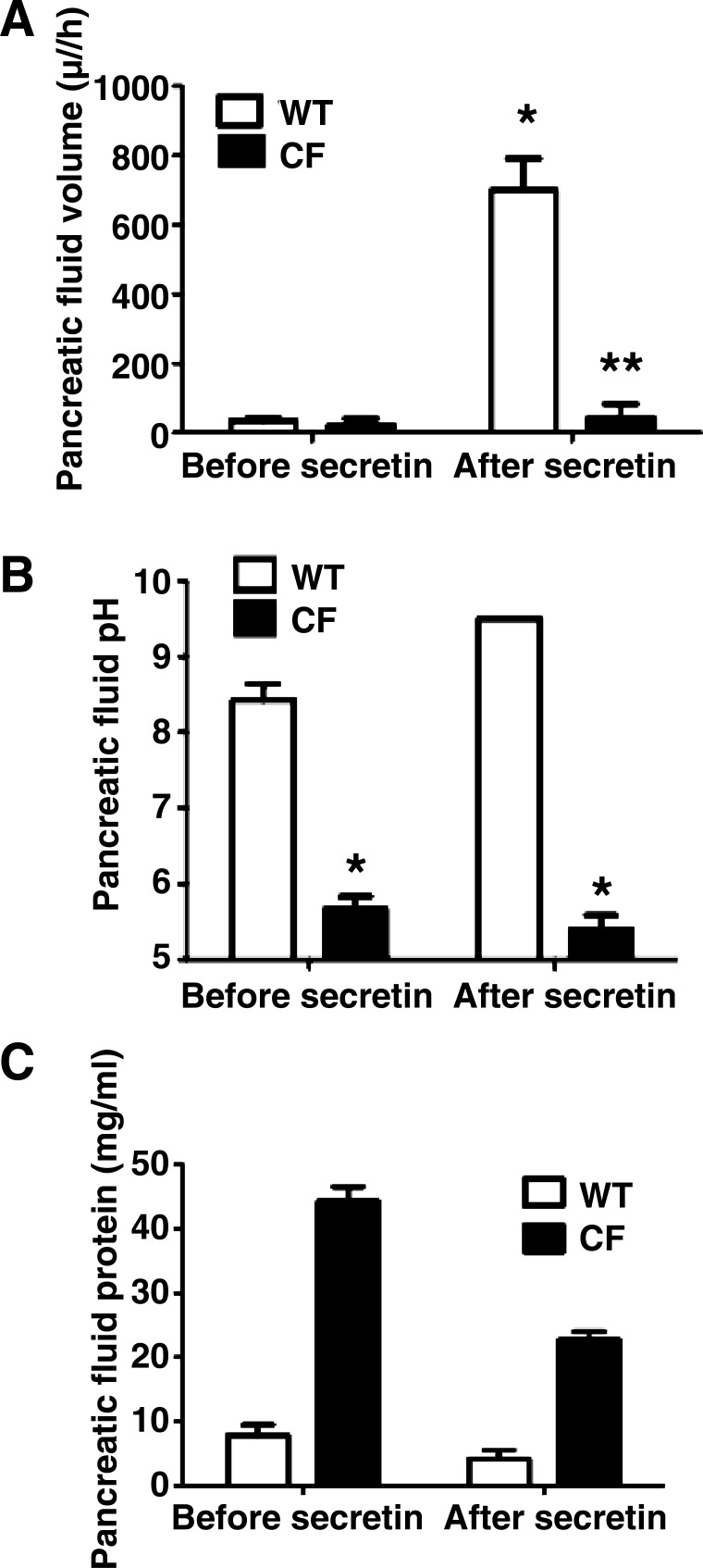
Because CFTR is localized to the pancreatic duct epithelia, it is expected that the function of pancreatic ducts would be most affected in CF. To determine whether pancreatic secretion in CF pigs was significantly different from that in WT pigs, we created intestinal blind loops to collect pancreatic and biliary fluids. Pancreatic fluid volume was 35 ± 9.5 and 22 ± 18 μl/h in WT and CF pigs, respectively, at baseline (P = 0.6). After stimulation with secretin, pancreatic fluid volume increased to 700 ± 91 μl/h in WT pigs (P < 0.01, before vs. after secretin), but it did not significantly increase in CF pigs (44 ± 36 μl/h, P = 0.3, before vs. after secretin; Fig. 4A).
Fig. 4.
Pancreatic fluid composition in WT and CF pigs. A: pancreatic fluid volume increased after secretin in WT, but not CF, pigs (n = 4 WT and 5 CF). *P < 0.01. After secretin, pancreatic fluid volume was lower in CF than WT pigs. **P < 0.01. B: pancreatic fluid pH was lower in CF than WT pigs at baseline and after secretin (n = 4 WT and 5 CF). *P < 0.01. C: protein concentration was >5 fold higher in pancreatic secretion from CF than WT pigs (n = 3 WT and 2 CF).
Pancreatic fluid pH was 8.4 ± 0.1 and 5.7 ± 0.1 in WT and CF pigs, respectively, at baseline (P < 0.001). After secretin stimulation, pH of WT pancreatic fluid increased to 9.5 ± 0 (P < 0.01, before vs. after secretin) but was unchanged in CF pigs (5.4 ± 0.1, P = 0.5, before vs. after secretin; Fig. 4B). We did not observe a higher secretion of pancreatic fluid volume or less dramatic pH changes in CFTRΔF508/ΔF508 pigs.
A sufficient quantity of pancreatic fluid was obtained from three WT and two CF pigs to measure the protein concentration. Protein concentration in pancreatic fluid was 7.9 and 4.2 mg/ml before and after secretin, respectively, in WT pigs and 44.2 and 22.7 mg/ml, respectively, in CF pigs (Fig. 4C). These results indicate defective pancreatic secretion in CF and are consistent with the reduced volume of secretions.
Biliary secretion in newborn CF pigs.
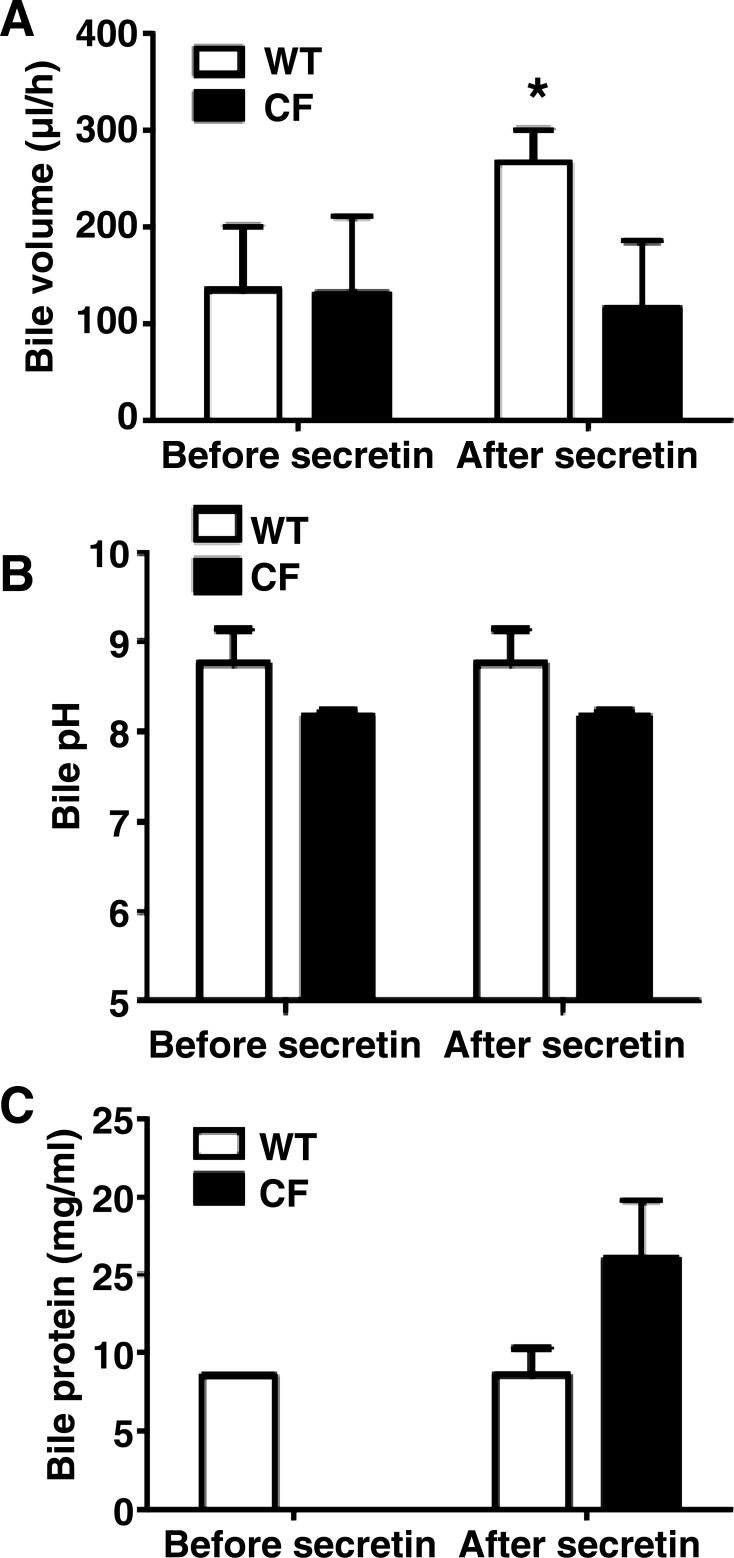
To assess biliary secretions, we took advantage of the separate openings of biliary and pancreatic systems in the pig to collect the bile separate from the pancreatic fluid. The baseline bile volume and pH were not significantly different between WT and CF pigs. At baseline, bile volume was 135 ± 65 and 131 ± 73 μl/h in WT and CF pigs, respectively (P = 0.82). After secretin, bile volume doubled in WT pigs to 267 ± 33 μl/h (P = 0.05, before vs. after secretin), but it was unchanged in CF pigs (118 ± 62 μl/h, P = 0.49, before vs. after secretin; Fig. 5A).
Fig. 5.
Bile composition in WT and CF pigs. A: at baseline, bile volume was not significantly different between WT and CF pigs. Bile volume increased after secretin in WT, but not CF, pigs (n = 4 WT and 5 CF). *P < 0.05. B: bile pH did not differ significantly by genotype before and after secretin (n = 4 WT and 5 CF). C: volume of bile in CF pigs was not sufficient to measure amount of protein at baseline. Protein concentration after secretin was 2-fold higher in CF than WT pig bile (n = 3 WT and 2 CF).
Although the differences were not as dramatic as in pancreatic fluid, bile pH was also lower in CF than WT pigs at baseline, but the difference did not reach statistical significance (8.7 ± 0.1 and 8.2 ± 0.05 in WT and CF, respectively, P = 0.18). After secretin, bile pH was unchanged in both groups: 8.7 ± 0.1 and 8.2 ± 0.05 in WT and CF pigs, respectively (Fig. 5B). We did not observe a higher secretion of biliary fluid volume or less dramatic pH changes in CFTRΔF508/ΔF508 pigs.
A sufficient quantity of bile was obtained from three WT and two CF pigs to measure protein concentration; CF samples were collected only after secretin stimulation. Bile protein concentration was, on average, 8.6 and 16.4 mg/ml in WT and CF pigs, respectively, after secretin (Fig. 5C).
These data indicate that the CF biliary system had impaired fluid secretion into the intestine with secretin stimulation. In addition, there was a tendency for a lower pH and increased protein concentration in the bile. These data show similarity to the data obtained with pancreatic secretions.
Contents of gallbladder are more acidic in CF pigs.
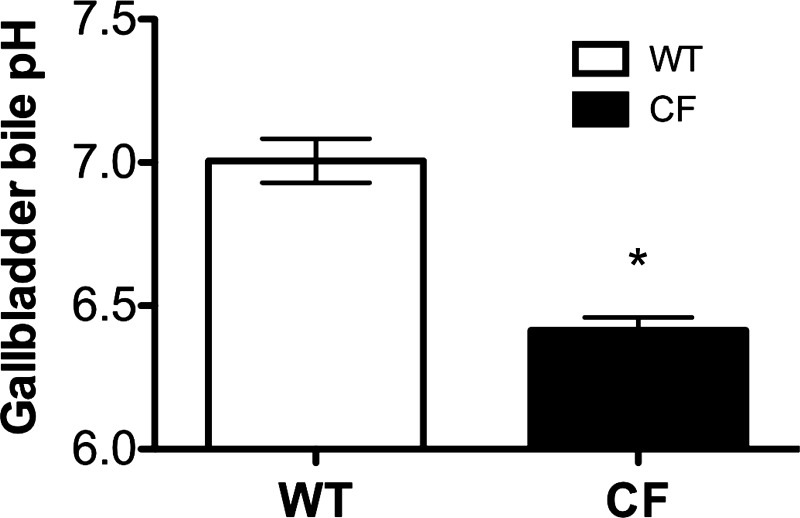
CFTR is expressed on the apical membrane of gallbladder epithelium and plays a significant role in fluid, Cl−, and HCO3− secretion (22, 25, 67). The gallbladder is affected in CF pigs, with an almost universal presence of microgallbladder (61, 66, 72). To determine whether the pH of gallbladder luminal contents was altered in CF pigs, we measured bile pH from the gallbladder of WT and CF pigs. Sampling was done at baseline only. WT pig gallbladder contents flowed easily, whereas they were very thick and tenacious in the CF pigs. The pH of the bile in the gallbladder was lower in newborn CF than WT pigs (6.42 ± 0.04 vs. 7.01 ± 0.08, P < 0.0001; Fig. 6). The pH was also lower in gallbladder bile than in bile collected from the intestinal blind loops, as expected on the basis of earlier studies (76).
Fig. 6.
Gallbladder bile pH. Gallbladder bile pH in newborn pigs at the time of euthanasia was measured using a needle-type fiber-optic pH meter. Gallbladder bile pH was lower in CF than WT pigs (n = 8 WT and 12 CF). *P < 0.0001 vs. WT.
DISCUSSION
In this study, we took advantage of the anatomically separate biliary and pancreatic drainage systems to assess differences between CF and non-CF secretions from the two organs. We found that secretions from both systems were altered in CF.
It is interesting that, under basal conditions, the protein concentration of pancreatic secretions was nearly fivefold greater in CF than WT pigs. This can be partially, but not completely, explained by a secretory rate that was reduced by ∼40% in CF. We do not know the reason for the rest of the difference in protein concentration. Given the destruction of the CF pancreatic acini and the resulting decreased pancreatic enzyme content of the pancreatic tissue, it is not likely due to increased enzyme secretion. However, it might have been due to intermittent fluid secretion by WT, but not CF pigs, that was missed in our assay and would have magnified a difference in secretory volume. Another possibility is the genotype-dependent difference in liquid absorption. Identifying the proteins in CF pancreatic fluid and further studying the pancreatic duct function might provide an explanation for, and an insight into, the pathogenesis of obstructive ductal plugs.
As suggested earlier in humans (41, 45, 50, 51) and mice (36) with CF, the volume of CF pig pancreatic secretions was reduced, and the fluid had decreased pH and increased protein concentration. The studies in humans used secretin (which induces volume- and HCO3−-rich secretions) and CCK (which induces pancreatic enzyme-rich secretions). We used only secretin in our studies, because pancreatic secretion does not increase in response to CCK in anesthetized pigs (20) and CCK induces pancreatic protein and trypsin, not volume secretion, only when directly delivered to the gastroduodenal circulation (gastric and right gastroepiploic arteries) in pigs (30). These arteries are very small and difficult to cannulate in the newborn pigs. Because loss of acinar mass does not lead to acidic and dehydrated pancreatic secretions [i.e., Shwachman-Diamond syndrome (47, 50)], these secretory defects in CF pigs are most likely due to the loss of CFTR function in the pancreatic ducts.
Bile volume and pH have not been individually analyzed in CF previously. Although there are differences between the mechanisms of fluid and electrolyte transport in biliary and pancreatic ducts, they do have some similarities, including stimulation by secretin and involvement of CFTR and Cl−/HCO3− exchange (11, 15, 33, 43, 54, 71, 79). In addition, constituents of bile, such as bile acids and ATP, also stimulate cholangiocyte secretion (4, 33, 58, 73). It has been proposed that a CFTR- and cAMP-independent Cl− secretory pathway in the biliary tract may account for the relatively low incidence of liver disease in patients with CF (32, 33). Studying the fluid and anion secretion in CF pig cholangiocytes and pancreatic duct epithelial cells will be an important approach to further understand the physiological differences between the liver and the pancreas.
The pH of gallbladder bile was significantly lower in CF pigs, similar to an observation in CF mice (38). Because CFTR is expressed on the apical membrane of gallbladder epithelium and plays a significant role in HCO3− secretion (22), a lower pH is expected in CF than WT pig gallbladder luminal contents. However, we do not know whether these pH changes are due to reduced HCO3− secretion from a defective CFTR vs. an increased H+ secretion from CF gallbladder epithelium. Nevertheless, our results are consistent with the evidence that CFTR is involved in HCO3− secretion in the pancreas (48) and other organs (14, 59, 78). We also found a trend for reduced bile pH from the CBD of CF pigs, although the difference was not statistically significant. This difference may be due to the lower number of pigs that underwent the blind-loop studies relative to the number of samples from gallbladders.
It is possible that the acidic pH during storage in the gallbladder may change physical characteristics of the bile, alter the epithelium to a mucus-producing phenotype, or produce other changes. Identification of pH differences between CF and non-CF gallbladder will allow studies of pathogenesis. Importantly, these findings may also be applicable to other gallbladder diseases.
Our study had advantages and limitations. A major advantage was the ability to separately obtain pancreatic and biliary secretions. Previous studies of human samples collected from the jejunum included a mixture of bile and pancreatic fluid (26, 34, 46, 51). Moreover, studies in CF mice have not separated pancreatic and biliary secretions (36). Another advantage is that CF pigs develop pancreatic and liver disease remarkably similar to that of humans with CF (2, 61, 66, 72, 81). We also were able to study newborn pigs, which minimizes the possibility that nutritional differences following birth might have altered hepatic or pancreatic function or disease state. Limitations of our study include the possibility that differences between pancreatic fluid and bile from CF pigs might be secondary to disease in these organs, because the newborn porcine CF liver shows only mild involvement (61, 66, 72). As in studies in humans, we also cannot exclude the possibility that the intestine might have altered the samples collected from the blind loops. However, the marked differences in fluid collected from adjacent pancreatic and biliary segments suggest that modifications by the intestine do not likely account for all the differences. Another consideration is that secretions were collected during laparotomy, which required general anesthesia. Anesthesia with pentobarbital sodium and chloralose-urethane reduces exocrine pancreatic responses to secretin and CCK in dogs (7), but it is not known whether the propofol or isoflurane we used has similar effects. Nevertheless, because anesthesia was the same for all the pigs, these anesthetics are not likely the cause of differences between CF and non-CF.
The difference in disease severity between the porcine CF pancreas and liver is striking. Results from our separate collections of their secretions may allow some speculation as to the pathogenic factor. Despite the large discrepancy in the degree of organ histopathology in CF pancreas and liver, secretin failed to increase secretion from either CF organ, and the protein concentration was increased in CF fluid from both organs. These results suggest that impaired fluid secretion may not be the key factor accounting for the different severity of liver and pancreas disease. The greatest difference between the pancreas and the liver was in the effect of CF on the pH of their secretions. With secretin stimulation, the pH of pancreatic secretions was 9.5 in non-CF and 5.4 in CF, an H+ concentration >10,000 times higher in CF. In contrast, bile pH was 8.7 in WT pigs and 8.2 in CF pigs, an approximately threefold difference. Specialization of the pancreatic duct for HCO3− secretion allows it to generate those very high pH values, and CFTR is critical for that HCO3− secretion. Thus we speculate that the marked difference in pH caused by loss of CFTR is responsible for the greater destruction in the CF pancreas than liver. The finding that an acute extracellular acid load sensitizes acinar cells to injury, causes pancreatitis, or worsens disease in animals (8, 62) would be consistent with our speculation. A reduced luminal pH and/or HCO3− concentration as the cause of pancreatic disease would also be congruent with data suggesting that such abnormalities initiate CF lung and intestinal disease (44, 68).
Beyond the conclusion that the CF pig mimics many aspects of the human disease, it may also be helpful in studying the mechanisms of chronic pancreatitis. Despite advances in understanding the pathogenesis of chronic pancreatitis, there are no well-established treatments to prevent the progression or treat complications of the disease (3). With features typical of chronic pancreatitis (chronic inflammation, acinar atrophy, and fibrosis) (1), the porcine CF model may be instrumental to further study this disease and possibly develop treatment options that could be applicable to humans.
In summary, we have shown that exocrine pancreatic secretion and bile were affected in CF vs. WT pigs. It is possible that the abnormal pancreatic and biliary secretion in CF may have important implications in disease pathogenesis. Additional studies in fetal pigs are required to elucidate the pathogenesis of the pancreatic and biliary disease in CF. The CF pig model may also be helpful in unraveling the role of ductal cell defects in acinar cell physiology and studying the mechanisms of chronic pancreatitis.
GRANTS
This work was supported by National institutes of Health Grants DK-084049 (A. Uc), HL-091842 (M. J. Welsh), and HL-51670 (M. J. Welsh) and Peregrine Charities (A. Uc).
DISCLOSURES
M. Welsh holds equity in Exemplar Genetics, which is licensing materials and technology related to this work.
AUTHOR CONTRIBUTIONS
A.U., R.G., D.K.M., M.A.E.H., P.J.T., and M.J.W. are responsible for conception and design of the research; A.U., R.G., D.K.M., M.G., L.S.O., X.T., M.A.E.H., D.A.S., P.L., A.A.P., and P.J.T. performed the experiments; A.U., D.K.M., M.G., L.S.O., X.T., and M.A.E.H. analyzed the data; A.U., R.G., D.K.M., M.G., L.S.O., X.T., M.A.E.H., and M.J.W. interpreted the results of the experiments; A.U., R.G., D.K.M., M.G., L.S.O., X.T., and M.A.E.H. prepared the figures; A.U. and D.K.M. drafted the manuscript; A.U., D.K.M., M.G., L.S.O., X.T., A.A.P., M.A.E.H., P.J.T., and M.J.W. edited and revised the manuscript; A.U., R.G., D.K.M., M.G., L.S.O., X.T., M.A.E.H., D.A.S., P.L., A.A.P., M.A.E.H., P.J.T., and M.J.W. approved the final version of the manuscript.
REFERENCES
- 1. Abu-El-Haija M, Ramachandran S, Meyerholz DK, Griffin M, Giriyappa RL, Stoltz DA, Welsh MJ, McCray PB, Jr, Uc A. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol 181: 499–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB, Jr, Butler J, Uc A. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology 11: 506–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aghdassi AA, Mayerle J, Christochowitz S, Weiss FU, Sendler M, Lerch MM. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair 4: 26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alpini G, Glaser S, Robertson W, Phinizy JL, Rodgers RE, Caligiuri A, LeSage G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol Gastrointest Liver Physiol 273: G518–G529, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: clinical and pathological study. Am J Dis Child 56: 344–399, 1938 [Google Scholar]
- 6. Beharry S, Ackerley C, Corey M, Kent G, Heng YM, Christensen H, Luk C, Yantiss RK, Nasser IA, Zaman M, Freedman SD, Durie PR. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 292: G839–G848, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Ben-Ari G, Rudick J, Kark AE, Dreiling DA. Effects of anesthesia on pancreatic exocrine secretion. Ann Surg 170: 747–752, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, Thrower EC, Gorelick FS. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology 137: 1083–1092, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, De Lisle RC, Lewindon P, Lichtman SM, Sinaasappel M, Baker RD, Baker SS, Verkade HJ, Lowe ME, Stallings VA, Janghorbani M, Butler R, Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr 41: 273–285, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Boue A, Muller F, Nezelof C, Oury JF, Duchatel F, Dumez Y, Aubry MC, Boue J. Prenatal diagnosis in 200 pregnancies with a 1-in-4 risk of cystic fibrosis. Hum Genet 74: 288–297, 1986 [DOI] [PubMed] [Google Scholar]
- 11. Boyer JL. Bile duct epithelium: frontiers in transport physiology. Am J Physiol Gastrointest Liver Physiol 270: G1–G5, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Bronstein MN, Sokol RJ, Abman SH, Chatfield BA, Hammond KB, Hambidge KM, Stall CD, Accurso FJ. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr 120: 533–540, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut 52: 1008–1016, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Hoglund P, Chan HC, Shi QX. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol Reprod 80: 115–123, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Choi JY, Lee MG, Ko S, Muallem S. Cl−-dependent HCO3− transport by cystic fibrosis transmembrane conductance regulator. JOP 2: 243–246, 2001 [PubMed] [Google Scholar]
- 16. Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3− transport in mutations associated with cystic fibrosis. Nature 410: 94–97, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med 13: 529–536, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr 131: 809–814, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Couper RT, Corey M, Moore DJ, Fisher LJ, Forstner GG, Durie PR. Decline of exocrine pancreatic function in cystic fibrosis patients with pancreatic sufficiency. Pediatr Res 32: 179–182, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Cuber JC, Corring T, Levenez F, Bernard C, Chayvialle JA. Effects of cholecystokinin octapeptide on the pancreatic exocrine secretion in the pig. Can J Physiol Pharmacol 67: 1391–1397, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Cui L, Aleksandrov L, Chang XB, Hou YX, He L, Hegedus T, Gentzsch M, Aleksandrov A, Balch WE, Riordan JR. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol 365: 981–994, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Curtis CM, Martin LC, Higgins CF, Colledge WH, Hickman ME, Evans MJ, MacVinish LJ, Cuthbert AW. Restoration by intratracheal gene transfer of bicarbonate secretion in cystic fibrosis mouse gallbladder. Am J Physiol Gastrointest Liver Physiol 274: G1053–G1060, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J 29: 522–526, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Dooley RR, Guilmette F, Leubner H, Patterson PR, Shwachman H, Weil C. Cystic fibrosis of the pancreas with varying degrees of pancreatic insufficiency. Am J Dis Child 92: 347–368, 1956 [DOI] [PubMed] [Google Scholar]
- 25. Dray-Charier N, Paul A, Scoazec JY, Veissiere D, Mergey M, Capeau J, Soubrane O, Housset C. Expression of ΔF508 cystic fibrosis transmembrane conductance regulator protein and related chloride transport properties in the gallbladder epithelium from cystic fibrosis patients. Hepatology 29: 1624–1634, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Durie PR. Pancreatic aspects of cystic fibrosis and other inherited causes of pancreatic dysfunction. Med Clin North Am 84: 609–620, ix, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Durie PR, Forstner GG. Pathophysiology of the exocrine pancreas in cystic fibrosis. J R Soc Med 82 Suppl 16: 2–10, 1989 [PMC free article] [PubMed] [Google Scholar]
- 28. Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol 164: 1481–1493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evilevitch L, Pierzynowski SG, Linderoth A, Ahren B, Erlanson-Albertsson C, Podgurniak M, Westrom BR. Three-day enteral exposure to a red kidney bean lectin preparation enhances the pancreatic response to CCK stimulation in suckling pigs. Biol Neonate 87: 20–25, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Evilevitch L, Westrom BR, Pierzynowski SG. CCK regulates pancreatic enzyme secretion via short duodenal-pancreatic reflexes in pigs. Scand J Gastroenterol 38: 201–206, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Feranchak AP. Hepatobiliary complications of cystic fibrosis. Curr Gastroenterol Rep 6: 231–239, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Feranchak AP, Sokol RJ. Cholangiocyte biology and cystic fibrosis liver disease. Semin Liver Dis 21: 471–488, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Fitz JG. Regulation of cholangiocyte secretion. Semin Liver Dis 22: 241–249, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Freedman SD, Blanco P, Shea JC, Alvarez JG. Mechanisms to explain pancreatic dysfunction in cystic fibrosis. Med Clin North Am 84: 657–664, x, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr−/− mice. Proc Natl Acad Sci USA 96: 13995–14000, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freedman SD, Kern HF, Scheele GA. Pancreatic acinar cell dysfunction in CFTR−/− mice is associated with impairments in luminal pH and endocytosis. Gastroenterology 121: 950–957, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Freedman SD, Scheele GA. Acid-base interactions during exocrine pancreatic secretion. Primary role for ductal bicarbonate in acinar lumen function. Ann NY Acad Sci 713: 199–206, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Freudenberg F, Leonard MR, Liu SA, Glickman JN, Carey MC. Pathophysiological preconditions promoting mixed “black” pigment plus cholesterol gallstones in a ΔF508 mouse model of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 299: G205–G214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaskin K, Gurwitz D, Durie P, Corey M, Levison H, Forstner G. Improved respiratory prognosis in patients with cystic fibrosis with normal fat absorption. J Pediatr 100: 857–862, 1982 [DOI] [PubMed] [Google Scholar]
- 40. Gaskin K, Waters D, Dorney S, Gruca M, O'Halloran M, Wilcken B. Assessment of pancreatic function in screened infants with cystic fibrosis. Pediatr Pulmonol Suppl 7: 69–71, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Gaskin KJ, Durie PR, Corey M, Wei P, Forstner GG. Evidence for a primary defect of pancreatic HCO3− secretion in cystic fibrosis. Pediatr Res 16: 554–557, 1982 [DOI] [PubMed] [Google Scholar]
- 42. Gholson CF, Provenza JM, Silver RC, Bacon BR. Endoscopic retrograde cholangiography in the swine: a new model for endoscopic training and hepatobiliary research. Gastrointest Endosc 36: 600–603, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol Cell Physiol 264: C591–C602, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hadorn B, Johansen PG, Anderson CM. Pancreozymin secretin test of exocrine pancreatic function in cystic fibrosis and the significance of the result for the pathogenesis of the disease. Can Med Assoc J 98: 377–385, 1968 [PMC free article] [PubMed] [Google Scholar]
- 46. Hadorn B, Zoppi G, Shmerling DH, Prader A, McIntyre I, Anderson CM. Quantitative assessment of exocrine pancreatic function in infants and children. J Pediatr 73: 39–50, 1968 [DOI] [PubMed] [Google Scholar]
- 47. Hill RE, Durie PR, Gaskin KJ, Davidson GP, Forstner GG. Steatorrhea and pancreatic insufficiency in Shwachman syndrome. Gastroenterology 83: 22–27, 1982 [PubMed] [Google Scholar]
- 48. Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 133: 315–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr 142: 624–630, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Kopelman H, Corey M, Gaskin K, Durie P, Weizman Z, Forstner G. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology 95: 349–355, 1988 [DOI] [PubMed] [Google Scholar]
- 51. Kopelman H, Durie P, Gaskin K, Weizman Z, Forstner G. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med 312: 329–334, 1985 [DOI] [PubMed] [Google Scholar]
- 52. Kraemer R, Rudeberg A, Hadorn B, Rossi E. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr Scand 67: 33–37, 1978 [DOI] [PubMed] [Google Scholar]
- 53. Laine J, Beattie M, LeBel D. Simultaneous kinetic determinations of lipase, chymotrypsin, trypsin, elastase, and amylase on the same microtiter plate. Pancreas 8: 383–386, 1993 [DOI] [PubMed] [Google Scholar]
- 54. Lenzen R, Alpini G, Tavoloni N. Secretin stimulates bile ductular secretory activity through the cAMP system. Am J Physiol Gastrointest Liver Physiol 263: G527–G532, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Maggee DF, Naruse S. Characteristics of secretin-stimulated pancreatic secretion in dogs. J Physiol 356: 391–399, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mall M, Kreda SM, Mengos A, Jensen TJ, Hirtz S, Seydewitz HH, Yankaskas J, Kunzelmann K, Riordan JR, Boucher RC. The ΔF508 mutation results in loss of CFTR function and mature protein in native human colon. Gastroenterology 126: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Marino CR, Matovcik LM, Gorelick FS, Cohn JA. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest 88: 712–716, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McGill JM, Basavappa S, Mangel AW, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology 107: 236–243, 1994 [DOI] [PubMed] [Google Scholar]
- 59. Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Meyerholz DK, Stabel TJ, Ackermann MR, Carlson SA, Jones BD, Pohlenz J. Early epithelial invasion by Salmonella enterica serovar Typhimurium DT104 in the swine ileum. Vet Pathol 39: 712–720, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol 176: 1377–1389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noble MD, Romac J, Vigna SR, Liddle RA. A pH-sensitive, neurogenic pathway mediates disease severity in a model of post-ERCP pancreatitis. Gut 57: 1566–1571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 373: 1891–1904, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol 2: 241–278, 1975 [PubMed] [Google Scholar]
- 65. Ornoy A, Arnon J, Katznelson D, Granat M, Caspi B, Chemke J. Pathological confirmation of cystic fibrosis in the fetus following prenatal diagnosis. Am J Med Genet 28: 935–947, 1987 [DOI] [PubMed] [Google Scholar]
- 66. Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB, Jr, Zabner J, Welsh MJ, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med 3: 74ra24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peters RH, van Doorninck JH, French PJ, Ratcliff R, Evans MJ, Colledge WH, Bijman J, Scholte BJ. Cystic fibrosis transmembrane conductance regulator mediates the cyclic adenosine monophosphate-induced fluid secretion but not the inhibition of resorption in mouse gallbladder epithelium. Hepatology 25: 270–277, 1997 [DOI] [PubMed] [Google Scholar]
- 68. Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB, Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Porta EA, Stein AA, Patterson P. Ultrastructural changes of the pancreas and liver in cystic fibrosis. Am J Clin Pathol 42: 451–465, 1964 [DOI] [PubMed] [Google Scholar]
- 70. Quinton PM. Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda) 22: 212–225, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Raeder MG. The origin of and subcellular mechanisms causing pancreatic bicarbonate secretion. Gastroenterology 103: 1674–1684, 1992 [PubMed] [Google Scholar]
- 72. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP release regulates Cl− secretion in cultured human and rat biliary epithelial cells. Am J Physiol Gastrointest Liver Physiol 276: G1391–G1400, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Roy CC, Weber AM, Morin CL, Lepage G, Brisson G, Yousef I, Lasalle R. Hepatobiliary disease in cystic fibrosis: a survey of current issues and concepts. J Pediatr Gastroenterol Nutr 1: 469–478, 1982 [DOI] [PubMed] [Google Scholar]
- 75. Scheele GA, Fukuoka SI, Kern HF, Freedman SD. Pancreatic dysfunction in cystic fibrosis occurs as a result of impairments in luminal pH, apical trafficking of zymogen granule membranes, and solubilization of secretory enzymes. Pancreas 12: 1–9, 1996 [DOI] [PubMed] [Google Scholar]
- 76. Shiffman ML, Sugerman HJ, Moore EW. Human gallbladder mucosal function. Effect of concentration and acidification of bile on cholesterol and calcium solubility. Gastroenterology 99: 1452–1459, 1990 [DOI] [PubMed] [Google Scholar]
- 77. Shumaker H, Amlal H, Frizzell R, Ulrich CD, Soleimani M. CFTR drives Na+-nHCO3− cotransport in pancreatic duct cells: a basis for defective HCO3− secretion in CF. Am J Physiol Cell Physiol 276: C16–C25, 1999 [DOI] [PubMed] [Google Scholar]
- 78. Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCOFormula secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol 298: C1057–C1065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Soleimani M, Ulrich CD. How cystic fibrosis affects pancreatic ductal bicarbonate secretion. Med Clin North Am 84: 641–655, x, 2000 [DOI] [PubMed] [Google Scholar]
- 80. Stern RC, Rothstein FC, Doershuk CF. Treatment and prognosis of symptomatic gallbladder disease in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 5: 35–40, 1986 [DOI] [PubMed] [Google Scholar]
- 81. Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29ra31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Villanger O, Veel T, Holthe MR, Cragoe EJ, Jr, Raeder MG. Secretin stimulates bile ductules to secrete both H+ and HCO3− ions. Acta Physiol Scand 146: 369–376, 1992 [DOI] [PubMed] [Google Scholar]
- 84. Waters DL, Dorney SF, Gaskin KJ, Gruca MA, O'Halloran M, Wilcken B. Pancreatic function in infants identified as having cystic fibrosis in a neonatal screening program. N Engl J Med 322: 303–308, 1990 [DOI] [PubMed] [Google Scholar]
- 85. Willi UV, Reddish JM, Teele RL. Cystic fibrosis: its characteristic appearance on abdominal sonography. AJR Am J Roentgenol 134: 1005–1010, 1980 [DOI] [PubMed] [Google Scholar]
- 86. Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut 56: 1153–1163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilschanski M, Fisher D, Hadas-Halperin I, Picard E, Faber J, Goldberg S, Branski D, Kerem E. Findings on routine abdominal ultrasonography in cystic fibrosis patients. J Pediatr Gastroenterol Nutr 28: 182–185, 1999 [DOI] [PubMed] [Google Scholar]