Abstract
Studies have shown that decreased mitochondrial content and function are associated with hepatic steatosis. We examined whether peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) overexpression and a subsequent increase in mitochondrial content and function in rat primary hepatocytes (in vitro) and Sprague-Dawley rats (in vivo) would comprehensively alter mitochondrial lipid metabolism, including complete (CO2) and incomplete (acid-soluble metabolites) fatty acid oxidation (FAO), tricarboxylic acid cycle flux, and triacylglycerol (TAG) storage and export. PGC-1α overexpression in primary hepatocytes produced an increase in markers of mitochondrial content and function (citrate synthase, mitochondrial DNA, and electron transport system complex proteins) and an increase in FAO, which was accompanied by reduced TAG storage and TAG secretion compared with control. Also, the PGC-1α-overexpressing hepatocytes were protected from excess TAG accumulation following overnight lipid treatment. PGC-1α overexpression in hepatocytes lowered expression of genes critical to VLDL assembly and secretion (apolipoprotein B and microsomal triglyceride transfer protein). Adenoviral transduction of rats with PGC-1α resulted in a liver-specific increase in PGC-1α expression and produced an in vivo liver phenotype of increased FAO via increased mitochondrial function that also resulted in reduced hepatic TAG storage and decreased plasma TAG levels. In conclusion, overexpression of hepatic PGC-1α and subsequent increases in FAO through elevated mitochondrial content and/or function result in reduced TAG storage and secretion in the in vitro and in vivo milieu.
Keywords: peroxisome proliferator-activated receptor-γ coactivator-1α, hepatocyte, mitochondria, steatosis
mitochondrial dysfunction and decreased fatty acid oxidation (FAO) have been proposed as mechanisms for the accumulation of triacylglycerol (TAG) in the liver (steatosis) and as early physiological insults that precipitate the development of nonalcoholic fatty liver disease, a condition that plays a crucial role in the development of the metabolic syndrome and type 2 diabetes. Hepatic steatosis could develop through any combination of increased liver free fatty acid (FFA) uptake and storage as TAG, increased de novo lipogenesis, decreased FAO, and decreased secretion of TAG as VLDL. Evidence from our group and others suggests that elevated mitochondrial number and function increase FAO, which may play a protective role by reducing hepatic TAG accumulation (28, 31, 38, 40).
The mitochondrial dysfunction associated with hepatic steatosis is observed as a variety of pathophysiologies, including deterioration of redox balance, decreased oxidative phosphorylation, damaged and/or decreased mitochondrial DNA (mtDNA), activation of mitochondrial proapoptotic events, and altered FAO (21). Previous studies examining genetic defects in mitochondrial FAO have demonstrated exaggerated development of steatosis in rodents and humans (13, 29). Rodent models of obesity and decreased intrinsic aerobic capacity demonstrate decreased hepatic mitochondrial content and lower mitochondrial FAO capacity, which are associated with increased liver TAG accumulation and fibrosis (31, 40).
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcription cofactor that interacts with numerous transcription factors and has been shown to be a potent activator of mitochondrial biogenesis and FAO in skeletal muscle in response to increased physical activity (12). The investigation of PGC-1α in liver physiology has focused on the integration of nutritional and hormonal signals resulting in the fed-to-fasted transition, primarily in the control of hepatic gluconeogenesis (19). However, as it relates to metabolic disease, reduced PGC-1α expression has been observed in the liver of obese, sedentary subjects compared with lean controls (6). Furthermore, hepatic PGC-1α protein expression and transcriptional activation of mitochondrial biogenesis have been reported to be lower in a rodent model with hepatic steatosis (4). Alternatively, increased physical activity has been observed to increase PGC-1α expression in the liver of a wheel-running rodent model (18), and PGC-1α has been shown to play a key role in exercise-induced hepatic mitochondrial adaptations (11). Since exercise is known to lower hepatic TAG and reduce circulating TAG levels, the activation of PGC-1α may play a critical role in these responses. However, whether a targeted overexpression of hepatic PGC-1α and subsequent increased FAO through increases in mitochondrial content and function can reduce TAG accumulation and secretion remains unknown.
In this study, we comprehensively investigate the effect of PGC-1α overexpression and associated increases in mitochondrial content and/or function on FAO, TAG storage, and TAG export in primary hepatocytes (in vitro) and liver (in vivo) of Sprague-Dawley rats. Initially, primary hepatocytes were utilized, so that hepatocyte-specific changes in FAO and lipid metabolism could be studied in isolation from peripheral factors (i.e., insulin, glucagon, and circulating lipids) known to influence liver metabolism and PGC-1α expression and activation in vivo. We then overexpressed PGC-1α in vivo to confirm the in vitro observations. We also examined changes in genes [apolipoprotein B (apoB) and microsomal triglyceride transfer protein (mttp)] that play a key role in triglyceride export. We hypothesized that overexpression of PGC-1α in primary hepatocytes and liver reduces TAG accumulation and export due to greater FAO from increased mitochondrial content and/or function.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (Charles River, Wilmington, MA) were used at 8–10 wk of age for cell culture experiments and at 12–14 wk of age for adenoviral transduction experiments. Purina standard rat chow and water were provided ad libitum, and the animals were kept on a 12:12-h light-dark cycle. The animal protocols were approved by the Institutional Animal Care and Use Committees at the University of Missouri and the Subcommittee for Animal Safety at the Harry S. Truman Memorial Veterans Affairs Hospital.
Hepatocyte isolation, culture, viral transduction, and lipid treatment.
Hepatocytes from 8- to 10-wk-old male Sprague-Dawley rats were isolated by the two-step collagenase perfusion method, as previously described with minor changes (10). Animals were fasted overnight and anesthetized with pentobarbital sodium (75 mg/kg). The hepatic portal vein was cannulated, and heparin was injected (1 U/g body wt). Infusion of EGTA perfusion solution [HBSS without Ca2+ and Mg2+ (Invitrogen), 15 mM HEPES, 100 U/ml penicillin-100 μg/ml streptomycin, and 0.5 mM EGTA, pH ∼7.2] was initiated, and the abdominal vena cava was transected. Once the liver was blanched, collagenase infusion was begun [HBSS (Invitrogen), 15 mM HEPES, 100 U/ml penicillin-100 μg/ml streptomycin, 10 mM l-glutamine, 0.1% collagenase (Invitrogen), and 0.1% soybean trypsin inhibitor (Invitrogen), pH ∼7.2]. The liver was removed and manually disrupted. The cell suspension was filtered through Miracloth (Calbiochem) and washed in Hepatocyte Wash Media (Invitrogen) three times, with the cells pelleted at 50 g for 5 min for the first wash and for 2 min for subsequent washes. Cells were resuspended in hepatocyte growth medium [Williams E (Invitrogen), 10% FBS, 4 mM l-glutamine, 100 U penicillin-100 mg streptomycin, 2 ng/ml rat EGF, 100 nM insulin, 100 nM dexamethasone, 0.1% BSA, and 10 mM sodium pyruvate], and viability was determined using Trypan blue. Cell viability was never observed to be <90%. To further reduce nonhepatocyte (e.g., fibroblasts and Kupffer cells) contamination of the culture, the crude cell isolate was preplated on 100-mm2 plates for 1 h. After 1 h, the unattached cells were collected, pelleted, and resuspended in a collagen matrix [DMEM-low glucose (Invitrogen) and rat tail collagen], and the cells were plated to produce a 10 μg/cm3 collagen culture matrix. After the collagen matrix solidified, the cells were fed hepatocyte growth medium daily. On the day following isolation, cells were transduced with ∼2.4 × 108 particles/cm2 of adenovirus expressing β-galactosidase (β-gal) or PGC-1α for 1 h; then the medium was replaced with fresh growth medium. In preliminary experiments, this viral load was sufficient to produce a physiologically relevant increase in PGC-1α protein expression with the smallest viral exposure. After 4 days of culture, the cells (12-well and 100-mm2 plates) were exposed to 50 μM palmitate-50 μM oleate or control in lipid medium (hepatocyte growth medium and lipids conjugated to BSA, 0.5% final concentration). The goal of utilizing a 1:1 ratio of long-chain saturated to unsaturated FFA during the lipid treatment was to more closely match in vitro the ratio of lipid species to which the liver is exposed in vivo. After overnight FFA exposure, the cells were serum-starved for 4 h. Starvation medium was reserved for determination of excreted lipids and stored at −80°C until analysis. Cells for mtDNA, mRNA, and lipid analysis and Western blotting were rinsed three times with ice-cold Krebs-Henseleit buffer, scraped, collected, and pelleted. The cell pellets for RNA and DNA analysis were placed on RNAlater (Ambion) and stored at −20°C until analysis. Samples for lipid analysis were lysed in 2% SDS buffer [surfactant, 100 mM NaCl, 20 mM Tris, 2 mM EDTA, 10 mM MgCl2, 10 mM NaF, 40 mM β-glycerol phosphate, protease inhibitors (Roche), and phosphatase inhibitors (Sigma)] and centrifuged (10 min, 8,000 g, 4°C). Cell pellets for Western blot analysis were lysed in 1% Triton X-100 lysis buffer [50 mM HEPES, 12 mM sodium pyrophosphate, 100 mM NaF, 10 mM EDTA, protease inhibitors (Roche), and phosphatase inhibitors (Sigma)] and centrifuged (10 min, 8,000 g, 4°C). All samples were stored at −80°C until analysis. The protein content of the cell lysates was determined by bicinchoninic acid (BCA) assay.
Sprague-Dawley rat adenoviral transduction and protocol.
Adenoviral transduction of male Sprague-Dawley rats was performed as previously described with minor changes (26). Briefly, on the day before adenoviral transduction, the animals were injected with cyclosporine (15 mg/kg ip) to reduce immune response. On the next day, the animals received an intravenous bolus through the tail vein of the appropriate adenovirus (3.33 × 109 virus particles/g body wt) that was diluted in normal saline to a final volume of 0.5 ml. At 0630 on day 9 after the adenoviral transduction, blood was collected from the tail vein for fed plasma analysis. The animals were fasted overnight, and on the morning of tissue collection (day 10) the animals were anesthetized by a bolus of pentobarbital sodium (75 mg/kg ip). Blood was collected by heart puncture, and a portion of the liver was quickly placed in ice-cold FAO buffer and mitochondrial isolation buffer. Plasma glucose (Sigma, St. Louis, MO), TAG (Sigma), and nonesterified fatty acids (NEFA, Wako Chemicals, Richmond, VA) were determined according to the manufacturer's instructions. Additional liver tissue segments were collected for RNA isolation and fixation, and the remaining liver was snap-frozen in liquid nitrogen. Red gastrocnemius, lung, kidney, and left ventricle were collected for RNA isolation.
Liver mitochondrial isolation.
Mitochondria were isolated from rat liver tissue, as previously described (33). Briefly, tissue was homogenized in cold liver mitochondrial isolation buffer (220 mannitol, 70 sucrose, 10 mM Tris, and 1 mM EDTA, adjusted to pH 7.4 with KOH) and kept on ice. The homogenate was transferred to a 15-ml conical tube and centrifuged (1,500 g, 10 min, 4°C), the supernatant was centrifuged (8,000 g, 10 min, 4°C), and the pellet was resuspended in liver mitochondrial isolation buffer by glass-on-glass homogenization and centrifuged at 6,000 g for 10 min at 4°C. The mitochondria were washed with liver mitochondrial isolation buffer containing 0.1% fatty acid-free BSA and pelleted (4,000 g, 10 min, 4°C). The mitochondria were resuspended in an appropriate buffer, and protein concentration was determined by BCA assay.
Primary hepatocyte FAO.
FAO by primary hepatocytes was determined as previously described with minor modifications (25). After serum starvation, 12-well plates were washed with warm PBS, and the cells were incubated with 14C-labeled FAO reaction medium consisting of DMEM-low glucose (Invitrogen), 0.25 μCi/ml [1-14C]palmitate, 0.25 μCi/ml [1-14C]oleate, 50 μM palmitate, 50 μM oleate, 0.5% BSA, 1 mM carnitine, and 12.5 mM HEPES (pH ∼7.4) at 37°C for 3 h in triplicate. A mixed 1:1 [1-14C]palmitate-[1-14C]oleate tracer was used in the FAO experiments to more closely mimic tissue lipid exposure and minimize individual enzyme preferences for specific FFA species; this provides additional novelty to the study. To approximate the carnitine palmitoyltransferase-1 (CPT-1)-mediated FAO, appropriate wells were treated with the CPT-1 inhibitor etomoxir (100 μM). CPT-1-mediated FAO is calculated as the difference between absolute FAO and FAO in the presence of etomoxir. After 3 h, the medium from each well was collected, and an aliquot of medium was dispensed into the sealed trapping device. The 14CO2 was driven from the media aliquot by addition of perchloric acid and trapped in NaOH, which was collected and analyzed by liquid scintillation counting for determination of complete FAO to CO2. The acidified medium was collected, refrigerated, and centrifuged (16,000 g, 4°C). An aliquot was analyzed by liquid scintillation counting for determination of the acid-soluble metabolites (ASMs) of FAO. The cells were rinsed three times with ice-cold Krebs-Henseleit buffer and lysed with SDS lysis buffer. The protein concentration of the lysate was determined by BCA assay.
Palmitate and pyruvate oxidation by liver homogenate and isolated mitochondria.
Oxidation of [1-14C]palmitate and [2-14C]pyruvate was measured in fresh liver homogenates and isolated mitochondria, as previously described (30). FAO was assessed by measurement of the production of 14CO2 and 14C-labeled ASMs in a sealed trapping device containing 200 μM palmitate, [1-14C]palmitate, tissue sample, and reaction buffer (100 mM sucrose, 10 mM Tris·HCl, 10 mM KPO4, 100 mM KCl, 1 mM 4 MgCl2·6H2O, 1 mM l-carnitine, 0.1 mM malate, 2 mM ATP, 0.05 mM CoA, and 1 mM DTT, pH 7.4) at 37°C. To estimate the FAO mediated by CPT-1-catalyzed FFA entry into the mitochondria, FAO was monitored in the presence of the CPT-1 inhibitor etomoxir (100 μM). The oxidation of 5 mM pyruvate ([2-14C]pyruvate) to 14CO2 by isolated liver mitochondria in the reaction buffer was used to monitor pyruvate oxidation and approximate tricarboxylic acid (TCA) cycle flux.
TAG quantification.
SDS cell lysates were used to isolate cellular and media lipid fractions by a modification of the chloroform-methanol extraction method described by Folch et al. (9). Aliquots from the SDS cell lysates and starvation medium were dispensed into one volume of TE buffer (50 mM Tris and 1 mM EDTA, pH ∼7.4), four volumes of chloroform-methanol-acetic acid (2:1:0.15) were added, and the samples were mixed by vortexing and allowed to stand for 10 min. The samples were centrifuged (50 g, 10 min, 10°C), and the bottom chloroform layer was pipetted off and filtered into a new tube. An additional 0.5 volume of chloroform-methanol was added to the first tube, and the above-described steps were repeated. The chloroform extract was blown to dryness under N2. The lipids were resuspended in chloroform-methanol (2:1) and resolved by TLC (hexane-diethyl ether-acetic acid, 70:30:1). The individual lipid species were visualized on the chromatography plate with 2,7-dichlorofluorscein and collected into glass tubes. Lipid species were scraped from the TLC plate and then methylated by incubation with toluene-0.5 M sodium methoxide (methanol) (1:2) for 1 min and separated in isooctane. Fatty acid methyl esters were analyzed by gas chromatography.
mRNA expression.
RNA was isolated from primary hepatocytes and tissue stored in RNAlater using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA), and cDNA was produced using the IMProm-II reverse transcriptase system (Promega, Madison, WI). RNA and cDNA concentration was determined using a spectrophotometer (model ND-1000, NanoDrop, Thermo Scientific, Wilmington, DE). Real-time quantitative PCR analysis was performed utilizing a Prism 7000 and TaqMan gene expression assay (Applied Biosystems, Foster City, CA). The relative mRNA expressions of pgc1a, mttp, and apoB were determined utilizing a predesigned 6-carboxyfluorescein (FAM)-labeled primer/probe assay supplied by Applied Biosystems. All gene-specific values were normalized to relative 18S rRNA expression values.
mtDNA content.
Total cellular DNA was isolated from stored RNAlater cell and liver tissue samples using DNeasy Blood and Tissue Kit (Qiagen). Total isolated cellular DNA concentration was determined using the Quant-iT PicoGreen dsDNA kit (Invitrogen). Total isolated DNA from the liver tissue was determined using a spectrophotometer (model ND-1000, NanoDrop). Real-time quantitative PCR analysis was performed utilizing a Prism 7000 and TaqMan gene expression assay (Applied Biosystems). For cell samples, the sequence-specific, FAM-labeled primer/probe assay for the rat mitochondrial D-loop and β-actin (Integrated DNA Technologies, Coralville, IA) is as follows: mitochondrial D-loop [5′-GGTTCTTACTTCAGGGCCATCA-3′ (forward) and 5′-GATTAGACCCGTTACCATCGAGT-3′ (reverse)], probe (5′-FAM-TTGGTTCATCGTCCATACGTTCCCCTTA), β-actin [5′-GGGATGTTTGCTCCAACCAA-3′ (forward) and 5′-GCGCCTTTTGACTCAAGGATTTAA-3′ (reverse)], and probe (5′-FAM-CGGTCGCCTTCACCGTTCCAGTT) (27). For tissue samples, the above-mentioned mitochondrial D-loop primer/probe assay was utilized and normalized relative to 18S rRNA values. The data were analyzed as follows: Rc = 2ΔCt and ΔCt = Ct(β-actin) − Ct(mtDNA), where Ct is the threshold cycle and Rc is the relative copy number (32).
Citrate synthase activity.
Citrate synthase activity was determined as previously described (37). Citrate synthase activity in whole cell lysates (primary hepatocytes) and liver homogenate (in vivo) was used as a general marker of mitochondrial content, while citrate synthase activity in isolated mitochondria from liver (in vivo) was used as a marker of mitochondrial function.
Western blot analysis.
Triton X-100 cell lysates were used to produce Western blot-ready Laemmli samples. Samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with primary antibodies. PGC-1α antibody was purchased from Calbiochem (EMD Chemicals, Gibbstown, NJ), apoB antibody from Abcam (Cambridge, MA), MTTP antibodies from Santa Cruz Biotechnology (Santa Cruz, CA), mitochondrial transcription factor A (mtTFA) antibody from Novus Biologicals (Littleton, CO), and oxidative phosphorylation complex I–V antibody cocktail from MitoScience (Eugene, OR). Individual protein bands were quantified using a densitometer (Bio-Rad), and protein loading was corrected by 0.1% amido-black (Sigma) staining to determine total protein, as previously described (30).
Statistics.
Comparison of in vivo β-gal with PGC-1α groups was performed using Student's t-test, with a minimum threshold of P < 0.05. In vitro data comparing β-gal with PGC-1α in the absence and presence of FFA were analyzed by analysis of variance, with a minimum threshold of P < 0.05. Fisher's least significant difference post hoc test was performed to determine differences between groups with SPSS (IBM, Somers, NY). Values are means ± SE.
RESULTS
Primary hepatocyte PGC-1α expression and markers of mitochondrial content.
Exposure of the primary hepatocytes to recombinant adenovirus expressing PGC-1α resulted in a dramatic increase in PGC-1α mRNA expression compared with β-gal controls (>1,500-fold, P < 0.05; Fig. 1A). This increase in mRNA levels following adenoviral transduction was sufficient to produce a 75% increase in PGC-1α protein compared with β-gal-transduced hepatocytes (P < 0.05; Fig. 1B). This increase in protein expression is comparable to increases in PGC-1α protein expression in hepatocytes isolated from exercised rats (unpublished data) and also within the range of PGC-1α expression in liver (19). Overexpression of PGC-1α in primary hepatocytes resulted in a 45% increase in mtTFA protein expression compared with β-gal cells (P < 0.05; Fig. 1C). This elevation in protein expression of mtTFA, a nuclear-encoded transcription factor critical for mtDNA transcription and replication (19), was associated with 33% higher relative mtDNA content in PGC-1α-overexpressing hepatocytes (P < 0.05; Fig. 1D). Citrate synthase activity, a classic marker of mitochondrial content, was ∼70% higher in PGC-1α-overexpressing than β-gal hepatocytes (P < 0.05; Fig. 1E). These increases in mtDNA following PGC-1α overexpression were associated with increases in the mtDNA-encoded protein cytochrome c oxidase IV subunit I (P < 0.05; Fig. 1F). Finally, primary hepatocytes demonstrated increased protein expression of nuclear-encoded members of the oxidative phosphorylation pathway complex II subunit Ip (succinate dehydrogenase) and complex V subunit a (ATP synthase) (P < 0.05; Fig. 1F). Together, these data clearly demonstrate the ability of PGC-1α overexpression to increase mitochondrial content, and potentially function, in isolated primary hepatocytes.
Fig. 1.
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) overexpression increases markers of primary hepatocyte mitochondrial biogenesis and content. Hepatocyte PGC-1α expression following adenoviral transduction was determined by RT-PCR and Western blot analysis, and protein expression of various markers of mitochondrial content was determined by Western blotting, as well as spectrophotometric determination of citrate synthase activity. A and B: PGC-1α mRNA and protein expression were significantly higher in cells transduced with adenovirus containing PGC-1α cDNA than in β-galactosidase (β-gal)-transduced cells. C: protein expression of mitochondrial transcription factor A (mtTFA) was greater in PGC-1α-overexpressing than β-gal cells. D: mitochondrial DNA (mtDNA) expression was greater in PGC-1α-overexpressing than β-gal hepatocytes. E: citrate synthase activity was higher in PGC-1α-overexpressing than β-gal primary hepatocytes. F: electron transport system (ETS) complex II, IV, and V protein expression was higher in PGC-1α-overexpressing than β-gal cells. Values are means ± SE; n = 8, except for mtDNA, where n = 5. +P < 0.05. G and H: representative Western blots.
Effects of PGC-1α on primary hepatocyte FAO.
FAO of PGC-1α-overexpressing primary hepatocytes was determined by monitoring the oxidation of [14C]palmitate-[14C]oleate (1:1). Complete FAO to CO2, ASM production, and total FAO (complete oxidation + ASMs) was determined. The overexpression of PGC-1α in primary hepatocytes resulted in a nearly twofold increase in complete FAO to CO2, which represents the oxidation of fatty acids from β-oxidation through the TCA cycle (P < 0.05; Fig. 2A). The β-oxidation of fatty acids produces metabolites that do not undergo further β-oxidation cycles or oxidation via the TCA cycle and are excreted from the mitochondria and the cell. The production of these ASMs was 25% higher in the PGC-1α-overexpressing than β-gal hepatocytes (data not shown). These increases in complete FAO and ASMs resulted in 30% higher total FAO in the PGC-1α-overexpressing primary hepatocytes (P < 0.05; Fig. 2B). As a marker of the overall efficiency of hepatocyte FAO, the percent FAO to CO2 was calculated as the complete FAO divided by the total FAO. This measure serves as a means to evaluate the coupling of β-oxidation of fatty acids to the oxidation of the produced acetyl-CoA via the TCA cycle. The percent FAO to CO2 was 45% higher in PGC-1α-overexpressing than β-gal hepatocytes (P < 0.05; Fig. 2C), suggesting greater FAO efficiency. The outer mitochondrial membrane-associated protein CPT-1 is the rate-limiting step for fatty acyl-CoA entry into the mitochondria as acylcarnitines. The CPT-1-selective inhibitor etomoxir can be utilized to estimate CPT-1-mediated FAO. The difference between the absolute FAO, described above, and the FAO in the presence of etomoxir can serve as an approximation of CPT-1-meditated FAO. Complete FAO was 40% greater in PGC-1α-overexpressing primary hepatocytes in the presence of etomoxir (P < 0.05; Fig. 2D), which is suggestive of increased FAO in other cellular organelles. From these data, the calculated complete FAO inhibited by etomoxir is 2.4-fold higher in PGC-1α-overexpressing primary hepatocytes than in β-gal cells (P < 0.05; Fig. 2D), suggesting greater mitochondrial complete FAO. Taken together, these data demonstrate that increased PGC-1α expression increases FAO efficiency in isolated primary hepatocytes.
Fig. 2.
Adenoviral overexpression of PGC-1α increases primary hepatocyte fatty acid oxidation (FAO). FAO rates of 14C-radiolabeled palmitate-oleate (50 μM palmitate-50 μM oleate) by primary hepatocytes were determined in the presence and absence of the carnitine palmitoyltransferase-1 (CPT-1) inhibitor etomoxir (100 μM). A: complete FAO to CO2 was elevated in PGC-1α-overexpressing hepatocytes. B: total FAO was greater in PGC-1α-overexpressing than β-gal hepatocytes. C: FAO efficiency (percent FAO to CO2) was greater in PGC-1α-overexpressing hepatocytes. D: complete FAO to CO2 in the presence of etomoxir was increased in PGC-1α-overexpressing hepatocytes, and CPT-1-mediated complete FAO, as approximated by complete FAO inhibited by etomoxir, was greater in PGC-1α-overexpressing than β-gal hepatocytes. Values are means ± SE; n = 8. +P < 0.05.
Effects of PGC-1α on primary hepatocyte lipid handling.
To determine if PGC-1α overexpression in primary hepatocytes altered lipid storage or secretion patterns, TAG levels were quantified by gas chromatography in extracts from cell lysate and starvation media samples in cells treated overnight with FFA (50 μM palmitate-50 μM oleate). Under the control conditions, overexpression of PGC-1α in hepatocytes resulted in decreased cellular TAG levels compared with β-gal cells (18%, P < 0.05; Fig. 3A). Lipid exposure resulted in 23% less cellular TAG accumulation in PGC-1α hepatocytes than in β-gal cells (P < 0.05). Importantly, the 100 μM overnight lipid exposure produced an increase in cellular TAG accumulation in β-gal cells (16%, P < 0.05), while no change in TAG accumulation was observed in PGC-1α-overexpressing hepatocytes. Also, the secretion rate of TAG into media was ∼30% lower in the PGC-1α-overexpressing primary hepatocytes than in β-gal cells under control and lipid-treated conditions (P < 0.05; Fig. 3B). Within adenoviral groups, the overnight exposure to FFA resulted in 25% and 20% increases in secretion rate of TAG in the PGC-1α-overexpressing and β-gal cells, respectively (P < 0.05). However, the increase in media TAG in PGC-1α-overexpressing hepatocytes was still less than the basal level in the β-gal cells (P < 0.05). Additionally, differences were observed in the absolute levels of FFA species in the cellular and secreted TAG between adenoviral groups under control and lipid-treated conditions (Fig. 3, C and D). However, no difference in the percent FFA species composition was observed in any lipid species in cell lysates or media samples under any of the experimental conditions (data not shown). These data demonstrate that PGC-1α overexpression in primary hepatocytes prevents TAG accumulation under control and lipid-treated conditions while also reducing the quantity of secreted TAG.
Fig. 3.
Decreased triacylglycerol (TAG) accumulation and secretion in PGC-1α-overexpressing primary hepatocytes. TAG accumulation and secretion in primary hepatocytes were determined following overnight treatment with control or free fatty acids (FFA; 50 μM palmitate-50 μM oleate). A: cellular accumulation of TAG was decreased in PGC-1α-overexpressing compared with β-gal hepatocytes under control conditions. After lipid treatment, cellular TAG accumulation was increased in β-gal hepatocytes compared with control, while no change was observed in PGC-1α-overexpressing hepatocytes. B: in the control state, TAG secretion into the medium was decreased in PGC-1α-overexpressing compared with β-gal hepatocytes. After overnight lipid exposure, TAG secretion into the medium was increased in PGC-1α-overexpressing and β-gal hepatocytes compared with control, with PGC-1α-overexpressing hepatocytes demonstrating decreased TAG secretion compared with β-gal cells. C and D: FFA constituents of TAG were elevated in both adenoviral groups following overnight lipid exposure in cell lysates and media samples. Con, control. Values are means ± SE; n = 8. +P < 0.05 between groups. †P < 0.05 within group.
Effects of PGC-1α on in vitro apoB assembly.
The packaging of TAG and cholesterol ester into an apolipoprotein for secretion and delivery of VLDL to peripheral tissues is a hallmark function of the liver. The primary lipoprotein produced and utilized by hepatocytes in the packaging and excretion of TAG is apoB (34). The mRNA expression of apoB was decreased 35% in PGC-1α-overexpressing primary hepatocytes compared with β-gal cells under control conditions (P < 0.05; Fig. 4A). Interestingly, overnight lipid treatment resulted in a 25% decrease in apoB mRNA expression in β-gal hepatocytes compared with control (P < 0.05), with no difference within PGC-1α-overexpressing cells or between adenoviral groups. In rodent hepatocytes, two proteins are produced from the apoB mRNA: the more highly expressed apoB-48, which is a truncated protein representing 48% of the NH2-terminal mRNA, and the full-length apoB-100. Under control conditions, the protein expression of apoB-48 is 30% less in the PGC-1α-overexpressing than β-gal cells (P < 0.05; Fig. 4B). This difference was mostly maintained in the PGC-1α-overexpressing cells after exposure to FFA (25%, P < 0.1). No alterations in apoB-48 protein expression were observed within adenoviral groups following lipid treatment. Protein expression of apoB-100 was suppressed to relatively the same level (30%) as apoB-48 in PGC-1α-overexpressing hepatocytes compared with β-gal control (P < 0.05; Fig. 4C). No changes in apoB-100 protein expression were observed in the β-gal hepatocytes after FFA treatment compared with control. However, overnight lipid treatment resulted in a nonsignificant increase (70%, P < 0.1) in apoB-100 protein expression in the PGC-1α-overexpressing hepatocytes compared with control. MTTP is responsible for chaperoning the translating apoB protein and transferring TAG to the apoB protein to form the immature lipoprotein (34). The overexpression of PGC-1α produced 25% and 30% decreases in MTTP protein expression in the absence and presence of lipid, respectively, compared with the appropriate β-gal group (P < 0.05; Fig. 4D). No differences were observed within adenoviral transduction groups following lipid exposure. These data suggest that the decrease in secreted TAG by primary hepatocytes overexpressing PGC-1α is due, in part, to changes in expression of the key genes involved in TAG packaging and secretion.
Fig. 4.
Decreased expression of apolipoprotein B (apoB) and microsomal triglyceride transfer protein (MTTP) in PGC-1α-overexpressing primary hepatocytes. Expression of genes involved in hepatocyte lipoprotein assembly was determined by RT-PCR and Western blot analysis in FFA (50 μM palmitate-50 μM oleate)-exposed and control primary hepatocytes. A: under control conditions, apoB mRNA expression was decreased in PGC-1α-overexpressing compared with β-gal hepatocytes. After overnight lipid treatment, expression of apoB mRNA was reduced in β-gal hepatocytes compared with control. B: apoB-48 protein expression was lower in PGC-1α-overexpressing than β-gal hepatocytes in the control state and tended to be lower following lipid treatment. C: apoB-100 protein expression was decreased in PGC-1α-overexpressing compared with β-gal hepatocytes in the control state. Expression of apoB-100 tended to be higher in lipid-treated than control PGC-1α-overexpressing hepatocytes. D: protein expression of MTTP was reduced in PGC-1α-overexpressing hepatocytes irrespective of treatment. Values are means ± SE; n = 8. +P < 0.05 between groups. †P < 0.05 within group.
Liver-specific PGC-1α overexpression and liver FAO.
To examine the effects of PGC-1α overexpression on hepatic lipid metabolism in vivo, Sprague-Dawley rats were studied 10 days after transduction with PGC-1α or β-gal adenovirus (AdvPGC1a or AdvBGAL) at 3.33 viral particles/g body wt. Liver PGC-1α mRNA expression was fivefold greater (P < 0.05; Fig. 5A) and PGC-1α protein expression was increased 20% in animals transduced with AdvPGC1a compared with those transduced with AdvBGAL (P < 0.05; Fig. 5B). Liver-specific, adenoviral overexpression of PGC-1α was verified, as no difference in PGC-1α mRNA expression was observed in soleus, kidney, lung, or heart-left ventricle between AdvPGC1a- and AdvBGAL-transduced rats (data not shown). Additionally, no difference was observed in fasting plasma glucose (156.6 ± 8.2 vs. 132.9 ± 7.4 mg/dl) or body weight (448.2 ± 23.9 vs. 404.3 ± 16.1 g) between AdvBGAL- and AdvPGC1a-transduced rats. The increase in liver PGC-1α mRNA expression was associated with an increase in citrate synthase activity, which is a classic marker of mitochondrial content in whole cell or tissue lysates (14%, P < 0.05; Fig. 5C). However, no differences were observed between the groups in liver mtDNA content, an additional marker of mitochondrial content (Fig. 5E). The production of CO2 and ASMs from [14C]palmitate was used to assess the FAO of fresh liver homogenates. The overexpression of PGC-1α in liver produced an 80% increase in complete FAO to CO2 in fresh tissue homogenates (P < 0.05; Fig. 6A), with no change in total FAO between adenoviral groups (Fig. 6B). This resulted in a greater than twofold increase in percent FAO to CO2 (P < 0.05; Fig. 6C), an index of the efficiency of FAO, in the liver of AdvPGC1a-transduced rats. As in the in vitro experiments, the selective CPT-1 chemical inhibitor etomoxir was used in a subset of rats to approximate CPT-1-mediated FAO in the liver homogenates. Complete FAO to CO2 in the presence of etomoxir was 50% lower in livers of AdvPGC1a- than AdvBGAL-transduced rats (P < 0.05; Fig. 6D). The amount of CPT-1-mediated complete FAO, estimated as the complete FAO inhibited by etomoxir, was threefold higher in the AdvPGC1a-transduced animals (P < 0.05; Fig. 6D). We also found that a classic marker of mitochondrial content in liver homogenate, citrate synthase activity, was positively correlated to complete FAO, despite the relatively small difference in citrate synthase activity between the groups. Although the correlation was not statistically significant (P = 0.058), it suggests that increased liver mitochondrial content is related to increased liver FAO. These data establish that the transduction of rats with AdvPGC1a produced liver-specific increases in PGC-1α expression, which produced increases in mitochondrial content and FAO similar to those in primary hepatocytes.
Fig. 5.
Adenoviral PGC-1α (AdvPGC1a) transduction increases hepatic PGC-1α expression and citrate synthase activity. Liver PGC-1α expression following adenoviral transduction was determined by RT-PCR and Western blot analysis. Citrate synthase activity was determined in liver homogenates and isolated liver mitochondria. A and B: liver PGC-1α mRNA and protein expression were higher in AdvPGC1a- than adenoviral β-gal (AdvBGAL)-transduced rats. C and D: hepatic overexpression of PGC-1α resulted in increased citrate synthase activity in liver homogenates (C) and isolated liver mitochondria (D). E: no difference was observed in liver mtDNA content between AdvBGAL- and AdvPGC1a-transduced rats. Values are means ± SE; n = 6. +P < 0.05.
Fig. 6.
Liver PGC-1α overexpression produces elevated liver FAO. After adenoviral transduction, FAO was determined in liver homogenates. A–C: AdvPGC1a-transduced animals had higher complete FAO to CO2, with no difference in total FAO, which results in elevated percent FAO to CO2 compared with AdvBGAL-transduced animals. Etomoxir (100 μM) was used to estimate carnitine palmitoyltransferase-1 (CPT-1)-mediated complete FAO. D: complete FAO in the presence of etomoxir and complete FAO inhibited by etomoxir were higher in liver homogenates from AdvPGC1a- than AdvBGAL-transduced rats. E: positive correlation between complete FAO and citrate synthase activity in liver homogenates from rats transduced with AdvPGC1a (●) and AdvBGAL (○). Values are means ± SE; n = 6, except etomoxir, where n = 3. +P < 0.05.
Effect of hepatic PGC-1α overexpression on liver mitochondria.
To evaluate if the increases in liver FAO following PGC-1α overexpression were due to changes in mitochondrial function and not just mitochondrial content, liver mitochondria were isolated and markers of mitochondrial function and FAO were assessed. The isolated mitochondria displayed a 40% increase in citrate synthase activity in isolated mitochondria from AdvPGC1a-transduced animals (P < 0.05; Fig. 5D), suggesting that mitochondrial enzyme activity, a marker of function, is higher per mitochondrion. As in whole homogenates, complete FAO to CO2 and total FAO were examined in isolated liver mitochondria. Complete FAO was 4.5-fold higher in isolated mitochondria from liver of PGC-1α-overexpressing rats (P < 0.05; Fig. 7A), which was matched with no difference in liver mitochondrial total FAO between adenoviral groups (Fig. 7B), as observed in the liver homogenates. As in the liver homogenates, this large difference in complete FAO to CO2 suggests greater efficiency of FAO, as observed by the nearly fivefold higher percent FAO to CO2 in the isolated mitochondria of the AdvPGC1a-transduced animals (P < 0.05; Fig. 7C). The increases in FAO to CO2 due to PGC-1α overexpression in primary hepatocytes, liver homogenates, and isolated mitochondria could be influenced by increased TCA cycle flux. To assess whether hepatic PGC-1α overexpression increased TCA cycle function, the oxidation of [2-14C]pyruvate to CO2 was measured in isolated liver mitochondria as a marker of TCA cycle flux, as the oxidation of 2-pyruvate to CO2 occurs after at least two passes through the TCA cycle. Oxidation of [2-14C]pyruvate was threefold higher in mitochondria from the liver of AdvPGC1a- than AdvBGAL-transduced rats (P < 0.05; Fig. 7D). As in the liver homogenates, the relationship between citrate synthase activity and complete FAO in isolated liver mitochondria was examined. In the context of isolated mitochondria, citrate synthase activity can be viewed as a marker of mitochondrial function. The analysis showed a significant, positive relationship between citrate synthase activity and complete FAO in isolated mitochondria (P < 0.002; Fig. 7E), suggesting that increased liver mitochondrial function is directly related to elevated FAO. These results suggest that increased hepatic PGC-1α expression not only increases mitochondrial content but also improves mitochondrial function.
Fig. 7.
Liver PGC-1α overexpression produces elevated mitochondrial FAO and tricarboxylic acid (TCA) cycle flux. After adenoviral transduction, FAO and pyruvate oxidation were determined in isolated liver mitochondria. A–C: in isolated liver mitochondria, AdvPGC1a-transduced animals had higher complete FAO to CO2, with no difference in total FAO, which results in elevated percent FAO to CO2 compared with AdvBGAL-transduced animals. D: TCA cycle flux was estimated from oxidation of [2-14C]pyruvate to CO2. Pyruvate oxidation was higher in isolated mitochondria from AdvPGC1a- than AdvBGAL-transduced rats. E: positive correlation between complete FAO and citrate synthase activity in isolated liver mitochondria from rats transduced with AdvPGC1a (●) and AdvBGAL (○). Values are means ± SE; n = 6. + P < 0.05.
Hepatic PGC-1α overexpression and TAG handling.
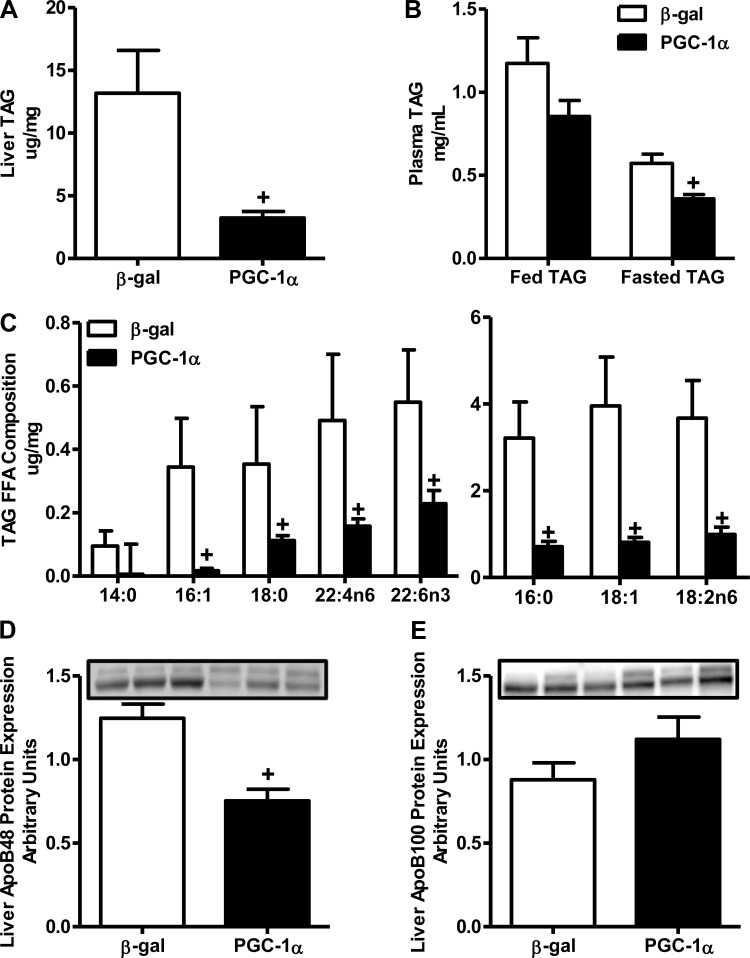
We measured hepatic TAG and the plasma lipid levels of TAG to determine if the AdvPGC1a-transduced animals displayed characteristics similar to those of the primary cells overexpressing PGC-1α. Hepatic overexpression of PGC-1α resulted in a dramatic 75% reduction in liver TAG accumulation (P < 0.05; Fig. 8A). This decrease in tissue TAG in AdvPGC1a-transduced animals was associated with decreased plasma TAG in the fasted state (∼40%, P < 0.05; Fig. 8B) and a trend toward lower plasma TAG in the fed state (∼30%) than in β-gal rats. These decreases in tissue and plasma TAG were not due to differences in FFA delivery to the liver from the adipose tissue, as evident by similar plasma nonesterified fatty acids in the plasma samples during fed and fasted conditions (data not shown). The decrease in liver TAG levels in the AdvPGC1a-transduced rats was mirrored by decreases in absolute levels of individual FFA species comprising the TAG (P < 0.05; Fig. 8C). However, similar to the in vitro experiments, no dramatic difference in the relative percentage of the FFA species composition of TAG was observed (data not shown). We also investigated the expression of genes necessary for appropriate hepatic TAG assembly in the AdvPGC1a-transduced animals. In contrast to the observations in PGC-1α-overexpressing primary hepatocytes, no difference in apoB mRNA expression or MTTP protein expression was observed following hepatic PGC-1α overexpression (data not shown). However, a 40% decrease in apoB-48 protein expression was observed in the liver of AdvPGC1a-transduced rats (P < 0.05; Fig. 8D), while no difference was observed in apoB-100 protein expression between groups. These data demonstrate that liver-specific PGC-1α overexpression results in decreased tissue TAG accumulation, with an associated decrease in plasma TAG levels potentially through decreased apoB-48 VLDL secretion.
Fig. 8.
Adenoviral overexpression of PGC-1α in liver reduces plasma and tissue TAG accumulation. Liver TAG accumulation and plasma TAG levels were determined to assess hepatic lipid handling. A and B: AdvPGC1a-transduced rats demonstrated decreased hepatic accumulation of TAG and decreased plasma TAG in fed and fasted states compared with AdvBGAL-transduced rats. After lipid treatment, cellular TAG accumulation was increased in β-gal hepatocytes compared with control, while no change was observed in PGC-1α-overexpressing hepatocytes. C: levels of FFA species were lower in liver TAG from AdvPGC1a- than AdvBGAL-transduced rats. D and E: hepatic overexpression of PGC-1α resulted in decreased apoB-48 protein expression, with no difference in apoB-100 protein expression between groups. Values are means ± SE; n = 6. +P < 0.05 between groups.
DISCUSSION
Mitochondrial dysfunction has been proposed as a cause for the initiation and progression of hepatic steatosis through decreases in mtDNA, reduced electron transport chain oxidative capacity, and reduced hepatic FAO in human patients and rodent models (21). To our knowledge, our study is the first to examine the impact of PGC-1α protein overexpression on primary hepatocyte mitochondrial content and function, FAO, and TAG storage and secretion in an intact in vitro cellular system independent of circulating peripheral factors. We show that these in vitro observations can be reproduced in vivo following liver-specific increases in PGC-1α expression. In the present study, we found that adenoviral overexpression of PGC-1α in isolated primary hepatocytes and in the liver of Sprague-Dawley rats resulted in 1) increased mitochondrial content and/or function, 2) elevated complete FAO, 3) decreased TAG accumulation, and 4) decreased TAG secretion, which is associated with changes in the expression of TAG assembly proteins.
The mitochondrion is unique among mammalian organelles, in that it contains DNA that encodes proteins required for appropriate function (21); as such, the relative mitochondrial DNA copy number can serve as an index for mitochondrial content under differing environmental or metabolic conditions (32). In skeletal muscle, PGC-1α increases mtDNA content through upregulation of the nuclear-encoded mtTFA via coactivation of nuclear respiratory factors 1 and 2 (12). Our observation of elevated mtDNA, in association with increased mtTFA protein expression, in PGC-1α-overexpressing primary hepatocytes supports the role of PGC-1α as a mediator of hepatic mitochondrial biogenesis and increased content. Furthermore, the role of PGC-1α in regulating hepatocyte mitochondrial content and function is supported by the increases in protein expression of the mitochondrial-encoded electron transport protein complex IV subunit I and in citrate synthase activity. However, the lack of data documenting the effect of in vitro PGC-1α overexpression on mitochondrial function in isolated primary hepatocytes is a limitation of this study. Our observation of increased citrate synthase activity in liver whole homogenates and isolated mitochondria from AdvPGC1a-transduced rats further supports PGC-1α involvement in hepatic mitochondrial homeostasis. The positive correlation between citrate synthase activity and FAO in liver homogenates and isolated mitochondria further supports the role of PGC-1α in modulating liver mitochondrial content and function. Nevertheless, the small increase in citrate synthase activity in the liver homogenate may represent only a dilution of increased mitochondrial citrate synthase activity and may not be representative of increased mitochondrial content in these animals, as suggested by the lack of difference in liver mtDNA content. However, a recent study in skeletal muscle demonstrated that mtDNA is a poor marker of mitochondrial content compared with citrate synthase (16), highlighting the difficulty in assessing mitochondrial content. Further work is necessary to fully elucidate the effect of PGC-1α overexpression on mitochondrial content and function in hepatocytes and liver and whether differential effects exist in various model systems (in vitro vs. in vivo).
Mitochondrial FAO represents the major route of acyl-CoA oxidative metabolism via β-oxidation to produce acetyl-CoA and chain-shortened acyl-CoAs, with the produced acetyl-CoA potentially entering the TCA cycle, culminating in complete oxidation of the carbon to CO2 (complete FAO). However, a portion of lipids are incompletely oxidized, a process that leads to the production of acid-soluble metabolites (e.g., ketone bodies, acyl-CoAs, and acylcarnitines). It has been suggested that an increase in the relative proportion of complete FAO to CO2 may be physiologically beneficial (24). The percentage of complete FAO to CO2 relative to total FAO can be viewed as a marker of FAO efficiency by estimation of the coupling of acetyl-CoA production by β-oxidation to the flux of acetyl-CoA through the TCA cycle. An increase in the efficient coupling of FAO could result in decreased accumulation of incomplete FAO metabolites, which has been observed in several metabolic disease states and is linked to insulin resistance (1, 24). Recently, we reported that elevated liver FAO efficiency was associated with increases in hepatic mitochondrial protein expression and/or function and decreased steatosis (30, 40). PGC-1α has been observed to functionally control FAO efficiency in skeletal muscle. Koves et al. (15) described increased complete FAO to CO2 and increased PGC-1α expression in skeletal muscle mitochondria in a comparison of exercised with sedentary mice, in addition to increased complete FAO in L6 rat skeletal muscle cells overexpressing PGC-1α. On the basis of these data and increases in oxidative phosphorylation and TCA pathway genes, Koves et al. suggested that increased PGC-1α expression produced coordinated increases in fatty acid β-oxidation and downstream mitochondrial pathways, resulting in elevated FAO to CO2. Our data show that increased hepatic PGC-1α expression produces a similar phenotype of increased complete FAO and increased percent FAO to CO2 in primary hepatocytes, isolated liver mitochondria, and liver homogenates. Additionally, the increases in estimated CPT-1-mediated complete FAO, TCA cycle flux (citrate synthase and pyruvate oxidation), and oxidative phosphorylation (complexes II, IV, and V) components further support the role of PGC-1α in coupling increased β-oxidation with increased TCA flux to promote efficient mitochondrial FAO. Considered together with the decrease in TAG accumulation, it could be suggested that PGC-1α-mediated increases in FAO efficiency can reduce TAG accumulation in hepatocytes and liver through more efficient mitochondrial oxidative metabolism of cellular FFA. In addition, our data suggest that this effect on TAG accumulation also impacts TAG secretion.
The packaging and secretion of TAG as VLDL particles represent a mechanism by which the liver can buffer plasma FFA concentration and deliver needed lipids to peripheral tissues for energy production (17). However, the dyslipidemia associated with the metabolic syndrome and type 2 diabetes is the result of increased VLDL secretion rate and is directly associated with liver fat content (2, 3, 7, 36, 39). Recently, it was suggested that hepatic TAG content is the most powerful modulator of VLDL-TAG secretion (20). Interestingly, PGC-1α overexpression resulted in decreased TAG levels in media samples from primary hepatocytes and decreased plasma TAG levels in rats, which are associated with decreased hepatic TAG levels in both cases. One possible explanation is that the amount of hepatic TAG and FFA available for packaging and secretion was reduced secondary to the increased FFA catabolism due to increased FAO following PGC-1α overexpression. Inhibition of hepatic FAO has been observed to stimulate increased VLDL-TAG and cholesterol secretion in perfused rat livers (14), while increased FAO in primary hepatocytes overexpressing CPT-1a resulted in decreased cellular TAG and TAG secreted into the media (38). Work done in a liver-specific pgc1a heterozygous mouse (8) further supports a role of PGC-1α controlling TAG secretion through altered liver lipid metabolism. A ∼50% reduction in liver PGC-1α expression, decreases in FAO genes, and elevated fasting steatosis, which was associated with increased plasma TAG levels, were observed in these mice. Additionally, in HepG2 cells and high-fat-fed LDL receptor knockout mice, a decrease in VLDL secretion following chemical MAPK activation was associated with increased PGC-1α mRNA expression, mitochondrial DNA content, elevated FAO, and decreased TAG accumulation (22, 23). Despite such evidence, it is possible that PGC-1α could play a direct role in modifying the expression of proteins involved in TAG secretion.
The assembly of VLDL in the hepatocyte is initiated within the endoplasmic reticulum by MTTP adding TAG to the ever-growing apoB peptide (2), which also results in protection of apoB protein from proteosomal degradation (34). In our study, the overexpression of PGC-1α in primary hepatocytes resulted in decreased expression of apoB and MTTP. A relationship between PGC-1α, apoB, and MTTP was also observed in the liver-specific pgc1a heterozygous mice mentioned above, where decreased PGC-1α expression resulted in increased apoB and MTTP expression (8). In our in vivo experiments, no difference in apoB and MTTP mRNA expression was observed, perhaps due to the smaller increase in PGC-1α protein expression. However, the reduction in apoB-48 protein could be sufficient to explain the decreases in plasma TAG. ApoB-48 represents the dominant apoB protein variant in rat liver, is less susceptible to intracellular degradation than apoB-100 (35), and has been observed to be preferentially increased over apoB-100 in animals with increased VLDL-TAG due to a chronic high-fat diet (5). However, no decrease in either apoB protein is required for reduced TAG secretion, as observed by decreased media TAG with no reduction in apoB-48 or apoB-100 protein expression in rat hepatocytes overexpressing CPT-1a (38). These reductions in genes critical to VLDL assembly and secretion could represent a direct influence of PGC-1α overexpression modifying the expression of genes that regulate VLDL secretion. More detailed studies are necessary to elucidate the precise mechanisms by which PGC-1α decreases TAG secretion. Interestingly, because exercise increases PGC-1α expression, PGC-1α may play a critical role in the ability of exercise to lower TAG secretion.
In conclusion, these data demonstrate that an elevation in hepatic PGC-1α expression results in increased mitochondrial content and/or function, increased complete FAO and TCA cycle flux, and reduced hepatic TAG content and secretion. Importantly, in the primary hepatocytes, this increase in mitochondrial content and FAO was sufficient to prevent cellular TAG accumulation following FFA treatment of the cells. These data provide insight into the role of PGC-1α expression and mitochondrial content and function in regulation of hepatocyte lipid metabolism and into how liver mitochondria influence the development of, and potentially protection from, excessive TAG accumulation and secretion.
GRANTS
This work was partially supported by National Institutes of Health Grants DK-068210 (J. A. Ibdah), DK-088940 (J. P. Thyfault), and 5T32 AR-48523-8 (E. M. Morris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.M.M. is responsible for conception and design of the research; E.M.M. and G.M.E.M. performed the experiments; E.M.M. and J.A.I. analyzed the data; E.M.M., F.W.B., K.L.F., C.D.H., J.P.T., and J.A.I. interpreted the results of the experiments; E.M.M. prepared the figures; E.M.M. drafted the manuscript; E.M.M., J.P.T., and J.A.I. edited and revised the manuscript; E.M.M. and J.A.I. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Deborah Muoio (Duke University) for the kind gift of the PGC-1α and β-gal adenovirus and Dorothy Slentz for technical assistance.
REFERENCES
- 1. Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adiels M, Olofsson SO, Taskinen MR, Boren J. Diabetic dyslipidaemia. Curr Opin Lipidol 17: 238–246, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 28: 1225–1236, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Aharoni-Simon M, Hann-Obercyger M, Pen S, Madar Z, Tirosh O. Fatty liver is associated with impaired activity of PPARγ coactivator 1α (PGC1α) and mitochondrial biogenesis in mice. Lab Invest 91: 1018–1028, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Cano A, Ciaffoni F, Safwat GM, Aspichueta P, Ochoa B, Bravo E, Botham KM. Hepatic VLDL assembly is disturbed in a rat model of nonalcoholic fatty liver disease: is there a role for dietary coenzyme Q? J Appl Physiol 107: 707–717, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Croce MA, Eagon JC, LaRiviere LL, Korenblat KM, Klein S, Finck BN. Hepatic lipin 1β expression is diminished in insulin-resistant obese subjects and is reactivated by marked weight loss. Diabetes 56: 2395–2399, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-γ coactivator-1α expression. Diabetes 58: 1499–1508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 10. Gugen-Guillouzo C. Isolation and culture of animal and human hepatocytes. In: Culture of Epithelial Cells, edited by Freshney RI, Freshney MG. New York: Wiley-Liss, 2002, p. 337–379 [Google Scholar]
- 11. Haase TN, Ringholm S, Leick L, Bienso RS, Kiilerich K, Johansen S, Nielsen MM, Wojtaszewski JF, Hidalgo J, Pedersen PA, Pilegaard H. Role of PGC-1α in exercise and fasting-induced adaptations in mouse liver. Am J Physiol Regul Integr Comp Physiol 301: R1501–R1509, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol 59 Suppl 7: 5–18, 2008 [PubMed] [Google Scholar]
- 13. Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology 128: 1381–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Ide T, Ontko JA. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem 256: 10247–10255, 1981 [PubMed] [Google Scholar]
- 15. Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci 63: 1393–1409, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol 106: 161–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Moon BC, Hernandez-Ono A, Stiles B, Wu H, Ginsberg HN. Apolipoprotein B secretion is regulated by hepatic triglyceride, and not insulin, in a model of increased hepatic insulin signaling. Arterioscler Thromb Vasc Biol 32: 236–246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris EM, Rector RS, Thyfault JP, Ibdah JA. Mitochondria and redox signaling in steatohepatitis. Antioxid Redox Signal 15: 485–504, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA, Huff MW. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 58: 2198–2210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulvihill EE, Assini JM, Lee JK, Allister EM, Sutherland BG, Koppes JB, Sawyez CG, Edwards JY, Telford DE, Charbonneau A, St-Pierre P, Marette A, Huff MW. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 60: 1446–1457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta 1801: 281–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL. Peroxisome proliferator-activated receptor-α regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 51: 901–909, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem 282: 14807–14815, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicklas JA, Brooks EM, Hunter TC, Single R, Branda RF. Development of a quantitative PCR (TaqMan) assay for relative mitochondrial DNA copy number and the common mitochondrial DNA deletion in the rat. Environ Mol Mutagen 44: 313–320, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Orellana-Gavalda JM, Herrero L, Malandrino MI, Paneda A, Sol Rodriguez-Pena M, Petry H, Asins G, Van Deventer S, Hegardt FG, Serra D. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation. Hepatology 53: 821–832, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Rector RS, Ibdah JA. Fatty acid oxidation disorders: maternal health and neonatal outcomes. Semin Fetal Neonatal Med 15: 122–128, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Ruiz-Ramirez A, Chavez-Salgado M, Peneda-Flores JA, Zapata E, Masso F, El-Hafidi M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am J Physiol Endocrinol Metab 301: E1198–E1207, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem Cell Biol 88: 251–267, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Salter AM, Wiggins D, Sessions VA, Gibbons GF. The intracellular triacylglycerol/fatty acid cycle: a comparison of its activity in hepatocytes which secrete exclusively apolipoprotein (apo) B100 very-low-density lipoprotein (VLDL) and in those which secrete predominantly apoB48 VLDL. Biochem J 332: 667–672, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sorensen LP, Andersen IR, Sondergaard E, Gormsen LC, Schmitz O, Christiansen JS, Nielsen S. Basal and insulin mediated VLDL-triglyceride kinetics in type 2 diabetic men. Diabetes 60: 88–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srere PA. Citrate synthase. Methods Enzymol 13: 3–11, 1969 [Google Scholar]
- 38. Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O'Doherty RM. A moderate increase in carnitine palmitoyltransferase-1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab 294: E969–E977, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 19: 291–302, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]