Abstract
Background
Previous studies suggest center volume is associated with outcome after the Norwood operation; however the impact of surgeon volume is less clear. We evaluated the relative impact of surgeon and center volume on mortality in a large Norwood cohort.
Methods
Patients in the Society of Thoracic Surgeons Congenital Heart Surgery Database undergoing the Norwood operation (2000–2009) were included. Using multivariable logistic regression, we evaluated the relationship between in-hospital mortality and annual center and surgeon volume adjusting for patient factors.
Results
2555 patients were operated on at 53 centers by 111 surgeons. Overall unadjusted mortality was 22.1%. When analyzed individually, both lower center and surgeon volume were associated with higher mortality [OR for centers with 0–10 vs. >20 cases/yr 1.56 (95% CI 1.05–2.31); OR for surgeons with 0–5 vs. >10 cases/yr 1.60 (95%CI 1.12–2.27)]. When analyzed together, the addition of surgeon volume to the center volume models attenuated but did not completely mitigate the association of center volume with outcome (relative attenuation of OR=34%). Adjusted mortality rates in low, medium, and high volume centers were 25.6%, 22.3%, and 17.7%, respectively. Across all center volume strata, lower volume surgeons had higher adjusted mortality rates.
Conclusions
Both center and surgeon volume appear to influence Norwood outcomes. These data suggest outcomes may potentially be improved through strategies that take advantage of the positive influence of both of these variables. This could include further investigation into the feasibility of regional collaborations, and the development of quality improvement initiatives within and across centers.
Keywords: CHD, Norwood, Outcomes
Introduction
Previous studies have shown that higher center volume is associated with improved outcome in patients undergoing congenital heart surgery, particularly for higher complexity cases [1–8]. This association has also been demonstrated in studies specifically evaluating patients undergoing the Norwood operation [6–8]. Little is known regarding the mechanisms underlying this association. It may be that higher volume centers have more resources dedicated to caring for these complex infants. On the other hand, it has been hypothesized for a variety of cardiac operations that outcome may be dependent not only on the resources available at each center but on how well the operation itself is performed, or the experience of the surgeon, and that higher volume centers may have more experienced or higher volume surgeons [9–12]. For example, Birkmeyer et al. used Medicare data to evaluate 474,108 adults undergoing surgery from 1998–1999, and found that surgeon volume accounted for a large proportion, or in some cases all, of the apparent effect of center volume (100% of the effect for aortic valve replacement, and 49% of the effect for coronary artery bypass grafting) [12]. The authors concluded that the observed associations between center volume and mortality were largely mediated by surgeon volume [12].
The relationship between center and surgeon volume and outcome for patients undergoing the Norwood operation is unclear. Previous studies have reported conflicting results, and have been limited by the use of administrative data and methodology used for calculating volume [6,13]. Thus, the purpose of this study was to evaluate the relative impact of surgeon and center volume on in-hospital mortality in a multicenter cohort of infants undergoing the Norwood operation utilizing a large clinical registry.
Patients and Methods
Data Source
The Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database contains information on >190,000 children undergoing heart surgery in North America since 1998 and represents ~80% of all US centers performing congenital heart surgery [14]. Data collected include peri-operative, operative, and outcomes data on all children undergoing heart surgery at participating centers. Data quality and reliability are evaluated through intrinsic verification of data (e.g. identification and correction of missing/out of range values and inconsistencies in values across fields), and a formal process of site visits and data audits conducted by a panel of independent data quality personnel and pediatric cardiac surgeons at 6 randomly chosen institutions annually [15, 16]. The Duke Clinical Research Institute serves as the data warehouse for the STS Databases. This study was approved by the Duke University Institutional Review Board with waiver of consent, and by the STS Access and Publications Committee.
Study Population
As described previously, a total of 78 US centers submitted data on at least one Norwood operation to the database from 2000–2009 [8]. Centers performing <5 Norwood operations during the entire 10-year study period (n=9), and centers with >15% missing data for any study variable (n=16) were excluded, leaving 53 centers available for analysis. While the STS Database contains nearly complete data for the standard core data fields required to calculate in-hospital mortality, not all centers submit complete data for the other variables in the STS Database. Therefore it is standard practice to exclude centers with >15% missing data for key study variables, in order to maximize data integrity and minimize missing data. We also excluded 1 surgeon (n=2 patients) who submitted less than 3 consecutive months of data during the study period. From the included centers/surgeons, patients with missing data on preoperative factors or mortality (n=32 patients) were excluded. Of note, overall in-hospital mortality was similar in the included cohort vs. all patients with available mortality data undergoing the Norwood operation in the STS Database during this time period (22% vs. 21%).
Data collection
Data collected included patient demographics and cardiac diagnosis. All patients undergoing the Norwood operation were included in the study regardless of underlying anatomy, and characterized by type of single ventricle: right dominant, left dominant, or undifferentiated [17]. Data were also collected on other secondary lesions such as total anomalous pulmonary venous return, along with the presence of any non-cardiac/genetic abnormality, pre-operative length of stay, and other pre-operative factors including mechanical ventilatory or circulatory support, shock, arrhythmia, and neurologic deficit as defined in the STS Database [18]. Center and surgeon average annual Norwood volume were also collected. In-hospital mortality following the Norwood operation, regardless of any subsequent operations performed during the hospitalization, was the primary outcome.
Analysis
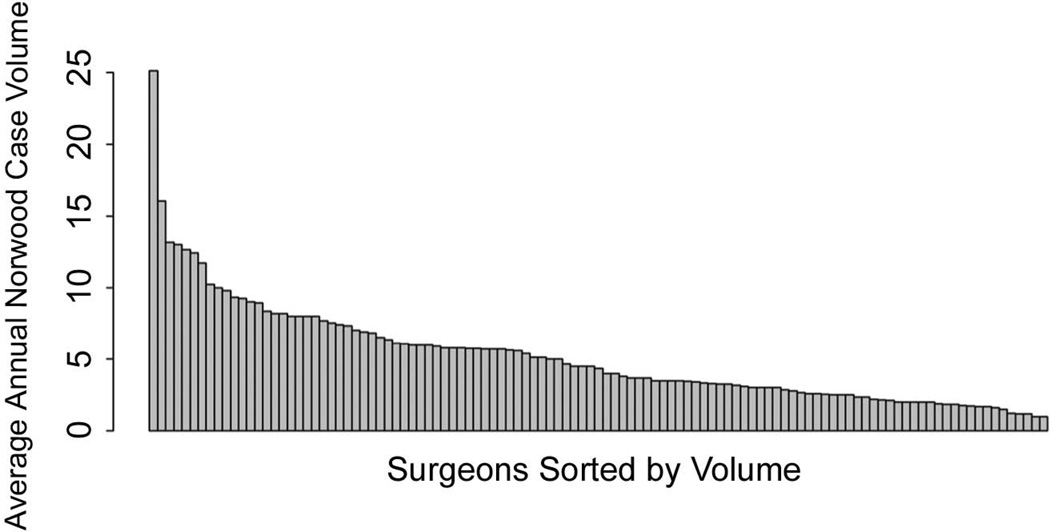
Study variables were described using standard summary statistics. Missing data were rare; pre-operative non-cardiac/genetic abnormality was the only variable with missing data (0.7%). For this variable, missing was imputed to none. Center and surgeon average annual Norwood volumes were analyzed both as continuous and categorical variables. Volume was categorized for descriptive purposes based on the distribution of the data. Center volume was categorized as 0–10, 11–20, and >20 cases/year. Surgeon volume was categorized as 0–5, 6–10, and >10 cases/year. The overall distribution of surgeon volume was plotted; the distribution of center volume in this patient population has been previously published [8]. Patient characteristics overall and across surgeon volume groups were described. Patient characteristics across the center volume groups have been previously published [8]. Multivariable logistic regression was utilized to evaluate the association of center and surgeon volume with in-hospital mortality adjusted for patient characteristics. Volume was analyzed both as a continuous (log-transformed) and categorical (as defined above) variable. The method of generalized estimating equations (GEE) was utilized to account for correlation between outcomes of patients at the same center. All models were adjusted for year of surgery, age, weight, sex, dominant ventricle, diagnosis of total anomalous pulmonary venous return, preoperative length of stay, the presence of any non-cardiac/genetic abnormality, and pre-operative shock, mechanical ventilatory or circulatory support, arrhythmia, or neurologic deficit. The impact of center and surgeon volume on outcome was first examined individually. Adjusted odds ratios and 95% confidence intervals are presented. To examine the relationship between center and surgeon volume, we then fit models including both volume variables and compared them to the models including only center or surgeon volume alone. The relative attenuation of the odds ratio for either center or surgeon volume (once the other volume variable was added into the model) was calculated using a previously described formula: (ORC-ORCS)/(ORC-1), where ORC is the odds ratio for mortality with a given center volume without consideration of surgeon volume, and ORCS is the odds ratio for mortality with a given center volume after adjustment for surgeon volume [12]. All models were adjusted for the patient characteristics listed above, and appeared well calibrated with a good match between observed and expected deaths across deciles of risk.
In addition, adjusted mortality rates in each center and surgeon volume group were calculated as: (observed mortality rate/expected mortality rate) * (overall sample observed mortality rate). The expected mortality rate for each group was obtained from the regression models noted above which were adjusted for the noted patient characteristics. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). A p-value <0.05 was considered statistically significant.
Results
Patient, center, and surgeon characteristics
A total of 111 surgeons from 53 centers performed 2555 Norwood operations during the study period. The median annual Norwood case volume was 4.3 cases/surgeon/year (interquartile range: 2.6–6.8). Overall, 55.9% of the 111 included surgeons performed 0–5 Norwood cases/year, 36.9% performed 6–10 Norwood cases/year, and 7.2% performed >10 Norwood cases/year. Regarding center volume, 64.2% of centers performed 0–10 Norwood cases/year, 24.5% performed 11–20 cases/year, and 11.3% performed >20 cases/year. The 53 included centers were diverse geographically (45.3% South, 26.4% Midwest, 17.0% West, 11.3% Northeast). Patient characteristics across surgeon volume categories are summarized in Table 1.
Table 1.
Study population characteristics
| Surgeon Volume (Norwood cases/year) | ||||
|---|---|---|---|---|
| Overall | 0–5 | 6–10 | >10 | |
| (n=2555) | (n=571) | (n=1285) | (n=699) | |
| Age, days | 6 (4–9) | 6 (4–9) | 6 (4–9) | 5 (3–8) |
| Weight, kg | 3.18 (2.80–3.50) | 3.16 (2.80–3.50) | 3.17 (2.80–3.50) | 3.20 (2.80–3.50) |
| Weight <2.5 kg | 247 (9.7%) | 60 (10.5%) | 106 (8.3%) | 81 (11.6%) |
| Gender, male | 1486 (58.2%) | 345 (60.4%) | 733 (57.0%) | 408 (58.4%) |
| Non-cardiac/genetic abnormality | 508 (19.9%) | 132 (23.1%) | 252 (19.6%) | 124 (17.7%) |
| Diagnosis | ||||
| Dominant ventricle | ||||
| Right | 2291 (89.6%) | 500 (87.5%) | 1147 (89.3 %) | 644 (92.1%) |
| Left | 203 (8.0%) | 54 (9.5%) | 107 (8.3%) | 42 (6.0%) |
| Undifferentiated | 61 (2.4%) | 17 (3.0%) | 31 (2.4%) | 13 (1.9%) |
| TAPVR | 33 (1.3%) | 6 (1.1%) | 20 (1.6%) | 7 (1.0%) |
| Other pre-operative factors | ||||
| Mechanical ventilatory support | 1020 (39.9%) | 200 (35.0%) | 454 (35.3%) | 366 (52.4%) |
| Mechanical circulatory support | 20 (0.8%) | 4 (0.7%) | 10 (0.8%) | 6 (0.9%) |
| Shock | 170 (6.7%) | 49 (8.6%) | 86 (6.7%) | 35 (5.0%) |
| Arrhythmia | 66 (2.6%) | 12 (2.1%) | 28 (2.2%) | 26 (3.7%) |
| Neurologic deficit | 33 (1.3%) | 9 (1.6%) | 17 (1.3%) | 7 (1.0%) |
Data are displayed as frequencies and percentages or median and interquartile range.
TAPVR = total anomalous pulmonary venous return, LOS = length of stay
Post-operative outcomes
Overall unadjusted mortality was 22.1%. Evaluation of the impact of center volume alone on outcome in multivariable analysis adjusted for patient characteristics (but without adjustment for surgeon volume), showed that lower center volume was associated with higher in-hospital mortality (p=0.03 when volume analyzed as a continuous variable; odds ratio for centers with 0–10 vs. >20 cases/year = 1.56, 95% CI 1.05–2.31, p=0.03; Table 2). When surgeon volume was examined alone (without adjustment for center volume) in multivariable analysis, lower surgeon volume was also associated with higher in-hospital mortality (p=0.02 when volume analyzed as a continuous variable, odds ratio for surgeons with 0–5 vs. >10 cases/year = 1.60, 95% CI 1.12–2.27, p=0.01; Table 2).
Table 2.
Adjusted odds ratios for in-hospital mortality associated with center and surgeon volume
| CENTER VOLUME |
Adjusted OR (95% CI) |
p-value | Adjusted OR with surgeon volume added to model |
p-value | Relative attenuation of odds ratio |
|---|---|---|---|---|---|
| 0–10 cases/year | 1.56 (1.05–2.31) |
0.03 | 1.37 (0.92–2.05) |
0.12 | 34% |
| 11–20 cases/year | 1.28 (0.83–1.99) |
0.26 | 1.20 (0.80–1.82) |
0.38 | -* |
| >20 cases/year | Reference | ||||
|
SURGEON VOLUME |
Adjusted OR (95% CI) |
p-value |
Adjusted OR with center volume added to model |
p-value |
Relative attenuation of odds ratio |
| 0–5 cases/year | 1.60 (1.12–2.27) |
0.01 | 1.47 (1.01–2.15) |
0.05 | 21% |
| 6–10 cases/year | 1.33 (0.94–1.87) |
0.11 | 1.26 (0.88–1.78) |
0.20 | -** |
| >10 cases/year | Reference | ||||
Results from models with center or surgeon volume entered individually, and those with both volume variables entered in, are displayed. The last column displays the relative attenuation in the odds ratio for center or surgeon volume when the other volume variable is entered into the models.
NA, the effect of center volume alone was not statistically significant.
NA, the effect of surgeon volume alone was not statistically significant.
When both center and surgeon volume were entered into the models, the impact of center volume on in-hospital mortality (with adjustment for surgeon volume) was attenuated, but not completely mitigated (relative attenuation in odds ratio = 34%). Conversely, similar results were found when center volume was added into the surgeon volume models. The impact of surgeon volume on outcome (with adjustment for center volume) was lessened but not also completely mitigated (relative attenuation in odds ratio = 21%).
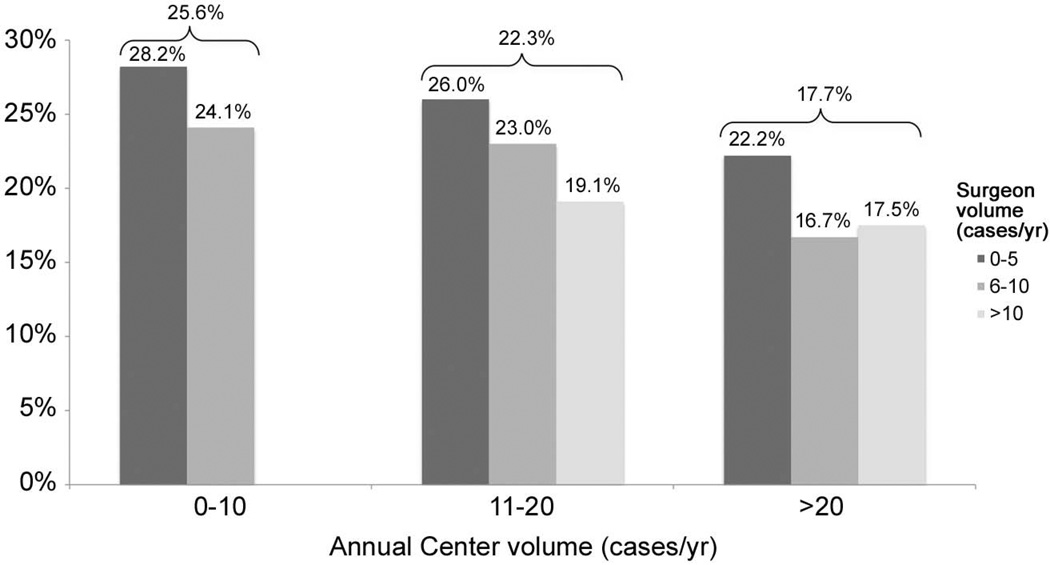
Adjusted mortality rates were also examined across center and surgeon volume categories (Figure 2). The overall relationship between lower center volume and higher in-hospital mortality is apparent. In addition, within each of the center volume strata, there is an association of surgeon volume with outcome, such that lower volume surgeons had higher adjusted in-hospital mortality rates across low, medium, and high volume centers. Formal interaction testing yielded nonsignificant results in all cases such that the impact of surgeon volume on outcome did not statistically differ across the three center volume categories.
Figure 2.
Adjusted mortality rates associated with center and surgeon volume.
Overall adjusted mortality rates for each center volume category are shown above the brackets. Note, there were no high volume surgeons in the low center volume group; therefore no data are displayed for this group.
Comment
This analysis suggests that both center and surgeon volume are significantly associated with outcome following the Norwood operation. Several previous studies have demonstrated variation in outcome across institutions, and the association between center volume and outcome in children undergoing heart surgery, including patients undergoing the Norwood operation [1–8, 16]. However, few studies have evaluated the impact of surgeon volume on outcome, or the relationship between center and surgeon volume. In adult cardiac surgery, it has been reported that surgeon volume accounts for a large proportion of the effect of center volume, and it has been suggested that patients can improve their chances of survival by selecting not only a high-volume center, but also a surgeon who performs the operation frequently [9–10,12]. In pediatric cardiac surgery, a previous analysis of a large administrative database of 801 neonates with hypoplastic left heart syndrome found that center, but not surgeon, volume was significantly associated with outcome [6]. However, there are limitations to using administrative data, which rely upon International Classification of Diseases, 9th revision (ICD-9) diagnosis and procedure codes. There is no ICD-9 code for the Norwood operation, such that a combination of other codes must be used, the accuracy of which is unknown. One other previous study analyzed neonates enrolled in a Congenital Heart Surgeons Society (CHSS) study, and found that neither center nor surgeon volume were associated with Norwood outcome [13]. However, volume estimates in the CHSS analysis were based on the number of patients from each center voluntarily enrolled in a cohort of patients with aortic atresia or stenosis undergoing the Norwood operation, rather than the overall number of patients at each center undergoing the Norwood operation [13].
In contrast, the present study evaluated all patients undergoing the Norwood operation at centers participating in a large clinical registry. Our results suggest that both center and surgeon volume are significantly associated with outcome. Interestingly, we found that while surgeon volume does appear to play a roll, the magnitude of this effect (relative to that associated with center volume) was smaller compared to what has been reported previously in adult cardiac surgery [12]. It has been hypothesized that for the Norwood operation, center-level variables impacting the ability to care for these complex infants both in regard to their pre-and post-operative cardiovascular physiology, as well as other common non-cardiac issues, may have an important impact on outcome in addition to technical issues pertaining to the surgery itself [13].
The results of our analysis suggest that outcomes may potentially be improved through strategies that take advantage of the positive influence of both center and surgeon volume on outcome. In some European countries, data such as these have been used to support regionalization of care to high volume or high performing centers [19]. For example, following regionalization of care in Sweden there was a significant reduction in 30-day mortality following congenital heart surgery from 9.5% to 1.9% [19]. In the US, a model based on affiliation of low volume programs with a larger academic program within the region, including program oversight and direct involvement of the senior surgeon from the larger program, and referral of high-complexity cases such as Norwood operations to the high volume program, has been shown to be associated with low overall mortality [20]. However, it remains under debate whether regionalization or selective referral is feasible or desirable on a more widespread basis in the US.
Other strategies may include the development and implementation of quality improvement initiatives, both within individual centers and across institutions, with the aim of reducing variation in care and improving outcome. Few studies have evaluated center or surgeon characteristics which may be subsequently targeted in such initiatives. In regard to surgeon characteristics, Bacha and colleagues designed a measure of surgical technical performance and showed that this can be an important variable impacting outcome following the Norwood operation [11]. However, variables influencing surgeon technical performance have not been elucidated. Our group and other investigators have previously evaluated center characteristics such as intensive care unit (ICU) structure, and found that the presence of a dedicated pediatric cardiac ICU vs. a general pediatric ICU did not appear to influence outcome [21,22]. Further study of other factors beyond structure alone is needed, such as training and availability of personnel, composition of the care team, use of standardized management protocols, and mechanisms to improve timely recognition and treatment of complications [23–26]. While data in the field of pediatric heart surgery are limited, Prager and colleagues have reported on recent quality improvement activities in adult cardiac surgery involving the Michigan Society of Thoracic and Cardiovascular Surgeons quality collaborative [27]. This group is composed of all adult cardiac surgery programs in Michigan and meets regularly to evaluate variation in practice and program outcomes, facilitate sharing of knowledge across sites, and encourage adoption of practices utilized by high performing sites. This initiative has been successful in reducing variation in care, improving outcome, and reducing costs [27]. In addition to multi-center quality improvement collaborations, our data also suggest that initiatives within individual institutions may also be useful. We found a wide range of surgeon volume across institutions, and a consistent association of low surgeon volume with higher mortality rates in all center volume categories. Thus, initiatives supporting enhanced mentoring by the senior or high-volume surgeon, or establishing a constant surgeon of record for Norwood operations, could be two potential mechanisms that may reduce variation and improve outcome.
Limitations
The limitations of this study are primarily related to the nature of the STS Database. While it is the largest pediatric heart surgery registry in North America, not all US centers participate. In addition while data for core data fields are nearly complete, not all centers submit complete data for all variables captured by the Database, and thus, are not included in the analysis. Results may therefore not be generalizable to all US centers or surgeons. We included all patients undergoing the Norwood operation regardless of underlying anatomy; this may lead to higher Norwood case volumes compared to studies limited to specific anatomic diagnoses. Regarding surgeon volume, there may be several other ways to characterize surgeon “experience” beyond volume alone which require further study.
In our adjustment for patient risk factors, certain variables are not captured in the database or had too much incomplete data to be analyzed. These included gestational age, the anatomic subtype of hypoplastic left heart syndrome, size of the ascending aorta, presence of a restrictive atrial septum, and source of pulmonary blood flow (modified Blalock-Tausig shunt vs. right ventricle-to-pulmonary artery conduit). However, a recent report from the Single Ventricle Reconstruction trial suggested that none of these variables were significant risk factors for inhospital mortality [28]. Nonetheless, we did attempt to account for these potential risk factors through adjusting for several variables that are available in the Database (and likely related to the above factors), including weight at surgery, pre-operative shock and mechanical ventilation, and year of surgery (with use of the right ventricle-to-pulmonary artery conduit becoming more prevalent in recent years). Previous analyses have shown that we are able to stratify patient pre-operative risk using these variables [8]. Finally, the Database currently does not capture information regarding personnel, or hospital structural or process measures. We were therefore not able to evaluate the relative contribution of these factors to the association between volume and outcome.
Conclusions
This analysis suggests that outcomes following the Norwood operation may potentially be improved through strategies that take advantage of the positive influence of higher center and surgeon volume on outcome. This could include further investigation into the feasibility and efficacy of regional collaboration and centralization of care for complex case, and/or the development of quality improvement initiatives within and across centers to reduce variation and improve quality of care and outcomes.
Figure 1.
Distribution of surgeon average annual Norwood case volume
Acknowledgments
This study was supported by an American Heart Association Mid-Atlantic Affiliate Clinical Research Award (PI: Pasquali), and Thrasher Research Fund Early Career Award (PI: Hornik).
Dr. Pasquali: Grant support, National Heart, Lung, and Blood Institute (1K08HL103631-01).
Dr. J Jacobs: Chair, STS Congenital Heart Surgery Database Task Force.
Dr. Peterson: Principal Investigator, STS National Databases Analytic Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welke KF, O’Brien SM, Peterson ED, et al. The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. J Thorac Cardiovasc Surg. 2009;137:1133–1140. doi: 10.1016/j.jtcvs.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins KJ, Newburger JW, Lock JE, Davis RB, Coffman GA, Iezzoni LI. In-hospital mortality for surgical repair of congenital heart defects: preliminary observations of variation by hospital caseload. Pediatrics. 1995;95:323–330. [PubMed] [Google Scholar]
- 3.Hannan EL, Racz M, Kavey R, et al. Pediatric cardiac surgery: the effect of hospital and surgeon volume on in-hospital mortality. Pediatrics. 1998;101:963–969. doi: 10.1542/peds.101.6.963. [DOI] [PubMed] [Google Scholar]
- 4.Bazzani LG, Marcin JP. Case volume and mortality in pediatric cardiac surgery patients in California, 1998–2003. Circulation. 2007;115:2652–2659. doi: 10.1161/CIRCULATIONAHA.106.678904. [DOI] [PubMed] [Google Scholar]
- 5.Welke KF, Diggs BS, Karamlou T, Ungerleider RM. The relationship between hospital surgical case volumes and mortality rates in pediatric cardiac surgery: a national sample, 1988–2005. Ann Thorac Surgery. 2008;89:889–896. doi: 10.1016/j.athoracsur.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 6.Checchia PA, McCollegan M, Daher N, et al. The effect of surgical case volume on outcome after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;129:754–759. doi: 10.1016/j.jtcvs.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch JC, Gurney JG, Donohue JE, et al. Hospital mortality for Norwood and Arterial Switch Operations as a function of institution volume. Pediatr Cardiol. 2008;29:713–717. doi: 10.1007/s00246-007-9171-2. [DOI] [PubMed] [Google Scholar]
- 8.Pasquali SK, Jacobs JP, He X, et al. The Complex Relationship between Center Volume and Outcome in Patients Undergoing the Norwood Operation. Ann Thorac Surg. doi: 10.1016/j.athoracsur.2011.07.081. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C, Hannan EL, Ryan TJ, et al. Is the impact of hospital and surgeon volumes on the in-hospital mortality rate for coronary artery bypass graft surgery limited to patients at high risk? Circulation. 2004;110:784–789. doi: 10.1161/01.CIR.0000138744.13516.B5. [DOI] [PubMed] [Google Scholar]
- 10.McPhee JT, Robinson WP, Eslami MH, et al. Surgeon case volume, not institution case volume, is the primary determinant of in-hospital mortality after elective open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:591–599. doi: 10.1016/j.jvs.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 11.Karamichalis JM, Thiagarajan RR, Liu H, et al. Stage 1 Norwood: optimal technical performance improves outcomes irrespective of preoperative physiologic status or case complexity. J Thorac Cardiovasc Surg. 2010;139:962–968. doi: 10.1016/j.jtcvs.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon Volume and Operative Mortality in the United States. NEJM. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 13.Karamlou T, McCrindle BW, Blackstone EH, et al. Lesion-specific outcomes in neonates undergoing congenital heart surgery are related predominantly to patient and management factors rather than institution or surgeon experience: A Congenital Heart Surgeons Society Study. J Thorac Cardiovasc Surg. 2010;139:569–577. doi: 10.1016/j.jtcvs.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs ML, Mavroudis C, Jacobs JP, et al. Report of the 2005 STS Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2006;82:1152–1158. doi: 10.1016/j.athoracsur.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Clarke DR, Breen LS, Jacobs ML, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;18(Suppl2):177–187. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JP, O'Brien SM, Pasquali SK, et al. Variation in outcomes for benchmark operations: An analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. doi: 10.1016/j.athoracsur.2011.06.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs JP, O'Brien SM, Chai PJ, Morell VO, Lindberg HL, Quintessenza JA. Management of 239 patients with hypoplastic left heart syndrome and related malformations from 1993 to 2007. Ann Thorac Surg. 2008;85:1691–1696. doi: 10.1016/j.athoracsur.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed 10/20/2011];STS Congenital Database Full Specifications. http://www.sts.org/documents/pdf/Congenital_DataSpecs_250.pdf.
- 19.Lundstrom NR, Berggren H, Bjorkhem G, Jogi P, Sunnegardh J. Centralization of pediatric heart surgery in Sweden. Pediatr Cardiol. 2000;21:353–357. doi: 10.1007/s002460010079. [DOI] [PubMed] [Google Scholar]
- 20.Mainwaring RD, Reddy VM, Reinhartz O, et al. Outcome analysis for a small, start-up congenital heart surgery program. J Card Surg. 2008;23:622–626. doi: 10.1111/j.1540-8191.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 21.Burstein DS, Jacobs JP, Li JS, et al. Care models and associated outcomes in congenital heart surgery. Pediatrics. 2011;127:1482–1489. doi: 10.1542/peds.2010-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheurer M, Gauvreau K, Laussen PC, Mayer JE, Atz AM, Newburger JW. The impact of a designated cardiac intensive care unit on outcomes after the Norwood procedure. J Am Coll Cardiol. 2011;57:E405. [Google Scholar]
- 23.Goh AY, Lum LC, Abdel-Latif ME. Impact of 24 hour critical care physician staffing on case-mix adjusted mortality in paediatric intensive care. Lancet. 2001;357:445–446. doi: 10.1016/S0140-6736(00)04014-9. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan C, Sachdeva R, Morrow WR, et al. Standardized management improves outcomes after the Norwood procedure. Congenit Heart Dis. 2009;4:329–337. doi: 10.1111/j.1747-0803.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Stukel TA, Siewers AE, et al. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 26.Pasquali SK, Li JS, Burstein DB, et al. The association of center volume with mortality and complications in pediatric heart surgery. Pediatrics. 2012 Jan 9; doi: 10.1542/peds.2011-1188. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prager RL, Armenti FR, Bassett JS, et al. Cardiac Surgeons and the Quality Movement: the Michigan Experience. Semin Thorac Cardiovasc Surg. 2009;21:20–27. doi: 10.1053/j.semtcvs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Tabbutt S, Ghanayem N, Cooper DS, et al. Risk factors for hospital mortality and morbidity following the Norwood procedure: Results from the multicenter Single Ventricle Reconstruction Trial. Circulation. 2011;124:A8160. [Google Scholar]