Abstract
Statin drugs are prescribed primarily for their ability to lower cholesterol, but may also exert beneficial side effects unrelated to cholesterol metabolism. Previous work has described a “statin paradox,” where statin treatment decreased osteoblastic markers in valve myofibroblasts while increasing those same markers in preosteoblasts. However, valvular interstitial cells (VICs) themselves are a multipotent cell type, capable of differentiating into activated, myofibroblastic VICs (aVICs) and osteoblastic VICs (obVICs), motivating the question of whether the statin paradox can exist within an individual valve containing these phenotypically distinct VIC subpopulations. In the current study, a heterogeneous initial population of porcine VICs was differentiated into aVICs or obVICs and treated with simvastatin. Gene expression analysis was conducted daily over an 8-day time course to capture temporally dynamic changes in cell phenotype induced by statin treatment. These studies demonstrated that the two VIC populations, aVICs and obVICs, exhibited differential responses to statin treatment. Specifically, simvastatin increased the expression of osteoblastic markers in obVICs, but not in aVICs, while also suppressing the myofibroblastic phenotype in both aVICs and obVICs. These results indicate that the statin paradox can exist within the heterogeneous VIC population of an individual diseased valve and that statin efficacy in the context of calcific aortic valve disease (CAVD) may be dependent upon the cellular composition of the valve. These findings may have implications for clinical usage of statins, shedding light on how statin efficacy in CAVD may be dependent upon the disease stage or why some individuals exhibit better responsiveness to statin therapy.
Keywords: valvular interstitial cell, 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor, osteoblast, myofibroblast, gene expression
the mechanisms of calcific aortic valve disease (CAVD) are not well understood, but it is believed to be an active process mediated by the valvular interstitial cell (VIC), during which VICs transdifferentiate into either a myofibroblastic or an osteoblastic phenotype (termed aVICs and obVICs, respectively) (8, 15). Although both phenotypes contribute to valve disease, each is thought to play a distinct role in disease development (16, 19), and so it is possible that each individual subpopulation could be differentially affected by any potential treatment. Although there is currently no approved treatment for valve disease, it has been suggested that treatment with 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase inhibitors (statins) may have a beneficial effect (11). A retrospective clinical analysis has indicated that statin efficacy in treating CAVD may be stage dependent, with statin treatment having a greater effect on earlier stages of valve disease (1), but the reason for this phenomenon is not known, nor have the temporal dynamics of VIC response to statin drugs been investigated.
Relatively few studies have examined the statin-valve relationship on a cellular level (2, 12, 23, 24). One previous publication documented a “statin paradox,” wherein the response of a clonal population of porcine aortic valve myofibroblasts to statin consisted of a decrease in mineralization markers [namely, nodule counts and alkaline phosphatase (ALP) production], whereas murine osteoblast precursor cells responded to statin by increasing these same markers (23). This approach was important in understanding the varied systemic effects of statin drugs, but also motivates the question of whether such paradoxical effects of statins could be experienced within the context of the valve alone.
Specifically, within a single diseased valve, one may find both aVICs and obVICs (13, 15, 16); if the aforementioned paradox holds true for these phenotypically distinct VIC subpopulations, then there is a possibility that the obVICs will respond to statins in a manner similar to osteoblasts, wherein statin treatment increases markers of calcification, whereas the aVICs in the same valve may respond in the same manner as an aortic valve myofibroblast population, wherein calcification markers are decreased by statin treatment. Evaluation of whether the statin paradox exists within the valve itself will help us to determine whether statin efficacy is dependent upon valve composition, a finding that could have significant consequences with respect to understanding existing clinical data and evaluating potential treatment options.
Moreover, evaluating the time-dependent response of VICs to statin treatment is also likely to shed light on the temporally dynamic nature of the valvular response to statin, which has not previously been characterized. Although statin treatment of valve cells has been evaluated in other studies performed to investigate different hypotheses (2, 12, 24), one limiting factor in all of these studies is that they all have only examined the effects of statin treatment at a single time point, thereby limiting the obtainable information about the relationship between valves and statin treatment.
Thus, to investigate the existence and temporal dynamics of the statin paradox among subpopulations of cells derived from the same VIC culture, this study describes how simvastatin treatment of aVICs and obVICs derived from the same starting cell population impacts the expression of both myofibroblastic and osteoblastic markers across multiple time points. Although performed in vitro, this investigation of whether the cellular composition of the valve may influence its response to a potential therapeutic agent has clinical implications with respect to both interpretation of clinical findings and identification of future anticalcific strategies.
MATERIALS AND METHODS
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Raw data were analyzed in Kaleidagraph (Synergy Software, Reading, PA) via either ANOVA with a Tukey's honestly significant difference posttest or an unpaired Student's t-test, as appropriate, and P values <0.05 were considered statistically significant. All data are presented as means ± SD.
All tissues used in this study were acquired postmortem from a commercial slaughterhouse and were, therefore, not subject to institutional animal protocol approval. The slaughterhouse follows United States Department of Agriculture and Humane Slaughter Act guidelines for the care and slaughter of swine.
Cell Culture
VICs were isolated from porcine aortic valves (Hormel, Austin, MN) by collagenase digestion and cultured as previously described (6), in growth medium containing M199, 15% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (all HyClone, Logan, UT). To induce aVIC or obVIC phenotypes, starting VICs (P2-P4) were seeded onto tissue culture polystyrene plates at a density of 50,000 cells/cm2 and cultured in either reduced-serum medium containing M199, 1% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine or mineralization medium containing M199, 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 10 mM β-glycerophosphate, and 50 μg/ml ascorbic acid, respectively. These cultures were performed with or without 1 μM simvastatin (EMD Biosciences, Gibbstown, NJ) applied on day 1 and replenished every 48 h thereafter.
Immunocytochemistry
To assess the starting population of cells, VICs were expanded in growth medium before being transferred to 24-well plates for fixation and staining. Cells from this starting population were also cultured in either reduced-serum medium or mineralization medium for 5 days before fixation and staining. Cell cultures were fixed in 10% neutral buffered formalin and were assayed for either α-smooth muscle actin (α-SMA) or ALP via immunocytochemical detection. Monoclonal mouse anti-α-SMA (Clone 1A4, 7.5 μg/ml) was used to detect α-SMA, whereas monoclonal mouse anti-ALP (Clone AP-59, 1 μg/ml) was selected to detect ALP. Each primary antibody was used in conjunction with AlexaFluor 488-labeled goat anti-mouse secondary antibody (Invitrogen; 2 μg/ml), and the cells were counterstained with 4′-6 diamidino-2-phenylindole (DAPI; 1 μg/ml). Fluorescence measurements were acquired using a Synergy HT plate reader (Biotek, Winooski, VT) to detect the α-SMA signal (488/528 nm ex/em), which was normalized to DAPI fluorescence (360/460 nm ex/em). Photomicrographs of the stained cells were also acquired to examine organization of α-SMA stress fibers as well as the distribution of the ALP protein (Olympus IX51; Olympus, Center Valley, PA).
Flow Cytometry
α-SMA production was also analyzed via flow cytometry on subconfluent aVICs and obVICs cultured in the presence or absence of 1 μM simvastatin for 5 days. Briefly, cells were detached from culture plates using 0.05% trypsin, fixed in 4% paraformaldehyde, permeabilized in 0.05% Triton X-100, blocked in 1% bovine serum albumin in 0.1% Tween-20, and then incubated with monoclonal mouse anti-α-SMA (Clone 1A4, 5 μg/ml) or isotype control overnight. Alexa Fluor 488-conjugated goat anti-mouse antibody (2 μg/ml) was then applied for 45 min, followed by data acquisition on an Accuri C6 flow cytometer and data analysis using CFlow Plus software.
ALP Quantification
ALP was detected in the media of subconfluent VICs treated with either low-serum or mineralization medium. Aliquots of the media were combined with 1-step PNPP (p-nitrophenyl phosphate; Thermo Scientific, Waltham, MA), and the color development of the reaction was stopped after 30 min by addition of 2N NaOH. The absorbance readings (405 nm) of sample wells were measured and compared against wells containing standard solutions composed of calf intestinal alkaline phosphatase (Promega, Madison, WI). Values are reported as units of alkaline phosphatase activity normalized to DNA content of the lysed cell culture (as measured via PicoGreen assay; Invitrogen).
Proliferation Assay
VICs were cultured in either reduced-serum or mineralization medium for 72 h before proliferation was assessed using the Click-It EdU assay kit (Life Technologies, Grand Island, NY). EdU (5-ethynyl-2′-deoxyuridine; 10 μM) was added to subconfluent cultures 18 h before fixation and staining, and all cells were counterstained with DAPI (1 μg/ml). The number of proliferating and total cells in photomicrographs of three randomly chosen fields of view per well (n = 4 wells per condition) was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Quantitative PCR
VICs were cultured in either reduced-serum or mineralization medium for 8 days. On each day of the experiment, 4–6 wells per condition were harvested for quantitative PCR (qPCR) analysis via the addition of TRI Reagent. RNA from all conditions was isolated according to the TRI Reagent protocol and DNAse-treated with 1 μl Turbo DNAse (Ambion, Austin, TX). RNA (200 ng) from each sample was reverse-transcribed with the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA), with thermal cycling at 25°C for 10 min, 37°C for 120 min, 85°C for 5 min, ending with 4°C. The samples were analyzed via qPCR (StepOnePlus Real-Time PCR System; Applied Biosystems) using TaqMan gene expression assays (Applied Biosystems) customized to detect the following genes: α-SMA, ALP, and osteocalcin (OCN). The qPCR cycling conditions consisted of initial heating to 50°C for 2 min followed by 95°C for 10 min, and 40–42 cycles of denaturing at 95°C for 15 s followed by annealing at 60°C for 1 min. Data were analyzed using the ΔΔCT method (18). All samples were initially normalized to GAPDH and subsequently normalized to day 1 values of the control VIC condition.
RESULTS
Characterization of Starting, Myofibroblastic, and Osteoblastic VIC Populations
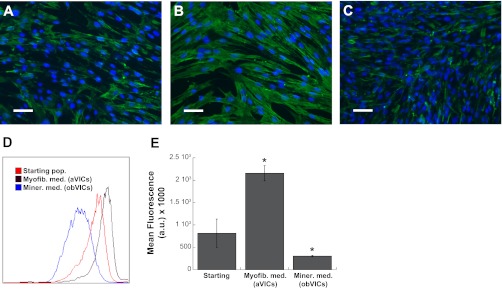
α-SMA protein organization and amount were assessed in VICs cultured in reduced serum or mineralization medium (Fig. 1). As evidenced by Fig. 1A, the starting VIC population (i.e., VICs that have been expanded in growth medium) was heterogeneous, with some cells staining positively for α-SMA, while other cells expressed little or no α-SMA protein. Figure 1B depicts the same stain in a culture of VICs that were cultured in myofibroblastic (reduced serum) conditions for 4 days, in which nearly all VICs stained positively for α-SMA, indicating a culture composed predominantly of myofibroblasts. α-SMA was also detected in VIC cultures treated with mineralization medium for 4 days (Fig. 1C), although its expression was found in a substantially decreased fraction of cells compared with control cultures. Analysis of samples via both immunocytochemistry and flow cytometry (Fig. 1, D and E) confirmed a mixed starting population (36.5% α-SMA-positive), an abundance of α-SMA expression (82.7% positive cells) in VICs cultured in reduced-serum conditions, and low α-SMA (7.4% of cells) in VICs cultured in mineralization medium.
Fig. 1.
α-Smooth muscle actin (α-SMA) protein expression by valvular interstitial cells (VICs) that have been cultured in growth medium (starting pop; A), low serum (myofibroblastic) medium (myofib med; B), and mineralization medium (miner med; C). Scale bar represents 50 μM. D: detection of α-SMA expression by starting VICs, VICs in myofibroblastic medium (aVICs), and VICs in mineralization medium [osteoblastic VICs (obVICs)] by flow cytometry, and mean fluorescence intensity of flow cytometry samples (E). *P < 0.0005 vs. starting population. AU, arbitrary units.
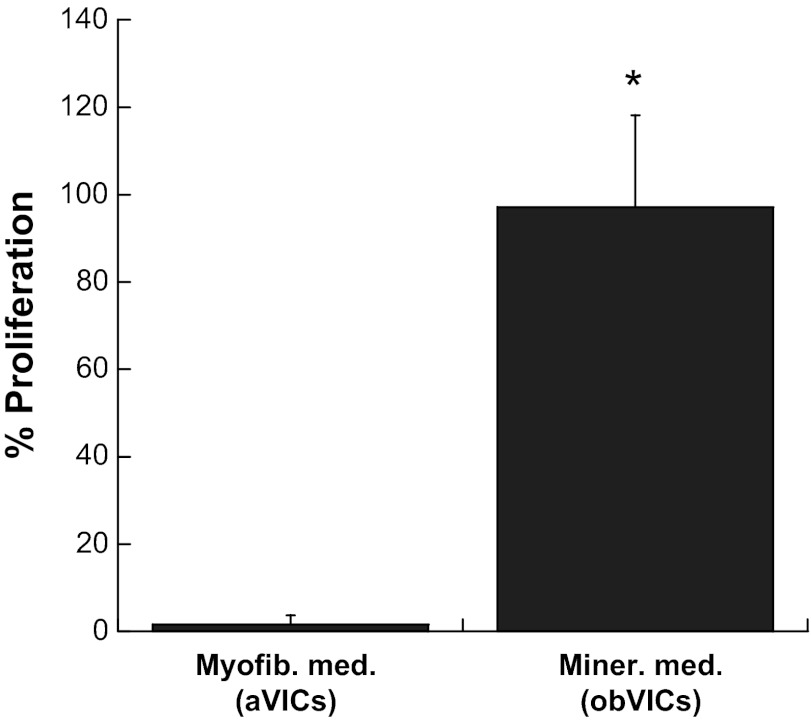
To confirm that the decreased expression of α-SMA in cultures treated with mineralization medium versus reduced-serum medium was due to a phenotypic switch rather than cellular quiescence, the fraction of proliferating cells was assessed in both cultures. These results are displayed in Fig. 2, in which nearly 100% of the cells treated with mineralization medium were found to be proliferating over an 18-h period, compared with less than 2% of the reduced-serum VICs, eliminating quiescence as a possibility for the mineralization medium-treated VICs. It should also be noted that this difference in proliferation is likely due to osteoblastic differentiation of the VICs, rather than differences in serum content of the media, as our previous work demonstrated a 2.5-fold increase in proliferation when comparing obVICs with VICs cultured in nonosteogenic medium containing the same amount (10%) of FBS (10).
Fig. 2.
Fraction of proliferating cells when VICs were cultured in either myofibroblastic medium (myofib med) or mineralization medium (miner med) conditions (aVICs and obVICs, respectively). *P < 0.0001 vs. aVICs.
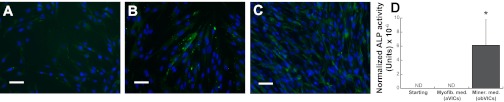
As anticipated, ALP staining of starting and reduced-serum VIC populations was weak (Fig. 3, A and B), whereas ALP production by cultures treated with mineralization medium was more robust (Fig. 3C). Quantification of ALP production in the VIC media confirmed this observation, as only VICs cultured in mineralization medium were found to produce detectable levels of ALP (Fig. 3D). Together with Figs. 1 and 2, these findings confirm that we commenced our experiments with a heterogeneous population of VICs that we then differentiate into two different subpopulations. These two subpopulations appear to be dominated by aVICs or obVICs upon treatment with reduced-serum or mineralization medium, respectively, and were subsequently used to investigate the “statin paradox.”
Fig. 3.
Alkaline phosphatase (ALP) protein expression in VICs that have been cultured in growth medium (starting pop; A), low serum (myofibroblastic) medium (myofib med; aVICs; B), and mineralization medium (miner med; obVICs; C). Scale bar represents 50 μM. D: quantification of ALP protein levels produced by the starting VIC population, VICs in myofibroblastic medium (aVICs), and VICs in mineralization medium (obVICs). ND, none detected. *P < 0.0001 vs. starting population.
Effect of Statin Treatment on aVICs Versus obVICs
α-SMA expression.
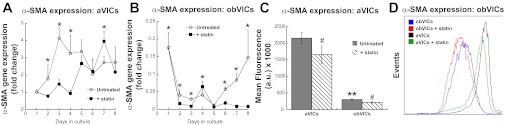
The most accepted marker of the myofibroblastic phenotype is α-SMA (22). Figure 4 shows the effect of statin treatment on α-SMA gene expression in VICs cultured in either myofibroblastic or osteogenic conditions (yielding aVICs or obVICs, respectively). Statin treatment caused a decrease in α-SMA gene expression by aVICs for the first 3 days of application (days 2–4; Fig. 4A), after which point statin treatment either had no effect or caused an increase in α-SMA expression in aVICs. The effect of statin treatment on VICs cultured in mineralization medium (obVICs) was similar to that observed in myofibroblastic conditions. Figure 4B shows decreased α-SMA expression throughout most of the 8-day time course, with the exception of days 4 and 5, in which statin treatment either had no effect or caused an increase in α-SMA expression. Despite the observation of similar patterns with statin treatment in both environments, the overall expression of α-SMA in mineralization medium was dramatically lower than the expression observed with nonosteogenic medium (<10% vs. >200% that of day 1 untreated control).
Fig. 4.
α-SMA gene expression by =aVICs (A) and obVICs (B) cultured with or without simvastatin. All values are expressed as fold-change relative to the day 1 aVIC condition. C: detection of α-SMA expression by aVICs and obVICs ± simvastatin by flow cytometry and quantification of mean fluorescence intensity in flow cytometry samples (D). *P < 0.05 compared with paired condition on same day, n = 3–6 per day per cell type; **P < 0.0001 compared with aVICs; #P < 0.01 compared with untreated condition in same media. AU, arbitrary units.
These gene expression results were confirmed by measurement of α-SMA protein in both untreated and statin-treated aVIC and obVIC samples via flow cytometry (Fig. 4, C and D). In the absence of statin treatment, the mean fluorescence intensity of α-SMA expression by aVICs was approximately sevenfold that found for obVICs. Statin treatment led to a slight, but statistically significant, downward shift in α-SMA production for both aVIC and obVIC populations.
ALP expression.
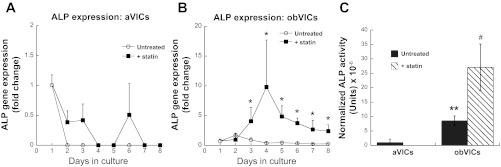
ALP is a commonly used osteoblastic marker generally accepted to signify early osteogenesis and bone formation (27). As seen in Fig. 5, culture conditions and statin treatment also effected differences in ALP expression. Detection of ALP gene expression in cultures of aVICs was challenging due to weak or absent signal (Fig. 5A). Upon statin treatment of aVICs, ALP expression was detected on only 3 days out of the 8-day experiment, and every detected CT was above 36 (i.e., very few copies were present). However, culture of VICs in mineralization medium yielded more consistent ALP expression compared with the control cultures (Fig. 5B). Furthermore, statin treatment of obVICs stimulated a sustained elevation of ALP gene expression over the untreated mineralization medium (Fig. 5B). For days 5–8, the statin-treated obVICs exhibited ALP expression that was ∼10-fold higher than that expressed by obVICs that did not receive statin, indicating a substantial increase in osteoblastic activity.
Fig. 5.
ALP gene expression by aVICs (A) and obVICs (B) cultured with or without simvastatin. All values are expressed as fold-change relative to the day 1 aVIC condition. C: detection of ALP activity in cell culture media of aVICs and obVICs ± simvastatin, normalized to DNA in lysate. *P < 0.02 compared with paired condition on same day; **P < 0.001 compared with aVICs; #P < 0.001 compared with untreated condition in same media.
Measurement of secreted ALP in the culture media confirmed these findings. ALP production by aVICs was negligible, and statin treatment did not increase these values (Fig. 5C). Meanwhile, obVICs exhibited significantly higher ALP production than aVICs, and this was further elevated (3-fold) by the addition of simvastatin to the culture medium (Fig. 5C).
OCN expression.
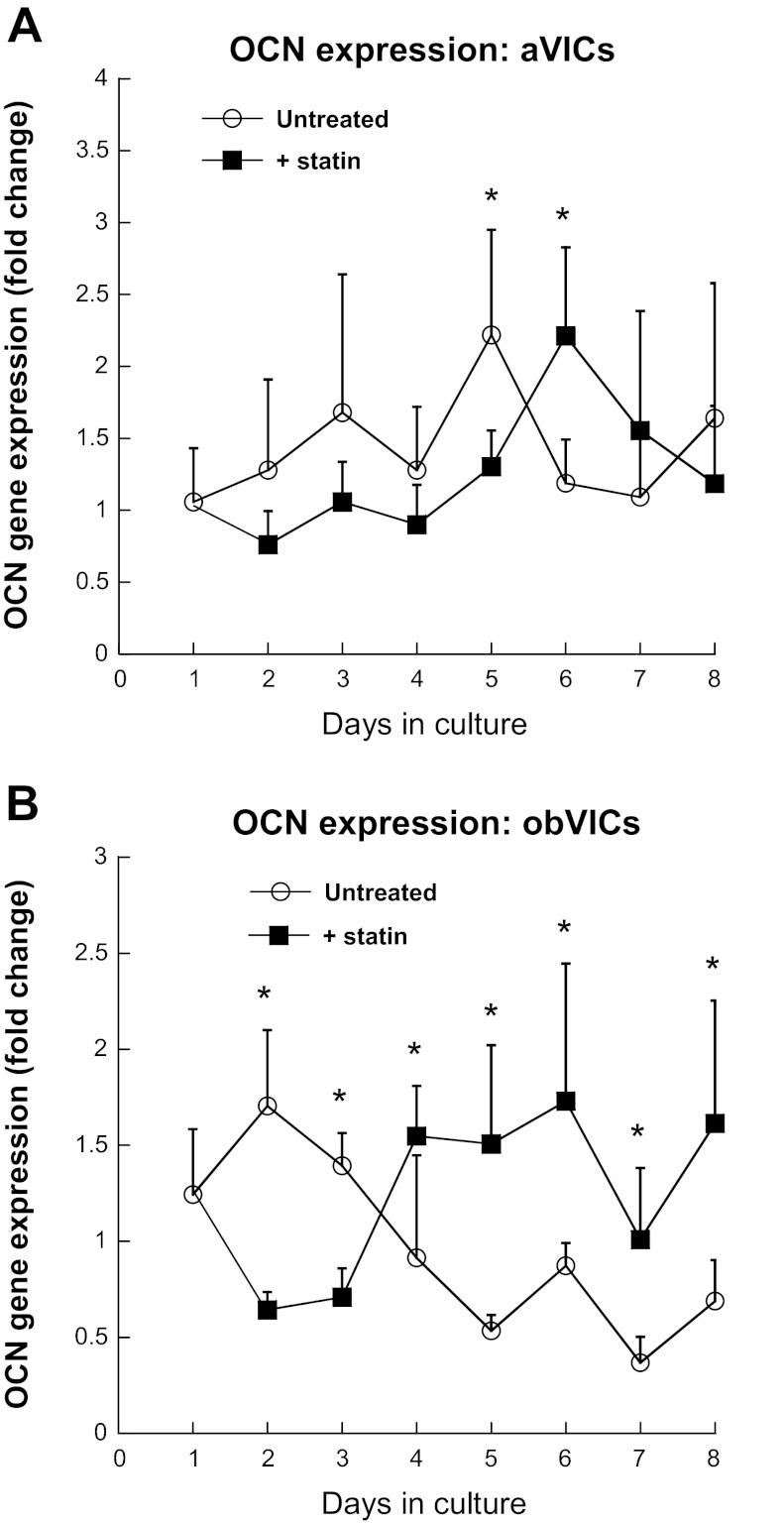
The expression of OCN, a more advanced marker of mineralization (7), is displayed in Fig. 6. aVICs did not exhibit any significant response to statin treatment with respect to expression of OCN (Fig. 6A). In contrast, obVICs experienced a two- to threefold increase in OCN gene expression upon statin treatment at all time points past day 3 (Fig. 6B), again indicating significantly increased osteogenic activity induced by simvastatin.
Fig. 6.
Osteocalcin (OCN) gene expression by aVICs (A) and obVICs (B) cultured with or without simvastatin. All values are expressed as fold-change relative to the day 1 aVIC condition. *P < 0.04 compared with paired condition on same day.
DISCUSSION
Although there have been numerous clinical studies examining the effect of statin drugs on heart valve disease (4, 5, 11, 17, 21), their conflicting results have not brought the scientific community closer to understanding the relationship between statin treatment and valve disease. The present study examined this relationship on a cellular level in vitro, with the goal of better understanding both the temporal response of VICs to statin treatment as well as determining whether statin efficacy in the context of valve calcification is dependent upon valve composition, a finding that could have significant consequences for both clinical and basic valve research. In this work, we report that, upon differentiation of a starting VIC population into either aVICs or obVICs, statin treatment exhibited a paradoxical effect between the two VIC subpopulations. Application of simvastatin elevated the expression of bone markers in the subpopulation of VICs already prone to mineralization (obVICs), whereas the same treatment did not exert this effect on the myofibroblastic aVIC population, thereby indicating that statin treatment can elicit widely differing effects within the heterogeneous VIC population of an individual valve.
Characterization of our cell populations confirmed that our experiments started with a phenotypically heterogeneous VIC population that was then differentiated to either aVICs or obVICs, depending upon the culture medium. The majority of this starting population was both ALP- and α-SMA-negative, suggesting that it was composed of quiescent and/or progenitor VICs (8). Approximately one-third of the starting population was aVICs, whereas no obVICs were detected. Thus, although the aVIC proportion was higher than that seen in a healthy valve in vivo (25), the absence of obVICs and minority of aVICs are consistent with the VIC phenotypes that one would expect in a healthy valve. Our data also suggest that our two differentiated cultures were enriched in aVICs or obVICs relative to the starting population, rather than containing the same number of aVICs and obVICs that then produced more α-SMA or ALP per cell.
In the myofibroblastic environment, statin treatment decreased expression of the myofibroblastic phenotype in VICs, with this inhibition being most robust during early time points. The effect of statin treatment was similar for VICs in mineralization medium, with statin treatment generally reducing α-SMA expression, although the baseline α-SMA expression by obVICs not receiving statin was already extremely low (also confirmed by protein results). These dramatically lower levels of α-SMA expression in obVICs relative to aVICs are consistent with our previous work that measured α-SMA expression in obVICs, early-stage osteoblast precursors, late-stage osteoblast precursors, and mature osteoblasts (10). We also confirmed that this decreased α-SMA expression in obVIC cultures was not indicative of quiescence, as the VICs treated with mineralization medium were found to be highly proliferative, a state that is also associated with early osteogenesis (14, 20). Despite differences in the level of α-SMA expression between aVICs and obVICs, statin treatment affected α-SMA expression in a similar manner, implying these subpopulations of VICs do share some commonalities in their response to simvastatin.
In mineralization medium, statin treatment induced a significant increase in ALP gene expression and ALP production by obVICs. This induction of ALP expression with statin treatment is consistent with findings in osteoblasts (23), but has not previously been reported for obVICs. Statin treatment of obVICs also tended to stimulate two- to threefold elevation of OCN, a result which is consistent with an obVIC population responding to statin treatment in a manner similar to bone-derived cells described in previous work (9, 23). Meanwhile, ALP production by aVICs was minimal and decreased to zero upon treatment with simvatatin.
Thus, in VIC cultures that were being driven toward an osteoblastic phenotype (i.e., cultured in mineralization medium), statin treatment tended to increase bone marker expression. This increase in osteogenic expression did not occur in aVIC cultures receiving the same statin treatment. These outcomes indicate that the paradoxical effects of statin treatment described previously for valvular myofibroblasts and osteoblast precursor cells also hold true for aVIC versus obVIC subpopulations of VIC cultures. Because diseased valves contain both aVICs and obVICs (13, 15, 16), this work suggests that treatment of a patient with a statin drug may be capable of producing both positive and negative outcomes with respect to stopping the progression of CAVD. Although even healthy valves are composed of a dynamically heterogeneous cell population, obVICs are more likely to be found in a valve that is already exhibiting some calcific disease (16). Thus, this study may also have consequences with respect to understanding how the effectiveness of statin treatment may change with the stage of heart valve disease. However, a major limitation of the current work is that it represents only in vitro results, which may not directly translate to prediction of aVIC versus obVIC response to simvastatin in vivo.
This study also serves to highlight the important differences between evaluating gene expression at a single time point versus over the course of an experiment. Even for the genes that had no apparent pattern in gene expression with statin treatment over control or mineralization medium alone, there was consistently at least 1 day out of the 8-day treatment course that statistical significance was reached with statin treatment. Additionally, many of the genes exhibited elevated expression with statin treatment for part of the time course, only to exhibit the opposite result within a different portion of the time course. These findings illustrate several significant consequences of single-time point gene expression analyses, ranging from inability to compare across studies to entirely missing important phenomena that may change the experimental conclusions. Although the hope is that a selected single time point will be representative of a particular cellular response, the choice of this point is often arbitrary or done in the absence of the temporal information that would be needed to make an informed selection. Thus, although the current standard continues to be sampling at a single time point for logistical or economic reasons, these data support the attempt to sample at least several time points over the course of an experiment for gene expression analysis.
Limitations
As noted above, one major limitation of this work is that it is yet unclear how the VIC/statin paradox will translate to the in vivo environment. Although some studies have demonstrated increased skeletal bone density upon clinical administration of statins, others have reported no effect (26). Additionally, although the statin dosage and treatment timing used in our work are consistent with previous in vitro investigations using simvastatin, they are indeed different from what is used in the clinical setting (3). Finally, the means by which our starting population of VICs was differentiated into aVIC and obVIC populations (via reduced-serum and osteogenic media, respectively) are unlikely to mimic the stimuli that would initiate this differentiation in vivo (although those in vivo initiators have not yet been identified). With that said, our statin paradox conclusions are not dependent upon the means by which our enriched aVIC and obVIC populations were achieved.
Conclusions
Different subpopulations of VICs that were derived from the same starting cell population exhibited a paradoxical response to statin treatment, suggesting that statin efficacy in the context of stopping the progression of CAVD will be dependent upon valve composition. Because obVICs tend to be present more in diseased heart valves, whereas aVICs are present in both healthy, remodeling valves as well as diseased valves, this differential response also carries significance regarding the CAVD stage-specificity of statin treatment and may contribute to the elucidation of underlying reasons for differences in patient responsiveness to statin therapy for CAVD. Finally, quantifying gene expression at daily time points showcases the dynamic nature of the VIC cell type and its response to statin treatment.
GRANTS
Funding support for this work was provided by the National Heart, Lung, and Blood Institute Grants R01-HL093281 (to K. S. Masters) and T32 HL007936-06 (to E. L. Monzack) for the Cardiovascular Training Program at the University of Wisconsin-Madison.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.L.M. and K.S.M. conception and design of research; E.L.M. and K.S.M. performed experiments; E.L.M. and K.S.M. analyzed data; E.L.M. and K.S.M. interpreted results of experiments; E.L.M. prepared figures; E.L.M. drafted manuscript; E.L.M. and K.S.M. edited and revised manuscript; E.L.M. and K.S.M. approved final version of manuscript.
REFERENCES
- 1. Antonini-Canterin F, Hirsu M, Popescu BA, Leiballi E, Piazza R, Pavan D, Ginghina C, Nicolosi GL. Stage-related effect of statin treatment on the progression of aortic valve sclerosis and stenosis. Am J Cardiol 102: 738–742, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting alpha-smooth muscle actin expression. Arterioscler Thromb Vasc Biol 29: 1950–1957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjorkhem-Bergman L, Lindh JD, Bergman P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br J Clin Pharmacol 72: 164–165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121: 306–314, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 352: 2389–2397, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Johnson CM, Hanson MN, Helgeson SC. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J Mol Cell Cardiol 19: 1185–1193, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Lian JB, Stein GS, Stein JL, van Wijnen AJ. Osteocalcin gene promoter: unlocking the secrets for regulation of osteoblast growth and differentiation. J Cell Biochem Suppl 30–31: 62–72, 1998 [PubMed] [Google Scholar]
- 8. Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171: 1407–1418, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lupattelli G, Scarponi AM, Vaudo G, Siepi D, Roscini AR, Gemelli F, Pirro M, Latini RA, Sinzinger H, Marchesi S, Mannarino E. Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism 53: 744–748, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Monzack EL, Masters KS. Can valvular interstitial cells become true osteoblasts? A side-by-side comparison. J Heart Valve Dis 20: 449–463, 2011 [PMC free article] [PubMed] [Google Scholar]
- 11. Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 49: 554–561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation 114: I547–I552, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O′Brien KD. Characterization of the early lesion of ‘degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 90: 844–853, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143: 420–430, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O′Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 124: 1783–1791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181–2184, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 359: 1343–1356, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol 35: 113–118, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Tsai KS, Kao SY, Wang CY, Wang YJ, Wang JP, Hung SC. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res A 94: 673–682, 2010 [DOI] [PubMed] [Google Scholar]
- 21. van der Linde D, Yap SC, van Dijk AP, Budts W, Pieper PG, van der Burgh PH, Mulder BJ, Witsenburg M, Cuypers JA, Lindemans J, Takkenberg JJ, Roos-Hesselink JW. Effects of rosuvastatin on progression of stenosis in adult patients with congenital aortic stenosis (PROCAS Trial). Am J Cardiol 108: 265–271, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 95: 253–260, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Wu B, Elmariah S, Kaplan FS, Cheng G, Mohler ER., 3rd Paradoxical effects of statins on aortic valve myofibroblasts and osteoblasts: implications for end-stage valvular heart disease. Arterioscler Thromb Vasc Biol 25: 592–597, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Yip CY, Blaser MC, Mirzaei Z, Zhong X, Simmons CA. Inhibition of pathological differentiation of valvular interstitial cells by C-type natriuretic peptide. Arterioscler Thromb Vasc Biol 31: 1881–1889, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Yip CY, Simmons CA. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc Pathol 20: 177–182, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Yue J, Zhang X, Dong B, Yang M. Statins and bone health in postmenopausal women: a systematic review of randomized controlled trials. Menopause 17: 1071–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Zhou H, Choong P, McCarthy R, Chou ST, Martin TJ, Ng KW. In situ hybridization to show sequential expression of osteoblast gene markers during bone formation in vivo. J Bone Miner Res 9: 1489–1499, 1994 [DOI] [PubMed] [Google Scholar]