Abstract
Calcium sensitivity of the force-pCa relationship depends strongly on sarcomere length (SL) in cardiac muscle and is considered to be the cellular basis of the Frank-Starling law of the heart. SL dependence may involve changes in myofilament lattice spacing and/or myosin crossbridge orientation to increase probability of binding to actin at longer SLs. We used the L48Q cardiac troponin C (cTnC) variant, which has enhanced Ca2+ binding affinity, to test the hypotheses that the intrinsic properties of cTnC are important in determining 1) thin filament binding site availability and responsiveness to crossbridge activation and 2) SL dependence of force in cardiac muscle. Trabeculae containing L48Q cTnC-cTn lost SL dependence of the Ca2+ sensitivity of force. This occurred despite maintaining the typical SL-dependent changes in maximal force (Fmax). Osmotic compression of preparations at SL 2.0 μm with 3% dextran increased Fmax but not pCa50 in L48Q cTnC-cTn exchanged trabeculae, whereas wild-type (WT)-cTnC-cTn exchanged trabeculae exhibited increases in both Fmax and pCa50. Furthermore, crossbridge inhibition with 2,3-butanedione monoxime at SL 2.3 μm decreased Fmax and pCa50 in WT cTnC-cTn trabeculae to levels measured at SL 2.0 μm, whereas only Fmax was decreased with L48Q cTnC-cTn. Overall, these results suggest that L48Q cTnC confers reduced crossbridge dependence of thin filament activation in cardiac muscle and that changes in the Ca2+ sensitivity of force in response to changes in SL are at least partially dependent on properties of thin filament troponin.
Keywords: Ca2+ sensitivity, sarcomere length, length-dependent activation, trabeculae, Frank-Starling relationship
myocardial performance is tightly regulated at the cardiomyocyte level by sarcomere length (SL), whereby increases in SL sharply increase force generation. This capability is the cellular basis of the Frank-Starling relationship, which allows the heart to match venous return and stroke volume of the right and left ventricles and to match systemic demand of the body on a beat-to-beat basis. The molecular mechanisms of this SL-dependent effect are currently debated but thought to result, at least in part, from changes in lattice spacing between the thin and thick filaments, and/or changes in myosin crossbridge orientation that increase the probability of myosin binding to actin at longer SLs (9, 11, 17, 20, 25, 41, 42). Furthermore, crossbridge binding enhances Ca2+ binding to troponin (cTn) in cardiac muscle, a unique form of cooperative thin filament activation whose mechanism is currently not well understood (10, 11, 19, 32). The net result of these interactions is an apparent increase in Ca2+ sensitivity of thin filament activation at longer SLs, whereby a given submaximal Ca2+ concentration ([Ca2+]) results in greater contractile force development. Thus SL-dependent changes in Ca2+-sensitivity of force generation result from the intrinsic properties of the thin filament (8, 42) as well as from complex interactions between thin and thick filament proteins. This is especially important to understand in cardiac muscle, since free intracellular [Ca2+] during activation is known to be submaximal (17, 35) and loss of SL dependence of contraction can occur during heart failure (22, 24, 39).
Under normal conditions, contractile activation is initiated by Ca2+-binding to the cTn complex on cardiac thin filaments, allowing tropomyosin movement that exposes myosin binding sites on actin. Myosin initially forms crossbridges with actin in a weak electrostatic conformation, then transitions to a strong hydrophobic conformation that generates force (15). Transition to a strong binding state further activates cardiac thin filaments and increases Ca2+ affinity for cTn, demonstrating a thick filament-mediated influence on thin filament Ca2+-binding properties (9, 33). This suggests the inherent Ca2+-binding properties of cTn may be relatively weak (compared with skeletal muscle Tn) and raises the question as to whether thin filaments containing cTn with greater Ca2+ affinity would be less dependent on myosin for activation. This, in turn, should confer Ca2+-sensitivity of force that is less responsive to changes in SL.
In the current study we tested the hypotheses that the intrinsic properties of cTn are important in determining Ca2+ sensitivity of thin filament activation in response to crossbridge binding, and thus the SL dependence of force in cardiac muscle. We compared the Ca2+ dependence of force generation at long (2.3 μm) and short (2.0 μm) SL for demembranated trabeculae exchanged with wild-type (WT) cTn versus cTn containing a mutant (L48Q) cTnC with enhanced Ca2+ affinity (27, 44). To separate the direct influence of strongly bound crossbridges, we compared changes in Ca2+ sensitivity at short SL (2.0 μm) with and without osmotic compression by 3% dextran, which is sufficient to compress trabeculae and increase maximal force to levels observed at SL 2.3 μm. Finally, we examined crossbridge influence on Ca2+ sensitivity of force at long SL using the crossbridge inhibitor 2,3-butanedione monoxime (BDM) at a concentration (7 mM) sufficient to decrease maximal force to levels approximately observed at short SL.
Our results, described below, imply that in cardiac muscle thin filament activation by Ca2+ alone is limited, perhaps due to a limited ability to expose crossbridge binding sites on F-actin. More complete activation requires strong binding crossbridges, and this crossbridge-dependent component of activation steepens the cardiac force-length relationship. Our data suggest that increasing the Ca2+ binding affinity and/or cTnC-cTnI interaction properties of cTn, via L48Q cTnC-cTn, can reduce or eliminate this limitation by improving thin filament activation at any given submaximal Ca2+. This, in turn, reduces or eliminates crossbridge-induced changes in Ca2+ sensitivity. Perhaps most important, the data suggest that the SL dependence of cardiac force development is greatly influenced by the properties of native cTn. This is likely to have implications for many familial inherited cardiomyopathies associated with mutations in thin filament proteins that result in altered Ca2+ affinity and/or Ca2+ sensitivity of force.
MATERIALS AND METHODS
Experimental animals and tissue preparations.
All animal procedures were conducted in accordance with the US National Institutes of Health Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Washington (UW) Animal Care Committee. Male Sprague-Dawley rats (200–250 g) were housed in the Department of Comparative Medicine at UW and cared for in accordance with the UW Institutional Animal Care and Use Committee procedures. Rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50–100 mg/kg). When animals had no reflexive response, the heart was rapidly excised and right ventricles were dissected in an oxygenated Ringer's solution containing (in mM) 100 NaCl, 24 NaHCO3, 2.5 KCl, 1 MgSO4*7H2O, 1 Na2HPO4, and 1 CaCl2. Trabeculae were permeabilized in situ by incubation of splayed ventricles overnight in a relaxing solution of (in mM) 100 KCl, 10 imidazole, 2 EGTA, 5 MgCl2, and 4 ATP containing 50% glycerol and 1% Triton X-100 (Sigma-Aldrich) at 4°C. Individual trabeculae were then dissected from ventricular free walls, pinned to the bottom of a Sylgard (R)-coated Petri dish, and stored for up to 1 wk in glycerinated relaxing solution at 4°C. All skinning and storage solutions contained protease inhibitor cocktail (P8340; Sigma-Aldrich).
Proteins and passive whole troponin exchange.
Cloning and expression of rat recombinant WT cTnC, cTnI, and cTnT were done as previously described (24a). Site-directed mutagenesis was performed using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) to substitute to produce L48Q cTnC variant. A pET-24 (Novagen, Madison, WI) vector containing the T7 promoter, lac operator, and a kanamycin-resistant gene was used for expression of WT and mutant proteins in E. coli (BL21). The DNA sequences of the expressing constructs were verified by DNA sequencing. The expressed protein was extracted from bacterial cells as previously described (4) and purified on DE 52 or CM 52 (Whatman) column equilibrated by 6M Urea, 25 mM Tris at pH 8.0, 1 mM EDTA, and 15 mM 2-mercaptoethanol. Proteins were eluted with a salt gradient washing in the same buffer from 0 to 0.3M NaCl. The fractions containing the desired protein and their concentrations were monitored by SDS-PAGE and DU 800 Spectrophotometer. Super pure proteins were stored in a −80° freezer before use.
Cardiac Tn (cTn) complex was reconstituted from isolated recombinant subunits (1:1:1), using a modification of published protocols (34). In brief, after subunits were complexed in a 1:1:1 molar ratio, cTn was gradually dialyzed into buffer containing (in mM) 200 KCl, 20 MOPS, 5 EGTA, 5 MgCl2, and 15 BME. Exchange of whole cTn complex into trabeculae was accomplished by incubating trabeculae overnight on a mechanical rocker (Beckman) at 4°C in a protein buffer solution with addition of 4 mM ATP, 1 mg/ml DTT, and protease inhibitor cocktail. Preparations were then washed several times in relaxing solutions containing 1 mg/ml BSA, transferred to the glycerinated (50% vol/vol) relaxing solution for storage at −20°C. Exchanged trabeculae were used within 2 days after the procedure.
Mechanical measurements.
Relaxing and activating solutions were prepared using a custom software package as described previously (31, 38). Solutions were maintained pH 7.0 at 15°C and contained (in mM) 15 phosphocreatine, 15 EGTA, 80 MOPS, 1 free Mg2+, 1 DTT, and 5 Mg2ATP. Ca2+ concentration (reported as pCa = −log[Ca2+]) was adjusted by varying amounts of CaCl2. Ionic strength was set to 0.17 M with KCl.
Trabeculae were mounted between a high-speed length controller and a force transducer via aluminum t-clips as previously described (27). SL was initially set to 2.3 μm and measured by laser diffraction, and trabeculae were fully activated in pCa 4.0 to determine maximum force (Fmax) before exposure to increasing Ca2+ concentrations (decreasing pCa). Passive force was determined at pCa 9.0 and subtracted from total force to obtain the active force values reported. After determining the passive force at SL 2.3 μm, trabeculae were exposed to increasing Ca2+ concentration (decreasing pCa) in a step-wise manner. At each pCa a slack-re-stretch maneuver was performed to measure steady state force and the rate of force redevelopment [ktr, (4)] followed by stiffness measurements obtained from applying small (0.1%) sinusoidal changes in length at 1,000 Hz for 0.5 sec. SL was then set to 2.0 μm, and the protocol was repeated. The order of initial SL was reversed in some trabeculae. Results from either SL sequence were rejected if force at pCa 4.0 (Fmax) declined by more than 15% at SL 2.3 μm at the end of the pCa curve. Force-pCa data were fitted with the Hill Equation to obtain values for pCa50 and nH (27). The ktr was determined from release-restretch transient, with the resulting force redevelopment trace fit using a monoexponential equation (4). For experiments with 3% dextran T500, SL was set to 2.0 μm and initial force-pCa data were obtained. The same trabeculae were then exposed to pCa solutions containing 3% dextran T500 to obtain additional force-pCa data. For experiments with 7 mM BDM, SL was set to 2.3 μm and initial force-pCa data were obtained. The same trabeculae were then exposed to pCa solutions containing 7 mM BDM to obtain additional force-pCa data. Reported pCa50 and nH values for force-pCa relationships are the average of individual fits for each experimental curve ± SE. Student's paired t-tests with statistical significance set at P < 0.05 were used to compare the means of reported data from the same cTnC-cTn exchange at different SL or with and without dextran T500 or BDM.
RESULTS
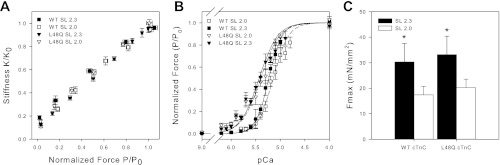
The Ca2+ dependence of isometric steady-state force and force redevelopment rate (ktr) at SL 2.0 (short) and 2.3 μm (long) were determined for demembranated cardiac trabeculae at 15°C (Fig. 1) after exchange of native cTn with WT cTn or cTn containing L48Q cTnC. We have previously demonstrated that our exchange protocol results in ∼90% exchange of native cTn and that excess cTn was completely removed before experiments (27). In each trabecula, after an initial activation at SL 2.3 μm, trabeculae were exposed to increasing Ca2+ concentration (decreasing pCa) at either long or short SL to obtain measurements of steady-state force, rate of force redevelopment (ktr), and stiffness; then SL was changed and the protocols were repeated. This varying of initial SL helps account for any run down of the preparation, and results from either SL sequence were rejected if force at pCa 4.0 (Fmax) declined by more than 15% at the end of the force-pCa curve.
Fig. 1.
Normalized sarcomere length (SL) dependence of Ca2+ sensitivity of force and stiffness after whole cardiac troponin (cTn) exchange containing wild-type (WT) or L48Q cardiac troponin C (cTnC). A: normalized force vs. stiffness as Ca2+ was varied at SL 2.0 μm (□ and ▽) and 2.3 μm (■ and ▼) for trabeculae containing WT (□ and ■) and L48Q cTnC-cTn (▽ and ▼). Normalized force-pCa (B) and absolute maximal force (Fmax; C) values for WT (□ and ■) and L48Q (▽ and ▼) cTnC-cTn exchanged trabeculae at SL 2.0 μm (□ and ▽) and 2.3 μm (■ and ▼) are also shown. Error bars represent ± SE and in some cases are contained within the symbol. The data were fit with the Hill equation, and corresponding fit values are included in Table 1. *P < 0.05 as compared with SL 2.0 μm.
SL dependence of steady-state force and stiffness.
Force-pCa relationships at SL 2.0 and 2.3 μm for trabeculae with either WT or L48Q cTnC-cTn are summarized in Fig. 1. A concomitant increase in stiffness with force occurred as Ca2+ was increased at short and long SL with both WT and L48Q cTnC-cTn (Fig. 1A). This suggests that force varied proportionally in all conditions as a result of changes in strong crossbridge binding and not a change in force produced per crossbridge. In Fig. 1B the data were compared by plotting force-pCa relationships normalized to Fmax at SL 2.0 or 2.3 μm. The absolute values for Fmax are summarized in Fig. 1C. The data from individual force-pCa curves were fit with the Hill equation for determination of pCa50 and nH, and the mean (±SE) of these values for each condition are listed in Table 1. Decreasing SL from 2.3 to 2.0 μm in WT trabeculae decreased Fmax from 30 ± 7 mN/mm2 to 17 ± 3 mN/mm2, and right shifted pCa50 by 0.09 ± 0.01 (ΔpCa50) (Fig. 1C; Table 1). These results are similar to our previous report (1).
Table 1.
Summary of steady-state force-pCa parameters after whole cTn exchange in cardiac trabeculae at SL 2.0 and 2.3 μm
| Type and SL, μm | Fmax, mN/mm2 | Fpassive, mN/mm2 | pCa50 | nH |
|---|---|---|---|---|
| WT cTnC | ||||
| 2.3 | 30 ± 7* | 4.8 ± 1.0* | 5.23 ± 0.03* | 3.2 ± 0.2 |
| 2 | 17 ± 3 | 0.9 ± 0.1 | 5.14 ± 0.04 | 2.8 ± 0.1 |
| L48Q cTnC | ||||
| 2.3 | 33 ± 4* | 7.6 ± 1.6* | 5.43 ± 0.03 | 2.3 ± 0.1 |
| 2 | 20 ± 3 | 1.8 ± 0.3 | 5.40 ± 0.03 | 2.2 ± 0.1 |
| Native | ||||
| 2.3 | 39 ± 4* | 3.8 ± 0.8* | 5.45 ± 0.03* | 4.2 ± 0.2 |
| 2 | 24 ± 2 | 1.3 ± 0.3 | 5.32 ± 0.03 | 4.2 ± 0.3 |
Values are means ± SE; n = 8 for wild-type cardiac troponin C (WT cTnC), 17 for L48Q cTnC, and 15 for native. SL, sarcomere length; F, maximal force; nH, Hill coefficient.
P < 0.05 as compared with SL 2.0 μm.
For trabeculae containing L48Q cTnC-cTn, decreasing SL from 2.3 to 2.0 μm reduced Fmax from 33 ± 7 mN/mm2 to 20 ± 3 mN/mm2 (Fig. 1C; Table 1). These values were not different from WT cTnC-cTn exchanged trabeculae. Increasing SL from 2.0 to 2.3 μm in L48Q cTnC-cTn trabeculae increased the passive force, similar to results for WT cTnC-cTn trabeculae (Table 1). Additionally, there was no difference in stiffness at 2.3 and 2.0 μm in relaxing solution between WT and L48Q cTnC-cTn trabeculae (data not shown), which indicates L48Q cTnC-cTn does not allow Ca2+-independent activation of the thin filament at pCa 9.0. However, in L48Q cTnC-cTn trabeculae, there was a dramatically different effect on the SL dependence of pCa50. As previously reported (27), L48Q cTnC-cTn increased Ca2+ sensitivity of force at SL 2.3 μm compared with WT. Interestingly, when SL was decreased from 2.3 to 2.0 μm, there was no significant change in pCa50 (Fig. 1B; Table 1), demonstrating a much larger effect of L48Q cTnC-cTn on pCa50 at short SL. In effect, this means that L48Q cTnC-cTn greatly reduced, and very likely eliminated, the SL dependence of Ca2+ sensitivity of thin filament activation in cardiac muscle. However, the influence of SL on maximal strong crossbridge binding (Fmax) remains. Similar results have been reported by others, i.e., a decrease in SL dependence of the Ca2+ sensitivity of force in myocardium containing L29Q-cTnC [decrease in ΔpCa50 (29)] or ssTnI [decrease in ΔEC50 (43)], both of which left-shifted the force-pCa curve.
Role of lattice spacing in the loss of SL dependence of force generation with L48Q cTnC-cTn.
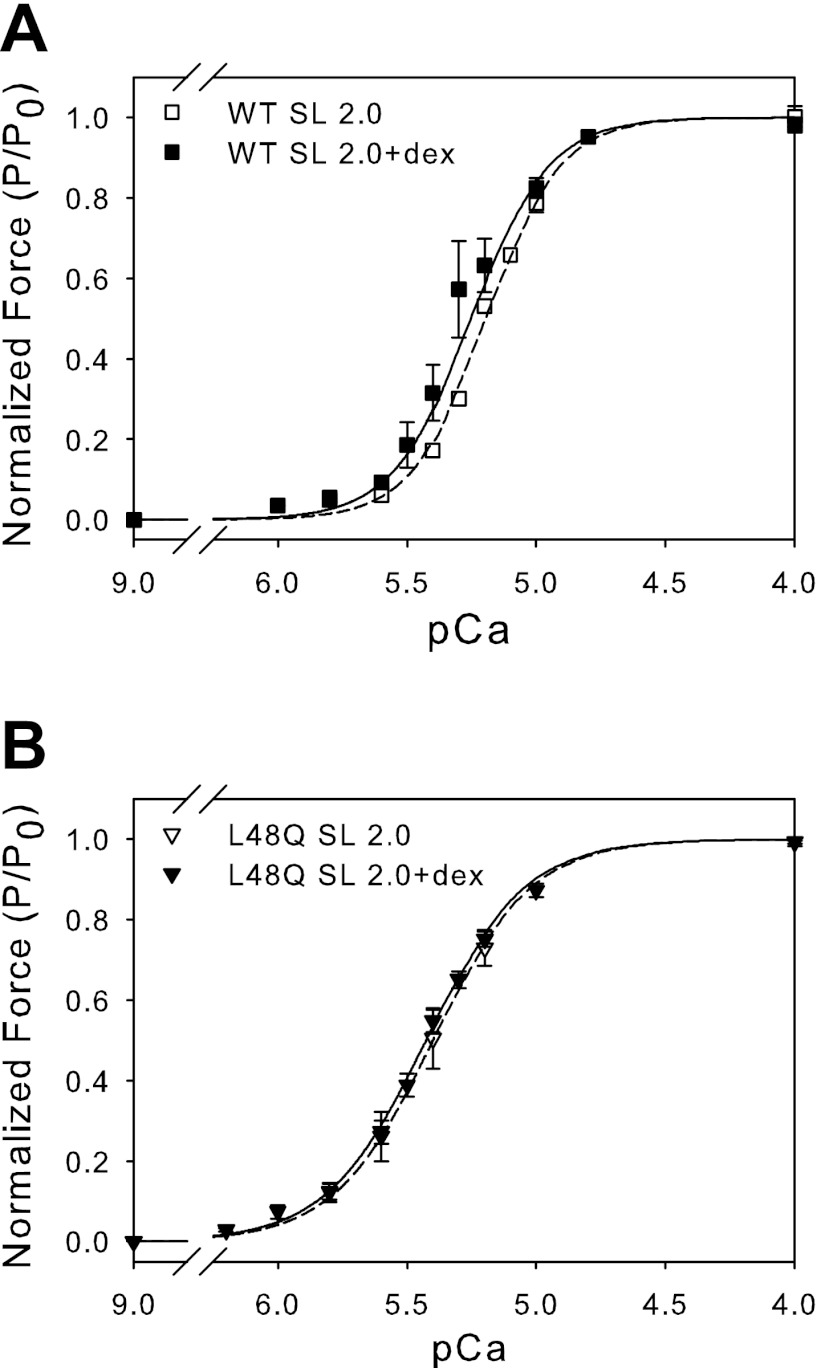
To determine whether loss of the SL dependence of Ca2+ sensitivity of force with L48Q cTnC-cTn is sensitive to changes in thin-thick filament spacing or actin-myosin proximity, myofilament lattice spacing was osmotically compressed using 3% dextran T500 (wt/vol) at SL 2.0 μm. This dextran concentration was selected because it increased Fmax values at SL 2.0 μm to those measured at SL 2.3 μm without dextran. Force-pCa relationships for these trabeculae are summarized in Fig. 2. Trabeculae with WT cTnC-cTn exhibited an increase in pCa50 and Fmax when osmotically compressed at SL 2.0 μm (Fig. 2A; Table 2). This is consistent with our previous work (1), which demonstrated that osmotic compression of lattice spacing at SL 2.0 μm results in force and Ca2+ sensitivity of force similar to that seen at SL 2.3 μm without dextran. However, with L48Q cTnC-cTn, 3% dextran increased Fmax at SL 2.0 μm without any significant change in pCa50 (Fig. 2B; Table 2). The increase in pCa50 with L48Q cTnC-cTn (relative to WT cTnC-cTn) was maintained, but there was no additional increase in pCa50 with osmotic compression. These results suggest that actin-myosin proximity may have little to no effect on myofilament Ca2+ sensitivity of force when L48Q cTnC-cTn is present in thin filaments.
Fig. 2.
Normalized force-pCa relationship at SL 2.0 μm ± 3% dextran (dex) for trabeculae containing WT or L48Q cTnC-cTn. A: WT cTnC-cTn at SL 2.0 (□) and SL 2.0 + 3% dextran (■). B: L48Q cTnC-cTn at SL 2.0 (▽) and SL 2.0 + 3% dextran (▼). Error bars represent ± SE and in some cases are contained within the symbol. The data were fit with the Hill equation, and corresponding fit values are included in Table 2.
Table 2.
Summary of steady-state force-pCa parameters after whole cTn exchange in cardiac trabeculae at SL 2.0 μm + 3% Dex or SL 2.3 μm + 7 mM BDM
| SL, μm | Fmax, mN/mm2 | Fpassive, mN/mm2 | pCa50 | nH | |
|---|---|---|---|---|---|
| WT cTnC | |||||
| Control | 2 | 15 ± 3 | 1.0 ± 0.2 | 5.19 ± 0.00 | 3.0 ± 0.1 |
| + 3% Dex | 2 | 23 ± 4* | 1.2 ± 0.2 | 5.25 ± 0.02 | 2.9 ± 0.1 |
| L48Q cTnC | |||||
| Control | 2 | 18 ± 3 | 1.7 ± 0.4 | 5.40 ± 0.05 | 2.3 ± 0.1 |
| + 3% Dex | 2 | 28 ± 5* | 2.2 ± 0.4 | 5.43 ± 0.02 | 2.3 ± 0.1 |
| WT cTnC | |||||
| Control | 2.3 | 27 ± 8 | 5.4 ± 2.0 | 5.27 ± 0.02 | 3.2 ± 0.3 |
| + 7 mm BDM | 2.3 | 17 ± 3 | 6.1 ± 2.8 | 5.09 ± 0.02* | 2.5 ± 0.1 |
| L48Q cTnC | |||||
| Control | 2.3 | 35 ± 5 | 10.6 ± 2.0 | 5.38 ± 0.03 | 2.6 ± 0.2 |
| + 7 mm BDM | 2.3 | 19 ± 3* | 8.2 ± 1.4 | 5.35 ± 0.03 | 2.9 ± 0.5 |
Values are means ± SE; n = 4 for WT cTnC control and + 3% dextran (Dex), and WT cTnC control and + 7 mm 2,3-butanedione monoxime (BDM); 9 for L48Q cTnC control; and 11 for L48Q cTnC + 7 mm BDM.
P < 0.05 as compared with control condition for WT or L48Q cTnC-cTn.
Role of strong crossbridge binding in the loss of SL dependence of force generation with L48Q cTnC-cTn.
To determine whether the loss of SL dependence of the Ca2+ sensitivity of force with L48Q cTnC-cTn is reliant on the number of strongly bound crossbridges, strong crossbridge formation was inhibited using BDM at SL 2.3 μm. The BDM concentration was selected such that Fmax at SL 2.3 μm with BDM was similar to Fmax values at SL 2.0 μm without BDM for each muscle preparation. Force-pCa relationships for these trabeculae are summarized in Fig. 3 and Table 2. The passive force in L48Q cTnC-cTn trabeculae was unchanged in the presence of BDM, indicating no active crossbridge cycling while at rest (pCa 9.0). With WT cTnC-cTn, BDM caused a right shift in force-pCa curve and a decrease of Fmax, both of which were similar to the levels observed when SL was reduced from 2.3 to 2.0 μm without BDM (Fig. 3A; Tables 1 and 2). Trabeculae containing L48Q cTnC-cTn also exhibited a decrease in Fmax with BDM (Fig. 3B; Tables 1 and 2); however, there was no significant right shift of the force-pCa curve. L48Q cTnC-cTn trabeculae still exhibited an increase in the Ca2+ sensitivity of force compared with WT cTnC-cTn at SL 2.3 μm, with and without BDM. These results suggest that improved myofilament Ca2+ binding, via incorporation of L48Q cTnC-cTn, reduces the dependence of thin filament activation on the number of strongly bound crossbridges.
Fig. 3.
Normalized force-pCa relationship at SL 2.3 μm ± 7 mM 2,3-butanedione monoxime (BDM) for trabeculae containing WT or L48Q cTnC-cTn. A: WT cTnC-cTn at SL 2.3 (□) and SL 2.3 + 7 mM BDM (■). B: L48Q cTnC-cTn at SL 2.3 (▽) and SL 2.3 + 7 mM BDM (▼). Error bars represent ± SE and in some cases are contained within the symbol. The data were fit with the Hill equation, and corresponding fit values are included in Table 2.
Ca2+ and SL dependence of force redevelopment (ktr).
In a previous study we demonstrated that L48Q cTnC-cTn enhances the Ca2+ sensitivity of the rate of force redevelopment (ktr), but not the maximal rate (27). In the current study, we determined how L48Q cTnC-cTn influences the SL dependence of the ktr-pCa and ktr-force relationships. These results are summarized in Fig. 4. Example representative traces of force redevelopment during the ktr slack-re-stretch protocol at pCa 4.0 for WT and L48Q cTnC-cTn trabeculae are shown in Fig. 4A. These traces and the summarized data demonstrate that at maximal Ca2+ (pCa 4.0) ktr was similar for both WT and L48Q cTnC-cTn at SL 2.0 and 2.3 μm. The Ca2+ dependence of ktr was not affected by SL for either WT (Fig. 4B) or L48Q (Fig. 4C). At submaximal Ca2+ (between pCa 6.0 and 5.4), ktr was significantly faster for L48Q cTnC-cTn (vs. WT cTnC-cTn) at both SL 2.0 and 2.3 μm. However, when ktr was compared at a given force level for WT and L48Q cTnC-cTn trabeculae, there was no difference between them at either SL 2.0 or 2.3 μm. This is demonstrated in Fig. 4D, where ktr values for WT and L48Q cTnC-cTn containing trabeculae at SL 2.3 μm are replotted versus the steady state force produced at each pCa. These results suggest that L48Q cTnC-cTn increases the activation level of the thin filament at submaximal forces independent of SL, but that at similar levels of thin filament activation, L48Q cTnC-cTn does not increase the rate of force development.
Fig. 4.
Rate of force redevelopment (ktr)-pCa and ktr-force relationships during steady-state activation for trabeculae containing WT or L48Q cTnC-cTn at SL 2.0 and 2.3 μm. A: WT (solid) and L48Q (dashed) cTnC-cTn example force traces after a slack-restretch procedure. The corresponding length trace is shown in dotted line. B: WT cTnC-cTn ktr-pCa relationships at SL 2.0 μm (□) and 2.3 μm (■). C: L48Q cTnC-cTn ktr-pCa relationships at SL 2.0 μm (▽) and 2.3 μm (▼). D: WT (■) and L48Q (▼) cTnC-cTn ktr-force relationships at SL 2.3 μm. Error bars represent ± SE and in some cases are contained within the symbol.
DISCUSSION
The SL dependence of the Ca2+ sensitivity of force is thought to be the molecular mechanism behind Frank-Starling relationship of the heart, and it has been shown to be influenced by various components of the contractile apparatus. Changes in thin filament properties, such as substitution of ssTnI for cTnI (3, 26, 43) or point mutations in cTnC (29) and cTnT (5, 6), as well as changes in thick filament properties (14) and factors affecting thin and thick filament interactions [lattice spacing (1, 11, 12, 14, 25, 30, 46), pH (13)] or the cross bridge cycle (10, 23) have been all shown to influence the SL dependence of contraction. However, no single mechanism has emerged as the primary determinant of the Frank-Starling relationship.
In this study we independently altered properties of the thin and thick filament that are responsible for thin filament activation and contraction of cardiac muscle. We demonstrated that a cTnC variant with increased Ca2+ affinity (L48Q) eliminated crossbridge-mediated effects on the apparent Ca2+ sensitivity of thin filament activation since the Ca2+ concentration required to produce half-maximal force (pCa50) for L48Q cTnC-cTn exchanged trabeculae was unaffected by perturbations of crossbridge activity/number. In contrast, these perturbations did produce changes in pCa50 of native or WT cTnC-cTn trabeculae. The concomitant increase in stiffness with force as Ca2+ was increased at short and long SL with both WT and L48Q cTnC-cTn (Fig. 1A) suggests that force varied proportionally in all conditions as a result of changes in strong crossbridge binding, and not a change in force produced by each crossbridge. These results corroborate the evidence presented by Sun et al. (42) that the intrinsic properties of the thin filament play a very significant role in the cooperative Ca2+ activation and regulation of cardiac contractility and suggest that by sensitizing the thin filament to Ca2+, L48Q cTnC greatly reduces the crossbridge assistance required for thin filament activation in cardiac muscle.
The reliance on strong crossbridges for thin filament activation is a hallmark of cardiac contraction. Ca2+ binding to troponin initiates thin filament activation exposing myosin binding sites on actin. However, unlike in skeletal muscle, thin filament activation in cardiac muscle is not achieved primarily by Ca2+ binding alone (16, 17, 37). Numerous studies have shown that crossbridge binding in cardiac muscle increases Ca2+ binding to cTnC (1, 11, 12, 17, 21, 41, 46) and induces structural changes in fluorescently tagged cTnC as measured by dichroism (40). Furthermore, rigor-myosin subfragment-1 crossbridges (NEM S-1) and Ca2+ can greatly increase the rate of product release from prepower stroke myosin bound to thin filaments over Ca2+ alone (10, 23). This effect was not observed in skeletal muscle, where Ca2+ alone was sufficient to achieve accelerated product release. A similar difference was observed between skeletal and cardiac myosin where S1-thin filament binding assays showed a Ca2+-induced fourfold change in closed-open equilibria of the skeletal versus cardiac system, which is indicative of a fundamental difference in the actin-TnI-TnC binding equilibria. Overall, the results suggest that situations that increase or decrease the number of strongly bound crossbridges (such as SL) increase or decrease the apparent Ca2+ sensitivity of cardiac muscle contraction.
We introduced perturbations to crossbridge binding that are generally accepted to affect pCa50, to determine whether they would have the same effect on pCa50 and SL dependence of the Ca2+ sensitivity of force in the presence of L48Q cTnC-cTn. Several studies (1, 11, 12, 14, 25, 46) have concluded that acto-myosin interaction distance, at least in part, is responsible for an increased contractile activation at longer SL in cardiac muscle. Martyn et al. (30) showed that compression with 3% osmotic solute dextran T-500 at SL 2.0 μm results in thick filament spacing observed at SL 2.3 μm with 0% dextran. In this manner, we matched the overall force generating capacity of myofilaments at a short SL to that at long SL via modulations in actin-myosin proximity. Despite increased crossbridge binding and force in the presence of dextran, pCa50 was unchanged for L48Q cTnC-cTn trabeculae, but was significantly increased for native and WT cTnC-cTn trabeculae, as previously demonstrated (1, 11, 12, 14, 25, 46). This suggests crossbridge binding induced increases of Ca2+ sensitivity with WT cTnC-cTn, but not with L48Q cTnC-cTn, and suggests that L48Q cTnC-cTn sufficiently increases the Ca2+ sensitivity of the thin filament to a level that no longer requires crossbridge contributions to achieve full activation.
To test this hypothesis, we inhibited crossbridge binding with BDM at SL 2.3 μm to decrease force to levels approximately equal to those seen at SL 2.0 μm. Thus a similar number of force generating crossbridges were likely involved at SL 2.0 and SL 2.3 μm in the presence of BDM (36), whereas the remaining myofilament environment characteristic of SL 2.3 μm was maintained (e.g., actin-myosin proximity, passive tension, etc.). With BDM, even though Fmax was reduced, little to no change in pCa50 was observed with L48Q cTnC-cTn, whereas there was a decrease in both Fmax and pCa50 with WT cTnC-cTn. All of these crossbridge perturbations resulted in the expected increases (longer SLs, dextran) and decreases (shorter SLs, BDM) in Fmax for both WT and L48Q cTnC-cTn exchanged trabeculae. This indicates that the numbers of strongly bound crossbridges were changing accordingly. Together our results and those of others (10) suggest that Ca2+ only partially activates thin filaments containing native cTnC, where more complete activation requires strongly bound crossbridges, and this crossbridge dependent component of activation may underlie the steep SL dependence of Ca2+ sensitivity in cardiac muscle. Furthermore, our results indicate that the crossbridge-induced feedback on thin filament Ca2+ sensitivity was eliminated with L48Q cTnC-cTn.
It is interesting to speculate on how L48Q cTnC might lessen the crossbridge dependent component of cardiac thin filament activation. In a recent study, we examined how the L48Q variant might affect the intrinsic properties of the thin filament by investigating the ability of L48Q cTnC to bind Ca2+ and cTnI using a combination of solution protein studies, nuclear magnetic resonance spectroscopy, and molecular dynamic simulation approaches (45). Our solution studies, and studies by others (44), have shown that L48Q cTnC has a significantly higher affinity for Ca2+ compared with WT cTnC alone or in cTn complex. We also demonstrated that L48Q cTnC has a higher affinity for cTnI in both the presence and absence of Ca2+. Both nuclear magnetic resonance spectroscopy and molecular dynamic simulation data indicate that the NH2-terminal lobe of L48Q cTnC has a more open structure and exposure of the cTnC hydrophobic patch is stabilized after Ca2+ binding. This should increase cTnC-cTnI interaction, allowing enhanced movement of Tm and access to myosin binding sites on actin for a given submaximal [Ca2+]. It is likely that this is the cause of increased Ca2+ sensitivity of thin filament activation and force development in cardiac muscle containing L48Q cTnC, and it may also reduce the requirement for crossbridges to stabilize the cTnC-cTnI state (7). This effect should be more pronounced at lower [Ca2+], where the slower dissociation rate of L48Q cTnC (27, 44) should result in Ca2+ being bound to more cTn in thin filaments at any given time. This is in contrast with behavior at higher [Ca2+] where thin filament activation is more complete, and thus dissociation rate of Ca2+ is not limiting. Indeed, Fmax appears to be similar with WT and L48Q cTnC at both long and short SL, and the greatest effect of L48Q cTnC on force is when pCa50 < 5.3 at both short and long SL. A recent study (29) examining L29Q cTnC, a mutation reported to be associated with hypertrophic cardiomyopathy, demonstrated increased Ca2+-binding affinity and, interestingly, also a decrease in Ca2+ sensitivity responsiveness to changes in SL. Additionally, others have shown that replacement of native TnI with ssTnI increased Ca2+ sensitivity of force and reduced SL-dependent activation (26). Our experiments using 2-deoxy-ATP that increases Ca2+ sensitivity of force production have shown no effect on the SL dependence of pCa50 (1). Because Ca2+ binding affinity per se and cTnC-cTnI interaction strength are coupled processes, it is difficult to discern which may be responsible for the loss of SL dependence of the Ca2+ sensitivity of thin filament activation and force development.
Further studies will be necessary to determine the relative importance of Ca2+-binding affinity and the strength of cTnC-cTnI interaction in the mechanism of the SL dependence of the force-pCa relationship in cardiac muscle. Use of other variants with altered Ca2+ sensitivity, Ca2+-sensitizing agents (1, 2), and/or altered strength of cTnC-cTnI interaction or factors changing C-I interactions, like PKA phosphorylation of TnI that lowers cTnC affinity for TnI, can be important tools in determining this. Regardless of the mechanism behind our results, this work demonstrates that alteration of intrinsic properties of troponin can have dramatic effect on thin filament activation in response to crossbridge binding, and thus, in determining SL dependence of force in cardiac muscle. Perhaps most important, the properties of cTn are likely to have implications for many familial inherited cardiomyopathies that are associated with mutations in thin filament proteins.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65497 and HL-091368 (to M. Regnier). F. S. Korte was supported in part by American Heart Association postdoctoral fellowship 09POST2310117 and NIH T32 HL007828. E. R. Feest was supported in part by a National Science Foundation Graduate Research Fellowship under Grant DGE-0718124. M. Regnier is an Established Investigator of the American Heart Association.
DISCLOSURES
M. Regnier holds a provisional patent and has filed an International Patent Application (PCT/US12/39897) on “Cell and Gene Based Methods to Improve Cardiac Function” that includes the L48Q cTnC protein.
AUTHOR CONTRIBUTIONS
Author contributions: F.S.K., E.R.F., M.V.R., and M.R. conception and design of research; F.S.K., E.R.F., M.V.R., and A.-Y.T. performed experiments; F.S.K., E.R.F., M.V.R., and A.-Y.T. analyzed data; F.S.K., E.R.F., M.V.R., and M.R. interpreted results of experiments; F.S.K., E.R.F., and M.V.R. prepared figures; F.S.K., E.R.F., and M.V.R. drafted manuscript; F.S.K., E.R.F., M.V.R., A.-Y.T., and M.R. edited and revised manuscript; F.S.K., E.R.F., M.V.R., A.-Y.T., and M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Zhaoxiong Luo for preparation of recombinant troponin complexes.
REFERENCES
- 1. Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J 87: 1784–1794, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arteaga GM, Kobayashi T, Solaro RJ. Molecular actions of drugs that sensitize cardiac myofilaments to Ca2+. Ann Med 34: 248–258, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I J Physiol 526: 541–549, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83: 3542–3546, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandra M, Rundell V, Tardiff J, Leinwand L, De Tombe P, Solaro R. Ca2+ activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol 280: H705–H713, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Chandra M, Tschirgi ML, Rajapakse I, Campbell KB. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys J 90: 2867–2876, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong WJ, Jayasundar JJ, An J, Xing J, Cheung HC. Effects of PKA phosphorylation of cardiac troponin I and strong crossbridge on conformational transitions of the N-domain of cardiac troponin C in regulated thin filaments. Biochemistry 46: 9752–9761, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farman GP, Allen EJ, Schoenfelt KQ, Backx PH, de Tombe PP. The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys J 99: 2978–2986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farman GP, de Tombe PP, Irving TC. Radial head position of cardiac muscle affects calcium sensitivity. Biophys J 84: 139a, 2003 [Google Scholar]
- 10. Fitzsimons DP, Moss RL. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ Res 83: 602–607, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Fuchs F, Martyn DA. Length-dependent Ca2+ activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil 26: 199–212, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Fuchs F, Wang YP. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol 28: 1375–1383, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Fukuda N, O-Uchi J, Sasaki D, Kajiwara H, Ishiwata S, Kurihara S. Acidosis or inorganic phosphate enhances the length dependence of tension in rat skinned cardiac muscle. J Physiol 536: 153–160, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J Physiol 553: 147–154, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem 68: 687–728, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Gillis TE, Martyn DA, Rivera AJ, Regnier M. Investigation of thin filament near-neighbour regulatory unit interactions during force development in skinned cardiac and skeletal muscle. J Physiol 580: 561–576, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon A, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Gordon AM, Ridgway EB. Cross-bridges affect both TnC structure and calcium affinity in muscle fibers. Adv Exp Med Biol 332: 183–192, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Hanft LM, Korte FS, McDonald KS. Cardiac function and modulation of sarcomeric function by length. Cardiovasc Res 77: 627–636, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Hofmann PA, Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol Cell Physiol 253: C90–C96, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Holubarsch C, Ruf T, Goldstein DJ, Ashton RC, Nickl W, Pieske B, Pioch K, Lüdemann J, Wiesner S, Hasenfuss G, Posival H, Just H, Burkhoff D. Existence of the Frank-Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation 94: 683–689, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Houmeida A, Heeley DH, Belknap B, White HD. Mechanism of regulation of native cardiac muscle thin filaments by rigor cardiac myosin-S1 and calcium. J Biol Chem 285: 32760–32769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacob R, Dierberger B, Kissling G. Functional significance of the Frank-Starling mechanism under physiological and pathophysiological conditions. Eur Heart J 13, Suppl E: 7–14, 1992 [DOI] [PubMed] [Google Scholar]
- 24a. Köhler J, Chen Y, Brenner B, Gordon AM, Kraft T, Martyn DA, Regnier M, Rivera AJ, Wang CK, Chase PB. Familial hypertrophic cardiomyopathy mutations in troponin I (K183D, G203S, K206Q) enhance filament sliding. Physiol Genomics 14: 117–128, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90: 59–65, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547: 951–961, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreutziger KL, Piroddi N, McMichael JT, Tesi C, Poggesi C, Regnier M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J Mol Cell Cardiol 50: 165–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang B, Chung F, Qu Y, Pavlov D, Gillis TE, Tikunova SB, Davis JP, Tibbits GF. Familial hypertrophic cardiomyopathy-related cardiac troponin C mutation L29Q affects Ca2+ binding and myofilament contractility. Physiol Genomics 33: 257–266, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Martyn DA, Adhikari BB, Regnier M, Gu J, Xu S, Yu LC. Response of equatorial x-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle. Biophys J 86: 1002–1011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martyn DA, Chase PB, Hannon JD, Huntsman LL, Kushmerick MJ, Gordon AM. Unloaded shortening of skinned muscle fibers from rabbit activated with and without Ca2+. Biophys J 67: 1984–1993, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morimoto S, Ohtsuki I. Ca2+ binding to cardiac troponin C in the myofilament lattice and its relation to the myofibrillar ATPase activity. Eur J Biochem 226: 597–602, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Moss R, Razumova M, Fitzsimons D. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ Res 94: 1290–1300, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Potter JD. Preparation of troponin and its subunits. Methods Enzymol 85: 241–263, 1982 [DOI] [PubMed] [Google Scholar]
- 35. Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J 87: 1815–1824, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Regnier M, Morris C, Homsher E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am J Physiol Cell Physiol 269: C1532–C1539, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Regnier M, Rivera A, Chen Y, Chase P. 2-Deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res 86: 1211–1217, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Regnier M, Rivera AJ, Wang CK, Bates MA, Chase PB, Gordon AM. Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca2+ relations. J Physiol 540: 485–497, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwinger RH, Böhm M, Koch A, Schmidt U, Morano I, Eissner HJ, Uberfuhr P, Reichart B, Erdmann E. The failing human heart is unable to use the Frank-Starling mechanism. Circ Res 74: 959–969, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Smith L, Tainter C, Regnier M, Martyn D. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys J 96: 3692–3702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun YB, Irving M. The molecular basis of the steep force-calcium relation in heart muscle. J Mol Cell Cardiol 48: 859–865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun YB, Lou F, Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol 587: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tachampa K, Wang H, Farman GP, de Tombe PP. Cardiac troponin I threonine 144: role in myofilament length dependent activation. Circ Res 101: 1081–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Tikunova SB, Davis JP. Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. J Biol Chem 279: 35341–35352, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Wang D, Robertson I, Li M, McCully M, Crane M, Luo Z, Tu AY, Daggett V, Sykes B, Regnier M. Structural and functional consequences of the cardiac troponin C L48Q Ca2+-sensitizing mutation. Biochemistry 51: 4473–4487, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang YP, Fuchs F. Osmotic compression of skinned cardiac and skeletal muscle bundles: effects on force generation, Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol 27: 1235–1244, 1995 [DOI] [PubMed] [Google Scholar]