Abstract
Reduced myofibrillar ATP availability during prolonged myocardial ischemia may limit post-ischemic mechanical function. Because creatine kinase (CK) is the prime energy reserve reaction of the heart and because it has been difficult to augment ATP synthesis during and after ischemia, we used mice that overexpress the myofibrillar isoform of creatine kinase (CKM) in cardiac-specific, conditional fashion to test the hypothesis that CKM overexpression increases ATP delivery in ischemic-reperfused hearts and improves functional recovery. Isolated, retrograde-perfused hearts from control and CKM mice were subjected to 25 min of global, no-flow ischemia and 40 min of reperfusion while cardiac function [rate pressure product (RPP)] was monitored. A combination of 31P-nuclear magnetic resonance experiments at 11.7T and biochemical assays was used to measure the myocardial rate of ATP synthesis via CK (CK flux) and intracellular pH (pHi). Baseline CK flux was severalfold higher in CKM hearts (8.1 ± 1.0 vs. 32.9 ± 3.8, mM/s, control vs. CKM; P < 0.001) with no differences in phosphocreatine concentration [PCr] and RPP. End-ischemic pHi was higher in CKM hearts than in control hearts (6.04 ± 0.12 vs. 6.37 ± 0.04, control vs. CKM; P < 0.05) with no differences in [PCr] and [ATP] between the two groups. Post-ischemic PCr (66.2 ± 1.3 vs. 99.1 ± 8.0, %preischemic levels; P < 0.01), CK flux (3.2 ± 0.4 vs. 14.0 ± 1.2 mM/s; P < 0.001) and functional recovery (13.7 ± 3.4 vs. 64.9 ± 13.2%preischemic RPP; P < 0.01) were significantly higher and lactate dehydrogenase release was lower in CKM than in control hearts. Thus augmenting cardiac CKM expression attenuates ischemic acidosis, reduces injury, and improves not only high-energy phosphate content and the rate of CK ATP synthesis in postischemic myocardium but also recovery of contractile function.
Keywords: creatine kinase, ischemia-reperfusion, metabolism, ATP
the creatine kinase (ck) reaction plays a central role in cardiac energy metabolism, maintaining cellular ATP levels during periods of increased demand by rapid and reversible conversion of phosphocreatine (PCr) and ADP to ATP and creatine (32). Severe ischemia reduces myocardial high-energy phosphates (HEP) with a rapid decline in PCr and a slower fall in ATP followed by a poor recovery of the rate of ATP synthesis via CK (i.e., CK flux) and contractile function during reperfusion (6, 17, 24). A critical role for ATP delivery by CK in ischemic-reperfused myocardium is suggested by impaired recovery of postischemic mechanical function in rabbit hearts subjected to pharmacological inhibition of CK (30) and in mouse hearts with CK gene deletion (36). Nonetheless, attempts to improve CK energy metabolism in the postischemic myocardium have had limited success. Although pharmacological agents improve HEP levels and mechanical function in postischemic hearts (27, 42), these effects are largely associated with reduced myocardial energy demands rather than direct augmentation of CK. Consequently, the possibility that direct CK augmentation might enchance contractile function in the ischemia-reperfusion setting is appealing but unproven.
Because the rate of turnover of HEPs through CK is decreased after ischemia and related to ventricular performance (6, 25), reduced CK flux may, in part, limit postischemic function (2, 3). Thus increasing cardiac CK expression is a logical approach to augment basal CK metabolism and to test whether CK overexpression increases ATP delivery via CK in ischemic-reperfused hearts and improves functional recovery. We generated cardiac-specific, conditional transgenic mice that overexpress the myofibrillar isoform of CK (CKM) (12) and subjected them to total global ischemia followed by reperfusion. We hypothesized that cardiac CKM overexpression delays ATP depletion during ischemia, and improves recovery of HEP, CK flux, and contractile function in postischemic myocardium. 31P-magnetic resonance (MR) spectroscopy (MRS) was used to quantify the ex vivo energetic consequences of cardiac CKM transgene expression throughout the ischemia-reperfusion protocol in the same hearts. We report here that myocardial CKM overexpression improves ATP kinetics, attenuates ischemic acidosis, and better preserves postischemic energetics and contractile function.
METHODS
Transgenic mice.
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. Double-transgenic mice overexpressing CKM and the regulatory protein tetracycline-controlled transactivator (tTA) were generated using the Tet-Off system as described previously (12). Briefly, mice encoding tTA under the control of α-myosin heavy chain promoter (α-MHC-tTA mice) were obtained first. Subsequently, mice expressing the gene of interest (i.e., CKM) under the control of tetracycline-responsive element were generated by microinjecting the CKM construct into fertilized mouse embryos (C57BL/6 strain) at the University of California, Los Angeles Transgenic Core facility. Double-transgenic mice were obtained by crossing tetracycline-responsive element-CKM mice with α-MHC-tTA mice, which allows conditional cardiac-specific transgene expression that was confirmed by genotyping. This allowed the CKM transgene to be expressed in a cardiac-specific conditional fashion. Double transgenic mice were chronically fed a diet containing doxycycline (650 mg/kg, Diet-2018 Teklad global). Gene induction was achieved by switching to a regular diet without doxycycline at least 4 wk before these studies. Age-matched littermates, expressing only tTA, were also fed a regular diet without doxycycline for at least 4 wk before study and were used as controls.
Heart perfusion.
Male mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (80 mg/kg body wt and repeated as necessary until a deep level of anesthesia was obtained as documented per toe pinch). After administration of a pentobarbital overdose, hearts were isolated and subjected to retrograde perfusion via the aortic cannula (Langendorff mode) at constant pressure of 100 cmH2O as previously described (12) using modified Krebs-Henseleit buffer at 37°C containing (in mM) 118.5 NaCl, 25 NaHCO3, 5.9 KCl, 1.2 MgSO4, 1.6 CaCl2, and 5 glucose. A water-filled latex balloon attached to a pressure transducer was inserted into the left ventricular cavity via the mitral valve. Left ventricular developed pressure [LVDP], change in pressure over time, and heart rate were recorded using a PowerLab system (AD Instruments) and the rate pressure product [RPP = LVDP × heart rate] was calculated.
Perfused hearts were placed inside a 10-mm diameter Nuclear Magnetic Resonance (NMR) tube and lowered into a Bruker Avance-500 (11.7T) vertical bore MR system interfaced with Xwin NMR software. Temperature was maintained at 37.0 ± 0.5°C by the variable temperature unit of the spectrometer. After baseline perfusion for 25 min, hearts were subjected to 25 min of no-flow ischemia at 37°C followed by 40 min reperfusion. Recovery of contractile function during reflow was expressed as a percentage of the preischemic LVDP and RPP.
31P-NMR spectroscopy.
Relative changes in the concentration of phosphorus metabolites were recorded during the ischemia-reperfusion protocol by acquiring consecutive 3-min 31P-NMR spectra at 202.5 MHz without proton decoupling by signal averaging 128 scans and using sweep width of 8,090 Hz (pulse width 12 μs, flip angle 67°, relaxation delay 1 s). The areas for PCr, β-ATP, and inorganic phosphate (Pi) were determined using National Institutes of Health Image analysis software (ImageJ) and expressed as a percentage of the respective peak areas of preischemic control spectra for each heart. Intracellular pH (pHi) was estimated from the chemical shift of Pi peak relative to PCr as described previously (8). Because hearts were perfused with a phosphate-free buffer, the Pi peak exclusively depicted intracellular phosphate.
Magnetization transfer (MT) was performed to measure the rate of ATP synthesis via CK (i.e., CK flux) by applying a continuous-wave saturating pulse (62 dB, 64 scans, pulse width 16 μs, flip angle 90°) centered at the γ-ATP for 8 s and measuring the PCr signal intensity (M0′). A control experiment was performed in which the selective irradiation was placed downfield at a frequency equidistant from the PCr resonance before measuring the PCr signal (M0). An entire MT experiment was completed in 18 min. MT experiments were performed both at baseline before ischemia and during reflow to determine the pseudo-first-order rate constant (kPCr→ATP) for the forward CK reaction (PCr + ADP + H+ → ATP + creatine). Intrinsic T1 PCr, the relaxation time of PCr in the absence of chemical exchange, was calculated in other studies in control and CKM hearts (12, 13) and used here to quantify kPCr→ATP [kPCr→ATP = (M0 − M0′)/(T1PCr × M0′)] as described previously (9). CK flux was determined as the product of kPCr→ATP and [PCr] (12, 13).
MT experiments were also used to quantify the unidirectional rates of ATP synthesis from Pi in the myocardium. kPi→ATP, the pseudo-first order rate constant for Pi→ATP synthesis, was determined by measuring the Pi signal in the presence (M0′Pi) and absence (M0Pi) of γ-ATP resonance saturation as described above. Intrinsic T1Pi, the spin lattice relaxation time for Pi in the absence of chemical exchange calculated in an earlier study (35), was used here to determine kPi→ATP [kPi→ATP = (M0Pi − M0′Pi)/ (T1Pi × M0′Pi)]. ATP synthesis from Pi was then determined as the product of [Pi] and kPi→ATP (35).
Biochemistry.
At the end of reperfusion, hearts were rapidly frozen in liquid nitrogen and weighed. Perchloric acid extracts of cardiac tissue were obtained (14) and myocardial [ATP] measured using a luciferase enzyme-based method (31).
In a separate series of experiments, isolated hearts from control and CKM mice were perfused for rapid freezing at baseline, end-ischemic, and end-reperfusion conditions to measure myocardial-free creatine levels, CKM and CK-mitochondrial (CK-mito) protein expression, in vitro total CK activity, and lactate dehydrogenase (LDH) release during reperfusion to quantify tissue injury. Myocardial-free creatine was determined in perchloric acid extracts of tissue using an enzyme-linked assay (37, 38), whereas total in vitro CK activity (12) and LDH release (23) were measured as described previously. Expression of CKM and CK-mito isoforms were determined by standard Western blotting techniques as reported previously (7) using antibodies specific to CKM and CK-mito as described recently in detail (12).
Calculation of [ADP], −ΔG∼ATP, and predicted CK velocity.
Intracellular free [ADP] was calculated from the CK reaction at equilibrium via [ADP] = ([ATP][free Cr])/([PCr][H+]Keq) (Eq. 1), where the cytosolic concentrations are in moles per liter, Keq is 1.66 × 109 l/mol for a [Mg+2] of 1.0 mmol/l (4, 33). A myocardial cytosolic volume of 0.48 ml/g wet wt (33) was used to convert metabolites measured in millimoles per kilogram wet weight to moles per liter. The free-energy change of ATP hydrolysis [-ΔG∼ATP (kJ/mol)] was determined from the formula ΔG∼ATP = ΔG0 + RT log [ADP] [Pi]/[ATP] (Eq. 2), where ΔG0 is the standard free energy change, R the universal gas constant, and T is the absolute temperature (11).
The theoretically predicted rate of the CK equation was calculated from the following relationship (4, 43): νfpred = [Vfmax [ADP][PCr]]/D Km(ADP)Ki(PCr) (Eq. 3), where Vmax is the reported maximum velocity of the CK reaction (4, 21); [PCr] was measured by 31P-MRS as described above; [ADP] was calculated per equation [1]; the Km of ADP was 0.07; and Ki of PCr is 1.13, both taken from Ref. 4; and D was determined from the relationship: D = 1 + [ADP]/Ki(ADP) + [PCr]/Ki(PCr) + [ATP]/Ki(ATP) + [Cr]/Ki(Cr) + [ADP][PCr]/Km(ADP)Ki(PCr) + [ATP][Cr]/Km(ATP)Ki(Cr) + [ADP][Cr]/Km(ADP)Ki(Cr) (Eq. 4).
Statistical analysis.
Data are expressed as means ± SE. Comparison of two groups was performed with paired or unpaired Student's t-test, as appropriate, whereas multiple comparisons were performed with ANOVA. Significance for repeated variables was determined by repeated-measures ANOVA. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of CKM overexpressing hearts.
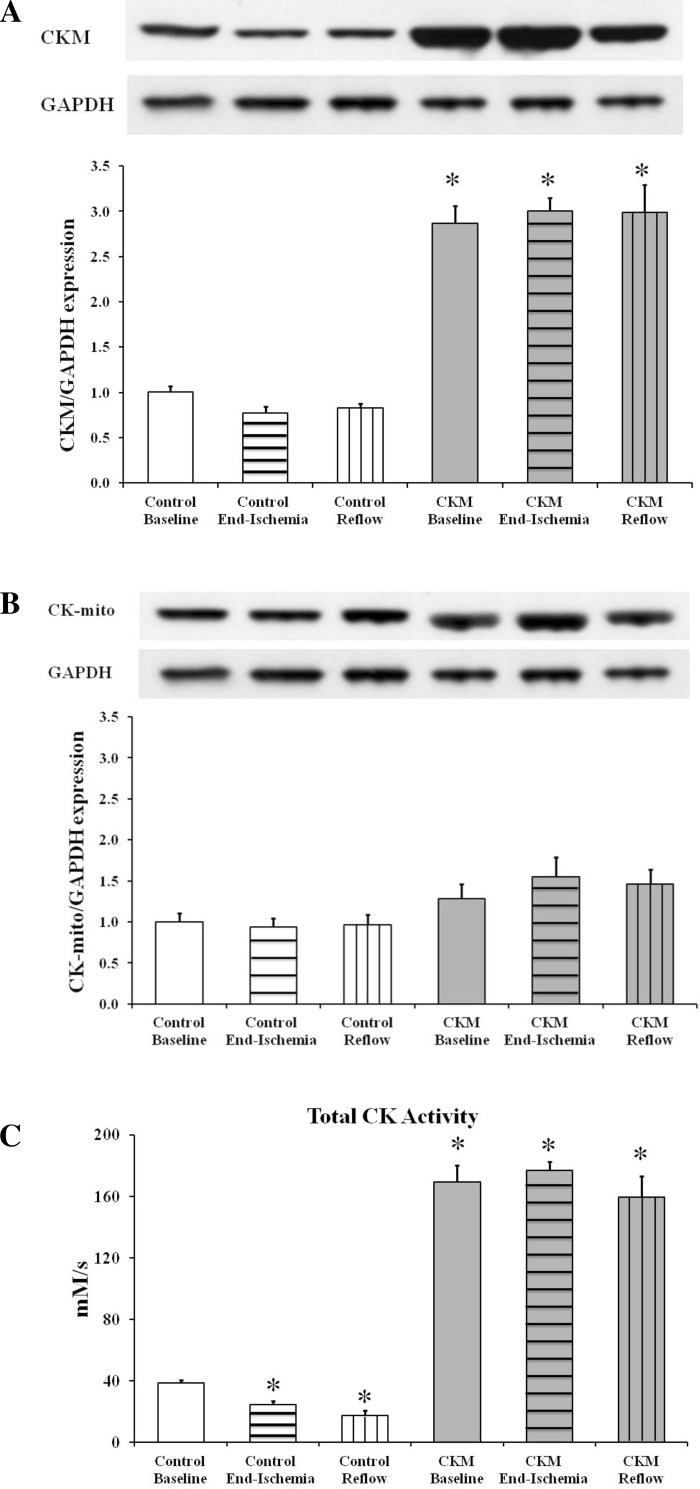
Myocardial CKM gene overexpression augmented CKM protein expression by ∼200% and increased total CK activity by about ∼300% but did not affect CK-mito protein expression in CKM versus control hearts (Fig. 1). Nonetheless, heart weight [181.6 ± 10.5 vs. 206.6 ± 10.9 mg, control vs. CKM; P = not significant (NS)] and heart weight-to-body weight ratios (5.0 ± 0.4 vs. 5.4 ± 0.4 mg/g, control vs. CKM; P = NS) were comparable in the two groups, as were baseline myocardial function (Table 1), mean cardiac PCr/ATP (1.77 ± 0.04 vs. 1.68 ± 0.07, control vs. CKM; P = NS) and [ATP] (12.4 ± 1.7 vs. 14.3 ± 1.7 mM; P = NS). Creatine (nonphosphorylated) was similar in control and CKM hearts at baseline (8.2 ± 1.2 vs. 8.8 ± 1.8 mM; P = NS) as was [ADP] (45.4 ± 1.3 vs. 51.5 ± 3.0 μM; P = 0.10).
Fig. 1.
Myofibrillar isoform of creatine kinase (CKM; A) and mitochondrial isoform of creatine kinase (CK-mito; B) protein expression as well as total CK activity (C) in control baseline, control end-ischemia, control reflow, CKM baseline, CKM end-ischemia, and CKM reflow hearts. Results expressed as means ± SE for n = 5 to 6 in each group. *P < 0.01 vs. control baseline.
Table 1.
Contractile function
| Heart Rate, beats/min | LVDP, cmH2O | RPP × 103, cmH2O/min | Maximum dP/dt, cmH2O/s | %LVDP recovery | %RPP recovery | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Control | 352 ± 55 | 91.4 ± 8.0 | 31.5 ± 4.4 | 2,491 ± 256 | – | – |
| CKM | 303 ± 28 | 97.6 ± 7.6 | 28.8 ± 1.7 | 1,928 ± 177 | – | – |
| At 40 min of reperfusion | ||||||
| Control | 435 ± 110 | 12 ± 3# | 4.1 ± 0.9# | 457 ± 112# | 13.1 ± 3.0# | 13.7 ± 3.4# |
| CKM | 352 ± 30 | 52 ± 9*† | 18.7 ± 4.0* | 1,522 ± 230* | 52.9 ± 8.0* | 64.9 ± 13.2* |
Values are means ± SE for n = 5 in each group. LVDP, left ventricular developed pressure; dP/dt, change in pressure over time; RPP, rate pressure product.
P < 0.01 vs. control during reperfusion;
P < 0.01 vs. control at baseline;
P < 0.05 vs. myofibrillar isoform of creatine kinase (CKM) at baseline.
Ischemia.
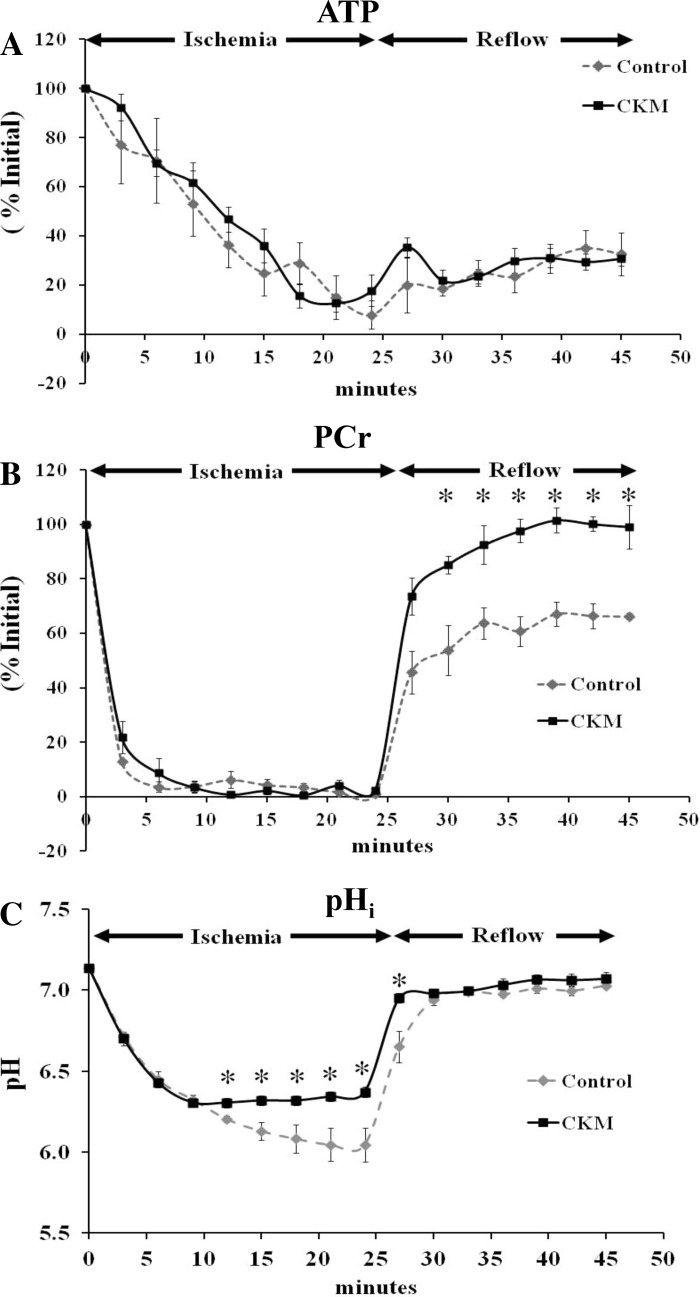
Within 5 min of the onset of global, total ischemia, LVDP was undetectable in both groups with no differences between the groups (data not shown). CKM expression was unchanged at the end of ischemia, as compared with baseline values in both groups, whereas mean in vitro CK activity declined by about 25–30% in control hearts (Fig. 1). Changes in myocardial ATP determined by 31P-MRS during ischemia in control and in CKM hearts are shown in Fig. 2A. ATP levels declined steadily but were not significantly different between the two groups, with levels reaching 8–18% of initial, baseline ATP values at the end of ischemia. PCr decreased rapidly during ischemia in both groups with levels falling to ∼10% of initial PCr in the first 5 min (Fig. 2B). The pHi fell more during ischemia, particularly after 12 min of ischemia, in control than in CKM hearts (Fig. 2C) with values reaching 6.04 ± 0.12 vs. 6.37 ± 0.04, respectively (P < 0.05), at the end of ischemia, suggesting better preservation of intracellular milieu in CKM hearts.
Fig. 2.
Effect of ischemia and reperfusion on percent changes in ATP (A) and phosphocreatine (PCr; B) as well as changes in intracellular pH (pHi; C) in control (gray) and CKM (black) mouse hearts. Results are means ± SE for n = 5 in each group. *P < 0.05 vs. control.
Reperfusion.
Toward the end of reperfusion, CKM hearts showed three- to fourfold better functional recovery (LVDP and RPP as % baseline) than control hearts (Table 1). In vitro CK activity trended below ischemic values in control but not CKM hearts (Fig. 1). There were no significant differences in ATP levels between control and CKM hearts (4.9 ± 0.5 vs. 6.3 ± 0.4 mM, control vs. CKM, determined by luciferase assay; P = 0.07) with levels reaching ∼30% of initial, baseline ATP values by the end of reperfusion (Fig. 2A). However, PCr levels increased in both groups and reached ∼66% of preischemic levels in control hearts and ∼99% of preischemic levels in CKM hearts (control vs CKM; P < 0.01; Fig. 2B). During reperfusion, pHi returned to preischemic levels in both groups (Fig. 2C). Creatine was similar in control and CKM hearts at the end of reperfusion (5.2 ± 0.6 vs. 6.1 ± 1.3 mM; P = NS) as were [ADP] (13.8 ± 0.8 μM vs. 13.8 ± 0.7 μM; P = NS) and ΔG∼ATP (−54.1 ± 0.4 vs. −54.7 ± 0.4 kJ/mol; P = NS). Cumulative LDH release over 40 min of reperfusion was significantly lower in CKM (28.8 ± 7.4 units/g) than in control (69.4 ± 10.5 units/g; P < 0.02) hearts, indicating less ischemic/reperfusion injury in the former.
Saturation transfer.
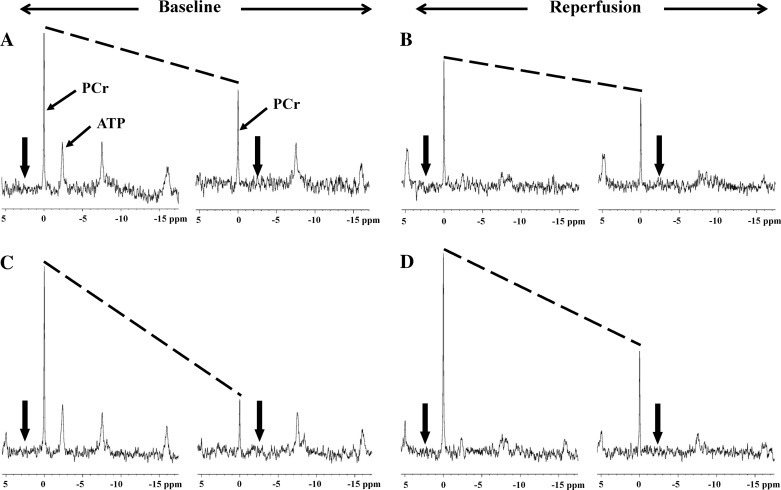
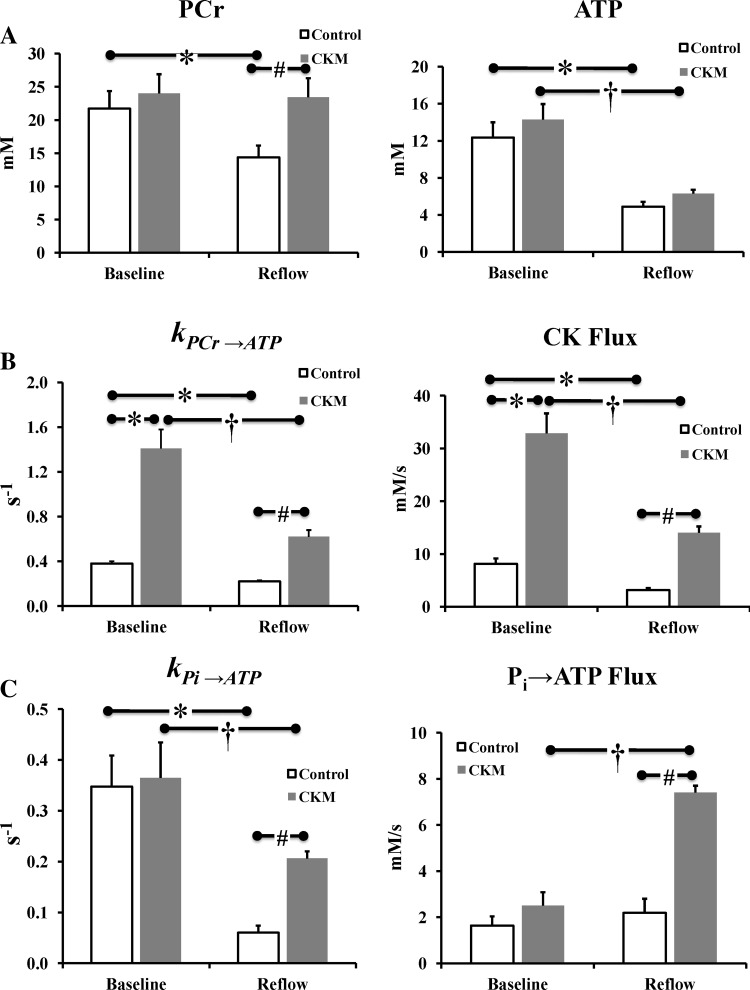
Figure 3, A and C, shows the representative MT spectra from control and CKM hearts at baseline. The reduction in PCr signal intensity with γ-ATP saturated (left vs. right spectrum in each pair) is directly proportional to the rate of ATP synthesis through CK (43). A greater decline in PCr signal observed in CKM versus control hearts indicates higher ATP flux through CK in the former hearts. The preischemic summary values for kPCr→ATP (0.38 ± 0.02 vs. 1.41 ± 0.17 s−1, control vs. CKM; P < 0.001) and ATP flux via CK (8.1 ± 1.0 vs. 32.9 ± 3.8 mM/s; P < 0.001; Fig. 4) were approximately threefold higher in CKM than those in control hearts. However, the preischemic values for kPi→ATP (0.35 ± 0.06 vs. 0.36 ± 0.07 s−1, control vs. CKM; P = 0.86) and unidirectional rates of ATP synthesis from Pi (1.64 ± 0.4 vs. 2.5 ± 0.6 mM/s; P = 0.26) were not different between the two groups.
Fig. 3.
Representative 31P-magnetization transfer (MT) spectra from control (A, baseline; B, reflow) and CKM (C, baseline; D, reflow) hearts. The spectra were acquired with saturating irradiation (thick arrows) in the control position (left spectrum in each panel) and γ-ATP position (right spectrum in each panel). The decrease in the height of PCr peak between control and γ-ATP saturation (slope of the dotted lines) is directly related to the rate of ATP synthesis through the CK reaction. A greater decline in PCr signal with γ-ATP saturation observed in CKM vs. control hearts (both at baseline and during reperfusion) indicates higher ATP flux through CK in CKM hearts.
Fig. 4.
Effect of ischemia and reperfusion on levels of PCr and ATP (A), the CK pseudo-first-order rate constant (kPCr→ATP), and rate of ATP synthesis via creatine kinase (CK flux; B) as well as Pi→ATP pseudo-first-order rate constant (kPi→ATP) and rate of ATP synthesis from Pi (C) in control (white) and CKM (gray) mouse hearts. Results are means ± SE for n = 5 in each group. *P < 0.01 vs. control (baseline); †P < 0.05 vs. CKM (baseline); #P < 0.01 vs. control (reflow).
Figure 3, B and D, shows the representative MT spectra from control and CKM hearts, respectively, during reperfusion. Mean kPCr→ATP (0.22 ± 0.01 vs. 0.62 ± 0.06 s−1, control vs. CKM; P < 0.001) and CK flux (3.2 ± 0.4 vs. 14.0 ± 1.2 mM/s; P < 0.001; Fig. 4) were severalfold higher in CKM than in control hearts during reperfusion. Indeed, ATP flux via CK was ∼70% higher in ischemic-reperfused CKM hearts than in control hearts at baseline. In addition, mean kPi→ATP (0.06 ± 0.01 vs. 0.21 ± 0.01 s−1, control vs. CKM; P < 0.001) and Pi→ATP flux (2.2 ± 0.6 vs. 7.4 ± 0.3 mM/s; P < 0.01) were approximately threefold higher in CKM than in control hearts during reperfusion (Fig. 4). Thus CKM overexpression increases the rates of ATP synthesis from both the CK reaction and from Pi during postischemic reperfusion.
DISCUSSION
Myocardial energy metabolism, and particularly that related to CK, is deranged during ischemia-reperfusion as evidenced by a decline in PCr and ATP during ischemia together with a decrease in the rate of ATP synthesis via CK during reperfusion (6, 17, 24). We report here that cardiac-specific overexpression of the myofibrillar isoform of CK limits the ischemic fall in pHi and during reperfusion enhances the rate of ATP synthesis via CK and from inorganic phosphate, reduces LDH loss, and improves contractile function. These changes demonstrate that augmenting cardiac CK expression improves ATP kinetics and mechanical function and reduces injury in postischemic myocardium.
CKM overexpression resulted in a twofold increase in mean myocardial CKM protein expression (vs. control hearts; Fig. 1) together with a two- to threefold increase in in vitro CK activity, kPCr→ATP, and ATP flux via CK but no change in [ATP] (Fig. 4), PCr/ATP, heart weight/body weight, or contractile function at baseline (Table 1). These observations suggest that CKM is at equilibrium in the normal heart since enhanced CKM expression augments ATP flux via CK without affecting the relative or absolute amounts of CK metabolites and/or cardiac morphology and function.
A role for CK in postischemic recovery of contractile function has been suggested by the blunted recovery of ventricular performance following an ischemic insult in CK-deficient mouse hearts (36) and in rabbit hearts pretreated with iodoacetamide, a CK inhibitor (30). Furthermore, increased susceptibility of hearts with genetic ablation of guanidinoacetate-N-methyltransferase, the final enzyme in the creatine synthesis pathway, to ischemia-reperfusion injury and to impaired contractile reserve also suggests that the CK system can affect contractile function in the ischemia-reperfusion setting (40). However, none of the prior approaches specifically augmented CK capacity to determine whether that could improve postischemic recovery. Thus we chose to augment CK expression and myofibrillar ATP synthesis through CK in murine myocardium rather than enhance CK metabolite pool sizes. It is noteworthy here that pharmacological strategies to preserve myocardial HEP levels and function postischemia have done so by primarily inhibiting cellular calcium overload, reducing myocardial energetic demand, or by increasing coronary flow (18, 27, 42) but not by directly augmenting CK. Furthermore, chronic creatine feeding in rats failed to augment myocardial HEP levels, ATP flux via CK, and contractile function after myocardial infarction (16). Indeed, a genetic approach to augment murine myocardial HEP levels by overexpressing sarcolemmal creatine-transporter resulted in left ventricular hypertrophy, dilatation, and contractile dysfunction together with increased ADP concentration and decreased free-energy levels (41). In contrast, our strategy to enhance CK expression in the heart improved ATP synthesis via CK and consequently function in the reperfused myocardium, a finding that is truly novel and provides a better understanding of the role of CK metabolism in the postischemic myocardium.
The CK reaction serves as spatial and temporal ATP buffer in the myocardium maintaining high cytosolic [ATP] at sites of ATP utilization and low ADP levels at sites of ATP synthesis (32). Both temporal and spatial buffering actions of CK may be important when energy demands vary widely during the cardiac cycle and with augmented cardiac work (15). The ∼60% reduction in CK flux in control hearts during reperfusion could limit contractile ATP supply or increase cytosolic-free ADP. The drop in CK flux in control hearts was due to a fall in both kPCr→ATP and [PCr]. An approximately fourfold higher ATP flux via CK observed in CKM hearts postischemia implies that these hearts replenish the ATP pool (assuming no change in oxidative phosphorylation) in a quarter of the time taken by control hearts thereby, rapidly generating myofibrillar ATP and maintaining a low myofibrillar ADP concentration. However, the global, time-averaged free cytosolic [ADP] was not different between control and CKM hearts during reperfusion. This suggests that the main affect of CKM overexpression during reperfusion is to increase rates of myofibrillar ATP delivery via CK rather than by further lowering cytosolic [ADP] diminished during reperfusion, a local subcellular effect notwithstanding (1, 19).
The attenuation of ischemic acidosis here in CKM overexpressing hearts compares with that observed in livers overexpressing the brain-isoform of CK (22). In contrast with control livers devoid of CK expression and activity, livers expressing CK maintained a significantly higher pHi and ATP during ischemia, suggesting better pH buffering by CK, and they also evidenced less cellular injury during reperfusion (22). The CK reaction consumes protons (PCr + ADP + H+ ↔ ATP + creatine). Myocardial CKM overexpression could therefore augment proton consumption, and this alkalinizing effect could, in addition to unidentified factors, attenuate acidosis in CKM hearts. Greater ischemic acidosis in control hearts (vs. CKM hearts) observed here could jeopardize functional recovery by augmenting reperfusion-related Na+/H+ exchange and hence intracellular Na+, resulting in higher Na+/Ca2+ exchange and a consequent rise in intracellular Ca2+ (24, 29, 39). Because intracellular pH was not independently modulated in control hearts during ischemia to that of CKM hearts, we cannot determine the extent to which the higher end-ischemic pH in CKM hearts per se contributed to their improved PCr recovery. However, in contrast, the more rapid recovery of pHi during the first minutes of reperfusion in CKM hearts as compared with control (Fig. 2C) would be expected to impair, rather than improve, their functional and PCr recovery (20, 26). Thus the attenuated ischemic acidosis and more rapid pH normalization during reperfusion with CKM overexpression may have offsetting effects on metabolic and functional recovery.
It is worth mentioning that the 31P-NMR saturation transfer method implemented here measures the unidirectional CK flux in the forward direction (i.e., in the direction of ATP synthesis) in the intact beating heart. The experiment can be performed with a different saturation scheme to measure the reverse reaction (i.e., in the direction of PCr synthesis) in which case the two can be summed to calculate net CK flux. In vitro CK activity in heart extracts correlates with, but is not the same as, CK flux in the intact heart since enzymatic conditions, pH, temperature, substrate-to-product ratios, post-translational modification of the enzyme, and other factors may not precisely replicate those of the beating heart. Changes in the rate of ATP synthesis through CK can be predicted from the CK rate equation (4) and compared with changes measured by 31P-MR in the beating heart to gain insight into factors controlling CK (4). Under baseline conditions, CKM overexpression increases the predicted rate of ATP synthesis through CK by 240% in agreement with the 260% increase in CK flux measured by 31P-MRS (Fig. 4 and Table 2). Reperfusion reduced the predicted rate of ATP synthesis through CK by ∼80% and ∼57% in control and CKM hearts, respectively, which is comparable with the 60% and 51% reductions, respectively, in measured cardiac CK flux. This suggests that under baseline and reperfusion conditions CK enzyme activity and metabolite concentrations are major determinants of CK flux in the beating heart, as previously shown for in vivo conditions in the rat (4). A way to look at the intrinsic properties of the CK protein and substrate control is to compare the rate of CK flux in the intact heart with the maximal CK activity in heart extracts (Vmax from Eq. 3) (33). The mean ratio of CK flux to CK Vmax (after both are converted to common units of millimoles per second; Table 2) was 0.21 and 0.18 for baseline and reperfusion conditions, respectively, in control mice. These are comparable with previously reported baseline values of ∼0.15 in wild-type mice (33) and suggest that, under control conditions for a given amount of CK enzyme, the CK reaction is primarily under similar substrate control during baseline and reperfusion. In CKM hearts, the ratio of CK flux to CK Vmax was 0.18 and 0.09 for baseline and reperfusion conditions, respectively, indicating substrate control for baseline conditions akin to that of control hearts, but reduced CK flux for the elevated amount of CK enzyme present in CKM mice during reperfusion. In summary, the CK enzyme has minimal nonsubstrate regulation during baseline and reperfusion conditions in control hearts, whereas the relative changes in rates of CK flux measured during CKM overexpression in intact hearts are in good agreement with those theoretically predicted. We cannot exclude the possibility that the intrinsic properties of the overexpressed CK are somewhat altered by post-translational modification (34) during reperfusion. Nevertheless, the net effect of CKM overexpression is a pronounced approximately threefold increase in the rate of ATP synthesis via CK in the reperfused heart along with a significant improvement in mechanical function.
Table 2.
Measured and predicted CK flux and CK Vmax and measured CK flux/Vmax
| Measured CK Flux, mM/s | Predicted CK Flux, mM/s | CK Vmax, mM/s | Measured CK Flux/Vmax | |
|---|---|---|---|---|
| Baseline | ||||
| Control | 8.1 ± 1.0 | 8.7 | 38.6 ± 1.8 | 0.21 |
| CKM | 32.9 ± 3.8 | 29.9 | 169.5 ± 10.8 | 0.17 |
| At 40 min of reperfusion | ||||
| Control | 3.2 ± 0.4 | 1.8 | 17.4 ± 3.2 | 0.18 |
| CKM | 14.0 ± 1.2 | 12.9 | 159.4 ± 13.8 | 0.09 |
Values are means ± SE. The measured creatine kinase (CK) flux (in mM) is the rate of ATP synthesis through CK measured by 31P-magnetic resonance spectroscopy (MRS) in intact hearts. Predicted CK flux is the predicted rate of ATP synthesis through CK calculated from the CK rate reaction. CK Vmax is the CK enzyme activity determined from heart extracts at 37°C (from Fig. 1C).
These 31P-NMR data were also analyzed to quantify the rate of phosphoryl exchange between inorganic phosphate and ATP in the direction of ATP synthesis. This rate was similar in control and CKM hearts at baseline, was unchanged during reperfusion in control hearts, but increased nearly fourfold during reperfusion in CKM hearts (Fig. 4C). The unidirectional rate of Pi→ATP exchange is influenced by all reactions in which ATP is generated from inorganic phosphate. In the perfused heart the main sources of this exchange are mitochondrial oxidative phosphorylation and GAPDH and phosphoglycerate kinase (PGK) (5, 10). The rate of oxidative phosphorylation has been related to postischemic functional recovery, whereas ATP flux through GAPDH/PGK has been closely linked to sarcoplasmic reticular calcium handling and homeostasis (44), critical during reperfusion. Although we cannot distinguish the relative contribution of these sources of Pi→ATP flux in these studies, it is clear that the fourfold increase (to ∼6 mM/s) provides significant additional ATP availability to support improved ionic homeostasis and contractile recovery during reperfusion.
Although cardiac-specific CKM overexpression improves the rate of ATP synthesis via CK and functional recovery postischemia, it does not completely normalize contractile function in this model. Nonetheless, CKM is the predominant isoform in the adult mammalian heart and, as such, was a rational primary target to test our hypothesis. Indeed, CKM overexpression improved CK function, as evidenced by enhanced ATP flux through CK in the postischemic myocardium. It is not clear whether the improvement in postischemic contractile function is due to the attenuation of ischemic acidosis, the improvement in myofibrillar ATP delivery during reperfusion, both, or another factor resulting from the improved metabolic status due to CKM overexpression. Nevertheless, this transgenic approach is an effective mode of augmenting CK capacity in the postischemic heart. However, future work is needed to delineate the mechanisms that impair CK flux during and following an ischemic insult and the impact of manipulations of other CK isoforms, so as to optimize physiologically favorable approaches to prevent and/or reverse the critical decline in CK capacity.
Limitations.
A key limitation of this study is that the isolated hearts were perfused with glucose as the only substrate, although long chain fatty acids are the dominant energy source in the in vivo well-oxygenated heart. Nevertheless, the role of glucose metabolism and glycolysis is increased during ischemia and glucose has been used as the sole substrate in isolated perfused heart studies over many years. Carbon substrate choice was not the focus of this study, but it is important to emphasize that exogenous substrate availability was identically matched between the control and CKM hearts.
Although the sample size used here was modest, nevertheless it was sufficient to identify statistically significant differences in metabolic and functional parameters and in line with American Physiological Society recommendations of the use of a minimum number of animals to produce scientifically valid results (http://www.the-aps.org/mm/SciencePolicy/Animal-Research/Publications/animals/quest3.html).
Summary.
In summary, we show that ATP synthesis via CK and contractile function are diminished in the postischemic myocardium and that augmenting cardiac CKM expression improves ATP kinetics and mechanical function in postischemic reperfused hearts. These findings highlight a crucial role for CK reaction in the pathogenesis of postischemic contractile dysfunction and suggest that enhancing CK expression offers an important strategy for improving myofibrillar ATP delivery and mechanical function and for reducing injury in reperfused myocardium.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-63030 (to R. G. Weiss) and HL-39752 (to C. Steenbergen) as well as funds from the Donald W. Reynolds Foundation and the Clarence Doodeman Endowment.
DISCLOSURES
Under a licensing agreement with NanoCor Therapeutics, Dr. Weiss is entitled to a share of royalty on sales of technology related to CK gene therapy. Dr. Weiss also is a paid consultant to and Scientific Advisory Board member of NanoCor Therapeutics. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
AUTHOR CONTRIBUTIONS
Author contributions: A.A., J.S., T.Y., A.G., M.K.L., V.P.C., and R.G.W. performed experiments; A.A., J.S., V.P.C., and R.G.W. analyzed data; A.A. drafted manuscript; A.A., T.Y., V.P.C., C.S., and R.G.W. edited and revised manuscript; A.A., J.S., T.Y., A.G., Y.W., M.K.L., V.P.C., C.S., and R.G.W. approved final version of manuscript; Y.W., C.S., and R.G.W. conception and design of research; A.A., C.S. and R.G.W. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Haiying Pu and Jing Gao for technical assistance in generating the CK transgenic mice.
REFERENCES
- 1. Aliev M, Guzun R, Karu-Varikmaa M, Kaambre T, Wallimann T, Saks V. Molecular system bioenergics of the heart: experimental studies of metabolic compartmentation and energy fluxes versus computer modeling. Int J Mol Sci 12: 9296–9331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banerjee A, Grosso MA, Brown JM, Rogers KB, Whitman GJ. Oxygen metabolite effects on creatine kinase and cardiac energetics after reperfusion. Am J Physiol Heart Circ Physiol 261: H590–H597, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Bittl JA, Balschi JA, Ingwall JS. Contractile failure and high-energy phosphate turnover during hypoxia: 31P-NMR surface coil studies in living rat. Circ Res 60: 871–878, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Bittl JA, DeLayre J, Ingwall JS. Rate equation for creatine kinase predicts the in vivo reaction velocity: 31P NMR surface coil studies in brain, heart, and skeletal muscle of the living rat. Biochemistry 26: 6083–6090, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Brindle KM, Radda GK. 31P-NMR saturation transfer measurements of exchange between Pi and ATP in the reactions catalysed by glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase in vitro. Biochim Biophys Acta 928: 45–55, 1987 [DOI] [PubMed] [Google Scholar]
- 6. Cave AC, Ingwall JS, Friedrich J, Liao R, Saupe KW, Apstein CS, Eberli FR. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation 101: 2090–2096, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Ebermann L, Piper C, Kuhl U, Klingel K, Schlattner U, Siafarikas N, Zeichhardt H, Schultheiss HP, Dorner A. Impact of myocardial inflammation on cytosolic and mitochondrial creatine kinase activity and expression. Basic Res Cardiol 104: 247–257, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Flaherty JT, Weisfeldt ML, Bulkley BH, Gardner TJ, Gott VL, Jacobus WE. Mechanisms of ischemic myocardial cell damage assessed by phosphorus-31 NMR. Circulation 65: 561–570, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Forsen S, Hoffmann R. Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys 39: 2892–2901, 1963 [Google Scholar]
- 10. From AH, Ugurbil K. Standard magnetic resonance-based measurements of the Pi–>ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles. Am J Physiol Cell Physiol 301: C1–C11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibbs C. The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol 17: 727–731, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Gupta A, Akki A, Wang Y, Leppo M, Chacko VP, Foster D, Cicares V, Kirk J, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy-starved. J Clin Invest 122: 291–302, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, Chacko VP, Schar M, Akki A, Weiss RG. Impaired ATP kinetics in failing in vivo mouse heart. Circ Cardiovasc Imaging 4: 42–50, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta A, Chacko VP, Weiss RG. Abnormal energetics and ATP depletion in pressure-overload mouse hearts: in vivo high-energy phosphate concentration measures by noninvasive magnetic resonance. Am J Physiol Heart Circ Physiol 297: H59–H64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heineman FW, Balaban RS. Phosphorus-31 NMR analysis of transient changes of canine myocardial metabolism in vivo. J Clin Invest 85: 843–852, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horn M, Remkes H, Dienesch C, Hu K, Ertl G, Neubauer S. Chronic high-dose creatine feeding does not attenuate left ventricular remodeling in rat hearts post-myocardial infarction. Cardiovasc Res 43: 117–124, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Jacobus WE, Taylor GJt Hollis DP, Nunnally RL. Phosphorus NMR of perfused working rat hearts. Nature 265: 756–758, 1977 [DOI] [PubMed] [Google Scholar]
- 18. Jolly SR, Menahan LA, Gross GJ. Diltiazem in myocardial recovery from global ischemia and reperfusion. J Mol Cell Cardiol 13: 359–372, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Joubert F, Hoerter JA, Mazet JL. Modeling the energy transfer pathways. Creatine kinase activities and heterogeneous distribution of ADP in the perfused heart. Mol Biol Rep 29: 177–182, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kusuoka H, Camilion de Hurtado MC, Marban E. Role of sodium/calcium exchange in the mechanism of myocardial stunning: protective effect of reperfusion with high sodium solution. J Am Coll Cardiol 21: 240–248, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Liao R, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res 78: 893–902, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Miller K, Halow J, Koretsky AP. Phosphocreatine protects transgenic mouse liver expressing creatine kinase from hypoxia and ischemia. Am J Physiol Cell Physiol 265: C1544–C1551, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Murphy E, LeFurgey A, Lieberman M. Biochemical and structural changes in cultured heart cells induced by metabolic inhibition. Am J Physiol Cell Physiol 253: C700–C706, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Murphy E, Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda) 23: 115–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neubauer S, Hamman BL, Perry SB, Bittl JA, Ingwall JS. Velocity of the creatine kinase reaction decreases in postischemic myocardium: a 31P-NMR magnetization transfer study of the isolated ferret heart. Circ Res 63: 1–15, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Panagiotopoulos S, Daly MJ, Nayler WG. Effect of acidosis and alkalosis on postischemic Ca gain in isolated rat heart. Am J Physiol Heart Circ Physiol 258: H821–H828, 1990 [DOI] [PubMed] [Google Scholar]
- 27. Pieper GM, Todd GL, Wu ST, Salhany JM, Clayton FC, Eliot RS. Attenuation of myocardial acidosis by propranolol during ischaemic arrest and reperfusion: evidence with 31P NMR. Cardiovasc Res 14: 646–653, 1980 [DOI] [PubMed] [Google Scholar]
- 28. Polimeni PI, Buraczewski SI. Expansion of extracellular tracer spaces in the isolated heart perfused with crystalloid solutions: expansion of extracellular space, trans-sarcolemmal leakage, or both? J Mol Cell Cardiol 20: 15–22, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Renlund DG, Gerstenblith G, Lakatta EG, Jacobus WE, Kallman CH, Weisfeldt ML. Perfusate sodium during ischemia modifies post-ischemic functional and metabolic recovery in the rabbit heart. J Mol Cell Cardiol 16: 795–801, 1984 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez P, Avellanal M, Felizola A, Barrigon S. Importance of creatine kinase activity for functional recovery of myocardium after ischemia-reperfusion challenge. J Cardiovasc Pharmacol 41: 97–104, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Ronner P, Friel E, Czerniawski K, Frankle S. Luminometric assays of ATP, phosphocreatine, and creatine for estimation of free ADP and free AMP. Anal Biochem 275: 208–216, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol 571: 253–273, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saupe KW, Spindler M, Hopkins JC, Shen W, Ingwall JS. Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. Reaction kinetics of the creatine kinase isoenzymes in the intact heart. J Biol Chem 275: 19742–19746, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Shen W, Spindler M, Higgins MA, Jin N, Gill RM, Bloem LJ, Ryan TP, Ingwall JS. The fall in creatine levels and creatine kinase isozyme changes in the failing heart are reversible: complex post-transcriptional regulation of the components of the CK system. J Mol Cell Cardiol 39: 537–544, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Spencer RG, Buttrick PM, Ingwall JS. Function and bioenergetics in isolated perfused trained rat hearts. Am J Physiol Heart Circ Physiol 272: H409–H417, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Spindler M, Meyer K, Stromer H, Leupold A, Boehm E, Wagner H, Neubauer S. Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol 287: H1039–H1045, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Steenbergen C, Deleeuw G, Rich T, Williamson JR. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ Res 41: 849–858, 1977 [DOI] [PubMed] [Google Scholar]
- 38. Steenbergen C, Deleeuw G, Williamson JR. Analysis of control of glycolysis in ischemic hearts having heterogeneous zones of anoxia. J Mol Cell Cardiol 10: 617–639, 1978 [DOI] [PubMed] [Google Scholar]
- 39. Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res 72: 112–125, 1993 [DOI] [PubMed] [Google Scholar]
- 40. ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, Sebag-Montefiore L, Watkins H, Clarke K, Isbrandt D, Wallis J, Neubauer S. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation 111: 2477–2485, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, Dawson D, Hulbert K, Zhang W, Zhang MH, Watkins H, Clarke K, Neubauer S. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: insights from creatine transporter-overexpressing transgenic mice. Circulation 112: 3131–3139, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Watts JA, Koch CD, LaNoue KF. Effects of Ca2+ antagonism on energy metabolism: Ca2+ and heart function after ischemia. Am J Physiol Heart Circ Physiol 238: H909–H916, 1980 [DOI] [PubMed] [Google Scholar]
- 43. Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA 102: 808–813, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res 77: 88–97, 1995 [DOI] [PubMed] [Google Scholar]