Abstract
We tested the responses of single, isolated lymphangions to selective changes in preload and the effects of changing preload on the response to an imposed afterload. The methods used were similar to those described in our companion paper. Step-wise increases in input pressure (Pin; preload) over a pressure range between 0.5 and 3 cmH2O, at constant output pressure (Pout), led to increases in end-diastolic diameter, decreases in end-systolic diameter, and increases in stroke volume. From a baseline of 1 cmH2O, Pin elevation by 2–7 cmH2O consistently produced an immediate fall in stroke volume that subsequently recovered over a time course of 2–3 min. Surprisingly, this adaptation was associated with an increase in the slope of the end-systolic pressure-volume relationship, indicative of an increase in contractility. Lymphangions subjected to Pout levels exceeding their initial ejection limit would often accommodate by increasing diastolic filling to strengthen contraction sufficiently to match Pout. The lymphangion adaptation to various pressure combinations (Pin ramps with low or high levels of Pout, Pout ramps at low or intermediate levels of Pin, and combined Pin + Pout ramps) were analyzed using pressure-volume data to calculate stroke work. Under relatively low imposed loads, stroke work was maximal at low preloads (Pin ∼2 cmH2O), whereas at more elevated afterloads, the optimal preload for maximal work displayed a broad plateau over a Pin range of 5–11 cmH2O. These results provide new insights into the normal operation of the lymphatic pump, its comparison with the cardiac pump, and its potential capacity to adapt to increased loads during edemagenic and/or gravitational stress.
Keywords: pressure-volume loop, isolated vessel, edema, heterometric regulation, homeometric regulation, stroke work, contractility
in our companion paper (10), we investigated the effects of selective afterload elevation on the pump function of single lymphangions isolated from the rat mesentery. Elevating output pressure (Pout), at constant preload [input pressure (Pin)], led to an intrinsic increase in lymphatic muscle contractility (i.e., positive inotropy), as characterized by a leftward shift in the end systolic pressure-volume relationship (ESPVR), increased peak dP/dt, and the development of higher peak systolic pressure. We concluded that the enhancement in contractility enables the vessel to eject against the adverse pressure gradient that normally exists in most parts of the lymphatic system and that becomes substantially elevated in lymphatic vessels of dependent extremities, including those of humans (31).
As in the heart, preload is a significant determinant of lymphatic pump function. Previous studies using isolated chains of series-coupled lymphatic segments from several species have provided evidence that lymphatic vessels are exquisitely sensitive to changes in filling pressure and accordingly modulate pump output using the Frank-Starling mechanism (13, 15, 26, 28). Notably, two studies have made use of cardiac-style analyses to study lymphatic pump function. Plots of intraluminal pressure (PL) as a function of volume (calculated from internal diameter measurements) were characterized by counterclockwise loops that shifted to the right after systemic volume expansion (3) or simultaneous elevation of Pin and Pout (26). While those studies provided valuable information about the adaptation of the intact lymphatic pump to edemagenic loads, the effects of selectively changing preload at low and high levels of afterload were not assessed. Furthermore, since only chains of multiple lymphangions were used in those and similar studies of lymphatic contractile function to date, preload and afterload were strictly controlled only for the first and last lymphangions of the chain. Thus, the “pure” effects of changing preload on lymphangion function have not been definitively resolved.
The goal of the present study was to determine how a single lymphangion adapts to a selective increase in preload. We posed the following questions: 1) what is the optimal preload associated with maximal stroke volume (SV) and other indexes of pump function? 2) does preload alter the adaptation to elevated afterload? 3) are there interactive effects between these two major determinants of lymphatic pump function? and 4) how does the response of the lymphangion compare with that of the cardiac ventricles? To answer these questions, we measured multiple pump parameters of single lymphangions isolated from the rat mesentery under well-defined conditions, where Pin and Pout could be independently manipulated while simultaneously measuring PL. The small size of these vessels enabled the use of videomicroscopic methods to measure diameter and track valve positions, thereby enhancing the analysis of single lymphangion function using the pressure-volume (P-V) relationship. The application of cardiac analyses allowed comparisons to be made between the pump behavior of lymphatic vessels and the isolated heart, thereby providing insights into the normal operation of the lymphatic pump and its potential capacity to adapt to increased loads during edemagenic stress.
METHODS
Detailed methods are given in our companion paper (10). Lymphatic vessels [inner diameter (ID): 80–220 μm] were identified and excised from mesenteric arcades of male rats (150–300 g) anesthetized with pentobarbital sodium (60 mg/kg ip). Animal protocols were approved by Animal Care and Use Committee of the University of Missouri and conformed with the Public Health Service Policy for the Humane Care and Use of Laboratory Animals (1996). After cannulation, a lymphatic vessel was transferred to the stage of an inverted microscope for subsequent study. Pressures were initially set to 3 cmH2O from standing reservoirs, and the vessel was bathed in physiological saline solution for a 30- to 60-min equilibration period at 36–37°C until a stable pattern of spontaneous contractions developed. Methods for pressure control, diameter tracking, detection of valve movements, and servo-null measurements of PL were the same as previously described (10). To control and evaluate the pump mechanics of single lymphangions, we used vessel segments containing exactly two functioning valves and exhibiting essentially synchronized contractions over the whole vessel length (valve-to-valve length of the central lymphangion: 1.16 ± 0.66 mm).
Protocols
To assess the responses of single lymphangions to step increases in preload, Pin was elevated to multiple levels at various rates at low and high levels of afterload. A selective increase in preload would probably not occur in vivo (without a concomitant increase in Pout), but this maneuver permitted the assessment of the response of the lymph pump to changes in preload alone. A single vessel was typically used for one to three of the following protocols.
Protocol 1: step increase in Pin at constant, low Pout.
Rapid, step-wise increases in Pin were imposed in ascending order to determine the speed of the adaptation and the time course over which intrinsic, secondary adjustments, if any, occurred. First, cumulative, step-wise increases in Pin from the baseline level to 1, 3, 5, 8, 12, and 16 cmH2O were imposed by the servo controller, with pressure elevation maintained for 1–2 min at each pressure. Second, the same Pin levels were tested (either randomized or sequential) but with Pin returned to the baseline level after each step; in these cases, each step and recovery period lasted ∼5 min. The order of the Pin steps made little difference as long as the vessel was permitted sufficient time to recover between steps. Pout was held constant at the baseline level throughout the protocols. Initial tests indicated that baseline Pin and Pout levels of 0.5 cmH2O were optimal for the assessment of the full range of responses to Pin steps; however, only ∼30% of the vessels contracted at a reasonable rate (>0.5 contractions/min) at 0.5 cmH2O, so that baseline pressures of 1 cmH2O, or higher were occasionally used.
Protocol 2: ramp increase in Pin at constant, low Pout.
As an alternative to a series of Pin steps, the response to a slow Pin ramp (lasting ∼4 min) starting from the baseline pressure and reaching a plateau at 8 or 16 cmH2O was tested while Pout was held constant at the baseline pressure. A selective, ramp-wise increase in preload allowed us to assess the adaptation of the vessel to a gradual rise in Pin to simulate the gradual changes in lymphangion filling that would occur under various conditions in vivo.
Protocol 3: ramp increase in Pout at different levels of Pin.
This protocol tested the effect of preload on the maximum level of Pout against which the lymphangion could eject [Plimit; as defined in our companion paper (10)]. This assessment of the effect of preload is therefore different from those used previously, where either contraction amplitude (19) or time-averaged flow (28) was used as an index of pump strength. The inclusion criteria for vessels used in this protocol are described in the results (in the context of describing the results shown in Fig. 5).
Fig. 5.
Effect of preload on the ability of an isolated lymphangion to pump against a ramp-wise elevation in Pout. A: recording showing the response of a single lymphangion to multiple Pout ramps, each beginning at a different level of Pin. The inset shows the expanded time scale for ramp 3. With each contraction during the ramp, the PL pulse amplitude increased to match Pout, until the ejection limit was reached (Plimit); after that point, the output valve failed to open during systole until the ramp was terminated. The output valve record is the top trace, and the gaps during the ramps denote the periods in which ejection fails. B: contraction AMP for each contraction cycle during each Pout ramp in A plotted as a single point against the instantaneous Pout level. Each curve represents the data set obtained for one Pout ramp at a given level of Pin. The family of curves is therefore analogous to a set of force-velocity curves (force vs. afterload) at different levels of preload. The color coding is similar to the scheme used in Fig. 1. C: difference between Plimit and Pin plotted as a function of Pin (filled circles) from the recording in A. The points were fit to a third-order polynomial. For comparison, the contraction AMP (averaged over at least 3 contraction cycles) before the beginning of each Pout ramp was plotted as a function of Pin (open circles; fit to a power function).
Protocol 4: ramp-wise, simultaneous increases in Pin and Pout.
Simultaneous increases in preload and afterload simulate changes that might occur during edemagenic stress in vivo (3, 18). A slow pressure ramp in both Pin and Pout, from baseline pressure to 12 or 16 cmH2O, was imposed. After the ramp was complete, Pin and Pout were returned to baseline until a stable contraction pattern redeveloped.
Protocol 5: ramp-wise Pin increase at constant, elevated Pout.
To test the range of preloads that were optimal for pumping against an elevated afterload, Pout was stepped to Plimit [as previously determined for each vessel using the method described in the companion paper (10)] and held there until a stable contraction pattern was established. A slow pressure ramp in Pin, from baseline to Plimit, lasting ∼4 min, was then imposed. Only vessels with Plimit > 8 cmH2O were used for this protocol.
Minimizing the Effects of Forward Flow
In protocols where Pin exceeded Pout (protocols 1, 2, and 4), pressure in the central chamber of the lymphangion (PL) rose to an intermediate value between Pin and Pout, depending on the position of the servo-null pipette and the relative resistances of the inflow and outflow cannulae; thus, some amount of net forward flow occurred under these conditions. Because significant flow could lead to inhibition of contraction frequency (FREQ) and changes in amplitude (AMP) through the production of nitric oxide (NO) from the endothelium (20, 21), two different sets of cannulating pipettes were used for protocols where Pin exceeded Pout. This strategy was preferred to the use of NO inhibitors, which sometimes led to irregular contraction patterns (and, if so, to possible changes in lusitropy). To minimize the effects of flow, a pair of relatively high-resistance pipettes (input pipette: 60 μm ID and output pipette: 80 μm ID) was used for all protocols except as specified. To demonstrate the effect of flow on contraction when Pin > Pout, a second pair of lower resistance pipettes, with larger and shorter tips, was used (input pipette: 120 μm ID and output: 140 μm ID; tip lengths approximately one-third those of the other cannulae). The outer pipette diameters were fashioned to the maximum diameter practical for cannulation.
The hydraulic resistance of the system with each pair of pipettes in place was determined in a set of calibration experiments in which a short, valve-less lymphatic vessel segment (ID: ∼180 μm and length: ∼600 μm) was cannulated with the respective sets of cannulae. The test vessel was then perfused at various flow rates (0.5–20 μl/min) from a syringe pump (model 22, Harvard Apparatus, Southnatick, MA) while the height of the Pout reservoir was manually adjusted to keep diameter constant (±2 μm). The pressure difference required to maintain each flow rate was determined from the readouts of the Pin and Pout transducers and used to calculate hydraulic resistance. The total resistance of the system using the larger diameter cannulae was 1/21 of that using the smaller diameter cannulae (slope: 0.04 cmH2O·μl−1·min−1 for low-resistance pipettes vs. 0.82 cmH2O·μl−1·min−1 for high-resistance pipettes), which suggests that flow rates through vessels perfused through the low-resistance cannulae would be ∼21 times higher at comparable diameters and lengths.
When the low-resistance cannulae were used for step or ramp protocols, significant flow was allowed to occur, and it exerted profound effects on contraction. For example, Pin ramps or steps of only 1 cmH2O in magnitude led to substantial inhibition of FREQ and AMP in two of five vessels and complete cessation of contraction in the other three vessels. The greater degree of inhibition of contraction than reported previously in response to flow alone (19, 20), despite a concomitant increase in PL, was presumably due to the much higher flow rates imposed here using relatively large-bore pipettes.
Data Analysis and Statistics
The following lymphatic pump parameters were calculated as described in our companion paper (10): FREQ, AMP, volume, end-diastolic diameter (EDD), end-systolic diameter (ESD), end-diastolic volume (EDV), end-systolic volume (ESV), SV, ejection fraction (EF), fractional pump flow (FPF), tone, and stroke work.
Data sets from one vessel per animal were used for analysis using Excel (Microsoft, Redman, WA), Igor, and/or JMP 8 (SAS, Cary, NC). ANOVAs were performed using JMP 8, with significance defined as P < 0.05. When appropriate, Dunnett's post hoc analyses were used to evaluate significant differences from a respective control value within each ANOVA data set, and Bonferroni post hoc tests were used for comparisons between data sets. For analysis of data obtained using pressure ramps, data were binned according to Pin and Pout values and designated as nominal variables for the JMP model. GraphPad Prism version 5.0 (La Jolla, CA) was used to fit curves to the P-V relationships.
RESULTS
Acute Response to a Step-Wise Elevation in Preload
The contraction patterns of a single lymphangion at six different levels of Pin (preload) in response to protocol 1 are shown in Fig. 1A. The inset in Fig. 1A is a diagram showing the relative locations of the diameter, pressure, and valve position measurements in all protocols, unless otherwise stated. For the lymphangion in Fig. 1A, contraction AMP was ∼65 μm [54% of passive diameter (passD)] with Pin and Pout initially set to 0.5 cmH2O and increased to ∼90 μm (69% of PassD) when Pin was elevated to 1 cmH2O (Pout remained fixed at 0.5 cmH2O). The increase in AMP was due both to an increase in EDD and a decrease in ESD. Further Pin elevation to 2 cmH2O increased AMP to ∼110 μm (84% of PassD) due solely to an increase in EDD. At all higher levels of Pin (3–12 cmH2O), AMP declined after the pressure step, with its magnitude being inversely proportional to the height of the step. FREQ progressively increased from ∼2 contractions/min at Pin = 0.5 cmH2O to ∼10 contractions/min at Pin = 12 cmH2O.
Fig. 1.
Response of an isolated lymphangion to short-lasting, step-wise elevations in input pressure (Pin). A: recording showing spontaneous lymphangion contractions at six different levels of preload (Pin), starting from 0.5 cmH2O, with output pressure (Pout) held constant. Pin was elevated in sequential steps (no return to baseline) and maintained for ∼5–10 contractions (cf. Fig. 2). The schematic diagram of the preparation shown in the inset shows where pressures and diameter were measured. The blue trace shows Pin (in cmH2O), the red trace shows Pout (in cmH2O), and the black trace shows intraluminal pressure (PL; in cmH2O), measured by the servo-null micropipette. End-diastolic diameter (EDD) was measured at the end of diastole, and end-systolic diameter (ESD) was measured at the peak of systole. B: pressure-volume (P-V) plots of data from A, showing three complete contractions at each Pin level; each set of contractions is color coded. Volume was calculated as described in methods. Stroke volume (SV) was maximal at Pin = 3 cmH2O in this vessel. Points a–d were used to quantify the loops, with point a showing the end of diastole, point b showing peak systolic pressure, point c showing the end of systole, and point d showing the minimal pressure during the contraction cycle. C: summary P-V diagram constructed from measurements of points a–d for 8 vessels. The solid and dotted black lines in B and C approximate the end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relationship (EDPVR), respectively. EDVPR was curvilinear. ESPVR was curvilinear for the lower range of Pin levels but linear over the higher range of Pin levels.
Changes in PL waveform height as a function of Pin are evident from the black trace on the pressure axis in Fig. 1A. Only a small systolic PL pulse (∼1 cmH2O) was apparent at the baseline pressure. In contrast to the effect of Pout elevation on the PL waveform, as described in the companion paper (10), pulse amplitude typically declined as Pin increased. This occurred because the output valve remained open continuously in the face of the prevailing Pin > Pout gradient, causing any pressure spikes to be blunted by the open output cannula. The small spikes indicate that at no time during the contraction cycle were both valves closed simultaneously (see the explanation of valve traces below). In response to a stepwise elevation in Pin, PL is predicted to rise to an intermediate value between Pin and Pout. For this protocol, the average equation relating mean PL to Pin was PL = 1.1 cmH2O + 0.45Pin (n = 8 vessels).
The corresponding changes in EDV, ESV, and SV at multiple levels of Pin between 0.5 and 14 cmH2O are evident on the P-V plot shown in Fig. 1B. Volumes were calculated as previously described (10). The P-V loops were characteristically broad and flat and remained so as Pin rose. Pin elevation from a baseline level of 0.5 to 1 or 3 cmH2O resulted in increases in EDV and small decreases in ESV that produced a net increase in SV. Further Pin elevation to 5, 8, or 14 cmH2O resulted in small increases in EDV but relatively larger increases in ESV, resulting in a progressive reduction in SV. In the short-duration Pin step protocol, the loops for consecutive contractions tended to overlay each other, and the variability in loop shape shown in the recording in Fig. 1A was representative of most vessels. The average responses of eight vessels to the short-duration Pin step protocol, with Pout held at 0.5 cmH2O, are shown in Fig. 1C. For analysis of individual P-V loops, measurements were made on each loop at the four points (points a-d) in Fig. 1B (for the P-V loops at Pin = 8 cmH2O). On average, the largest SV (point a − point c) was recorded at Pin = 1 cmH2O and Pout = 0.5 cmH2O.
Time Course of the Response to a Sustained Pin Elevation
After a step-wise increase in Pin, contraction AMP immediately declined (except in response to the smallest pressure steps) followed by a trend of a secondary increase over time as Pin was held constant at the elevated level; this pattern is evident in Fig. 1A (time: 222–225 min). The time course of these changes suggested that the lymphangion adapted dynamically to elevated Pin. To specifically assess these changes, we used a variation of protocol 1, where Pin was elevated for 5 min at each new level and returned to control for 5 min before the next step. An example of the lymphangion response is shown in Fig. 2. After a step-wise increase in Pin, EDD increased and AMP decreased (Fig. 2A). Within the first few contractions, AMP progressively recovered due to a progressive fall in ESD (arrow). This adaptation occurred over the course of ∼2 min, until AMP had returned nearly to its control value. A summary of the progression of AMP and FREQ changes over time after each Pin step is shown in Fig. 2, B and C (7 vessels). The general pattern was similar to the example shown in Fig. 2A: an immediate reduction in AMP after the Pin step followed by a significant, secondary recovery accomplished primarily by a time-dependent fall in ESD. The degree of recovery depended on the size of the Pin step and appeared to be largest for intermediate Pin steps (step size: 2–7 cmH2O). FREQ increased initially after all Pin steps (Fig. 2C) but then declined over time, stabilizing at a level slightly higher than control. This behavior likely reflects a rate-sensitive burst in FREQ associated with the rapid pressure increase, similar to that reported in response to a simultaneous increase in Pin + Pout (8).
Fig. 2.
Time course of responses to sustained, step-wise Pin elevation. A: recording showing the pressure and diameter responses that occurred in response to a sustained Pin step. Initially, Pin was set to 0.5 cmH2O and Pout to 1 cmH2O (to prevent forward flow). Pin was then increased in steps to 1, 5, 8, and 12 cmH2O for 5 min at each level, returning to the control level after each step (for this vessel, Pin was elevated using the reservoir system); Pout was held constant. Time-dependent, secondary changes in EDD, amplitude (AMP), and ESV occurred that were not evident in Fig. 1 (denoted by arrow for the first Pin step). In this particular vessel, the servo-null pipette was positioned closer to the input valve than in Fig. 1 so that PL was closer to Pin than Pout. *Diameter tracking artifact. The third step in this recording is available as a movie (see Supplemental Movie S1, available at the American Journal of Physiology-Heart and Circulatory Physiology website). B and C: summary analysis of AMP and frequency (FREQ) changes over time for the protocol used in A plotted as a function of contraction number. Data are means ± SE. Time is relative to the Pin step. The color coding scheme is same as in Fig. 1. The open symbols indicate points that were significantly different from the point at time 0 (immediately after the respective Pin step) using ANOVA followed by Dunnett's test. The color denotations in C apply to both B and C. D: P-V plot showing selected contractions from the recording in A. The blue loops correspond to three contractions at the control Pin level (marked by the blue dot in A). Each black loop corresponds to the first contraction after the Pin step (black dot in A), and each gold loop corresponds to the contraction in which the minimal value of ESV was first reached (gold dot in A). The dotted black line approximates EDPVR, whereas the dotted lines approximate ESPVR for the initial contraction and for the contraction when a steady-state AMP was reached (ESPVR′). E: summary plot showing the average shape of P-V loops from seven vessels at various levels of Pin (afterload was held at the baseline level). Because of the unusual loop shapes in several vessels, the six standard points described in the companion paper (10) were measured for each P-V loop. The color scheme is similar to that used in Fig. 1, B and C. The open symbols represent contractions immediately after a Pin step, whereas the filled symbols represent contractions when a steady-state response had been achieved. The black and gold lines were fitted to the ESPVR (solid lines) and EDPVR (dotted lines) for the initial and steady-state time points, respectively. A modified hyperbolic function was used to fit ESPVR, whereas an exponential function was used to fit EDPVR. The steady-state curves significantly differed from the initial curves for both ESPVR (P < 0.0001) and EDPVR (P < 0.05).
P-V loop analysis for the lymphangion response to a 5-min Pin step is shown in Fig. 2D. Pairs of P-V loops were plotted for each of the four Pin steps shown in Fig. 2A. After each step increase in Pin, the P-V data in Fig. 2C are shown for one of the initial loops after the pressure step (black trace) and one of the loops after completion of the secondary adaptation (gold trace). After the Pin step, the initial P-V loop shifted up and to the right, after which the left edge of the loop progressively shifted further to the left with time, restoring or increasing SV at a higher EDV. The right edge of the loop also shifted slightly to the right over time after a Pin step. Surprisingly, ESPVR appeared to substantially increase in slope with time after the initial Pin step (ESPVR to ESPVR′; solid lines). There was also a small, rightward shift in the end-diastolic pressure-volume relationship (EDPVR; dotted line) over time. ESPVR and EDPVR reflect the mechanical properties of the contracted and relaxed lymphangion, respectively, by analogy to the properties of cardiac ventricular chambers.
The shapes of the P-V loops under these conditions were artificially flattened by our measurement conditions (both valves were never closed when Pin > Pout so that generated PL spikes were blunted by the open pipettes). However, if a small, constant level of afterload was imposed, the loops became more “cardiac like” in appearance and the left shift in ESPVR still occurred (not shown). A summary graph showing the shape of the average P-V loops from seven vessels at various levels of Pin (with afterload held at 0.5 cmH2O) is shown in Fig. 2E. The color scheme in Fig. 2E is similar to that used in Fig. 1, B and C. The open symbols represent contractions immediately after the Pin step, and the filled symbols represent contractions after a steady-state response was achieved. The black and gold lines were fitted to the ESPVR (solid lines) and EDPVR (dotted lines) for the initial and steady-state time points, respectively. Not only was there a statistically significant leftward shift in ESPVR with time after the Pin step, but there was also a significant, small rightward shift in EDPVR.
Figure 3 summarizes the changes in key contraction parameters and calculated indexes of pumping ability as a function of Pin for protocol 1 (with a step duration of 5 min). Changes in each parameter immediately (0.5 min) after the Pin step are shown by black symbols; changes 4.5 min after the Pin step are shown by open symbols. A step increase in Pin was associated with an abrupt jump in EDD and ESD, with a greater rise in ESD that resulted in a net reduction in AMP for the early time points (filled symbols). The severity of the immediate drop in AMP was proportional to the magnitude of the pressure step. Over time, a progressive fall in ESD was observed, which resulted in a net increase in AMP over the Pin range of 3–12 cmH2O (open symbols). SV showed a similar trend, although there was more scatter in the data. EF fell with increasing Pin over the entire pressure range for the early time points, but, at steady state, EF was maintained near control levels until Pin exceeded 8 cmH2O. When measured immediately after a Pin step, FREQ increased proportionally with the magnitude of the step, showing an approximately fivefold increase over control at the highest step to 16 cmH2O. However, FREQ slowed after 1–2 min such that FREQ at the steady state was only approximately twofold higher than control for steps to 8 cmH2O and lower. Immediate changes in FPF, an index of pumping ability, included a progressive rise with increasing pressure until Pin reached 8 cmH2O, after which it dropped sharply. The steady-state changes in FPF reflect its maintenance at a level consistently twofold higher than the control at all levels of Pin.
Fig. 3.
Summary of lymphangion contraction parameters as a function of Pin obtained using 5-min step-wise increases in Pin with Pout held constant at 1 cmH2O. A: AMP. B: normalized (norm) AMP. C: SV. D: EDD. E: ESD. F: tone. G: ejection fraction (EF). H: FREQ. I: fractional pump flow (FPF). Each point represents the mean ± SE. Filled black symbols represent the initial response (within 0.5 min after the pressure step), open symbols represent steady-state responses (4.5 min after the pressure step), and gray symbols indicate baseline values. n = 7 vessels except for F, where n = 4 and valid calculations of tone could not be calculated at some Pin levels for three vessels. *P < 0.05 between the 0.5- and 4.5-min time points using Bonferroni's post hoc test after two-way ANOVA.
Response to a Ramp-Wise Preload Elevation
Using protocol 2, we tested the response of a single lymphangion to a gradual and progressive increase in Pin from 2 to 16 cmH2O using a ramp waveform at constant Pout. The response of a representative lymphangion is shown in Fig. 4A. A higher baseline pressure of 1 cmH2O was used for this particular vessel because it contracted only infrequently at 0.5 cmH2O. Ramp-wise elevation in Pin, using the high-resistance cannulae to minimize forward flow, led to a progressive increase in FREQ and decrease in AMP. PL increased during the ramp by approximately half as much as Pin, which is consistent with the servo-null pipette being positioned near the middle of the vessel segment. A short, imposed pulse in Pout (denoted by the asterisk), preceding the ramp, evoked a single contraction at the peak of which PL= Pout; the agreement between these pressures confirmed accurate calibration of the servo-null pipette. Similar to the vessels shown in Figs. 1 and 2, PL pulse AMP was ∼1 cmH2O at the baseline pressure and progressively declined as the Pin ramp proceeded. At the conclusion of the ramp, there was a considerable quiescent period before contractions resumed. This delay most likely reflects rate-sensitive inhibition of FREQ in response to the rapid, downward pressure step from 16 to 2 cmH2O (8) rather than a possible effect of endothelium-derived vasodilators, because a similar quiescent period was also observed in the same vessel when no imposed gradient for flow occurred (see Fig. 4B). Figure 4C shows a plot of the P-V loops for the contractions shown in the middle of the recording in Fig. 4A. The blue loops correspond to three contractions at the control Pin level and the multicolored loops correspond to the contractions during the Pin ramp using the same, time-based color scheme as in Fig. 4A, as per the convention introduced in our companion paper (10). As in Fig. 2D, ESPVR and EDPVR are denoted by the solid and dotted black lines, respectively.
Fig. 4.
Response to ramp-wise Pin elevation under conditions where forward flow is minimized. A: response of an isolated lymphangion to a ramp-wise increase in Pin from 2 to 16 cmH2O with Pout held constant at 2 cmH2O. The long pause before contractions resumed after the end of the ramp (time: 162–164 min) is consistent with rate-sensitive inhibition (8). *Brief simultaneous pulse of Pout to 12 cmH2O to check the servo-null calibration. The dotted horizontal line shows the maximal EDD for reference (obtained at Pin = 16 cmH2O). Valve position traces show the relative positions of the input (blue trace) and output (red trace) valve leaflets, where 1 = open and 0 = closed (for tracking details, see methods). B: response of the same lymphangion in A to a combined ramp-wise increase in Pin and Pout from 2 to 16 cmH2O. For reference, the dotted horizontal line shows the maximal EDD (obtained at Pin = Pout ∼ 8 cmH2O); subsequently, a slight constriction (arrow) occurred at higher levels of Pin and Pout. C: P-V relationship for the contractions during the Pin ramp in A. The blue loops correspond to three contractions at the control Pin level, and the color-coded loops correspond to the contractions shown in the corresponding colors of the trace in A. D: P-V relationship for the contractions shown in the first half of the Pin ramp in B. The blue loops indicate five contractions at the control Pin level, and the color-coded loops correspond to the corresponding colors of the trace in B. Only the contractions for the first half of the combined ramp are shown so that the ranges of PL values are matched between C and D. The solid and dotted black lines in C and D define ESPVR and EDPVR, respectively. The slight leftward bowing of the ESPVR in C is discussed in the text.
As a comparison, Fig. 4B shows typical responses of the same lymphangion to a simultaneous Pin + Pout ramp (protocol 4). Mean PL was equal to Pin and Pout before the start of the ramp and closely followed Pin and Pout. Contraction AMP declined, and FREQ increased with increasing Pin + Pout, as it did during the Pin ramp. The change in FREQ was 3.2-fold compared with 4.4-fold for the Pin ramp in Fig. 4A (measured at comparable values of PL); AMP was reduced to 44% of control during the Pin + Pout ramp compared with 41% of control for the Pin ramp (measured at comparable values of PL). Interestingly, the change in EDD with increasing pressure was also different from that observed in Fig. 4A. EDD rose with pressure up to ∼10 cmH2O and then declined slightly as pressure continued to increase to 16 cmH2O. This effect reflected a slight myogenic constriction (7), which was usually evident with ramp increases in pressure in the absence of forward flow (25). Figure 4D shows a plot of the P-V loops for the contractions shown in the middle of the recording in Fig. 4B. The blue loops correspond to five control contractions, and the color-coded loops correspond to the contractions during the first half of the combined Pin + Pout ramp (the ranges of PL values were matched to those in Fig. 4C). The solid and dotted black lines in Fig. 4D denote ESPVR and EDPVR, respectively. ESPVR was quite linear under these conditions and bowed leftward, likely reflecting the effect of the fixed, lower level of Pout.
The top traces in Fig. 4, A and B, show the valve gating patterns that occurred during Pin ramps, as determined from densitometer windows positioned over the bases of the input and output valve leaflets (9). Here, and in subsequent figures, the red trace corresponds to the output valve and the blue trace corresponds to the input valve. When Pin and Pout were equal, both valves were typically open during diastole, for reasons previously described (9). For these protocols, however, Pout was typically set to a value 0.1–0.2 cmH2O higher than Pin to close the valve in diastole and prevent forward flow (to the extent that flow occurred) from altering the baseline contraction pattern. At the beginning of each systole, PL rose slightly, leading to closure of the input valve (which opened in diastole) and then opening of the output valve; at the end of systole, the output valve typically closed first as PL fell, followed by opening of the input valve. Shortly after the onset of the Pin ramp, both valves opened and remained open for the duration of the ramp due to the increasing gradient for forward flow and/or the requirement for a higher reverse pressure gradient for closure of the valves as the vessel became more distended (9).
Changes in key contraction parameters (e.g., SV, EF, and FPF) during Pin ramps for 10 vessels were very similar in pattern and magnitude to those at 4.5–5 min in response to the Pin steps shown in Fig. 3 (not shown).
Effect of Preload on the Response to a Pout Ramp
Next, we assessed the interactions of preload and afterload using protocol 3. A representative example of the response is shown in Fig. 5. Pin was initially set to 2 cmH2O, with Pout slightly higher to ensure that the output valve remained closed. PL pulse AMP was ∼3 cmH2O. A ramp-wise increase in Pout (ramp 1) led to a progressive increase in peak PL that matched Pout in the systolic phase of each contraction, until Pout reached ∼7 cmH2O, when Plimit was reached; the Pout ramp was terminated at 9 cmH2O. The range of Pout levels associated with failure of ejection is evident by the gap in the position trace for the output valve (top trace in Fig. 5A), indicating that the valve remained closed even though contractions continued. There was no imposed flow during this protocol, since flow was prevented by the closed output valve when Pout ≥ Pin. Contraction AMP was ∼90 μm initially and progressively declined as Pout was raised, due both to a decline in EDD (myogenic constriction) and rise in ESD. FREQ increased from 11 to 29 contractions/min as the ramp progressed, although the change was not evident on the compressed timescale. Pin was subsequently lowered to 0.5 cmH2O (time: 148 min), which led to an immediate decrease in FREQ. A second Pout ramp (ramp 2) led to similar changes in AMP, FREQ, and PL pulse AMP, but Plimit was only 3.5 cmH2O. The protocol was repeated at increasing levels of Pin (except that Pin was the same for ramps 4 and 5), up to Pin = 10 cmH2O (ramp 9), when the difference between Plimit and Pin declined precipitously. An example of the response on an expanded timescale is shown at the right for ramp 3. Clearly, the vessel was able to pump against a relatively high Pout level over a wide range of preloads.
Although the Pout ramps for this protocol were usually terminated shortly after Plimit was reached to minimize the load on the vessel, this was a demanding protocol with a low success rate. The results shown in Fig. 5 are representative of only three vessels (of ∼50 vessels total) that successfully completed protocol 3. The low success rate reflects the stringent criteria required: robust pumping with a value of Plimit equal to or exceeding the group median (10), spontaneous contractions at low Pin levels (0.5 and 1 cmH2O), and lack of dysfunction in either valve over the entire ranges of both Pin and Pout.
Figure 5B shows an analysis of contraction AMP as a function of Pout, with each curve representing the relationship for a given level of Pin. Thus, this graph shows AMP as a function of afterload and preload. The vertical set of points for ramp 9 (at the highest preload) reflect the fact that the input valve became dysfunctional. An alternative analysis of the data for this protocol is shown in Fig. 5C, where (Plimit − Pin) is plotted as a function of Pin. The quantity (Plimit − Pin) represents the adverse pressure gradient against which the vessels could successfully eject (10). For comparison, the maximum contraction AMP at each level of Pin, taken just before the beginning of the Pout ramp, is also plotted as a function of Pin; the relevance of using AMP in this context is that it was used previously as a contraction index in analyses of isolated lymphatic vessels from the rat mesentery (19–21). Contraction AMP was determined before each Pout ramp (i.e., in a relatively unloaded vessel). Note that the curves for Plimit and AMP were generally similar, but that there was a difference in the Pin associated with the respective peak values, suggesting that the optimal preload shifts to a higher pressure when the vessel is subjected to an imposed afterload.
Stroke Work Under Various Combinations of Elevated Preload and/or Afterload
The P-V data derived from individual contractions allowed the calculation of stroke work for the various protocols. Stroke work was computed according to the equation stated in methods for individual contractions at defined levels of preload or afterload. The data for the various pressure ramp protocols are shown in Fig. 6, binned in 1-cmH2O intervals. The results shown in Fig. 6, A, B, C, and D, correspond to protocols 3, 2, 4, and 5, respectively. One general observation from these results is that, under relatively low imposed afterloads, work was maximal at low preloads (Pin ∼2 cmH2O) (e.g., Fig. 6, B and C), whereas at high loads, the optimal preload for maximal work displayed a broad plateau over a Pin range of ∼5–11 cmH2O, (e.g., Fig. 6, A and D). These results suggest that the lymphangion can increase its stroke work over ∼10-fold range and that it is able to adapt to relatively high levels of imposed afterload. The difference between the results shown in Fig. 6, C and D, suggests that more stroke work must be performed in the presence of an elevated afterload, unless preload is elevated along with the afterload at the same rate. An increase in afterload at fixed preload therefore elicits a greater increase in stroke work.
Fig. 6.
Stroke work for the various protocols calculated from the area of the P-V loops for individual contractions. A: protocol 3. B: protocol 2. C: protocol 4. D: protocol 5. See methods for protocol descriptions. A: effects of elevated Pout at two levels of Pin. B: effects of Pin at two levels of Pout. *Significantly different from control (Pin = 1 cmH2O except in B, where Pout = 1 cmH2O) using Dunnett's post hoc tests; ≠significantly different from control (Pin = 3 cmH2O in A and Pout = 3 cmH2O in B) using Dunnett's post hoc tests.
Preload Accommodation
We observed an additional and interesting phenomenon in a subset of vessels subjected to step-wise elevation in Pout. An example is shown in Fig. 7. When Pout was elevated step wise to a level that exceeded the initial capacity of the vessel to eject (in this case, 8 cmH2O), the initial peak systolic PL was only ∼3.5 cmH2O, which was insufficient to open the output valve during systole. Peak systolic PL then began to increase with time, similar to the behavior described in our companion paper (see Fig. 8 in Ref. 10), but, in this case, after a slight delay, a gradual rise in diastolic PL also occurred (arrow 1). Diastolic PL rose to a value of 6 cmH2O, at which point peak systolic PL exceeded Pout, opening the output valve and resulting in partial ejection. This response was different from that shown in Fig. 8 in Ref. 10, where peak systolic PL rose but diastolic PL remained near Pin levels because the input valve opened during each period of diastole, allowing PL to equalize with Pin. Here, the lymphangion appeared to be accommodating (by regulating diastolic filling) to increase its strength of contraction and overcome the imposed afterload. After diastolic PL peaked, it subsequently declined, as the lymphangion continued to eject successfully, until diastolic PL returned near the baseline level and the input valve again opened transiently during each period of diastole (arrow 2). Presumably, the time-dependent increase in contractility evoked by afterload elevation (10) allowed diastolic PL to return toward control levels while maintaining sufficiently strong contractions, thereby enabling the pump to recover diastolic reserve and thus SV. Note the parallel declines in EDD and ESD that were associated with the secondary fall in diastolic PL. This behavior, therefore, appeared to reflect both an intrinsic increase in contractility and “preload accommodation” to maintain effective pump function.
Fig. 7.
Example of a lymphangion that exhibited preload accommodation when contraction strength was insufficient to pump against an elevated afterload. A: recordings of pressure, diameter, and valve position of an isolated lymphangion in response to a step-wise Pout increase to a relatively high value (8 cmH2O). Control contractions at equal levels of Pin and Pout are shown in blue. After the Pout step, the output valve closed and remained closed for ∼10 contractions. Initially, the lymphangion only developed a peak systolic pressure of ∼3.5 cmH2O. PL pulse amplitude began to subsequently increase (to ∼6 cmH2O), but this was insufficient to achieve ejection as the output valve continued to remain closed during systole. The input valve then closed (arrow 1), after which diastolic volume and pressure increased. At time = 104 min, ejection resumed as the resulting peak systolic pressure exceeded Pout. Subsequently, contractions became stronger, PL pulse amplitude increased, and ejection continued as diastolic pressure returned toward control levels. At arrow 2, diastolic PL essentially returned to control because the input valve began to open and close with each contraction cycle (indicating that PL equalized with Pin). After Pout returned to the control level, contraction AMP (green trace) remained elevated (compared with its initial level before the Pout step), suggesting that contraction strength continued to remain elevated. B: P-V loop analysis for the first half of the trace in A. The blue loops are control contractions, corresponding to the blue portion of trace in A. The other loops correspond to the multicolored portion of the trace labeled “B,” with the time of the loops indicated by the color of the trace. The line labeled ESPVR was drawn from ESV of the control loops to ESV of the final loops (i.e., when EDP was maximal) for this portion of the trace. The lines for ESPVR′ and EDVPR are redrawn from the plot in C. C: P-V loop analysis for the second half of the trace in A. The blue loops show the control contractions for reference (corresponding to the blue portion of the trace in A). The other loops correspond to the multicolored portion of the trace in A, with the time of the loops indicated by the color of the trace. The green traces show several loops immediately after the return of Pout to its control level (corresponding to the green portion of the trace in A). The line representing EDPVR was drawn through the diastolic points for the yellow portion of the trace and then also overlaid on the curve in B for reference. The line representing ESPVR′ was drawn from the ESV for the control loops and ESV for the final loops for the yellow portion of the trace and then also overlaid on the curves in B. The curve labeled “passPVR” is the passive P-V relationship obtained with a simultaneous Pin + Pout ramp in Ca2+-free physiological saline solution at the end of the experiment.
Fig. 8.
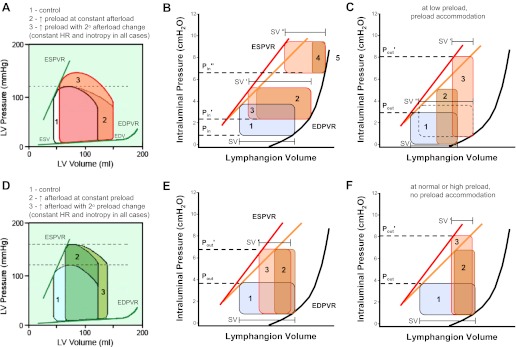
Schematic diagram comparing the responses of the heart and the isolated lymphangion to changes in preload (A–C) and afterload (D–F). A: response of the cardiac ventricle to increased preload. The control cardiac cycle is loop 1 (black outline). In response to an increase in preload, the isolated heart adjusts to loop 2 (on the second and subsequent contractions, given the stated conditions). The intact heart subsequently adjusts to loop 3 on the third contraction because of a secondary increase in afterload. B: response of the lymphangion to preload elevation (Pin to P′out),). The P-V loop initially contracts (loop 1 to loop 2) and then subsequently expands to loop 3 over the course of 5–20 contractions such that SV′ > SV. At much higher levels of preload (P″out),), the same adaptation occurs, but the resulting SV is reduced from the control value (SV″ < SV). C: if afterload is elevated within a certain range, the P-V loop narrows (loop 1 to loop 2), but the isolated lymphangion can subsequently accommodate by increasing diastolic filling and EDP to increase contraction strength (loop 2 to loop 3). See text for discussion. D: the isolated heart responds to an increase in aortic pressure by shifting from loop 1 to loop 2, with SV decreasing in proportion to the increase in ESV. The intact heart undergoes a subsequent increase in preload (loop 2 to loop 3) due to incomplete emptying of the ventricle; over a longer time interval, the Anrep effect may also be invoked to produce a modest decrease in ESV (not shown). E: the response of the lymphangion to afterload elevation (Pout to P′out) involves an initial narrowing of the P-V loop (loop 1 to loop 2) followed by subsequent expansion (loop 2 to loop 3) as an intrinsic increase in contractility occurs. ESPVR shifts to a higher slope. F: if afterload is elevated within a certain range, the lymphangion is initially unable to eject and the P-V loop narrows (loop 1 to loop 2); subsequently, the strength of contraction increases (loop 2 to loop 3) until ejection resumes. The results shown in E and F are based in part on data from the companion paper (10). A and D were modified from R. Klabunde (http://www.cvphysiology.com/CardiacFunction/CF025.htm).
An analysis of the same data using the P-V relationship supported these conclusions. Figure 7B shows a plot of the P-V loops for the first portion of the trace shown in Fig. 7A. The blue loops represent the control contractions at equal levels of Pin and Pout, corresponding to the blue portion of the trace in Fig. 7A. The other loops correspond to the multicolored portions of the same trace labeled “B” in Fig. 7A, with the timing of the loops in Fig. 7B indicated by the color of the trace. Over the first four contraction cycles after the Pout step, the loop increases in height and then shifts slightly to the left. The line labeled ESPVR is drawn from ESV of the control loops to ESV of the final loops [when end-diastolic pressure (EDP) was maximal] for that portion of the trace. The deviation to the left of the ESPVR line for those contractions is probably not indicative of an increase in contractility because EDV also shifted to the left. The P-V loop analysis for the middle portion of the trace is shown in Fig. 7C. The blue loops again show the control contractions. The other loops correspond to the multicolored portion of the trace labeled “C” in Fig. 7A, where EDP declines from ∼6 to 1 cmH2O. The timescale in Fig. 7C is color coded as described above. The line representing EDPVR was drawn through the diastolic points for the trace and then also overlaid on the data in Fig. 7B. Based on the results shown in Fig. 7C, there are several indications that an increase in contractility had occurred by the time EDP returns to control (arrow 2 in Fig. 7A). First, when EDP and EDV had returned to control, peak systolic pressure was substantially higher than it was for the initial contractions after Pout elevation (compare the yellow loops in Fig. 7C with the black loops in Fig. 7B). Second, ESV was shifted substantially to the left of its value at peak EDP (compared with ESV for the yellow loops in Fig. 7B). The new ESPVR is labeled ESPVR′ and is reproduced in Fig. 7B to facilitate comparisons. Finally, when Pout was rapidly returned to control, contraction AMP and SV were transiently much larger than during the control period (ESV was lower and EDV was higher), as evident by the green traces in Fig. 7C, which correspond to the green portion of the trace in Fig. 7A. We did not perform dPL/dt analysis in this context because changes in dP/dt are not thought to be meaningful when preload is changing (35).
Vessels showing the combined response involving an increase in contractility plus a transient increase in diastolic filling were only able to increase their strength of contraction over a limited range of Pout. Several variations of this behavior were observed. In some vessels, diastolic Pin only rose spontaneously if a vessel successfully ejected against low afterloads and was then subjected to a higher afterload. In other vessels (data not shown), the increase in diastolic PL was sustained, rather than transient, and remained elevated throughout the observation period, or only declined somewhat, but did not return back to control (thus the input valve never opened until Pout was lowered). In other cases, diastolic PL increased but ejection still failed (data not shown). Preload accommodation (with an accompanying rise in EDP) was also observed during some Pout ramps but led to valve dysfunction if the ramp continued; however, if the ramp was halted (i.e., Pout maintained without a further increase), then diastolic PL would often decline toward control values.
DISCUSSION
Our results, taken together with those of the companion paper (10), comprise the first detailed analysis of how the single, isolated lymphangion adapts to changes in preload at low and high levels of imposed afterload. We found that a step-wise increase in preload induced immediate decreases in AMP and SV that subsequently recovered over the time course of 2–3 min (Fig. 2). At preloads < 4 cmH2O, the resulting steady-state SV was equal to or larger than the initial SV, but at higher preload levels, the underlying compensatory mechanism was insufficient to prevent a net fall in SV. Ramp-wise increases in preload induced an adaptation that occurred on an incremental, beat-to-beat basis, masking the time-dependent, secondary compensation in SV; the adaptation during ramps of the duration used in our protocols is likely to be incomplete. Analysis of the ESPVR from P-V loops at multiple levels of Pin was strongly suggestive of a preload-induced increase in contractility (Fig. 2, D and E), which was unexpected. In contrast, gradual afterload elevation at a fixed preload led to a progressive reduction in SV (Fig. 5B) but an increase in peak systolic pressure; when afterload became sufficiently high, ejection failed. The limiting afterload at which successful ejection occurred was highly dependent on preload, with an optimal preload level slightly higher than that measured under conditions of minimal afterload (Fig. 5C). At a fixed preload, a step-wise elevation in afterload to a relatively high level elicited a time-dependent increase in contractility that resulted in a progressive increase in peak systolic pressure and eventual restoration of ejection, as shown in the companion paper (10). A similar increase in afterload at a very low preload level produced an increase in contractility that was insufficient to achieve ejection; however, the lymphangion could transiently accommodate preload (i.e., increased EDP at constant Pin) to a more optimal level (Fig. 7), thereby enhancing its contractile function and improving the likelihood of successful ejection. Collectively, these results argue that muscular lymphatic vessels possess several mechanisms to maintain lymph flow under various filling states and pressures, a capacity that is likely necessary for the health of the organism.
In the following sections, we discuss these issues in detail and compare the behavior of the isolated lymphangion with that of the isolated and intact heart. Finally, we address the relevance of our findings in the context of lymphatic vessel responses to edemagenic and gravitational loads.
Comparison With Previous Lymphatic Studies
Our results suggest that the lymphatic vessel normally obeys the Frank-Starling relationship between diastolic pressure/volume and output and is optimized to operate over a low and rather limited range of EDP (1–8 cmH2O), above which pump function becomes progressively compromised. Our conclusion is consistent with previous studies (3, 14, 15) of rat mesenteric lymphatics, where calculated pump output was maintained over a pressure range from 1 to 5 cmH2O because increases in contraction FREQ counterbalanced decreases in SV; likewise, measurements of SV, EF, and/or flow from bovine and ovine lymphatics suggest that optimal EDP in those vessels is between 2 and 8 cmH2O (12, 14, 15, 22, 24, 26, 28, 34). An upper end of 8 cmH2O is likely to correlate with the diminished pump capacity observed in human peripheral lymphatic vessels during chronic lymphedema (31). The extent to which a rat mesenteric lymphangion can adapt to sustained levels of higher pressures past the 5-min test period used in our protocols is an important issue that remains to be resolved.
Our present results extend these studies and provide several new insights into the control of lymphatic vessel function by intraluminal pressure, as our servo pressure controller allowed us to manipulate Pin selectively (holding afterload at a very low value), gradually raise Pout at any given level of preload, or increase Pin + Pout together (to maintain a zero transaxial pressure difference). Novel aspects of our study include the following findings. First, this is one of only three studies to characterize the relationship between diastolic pressure and function for a single, isolated lymphangion. With the exception of two short reports in untranslated Russian journals (18, 27), previous studies (3, 12, 14, 15, 26, 28) used multiple chains of lymphangions where, due to intrinsic contractile behavior that could be synchronized or nonsynchronized, Pin was controlled only for the first segment of the chain and Pout only for the last segment. Hence, the results presented here provide insights into the effect of preload on the function of the fundamental pumping unit of the lymphatic system: the lymphangion. Second, we uncovered a time-dependent increase in ESV and SV (after an initial fall) in response to changes in Pin (preload). This adaptation appears to reflect an underlying change in contractility that may be important in compensating for the initial drop in SV that otherwise occurs with each progressive increase in pressure (discussed further below). Finally, we found that the optimal preload depends on the index of contractile function used, such that peak SV, FPF, and work each occur at slightly different levels of preload and, importantly, that optimal preload shifts to a higher pressure when the lymphangion is subjected to an imposed afterload.
Comparison of the Effects of Preload and Afterload on the Heart and Lymphangion
Our results, combined with previous work from multiple laboratories (29, 36), suggest that lymphangion contractile function is modulated by the same four primary determinants of left ventricular function (37): preload, afterload, contraction FREQ, and inotropic state. Here, the term “inotropy” is used in a conservative sense that is consistent with the cardiac literature (rather than the lymphatic literature) to refer to an increase in muscle contractility (including a leftward shift in the ESPVR) induced by an applied agonist. In the present protocols, we did not specifically assess the effects of purported inotropic agents on lymphatic muscle contractility, although those effects might be instructive when evaluated using P-V analysis. However, our results indicate that the lymphangion adapts in several unique ways to changes in preload and afterload. Based on results from the present study and in Figs. 7 and 8 of the companion study (10), we summarized the effects of preload and afterload on the isolated lymphangion in Fig. 8 to facilitate comparisons with their respective effects on the cardiac ventricles.
The isolated heart responds to increased filling (e.g., increased venous return) with an increase in EDV, shifting to the right along the EDPVR (Fig. 8A, loop 1 to loop 2); ESV is maintained, resulting in increased SV and increased output without a shift in the ESPVR (i.e., heterometric autoregulation). The normal heart can make these adjustments over a relatively wide range of EDV before the descending limb of the Frank-Starling relationship is reached (37). An increase in preload in the isolated heart can occur in the absence of a change in afterload, but a preload-induced increase in SV in the intact heart leads to an increase in cardiac output, resulting in an increase in afterload beginning with the next cardiac cycle (Fig. 8A, loop 2 to loop 3).
For the isolated lymphangion, an increase in preload (Pin to P′in) shifts EDV to the right according to the EDPVR relationship for the lymphangion (Fig. 8B, loop 1 to loop 2), which is steeper than the EDPVR for the heart (see Figs. 1B, 2E, and 4C for examples). The apparently lower compliance of the lymphangion relative to the cardiac chambers dictates that EDP rises more steeply with a rise in EDV than in the heart. A step-wise increase in EDV causes an immediate reduction in SV, which subsequently recovers to or toward the control level over the course of 5–20 contractions (Fig. 8B, loop 2 to loop 3). A net increase in SV occurs consistently with increasing preload only at very low values of EDP (0.5∼4 cmH2O); from EDP = 4–8 cmH2O (Pin′) SV is maintained (SV′ = SV), and at EDP > 8 cmH2O (P″in) SV shows only a limited secondary recovery (Fig. 8B, loop 4 to loop 5). Importantly, it appears that these changes in preload (at least over the lower range of Pin levels) result in a leftward bowing of the ESPVR with an increase in slope (based on the results shown in Fig. 2, D and E). It should be noted that the height of the idealized loops shown in Fig. 8, B, C, E, and F, are “corrected” for underestimation of the actual PL pulse height due to the open cannulae and the low level of afterload that was maintained intentionally.
In response to increased afterload, the left ventricle must generate a higher pressure to open the aortic valve (37). Initially, ESV increases (and SV falls), as shown in Fig. 8D, loop 1 to loop 2. In the intact heart, compensation for the fall in SV is accomplished in two ways: 1) by a secondary increase in EDV due to reduced ejection volume (Fig. 8D, loop 2 to loop 3) and 2) by an increase in contractility [homeometric autoregulation (37)], which leads to a secondary, parallel decline in EDV and ESV without a change in SV [the Anrep effect (37); not shown].
The isolated lymphangion shows a similar, but limited, response to a step-wise increase in afterload (Fig. 8E, Pout to P′out On the first contraction after the Pout increase, ESV increases and SV falls (Fig. 8E, loop 1 to loop 2). Subsequently, ESV decreases over time (and SV partially recovers), so that the loop shifts leftward to a new ESPVR with a higher slope [Fig. 8E, loop 2 to loop 3; see Fig. 7 in the companion paper (10) for examples]. This response occurs in conjunction with a slight decrease in EDV (and EDD, an apparent myogenic constriction). Like the heart, a net decrease in lymphangion SV is a trade off for being able to pump against a higher afterload. But, because of an increase in contractility, the net reduction in SV (SV′) is not as severe as it might have been otherwise. An important difference between the lymphangion and the cardiac ventricles is that the isolated lymphangion can make this adjustment in contractility, whereas the isolated heart cannot.
Figure 8, C and F, shows additional ways in which the isolated lymphangion can adapt to an elevated afterload. When the lymphangion is subjected to a higher afterload against which it cannot initially eject (Fig. 8F, Pout to P′out), diastolic PL remains constant, but a time-dependent increase in peak systolic PL (and increase in ESPVR slope) occurs (at least in some cases) that allows the lymphangion to eject when peak systolic PL eventually exceeds P′out [see Fig. 7 in the companion paper (10) for examples]. This response can occur with or without a leftward shift in ESV or myogenic constriction, but because dPL/dt increases at constant preload (10), we presume that the slope of ESPVR increases. In other vessels, peak systolic PL begins to increase in response to the elevated afterload but apparently is insufficient to produce ejection. This response is shown in Fig. 8C, where a Pout increase from ∼4 to 8 cmH2O P″out causes a shift from loop 1 to loop 2 that allows ejection only against Pout levels up to ∼5 cmH2O. The lymphangion accommodates by increasing diastolic PL and at the same time also increasing contractility (i.e., in conjunction with an increase in ESPVR slope; see Fig. 7, B and C). The combination of these two mechanisms thereby enables the generation of a peak systolic PL exceeding 8 cmH2O, opening the output valve. The dotted loop in Fig. 8C shows the larger SV that would be associated with the new level of preload if afterload had not increased (SV″ > SV). Note also that the initial preload level is lower in Fig. 8C than in Fig. 8, A, E, and F, to reflect the observation (Fig. 7) that preload accommodation only seems to occur when the initial preload level is quite low (<2 cmH2O). While we noted several variations of this response (see Supplemental Movie S2),1 the collective behavior of several vessels suggested that, if a time-dependent increase in contractility was insufficient to increase the strength of contraction to overcome an imposed afterload, then the vessel adapted by increasing preload as well, in an attempt to achieve or maintain successful ejection. Ejection failed once the imposed load exceeded the capacity of the combination of these two compensatory mechanisms. Preload accommodation at low volumes/pressures would allow the vessel to pump at suboptimal filling pressures that otherwise might severely compromise SV and output. Such low volumes/pressures normally may be associated with low hydration states in which lymphatic filling is minimal (24). This adaptation may be particularly relevant in peripheral lymphatic vessels subjected to gravitational loads, i.e., relatively high levels of afterload (32).
Secondary Adaptation in ESV Induced by an Increase in Preload
As mentioned above, a unique aspect of the lymphatic vessel response to elevated preload that has not been reported in previous studies is the secondary adaptation in ESV and SV over time. In the lymphangion, the time-dependent increase in ESV after a step-wise increase in preload takes 5–20 contractions (Fig. 2) over a time course of 2–3 min (depending on the intrinsic contraction rate). In the heart, the Frank-Starling response is immediate, within one cardiac cycle (37). Are the viscoelastic properties of lymphatic vessels such that the lymphangion requires a much longer time to equilibrate to increased filling than the ventricular chambers? The answer is not known, although compliance of the lymphangion must be lower than that of the heart since the heart shows very little increase in EDP for a relatively large change in EDV. Surprisingly, our results suggest that the adaptation of the lymphangion to increased preload involves an increase in contractility. It is interesting that the time course of the secondary decrease in ESV is approximately the same as for the increase in lymphangion contractility in response to increased afterload (10). ESPVR appeared to undergo a leftward shift under these conditions, but a possibly significant increase in the slope was difficult to establish due to the simultaneous rightward shift in EDV [although it was more than simply a parallel shift, as reported by Li et al. (26)]. It should be noted that shifts in the P-V relationships of the mouse cardiac ventricle similar to those shown in Fig. 2, D and E, have been recently discussed in detail, where the authors conclude that a leftward shift of a curvilinear ESPVR can, indeed, reflect an increase in contractility (6). In the heart, changes in contractility appear to involve changes in myofilament Ca2+ concentration, Ca2+ sensitization, and/or changes in cross-bridge cooperativity (35), whereas the Frank-Starling mechanism was originally thought to reflect changes in the degree of actin-myosin filament overlap. However, even changes in preload alone have been shown to be associated with changes in myofilament Ca2+ concentration, thus blurring the distinction between the Frank-Starling mechanism and changes in contractility (17). Although comparatively little is known about myofilament Ca2+ concentration dynamics in lymphatic muscle, intracellular Ca2+ concentration increases during the lymphatic vessel response to increased preload (33), and this may provide a mechanism to explain the apparent increase in contractility that we observed upon changing preload. If analogies to cardiac muscle hold, contractility changes in the lymphangion are likely to involve a combination of electrophysiological, Ca2+ handling, and contractile protein phosphorylation mechanisms. However, our knowledge of these processes in lymphatic muscle is too incomplete at this time to offer worthwhile speculation regarding the exact mechanism.
At least two additional effects of a “pure” increase in preload are not shown in Fig. 8. First, an intrinsic increase in FREQ occurs in the lymphangion when preload increases, with significant effects on pump function. This inherent increase in contraction FREQ of the lymphangion is critical to maintaining output (e.g., FPF) relatively constant over a wide preload range because it compensates for the inability of the lymphangion to increase SV when EDP exceeds ∼3–4 cmH2O (Fig. 2). However, the increase in FREQ also leads to a decrease in the time available for diastolic filling (i.e., negative lusitropy). These effects of FREQ on filling occur over a lower working range for lymphatic vessels than for the heart (37) and may possibly aid the lymphangion in compensating for the narrower working range. Second, in contrast to the heart, changes in lymphatic preload (when afterload is held at a low level) will also lead to changes in flow. Flow has multiple effects on collecting lymphatic vessel contraction depending on whether the flow is transient or sustained. Pulsatile changes in flow and NO production are associated with spontaneous contractions (1, 4, 5, 11) and result in a positive lusitropic effect (at least in thoracic duct), leading to an increase in the time available for diastolic filling and subsequent increase in SV (21). As a result, FPF can be maintained at a lower FREQ. On the other hand, sustained flow, which would occur if Pin exceeds Pout for an extended period of time, leads to reductions in FREQ and AMP when a flow increase occurs in the absence of a pressure change (19, 20, 30) and can result in complete cessation of contractions; in some cases, flow can even override a simultaneous stimulatory effect of increased pressure. Although we minimized the influence of flow in protocols where Pin was elevated above Pout, we cannot exclude a small effect on the results shown in Figs. 1–4. However, the hallmark effects of flow, decreases in FREQ and AMP, after Pin elevation (20), were consistently absent under those conditions. The slight bowing of ESPVR shown in Fig. 4A (compared with Fig. 4B) likely reflects an increase in contractility when afterload is held low rather than a subtle positive lusitropic effect due to flow, because we never observed a decrease in FREQ when Pin was elevated (Figs. 1A, 2A, 3H, and 4). Nevertheless, the modulation of the responses shown in Fig. 8, B and C, by flow under in vivo conditions is potentially one of the distinct differences between the heart and lymphangion response to a preload change.
Physiological Significance
The lymphatic vasculature plays an important role in the physiological adaptation of the cardiovascular system to interstitial edema, where increases in interstitial volume and pressure lead to increased lymph formation in the lymphatic capillaries and then to increased filling and pressure in collecting lymphatic vessels (23, 24). The lymphangion must adapt to this increase in preload to maintain sufficient output. Lymph flow in the lower limbs of human subjects increases 10- to 15-fold after a transition from supine to quiet standing and up to 63-fold if the foot is heated while standing (32). The increase in lymph flow is primarily due to active pumping of the lymphatic vessels in the lower extremities, with minimal contribution of external compressive forces (2, 32). The present results advance our understanding of how the lymphangion adapts to changes in preload at both low and high levels of afterload. Depending on the region of the body, a lymphatic vessel might experience a preload change with or without a parallel change in afterload. For example, in a supine body position, the afterload imposed on intestinal lymphatic vessels would be quite low, with preload and afterload rising together if the intestine becomes edematous; in contrast, afterload always would be higher than preload in a dependent extremity (even higher if the valves are insufficient) and interstitial edema would lead to parallel increases in both preload and afterload. Our results suggest that parallel increases in afterload and preload (over a limited range) will not change SV substantially, due to opposing effects on EDV and ESV.
Ultimately, our results must be understood in the context of the in vivo lymphatic vessel, which is composed of chains of lymphangions connected in series. Thus, the afterload of one lymphangion determines the preload of the next lymphangion downstream, depending on whether their contractions are in phase. Our protocol of holding afterload at a low, fixed level while preload was altered was most likely artificial and may not occur in vivo; however, that protocol was designed to assess the effect of changing preload selectively. Preload might increase transiently with afterload remaining low in vivo in a chain of lymphangions if the downstream segment has a negative dip in pressure below baseline during diastole [as shown in Figs. 2 and 5 of the present paper and in other figures of the companion paper (10)] as the upstream segment ejects. However, it is unlikely that Pin can increase for an extended time without an increase in afterload. The experiments of Eisenhoffer (12) provide additional insights into the behavior of chains of lymphangion as those authors measured pressures through t-tubes at multiple points along the lengths of the chains. Under an imposed afterload (i.e., elevated downsteam pressure), EDP was elevated in the most downstream segments but was sequentially lower in upstream lymphangion chains due to competent valves and spontaneous vessel contractions. The authors concluded that the lymphatic vessel was entering a decompensation phase when EDP in the most upstream lymphangion of a lymphatic chain increased in response to elevated Pout (12). While we agree with this conclusion for vessels undergoing output valve dysfunction, we do not think it applies to instances such as that shown in Fig. 7 (preload accommodation) because in such cases the increase in EDP is only transient. Based on our observations, individual lymphangions are able to compensate to some degree under these conditions. Our methods could potentially be used to test this idea in isolated segments containing three or more lymphangions, where analyses of the time course of diameter (both EDD and ESD) and P-V loop changes would be informative.
Finally, the extent to which the behavior of vessels shown in Fig. 8 is generally applicable to collecting lymphatic vessels in other body regions, e.g., peripheral vessels in dependent extremities, remains to be determined. Gashev et al. (19) showed significantly different behavior (in terms of the effect of preload on SV and FPF) between vessels from different regions (cervical femoral, mesenteric, and thoracic duct), with each type of vessel optimized to operate around the pressure level it normally experiences in vivo. However, those experiments were all conducted at equal levels of Pin and Pout. It is not known if differences in the response of the lymphangion to imposed afterloads will exist in different regions, but a reasonable prediction is that vessels from more peripheral body regions would be able to pump against higher afterloads. Whether this occurs and, if so, what underlying mechanisms are involved would be topics for further investigation that might ultimately lead to therapeutic approaches for reversing lymphatic pump dysfunction in pathological states such as secondary lymphedema.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-089784 (to M. J. Davis), HL-070308 (to D. C. Zawieja), and HL-080526 (to M. Muthuchamy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S. and M.J.D. conception and design of research; J.S. and M.J.D. performed experiments; J.S., J.H.W., and M.J.D. analyzed data; J.S., M.M., D.C.Z., A.A.G., and M.J.D. interpreted results of experiments; J.S. and M.J.D. drafted manuscript; J.S., J.H.W., M.M., D.C.Z., A.A.G., and M.J.D. edited and revised manuscript; J.S., J.H.W., M.M., D.C.Z., A.A.G., and M.J.D. approved final version of manuscript; M.J.D. prepared figures.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Shanyu Ho.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Akl TJ, Nagai T, Cote G, Gashev AA. Mesenteric flow in adult and aged rats. Am J Physiol Heart Circ Physiol 301: H1828–H1840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aukland K. Why don't our feet swell in the upright position? News Physiol Sci 9: 214–219, 1994 [Google Scholar]
- 3. Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol 301: H1897–H1906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol 301: H2198–H2206, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296: H293–H302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587: 165–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301: H48–H60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphatic muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol; doi:10.1152/ajpheart.01097.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Eisenhoffer J, Elias RM, Johnston MG. Effect of outflow pressure on lymphatic pumping in vitro. Am J Physiol Regul Integr Comp Physiol 265: R97–R102, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Eisenhoffer J, Kagal A, Klein T, Johnston MG. Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc Res 49: 97–110, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Elias RM, Eisenhoffer J, Johnston MG. Role of endothelial cells in regulating hemoglobin-induced changes in lymphatic pumping. Am J Physiol Heart Circ Physiol 263: H1880–H1887, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Elias RM, Wandolo G, Ranadive NS, Eisenhoffer J, Johnston MG. Lymphatic pumping in response to changes in transmural pressure is modulated by erythrolysate/hemoglobin. Circ Res 67: 1097–1106, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Engeset A, Olszewski W, Jaeger PM, Sokolowski J, Theodorsen L. Twenty-four hour variation in flow and composition of leg lymph in normal men. Acta Physiol Scandinavica 99: 140–148, 1977 [DOI] [PubMed] [Google Scholar]
- 17. Fuchs F, Smith SH. Calcium, cross-bridges and the Frank-Starling relationship. News Physiol Sci 16: 5–10, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Gashev AA. [The pump function of the lymphangion and the effect on it of different hydrostatic conditions]. Fiziol Zh SSSR Im I M Sechenova 75: 1737–1743, 1989 [PubMed] [Google Scholar]
- 19. Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 450: 1023–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol 233: H57–H65, 1977 [DOI] [PubMed] [Google Scholar]
- 23. Hogan RD, Unthank JL. The initial lymphatics as sensors of interstitial fluid volume. Microvasc Res 31: 317–324, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Hogan RD, Unthank JL. Mechanical control of initial lymphatic contractile behavior in bat's wing. Am J Physiol Heart Circ Physiol 251: H357–H363, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol 261: H1706–H1715, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Li B, Silver I, Szalai JP, Johnston MG. Pressure-volume relationships in sheep mesenteric lymphatic vessels in situ: response to hypovolemia. Microvasc Res 56: 127–138, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Lobov GI. [Self-regulation of the pump function of the lymphangion]. Fiziol Zh SSSR Im I M Sechenova 74: 977–986, 1988 [PubMed] [Google Scholar]
- 28. McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann NY Acad Sci 1131: 89–99, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18: 463–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olszewski WL. Contractility patterns of normal and pathologically changed human lymphatics. Ann NY Acad Sci 979: 52–63, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Olszewski W, Engeset A, Jaeger PM, Sokolowski J, Theodorsen L. Flow and composition of leg lymph in normal men during venous stasis, muscular activity and local hyperthermia. Acta Physiol Scandinavica 99: 149–155, 1977 [DOI] [PubMed] [Google Scholar]
- 33. Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol 285: H2573–H2577, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Smith RO. Lymphatic contractility; a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med 90: 497–509, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solaro RJ. Regulation of Cardiac Contractility. San Rafael, CA: Morgan & Claypool Life Sciences, 2011 [PubMed] [Google Scholar]
- 36. von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol 36: 1147–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 37. West JB. Best and Taylor's Physiological Basis of Medical Practice. Baltimore, MD: Williams & Wilkins, 1991 [Google Scholar]