Abstract
The polarized organization of epithelial cells is required for vectorial solute transport and may be altered in renal cystic diseases. Vesicle integral protein of 17 kDa (VIP17/MAL) is involved in apical vesicle transport. VIP17/MAL overexpression in vivo results in renal cystogenesis of unknown etiology. Renal cystogenesis can occur as a consequence of defects of the primary cilium. To explore the role of VIP17/MAL in renal cystogenesis and ciliogenesis, we examined the polarization and ciliary morphology of wild-type and VIP17/MAL overexpressing Madin-Darby canine kidney renal epithelial cells grown in two-dimensional (2D) and three-dimensional (3D) cyst culture. VIP17/MAL is apically localized when expressed in cells maintained in 2D and 3D culture. VIP17/MAL overexpressing cells produce more multilumen cysts compared with controls. While the distributions of basolateral markers are not affected, VIP17/MAL expression results in aberrant sorting of the apical marker gp135 to the primary cilium. VIP17/MAL overexpression is also associated with shortened or absent cilia. Immunofluorescence analysis performed on kidney sections from VIP17/MAL transgenic mice also demonstrates fewer and shortened cilia within dilated lumens (P < 0.01). These studies demonstrate that VIP17/MAL overexpression results in abnormal cilium and cyst development, in vitro and in vivo, suggesting that VIP17/MAL overexpressing mice may develop cysts secondary to a ciliary defect.
Keywords: cystogenesis, polycystic kidney disease, epithelial trafficking
vesicular integral protein of 17 kDa (VIP17/MAL), also known as myelin and lymphocyte protein (MAL) and myelin vesicular protein of 17 kDa (MVP), is a 17-kDa proteolipid involved in the transport and delivery of carrier vesicles to the apical plasma membrane domains of polarized epithelial cells (9, 42, 61). Originally identified over 20 years ago through a search for genes that are differentially expressed during human T-cell development, VIP17/MAL is also produced in oligodendrocytes and Schwann cells and in epithelial cells of the thyroid, kidney, stomach, and large intestine (25, 32). In addition, VIP17/MAL is expressed in cultured polarized epithelial cells, including the renal Madin-Darby canine kidney (MDCK) cell line, a model system for studying epithelial polarity (36, 61).
The VIP17/MAL gene encodes a nonglycosylated integral membrane protein with four predicted transmembrane domains. In epithelial cells, VIP17/MAL is localized predominantly at the apical plasma membrane (19). VIP17/MAL contains multiple hydrophobic segments and, in contrast to most other integral membrane proteins, is highly soluble in organic solvents. This behavior accounts for the designation of VIP17/MAL as a proteolipid (1). Moreover, in all of the cell types in which it is expressed, VIP17/MAL is present in glycolipid- and cholesterol-enriched membrane (GEM) domains or “rafts” (25, 32, 35, 61). As incorporation into GEM domains at the level of the TGN may be responsible for the apical sorting of a number of proteins, it is possible that VIP17/MAL may facilitate the organization of apical cargo into microdomains to facilitate their targeting and transport (9, 42, 47, 61).
When VIP17/MAL levels are suppressed in vitro through treatment of cultured epithelial cells with RNAi, the ordinarily apically localized influenza hemagglutinin protein accumulates in the Golgi complex with diminished apical expression and partial missorting to the basolateral membrane (9, 42, 61). In contrast, when VIP17/MAL is overexpressed in vitro, net membrane delivery to the apical surface appears to be enhanced, and as a consequence VIP17/MAL overexpressing MDCK cells have excess or enlarged apical membranes (9). When grown in three-dimensional culture, MDCK cells in which VIP17/MAL expression has been decreased by RNAi manifest abnormal cyst formation as well as atypical ciliogenesis (53). These studies collectively suggest that VIP17/MAL is involved in the accurate trafficking of a subgroup of proteins to the apical surface in MDCK cells as well as in epithelial morphogenesis.
When VIP17/MAL is overexpressed under the control of its own promoter in transgenic animals in vivo, the epithelial cells lining distal nephron segments in the mouse kidney also appear to exhibit abnormal morphology. They manifest a pseudostratified appearance with amplified apical membranes that balloon into the tubule lumina. In addition, these animals develop renal cysts, although the histology of these cysts differs from that characteristic of autosomal dominant polycystic kidney disease (ADPKD; Ref. 18). In these transgenic mice, renal expression of VIP17/MAL is greatest in the collecting duct, although staining can also be detected in other tubule segments (19, 23). The collecting duct also expresses aquaporin-2 (AQP2), the water channel that is trafficked from an intracellular vesicular compartment to the apical plasma membrane in response to the antidiuretic hormone arginine vasopressin. VIP17/MAL colocalizes and interacts with AQP2 in the renal collecting duct (23). In LLC-PK1 cells, expression of VIP17/MAL increases surface expression of AQP2 by decreasing the rate of the channel's endocytic internalization (23). Moreover, in cultured cells in vitro, vasopressin induces the expression of VIP17/MAL, which may facilitate the apical trafficking or retention of newly synthesized AQP2 (24). The VIP17/MAL-mediated increase in apical AQP2 expression may be due to decreased endocytosis from the apical surface and/or the increased exocytosis of AQP2 from recycling endosomes (6). These studies collectively suggest that VIP17/MAL overexpression results in an imbalance between apical membrane formation and internalization.
It is unclear why VIP17/MAL transgenic mice develop renal cystic disease. However, it is well established that renal cystogenesis occurs in association with defects of the primary cilium, a specialized compartment of the apical membrane with a protein and phospholipid composition that is distinct from that of the remainder of the apical membrane (44). A number of genetic diseases are attributable to mutations in the genes encoding proteins involved in cilia function. Many of these “ciliopathies” include among their rosters of characteristic symptoms the presence of renal cysts (29, 38, 48, 52, 56, 57, 59). We wondered, therefore, whether the cystic phenotype observed in VIP17/MAL overexpressing mice might be attributable to possible effects of this overexpression on the formation or function of primary cilia.
To explore the role of VIP17/MAL in renal cystogenesis and ciliogenesis, we examined the morphology and polarization of wild-type (WT) and VIP17/MAL overexpressing MDCK cells grown in two-dimensional and three-dimensional culture. In addition, we evaluated the cilia in both WT and VIP17/MAL overexpressing MDCK cells. Our in vitro and in vivo studies demonstrate that both epithelial morphogenesis and ciliary structure are profoundly perturbed in cells that overexpress VIP17/MAL. These observations suggest an explanation for the renal cystic phenotype observed in VIP17/MAL transgenic mice.
MATERIALS AND METHODS
Cell culture, cell lines, and transfection.
MDCK type II cells were cultured in α-MEM (GIBCO) supplemented with 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin, and 50 ug/ml streptomycin in a humidified incubator with 5% CO2. VIP17/MAL overexpressing MDCK cells were similarly cultured. Three independent clones of VIP17/MAL overexpressing MDCK cells were generated and examined for these studies.
The coding sequence for VIP17/MAL (NM_005434.4) was inserted into pcDNA 3.1+ vector (Invitrogen, Carlsbad, CA) using Nhe1 and XbaI. A Flag sequence was subsequently inserted at the amino-terminus by PCR amplification using the primers F-5′-GCCCAAGCTTATGGACTACAAGGACGACGATGACAAGGCCCCCGCAGCGGCGACGGGG-3′ (Flag epitope in bold, HindIII site underlined) and R-5′-GCGCCTCGAGTTATGAAGACTTCCATCTGAT-3′ (KpnI site underlined) and subcloned as previously described (23).
MDCK cells were stably transfected with Lipofectamine 2000 (Invitrogen). Clones were selected by growth in medium supplemented with 1.6 mg/ml Zeomycin and screened by immunofluorescence.
In vitro cystogenesis.
Briefly, 1 × 105 MDCK or VIP17/MAL overexpressing cells were suspended in Matrigel (BD Biosciences) per the protocol previously described by Mangoo-Karim et al. (31). All suspensions were coincubated with MDCK culture medium as described above, with media being replaced every other day. Matrigel suspensions were then placed at 95°C until melted, at which point the cysts were allowed to settle to the bottom of the well for image capture by light microscopy. For cyst immunofluorescence, cysts were retained within the gel.
Immunofluorescence in two-dimensions and three-dimensions.
Cells on coverslips were washed twice with cold PBS (Sigma-Aldrich) with 1 mM MgCl2 and 100 μM CaCl2 (PBS2+) and fixed for 30 min in 4% PFA at room temperature. Cells were subsequently washed with PBS2+ and then permeabilized with permeabilization buffer (0.3% Triton X-100/0.1% BSA in PBS). Cells were incubated for 1 h with anti-acetylated α-tubulin (Sigma) followed by incubation with fluorescein isothiocyanate- or rhodamine-conjugated anti-rat IgG (Molecular Probes). Nuclei were stained with propidium iodide when required. Cells were visualized on a Zeiss LSM 780 confocal microscope.
Immunofluorescence on three-dimensional cysts was performed as previously described by O'Brien et al. (37). Briefly, cysts were fixed with 4% PFA for 30 min, permeabilized in 0.025% saponin for 30 min, quenched for 10 min with 7 mM NH4Cl, 20 mM glycine, in PBS2+ (pH 8), and then incubated overnight with primary antibody to anti-zona occludens 1 (anti-ZO-1; Chemicon), anti-E-cadherin (Sigma), anti-β-catenin (Sigma), anti-FLAG (Sigma), anti-gp58 (gift of Ira Mellman), or anti-gp135 (gift of Ira Mellman) as indicated, diluted in goat serum. Cysts were washed for 2–4 h with saponin in PBS2+ and incubated with secondary antibodies overnight. All other details of three-dimensional immunofluorescence are the same as those outlined in the protocol for immunofluorescence analysis of cells grown in two-dimensions that is described above. Cells were visualized using confocal microscopy (Zeiss LSM 780) at ×40 and ×63. Images were processed using LSM Image Viewer (Carl Zeiss).
Quantitative RT-PCR.
RNA from WT and VIP17 overexpressing MDCK cells was purified using the RNeasy kit (Qiagen, Germany). cDNA was synthesized using Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Quantitative RT-PCR was performed using the QuantiTect SYBR Green PCR kit (Qiagen). Initial denaturation was performed for 10 min at 95°C, and amplification was performed for 40 cycles, at 95°C for 30 s, 57°C for 1 min, and 72°C for 1 min. To amplify exogenous human VIP17, the primers 5′-GTACATAATTGGAGCCCACGGTGGA-3′ (sense) and 5′-AAGCCGTCTTGCATCGTGATGGT-3′ (antisense) were used, resulting in a 135-bp product. To amplify endogenous canine VIP17, the primers 5′-CACCACAGCCCTGCTTGTCCTGT-3′ (sense) and 5′-TCCCAATGGTGGCCAAAGCTTCC-3′ (antisense) were used, resulting in a 143-bp product. The products were confirmed by agarose gel electrophoresis and quantified using the ΔΔCT method with β-actin as a reference gene. VIP17 expression in WT MDCK cells was normalized to 1.
Immunohistochemistry.
Both WT and VIP-17/MAL transgenic mouse kidneys were fixed in 4% paraformaldehyde before being immersed in 30% sucrose and then embedded in optimum cutting temperature compound (19). Cryosections (5 μm) were obtained, and sections were then processed for immunofluorescence experiments. Briefly, sections were washed three times in TBS and then incubated for 5 min in TBS with 1% SDS before being washed in TBS again. Following blocking in goat serum dilution buffer [GSDB; 10% goat serum, 1% Triton X-100, and 10 mM glycine in PBS supplemented with 100 μM CaCl2 and 1 mM MgCl2 (PBS++)], sections were incubated with a rabbit polyclonal antibody to ADP-ribosylation factor-like protein 13B (ARL13B; Ref. 7; kind gift of Dr. S. Weatherbee, Yale University, used at 1:1,000) and a mouse monoclonal antibody to Na-K-ATPase (41; Millipore, used at 1:400) diluted in GSDB. Sections were then washed again in TBS before being incubated with an anti-rabbit rhodamine and anti-mouse FITC secondary antibody (also diluted in GSDB) for 1 h at room temperature. After a final set of washes in TBS, sections were examined and pictures were taken using a Leica confocal microscope.
Ciliary length was quantitated using NIS Elements Imaging Software (Nikon). Only unfolded cilia were used for quantitation, with three cilia measured per mouse from three control and three VIP17/MAL transgenic mice for a total n of nine per condition.
Statistical analysis.
We calculated the statistical significance of differences in results using unpaired Student's t-tests. We considered a P value <0.05 to be statistically significant.
RESULTS
VIP17/MAL overexpressing MDCK cells display apical localization of VIP17/MAL.
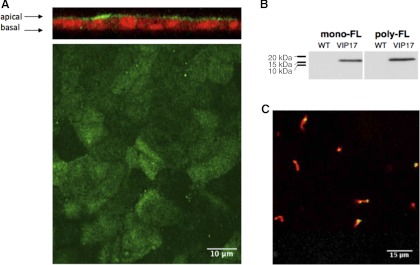
To investigate the role of VIP17/MAL in epithelial polarization and cyst formation, we used MDCK cells, a model system extensively employed for in vitro studies of epithelial membrane polarization. These cultured renal epithelial cells were stably transfected with a cDNA encoding the VIP17/MAL protein tagged with a FLAG epitope at its amino terminus. Three independent clones of VIP/MAL overexpressing MDCK cells were examined. Quantitative PCR using primers sets designed to detect expression of either the endogenous canine VIP17/MAL transcript or the transcript product from the transfected human VIP/17 MAL construct indicated that the transfected cells express ∼175 fold higher levels of VIP17/MAL mRNA than do untransfected cells. To determine the subcellular localization of the VIP17/MAL protein, we performed immunofluorescence studies on fully differentiated and intact monolayers of MDCK cells (Fig. 1A). Using an antibody directed against the FLAG-epitope, we observed that the VIP17/MAL protein is present primarily on the apical membranes of MDCK cells, consistent with prior reports (9, 61). Interestingly, the VIP17/MAL protein was detected over the entire apical surface and was apparently able to access the “exclusion zones” that surround the bases of cilia (17). Consistent with this observation, the overexpressed VIP17/MAL protein could also be detected in a punctate distribution in some but not all cilia (Fig. 1C). Western blot analysis of protein extracts prepared from these cells (Fig. 1B) using both monoclonal and polyclonal anti-FLAG antibodies detects a protein with the anticipated size of 17 kDa.
Fig. 1.
Stable vesicle integral protein of 17 kDa (VIP17/MAL) expression. A: immunofluorescence analysis of Madin-Darby canine kidney (MDCK) cells stably transfected with Flag-VIP17/MAL. Apical localization of VIP17/MAL is revealed with poly-Flag (green) in both en face extended depth of focus (bottom) and in XZ cross-section (top) images. B: Western blot analysis of MDCK cell lines stably transfected with VIP17/MAL. A specific band is detected at 17 kDa. C: VIP17/MAL (green) is detectable in a punctate pattern in some but not all cilia (labeled with anti-acetylated tubulin; red) in transfected MDCK cells. WT, wild type.
VIP17/MAL overexpressing cysts have altered morphology.
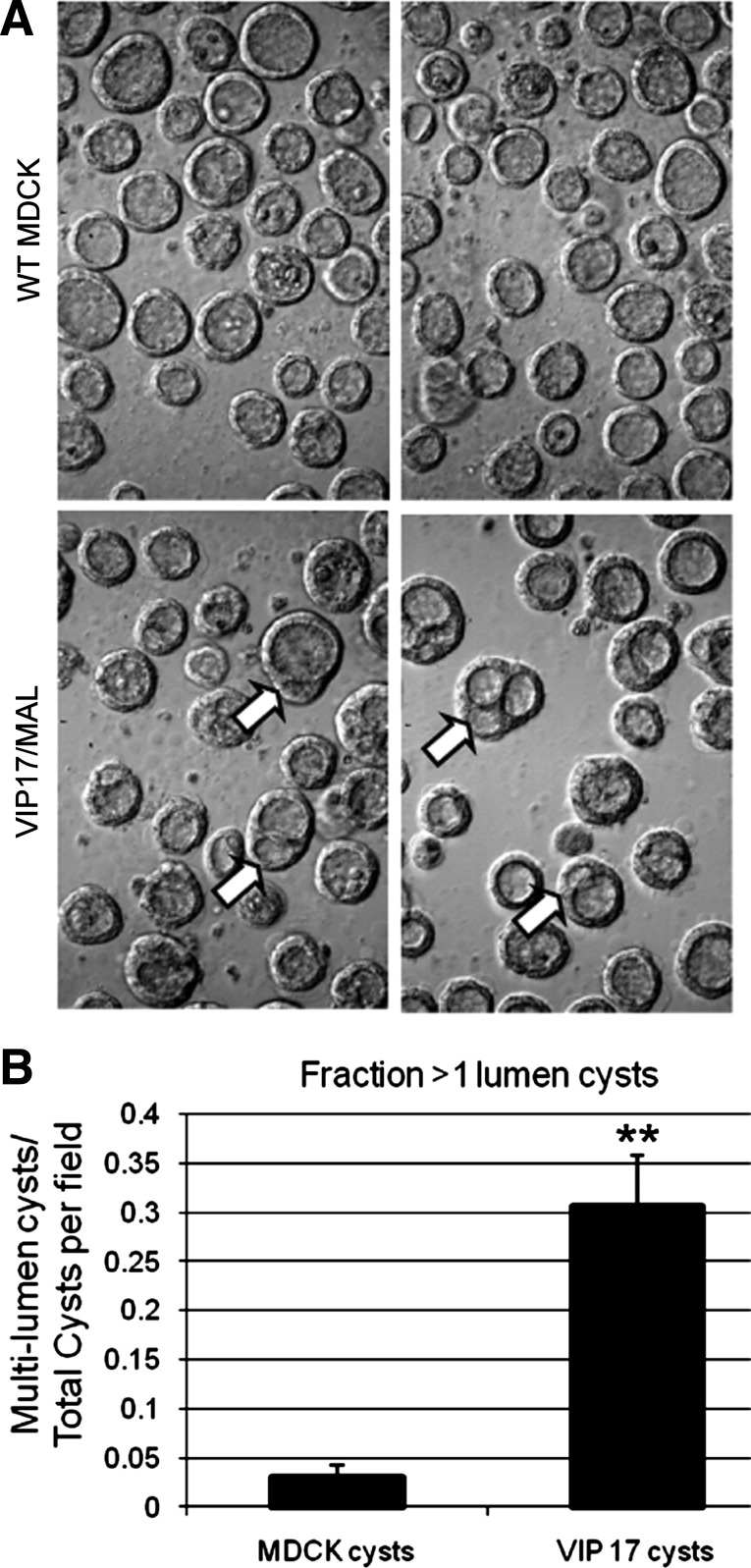
The presence of amplified apical membranes in VIP17/MAL overexpressing MDCK cells grown in two-dimensional culture suggests that VIP17/MAL plays a role in regulating vesicular traffic to and from the apical membrane (9, 42, 61). To evaluate the effect of VIP17/MAL on epithelial morphogenesis, VIP17/MAL overexpressing MDCK cells and WT MDCK cells were suspended in a three-dimensional collagen matrix and allowed to form cysts spontaneously as previously described (31). While untransfected MDCK cells generally formed cysts surrounding a single lumen, cysts produced by VIP17/MAL overexpressing cells were significantly more likely to exhibit multiple lumens (Fig. 2A). The difference in lumen number is quantified in Fig. 2B (P < 0.01). Both proliferative rate and the level of the mammalian target of rapamycin activity have been shown to be elevated in the context of renal cystic disease (8, 51), so we assessed the proliferation rate and mammalian target of rapamycin activity in VIP17/MAL overexpressing MDCK cells by immunofluorescence staining for Ki67 and Western blotting for phospho-S6 kinase, respectively. This analysis revealed no differences between the WT and VIP17/MAL overexpressing cells (not shown). It is interesting and perhaps somewhat surprising to note that a similar multiple lumen phenotype is observed with MDCK cells in which VIP17/MAL expression has been reduced through treatment with RNAi (53). While the mechanisms responsible for multiple lumen formation in the context of VIP17/MAL knockdown and overpression may not be identical, together these results suggest that a specific level of VIP17/MAL expression is required to drive the cavitation process responsible for MDCK cyst lumen formation towards the production of a single central lumen.
Fig. 2.
VIP17/MAL overexpression alters morphology of cultured MDCK cysts. A: representative images of WT MDCK (top) and VIP17/MAL overexpressing MDCK (bottom) cysts, acquired by light microscopy, demonstrating more multilumen (arrows) cysts produced by cells overexpressing VIP17/MAL. B: quantitation of microscopic images revealing an increased incidence of multilumen cysts derived from VIP17/MAL overexpressing MDCK cells. **P < 0.01 (n = 100 cysts).
E-cadherin, β-catenin, gp58, and ZO-1 expression in VIP17/MAL overexpressing cysts is similar to that of control MDCK cysts.
The multiple lumen formation that we observed in VIP17/MAL overexpressing cell cysts could, in principle, arise as a consequence of a defect in the function of membrane protein polarity or sorting pathways. To assess this possibility, we examined the distribution and expression of several markers of epithelial polarity. E-cadherin is a calcium-dependent cell adhesion molecule that binds to a catenin protein complex (α and β) to mediate cell-cell adhesion and polarized epithelium formation (54, 58). Polycystin-1, one of two proteins implicated in ADPKD, interacts with the E-cadherin complex containing β-catenin, and a large body of evidence suggests that aberrant β-catenin-mediated Wnt signaling may be involved in cystogenesis (4, 22, 27, 43, 45, 46, 50).
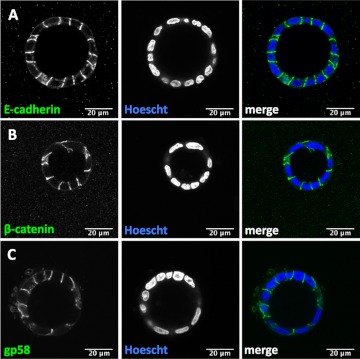
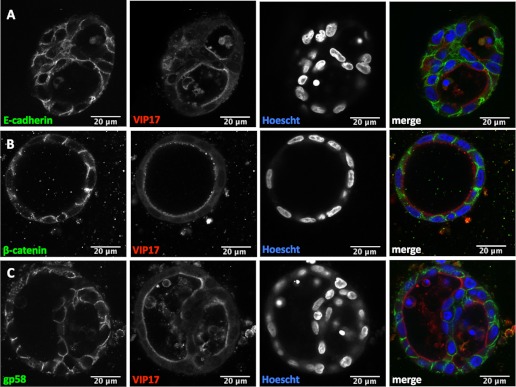
To test whether the localization of E-cadherin and β-catenin is disrupted in VIP17/MAL overexpressing cell cysts, immunofluorescence studies were performed, and protein localization was evaluated by laser scanning confocal microscopy. There is normal basolateral localization of E-cadherin in both WT (Fig. 3A) and VIP17/MAL overexpressing cell cysts (Fig. 4A). Under normal circumstances, β-catenin localizes to the perinuclear cytoplasm or to the basolateral membrane, where it engages with E-cadherin (26). In cysts formed from WT MDCK cells (Fig. 3B) or VIP17/MAL overexpressing cells (Fig. 4B), β-catenin retains its classic distribution along the basolateral membrane. Finally, immunofluorescence was used to assess the distribution of the basolateral marker antigen identified as gp58, which is the β-subunit of the Na-K-ATPase. This protein also retains a basolateral distribution independent of VIP17/MAL overexpression (Figs. 3C and 4C; Refs. 3, 20). We conclude that VIP17/MAL overexpression does not affect the distribution of E-cadherin, β-catenin, or gp58 in an in vitro model of cystogenesis.
Fig. 3.
Localization of basolateral membrane markers in three-dimensional WT MDCK cell cysts. Immunofluorescence analysis of representative WT MDCK cell cysts demonstrates the localization of E-cadherin (A), β-catenin (B), and gp58 (C; green) at the basolateral membrane. Hoescht (blue) was used to label nuclei. Merged color images are depicted at right. All scale bars depicted are 20 μm.
Fig. 4.
Basolateral membrane marker expression is unchanged by overexpression of VIP17/MAL. Immunofluorescence analysis of representative VIP17/MAL overexpressing MDCK cell cysts with antibodies to the basolateral markers E-cadherin (A), β-catenin (B), and gp58 (C; green) demonstrates normal basolateral localization, similar to that detected in control MDCK cysts. Hoescht staining (blue) was used to label nuclei. Mono-FLAG (red) indicates VIP17/MAL expression. Merged color images are depicted at right. All scale bars depicted are 20 μm.
ZO-1 is a classic structural component of tight junctions in MDCK cells (2). The integrity of the tight junction is important for establishing the extent and nature of the paracellular ion permeability barrier, which in turn plays a role in the mechanisms responsible for luminal fluid secretion (13, 60). We therefore examined the localization of ZO-1 in cysts composed of WT MDCK cells or VIP17/MAL overexpressing MDCK cells. By immunofluorescence and confocal imaging, ZO-1 was abundantly expressed and properly localized to tight junctions in both cyst types (data not shown). We conclude that VIP17/MAL expression does not have an obvious effect on ZO-1 localization.
VIP17/MAL overexpression results in apical accumulation of gp135 with aberrant localization of this protein to the primary cilium.
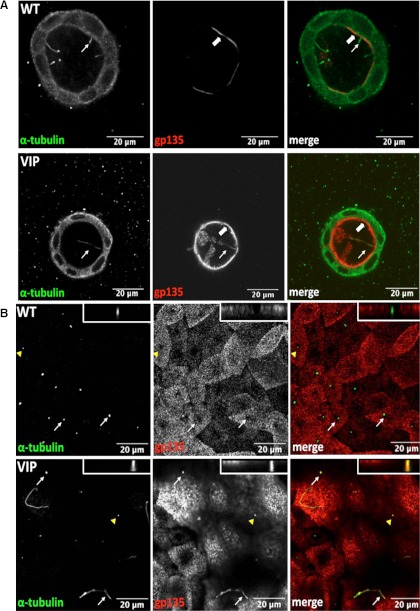
gp135, also known as podocalyxin, is endogenously expressed and localized to the apical membrane in MDCK cells (33). VIP17/MAL overexpression results in increased apical expression of the influenza virus hemagglutinin, among other proteins (9). In VIP17/MAL overexpressing MDCK cysts, immunofluorescence localization of gp135 reveals a surprising alteration in this protein's distribution. In control MDCK cells, gp135 is not present in the cilium that extends from the apical surface, accounting for the characteristic pattern of “ciliary exclusion” that is observed when the pattern of gp135 localization is observed in the apical surfaces of MDCK cells viewed en face (17). Interestingly, it appears that gp135 localizes to the primary cilium in VIP17/MAL overexpressing MDCK cells grown in three-dimensional cyst culture (Fig. 5A). This behavior was also detected in two-dimensional cultures in which we determined that VIP17/MAL overexpression correlates with ciliary localization of gp135 (Fig. 5B). The gp135 is notably absent from the cilia of control MDCK cells grown either under two- or three-dimensional culture conditions (Fig. 5, A and B).
Fig. 5.
VIP17/MAL overexpression results in gp135 apical localization and ciliary localization in an in vitro cyst culture model. A: representative WT MDCK (top) and VIP17/MAL overexpressing cell cysts (bottom) depicting expression of the apical marker gp135 (red) at the apical membrane (thick arrow) and in the cilium (thin arrow; α-tubulin; green) in cells expressing VIP17/MAL. B: gp135 (red) is present in the primary cilia of VIP17/MAL overexpressing MDCK cells grown in 2D (thin arrows, bottom), but is notably absent from WT MDCK cilia and from the ciliary exclusion zones in the apical membranes of WT MDCK cells (thin arrows, top). Orthogonal images of the individual cilia indicated by the arrow heads are shown in the top right corners at top and bottom. All scale bars depicted are 20 μm.
VIP17/MAL overexpression results in shortened or absent cilia in vitro and in vivo.
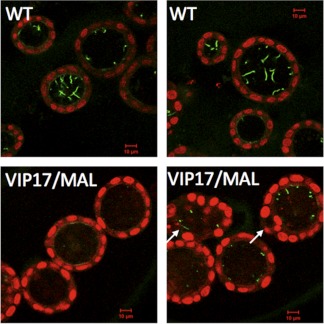
Since VIP17/MAL transgenic mice are reported to develop renal cysts, and susceptibility to the formation of renal cysts is frequently associated with perturbations that produce ciliary defects, we next examined the cilia of MDCK cysts grown in vitro. Untransfected MDCK cells and VIP17/MAL overexpressing MDCK cells were both suspended in a collagenous matrix, and immunofluorescence analysis, employing an antibody directed against acetylated tubulin, was used to visualize the ciliary axoneme. As shown in Fig. 6, we found that MDCK cell cysts demonstrated long cilia that extended well into the lumen, consistent with our previous observations and those of others (55). In contrast, cysts produced by VIP17/MAL overexpressing cells displayed stunted or absent cilia. Moreover, even when present, primary cilia did not appear to be protruding extensively into the lumen. Therefore, we conclude that VIP17/MAL overexpression perturbs cilia formation, extension, or stability in a model of renal epithelial cells grown in three-dimensional culture in vitro.
Fig. 6.
VIP17/MAL overexpression results in aberrant ciliogenesis in an in vitro three-dimensional cyst culture model. MDCK cells suspended in Matrigel were analyzed by immunofluorescence to localize acetylated tubulin (green). Nuclei are labeled with propidium iodide (red). Prominent cilia are seen protruding into the apical lumen in WT MDCK cysts (top), while very few, shortened cilia are seen in VIP17/MAL overexpressing cysts (bottom). Arrows point to multilumen cysts.
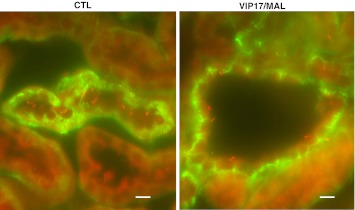
We also evaluated primary cilia in kidneys isolated from transgenic VIP17/MAL overexpressing mice (Fig. 7). Immunofluorescence analysis was performed on the kidneys of adult mice using an anti-ARL13B antibody (7) to label the cilium and an anti-Na-K-ATPase antibody (41) to identify the basolateral membrane. ARL13B is the ciliary protein mutated in Joubert syndrome (5). In parallel with our in vitro findings, we readily observed the presence of long, protruding cilia within the lumina of the renal tubules of WT control mice of the same genetic strain. In contrast, cilia were significantly stunted or absent in the dilated lumina/cysts of the renal tubules of in tissue from a VIP17/MAL overexpressing transgenic mouse model. The average lengths of these cilia were roughly half of those measured in similar renal tubule segments of WT animals (6 vs. 12 μM; P < 0.01). These studies suggest that VIP17/MAL overexpression results in the development of abnormal cilia.
Fig. 7.
VIP17/MAL transgenic mice have abnormal cilia. Immunofluorescence analysis to detect cilia in control (CTL) and VIP17/MAL transgenic mice. Renal sections were labeled with antibody directed against the ciliary protein Arl13b (red) and the Na-K-ATPase (green). Control mice display readily detectable, long cilia protruding into the distal tubule lumen, while VIP17/MAL transgenic mice display fewer and smaller cilia within their dilated lumina/cysts. Scale bars (bottom right) are 10 μm.
DISCUSSION
We find that VIP17/MAL overexpression results in abnormal cilia and cyst development in renal epithelial cells, in vitro and in vivo. In the well-studied MDCK cell culture model of in vitro epithelial cystogenesis, VIP17/MAL overexpressing cells formed multilumen cysts more frequently than did control cells, and their cilia were either shortened or absent. The VIP17/MAL transgenic mice are known to develop renal cysts (18), and we show that the cilia in these structures are also diminished in number and size compared with those found in association with the epithelial cells of renal tubules from WT animals. These phenotypes are present in the context of normal sorting of basolateral membrane proteins, including E-cadherin, β-catenin, Na-K-ATPase, and ZO-1. In contrast, the distribution of the apical marker protein gp135 is markedly abnormal in the VIP/MAL overexpressing cells. The presence of this polypeptide on the apical surface appears to be increased, and, most importantly, it is no longer excluded from the cilium in the VIP17/MAL overexpressing cells. This observation suggests that overexpression of VIP17/MAL perturbs the formation or maintenance of the barrier that normally maintains the unique composition of the ciliary membrane by preventing intermixing of apical and ciliary membrane components (30).
VIP17/MAL has been proposed to facilitate the apical delivery of several newly synthesized membrane proteins, including influenza hemagglutinin, gp80, and gp114 (9, 42). This function is supported by in vitro studies in which decreased levels of VIP17/MAL result in aberrant trafficking of these apical proteins to the basolateral membrane in MDCK monolayers. In contrast, overexpression of VIP17/MAL in this same cultured epithelial model system leads to expansion of the apical plasma membrane surface area, suggesting increased delivery or decreased retrieval of apical components (9, 42, 61).
VIP17/MAL has been shown to decrease the rate of endocytosis of the AQP2 water channel and thus to stabilize its residence in the apical plasma membrane (23). It is therefore possible that an imbalance in the rates of endocytosis and exocytosis is responsible for the capacity of VIP17/MAL overexpression to induce the development of amplified renal epithelial cell membranes (18). In contrast to the flattened cyst lining epithelia observed in the most common renal cystic disease ADPKD, the cells lining the cysts of VIP17/MAL overexpressing mice exhibit ballooning or blebbing of the apical membrane into the cyst lumen, with a pseudostratified morphology (18). Collectively, these data support the premise that VIP17/MAL may contribute to cystogenesis through perturbations in the structure of the apical membrane induced by disrupting the strict balance between endocytosis and exocytosis that normally determines the surface area of the apical plasma membrane domain.
In genetic polycystic kidney diseases, including autosomal dominant polycystic kidney disease, autosomal recessive polycystic kidney disease, and the nephronopthises, the proteins encoded by the responsible genes are often localized to the primary cilium (49). Renal cysts are therefore common features of the “ciliopathies,” the family of genetic diseases attributable to perturbations of genes whose protein products contribute to ciliary structure or function. The common mechanism underlying these diseases involves disruption of the primary cilium or proteins that colocalize with it (39). The primary cilium is formed when the designated mother centriole is modified to assume its role as the basal body beneath the cell's apical surface (40). Ciliogenesis ensues, with the cell directing necessary cargo to the tip of the cilium in a process known as intraflagellar transport.
Ciliopathies can result from either abnormal transport of proteins within the cilium or from disrupted delivery of vesicles from intracellular compartments to the base of the cilium (10, 16). Recent data suggest that while targeting of ciliary proteins to the cilium may occur via lateral transport from within the cell's apical membrane (34), there is also a component of targeted exocytosis to the base of the cilium (15). This cargo navigates through or across the peri-ciliary membrane or ciliary necklace and is subsequently trafficked into the cilium (28). While VIP17/MAL has not previously been implicated in ciliary trafficking, a number of other proteins similarly implicated in epithelial polarity have been shown to be involved in ciliogenesis (11, 12, 14, 62). It is also worth noting that recent studies (21) have shown that Septin 2, a guanosine triphosphatase is located at the base of the ciliary membrane and is thought to be part of a diffusion barrier that maintains the unique composition of the ciliary membrane. When Septin 2 is absent, the structure of the ciliary membrane is destroyed and abnormal or abrogated ciliary growth occurs.
When VIP17/MAL was overexpressed in cultured MDCK cells in vitro, we noted that cilia were also shortened and compositionally altered, with aberrant sorting of gp135 to the cilium. These observations suggest the interesting interpretation that the barrier regulating ciliary protein trafficking had been disrupted. According to this model, VIP17/MAL overexpression may either perturb the sorting of those proteins to the cilium that are required for maintaining the ciliary barrier or VIP17/MAL may itself directly disturb this boundary. Regardless, given this disturbance, it is perhaps expected that ciliary structure and thereby function is altered, leading to shortened and developmentally altered cilia, such as those seen in Fig. 6, and to an in vivo phenotype that bears the hallmarks of a ciliopathy (Fig. 7). It is important to note that our analysis of ciliary length in WT and VIP17/MAL overexpressing kidneys was limited to a relatively small number of specimens, and thus the results need to be interpreted with caution. Nevertheless, our data suggest the novel possibility that VIP17/MAL overexpression produces renal cysts in vivo by inducing the production of overabundant apical membrane, which in turn disrupts the composition and structure of the cilium. Thus the cysts observed in VIP17/MAL overexpression may be seen as arising as the consequence of a “secondary ciliopathy.”
These studies highlight the relationship between epithelial cell polarization and ciliogenesis, which results in cystogenesis when disrupted. VIP17/MAL appears to be a central component involved in both of these processes. When evaluated collectively, these data suggest a connection between cilia formation, post-Golgi sorting mechanisms, and epithelial cell polarization in renal cystogenesis.
GRANTS
This work was supported by National Institutes of Health Grants MSTP TG 5T32-GM-07205 and F30-DK-083221 (to V. Takiar) and DK-57328 and DK17433 (to M. J. Caplan). These studies made use of imaging resources provided through the Center for Polycystic Kidney disease Research at Yale (DK-090744).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.T., K.M., M.C., N.S.-W., and M.J.C. conception and design of research; V.T., K.M., M.C., and N.S.-W. performed experiments; V.T., K.M., M.C., and M.J.C. analyzed data; V.T., K.M., M.C., and M.J.C. interpreted results of experiments; V.T. and K.M. prepared figures; V.T. drafted manuscript; V.T. and M.J.C. edited and revised manuscript; V.T. and M.J.C. approved final version of manuscript.
REFERENCES
- 1.Alonso MA, Weissman SM. cDNA cloning and sequence of MAL, a hydrophobic protein associated with human T-cell differentiation. Proc Natl Acad Sci USA 84: 1997–2001, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol 106: 1141–1149, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcarova-Stander J, Pfeiffer SE, Fuller SD, Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J 3: 2687–2694, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boca M, D'Amato L, Distefano G, Polishchuk RS, Germino GG, Boletta A. Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Mol Biol Cell 18: 4050–4061, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83: 170–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmosino M, Rizzo F, Procino G, Basco D, Valenti G, Forbush B, Schaeren-Wiemers N, Caplan MJ, Svelto M. MAL/VIP17, a new player in the regulation of NKCC2 in the kidney. Mol Biol Cell 21: 3985–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 12: 767–778, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. J Cell Biol 191: 701–710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA 96: 6241–6248, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dentler W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J Cell Biol 170: 649–659, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol 178: 387–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol 14: 1451–1461, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol 10: 1337–1345, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH. The exocyst protein Sec10 interacts with polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7: e1001361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol 188: 21–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17: 3781–3792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol 193: 219–233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank M, Atanasoski S, Sancho S, Magyar JP, Rulicke T, Schwab ME, Suter U. Progressive segregation of unmyelinated axons in peripheral nerves, myelin alterations in the CNS, and cyst formation in the kidneys of myelin and lymphocyte protein-overexpressing mice. J Neurochem 75: 1927–1939, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Frank M, van der Haar ME, Schaeren-Wiemers N, Schwab ME. rMAL is a glycosphingolipid-associated protein of myelin and apical membranes of epithelial cells in kidney and stomach. J Neurosci 18: 4901–4913, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fullekrug J, Shevchenko A, Simons K. Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics. BMC Biochem 7: 8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329: 436–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest 104: 1459–1468, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA 104: 16696–16701, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang DY, Park JI, Cho WS, Jeong MH, Cho GW, Park HT, Bae HR. Identification of vasopressin-induced genes in AQP2-transfected MDCK cells by suppression subtractive hybridization. Biochem Biophys Res Commun 324: 1234–1241, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kim T, Fiedler K, Madison DL, Krueger WH, Pfeiffer SE. Cloning and characterization of MVP17: a developmentally regulated myelin protein in oligodendrocytes. J Neurosci Res 42: 413–422, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Kugoh H, Kleymenova E, Walker CL. Retention of membrane-localized beta-catenin in cells lacking functional polycystin-1 and tuberin. Mol Carcinog 33: 131–136, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 17: 3105–3117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell 103: 209–221, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magal LG, Yaffe Y, Shepshelovich J, Aranda JF, de Marco Mdel C, Gaus K, Alonso MA, Hirschberg K. Clustering and lateral concentration of raft lipids by the MAL protein. Mol Biol Cell 20: 3751–3762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangoo-Karim R, Uchic M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci USA 86: 6007–6011, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Belmonte F, Kremer L, Albar JP, Marazuela M, Alonso MA. Expression of the MAL gene in the thyroid: the MAL proteolipid, a component of glycolipid-enriched membranes, is apically distributed in thyroid follicles. Endocrinology 139: 2077–2084, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Meder D, Shevchenko A, Simons K, Fullekrug J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol 168: 303–313, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol 187: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millan J, Alonso MA. MAL, a novel integral membrane protein of human T lymphocytes, associates with glycosylphosphatidylinositol-anchored proteins and Src-like tyrosine kinases. Eur J Immunol 28: 3675–3684, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Millan J, Puertollano R, Fan L, Alonso MA. Caveolin and MAL, two protein components of internal detergent-insoluble membranes, are in distinct lipid microenvironments in MDCK cells. Biochem Biophys Res Commun 233: 707–712, 1997 [DOI] [PubMed] [Google Scholar]
- 37.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3: 831–838, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Ong AC, Wheatley DN. Polycystic kidney disease–the ciliary connection. Lancet 361: 774–776, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol 12: 551–555, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Pearson CG, Culver BP, Winey M. Centrioles want to move out and make cilia. Dev Cell 13: 319–321, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Pietrini G, Matteoli M, Banker G, Caplan MJ. Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc Natl Acad Sci USA 89: 8414–8418, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puertollano R, Martin-Belmonte F, Millan J, de Marco MC, Albar JP, Kremer L, Alonso MA. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J Cell Biol 145: 141–151, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 280: 3938–3945, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Reiter JF, Mostov K. Vesicle transport, cilium formation, and membrane specialization: the origins of a sensory organelle. Proc Natl Acad Sci USA 103: 18383–18384, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell 15: 1334–1346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972–5981, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell 62: 207–210, 1990 [DOI] [PubMed] [Google Scholar]
- 48.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313: 629–633, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Sorenson CM. Nuclear localization of beta-catenin and loss of apical brush border actin in cystic tubules of bcl-2−/− mice. Am J Physiol Renal Physiol 276: F210–F217, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Takiar V, Caplan MJ. Polycystic kidney disease: Pathogenesis and potential therapies. Biochim Biophys Acta 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tammachote R, Hommerding CJ, Sinders RM, Miller CA, Czarnecki PG, Leightner AC, Salisbury JL, Ward CJ, Torres VE, Gattone VH II, Harris PC. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum Mol Genet 18: 3311–3323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torkko JM, Manninen A, Schuck S, Simons K. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci 121: 1193–1203, 2008 [DOI] [PubMed] [Google Scholar]
- 54.van Adelsberg J. Protein targeting: the molecular basis of vectorial transport in the kidney. Semin Nephrol 18: 152–166, 1998 [PubMed] [Google Scholar]
- 55.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA 103: 18556–18561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol 15: 592–602, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Watnick T, Germino G. From cilia to cyst. Nat Genet 34: 355–356, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 79: 73–98, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Yu AS, Kanzawa SA, Usorov A, Lantinga-van Leeuwen IS, Peters DJ. Tight junction composition is altered in the epithelium of polycystic kidneys. J Pathol 216: 120–128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zacchetti D, Peranen J, Murata M, Fiedler K, Simons K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett 377: 465–469, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Zuo X, Fogelgren B, Lipschutz JH. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem 286: 22469–22477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]