Abstract
The blood-testis barrier (BTB) divides the seminiferous epithelium into the basal and the adluminal compartment. It restricts paracellular diffusion of molecules between Sertoli cells, confers cell polarity, and creates a unique microenvironment in the adluminal compartment for spermatid development. However, it undergoes restructuring during the epithelial cycle so that preleptotene spermatocytes differentiated from type B spermatogonia residing in the basal compartment can traverse the BTB at stage VIII of the cycle, while the immunological barrier is maintained. Herein, coxsackievirus and adenovirus receptor (CAR), a tight junction (TJ) integral membrane protein in the testis and multiple epithelia and endothelia, was found to act as a regulatory protein at the BTB, besides serving as a structural adhesion protein. RNAi-mediated knockdown of CAR in a Sertoli cell epithelium with an established TJ-permeability barrier that mimicked the BTB in vivo resulted in a disruption of the TJ barrier and an increase in endocytosis of the TJ-protein occludin. Furthermore, such an enhancement in occludin endocytosis was accompanied by a downregulation of Thr-phosphorylation in occludin and an increase in the association of endocytosed occludin with early endosome antigen-1. These findings were confirmed by overexpressing CAR in Sertoli cells, which was found to “tighten” the Sertoli cell TJ barrier, promoting BTB function. These findings support the emerging concept that CAR is not only a structural protein, it is involved in conferring the phosphorylation status of other adhesion proteins at the BTB (e.g., occludin) possibly mediated via its structural interactions with nonreceptor protein kinases, thereby modulating endocytic vesicle-mediated protein trafficking.
Keywords: testis, spermatogenesis, seminiferous epithelial cycle, CAR, Sertoli cell, blood-testis barrier, tight junction, ectoplasmic specialization
coxsackievirus and adenovirus receptor (CAR), a 46-kDa integral membrane protein, was first reported in 1997 as a common receptor for coxsackie virus group B and adenovirus groups 2 and 5 (2). CAR allows viral attachment and entry into cells (3). Subsequent studies (10–12, 61) have shown that CAR is found mostly at the tight junction (TJ) in multiple tissues and organs including brain, heart, muscles, and testes. Interestingly, it is highly expressed in both Sertoli cells and metabolically quiescent germ cells including highly differentiated haploid spermatids (36, 37, 62), and it is also a component of the apical ectoplasmic specialization [apical ES, a testis-specific atypical adherens junction (AJ)] besides the Sertoli cell TJ barrier (62). Since the presence of CAR on cell surface is a crucial determining factor for the entry of adenoviruses and coxsackieviruses (3), CAR has been extensively studied and targeted for gene therapy (47, 50). Recent studies (10, 18, 45) have shown that CAR is a structural adhesion protein at the TJ in multiple cell epithelia via homotypic interactions between neighboring epithelial cells. CAR also possesses a PSD-95/Dlg1/zonula occludens 1 (ZO-1)-binding (PDZ-binding)motif to facilitate protein-protein interactions, as such, CAR has been shown to interact with adaptor proteins ZO-1 and β-catenin (10, 60) and gap junction protein connexin 45 (Cx45) (31), forming a protein complex. In the testis, among more than a dozen BTB-associated structural and regulatory proteins that were examined, CAR was also found to interact with JAM-C (36), β-catenin, vinculin, and most importantly non-receptor protein tyrosine kinase c-Src based on studies (62) using coimmunoprecipitation (Co-IP), suggesting CAR may have other functional and/or regulatory roles. In fact, CAR is important in atrioventricular-node conduction and cardiac function as demonstrated in studies (31) using cardiac-specific CAR knockout (CAR-KO) mice, since CAR was shown to be involved in the localization of Cx45, ZO-1, and β-catenin at the intercalated disc in the heart and Cx45 was known to be involved in atrioventricular node conduction. Its tissue/organ-specific knockout in adult mice also led to dilated intestinal tract and atrophy of the exocrine pancreas, illustrating its inactivation led to multiple phenotypic changes (42). CAR may also act as a tumor suppressor (54). Collectively, these recent findings illustrate the critical role of CAR in multiple cellular functions besides serving as a receptor for viral entry and as a structural adhesion protein at the TJ and/or AJ, possibly mediated via its binding partner(s) such as c-Src.
Since the findings of CAR as a putative integral membrane proteins in Sertoli and germ cells in the testis (36, 62), it was speculated that CAR may serve as regulatory molecule to allow the transit of preleptotene spermatocytes at the BTB at stage VIII of the epithelial cycle (37, 61); however, the molecular mechanism(s) underlying this event remains elusive. Herein, we report findings using techniques of gene silencing by RNAi and transient overexpression that CAR plays a regulatory role at the Sertoli cell BTB via its involvement in modulating the phosphorylation status of integral membrane proteins at the BTB (e.g., occludin), which, in turn, modulates endocytic vesicle-mediated protein trafficking events at the site, thereby affecting cell adhesion status at the Sertoli cell-cell interface. In short, CAR appears to take part in a dynamic and efficient regulatory mechanism so that germ cells can be in transit at the BTB while the immunological barrier function remains intact during the epithelial cycle of spermatogenesis.
MATERIALS AND METHODS
Animals and antibodies.
Male Sprague-Dawley rats, both adults (∼250–300 g body wt) and 20-day-old pups, were purchased from Charles River Laboratories (Kingston, NY). The use of animals for the studies reported herein was approved by the Rockefeller University Laboratory Animal Care and Use Committee with Protocol No. 09–016 and 12–506. Antibodies were commercially obtained except for anti-desmoglein-2, which was prepared in our laboratory as earlier described (28) (Table 1).
Table 1.
Antibodies used for different experiments in this study
| Working Dilution |
|||||||
|---|---|---|---|---|---|---|---|
| Antibody | Catalog No. | Lot No. | Host | V Vendor | IB | IF | IP |
| CAR | sc-15405 | B2808 | Rabbit | Santa Cruz Biotechnology | 1:200 | 1:50 | |
| Occludin | 71-1500 | 00250207 | Rabbit | Zymed/Invitrogen | 1:400 | 1:50 | 1:40 |
| JAM-A | 36-1700 | 370923A | Rabbit | Zymed/Invitrogen | 1:250 | ||
| Claudin-11 | 36-4500 | 387613A | Rabbit | Zymed/Invitrogen | 1:125 | ||
| ZO-1 | 61-7300 | 389452A | Rabbit | Zymed/Invitrogen | 1:250 | 1:50 | |
| N-Cadherin | sc-7939 | H0907 | Rabbit | Santa Cruz Biotechnology | 1:200 | ||
| α-Catenin | sc-7894 | G3003 | Rabbit | Santa Cruz Biotechnology | 1:100 | ||
| β-Catenin | 71-2700 | 60806848C2 | Rabbit | Zymed/Invitrogen | 1:250 | ||
| Desmoglein-2* | — | — | Rabbit | Cheng Lab | |||
| Desmocollin-2 | sc-66863 | A0108 | Rabbit | Santa Cruz Biotechnology | 1:200 | ||
| γ-Catenin | 610254 | 0000074819 | Mouse | BD Transduction Laboratories | 1:1000 | 1:50 | |
| EEA-1 | 610457 | 87095 | Mouse | BD Transduction Laboratories | 1:2000 | 1:100 | 1:40 |
| Phospho-Ser | 61-8100 | 40487618 | Rabbit | Zymed/Invitrogen | 1:500 | ||
| Phospho-Thr | 71-8200 | 681682A | Rabbit | Zymed/Invitrogen | 1:500 | ||
| Phospho-Tyr | 61-5800 | 11067406 | Rabbit | Zymed/Invitrogen | 1:500 | ||
| Actin | sc-1616 | F2007 | Goat | Santa Cruz Biotechnology | 1:200 | ||
Anti-desmoglein-2 antibody was prepared in our laboratory against a rat desmoglein-2 recombinant protein of ∼17.5 kDa as described previously (28). CAR, coxsackievirus and adenovirus receptor; ZO-1, zonula occludens 1; EEA-1, early endosome antigen 1; IB, immunoblotting, IF, immunofluorescence analysis; IP, immunoprecipitation.
Primary Sertoli cell cultures.
Sertoli cells were isolated from 20-day-old rat testes as described previously (39). Sertoli cells were cultured in serum-free F-12/DMEM supplemented with sodium bicarbonate, gentamicin, epidermal growth factor, insulin, transferrin, and bacitracin at 35°C with 5% CO2-95% air (vol/vol) in a humidified CO2 incubator as described previously (39). An incubation temperature of 35°C was used for testicular cells, such as Sertoli cells, since in rodents and humans testicles are located outside the abdominal cavity, enclosed in the scrotum to maintain a temperature at 35°C, cooler than the body temperature of 37°C, necessary to maintain spermatogenesis and fertility (21, 49, 55). It is noted that these Sertoli cells were fully differentiated and ceased to divide (41). Furthermore, Sertoli cells isolated from 20-day-old rats were indistinguishable from cells isolated from 90-day-old rat testes both morphologically and functionally (26, 33). However, the purity of Sertoli cells isolated from adult rat testes was ∼85 vs. >98% from 20-day-old rat testes because of the lack of round/elongating/elongated spermatids in the latter group, which were tightly embedded in Sertoli cells and difficult to be removed including the use of hypotonic treatment (26, 33). Depending on the type of experiments, Sertoli cells were plated at different cell density for various applications. First, cells were plated at 0.45–0.5 × 106 cells/cm2 on Matrigel-coated 12-well dishes [Matrigel diluted 1:7 in serum-free F-12/DMEM (Sigma-Aldrich, St Louis, MO)] with each well containing 3-ml F-12/DMEM, and cultures were harvested at specified time points to obtain enough protein in cell lysates for immunoblotting or for nucleic acid extraction for RT-PCR or quantitative PCR. Second, Sertoli cells were plated at 0.04 × 106 cells/cm2 on Matrigel-coated coverslips for immunofluorescence analysis to assess changes in protein distribution and/or localization with each coverslip placed in 6-well dishes, and each well contained 5-ml F-12/DMEM. Thus Sertoli cells were evenly spaced, and changes in protein localization/distribution at the Sertoli cell-cell interface in treatment vs. control groups could be easily detected. Third, Sertoli cells were plated at 1.2 × 106 cells/cm2 on Matrigel-coated bicameral units (Millicell cell culture inserts, 12-mm diameter, 0.45-μm pore size, effective surface area, ∼0.6 cm2; Millipore, Bedford, MA), and units were placed in 24-well dishes with 0.5-ml F-12/DMEM each in the apical and basal compartment. About 48 h after plating, cells were subjected to a brief hypotonic treatment (20 mM Tris, pH 7.4 at 22°, 2.5 min) to lyse residual germ cells as described previously (14), and cultures were rinsed twice with F-12/DMEM to remove germ cell debris before replaced with fresh F-12/DMEM containing the necessary supplements (39). As such, Sertoli cell cultures used for our studies had a purity of >98% with negligible contaminations of germ, Leydig, and/or peritubular myoid cells when markers of these cells were assessed by RT-PCR using specific primer pairs as described previously (25). It is noted that germ cells, namely spermatogonia and spermatocytes (note: round/elongating/elongated spermatids are absent in 20-day-old rat testes; Ref. 9), are susceptible, but not Sertoli cells, to this brief hypotonic treatment. This treatment was found not to impede the TJ-permeability barrier function when the transepithelial electrical resistance (TER) was assessed ∼12–24 h after Sertoli cells were rinsed and returned to normal culture conditions vs. Sertoli cell cultures not subjected to the hypotonic treatment. It is also noted that using this in vitro model, Sertoli cells assembled a functional TJ-permeability barrier, and ultrastructures of both TJ, basal ES, gap junction (GJ), and desmosome were found when examined by electron microscopy, mimicking the BTB in vivo as described previously (27, 28, 53). In fact, this system has been used by investigators to study the biology and regulation of Sertoli cell BTB dynamics (4, 5, 15, 19, 20, 22, 40, 46).
CAR knockdown in Sertoli cells by RNAi using CAR-specific small interfering RNA duplexes.
Sertoli cells obtained from 20-day-old rat testes were cultured alone for 3 days to allow the establishment of a functional TJ-permeability barrier, which was manifested by the presence of a stable TER across the cell epithelium. Thereafter, cells were transfected with 100 nM nontargeting or CAR-specific small interfering (si)RNA duplexes by using RiboJuice siRNA Transfection Reagent (Novagen, EMD Biosciences) for 24 h according to the instructions of the manufacturer. To silence CAR, a mixture of 100 nM CAR (5′-CCUGAACAGAGGAUCGAAAtt, s137152; 5′-GAAAUGACUUCACCGGUUAtt, s137153; 5′-CGAGUACACUUUACGAGUAtt, s137154; Ambion) siRNA duplexes vs. 100 nM nontargeting control duplexes (Ambion) was used. After transfection, reaction mixture was removed, washed, and replaced with fresh F-12/DMEM, and cells were cultured for another 48 h before termination. For dual-labeled immunofluorescence analysis, 2 nM siGLO Red Transfection Indicator (Dharmacon/Thermo Fisher Scientific) were used to cotransfect with siRNA duplexes to confirm successful transfection.
Overexpression of CAR in Sertoli cells.
The full-length cDNA encoding CAR was obtained by PCR as earlier described (64, 65) using cDNAs derived from Sertoli cell total RNA via a reverse transcription step that served as the template and a CAR-specific primer pair designated ex-CAR (Fig. 1 and Table 2). The full-length CAR cDNA was cloned into pCI-neo mammalian expression vector (Promega) at the restriction enzyme sites between XhoI and NotI by using specific primers of CAR (see Table 2). The pCI-neo mammalian expression vector carries the human cytomegalovirus immediate early enhancer/promoter region that promotes constitutive expression of the CAR insert in Sertoli cells. The authenticity of these clones was confirmed by direct nucleotide sequencing (Genewiz). On day 3 after isolation, Sertoli cells plated on Matrigel-coated 12-well dishes or bicameral units at a cell density of 0.5 × 106 or 1.2 × 106 cells/cm2, respectively, were transfected with 1 or 0.5 μg of plasmid DNA per well or insert by using Effectene Transfection Reagent (Qiagen) at a ratio of 1 μg DNA to 15 μl transfection reagent. Transfection mixture was removed 24 h thereafter and replaced with fresh F-12/DMEM. RNA and protein lysates were extracted from these Sertoli cell cultures 2-day thereafter (i.e., 3-day after transfection began), as described previously (58). The Sertoli cell-TJ barrier function after transient expression of CAR vs. pCIneo vector alone was also assessed by TER measurement. To assess the transfection efficiency using the Mammalian Expression Vector pCI-neo in Sertoli cells, luciferase reporter plasmid (pGL3-Control and pRL-TK, Promega) was cotransfected into Sertoli cells with plasmid DNAs at ∼0.1–3 μg and different cell densities at 0.5 or 1.2 × 106 cells/cm2 for 24-h by assaying the luciferase reporter gene activity as described previously (64). With the use of this approach, the transfection efficacy was estimated to be ∼15–20%.
Fig. 1.
Primary nucleotide sequence of coxsackievirus and adenovirus receptor (CAR) (GenBank accession no.: NM_053570) that was used to clone the full-length rat testicular CAR. Rat testicular CAR was cloned by PCR using a CAR primer pair designated ex-CAR (see Table 2) based on the known CAR sequence as shown herein, annotated by the “black” box, utilizing rat Sertoli cell total cDNAs as the template, which were reverse transcribed from Sertoli cell RNA, and the cycling parameters were shown in Table 2. This rat testicular CAR cDNA was then used to prepare the full-length cDNA construct and its subsequent ligation into the mammalian pCI-neo vector, and XhoI and NotI restriction sites were added to its 5′- and 3′-end, respectively, by PCR using a CAR primer pair designated CAR (Table 2) and annotated by the “dash-lined” box. Numbers at left represent the nucleotide sequence of NM_053570. This rat testicular CAR cDNA clone was verified by direct nucleotide sequencing at Genewiz (South Plainfield, NJ).
Table 2.
Primer sequences used to clone the rat Sertoli cell CAR full-length cDNA and its insertion into pCI-neo mammalian expression vector*
| Gene | Primer Sequence | Orientation | Position | Length, bp | Tm, °C | Cycle No. | GenBank Accession No. |
|---|---|---|---|---|---|---|---|
| Ex-CAR | 5′-CTGAGAGCGTTTACCTGC-3′ | Sense | (−40)–(−23) | 1,288 | 54.3 | 35 | NM_053570 |
| 5′-TGAGGCAGACAACAGGAT-3′ | Anti-sense | 1,231–1,248 | |||||
| CAR | 5′-ACCTCGAGATGGCGCTCCTACTGTG-3′ | Sense | 1–17 | 1,059 + 8 + 10 = 1,077 | 56 | 35 | NM_053570 |
| 5′- ACGCGGCCGCTTATACCACTGCAATGCCATCG-3′ | Anti-sense | 1,038–1,059 |
CTCGAG, restriction site for XhoI; GCGGCCGC, restriction site for NotI; ATG, start codon; TTA, stop codon; Tm, annealing temperature (see Fig. 1 for the primary sequence of rat testicular CAR).
Functional assessment of the Sertoli cell TJ-permeability barrier.
The Sertoli cell TJ-permeability barrier was quantified by the ability of the cell epithelium to restrict the flow of current (i.e., quantified as conductivity in ohm, Ω) that was sent across the Sertoli cell epithelium when two electrodes of a Millipore Millicell-ERS were placed in the corresponding apical and basal chamber of the bicameral unit as earlier described (16). In short, Sertoli cells cultured in F-12/DMEM were plated on Matrigel-coated bicameral units (in triplicates) at 1.2 × 106 cells/cm2 at time 0, and TER was recorded daily, with fresh F-12/DMEM replenished after the TER measurement. On day 3, when the TJ-permeability barrier was established as manifested by a stable TER across the Sertoli cell epithelium, transfection was performed using siRNA duplexes to silence CAR or using pCI-neo/CAR to overexpress CAR vs. corresponding controls to assess the effects of CAR knockdown or its overexpression on the Sertoli cell TJ-permeability barrier function.
Endocytosis assay.
Endocytosis assay was performed essentially as described previously (63, 67) and detailed in an earlier report (24). In brief, Sertoli cells were cultured at 0.5 × 106 cell/cm2 on Matrigel-coated 6-well plates for 3 days. Thereafter, cells were transfected with CAR-specific siRNA duplexes vs. nontargeting control siRNA duplexes for 24 h. Three days after transfection when CAR was knockdown by ∼70%, cell surface proteins were biotinylated with 0.5 mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford, IL; note: sulfo-NHS-SS-biotin is a cell-impermeable, cleavable, biotinylation reagent) in PBS/CM buffer (PBS containing 0.9 mM CaCl2 and 0.33 mM MgCl2) at 4°C for 30 min (at this temperature, protein internalization did not occur). Free biotin was then quenched with 50 mM NH4Cl in PBS/CM buffer at 4°C for 15 min. To initiate endocytosis, Sertoli cell cultures were transferred from 4°C to a CO2 incubator in humidified atmosphere with 95% air-5% CO2 (vol/vol) at 35°C and incubated for the specified time points to assess the kinetics of biotinylated protein internalization in Sertoli cells transfected with CAR-specific siRNA duplexes vs. nontargeting control siRNA duplexes. At termination (i.e., 0, 15, 30, 60, and 90-min), biotins on uninternalized cell surface proteins were stripped with 50 mM sodium 2-mercaptoethanesulfonate (MESNA, a reducing agent that cleaved biotin from biotinylated Sertoli cell surface proteins; Sigma-Aldrich) in 100 mM Tris·HCl, 100 mM NaCl, and 2.5 mM CaCl2, pH 8.6, at 4°C for 30 min and quenched with 5 mg/ml iodoacetamide (Sigma-Aldrich) in PBS/CM buffer at 4°C for 15 min. Cell lysates were then obtained using IP lysis buffer [10 mM Tris, 0.15 M NaCl, 1% NP-40, and 10% glycerol (vol/vol), pH 7.4, at 22°C] supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Endocytosed biotinylated proteins were pulled down with UltraLink Immobilized NeutrAvidin Plus beads (Pierce) from each sample (note: each sample contained ∼300 μg protein of Sertoli cell lysates). The complexes were then washed, and the proteins were extracted in SDS-sample buffer containing 2-mercaptoethanol (6) at 100°C for 5 min (to cleave proteins from biotin, Mr 244.31, that bound to NeutrAvidin Plus beads) and subjected to SDS-PAGE and immunoblot analysis using anti-occludin antibody. All samples within an experimental group were processed simultaneously to avoid interexperimental variations. All endocytosis experiments reported herein were repeated at least three times using different batches of Sertoli cells, and each experiment yielded similar results.
Dual-labeled immunofluorescence analysis.
Distribution and/or localization of target proteins in Sertoli cells following CAR knockdown vs. its corresponding control were examined by immunofluorescence microscopy. In brief, Sertoli cells were isolated and cultured at a density of 0.04 × 106 cells/cm2 on Metrigel-coated coverslips and placed in 6-well dishes with each well containing 5-ml F-12-DMEM. On day 3, cells were transfected with 100 nM nontargeting or CAR-specific siRNA duplexes with Ribojuice siRNA transfection reagent (Novagen) served as a transfection medium for 24 h. Thereafter, the transfection mixture was removed and cells were cultured for an additional 48 h. Sertoli cells were fixed in 4% paraformaldehyde in PBS (10 mM NaH2PO4, pH 7.4, at 22°C containing 0.15 M NaCl; vol/vol) for 10-min at room temperature (22°C). Paraformaldehyde-fixed cells were permeabilized with 0.1% Triton X-100 in PBS (vol/vol). After blocking with 10% normal goat serum in PBS (vol/vol), cells were incubated with a target antibody at appropriate dilution in PBS (see Table 1). Following overnight incubation, cells were incubated with secondary antibodies conjugated to Cy3 or FITC (Invitrogen; 1:100 dilution in PBS) for 1 h. Then, cells were mounted with ProLong anti-fade reagent containing DAPI for nuclei staining (Molecular Probes, Eugene, OR), and fluorescence micrographs were obtained using an Olympus BX61 fluorescence microscope, and images were acquired using Olympus MicroSuite FIVE (Version 1224) software package and saved in TIFF format. Dual-labeled immunofluorescence images were subsequently merged for analysis to assess colocalization using PhotoShop in Adobe Creative Suite (Version 3.0).
RNA extraction and RT-PCR.
Total RNAs were extracted from Sertoli cells 3 days after transfection with siRNA duplexes using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Contaminating genomic DNA in each RNA sample, if any, was digested with RNase-free DNase I (Invitrogen) before their use for reverse transcription into cDNAs using Moloney murine leukemia virus reverse transcriptase (MMLV RT) reagent (Invitrogen). PCR was performed as earlier described (56, 58) using primer pairs specific to corresponding target genes (Tables 2 and 3). For RT-PCR, a target gene was coamplified with ribosomal S16 (Table 3), which served as an internal control for equal sample processing and RNA loading.
Table 3.
Primer sequences used for RT-PCR experiments
| Gene | Primer Sequence | Orientation | Position | Length, bp | Tm, °C | Cycle No. | GenBank Accession No. |
|---|---|---|---|---|---|---|---|
| CAR | 5′-CGCTCCTACTGTGCTTC-3′ | Sense | 5–21 | 195 | 54 | 30 | NM_053570 |
| 5′-CTTTCTGGTTATCGGACGG-3′ | Anti-sense | 181–199 | |||||
| OAS1A | 5′-GAGTGAAGTTTGAGGTCCAGA-3′ | Sense | 350–370 | 230 | 54.9 | 30 | NM_138913 |
| 5′-CTCCGTGAAGCAGGTAGA-3′ | Anti-sense | 562–579 | |||||
| STAT1 | 5′-GAGTGGAAGCGAAGACAG-3′ | Sense | 712–729 | 218 | 54.6 | 30 | NM_032612 |
| 5′-TGGAAGAGGACGAAGGTG-3′ | Anti-sense | 912–929 | |||||
| S-16 | 5′-TCCGCTGCAGTCCGTTCAAGTCTT-3′ | Sense | 15–38 | 385 | XM_341815 | ||
| 5′-GCCAAACTTCTTGGTTTCGCAGCG-3′ | Anti-sense | 376–399 |
Immunoblot analysis.
Lysates from Sertoli cell cultures were obtained by treating cells in IP lysis buffer (10 mM Tris, 0.15 M NaCl, 1% NP-40, and 10% glycerol, pH 7.4, at 22 °C) supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich), sonicated, and centrifuged at 15,000 g for 45 min at 4°C to obtain clear supernatant. Lysates were stored at −20°C until use. Forty micrograms of Sertoli cell lysate protein from each sample were resolved by SDS-PAGE for immunoblot analysis with target proteins being probed by the corresponding primary antibodies (see Table 1). Protein estimation was performed by spectrophotometry with a Bio-Rad Dc (detergent compatible) protein assay kit using BSA as a standard and a Bio-Rad Model 680 Plate Reader.
Co-IP.
Co-IP was used to monitor changes in protein-protein interaction as well as changes in occludin phosphorylation status. In brief, 2 μg normal mouse or rabbit IgG were added to 300 μg Sertoli cell protein lysate and incubated for 1 h before precipitated with 10 μl protein A/G agarose beads (Santa Cruz) for 1 h, and the supernatant was obtained (1,000 g, 5 min at 4°C) for subsequent Co-IP. This precleaning step was important to remove nonspecific IgG-interacting proteins from cell lysates. Thereafter, the supernatants were incubated with 2 μg normal mouse or rabbit IgG to serve as negative control, or anti-early endosome antigen 1 (anti-EEA-1) or anti-occludin for Co-IP on a Labnet MiniLab Roller overnight at room temperature (∼22 ± 1°C), to be followed by an incubation with 20 μl protein A/G agarose beads to precipitate the immunocomplexes. Thereafter, beads were washed with IP lysis buffer and immunocomplexes were extracted in an SDS-PAGE sample buffer (6) at 100°C for SDS-PAGE and immunoblot analysis as described previously (57).
Statistical analysis.
GB-STAT statistical analysis software (Version 7.0, Dynamic Microsystems) was used for statistical analyses. Each experiment was repeated at least three times, and data are means ± SD. Statistical significance was analyzed with Student's t-test or one-way ANOVA coupled with two-tailed Dunnett's test.
RESULTS
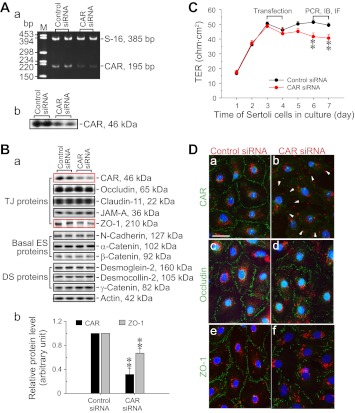
Knockdown of CAR by RNAi perturbs Sertoli cell TJ-permeability barrier function via changes in the localization and/or distribution of TJ proteins at the Sertoli cell BTB.
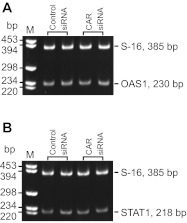
Figure 1 shows the primary sequence of rat testicular CAR that was cloned from the cDNAs derived from Sertoli cell RNA by PCR (Table 2), and the CAR-specific siRNA duplexes were prepared based on this rat testicular CAR primary sequence (see materials and methods). RNAi was used to knockdown CAR expression by ∼70% by transfecting Sertoli cells in vitro with an established functional TJ-permeability barrier that mimicked the Sertoli cell BTB in vivo using CAR-specific siRNA duplexes vs. the nontargeting control siRNA duplexes (Fig. 2, A, a and b, and B, a and b). The knockdown of CAR by ∼70% was found to associate with a downregulation of ZO-1 expression, but not other TJ, basal ES, and desmosome proteins examined (Fig. 2B, a and b). This CAR knockdown also perturbed the Sertoli cell TJ-permeability barrier function (Fig. 2C). We next investigated any changes in protein localization and/or distribution at the Sertoli cell-cell interface by dual-labeled immunofluorescence analysis in these cultures (Fig. 2D, a-f). Consistent with findings shown in Fig. 2, A and B, a knockdown of CAR by RNAi considerably abolished the expression of CAR at the Sertoli cell-cell interface (see “white” arrowheads in Fig. 2D, b vs. a). This also led to changes in the localization and distribution of occludin (a TJ-integral membrane protein) and ZO-1 (a TJ-associated adaptor protein), with these proteins being redistributed, moving from near the Sertoli cell surface, and into the cell cytosol (Fig. 2D, d and f vs. Fig. 2D, c and e). Such changes in distribution and/or localization likely contributed to a destabilization of cell adhesion at the TJ barrier, perturbing the Sertoli cell TJ-permeability barrier as shown in Fig. 2C. However, it is also possible that such changes contributed to an alteration of the paracellular transport of the TJ barrier. The disruptive effects following the knockdown of CAR in Sertoli cells by RNAi on the TJ barrier function (Fig. 2C) and on protein distribution/localization at the cell-cell interface (Fig. 2D) did not appear to be the results of off-target effects, since the steady-state levels of several BTB-associated proteins that were examined, including occludin, remained unaltered, with the exception of ZO-1, which was downregulated (Fig. 2B, a and b). Furthermore, the expression of two interferon-stimulated genes (ISG), such as oligoadenylate synthetase 1 (OAS1) and signal transducer and activator of transcription 1 (STAT1) that were known to be upregulated due to off-target effects when cells were transfected by RNAi vectors for gene knockdown (1, 34, 43), were found to be unaffected with S16 served as a loading control in this semi-quantitative RT-PCR experiment (Fig. 3, A and B). In short, small regulatory RNAs, such as siRNA used here, could trigger cellular immune responses in Sertoli cells, such as by activating interferon-stimulated gene expression, which in turn led to nonspecific cellular phenotypes and side effects (e.g., TJ-barrier disruption, altering protein distribution/localization) known as off-target effects. Since neither one of the two ISGs, namely OAS1 and STAT1, was upregulated in the CAR knockdown Sertoli cells vs. control cells, these findings (Fig. 3, A and B) coupled with immunoblotting data shown in Fig. 2B thus support our conclusion that the changes shown in Fig. 2, C and D, were the results of CAR knockdown. Attempts were made by immunofluorescence microscopy to assess changes in other BTB-associated proteins at the Sertoli cell-cell interface besides occludin and ZO-1 following CAR silencing, such as JAM-A and claudin-11; however, working antibodies were not available.
Fig. 2.
Study to assess the effects of CAR knockdown by RNAi on the Sertoli cell tight junction (TJ)-permeability barrier function in vitro. Sertoli cells were cultured alone on Matrigel-coated dishes (A and B), bicameral units (C), or coverslips (D) for 3 days to allow the assembly of a functional TJ permeability, possessing the ultrastructures of TJ, basal ectoplasmic specialization (ES), gap junction (GJ), and desmosome (DS) as described previously (27, 28, 53). Thereafter, cells were transfected with either CAR-specific siRNA duplexes vs. nontargeting control siRNA duplexes for 24 h, and cultures were then terminated for RT-PCR/immunoblotting (IB; A), immunoblotting (B), Sertoli TJ-permeability barrier assessment (C), and dual-labeled immunofluorescence analysis (IF; D). A: knockdown of CAR by RNAi using CAR-specific small interfering (si)RNA duplexes in Sertoli cells terminated 3 days after transfection was verified by RT-PCR (a) and immunoblotting (b). M, DNA size markers in basepair (bp). B: following the knockdown of CAR by ∼70% (a and b), the levels of TJ [e.g., occludin, claudin-11, JAM-A, and zonula occludens 1 (ZO-1)], basal ES (e.g., N-cadherin, α-catenin, and β-catenin), and desmosome proteins (e.g., desmoglein-2, desmocollin-2, and γ-catenin) at the Sertoli cell blood-testis barrier (BTB) were also examined by immunoblotting 3 days after transfection. It was noted that except for the level of ZO-1, which was downregulated by ∼30% following the knockdown of CAR in Sertoli cells by ∼70%, the levels of other BTB proteins remained relatively unaltered (a). Densitometric analyses of CAR and ZO-1 immunoblotting data that were normalized against actin wherein the control was arbitrarily set at 1 (b). Each bar is a mean ± SD of n = 3 experiments. C: knockdown of CAR was found to transiently perturb the Sertoli cell TJ barrier in vitro. PCR, immunoblotting (IB), and dual-labeled immunofluorescence analysis (IF) were performed 3 days after transfection. D: to investigate changes in protein distribution/localization at the cell-cell interface, Sertoli cells cultured on Matrigel-coated coverslips were cotransfected with siGLO Red (a transfection indicator) with either nontargeting or CAR-specific siRNA duplexes and stained for CAR (green fluorescence), occludin (green fluorescence), or ZO-1 (green fluorescence) on day 3. Sertoli cells were processed for IF 3 days after transfection (i.e., day 6 in culture). Loss of CAR expression at the Sertoli cell-cell interface was detected in the CAR knockdown cells (see “white” arrowheads in b vs. a). Although CAR RNAi had no apparent effects on the steady-state level of occludin (B), its knockdown was found to induce mis-localization of occludin, causing it to move from near the cell surface into the cell cytosol. Analogous to occludin, CAR knockdown also impaired the localization/distribution of ZO-1 at the Sertoli cell interface, besides downregulating ZO-1 expression. Scale bars = 20 μm in a, which applies to b–f. **P < 0.01.
Fig. 3.
Study to monitor off-target effects during CAR knockdown in Sertoli cells by RNAi. Two interferon-stimulated genes (ISG), namely OAS1 (oligoadenylate synthetase 1) and STAT1 (signal transducer and activator of transcription 1), were used as markers to assess off-target effects since these genes were known to be upregulated nonspecifically when cells transfected by RNAi vectors for gene silencing. Neither OAS1 nor STAT1 was upregulated in Sertoli cells following the knockdown of CAR vs. control. M, DNA size markers in basepair (bp).
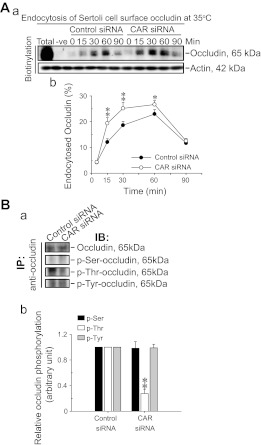
CAR knockdown results in increased endocytic vesicle-mediated protein trafficking and changes in occludin phosphorylation.
Since CAR knockdown that led to a partial disruption of the Sertoli cell TJ-permeability barrier shown in Fig. 2C appeared to be the result of changes in protein localization/distribution due to an increase in the internalization of occludin (Fig. 2D), protein endocytosis assay was used to explore this possibility. It was noted that the knockdown of CAR indeed significantly enhanced but not in the control group where Sertoli cells were transfected with nontargeting control siRNA duplexes, the kinetics of occludin endocytosis (Fig. 4A, a and b). It is noted that in this endocytosis assay of Le et al. (24), the use of a cell-impermeable but thiol-cleavable biotinylation reagent, sulfo-NHS-SS-biotin, only Sertoli cell surface proteins with exposed primary amines, including occludin, were biotinylated. In the lane designated “Total,” it represents total biotinylated cell surface proteins in the control group where Sertoli cells subjected to biotinylation for 30 min without stripping with MESNA buffer (i.e., before endocytosis; ∼300 μg protein) were pulled down by NeutraAvidin Plus beads and subjected to immunoblotting to visualize occludin, which was used to estimate the percentage of endocytosed occludin. Since at the time of biotinylation before the endocytosis assay, total cell surface occludin was similar in both control and CAR siRNA groups; thus a single total lane from the control group was shown in Fig. 4A, a. Only a small fraction of biotinylated occludin was endocytosed (Fig. 4A, a and b). In short, the purpose of this assay was not attempted to quantify differences between cell surface occludin in the CAR knockdown vs. the control group but to assess any alteration in the kinetics of protein endocytosis. Since protein endocytosis is known to be regulated, at least in part, via changes in the protein phosphorylation of an integral membrane protein, such as occludin and E-cadherin (7, 17), we next examined any alterations in the phospho-Ser, -Thr, and -Tyr content in occludin between the knockdown and control groups. While the levels of occludin between the control and CAR-silenced Sertoli cells by Co-IP were similar (Fig. 4B), consistent with data shown in Fig. 2B, the level of phospho-Thr-occludin was significantly lowered, but not phospho-Ser- nor phospho-Tyr-occludin, in the CAR knockdown cells vs. control cells, illustrating there was a reduction of phospho-Thr content in occludin following CAR silencing (Fig. 4B, a and b).
Fig. 4.
A knockdown of CAR in Sertoli cells with an established TJ-permeability barrier by RNAi enhances endocytosis of occludin and alters the phosphorylation status of occludin. A: Sertoli cells (0.45 × 106 cells/cm2) were cultured alone for 3 days before RNAi in which CAR was knockdown by transfecting cells with 100 nM CAR-specific siRNA duplexes vs. nontargeting control siRNA duplexes for 24 h (see Fig. 2C). Three days thereafter, endocytosis assay was performed. Total biotinylated cell surface proteins (Total, from control group as shown herein, which was similar in CAR siRNA group), without stripping with 2-mercaptoethanesulfonate, were used to assess the percentage of occludin that have been endocytosed. Equal amount of proteins (∼300 μg) were used for extracting the biotinylated proteins by using Ultralink Immobilized NeutrAvidin Plus beads (Pierce, including the “Total,” as illustrated by the corresponding actin blot). The “-ve” represents cell surface proteins (∼300 μg) without biotinylation but subjected to extraction with NeutrAvidin Plus beads and immunoblotting using an anti-occludin (see Table 1). Knockdown of CAR in Sertoli cells by ∼70% was found to accelerate the internalization of occludin, a TJ-integral membrane protein, significantly vs. control cells (a). Actin shown in a served as a protein loading control, representing actin from Sertoli cell lysates before NeutrAvdin pull-down. Composite data of this endocytosis experiment were shown in b in which each data point is a mean ± SD of n = 3 independent experiments. Statistical analysis was performed by one-way ANOVA. **P < 0.01. B: Sertoli cell lysates (∼300 μg of protein) were first subjected to coimmunoprecipitation (Co-IP) with an anti-occludin antibody to immunoprecipitate occludin, and the levels of occludin in the samples between the nontargeting control and the CAR knockdown groups were similar (top panel), consistent with findings shown in Fig. 1B, which also illustrated equal protein loading between the two groups. These blots were then probed with anti-phospho-Ser, -Thr, or -Tyr antibodies (see Table 1) and shown in the subsequent panels (a). These findings were densitometrically scanned and normalized against occludin level with the level in the control arbitrarily set at 1, against which statistical comparison was performed. Each bar is a mean ± SD of n = 3–4 experiments. *P < 0.05; **P < 0.01.
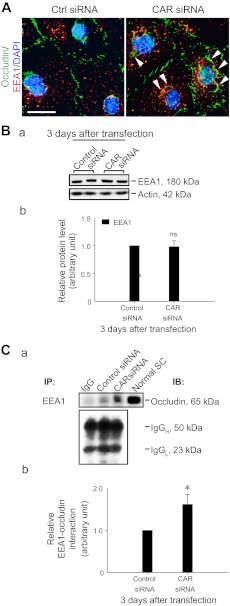
Knockdown of CAR in Sertoli cells enhances association of occludin with endosomes.
To further expand and confirm the observations shown in Figs. 2 and 4 that there was an increase in protein endocytosis following CAR knockdown, we examined any changes in the protein expression of endocytic vesicle-associated proteins, such as EEA-1 (an endosome marker; Ref. 35), or its association with occludin in Sertoli cells after CAR knockdown. It is noted that following CAR knockdown, occludin was internalized and it appeared to become better associated with EEA1 (Fig. 5A). While the steady-state level of EEA-1 in the CAR knockdown Sertoli cells vs. control cells was similar (Fig. 5B, a and b), a statistically significant increase in the association between occludin and EEA-1 was detected by Co-IP (Fig. 5C, a and b), supporting findings obtained by dual-labeled immunofluorescence analysis (Fig. 5A).
Fig. 5.
Effects of CAR knockdown on the endocytic vesicle-mediated protein trafficking in Sertoli cell epithelium. A: cellular localization of EEA-1 (red, an endosome marker) and occludin (a TJ-integral membrane protein) in Sertoli cell epithelium and their changes following CAR knockdown were examined by dual-labeled immunofluorescence analysis 3 days after transfection of cells with CAR-specific siRNA duplexes (CAR siRNA) vs. nontargeting control duplexes (Ctrl siRNA). After CAR knockdown, more occludin (green fluorescence) was found to be endocytosed in Sertoli cells, consistent with findings shown in Fig. 1D. Also, occludin and EEA-1 (red fluorescence) appeared to co-localize more extensively in the CAR knockdown cells (see “white” arrowheads which denote an increase in the colocalization of occludin with EEA-1 after CAR knockdown). DAPI (blue) was used to visualize nuclei. Scale bar = 20 μm, which applies to the other micrograph. B: Sertoli cells cultured for 3 days were transfected with CAR-specific siRNA duplexes vs. the nontargeting control siRNA duplexes for 24 h. Cells were rinsed and cultured with fresh F-12/DMEM for an additional 48 h and terminated thereafter and used for immunoblotting. CAR knockdown had no apparent effects on the steady-state level of EEA-1 in Sertoli cells (a), and the composite results of n = 3 experiments were shown in (b); ns, nonsignificantly different. C: Co-IP was used to assess changes in protein-protein interactions between occludin with EEA-1 following CAR knockdown by using ∼300 μg protein lysate for immunoprecipitation as shown in a. Normal Sertoli cell lysate (Normal SC, 40 μg protein) without Co-IP served as a positive control (a). Bottom: blot of IgGH and IgGL chains, illustrating equal protein loading in this Co-IP experiment (a). An increase in interaction between occludin with EEA-1 was detected following CAR knockdown in Sertoli cells (a). Composite data, with each bar represents a mean ± SD of n = 3 experiments shown in b in which the relative association of occludin with EEA-1 in control group was arbitrarily set at 1, against which statistical comparison was performed. *P < 0.05 by ANOVA.
Overexpression of CAR in Sertoli cells promotes TJ-permeability barrier function via upregulation of TJ protein adaptor ZO-1 and desmosome proteins at the Sertoli cell BTB.
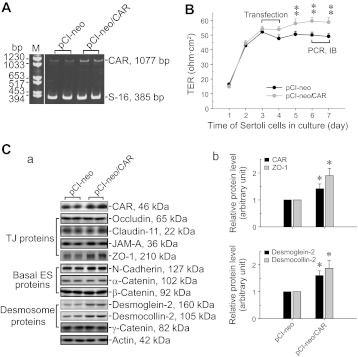
To further validate data obtained by RNAi that illustrate the regulatory role of CAR in BTB dynamics, the full-length CAR cDNA was isolated by PCR (Fig. 1) using cDNAs derived from Sertoli cell RNA as the template, ligated to a mammalian expression vector pCI-neo (Table 2). This pCI-neo/CAR plasmid was used for overexpression of CAR in Sertoli cell epithelium with an established TJ-permeability barrier (Fig. 6, A and B). Consistent with findings using RNAi in which the knockdown of CAR perturbed the Sertoli TJ-barrier function, overexpression of CAR (Fig. 6A) by ∼50% (Fig. 6C) led to a “tightening” of the Sertoli cell TJ-permeability barrier (Fig. 6B). Studies by immunoblotting also illustrated that an overexpression of CAR led to an upregulation of ZO-1 expression, consistent with data shown in Fig. 1 wherein its knockdown could downregulate ZO-1 expression, but also an upregulation of desmoglein-2 and desmocollin-2, but not other BTB-associated TJ and basal ES proteins (Fig. 6C, a and b).
Fig. 6.
Effects of CAR overexpression on the Sertoli cell TJ-permeability barrier and the steady-state levels of BTB constituent proteins in Sertoli cells in vitro. To confirm findings regarding the physiological role of CAR in regulating BTB function based on the use of RNAi, Sertoli cells were transfected with pCI-neo/CAR vector vs. empty pCI-neo vector (control) to examine changes in TJ-barrier function following transient expression of CAR. A: increase in the expression of CAR in Sertoli cells transfected with pCI-neo/CAR vector containing the full-length CAR construct vs. empty vector (pCI-neo) was detected by RT-PCR. B: Sertoli cells at 1.2 × 106 cells/cm2 were cultured alone for 3 days to allow the establishment of a functional TJ-permeability barrier and were then transfected with pCI-neo vector vs. pCI-neo/CAR vector. Changes in the TJ-barrier function was monitored by quantifying TER across the Sertoli cell epithelium. Each data point is a mean ± SD of n = 3 replicates of a representative experiment, similar results were obtained from 3 independent experiments. **P < 0.01 by one way ANOVA followed by Dunnett's test. C: increase in CAR expression following transfection of Sertoli cells with pCI-neo/CAR vector was further confirmed by immunoblotting (a). Also noted is an upregulation of ZO-1, consistent with findings shown in Fig. 2B, in which a knockdown of CAR was found to associate with a downregulation of ZO-1 (a). An upregulation of desmosome proteins desmogelin-2 and desmocollin-2 was consistently detected following CAR overexpression in Sertoli cells as shown in a. Data of immunoblotting were summarized in histograms shown in b, illustrating a significant upregulation on ZO-1 (top), desmoglein-2, and desmocollin-2 (bottom) expression following CAR overexpression. Each bar is a mean ± SD of n = 4–5 experiments in which the expression of a target gene was normalized against the corresponding actin level, and the protein level in Sertoli cells transfected with pCI-neo empty vector was arbitrarily set at 1, against which statistical comparison was performed. *P < 0.05.
DISCUSSION
CAR was initially identified in 1997 (2) as the receptor for coxsackievirus and adenovirus (3, 12). It is a constituent protein and cell adhesion molecule of the TJ in multiple epithelia and endothelia (10, 32, 61). It is now established that CAR is an important TJ integral membrane protein between cardiomyocytes known as the intercalated disc in the heart via homotypic interactions of CAR between adjacent cells, crucial to regulate cardiac remodeling and electrical conductance between atria and ventricle (11, 42). Because CAR is a specific receptor for adenovirus, it has also been extensively investigated to explore the use of CAR for adenovirus-based gene therapy (23, 50, 68). However, recent studies have shown that CAR is a molecule with diversified functions; its contribution to cellular physiology is well beyond its structural and cell adhesion role at the TJ and other intercellular junctions. For instance, CAR was shown to be a metastatic tumor suppressor since its expression reduces lung metastatic potential in murine cancer cells (66). However, CAR expression is also needed for the efficient formation of tumors in a subset of lung cancer cells (44), and its expression is necessary to support the establishment of distant metastases and reducing apoptosis in adenomas in colon cancer (54). Collectively, these findings thus illustrate its involvement in tumorigenesis. Furthermore, CAR was recently shown to play a supporting role in regeneration of damaged myocardium in the heart (59), in neuronal homeostasis by involving in axonal transport of clathrin-mediated organelles via endocytosis in the brain (48), and in mediating signaling function at the ventricular intercalated disc in the heart by recruiting connexin 45 (a GJ integral membrane and structural protein), β-catenin, and ZO-1 to the site for proper atrioventricular conduction and cardiac function (31). In short, there is mounting evidence based on studies in multiple tissues and/or organs that support the expanding physiological roles of CAR in different aspects of cellular physiology.
In this report, CAR was shown to be more than a structural component of the TJ at the Sertoli cell BTB. First, its knockdown without any apparent off-target effects was found to associate with a downregulation of ZO-1 expression in Sertoli cell epithelium with an established TJ barrier that mimicked the Sertoli cell BTB in vivo, suggesting CAR may play a regulatory role in protein expression at the BTB. Our observation is also consistent with a recent report (31) using cardiac-specific CAR conditional knockout (CAR-cKO) mice, in which CAR KO in the heart also led to a marked decline in β-catenin and ZO-1 expression at the intercalated disc, which is an ultrastructure between cardiomyocytes composed of TJ, AJ, and GJ. More importantly, its knockdown was found to associate with a disruption of the Sertoli cell TJ-permeability barrier, and an increase in endocytosis of TJ protein occludin at the Sertoli cell BTB. This increase in endocytic vesicle-mediated protein trafficking is apparently the result of a loss of phospho-Thr in occludin (but not phospho-Ser or phospho-Tyr), which in turn leads to an increased association between occludin and EEA-1, an endosome marker, as supported by a Co-IP experiment. The mechanism by which CAR regulates the phosphorylation content of occludin is likely mediated via c-Src, a nonreceptor protein tyrosine kinase at the BTB (8), since c-Src is one of the few regulatory kinases that was shown to form a protein complex with CAR in the testis (62). For instance, neither phosphoinositide 3-kinase nor focal adhesion kinase (FAK) was found to structurally associate with CAR (62) even though both phosphoinositide 3-kinase (53) and FAK (51, 52), in particular p-FAK-Tyr407 (30), have been localized to the BTB in the rat testis and expressed stage specifically during the epithelial cycle. In this context, it is of interest to note that the use of Co-IP that demonstrated interactions between occludin and EEA-1; this procedure may bring down other components of the endocytic vesicles (but not the entire endocytic vesicles because lysates of Sertoli cells, instead of intact cells, were used), such as protein involving in recycling or protein degradation, which may also be involved in perturbing the Sertoli cell TJ-barrier function. However, it is unlikely that the silencing of CAR would enhance endosome-mediated occludin degradation since the steady-state level of protein remained unaltered during the experimental period. Collectively, these data support the notion that CAR plays a possible regulatory role at the BTB to coordinate the proper functioning of different adhesion protein complexes at the site. In this context, it is of interest to note that at the TJ in multiple epithelia CAR is known to associate with adaptor proteins ZO-1 and β-catenin (10, 60), which may serve as linkers for tethering CAR to the underlying actin-based cytoskeleton. Since ZO-1 also structurally interacts with the occludin at a stoichiometric ratio of 1:1 (13); thus a downregulation of ZO-1 can lead to a loss of occludin-ZO-1-based adhesion function at the BTB, and the “free” occludin not binding to ZO-1 may lead to its “unwanted” phosphorylation, possibly via c-Src, which triggers an increase in its internalization as shown herein. However, the precise molecular signaling event(s) and/or mechanism(s) that leads to a downregulation of ZO-1 expression following a knockdown of CAR remains to be identified. Second, these findings were supported in studies by overexpressing CAR in Sertoli cell epithelium, which was found to “tighten” and “promote” the Sertoli cell TJ-permeability barrier. Overexpression of CAR was also found to upregulate ZO-1 in Sertoli cells, consistent with findings in RNAi experiments. Interestingly, overexpression of CAR also upregulated the expression of desmoglein-2 and desmocollin-2, a putative integral membrane protein and adaptor, respectively, at the desmosome, which together with coexisting TJ, basal ES, and GJ constitute the BTB (8, 29), suggesting that CAR, being a TJ protein, may act as a “signaling molecule” that regulates the expression of constituent proteins of desmosome. However, it is noted that a knockdown of CAR failed to downregulate the expression of desmoglein-2 and desmocollin-2 as shown herein based on multiple experiments, suggesting that only its overexpression can trigger some unintended signaling pathway(s) that leads to an upregulation of these two desmosomal proteins. Nonetheless, these findings illustrate the likely role of CAR in coordinating other adhesion protein complexes to maintain the barrier function at the BTB. This finding is also consistent with a recent report (31) illustrating that in cardiac CAR-cKO mice the loss of CAR also affects the expression of connexin 45 (a GJ protein) at the intercalated disc, perturbing atrioventricular conduction to coordinate cardiac function. It is highly likely that there is a similar underlying molecular mechanism(s) that induces a downregulation of ZO-1, β-catenin, and connexin 45 in these different organs following the knockdown or knockout of CAR, which should be vigorously evaluated in future studies.
Based on the established cell adhesion properties of CAR, and the fact that CAR is found in both Sertoli and germ cells (36, 37, 62), it was postulated that during the transit of preleptotene spermatocytes at the BTB at stage VIII-IX of the epithelial cycle, CAR residing in spermatocytes (SP) can form homotypic interactions with CAR in Sertoli cells (SC), such that the barrier function can be maintained in which CAR residing in spermatocytes “replaces” one of the two interacting CARs between adjacent Sertoli cells (SC/CAR-CAR/SC) forming a transient SP/CAR-CAR/SC barrier (61). Interestingly, it is now generally accepted that to preserve the BTB integrity during the transit of preleptotene spermatocytes at the BTB at stage VIII-IX of the epithelial cycle, “new” BTB (e.g., TJ fibrils) is first assembled behind migrating preleptotene spermatocytes, which are connected in “clones” via intercellular bridges, before the “old” BTB is “disassembled” (7, 8, 38). Thus, besides serving as homotypic interacting adhesion molecules between spermatocytes and Sertoli cells during the transit of spermatocytes across the BTB, CAR may also take part in the assembly of “new” BTB and the disassembly of “old” BTB near the basal- and apical-region of migrating spermatocytes, respectively, via its corresponding “overexpression” and “silencing” at the two sites as illustrated in this report. This possibility must be carefully evaluated in future studies. However, findings reported herein have provided the framework upon which functional experiments can be designed.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.S. and C.Y.C. performed experiments; L.S., D.D.M., and C.Y.C. analyzed data; L.S. and C.Y.C. prepared figures; L.S., D.D.M., and C.Y.C. approved final version of manuscript; D.D.M. and C.Y.C. conception and design of research; D.D.M. and C.Y.C. interpreted results of experiments; C.Y.C. drafted manuscript; C.Y.C. edited and revised manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Child Health and Human Development Grants R01-HD-056034 (to C. Y. Cheng) and U54-HD-029990 Project 5 (to C. Y. Cheng) and National Natural Science Foundation of China Grant 31101043 (to L. Su).
REFERENCES
- 1.Bauer M, Kinkl N, Meixner A, Kremmer E, Riemenschneider M, Forstl H, Gasser T, Ueffing M. Prevention of interferon-stimulated gene expression using microRNA-designed hairpins. Gene Ther 16: 142–147, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J, Cunningham J, Droguett G, Kurt-Jones E, Krithivas A, Hong J, Horwitz M, Crowell R, Finberg R. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275: 1320–1323, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bergelson JM. Receptors mediating adenovirus attachment and internalization. Biochem Pharmacol 57: 975–979, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl 7: 59–68, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Fok KL, Chen H, Zhang XH, Xu WM, Chan HC. Cryptorchidism-induced CFTR downregulation results in disruption of testicular tight junctions through upregulation of NF-κB/COX-2/PGE2. Hum Reprod 27: 2585–2597, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Cheng CY, Musto NA, Gunsalus GL, Frick J, Bardin CW. There are two forms of androgen binding protein in human testes: comparison of their protomeric variants with serum testosterone-estradiol-binding globulin. J Biol Chem 260: 5631–5640, 1985 [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 82: 825–874, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 64: 16–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clermont Y, Perry B. Quantitative study of the cell population of the seminiferous tubules in immature rats. J Anat 100: 241–267, 1957 [DOI] [PubMed] [Google Scholar]
- 10.Cohen C, Shieh J, Pickles R, Okegawa T, Hsieh J, Bergelson J. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA 98: 15191–15196, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer R, Poller W, Schultheiss HP, Gotthardt M. CAR-diology–a virus receptor in the healthy and disease heart. J Mol Med 87: 879–884, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. Curr Top Microbiol Immunol 323: 67–87, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127: 1617–1626, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl 5: 249–259, 1981 [Google Scholar]
- 15.Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol 89: 127–140, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Grima J, Wong CC, Zhu LJ, Zong SD, Cheng CY. Testin secreted by Sertoli cells is associated with the cell surface, and its expression correlates with the disruption of Sertoli-germ cell junctions but not the inter-Sertoli tight junction. J Biol Chem 273: 21040–21053, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner B. Regulation of cadherin adhesive activity. J Cell Biol 148: 399–403, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, Kozakai I, Kobayashi K, Kumanishi T, Watanabe YG, Odani S, Kuwano R. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Mol Brain Res 77: 15191–15196, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood-testis barrier. Endocrinology 129: 1489–1496, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures–a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol 112: 51–57, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril 49: 1–23, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Kato R, Maeda T, Akaike T, Tamai I. Characterization of novel Na+-dependent nucleobase transport systems at the blood-testis barrier. Am J Physiol Endocrinol Metab 290: E968–E975, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kolawole AO, Sharma P, Yan R, Lewis KJ, Hostetler HA, Excoffon KJ. The PDZ1 and PDZ3 domains of MAGI-1 regulate the eight exon isoform of the coxsackievirus and adenovirus receptor. J Virol 86: 9244–9254, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: A potential mechanism for regulating cadherin dynamics. J Cell Biol 146: 219–232, 1999 [PMC free article] [PubMed] [Google Scholar]
- 25.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl 25: 200–215, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Li JCH, Lee WM, Mruk DD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl 22: 266–277, 2001 [PubMed] [Google Scholar]
- 27.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41: 2302–2314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol 42: 975–986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol 286: 223–269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 109: 12562–12567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim BK, Xiong D, Dorner A, Youn TJ, Yung A, Liu TI, Gu Y, Dalton ND, Wright AT, Evans SM, Chen J, Peterson KL, McCulloch AD, Yajima T, Knowlton KU. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J Clin Invest 118: 2758–2770, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisewski U, Shi Y, Wrackmeyer U, Fischer R, Chen C, Schirdewan A, Juttner R, Rathjen F, Poller W, Radke MH, Gotthardt M. The tight junciton protein CAR regulats cardiac conduction and cell-cell communication. J Exp Med 205: 2369–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 68: 1597–1612, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Meng Z, Zu Y, Wu J, Tian Y, Kemper T, Bleekmann B, Roggendorf M, Yang D, Lu M. Inhibition of hepatitis B virus gene expression and replication by endoribonuclease-prepared siRNA. J Virol Methods 150: 27–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills IG, Jones AT, Clague MJ. Regulation of endosome fusion. Mol Membr Biol 16: 73–79, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Mirza M, Hreinsson J, Strand ML, Hovatta O, Soder O, Philipson L, Pettersson RF, Sollerbrandt K. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res 312: 817–830, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Mirza M, Petersen C, Nordqvist K, Sollerbrandt K. Coxsackievirus and adenovirus receptor is upregulated in migratory germ cells during passage of the blood-testis barrier. Endocrinology 148: 5459–5649, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25: 747–806, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol 763: 237–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl 17: 249–255, 1996 [PubMed] [Google Scholar]
- 41.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203: 485–492, 1982 [DOI] [PubMed] [Google Scholar]
- 42.Pazirandeh A, Sultana T, Mirza M, Rozell B, Hultenby K, Wallis K, Vennstrom B, Davis B, Arner A, Heuchel R, Lohr M, Philipson L, Sollerbradt K. Multiple phenotypes in adult mice following inactivation of the coxsackievirus and adenovirus receptor (CAR) gene. PLos One 6: e20203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pebernard S, Iggo RD. Determinants of interferon-stimulated gene induction by RNAi vectors. Differentiation 72: 103–111, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Qin M, Escuadro B, Dohdwala M, Sharma S, Batra RK. A novel role for the coxsackie adenovirus receptor in mediating tumor formation by lung cancer cells. Cancer Res 64: 6377–6380, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, Pettersson RF. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight juncitons, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res 312: 1566–1580, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Robillard KR, Hoque MT, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther 340: 96–108, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Sakurai F. Development and evaluation of a novel gene delivery vehicle composed of adenovirus serotype 35. Biol Pharm Bull 31: 1819–1825, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, Kremer EJ. CAR-associated vesicular transport of an aenovirus in motor neuron axons. PLoS Pathog 5: e1000442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setchell BP. The Parkes lecture: heat and the testes. Fertil Sertil 114: 179–194, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Sharma P, Kolawole AO, Wiltshire SM, Frondorf K, Excoffon KJ. Accessibility of the coxsackievirus and adenovirus receptor and its importance in adenovirus gene transduction efficency. J Gen Virol 93: 155–158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology 150: 3336–3344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology 144: 2141–2163, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 280: 25029–25047, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Stecker K, Vieth M, Koschel A, Wiedenmann B, Rocken C, Anders M. Impact of the coxsackievirus and adenovirus receptor on the adenoma-carcinoma sequence of colon cancer. Br J Cancer 104: 1426–1433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinberger E, Dixon WJ. Some observations on the effect of heat on the testicular germinal epithelium. Fertil Sertil 10: 578–595, 1959 [Google Scholar]
- 56.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol 41: 2578–2587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su L, Mruk DD, Lee WM, Cheng CY. Drug transporters and blood-testis barrier function. J Endocrinol 209: 337–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK). Proc Natl Acad Sci USA 108: 19623–19628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tatrai E, Bedi K, Kovalszky I, Hartyanszky I, Laszik A, Acsady G, Sotonyi P, Hubay M. No mutation but high mRNA expression of coxsackie-adenovirus receptor was observed in both dilated and ischemic cardiomyopathy. Forensic Sci Int 212: 47–50, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowking virus escape. Cell 110: 789–799, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Wang CQF, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol 178: 549–556, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res 313: 1373–1392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA 107: 11399–11404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem 281: 16799–16813, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood-testis barrier dynamics in adult rat testes. Proc Natl Acad Sci USA 104: 3841–3846, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashita M, Ino A, Kawabata K, Sakurai F, Mizuguchi H. Expression of coxsackie and adenovirus receptor reduces the lung metastatic potential of murine tumor cells. Int J Cancer 121: 1690–1696, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 22: 1945–1959, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu D, Jin C, Leja J, Majdalani NN, B , Eriksson F, Essand M. Adenovirus with hexon Tat-protein transductrion domain modification exhibits increased therapeutic effect in experimental neuroblastoma and neuroendocrine tumors. J Virol 85: 13114–13123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]