Abstract
Sepsis has deleterious effects on cardiac function including reduced contractility. We have shown previously that lipopolysaccharides (LPS) directly affect HL-1 cardiac myocytes by inhibiting Ca2+ regulation and by impairing pacemaker “funny” current, If. We now explore further cellular mechanisms whereby LPS inhibits excitability in HL-1 cells. LPS (1 μg/ml) derived from Salmonella enteritidis decreased rate of firing of spontaneous action potentials in HL-1 cells, and it increased their pacemaker potential durations and decreased their rates of depolarization, all measured by whole cell current clamp. LPS also increased action potential durations and decreased their amplitude in cells paced at 1 Hz with 0.1 nA, and 20 min were necessary for maximal effect. LPS decreased the amplitude of a rapidly inactivating inward current attributed to Na+ and of an outward current attributed to K+; both were measured by whole cell voltage clamp. The K+ currents displayed a resurgent outward tail current, which is characteristic of the rapid delayed-rectifier K+ current, IKr. LPS accordingly reduced outward currents measured with pipette Cs+ substituted for K+ to isolate IKr. E-4031 (1 μM) markedly inhibited IKr in HL-1 cells and also increased action potential duration; however, the direct effects of E-4031 occurred minutes faster than the slow effects of LPS. We conclude that LPS increases action potential duration in HL-1 mouse cardiomyocytes by inhibition of IKr and decreases their rate of firing by inhibition of INa. This protracted time course points toward an intermediary metabolic event, which either decreases available mouse ether-a-go-go (mERG) and Na+ channels or potentiates their inactivation.
Keywords: delayed rectifier K+ current, patch-clamp electrophysiology, Salmonella enteritidis, E-4031
sepsis is a complex clinical problem that can progress into multiorgan dysfunction syndrome (MODS) and death. At least one-half of the high mortality rates of severe-sepsis, sepsis, and MODS result from cardiovascular failure (27). Approximately 40% of patients in septic shock also experience myocardial dysfunction (36, 44), which comprises reduced systolic contractility, impaired diastolic relaxation, and ventricular dilatation. The initial clinical pattern, however, ironically is termed “hyperdynamic,” because it often presents with high cardiac output, elevated heart rate, and vasodilatation of the dermis (27). This is attributable in part to the volume loading that is frequently the initial treatment for septic shock patients to maintain cardiac output and organ perfusion (15, 28). As is often the case in the complexity of sepsis, the prevailing symptoms can mask organ and system failure occurring at a deeper level in the clinical spectrum.
The most prevalent sign of myocardial depression in the septic patients, in spite of their increased heart rates, is reduced heart rate variability (HRV), which is prodromal to unfavorable outcome (8, 30, 31, 38). Based on telemetry studies in mice, this reduction in HRV is attributable to lipopolysaccharide (LPS; Ref. 5); however, it remains uncertain whether this action of LPS results from its direct effect on heart cells (41, 47) or broader effects involving the central and autonomic nervous systems (6).
We have reported that bacterial LPS added to an immortalized mouse myocardial cell line, HL-1 cells (4), reduced and even eliminated oscillations of intracellular Ca2+ ([Ca2+]i) and also reduced total [Ca2+]i (41). LPS also inhibited activation of the cardiac pacemaker, “funny” current, If (41), which is attributable to direct inhibition of human HCN2 ion channel current (19). These results were in keeping with reports in both isolated rat cardiomyocytes and a modified Langendorff isolated rat heart preparation showing that LPS markedly reduced various hemodynamic characteristics including cardiomyocyte shortening, left ventricular (LV) developed pressure, heart rate, LV ejection fraction, and cardiac output (17). Interestingly, LPS treatment of rats also increased the time constant of LV relaxation and blunted its decrease with pacing (17). The authors attributed these results to impaired Ca2+ uptake by the sarcoplasmic reticulum in LPS treated animals. Others (16) have shown that endotoxin impairs cardiac hemodynamics by affecting loading conditions, which could indicate inadequate myocardial relaxation, but not by reducing cardiac ionotropism. Taken together, these studies show that LPS as a circulating constituent of sepsis markedly reduces cardiac function via Toll-like receptor 4 (TLR4)-mediated alterations in cellular metabolism. It appears that the heart, although metabolically depressed during sepsis, has sufficient physiologic reserve to respond and handle volume loading and inotropic stimulation as part of therapy; however, cardiac depression persists and complicates the clinical picture during recovery.
We conducted the present study to determine whether LPS alters additional electrophysiologic variables of cardiomyocytes that may account for myocardial depression. We show here that LPS added to HL-1 cells increases duration on the action potential resulting from inhibition of the rapid, delayed rectifier K+ current, IKr.
METHODS AND MATERIALS
HL-1 cell culture.
HL-1 atrial cardiomyocytes were a gift of Dr. William Claycomb (Louisiana State University Medical Center). They were grown in 5% CO2 at 37°C in Claycomb media (Sigma) supplemented with batch-specific 10% FBS (Sigma), 100 U/ml:100 μg/ml penicillin/streptomycin (Invitrogen), 0.1 mM norepinephrine (Sigma), and 2 mM l-glutamine (Invitrogen). Before culturing cells, flasks were treated overnight with 0.02% Bacto gelatin (Fisher Scientific):0.5% fibronectin (Invitrogen). For electrophysiologic or calcium measurements cells were plated at a density of 3 × 105 cells/35-mm culture dish on glass coverslips (12-mm diameter) that had been briefly flamed to enhance coating and then transferred to a 35-mm culture dish where they were treated with gelatin/fibronectin overnight.
Whole cell voltage-clamp measurements.
Cells were grown for 1–2 days on 12-mm diameter glass plastic coverslips, which resulted in islands of juxtaposed cells. These were transferred to an acrylic chamber (Warner, New Haven, CT) on the stage of an inverted microscope (Olympus IMT-2) equipped with Hoffman modulation contrast optics. Cells were superfused at room temperature with a standard external salt solution. Patch pipettes (3–6 MΩ in the bath solution) were fabricated from glass capillaries (1.1- to 1.2-mm inner diameter, 0.2-mm wall thickness, nonheparinized micro-hematocrit capillary tubes; Fisher Scientific) with a Brown-Flaming horizontal micropipette puller (P-97; Sutter Instruments, Novato, CA). A micromanipulator (MO-202; Narishige, Tokyo) fixed to the microscope was used to position pipettes. The whole cell patch configurations were obtained by standard patch-clamp technique (14). Voltage-clamp currents and current-clamp voltages were measured with a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Foster City, CA) with the lowpass, Bessell filtering (−3 dB) set at 5 kHz. Signals from the patch-clamp amplifier were fed into a computer via a digital interface (Digidata 1322A) and processed by Clampex 8 software (Axon Instruments). In some instances of current-clamp recordings cells, were stimulated with 0.1 nA (Grass S44; Grass Instruments) through the patch pipette. Ag/AgCl half-cells constituted the electrodes, and an agar bridge (4% wt/vol in external solution) connected the reference electrode to the bath solution. Series resistances were compensated following whole cell access before recordings.
Solutions and chemicals.
Standard external salt solution contained the following (mM): 150 NaCl, 6 KCl, 1 MgCl2, 1.5 CaCl2, 10 HEPES, and 10 glucose (pH adjusted to 7.41 with NaOH). Pipette solution contained the following (mM): 120 aspartic acid, 20 KCl, 4 Na2ATP, 1 MgCl2, 5 EGTA, 0.1 CaCl2, and 10 HEPES (pH adjusted to 7.2 with KOH). In some instances, Cs+ was substituted for K+ for the pipette solution (pH adjusted to 7.2 with CsOH). LPS from Salmonella enteritidis was obtained from Sigma Chemical (St. Louis, MO); recombinant human LPS binding protein (LBP) was obtained from R&D Systems (Minneapolis, MN). LBP is an acute-phase protein that binds to LPS and presents it to the pattern recognition receptor TLR4 (25). E-4031 was obtained from Alomone Labs (Jerusalem, Israel).
Data analysis.
Results were expressed as means ± SE and differences between means were computed by Student's paired or unpaired t-test, or they were expressed as box and whisker plots showing the mean, minimum and maximum, 25th percentile, median, and 75th percentile. Some electrophysiological data were analyzed using WinASCD software (Guy Droogmans, Katholieke Universiteit, Leuven, BE; ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/winascd.zip).
RESULTS
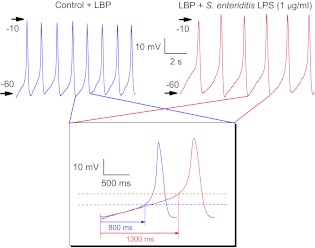
Action potentials occur spontaneously in ∼40% of nonconfluent immortalized mouse Hl-1 cardiomyocytes, accompanied by transient increases in intracellular Ca2+ (29, 41). These action potentials display depolarizing, diastolic pacemaker potentials in phase 4 followed by rapid upstrokes and repolarizations, which are characteristic of sinoatrial node cardiomyocytes in situ, (Fig. 1). Under control conditions with added LBP (10 ng/ml), the cell in Fig. 1 fired action potentials at 49/min (blue traces). Twenty minutes following added LPS endotoxin (S. enteritidis; 1 μg/ml), the rate of firing had decreased to 36/min (Fig. 1, red traces). Selected action potential traces from both trains were overlayed for further comparisons (Fig. 1, inset). These showed that LPS treatment prolonged duration of the pacemaker potential (which is taken as the duration from the maximal diastolic potential until the foot of the action potential; Fig. 1, red trace; 1,300 ms) compared with that obtained from the cell under LBP-control condition (Fig. 1, blue trace; 800 ms), as well as shifted the threshold potential (Fig. 1, dashed lines) to a more positive voltage (Fig. 1, inset). In summary, LPS treatment significantly increased the duration of the pacemaker potential from 694 ± 20 to 1,004 ± 96 ms (n = 10; P < 0.004). Moreover, LPS treatment also reduced the slope of the depolarizing potential of the LBP control from 12.4 ± 0.6 to 8.9 ± 0.9 V/s (n = 10; P < 0.001).
Fig. 1.
Spontaneous action potentials recorded in current-clamp mode from HL-1 mouse cardiomyocytes. Control traces (blue) contained only lipopolysaccharide (LPS) binding protein (LBP; 10 ng/ml) in the external solution. Traces (red) were recorded 20 min after LPS endotoxin (Salmonella enteritidis) plus LBP were added to external solution at 1 μg/ml. Arrows and numbers indicate the magnitude of transmembrane voltage. Inset: overlay of actions potentials selected from each set to compare pacemaker potential and action potential duration. Dashed lines indicate thresholds at the foot of the action potentials.
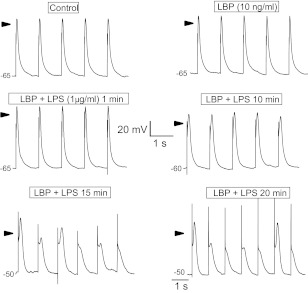
Prolongation and reduced rate of depolarization of the pacemaker potential by LPS is consistent with our report that LPS inhibits the hyperpolarization-activated cationic current, If, (41) and HCN ion channels (19). This, however, does not account completely for the extended action potential duration. To assess this effect of LPS, we paced HL-1 cells in current-clamp mode by passing 0.1 nA current through the pipette at 1 Hz. Representative action potential traces under these conditions included control, added LPB, and various times after added LPB plus LPS (1 μg/ml; Fig. 2). The added LPS increased action potential duration; however, this prolongation took ≥10 min to develop (Fig. 2). In addition, LPS reduced the amplitude of the action potentials, which is addressed (below) by the measurement of the effect of LPS on inward current attributable to Na+.
Fig. 2.
Action potentials recorded in current-clamp mode of a mouse HL-1 cardiomyocyte paced at 1 Hz by 0.1-nA current pulse (2.5-ms duration; 10 ng/ml LBP). Times indicate period of exposure to LPS endotoxins when the trains of action potentials were recorded. Time and voltage calibrations apply to all traces except at 15- and 20-min LPS where the traces were slightly protracted. Arrows indicate 0 mV.
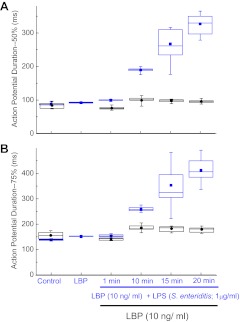
To quantify this effect of LPS on action potentials, we measured action potential durations at 50 and 75% repolarization for the groups shown in Fig. 2. LBP alone had no effect compared with the untreated control condition, whereas LPS treatment increased action potential duration at both 50 and 75% repolarization (Fig. 3, A and B, respectively). The effect, however, was not evident until ≥10 min following added LPS, and then duration increased further up to 20 min following the addition (Fig. 3, A and B). LBP alone had no significant effect on action potential durations measured under identical time course and perecent repolarization (Fig. 3, A and B).
Fig. 3.
Action potential durations recorded by current-clamp mode of a mouse HL-1 cardiomyocyte at various times after added LPS endotoxin (S. enteritidis; blue) compared with controls (untreated and added 10 ng/ml LBP; black) as in Fig. 2. A: action potential duration at 50% repolarization. B: action potential duration at 75% repolarization. Box and whisker plots: ■, mean; T-bars, minimum and maximum; box, 25th percentile, median, and 75th percentile; n = 10.
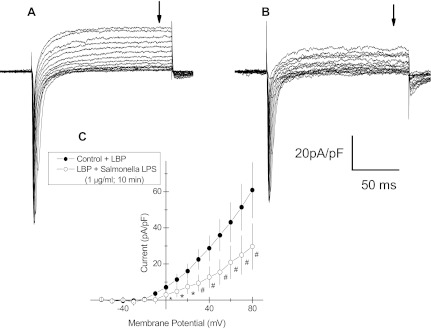
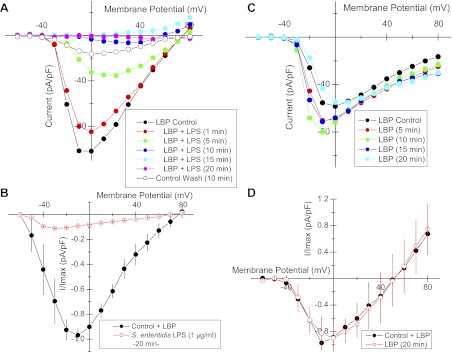
To explore the mechanism by which LPS affected action potential duration, an HL-1 cell was voltage-clamped and whole cell currents were measured, first under LBP-control conditions and then 10 min following added S. enteritidis LPS (1 μg\ml; Fig. 4). The holding potential was −80 mV with an initial voltage step to −60 followed by successive 10-mV increments to 80 mV. Under control condition (Fig. 4A), inward currents activated rapidly at −60 mV and were evident at additional depolarized voltage steps. These inward currents quickly inactivated. Outward currents (Fig. 4, A and C) were first evident at ∼−10 mV and increased in magnitude up to 80 mV. These showed no inactivation. LPS (10 min) reduced inward current magnitudes at all depolarizations compared with LBP control (Fig. 4B). LPS also inhibited voltage-activated outward currents compared with those measured under the LPB-control condition (Fig. 4, A and B), and current/voltage plots from repeated measures taken at the arrows in Fig. 4, A and B, are shown in Fig. 4C.
Fig. 4.
Whole cell voltage-clamp recordings from an HL-1 mouse cardiomyocyte. A: time-dependent inward and outward currents recorded in response to voltage steps from −60 to 80 mV (10-mV increments) and a holding potential of −80 mV. B: repetition of the voltage protocol in the same cell as in A but 10 min after added LPS endotoxin (S. enteritidis; 1 μg/ml). C: amplitude of steady-state outward currents (at arrows in A and B) vs. the clamped voltage before voltage-step back to holding potential = −80 mV. ●, Control; ○, 10 min following added LPS endotoxin (S. enteritidis; 1 μg/ml); means ± SE (n = 3); *P < 0.05; #P < 0.01. Time and current calibration applies to A and B. LBP (10 ng/ml) was added in all measurements.
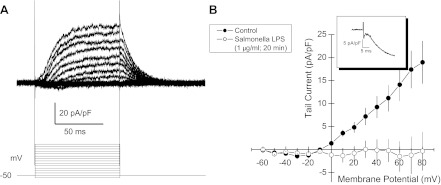
The voltage-clamp protocol and the composition of external and pipette solutions did not allow discrimination among cationic currents. Aspartate was the predominant anion in the pipette solution; thus the inward currents are attributable to Na+ and Ca2+. These were eliminated when methy-d-glucamine (NMDG+) was substituted for external Na+ and Ca2+ (Fig. 5A), which left only outward currents attributable to K+. Delayed rectifier K+ current, IKr, constitutes the predominant outward current in HL-1 cells (4, 39). Moreover, IKr, inactivation contributes to the extended duration of the cardiac action potential and release from inactivation contributes to its repolarization (12). By virtue of the rapid release of channel inactivation on partial repolarization of the plasma membrane, IKr displays a resurgent outward tail current due to the relatively slow ion channel deactivation (33) (Fig. 5B, inset). Thus we evaluated the effects of LPS on tail currents. LPS completely inhibited HL-1 cell tail currents within 20 min of addition (Fig. 5B). Control measurements in untreated HL-1 cells demonstrated no “rundown” of this current over 20 min (Wondergem R., unpublished observations, and F. Toyoda, personal communication).
Fig. 5.
K+ tail current measured in voltage-clamped HL-1 cells. A: whole cell voltage-clamp recordings from HL-1 mouse cardiomyocytes under conditions in which external Na+ and Ca2+ were replaced with isomolar concentrations of N-methyl-d-glucamine to minimize inward currents and to maximize outward K+ current and tail currents on return of holding potential B: amplitude of peak tail current (inset) vs. the clamped voltage before the voltage step back to holding potential = −80 mV. ●, Control with LBP (10 ng/ml); ○, 20 min following added LPS (1 μg/ml S. enteritidis plus 10 ng/ml LBP); means ± SE (n = 9). Each experiment differs from control at P = 0.03 or less.
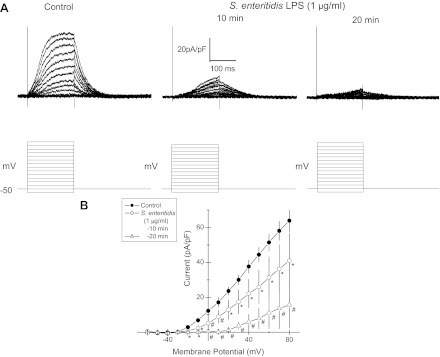
The Ikr is prevalent in HL-1 cells (4, 34, 39); however, outward currents measured here activated ∼0 mV and did not display the characteristic inactivation at >20 mV. Neither is in keeping with IKr. This may have resulted from the relatively short duration of our voltage steps. Still, various K+ currents have been reported for HL-1 cells (9, 26). Thus, to isolate IKr and to assess the likelihood that LPS treatment inhibited the delayed rectifier K+ current, we repeated voltage-clamp measurements with pipettes filled with Cs+ substituted for K+, since Cs+ is excluded by many K+ channels but conducted by IKr (32, 45). The external solution here contained nifedipine (1 μM). Outward currents were evident at voltage steps greater than −30 mV, and these diminished in amplitude sequentially at 10 and 20 min following LPS (1 μg/ml; Fig. 6A). Repeated measures of this inhibition of outward currents by LPS are shown in current/voltage plots in Fig. 6B.
Fig. 6.
Outward currents were measured by voltage clamp of HL-1 cells in which external solution contained nifedipine (1 μM) and the pipette was filled with Cs+ substituted for K+ to maximize delayed-rectifier K+ currents. A, top left: control; A, top right: LPS (1 μg/ml) for 10 and 20 min. LBP was added throughout (10 ng/ml). A, bottom: voltage protocols for each clamp; holding potential = −50 mV. B: steady-state outward current-voltage (I/V) plots for repeated measurements for control conditions and for added LPS (1 μg/ml) at 10 and 20 min following addition. Means ± SE (n = 8) ; *P < 0.05; #P < 0.01.
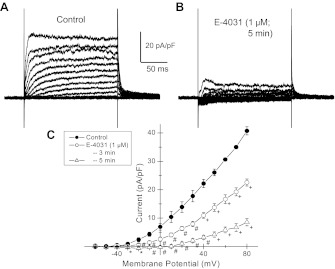
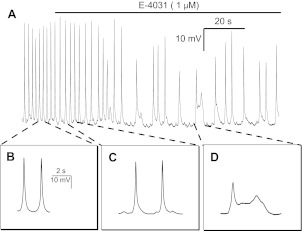
To determine whether inhibition of IKr in HL-1 cells increases action potential duration, we examined the effects of E-4031, a known inhibitor of delayed-rectifier K+ current in HL-1 cells (39). Here, NMDG+ again was substituted for external Na+ and Ca2+ to eliminate inward currents. E-4031 (1 μM) markedly inhibited the outward currents in HL-1 cells recorded over 3–5 min (Fig. 7). In addition, E-4031 treatment extended spontaneous action potential durations at 50% repolarization from 679 ± 29 to 951 ± 38 ms (n = 10; P < 0.001) and at 75% repolarization from 768 ± 32 to 1085 ± 38 ms (n = 10; P < 0.001; Fig. 8, A-C). Moreover, E-4031 decreased action potential amplitude and elicited early afterdepolarizations (Fig. 8D).
Fig. 7.
Whole cell voltage-clamp recordings from HL-1 mouse cardiomyocytes under conditions in which external Na+ and Ca2+ were replaced with isomolar concentrations of N-methyl-d-glucamine to minimize inward currents and to maximize outward K+ current. A: control recordings. B: same cell following E-4031 (1 μM) for 5 min. C: steady-state outward I/V plots for repeated measurements for control conditions and for added E-4031 (1 μM) at 3 and 5 min following addition. Data are means ± SE (n = 4); *P < 0.05; #P < 0.01; +P < 0.001.
Fig. 8.
Spontaneous action potentials recorded by current-clamp mode from HL-1 mouse cardiomyocyte. A: readout of spontaneous action potential recordings, initially under control conditions and then ∼2-min of recording following added E-4031 (1 μM). B: expanded time-base of 2 action potentials under control conditions. C: expanded time-base of two action potentials within 1 min of added E-4031 (1 μM) showing slowing or rate of firing. D: expanded time-base of 2 action potentials in which the second occurred as bi-geminal early afterdepolarization.
The amplitude of the paced action potentials and inward currents diminished variously between 10 and 20 min after added LPS (Figs. 2 and 4, A and B), which most likely was not accounted for by reduced K+ currents and prolonged repolarization. We also noted that added LPS resulted in a positive shift in threshold potential, Fig. 1. Both suggest a diminution of the inward, cationic currents activated at the more negative voltages in HL-1 cells, i.e., INa and ICa,T (43). The magnitude of these inward currents is in keeping with that of INa (43), and we have shown that they are eliminated by substituting NMDG+ for external Na+ (41). We also have reported that LPS does not inhibit HL-1 cell l-type Ca2+ currents within this time course (41). Although it is apparent that voltage control of INa is problematic in HL-1 cells (43) and other atrial myocytes (40), carefully controlled measurements demonstrated that LPS over 20 min reduced the magnitude of the largest and most rapid of the voltage-activated inward currents in HL-1 cells (Fig. 9, A and B), whereas inward current did not decrease during this time course in LBP-control cells (Fig. 9, C and D). These large inward currents were present in only 20% of HL-1 cells recorded in cells 1–2 days beyond passage.
Fig. 9.
I/V plots of rapidly activating/inactivating inward currents in HL-1 cells as shown in Fig. 4. A: I/V plots of inward current as a function of time following added LPS (S. enteritidis; 1 ug/ml). B: I/V plots of inward currents in control (LBP; 10 ng/ml) and after LPS (20 min). Data are means ± SE; n = 6. C: I/V plots of inward current of untreated LBP control as a function of time. D: I/V plots of inward currents in untreated control and at 20 min with LBP (10 ng/ml) added at both times. Data are means ± SE; n = 6. Holding potential = −80 mV in all recordings.
DISCUSSION
We have shown that LPS increases duration of the action potential in HL-1 cells by as much as 250 ms. Considering that murine heart rates range from 400 to 800 beats/min (20), an increase in action potential duration of this magnitude could account for LPS-induced bradycardia (22). Projecting in vitro observations to heart function in situ, these findings account at least in part for reduced heart rate variability (8, 30, 31, 38) and depressed cardiac function that accompanies sepsis (36, 44). This may be particularly relevant to neonatal sepsis where mortality is often preceded by reduced heart rate variability and transient decelerations (10, 11).
Pathogen activation of TLRs initiates a complex response by the innate immune system with subsequent release of proinflammatory cytokines from macrophages. These in turn activate neutrophils to produce various antimicrobial molecules to counter the primary infection (1, 24). Notwithstanding, many nonimmune cell types also contain TLRs, and their coactivation can have deleterious effects on normal organ function. For example, sepsis can depress cardiac function via TLR signaling (23); however, the multiple array of effectors in situ can impede our understanding of the specific mechanism of cardiac damage. Thus in vitro cell culture systems can be useful in elucidating particular effector responses. Here, we have demonstrated that endotoxin directly diminishes excitability of immortalized mouse HL-1 cardiomyocytes in culture. Prolongation and decreased rate of the phase 4 pacemaker potential by LPS are consistent with our previous report that LPS inhibits the “funny current,” If, in HL-1 cells (41) and that it also inhibits HCN channels in native murine sino-atrial node cells and those expressed in HEK293 cells (19). This, as well as an LPS inhibition of INa and positive shift in threshold voltage, explains, in part, the bradycardia in mice following experimental sepsis by cecal ligation and puncture (7). Prolongation of the action potential duration in HL-1 cells by LPS most likely results from its inhibition of the rapid, delayed rectifier K+ current, IKr. This current appears as the predominant K+ current in HL-1 cells (4, 39); however, we cannot rule out involvement of additional K+ currents in HL-1 cells (26). To this end we focused measurements on outward “tail currents,” which are the signature element of IKr and on outward currents carried by Cs+, which is conducted by IKr channels (32, 45). Although not definitive, our findings are consistent with known characteristics of the IKr. Thus, to the best of our knowledge, this is the first demonstration that LPS inhibits the IKr. The ion channel protein that accounts for the murine IKr is the mouse ether-a-go-go (mERG), which is encoded by the Kcnh2 mouse gene. The latter is an orthologue of KCNH2, which is the gene encoding the human ether-a-go-go (hERG) ion channel. HL-1 cells express mouse ERG1 (39), and these authors have shown that knockdown of mERG1 transcripts in HL-1 cells with small interfering RNA reduces IKr, which confirms the linkage (39). Others (21) have reported that selective knockout of mouse ERG1 B K+ channel eliminates IKr in adult ventricular myocytes and elicits episodes of abrupt sinus bradycardia, which is consistent with the effects of LPS on IKr and action potential duration.
The increase in action potential duration secondary to inhibition of IKr by LPS also accounts in part for the decreased in firing rate of spontaneous action potentials in HL-1 cells. Inihibition of IKr by dofetilde increased HL-1 cell action potential duration significantly and decreased their beat rate by 42% (43). These investigators also reported that tetrodotoxin and lidocaine decreased HL-1 cell beat rate, which resulted from an increase in diastolic depolarization rather that increased action potential duration (43). Thus the increased duration of the HL-1 cell pacemaker potential by LPS reported here, as well as the shift in threshold potential to more positive voltage, may well have resulted from its inhibition of INa. Others (13) have reported that pharmacologic LPS concentrations (>50 ng/ml) reduces the availability of human skeletal muscle voltage-gated Na+ channels at depolarized membrane potentials by enhancing channel inactivation.
The effect of LPS to increase action potential duration and decreased frequency of firing occurred slowly over 20 min and correlated with the time course over which LPS reduced IKr tail currents and INa. This protracted inhibition, however, points to a mechanism of LPS action that involves intermediary steps of cell signaling or metabolism. Methanesulfonanilides, such as E-4031, reportedly block open hERG IKr channels in myocardium (35), which explains their rapid block of IKr tail currents in HL-1 cells and suggests an alternate mechanism for inhibition by LPS, which occurs slower. Others (34) have shown that mdivi-1, an inhibitor of mitochondrial fission, also increases action potential duration and inhibits IKr in HL-1 cells, and this occurred within min. Thus we have no further information that might elucidate the cellular mechanisms by which LPS might inhibit IKr other than to conjecture, based on other reports, that phosphatidylinositol 4,5-bisphosphate (PIP2) may be involved, since it is a cofactor for function of many ion channels (37), including HCN (46) and hERG (2, 3). It is of interest that TLR4 downstream signaling involves recruitment of the adaptor protein TIRAP, which in turn is recruited to the plasma membrane via a PIP2 binding domain (18). This suggests that TLR4 activation of the myocardium conscripts PIP2 and reduces its availability for IKr activation; however, we not aware of reports demonstrating a role for PIP2 in the regulation of INa and voltage-regulated Na+ channels (37).
Finally, the magnitude and expression of various ionic currents varied markedly among cells reported herein and by others (29, 42, 43). This may reflect variability in the differentiation of the contractile phenotype among HL-1 cells depending on the age of the culture and the degree of cell confluence. We conducted our experiments on small islands of cells 1–2 days in culture to correspond with our previous measurements of the effects of LPS on Ca2+ oscillations and [Ca2+]i and to obviate space-clamp errors that can occur in cells electrically coupled via syncytial connexins (4). Thus, as often as possible, we compared control and experimental variables measured in the same cell.
In summary, we have demonstrated that LPS increases action potential duration and slows rate of firing in mouse HL-1 cardiomyocytes and that this results secondarily to inhibition of IKr and INa by LPS. This inhibition most likely results from a primary metabolic effect of LPS that reduces available mERG and voltage-gated Na+ channels or extends their inactivation. Taken together, these findings elucidate cellular mechanisms that account for myocardial depression that accompanies sepsis.
GRANTS
This work was supported by the National Institute of General Medical Sciences Grants GM-083016 (to C. Li and D. L. Williams) and GM-53522 (to D. L. Williams).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.W., C.L., and D.L.W. conception and design of research; R.W. and B.M.G. performed experiments; R.W. and B.M.G. analyzed data; R.W., B.M.G., C.L., and D.L.W. interpreted results of experiments; R.W. prepared figures; R.W., B.M.G., C.L., and D.L.W. drafted manuscript; R.W., B.M.G., C.L., and D.L.W. edited and revised manuscript; R.W., B.M.G., C.L., and D.L.W. approved final version of manuscript.
REFERENCES
- 1. Beutler BA. TLRs and innate immunity. Blood 113: 1399–1407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bian JS, Kagan A, McDonald TV. Molecular analysis of PIP2 regulation of HERG and IKr. Am J Physiol Heart Circ Physiol 287: H2154–H2163, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bian JS, McDonald TV. Phosphatidylinositol 4,5-bisphosphate interactions with the HERG K(+) channel. Pflügers Arch 455: 105–113, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fairchild KD, Saucerman JJ, Raynor LL, Sivak JA, Xiao Y, Lake DE, Moorman JR. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol 297: R1019–R1027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol 300: R330–R339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ganopolsky JG, Castellino FJ. A protein C deficiency exacerbates inflammatory and hypotensive responses in mice during polymicrobial sepsis in a cecal ligation and puncture model. Am J Pathol 165: 1433–1446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garrard CS, Kontoyannis DA, Piepoli M. Spectral analysis of heart rate variability in the sepsis syndrome. Clin Auton Res 3: 5–13, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Goldoni D, Zhao Y, Green BD, McDermott BJ, Collins A. Inward rectifier potassium channels in the HL-1 cardiomyocyte-derived cell line. J Cell Physiol 225: 751–756, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics are associated with neonatal mortality. Pediatr Res 55: 782–788, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res 53: 920–926, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Grunnet M. Repolarization of the cardiac action potential. Does an increase in repolarization capacity constitute a new anti-arrhythmic principle? Acta Physiol (Oxf) 198, Suppl 676: 1–48, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Haeseler G, Foadi N, Wiegand E, Ahrens J, Krampfl K, Dengler R, Leuwer M. Endotoxin reduces availability of voltage-gated human skeletal muscle sodium channels at depolarized membrane potentials. Crit Care Med 36: 1239–1247, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Hollenberg SM, Dumasius A, Easington C, Colilla SA, Neumann A, Parrillo JE. Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med 164: 891–895, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Jianhui L, Rosenblatt-Velin N, Loukili N, Pacher P, Feihl F, Waeber B, Liaudet L. Endotoxin impairs cardiac hemodynamics by affecting loading conditions but not by reducing cardiac inotropism. Am J Physiol Heart Circ Physiol 299: H492–H501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joulin O, Marechaux S, Hassoun S, Montaigne D, Lancel S, Neviere R. Cardiac force-frequency relationship and frequency-dependent acceleration of relaxation are impaired in LPS-treated rats. Crit Care 13: R14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125: 943–955, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Klockner U, Rueckschloss U, Grossmann C, Ebelt H, Muller-Werdan U, Loppnow H, Werdan K, Gekle M. Differential reduction of HCN channel activity by various types of lipopolysaccharide. J Mol Cell Cardiol 51: 226–235, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Kramer K, van Acker SA, Voss HP, Grimbergen JA, van der Vijgh WJ, Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods 30: 209–215, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Lees-Miller JP, Guo J, Somers JR, Roach DE, Sheldon RS, Rancourt DE, Duff HJ. Selective knockout of mouse ERG1 B potassium channel eliminates I(Kr) in adult ventricular myocytes and elicits episodes of abrupt sinus bradycardia. Mol Cell Biol 23: 1856–1862, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin SL, Lee YM, Chang HY, Cheng YW, Yen MH. Effects of naltrexone on lipopolysaccharide-induced sepsis in rats. J Biomed Sci 12: 431–440, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res 108: 1133–1145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol 21: R488–493, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem 268: 4580–4589, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Nobles M, Sebastian S, Tinker A. HL-1 cells express an inwardly rectifying K+ current activated via muscarinic receptors comparable to that in mouse atrial myocytes. Pflügers Arch 460: 99–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parrillo JE. The cardiovascular pathophysiology of sepsis. Annu Rev Med 40: 469–485, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Rackow EC, Kaufman BS, Falk JL, Astiz ME, Weil MH. Hemodynamic response to fluid repletion in patients with septic shock: evidence for early depression of cardiac performance. Circ Shock 22: 11–22, 1987 [PubMed] [Google Scholar]
- 29. Sartiani L, Bochet P, Cerbai E, Mugelli A, Fischmeister R. Functional expression of the hyperpolarization-activated, non-selective cation current I(f) in immortalized HL-1 cardiomyocytes. J Physiol 545: 81–92, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt H, Muller-Werdan U, Hoffmann T, Francis DP, Piepoli MF, Rauchhaus M, Prondzinsky R, Loppnow H, Buerke M, Hoyer D, Werdan K. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med 33: 1994–2002, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt H, Saworski J, Werdan K, Muller-Werdan U. Decreased beating rate variability of spontaneously contracting cardiomyocytes after co-incubation with endotoxin. J Endotoxin Res 13: 339–342, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Schonherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol 493: 635–642, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shepard PD, Canavier CC, Levitan ES. Ether-a-go-go-related gene potassium channels: what's all the buzz about? Schizophr Bull 33: 1263–1269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. So EC, Hsing CH, Liang CH, Wu SN. The actions of mdivi-1, an inhibitor of mitochondrial fission, on rapidly activating delayed-rectifier K(+) current and membrane potential in HL-1 murine atrial cardiomyocytes. Eur J Pharmacol 683: 1–9, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Spector PS, Curran ME, Keating MT, Sanguinetti MC. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ Res 78: 499–503, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Stahl W, Bracht H, Radermacher P, Thomas J. Year in review 2009: critical care–shock. Crit Care 14: 239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, Hirasawa H. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock 28: 549–553, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Toyoda F, Ding WG, Zankov DP, Omatsu-Kanbe M, Isono T, Horie M, Matsuura H. Characterization of the rapidly activating delayed rectifier potassium current, I (Kr), in HL-1 mouse atrial myocytes. J Membr Biol 235: 73–87, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Wendt DJ, Starmer CF, Grant AO. Na channel kinetics remain stable during perforated-patch recordings. Am J Physiol Cell Physiol 263: C1234–C1240, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Wondergem R, Graves BM, Ozment-Skelton TR, Li C, Williams DL. Lipopolysaccharides directly decrease Ca2+ oscillations and the hyperpolarization-activated nonselective cation current If in immortalized HL-1 cardiomyocytes. Am J Physiol Cell Physiol 299: C665–C671, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xia M, Salata JJ, Figueroa DJ, Lawlor AM, Liang HA, Liu Y, Connolly TM. Functional expression of L- and T-type Ca2+ channels in murine HL-1 cells. J Mol Cell Cardiol 36: 111–119, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Yang Z, Murray KT. Ionic mechanisms of pacemaker activity in spontaneously contracting atrial HL-1 cells. J Cardiovasc Pharmacol 57: 28–36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care 15: 392–397, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Zhang S. Isolation and characterization of IKr in cardiac myocytes by Cs+ permeation. Am J Physiol Heart Circ Physiol 290: H1038–H1049, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 52: 1027–1036, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Zorn-Pauly K, Pelzmann B, Lang P, Machler H, Schmidt H, Ebelt H, Werdan K, Koidl B, Muller-Werdan U. Endotoxin impairs the human pacemaker current If. Shock 28: 655–661, 2007 [PubMed] [Google Scholar]