Abstract
To define the stoichiometry and molecular identity of the Cl−/HCO3− exchanger in the apical membrane of pancreatic duct cells, changes in luminal pH and volume were measured simultaneously in interlobular pancreatic ducts isolated from wild-type and Slc26a6-null mice. Transepithelial fluxes of HCO3− and Cl− were measured in the presence of anion gradients favoring rapid exchange of intracellular HCO3− with luminal Cl− in cAMP-stimulated ducts. The flux ratio of Cl− absorption/HCO3− secretion was ∼0.7 in wild-type ducts and ∼1.4 in Slc26a6−/− ducts where a different Cl−/HCO3− exchanger, most likely SLC26A3, was found to be active. Interactions between Cl−/HCO3− exchange and cystic fibrosis transmembrane conductance regulator (CFTR) in cAMP-stimulated ducts were examined by measuring the recovery of intracellular pH after alkali-loading by acetate prepulse. Hyperpolarization induced by luminal application of CFTRinh-172 enhanced HCO3− efflux across the apical membrane via SLC26A6 in wild-type ducts but significantly reduced HCO3− efflux in Slc26a6−/− ducts. In microperfused wild-type ducts, removal of luminal Cl−, or luminal application of dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid to inhibit SLC26A6, caused membrane hyperpolarization, which was abolished in Slc26a6−/− ducts. In conclusion, we have demonstrated that deletion of Slc26a6 alters the apparent stoichiometry of apical Cl−/HCO3− exchange in native pancreatic duct. Our results are consistent with SLC26A6 mediating 1:2 Cl−/HCO3− exchange, and the exchanger upregulated in its absence, most probably SLC26A3, mediating 2:1 exchange.
Keywords: isolated pancreatic duct, micropuncture, microperfusion, stoichiometry
the pancreatic duct system of humans produces ∼2.5 liters of isotonic fluid per day, containing ∼140 mM HCO3−. This process is dependent on the activity of the cystic fibrosis transmembrane conductance regulator (CFTR) and can be seriously disrupted in cystic fibrosis. The HCO3−-rich ductal secretion acts as a vehicle for acinus-derived digestive enzymes and as a buffer for duodenal acidity. Our previous studies (2, 11, 23) suggest that HCO3− secretion across the apical membrane is mediated by Cl−/HCO3− exchange and also occurs via the HCO3− conductance of CFTR. However, the relative contributions of these two mechanisms depend upon the anion composition of the luminal fluid. Cl−/HCO3− exchangers are thought to provide a major route for HCO3− secretion in the proximal part of the ductal tree, close to the acini, where luminal Cl− concentration ([Cl−]L) is high. The luminal HCO3− concentration ([HCO3−]L) then increases, due to HCO3− secretion and Cl− absorption, with increasing distance along the duct. Once [HCO3−]L reaches 130–140 mM, theory predicts that apical Cl−/HCO3− exchange will approach equilibrium and may even reverse (22, 23). Under these conditions CFTR, although primarily a Cl− channel, is believed to provide the major pathway for HCO3− secretion (11).
It is now generally accepted that in epithelial tissues, including pancreatic duct, apical Cl−/HCO3− exchange is mediated by members of the SLC26 family of anion transporters (19). Although SLC26A3, SLC26A6, and SLC26A11 have all been identified in pancreatic duct cells (7, 24), there is evidence to suggest that SLC26A6 normally mediates the majority of the Cl−/HCO3− exchange occurring at the apical membrane. This is because 1) the apical Cl−/HCO3− exchanger, like SLC26A6 but not SLC26A3, is inhibited by dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (H2DIDS; 200 μM; Refs. 4, 10, 25); 2) SLC26A6 and CFTR are colocalized at the apical membrane of rat intralobular ducts (15); and 3) HCO3− efflux upon removal of luminal Cl− is significantly reduced in ducts isolated from Slc26a6-null mice (8).
It has been suggested that the stoichiometry of SLC26A6 may be an important factor in achieving high secreted HCO3− concentrations (23). Mouse SLC26A6 is reported to be electrogenic and to mediate 1:2 Cl−/HCO3− exchange when expressed in heterologous expression systems, while SLC26A3 mediates 2:1 Cl−/HCO3− exchange (14, 21). Furthermore, recent measurements of membrane potential in guinea-pig pancreatic duct cells are consistent with a 1:2 stoichiometry (25) for the apical Cl−/HCO3− exchanger: removal of luminal Cl− or application of H2DIDS to the luminal membrane both lead to membrane hyperpolarization. However, it should be noted that mouse SLC26A6 has been reported to be electroneutral in some heterologous expression system experiments (3). In theory, a 1:2 exchanger would be able to achieve a slightly higher [HCO3−]L than a 1:1 or 2:1 exchanger when the membrane potential is around −60 mV, as in guinea-pig duct cells (23). In addition, a 1:2 stoichiometry would help to prevent HCO3− reabsorption in the more distal ducts of species that secrete high HCO3− concentrations.
To examine the stoichiometry of mouse SLC26A6 in native pancreatic duct cells, we have examined the net transepithelial movements of HCO3− and Cl− that accompany apical Cl−/HCO3− exchange and fluid secretion in interlobular pancreatic ducts isolated from Slc26a6-null mice. We have also studied the interactions between Cl−/HCO3− exchange and CFTR by examining HCO3− efflux across the apical membrane after alkali-load in Slc26a6-null mice and ΔF cystic fibrosis mice.
METHODS
The following study was approved by the Ethical Committee on Animal Use for Experiment and the Recombinant DNA Experiment Safety Committee of Nagoya University.
Animals.
Slc26a6-null mice (29), F508del mice (a cystic fibrosis model in which the ΔF508 mutation was introduced in mouse CFTR by gene targeting in embroynic stem cells, ΔF mice; Ref. 31), and their wild-type littermates were used for the experiments. The mice were maintained on a standard diet, and genotyping was carried out on day 14 postpartum.
Isolation of interlobular pancreatic ducts.
Animals were killed by cervical dislocation. As described previously (10), the pancreas was removed and interlobular ducts (∼100 μm in diameter) were isolated and cultured overnight.
Measurement of luminal pH and fluid secretory rate in isolated pancreatic ducts.
During culture, the ends of the ducts sealed spontaneously, thus isolating the luminal space from the bathing medium. Changes in luminal pH (pHL) and luminal volume were measured simultaneously at 37°C as described previously (10, 13). Briefly, the duct lumen was micropunctured and the luminal fluid was replaced with a solution containing the dextran conjugate of 2′7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-dextran; 20 μM). pHL was calculated from the fluorescence ratio at 530 nm (F480/F430). Images of the luminal fluorescence were obtained at 1-min intervals using a charged-coupled device camera. The luminal volume (VL) was calculated from the area of fluorescence in each image assuming cylindrical geometry. The rate of fluid secretion was then calculated from the increment in the luminal volume and expressed as secretory rate per unit area of epithelium (nl·min−1·mm−2).
[HCO3−]L was estimated from pHL assuming that the CO2 solubility was 0.03 mM mmHg−1 and the pK′a of the HCO3−-CO2 buffering system was 6.1 (10). The transepithelial flux of HCO3− (JHCO3−) was calculated for each 1-min time interval (Δt) from the change in the luminal HCO3− content using:
where A is the luminal surface area of the epithelium. To calculate the Cl− flux, the [Cl−]L was estimated at each time point by assuming that [HCO3−]L + [Cl−]L = 150 mM. This relies on the fact that salt-water coupling in the pancreatic duct achieves near isotonicity. The transepithelial flux of Cl− was then calculated in the same way, using:
Measurement of intracellular pH and base flux in microperfused pancreatic ducts.
The lumen of each interlobular duct segment was microperfused as described previously (11, 13). Both ends of the duct were cut open using sharpened needles, and one end was cannulated with concentric holding and perfusion pipettes. The bath and lumen were perfused separately and the bath was maintained at 37°C.
Intracellular pH (pHi) in the duct cells was estimated by microfluorometry (9). After the duct for luminal perfusion was cannulated, the duct cells were loaded with BCECF for 10 min by addition of the acetoxymethyl ester BCECF-AM (2 μM) to the bathing solution. Small regions of the duct epithelium (10–20 cells) were selected, and pHi was calculated from the fluorescence ratio at 530 nm (F480/F430).
In the acetate prepulse experiments, base efflux was calculated from the rate of change of pHi and taking into account the pH dependence of the intracellular buffering capacity (26). Cell height was assumed to be 10 μm.
Measurement of intracellular potential in microperfused pancreatic ducts.
The membrane potential was measured by impaling the basolateral membrane of the microperfused ducts with glass microelectrodes (12). Pancreatic duct epithelium is a low-resistance epithelium (11, 17), and thus the basolateral membrane potential is very similar in magnitude to the apical membrane potential.
Solutions.
The standard HEPES-buffered solution contained the following (in mM): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES and was equilibrated with 100% O2. The standard HCO3−-buffered solution contained the following (in mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 d-glucose, and 25 NaHCO3− and was equilibrated with 95% O2-5% CO2. The solution containing acetate (80 mM) was made by equimolar substitution for Cl−. The Cl−-free, HCO3−-buffered solution was made by replacing Cl− with glucuronate. The high-HCO3− solution contained 125 mM NaHCO3 and 24 mM NaCl and was equilibrated with 95% O2-5% CO2 (pH ∼8.2). All solutions, apart from the high-HCO3− solution, were adjusted to pH 7.4 at 37°C.
Materials.
BCECF-AM, BCECF-dextran, and H2DIDS were obtained from Invitrogen (Carlsbad, CA). CFTRinh-172, forskolin, and other standard laboratory chemicals were from Sigma (St. Louis, MO).
Statistics.
Data are presented as the means ± SE. Tests for statistical differences were made with Student's t-test and significance was taken as P < 0.05.
RESULTS
Effects of Slc26a6 deletion on Cl−-dependent HCO3− secretion.
To examine the stoichiometry of apical Cl−/HCO3− exchange, changes in pHL and luminal volume were measured simultaneously in sealed isolated ducts. From these changes, net fluxes of Cl− and HCO3− were calculated at 1-min intervals. The duct lumen was filled with a Cl−-rich (149 mM), HCO3−-free, HEPES-buffered solution, and the bath was initially perfused with the same solution. Duct cells were stimulated with forskolin (1 μM), and, after a 5-min control period, the bath solution was switched to a HCO3−-buffered solution containing 25 mM HCO3− and 124 mM Cl−. At this point, the HCO3− in the bath solution was taken up rapidly into the duct cells via the basolateral Na+-HCO3− cotransporter (9), thus quickly establishing anion gradients favoring exchange of intracellular HCO3− with luminal Cl− at the apical membrane. This protocol allows us to examine HCO3−, Cl−, and fluid movements across the apical membrane that result mainly from apical Cl−/HCO3− exchange.
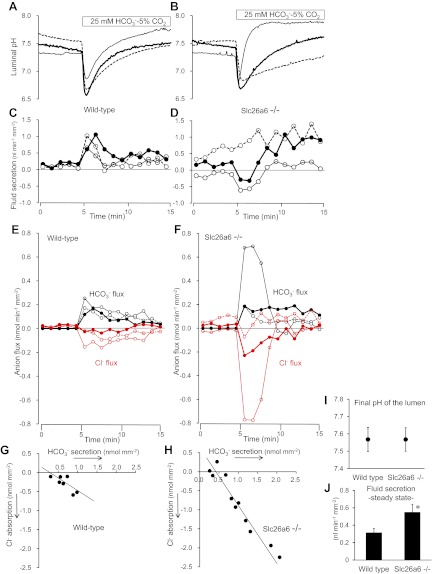
Figure 1, A and C, shows individual time courses of pHL and fluid secretion in three representative ducts from wild-type mice. Switching the bath solution to the HCO3−-buffered solution caused a transient luminal acidification (due to CO2 diffusion into the lumen) followed by a progressive increase in pHL due to HCO3− secretion (10). The changes in pHL were accompanied by a transient increase in fluid secretion followed by a smaller sustained secretion. Figure 1E shows time courses of the corresponding net fluxes of Cl− and HCO3−, which were calculated from the changes in pHL and luminal volume as described in methods.
Fig. 1.
Cl−-dependent HCO3− and fluid secretion in interlobular pancreatic ducts isolated from wild-type and Slc26a6−/− mice. Sealed, isolated ducts from wild-type and Slc26a6−/− mice were filled with Cl−-rich (149 mM) HCO3−-free HEPES-buffered solution containing 2′7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)-dextran (20 μM). The bath was first perfused with the same HCO3−-free solution, and then the bath solution was switched to the standard HCO3−-buffered solution containing 25 mM HCO3− and 124 mM Cl− as indicated. Cells were stimulated with forskolin (1 μM) throughout the experiments. Luminal pH and luminal volume were measured simultaneously. A and B: changes in luminal pH in 3 representative ducts isolated from wild-type (A) and Slc26a6−/− (B) mice respectively. C and D: changes in fluid secretory rate in the same wild-type (C) and Slc26a6−/− (D) ducts. Negative values indicate fluid absorption. E and F: changes in calculated fluxes of Cl− (red) and HCO3− (black) in the same wild-type (E) and Slc26a6−/− (F) ducts. Positive and negative values indicate secretory and absorptive fluxes, respectively. G and H: plot of cumulative fluxes of Cl− and HCO3− during the first 5 min after bath application of HCO3− in wild-type (G, n = 7) and Slc26a6−/− ducts (H, n = 11). I and J: final values of luminal pH (I) and steady-state fluid secretory rate (J) in wild-type and Slc26a6−/− ducts. Data are means ± SE. *P < 0.05, significant difference.
The cumulative fluxes of Cl− and HCO3− over the first 5 min following the application of HCO3− are plotted against each other in Fig. 1G for each of seven ducts. Although these fluxes, which we attribute mainly to apical Cl−/HCO3− exchange, varied between ducts, it is possible to see a correlation between the secretory HCO3− flux and the absorptive Cl− flux. The slope of the fitted line is −0.68 ± 0.25 (r2 = 0.603; n = 7). If these fluxes were due solely to 1:2 Cl−/HCO3− exchange via SLC26A6, the expected slope would be −0.5.
Figure 1, B, D, and F, shows data from three representative ducts isolated from Slc26a6−/− mice. The changes in pHL, fluid movement, and anion fluxes following application of HCO3− varied even more widely between ducts from different animals. The experiment represented by the thin solid line, for example, showed a steep increase in pHL (Fig. 1B), which was accompanied by a transient absorption of luminal fluid (Fig. 1D) and large HCO3− and Cl− fluxes during the first 3–4 min (Fig. 1F). These data can only be explained by a marked upregulation of some other pathway for apical Cl−/HCO3− exchange (not via SLC26A6) and with a stoichiometry that initially resulted in net anion absorption, hence fluid absorption.
The experiment represented by the dotted line, in contrast, shows a slower increase in pHL (Fig. 1B) indicating a lower level of apical Cl−/HCO3− exchange. The introduction of HCO3− caused a modest increase in secretory rate from an already raised basal level (Fig. 1D). There was also a small, transient drop in Cl− secretion (Fig. 1F). These observations are more likely due to upregulation of HCO3−-independent Cl− secretion (probably via CFTR) rather than apical Cl−/HCO3− exchange. The experiment represented by the thick solid line shows an intermediate pattern of changes, but once again there was a small, transient absorption of luminal fluid, driven by an absorptive Cl− flux that exceeded the secretory HCO3− flux during the first few minutes after the introduction of HCO3− (Fig. 1, D and F).
Six of the 11 Slc26a6−/− ducts absorbed luminal fluid for the first 2–3 min after introduction of HCO3− to the bath (two are shown in Fig. 1D). This contrasts with the wild-type ducts where there was normally an increased secretion of fluid during this period. Because fluid transport is near isosmotic, the direction of fluid flow is determined by the direction of net anion transport. In these conditions, the Cl− and HCO3− fluxes will be mediated mainly by anion exchange. Net secretion, as observed in the wild-type ducts would be consistent with a 1:2 Cl−/HCO3− exchange stoichiometry, as proposed for SLC26A6. Net absorption, as observed in the Slc26a6−/− ducts, would be consistent with a 2:1 stoichiometry, as proposed for SLC26A3.
Cumulative fluxes of Cl− and HCO3− during the first 5 min following application of HCO3− are plotted in Fig. 1H for the 11 individual Slc26a6−/− ducts. The slope of the fitted line is −1.38 ± 0.12 (r2 = 0.937), which is significantly different (P < 0.05) from the slope (−0.68) in the wild-type ducts (Fig. 1G). If these fluxes were due solely to 2:1 exchange via SLC26A3, the expected slope would be −2.
The wide range of Cl− and HCO3− fluxes that we observed in these experiments suggests that the activity of the alternative Cl−/HCO3− exchanger was quite variable among the Slc26a6−/− ducts. Also, the fact that the fitted line in Fig. 1H was shifted to the right may indicate the involvement of a Cl−-independent HCO3−-secretory pathway, possibly via CFTR. Nonetheless the difference in slope between Fig. 1, G and H, strongly suggests that the Cl−/HCO3− exchanger that replaces SLC26A6 in the Slc26a6−/− ducts has a different stoichiometry.
Figures 1I and 1J show the final values of pHL and the steady-state fluid secretory rates at the end of these experiments (mean values from the last 3 min). pHL reached ∼7.57 (thus [HCO3−]L was ∼37 mM) in both wild-type and Slc26a6−/− ducts while steady-state fluid secretion was significantly greater in the Slc26a6−/− ducts (P < 0.05). At this final stage in the experiments, the Cl− and HCO3− gradients favoring Cl−/HCO3− exchange are reduced (Fig. 1, E and F) and it is likely that CFTR now contributes more to HCO3− secretion. Thus the higher rate of steady-state fluid secretion in the Slc26a6−/− ducts can probably be explained by the previously reported upregulation of CFTR (28).
Effects of Slc26a6 deletion and CFTR inhibition on HCO3− efflux in ducts perfused with high Cl− solutions.
Another way to measure apical Cl−/HCO3− exchange is to examine the recovery of pHi following intracellular alkalinization. Using luminally perfused ducts for these experiments, we were able to control more precisely the conditions, for example, by changing the [HCO3−]L or [Cl−]L or by applying the CFTR channel blocker CFTRinh-172 to the luminal membrane.
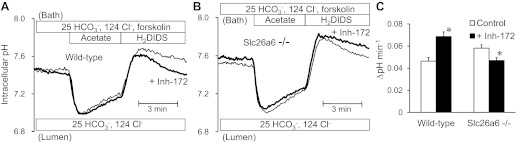
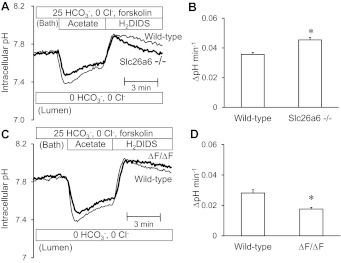
Intracellular pH was measured in duct cells that were alkali-loaded with a 4-min prepulse of 80 mM acetate (25). Ducts were superfused with the standard HCO3−-buffered solution and stimulated with 1 μM forskolin. Experiments were performed with 500 μM H2DIDS present in the bath solution to inhibit basolateral HCO3− fluxes via the AE2 Cl−/HCO3− exchanger and NBC1 Na+-HCO3− cotransporter (25). Addition of acetate to the bath led to intracellular acidification, and its subsequent removal caused intracellular alkalinization to a higher than normal pHi value (Fig. 2). HCO3− efflux across the apical membrane was quantified as the rate of recovery of pHi from the alkali load.
Fig. 2.
Effects of cystic fibrosis transmembrane conductance regulator (CFTR) inhibition on apical HCO3− efflux in wild-type and Slc26a6−/− ducts luminally perfused with high Cl− solution. Bath and lumen were perfused with the standard HCO3−-buffered solution containing 25 mM HCO3− and 124 mM Cl−. Cells were stimulated with forskolin and alkali-loaded with an acetate prepulse (80 mM). HCO3− efflux across the apical membrane was estimated from the recovery rate of intracellular pH (pHi) with dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (H2DIDS; 500 μM) present in the bath to inhibit basolateral HCO3− fluxes. A: changes in pHi in the presence (thick line) and absence (thin line) of luminal CFTRinh-172 (10 μM) in wild-type ducts. Representative traces from 9 and 7 experiments respectively. B: changes in pHi in the presence (thick line) and absence (thin line) of luminal CFTRinh-172 in Slc26a6−/− ducts. Representative traces from 6 and 8 experiments, respectively. C: recovery rate of pHi in the presence (solid bar) and absence (open bar) of luminal CFTRinh-172 in wild-type and Slc26a6−/− ducts. Data are means ± SE of 6–9 experiments. *P < 0.05, compared with control (absence of CFTRinh-172).
When the lumen of wild-type ducts was microperfused with the standard HCO3−-buffered solution containing 25 mM HCO3− and 124 mM Cl−, the mean rate of recovery from the alkali load was 0.046 ± 0.003 pH U/min (n = 9, Fig. 2, A and C). The corresponding base efflux was calculated to be 0.040 ± 0.003 nmol·min−1·mm−2 which is comparable with the HCO3− fluxes shown in Fig. 1E. As in those experiments, the luminal fluid in the acetate prepulse experiments was Cl−-rich and the efflux of HCO3− across the apical membrane was probably mediated largely by Cl−/HCO3− exchange.
Interestingly, luminal application of CFTRinh-172 (10 μM) to block CFTR significantly accelerated the efflux of HCO3− (Fig. 2, A and C). This finding is consistent with our previous data from guinea-pig pancreatic ducts (25). It suggests first that HCO3− efflux via CFTR is not a major contributor to the recovery from alkalinization. Second, since CFTR inhibition leads to hyperpolarization of the apical membrane, the observed acceleration of Cl−/HCO3− exchange is consistent with SLC26A6 having a 1:2 stoichiometry.
In Slc26a6−/− ducts, the recovery of pHi from alkali load was slightly faster than in wild-type ducts (P < 0.05; n = 6; Fig. 2, B and C). Also, in contrast to wild-type ducts, application of luminal CFTRinh-172 significantly reduced the recovery rate in Slc26a6−/− ducts. This could indicate that CFTR, which is upregulated in the Slc26a6−/− ducts, mediates some of the compensatory HCO3− efflux that follows alkalinisation. Alternatively, or in addition, the hyperpolarization caused by CFTRinh-172 may slow the Cl−/HCO3− exchanger that replaces SLC26A6 in the knockout animals. This is exactly what would be expected if that exchanger has a 2:1 Cl−/HCO3− stoichiometry as proposed for SLC26A3.
Apical HCO3− efflux in ducts perfused with high HCO3− solutions.
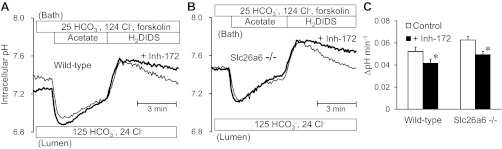
We repeated these experiments in ducts luminally perfused with a high-HCO3− solution containing 125 mM HCO3− and 24 mM Cl−. In this condition, HCO3− efflux across the apical membrane is assumed to be mediated largely by the HCO3− conductance of CFTR rather than by Cl−/HCO3− exchange. This is because the Cl− and HCO3− gradients across the apical membrane are close to equilibrium values for Cl−/HCO3− exchangers (23).
As expected, luminal application of CFTRinh-172 significantly inhibited HCO3− efflux in both the wild-type and Slc26a6−/− ducts (Fig. 3). In the absence of the inhibitor, the rate of pHi decrease in Slc26a6−/− ducts was significantly greater than in wild-type ducts (P < 0.05), which would be consistent with the increased activity of CFTR in Slc26a6−/− ducts as previously reported (28).
Fig. 3.
Effects of CFTR inhibition on apical HCO3− efflux in wild-type and Slc26a6−/− ducts luminally perfused with high HCO3− solution. The lumen was perfused with the high-HCO3− solution containing 125 mM HCO3− and 24 mM Cl−. Cells were stimulated with forskolin and alkali-loaded with an acetate prepulse. A: changes in pHi in the presence (thick line) and absence (thin line) of luminal CFTRinh-172 in wild-type ducts. Representative traces from 8 experiments. B: changes in pHi in the presence (thick line) and absence (thin line) of luminal CFTRinh-172 in Slc26a6−/− ducts. Representative traces from 7 and 9 experiments, respectively. C: The recovery rate of pHi in the presence (solid bar) and absence (open bar) of luminal CFTRinh-172 in wild-type and Slc26a6−/− ducts.
Apical HCO3− efflux in ΔF/ΔF cystic fibrosis ducts.
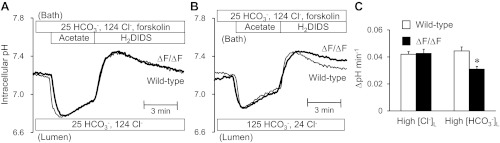
To examine the role of CFTR in Cl−-dependent and Cl−-independent HCO3− efflux across the apical membrane, changes in pHi were examined using the same protocol in cAMP-stimulated ducts isolated from ΔF/ΔF mice. When the lumen was perfused with the high-Cl− solution containing 25 mM HCO3− and 124 mM Cl− (Fig. 4A), there was no significant difference in the rate of pHi decrease after alkali load between wild-type and ΔF/ΔF ducts (Fig. 4C). Assuming that pHi recovery here is entirely due to Cl−/HCO3− exchange via SLC26A6, this result suggests that the absence of functional CFTR has no effect on the activity of SLC26A6, contrary to previous reports (16).
Fig. 4.
Apical HCO3− efflux in wild-type and ΔF ducts luminally perfused with high Cl− or high HCO3− solution. The lumen was perfused with the high-Cl− (25 mM HCO3− and 124 mM Cl−; A) or high-HCO3− (125 mM HCO3− and 24 mM Cl−; B) solution. Cells were stimulated with forskolin and alkali-loaded with an acetate prepulse. A and B: changes in pHi in wild-type (thin line) and ΔF/ΔF ducts (thick line) with high [Cl−]L (A) or high [HCO3−]L (B). Representative traces from 6–10 experiments. C: recovery rate of pHi in wild-type (open bar) and ΔF/ΔF ducts (solid bar) with high luminal Cl− concentration ([Cl−]L) or high luminal HCO3− concentration ([HCO3−]L). Data are means ± SE. *P < 0.05, compared with wild-type ducts.
In contrast, when the lumen was perfused with the high-HCO3− solution containing 125 mM HCO3− and 24 mM Cl− (Fig. 4B), the rate of pHi decrease in the ΔF/ΔF ducts was significantly slower than in the wild-type ducts (P < 0.05; Fig. 4C). This is consistent with our assumption that CFTR, when present, makes a significant contribution to the compensatory HCO3− efflux when the [HCO3−]L is high and SLC26A6 is close to equilibrium.
Apical HCO3− efflux in the absence of Cl−.
To establish whether CFTR activity is upregulated in Slc26a6 null pancreatic ducts, duct cells were next alkali loaded in the absence of Cl− to examine Cl−-independent HCO3− efflux across the apical membrane (Fig. 5). Ducts were superfused with a Cl−-free, HCO3−-buffered solution and stimulated with forskolin. The lumen was microperfused with a Cl−-free and HCO3−-free solution. In these conditions, we expect CFTR to provide the main pathway for HCO3− efflux across the apical membrane.
Fig. 5.
Cl−-independent apical HCO3− efflux in Slc26a6−/− and ΔF ducts. Ducts were superfused with the Cl−-free HCO3−-buffered solution containing forskolin. Lumen was microperfused with the Cl−-free, HCO3−-free solution. Cells were alkali loaded with an acetate prepulse. A: changes in pHi in wild-type (thin line) and Slc26a6−/− ducts (thick line). Representative traces from 7 and 8 experiments respectively. B: data are means ± SE of the recovery rate of pHi. *P < 0.05, significant difference compared with wild-type ducts. C: changes in pHi in wild-type (thin line) and ΔF/ΔF ducts (thick line). Representative traces from 7 experiments. D: data are means ± SE of the recovery rate of pHi. *P < 0.05, compared with wild-type ducts.
Owing to the absence of HCO3− efflux via Cl−/HCO3− exchange, the basal level of pHi was already relatively high in both wild-type and Slc26a6−/− ducts (7.7∼7.8; Fig. 5, A and B). After alkali loading, the rate of pHi decrease in the Slc26a6−/− ducts was significantly greater than in the wild-type ducts (P < 0.05; Fig. 5, A and B). This is consistent with the upregulation of CFTR in Slc26a6−/− ducts reported by others (28) and suggested in our own experiments as described above.
In ΔF/ΔF ducts, the rate of pHi decrease following alkali loading in the absence of Cl− was significantly slower than in wild-type ducts (P < 0.05; Fig. 5, C and D). This again supports the idea that CFTR provides a significant pathway for HCO3− efflux across the apical membrane in the absence of apical Cl−/HCO3− exchange.
Effects of luminal H2DIDS and Cl− removal on membrane potential.
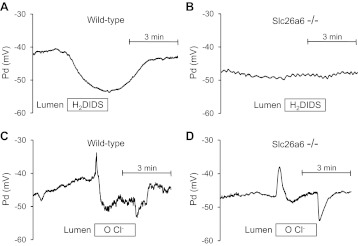
To explore further the electrogenicity of SLC26A6-mediated Cl−/HCO3− exchange, we next examined the effects of luminal H2DIDS and Cl− removal on membrane potential recorded with conventional glass microelectrodes in Slc26a6−/− and wild-type ducts. The bath was perfused with the standard HCO3−-buffered solution containing forskolin. The lumen was microperfused with the Cl−-rich (25 mM HCO3−-124 mM Cl−) solution so that apical Cl−/HCO3− exchange was active.
Luminal application of H2DIDS (200 μM), which inhibits SLC26A6 activity (25), resulted in a reversible hyperpolarization in wild-type ducts (10.5 ± 1.7 mV; n = 4) but had no effect in Slc26a6−/− ducts (Fig. 6, A and B). If, as we believe, SLC26A6 has a 1:2 Cl−/HCO3− exchange stoichiometry, its activity will have a depolarizing effect on membrane potential in the wild-type ducts. Thus inhibition by H2DIDS would be expected to cause hyperpolarization, exactly as was observed. The absence of any effect of H2DIDS on membrane potential in the Slc26a6−/− ducts suggests either that the exchanger that replaces SLC26A6 is H2DIDS insensitive, which would be true for SLC26A3 (4), or that it is electroneutral.
Fig. 6.
Effects of luminal H2DIDS and Cl− removal on membrane potential in wild-type and Slc26a6−/− ducts luminally-perfused with high Cl− solution. Bath and lumen were perfused with the standard HCO3−-buffered solution containing 25 mM HCO3− and 124 mM Cl−. Cells were stimulated with forskolin. Membrane potential (Pd) was measured by conventional microelectrodes. A and B: H2DIDS (200 μM) was applied to the lumen as indicated in wild-type (A) and Slc26a6−/− (B) ducts. Representative of 4 experiments. C and D: Cl− in the luminal solution was replaced with glucuronate as indicated in wild-type (C) and Slc26a6−/− (D) ducts. Representative of 4 experiments.
In wild-type ducts, removal of luminal Cl− resulted in a transient depolarization (4.6 ± 1.2 mV; n = 4) followed by a more sustained hyperpolarization of 9.8 ± 1.9 mV (Fig. 6C), which is similar to what has been observed in guinea-pig pancreatic duct (25). The rapid, transient depolarization is due to Cl− efflux via CFTR, and the slower hyperpolarization that follows is likely due to reversal of an electrogenic Cl−/HCO3− exchanger, presumably SLC26A6.
In the Slc26a6−/− ducts, luminal Cl− removal resulted in a transient depolarization (6.3 ± 1.8 mV; n = 4) that was slightly more prolonged than in the wild-type ducts, but the subsequent hyperpolarization was completely abolished (Fig. 6D). This result is easily explained by the absence of SLC26A6 if it has a 1:2 stoichiometry and therefore normally hyperpolarizes the cell when reversed. If the Cl−/HCO3− exchanger that replaces SLC26A6 in the Slc26a6−/− ducts is SLC26A3, and if this has a 2:1 stoichiometry, it may have contributed to the depolarization that followed the substitution of luminal Cl−. Its contribution would have been transient, however, as intracellular Cl− would be quickly depleted by Cl− efflux through the upregulated CFTR conductance.
DISCUSSION
Stoichiometry of SLC26 exchangers.
Mouse SLC26A6 and SLC26A3 have both been reported to be electrogenic and to mediate 1:2 and 2:1 Cl−/HCO3− exchange, respectively, when expressed in heterologous systems (14, 21). Others (3), however, have found that Cl−/HCO3− exchange by mouse and human SLC26A6 is insensitive to voltage. More recently, SLC26A6 in mouse duodenum has been shown to be sensitive to voltage but only in the presence of CFTR (27). Given that mouse and human SLC26A6 share only 78% amino acid identity, these functional differences are perhaps not surprising (5). The stoichiometry of SLC26A3 is also not yet firmly established. For example, a recent study (1) of mouse cecum failed to support the proposed 2:1 stoichiometry and the E367A mutation of SLC26A3 was found to change its stoichiometry from 2:1 to 1:1 (18). It therefore seems that the stoichiometry of SLC26 Cl−/HCO3− exchangers may vary among species and between tissues and may also change with altered experimental conditions.
We (8) have previously examined apical Cl−/HCO3− exchange in Slc26a6−/− ducts by measuring changes in pHi upon removal or addition of luminal Cl−. Measured in this way, deletion of Slc26a6 had opposite effects on apical Cl−/HCO3− exchange: Cl−o/HCO3−i exchange was reduced as expected, while Cl−i/HCO3−o exchange was unexpectedly enhanced (where o is extracellular and i is intracellular). This result was consistent with SLC26A6 having an electrogenic 1:2 stoichiometry, and the exchanger that was upregulated in its absence having the reverse, 2:1 stoichiometry. Significantly, in our hands, the enhancement of Cl−i/HCO3−o exchange was associated with an increase in mRNA expression of SLC26A3.
Other support for the 1:2 stoichiometry of SLC26A6 in pancreatic duct epithelium includes a recent study showing that isolated ducts from Cftr null mice still secrete fluid in response to cAMP stimulation (20). In this situation, only a 1:2 stoichiometry could support net anion and fluid secretion in the absence of CFTR. In guinea-pig ducts, measurements of membrane hyperpolarization and fluid secretion associated with apical Cl−/HCO3− exchange also support the 1:2 stoichiometry (25). As in the present study, removal of luminal Cl− (favoring Cl−i/HCO3−o exchange) led to an H2DIDS-sensitive hyperpolarization. Also, when CFTR was blocked with luminal CFTRinh-172, net secretion of HCO3−-rich fluid persisted, which was likely mediated largely by H2DIDS-sensitive 1:2 Cl−/HCO3− exchange. However, it should be noted that SLC26A11, which is now known to be present in guinea-pig pancreatic ducts, is also inhibited by DIDS (24). SLC26A11 appears to be an electroneutral Cl−/HCO3− exchanger, but it also exhibits a Cl− conductance in mouse kidney intercalated cells (30). Its contribution to HCO3− secretion in the pancreatic duct certainly demands further investigation.
Cl−/HCO3− exchange fluxes in mouse ducts.
Simultaneous measurement of luminal pH and volume in sealed, isolated ducts enables us to estimate net transepithelial fluxes of HCO3− and Cl− (10). In the present study, we adopted this method to estimate the stoichiometry of apical Cl−/HCO3− exchange under physiological conditions where cAMP-stimulated mouse duct cells were actively secreting HCO3−. The transient changes in fluid secretion/absorption that accompanied the rapid exchange of intracellular HCO3− and luminal Cl−, following addition of HCO3− to the bath, were clearly different between the wild-type and Slc26a6−/− ducts (Fig. 1). In wild-type ducts, we observed net fluid secretion, which gradually declined over a 10-min period (Fig. 1C), likely due to the gradual decrease in the anion gradients for Cl−o/HCO3−i exchange across the apical membrane. Calculation of the Cl− and HCO3− fluxes revealed Cl− absorption and HCO3− secretion with a flux ratio of ∼0.7 (Fig. 1G). In contrast, in Slc26a6−/− ducts, we observed net fluid absorption in 6 of the 11 ducts examined (Fig. 1D). Calculation of the accompanying Cl− and HCO3− fluxes (Fig. 1F) revealed a good correlation of Cl− influx and HCO3− efflux with a Cl− absorption-to-HCO3− secretion ratio of ∼1.4 (Fig. 1H).
Most of the observed anion fluxes in these experiments were probably mediated by Cl−/HCO3− exchangers. Cl− secretion via CFTR was negligible in the wild-type ducts and only detectable in some of the Slc26a6−/− ducts (in 7 of the 11 ducts examined). It may have increased slightly, when HCO3− was introduced, as a result of increased Cl− uptake at the basolateral membrane via the basolateral Cl−/HCO3− exchanger AE2. However, one thing is certain: the net absorption of Cl− that we observed following the introduction of HCO3− cannot be explained by the reversal of the Cl− flux through CFTR. This would require both a large depolarization and a fall in the intracellular Cl− concentration to reverse the electrochemical gradient. More likely, a small increase in Cl− secretion via CFTR will have caused us to underestimate Cl− absorption via Cl−/HCO3− exchange.
There may also have been some HCO3− secretion via CFTR when HCO3− was introduced to the bath. It is unlikely to have been very much in the wild-type ducts because CFTRinh-172 enhanced rather than reduced the HCO3− efflux following alkalinization in the acetate experiments (Fig. 2, A and C). CFTR probably contributed significantly more to HCO3− secretion in the Slc26a6−/− ducts in which CFTR activity appeared to be upregulated as previously reported (28).
Large duct-to-duct variations in the anion fluxes were observed in both wild-type and Slc26a6−/− ducts, particularly the latter. In mouse pancreatic duct, we believe that the principal apical Cl−/HCO3− exchangers are SLC26A6 and SLC26A3 (14) and we (8) have previously presented evidence that SLC26A3 is upregulated in Slc26a6−/− pancreas. This could account for the large Cl− absorption/HCO3− secretion fluxes in Fig. 1H and may also have contributed to the increased pHi recovery rate in Fig. 2. We can only suppose that the particularly wide variability among the ducts from the knockout animals reflects different levels of SLC26A3 expression.
At the very least, we have to presume that the apical Cl− and HCO3− fluxes measured in this study are due to the combined activities of SLC26A6, SLC26A3, and CFTR in the wild-type ducts and due to the upregulated SLC26A3 and CFTR activities in the Slc26a6−/− ducts. When subjected to gradients favoring rapid Cl−/HCO3− exchange, deletion of Slc26a6 altered the overall ratio of Cl− absorption to HCO3− secretion from ∼0.7 to ∼1.4. Our data therefore suggest the presence of multiple Cl−/HCO3− exchangers operating with different stoichiometries and are consistent with a 1:2 stoichiometry for SLC26A6 and a 2:1 stoichiometry for SLC26A3. Measurements of membrane potential support the electrogenicity of SLC26A6 (Fig. 6): the hyperpolarization induced in wild-type mouse ducts by substitution of luminal Cl− or application of H2DIDS to the apical membrane was similar to that seen in guinea-pig ducts (25) and was abolished in Slc26a6−/− ducts.
Physiological significance of 1:2 Cl−/HCO3− exchange in pancreatic duct.
With a 1:2 stoichiometry, SLC26A6 will make a significant contribution to HCO3− secretion in the pancreatic duct system. In the proximal part of the ductal tree, close to the acini where the luminal [Cl−]L is high, a 1:2 exchanger can produce a HCO3−-rich fluid secretion, even without the support of CFTR. It will dilute and alkalinize the protein-rich acinar secretion more quickly and effectively than a 1:1 or 2:1 exchanger.
Despite this, we found that the final, steady-state pHL value (∼7.6) and [HCO3−]L (∼40 mM) in the stimulated sealed ducts were not significantly different between the wild-type and the Slc26a6−/− ducts (Fig. 1I). Given the moderate [HCO3−]L typical of the mouse pancreas, an upregulated SLC26A3 exchanger would still secrete HCO3− because it is some way from equilibrium. With a 2:1 stoichiometry, it would also promote fluid absorption as a result of its net absorption of anions. These factors, together with increased Cl− and HCO3− secretion via the upregulated CFTR conductance, probably account for the moderately HCO3−-rich secretion that is still observed in the Slc26a6−/− ducts. The upregulation of CFTR has been reported previously in the ducts of these animals (28) and is also supported by our observation that Cl−-independent HCO3− efflux following alkalinization was enhanced in the Slc26a6−/− ducts (Fig. 5).
The fact that the steady-state fluid secretory rate was greater in Slc26a6−/− ducts than in the wild-type ducts (Fig. 1J) suggests that increased anion secretion via CFTR more than compensates for the loss of SLC26A6-mediated anion secretion and the possible increase in net anion absorption via SLC26A3. These results are consistent with our previous findings (8) that forskolin-stimulated fluid secretion in sealed ducts, and also secretin-stimulated HCO3− and fluid secretion in vivo, were not significantly affected by deletion of Slc26a6. However, it should be noted that another group (28) has reported that stimulation with secretin failed to enhance fluid secretion in Slc26a6−/− ducts so there may be significant differences between knockout animal strains.
As [HCO3−]L rises with time and distance along the pancreatic duct, the contribution of Cl−/HCO3− exchange becomes smaller and the HCO3− conductance of CFTR is thought to provide the dominant route for HCO3− secretion (11). We simulated this situation, although unphysiological for mouse ducts, by perfusing them with a high HCO3− solution (125 mM; Fig. 3). Surprisingly, the efflux of HCO3− following an alkali load was reduced only ∼20% by luminal CFTRinh-172 (Fig. 3). This may be due partly to incomplete block (∼60%) of the CFTR-mediated HCO3− conductance by CFTRinh-172 (11), but it also suggests that there is a significant HCO3−-independent component in the recovery of pHi (25). The residual pHi recovery after alkali load in ΔF/ΔF ducts perfused with high [HCO3−]L (Fig. 4, B and C) and in the absence of Cl− (Fig. 5, C and D) is also compatible with there being a HCO3−-independent component.
In human and guinea-pig pancreas, the [HCO3−]L probably already exceeds 100 mM in the proximal part of the ductal tree and the HCO3− conductance of CFTR provides the major route for apical HCO3− secretion in the distal part (11). In contrast, the HCO3− concentration of mouse pancreatic juice may never exceed 50 mM (8). This reflects the larger driving force for Cl− secretion that is generated at the basolateral membrane in the mouse ducts (6). Consequently Cl−/HCO3− exchange via SLC26A6 probably provides a major route for apical HCO3− secretion all along the duct system. The 1:2 stoichiometry of SLC26A6 is therefore beneficial for its efficiency in mouse ducts, rather than for preventing HCO3− reabsorption, which is more important in high HCO3− secretors such as human or guinea-pig pancreas (23).
Interaction of SLC26A6 and CFTR in pancreatic duct.
Heterologous expression studies (14, 15) have revealed that SLC26 transporters interact with CFTR through the STAS domain of the SLC26s and the R domain of CFTR, thereby mutually enhancing each other's transport activity. Furthermore, SLC26A6 and CFTR colocalize at the apical membrane of rat intralobular ducts (15), and the approximately twofold activation of apical Cl−/HCO3− exchange by cAMP in the mouse main duct is dependent on CFTR and is abolished in ΔF/ΔF cystic fibrosis mice (16). In the present study, however, we could not find strong evidence for the activation of SLC26A6 in interlobular ducts being dependent on the presence of functional CFTR. There was no difference in Cl−-dependent HCO3− efflux across the apical membrane under cAMP stimulation (largely mediated by SLC26A6) between ΔF/ΔF ducts and wild-type ducts (Fig. 4, A and C). This may indicate significant functional differences between the interlobular ducts and main duct in mouse pancreas.
Apart from direct protein-protein interactions, SLC26 exchangers and CFTR also interact through their effects on the electrochemical gradients for Cl− and HCO3− at the apical membrane. For example, when we blocked CFTR in the wild-type mouse ducts with CFTRinh-172, we observed enhanced apical Cl−/HCO3− exchange (Fig. 2, A and C) as previously reported in guinea-pig ducts (25). In the Slc26a6−/− ducts, where there is increased expression of an alternative exchanger, most likely SLC26A3, inhibition of CFTR had the opposite effect, slowing the apical Cl−/HCO3− exchanger (Fig. 2, B and C). These results may be explained by the difference in stoichiometry between SLC26A6 (1:2) and SLC26A3 (2:1) and do not necessarily indicate any direct molecular interaction. CFTR and SLC26A6 share the same inside-negative potential difference as a component of their driving force, and thus membrane hyperpolarization induced by blocking CFTR with luminal CFTRinh-172 should enhance 1:2 Cl−/HCO3− exchange, as was observed. Conversely, SLC26A3, with a 2:1 stoichiometry, would be more active under depolarized conditions and its activity would be expected to be reduced by hyperpolarization following luminal application of CFTRinh-172. This difference was lost when the lumen was perfused with the high-HCO3− solution (Fig. 3), most probably because the HCO3− conductance of CFTR contributes more than Cl−/HCO3− exchange to the apical HCO3− efflux seen in this condition.
In conclusion, we have demonstrated that deletion of Slc26a6 alters the overall stoichiometry of apical Cl−/HCO3− exchange in native mouse interlobular ducts. Our results are compatible with the reported 1:2 stoichiometry of mouse SLC26A6 and provide further support for the upregulation of a Cl−/HCO3− exchanger with a different stoichiometry, most probably SLC26A3, in the Slc26a6−/− animals.
GRANTS
This work was supported by grants from the Japanese Society for the Promotion of Science and the Research Committee of Intractable Pancreatic Diseases (principal investigator: Tooru Shimosegawa) provided by the Ministry of Health, Labor, and Welfare, Japan. Y. Song was supported by Uehara Memorial Foundation and Daiko Foundation. A. Stewart was supported by a Long Term Fellowship from the Japanese Society for the Promotion of Science and support from National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-43495 (Seth L. Alper) and DK-34854 (Harvard Digestive Diseases Center Core C and Pilot/Feasibility Grant).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.S., A.Y., and H.I. performed experiments; Y.S., A.Y., M.C.S., S.B.K., A.K.S., B.-C.L., and H.I. analyzed data; Y.S., A.Y., M.C.S., S.B.K., A.K.S., M.S., B.-C.L., T.K., C.-X.J., and H.I. interpreted results of experiments; Y.S. and H.I. prepared figures; Y.S. and H.I. drafted manuscript; Y.S., A.Y., M.C.S., S.B.K., A.K.S., M.S., B.-C.L., T.K., C.-X.J., and H.I. approved final version of manuscript; A.Y., M.C.S., S.B.K., A.K.S., M.S., T.K., C.-X.J., and H.I. conception and design of research; M.C.S., A.K.S., and H.I. edited and revised manuscript.
REFERENCES
- 1. Alper SL, Stewart AK, Vandorpe DH, Clark JS, Horack RZ, Simpson JE, Walker NM, Clarke LL. Native and recombinant Slc26a3 (downregulated in adenoma, Dra) do not exhibit properties of 2Cl−/1HCO3− exchange. Am J Physiol Cell Physiol 300: C276–C286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Argent B, Gray M, Steward M, Case M. Cell physiology of pancreatic ducts. In: Physiology of the Gastrointestinal Tract, edited by Johnson LB, Ghishan KE, Merchant FK, Said JL, Wood HM: Philadelphia, PA: Elsevier Academic, 2006, p. 1371–1396 [Google Scholar]
- 3. Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark JS, Vandorpe DH, Chernova MN, Heneghan JF, Stewart AK, Alper SL. Species differences in Cl− affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl− exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis. J Physiol 586: 1291–1306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández-Salazar MP, Pascua P, Calvo JJ, López MA, Case RM, Steward MC, San Román JI. Basolateral anion transport mechanisms underlying fluid secretion by mouse, rat and guinea-pig pancreatic ducts. J Physiol 556: 415–428, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281: G1301–G1308, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl−/HCO3− exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol 292: G447–G455, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol 528: 305–315, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol 511: 407–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 133: 315–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishiguro H, Steward MC, Sohma Y, Kubota T, Kitagawa M, Kondo T, Case RM, Hayakawa T, Naruse S. Membrane potential and bicarbonate secretion in isolated interlobular ducts from guinea-pig pancreas. J Gen Physiol 120: 617–628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishiguro H, Steward MC, Yamamoto A. Microperfusion and micropuncture analysis of ductal secretion. In: The Pancreapedia Exocrine Pancreas Knowledge Base 2011. Ann Arbor, MI; University of Michigan Library, 2011 [Google Scholar]
- 14. Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J 21: 5662–5672, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3− exchange in mouse submandibular and pancreatic ducts. J Biol Chem 274: 14670–14677, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Novak I, Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflügers Arch 411: 546–553, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol 137: 239–251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587: 2179–2185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pascua P, García M, Fernández-Salazar MP, Hernández-Lorenzo MP, Calvo JJ, Colledge WH, Case RM, Steward MC, San Román JI. Ducts isolated from the pancreas of CFTR-null mice secrete fluid. Pflügers Arch 459: 203–214, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol 127: 511–524, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sohma Y, Gray MA, Imai Y, Argent BE. HCO3− transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol 176: 77–100, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67: 377–409, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko SB, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol 301: C289–C303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol 296: G1307–G1317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szalmay G, Varga G, Kajiyama F, Yang XS, Lang TF, Case RM, Steward MC. Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J Physiol 535: 795–807, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL. Functional activity of Pat-1 (Slc26a6) Cl−/HCO3− exchange in the lower villus epithelium of murine duodenum. Acta Physiol (Oxf) 201: 21–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J 25: 5049–5057, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Xu J, Barone S, Li H, Holiday S, Zahedi K, Soleimani M. Slc26a11, a chloride transporter, localizes with the vacuolar H+-ATPase of A-intercalated cells of the kidney. Kidney Int 80: 926–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB, Jr, Capecchi MR, Welsh MJ, Thomas KR. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest 96: 2051–2064, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]