Abstract
Background
Serum potassium levels affect insulin secretion by pancreatic beta-cells, and hypokalemia associated with diuretic use has been associated with dysglycemia. We hypothesized that adults with lower serum potassium levels and lower dietary potassium intake are at higher risk for incident diabetes, independent of diuretic use.
Methods
We analyzed data from 12,209 participants from the Atherosclerosis Risk in Communities (ARIC) Study, an on-going prospective cohort study beginning in 1986, with 9 years of in-person follow-up and 17 years of telephone follow-up. Using multivariate Cox proportional hazard models, we estimated the relative hazard (RH) of incident diabetes associated with baseline serum potassium levels.
Results
During 9 years of in-person follow-up, 1475 participants developed incident diabetes. In multivariate analyses, we found an inverse association between serum potassium and risk of incident diabetes. Compared to those with a high-normal serum potassium (5.0-5.5 mEq/l), adults with serum potassium levels of < 4.0, 4.0-<4.5, and 4.5-<5.0, (mEq/L) had adjusted relative hazards (RH) (95% CI) of incident diabetes of 1.64 (1.29-2.08), 1.64 (1.34-2.01), and 1.39 (1.14-1.71) respectively. An increased risk persisted during an additional 8 years of telephone follow-up based on self-report with RHs of 1.2-1.3 for those with a serum potassium less than 5.0 mEq/L. Dietary potassium intake was significantly associated with risk of incident diabetes in unadjusted models but not in multivariate models.
Conclusions
Serum potassium is an independent predictor of incident diabetes in this cohort. Further study is needed to determine if modification of serum potassium could reduce the subsequent risk of diabetes.
Introduction
Several lines of evidence point to hypokalemia as a possible risk factor for type 2 diabetes. First, in analyses of data collected from randomized controlled trials of thiazide diuretics, serum potassium was inversely related to glucose—an effect that is blunted by oral potassium supplementation (1). Second, experimental studies provide biological plausibility by showing that thiazide-induced hypokalemia leads to diminished insulin secretion (2, 3). Third, in some randomized control trials, angiotensin converting enzyme-inhibitors (ACE-I), which increase serum potassium as well as have several other effects along with their effects on blood pressure, were associated with a decreased risk of diabetes mellitus (4). Most recently, a re-analysis of data from the Systolic Hypertension in Elderly Program (SHEP) Study identified hypokalemia as a mediator of thiazide-related risk of incident diabetes (5). However, no epidemiologic studies have evaluated the risk of diabetes associated with serum potassium levels independent of thiazide use. We, therefore, analyzed data from the Atherosclerosis Risk in Communities (ARIC) Study to test the hypothesis that adults with lower serum potassium levels within the ‘normal range’ are at higher risk for incident diabetes, even without diuretic use. We also sought to determine whether higher dietary potassium intake was associated with lower diabetes risk. If low serum potassium is indeed a risk factor for diabetes, then a strategy to increase serum potassium--either with medications, supplements, or dietary modifications—might represent a novel approach to diabetes prevention.
Methods
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort study involving 15,792 adults aged 45 to 65 years at the baseline visit, recruited based on population-based probability sampling in 1986-1989 from four US communities: Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Participants came to clinic visits every three years through 1998 for approximately 9 years of follow up. They were then followed yearly for an additional 8 years (through 2006), primarily through telephone contact. Details of the design and conduct of the ARIC study have been published previously (6). Institutional review boards at each of the participating institutions approved the study.
Study participants
We excluded participants sequentially from this analysis if, at the baseline visit, they had diabetes (n=1870), defined as 1) fasting glucose ≥ 126mg/dL, 2) non-fasting glucose of ≥ 200mg/dL, 3) participant report of a physician diagnosis, or 4) use of medications to treat diabetes (7). We excluded participants with missing baseline diabetes information or missing serum potassium levels (n=148), high serum potassium level (>5.5 mEq/L) (n= 156), ethnicity other than white or African American (n=44), fasted less than 8 hours (n=257), had a serum creatinine > 1.7 mg/dL (n=75), or had missing information on incident diabetes or covariates outside of the main exposure (n=1033). These exclusions produced a cohort of 12,209 subjects for this analysis. For the dietary analyses, we further excluded participants if they had missing or incomplete dietary information (n=364), other missing covariates (n=117), or if they had extreme values in total daily caloric intake. We defined extreme values for total daily caloric intake as < 600 or > 4000 kcal/day for females and < 800 or > 5000 kcal/day for males (n=204), producing a cohort of 11,530 for the dietary analyses.
Serum and Dietary Potassium
The primary exposure of interest was serum potassium. Blood samples were aliquoted, centrifuged, frozen, and stored at −70°C in central laboratories (6). Serum potassium from the baseline visit was measured with a direct electrochemical technique on undiluted serum (8). Dietary potassium intake was estimated from an interviewer-administered, modified version of the 61 item food frequency questionnaire developed by Willett et al (9). Dietary potassium intake was analyzed as milligram (mg) of intake per total kilocalories consumed per day.
Incident Type 2 Diabetes Mellitus
The main outcome was a diagnosis of diabetes assessed in participants at each of the three follow-up visits. A visit-based definition of diabetes was defined, as above, by any one of the following 4 conditions: 1) fasting glucose ≥ 126mg/dL, 2) non-fasting glucose of ≥ 200mg/dL, 3) participant report of a physician diagnosis, or 4) use of medications to treat diabetes (7). Since those with diabetes at the baseline visit were excluded, participants who met these criteria at subsequent visits were considered to have incident diabetes. For this definition, the date of onset of diabetes was estimated by linear interpolation using fasting glucose values at the visit at which diabetes was ascertained and the immediately preceding visit (7).
To confirm the robustness of the main findings, we conducted analyses with an alternative definition of ‘incident diabetes’ and longer duration of follow-up. First, we limited the definition of diabetes to self-report of physician diagnosis or self-reported use of diabetes medications through visit 4 (year 9 of follow-up), thereby excluding cases of ‘undiagnosed diabetes’. This interview-based approach allowed us to extend follow-up beyond the final ‘in person’ clinic visit to include data from annual telephone calls. Thus, the second analysis defined ‘incident diabetes’ as self-report of physician diagnosis or self-reported use of diabetes medications through 2006 (year 17-20 of follow-up). For both the first and second analyses, the date of onset of diabetes was defined as the date of the interview in which diabetes was first reported.
Covariates
Potential confounding factors included different demographic variables, anthropometric values, laboratory values, and use of medications which were assessed at the baseline visit. We included the following covariates in our models: age, sex, race, center, body-mass index (BMI), waist circumference, serum magnesium, calcium, and creatinine levels, physical activity level, parental history of diabetes, presence of hypertension, average of systolic blood pressure readings from a second and third measurement, fasting glucose, fasting insulin, income, use of beta-blockers, use of diuretics, and use of ACE-I. Demographic information and information on parental history, physical activity, and medication use were obtained during an in-person interview; anthropometric and laboratory measurements were obtained in a standardized fashion and by trained personnel (8, 10).
Statistical analyses
Serum potassium was categorized into 4 clinically meaningful groups--<4.0, 4.0-<4.5, 4.5-<5.0 and 5.0-5.5 (mEq/L). We determined and compared the mean and standard deviation or frequency of baseline characteristics of the study population by categories of potassium level using one-way analysis of variance F tests for continuous variables and Pearson's chi-square tests for categorical variables. We performed multivariable linear regression to assess cross-sectional relationships between serum potassium and glycemic parameters including fasting glucose, fasting insulin, and HOMA-IR (11), after adjustments for potential covariates. We used Cox proportional hazard regression models to investigate the association between baseline serum potassium levels and incident diabetes after adjustment for potential confounding variables, chosen based on a priori knowledge, using the highest potassium category (5.0-5.5 mEq/L) as the reference group. The proportional hazards assumption was tested on categories of serum potassium and all potential confounders using log-log survival curves and goodness of fit tests. We also conducted analyses using serum potassium as a continuous variable and categorizing measures into quintiles and deciles to ensure that the categorization by clinically relevant cut-points was robust. We tested for interaction between serum potassium and age, sex, race, BMI, and income, as categorical variables on risk of diabetes. In the analyses of dietary potassium, we also used Cox regression models. We categorized potassium intake per kilocalorie (kcal) into quartiles with cutoffs of <1.37, 1.37-1.63, 1.63-1.93, and ≥ 1.93 mg/kcal and used the highest quartile of intake (≥ 1.93 mg/kcal) as the reference group. We also used dietary potassium intake as a continuous variable and as a categorical variable by itself, with total kilocalorie intake as a separate independent variable.
Finally, we performed other sensitivity analyses using subgroups of participants based on their use of medications, excluding participants that were on diuretics, as well as excluding participants that were on diuretics, beta-blockers, ACE-I, potassium or magnesium supplements. Finally we performed subsidiary analyses with additional covariates including estimated glomerular filtration rate (eGFR), HOMA-IR, smoking status (never, current, former), and amount of alcohol consumption.
Tests of significance were two-tailed, with an alpha level of 0.05. We performed all analyses using SAS, version 9.1.3 (SAS Institute, Cary, NC).
Results
The mean age of participants at baseline was 54 years. 56% were female, 22% were African American; mean BMI was 27 kg/m2, and mean serum potassium was 4.4 mEq/L with a range of 2.5-5.5 mEq/L.
Table 1 shows the characteristics of the participants based on their level of serum potassium. These groups differed significantly for all baseline covariates assessed except for percentages of participants with parental history of diabetes and use of ACE-I, which was low for all groups. A higher percentage of African Americans and females had potassium levels in the lower range of the spectrum. Lower potassium levels were associated with higher BMI, larger waist circumference, lower serum magnesium levels, higher fasting insulin levels, higher use of beta-blockers, and higher use of diuretics. Multivariate cross-sectional analyses revealed a significant inverse relationship between serum potassium and fasting insulin levels. A non-linear association was found between serum potassium and fasting glucose (Table 1).
Table 1. Baseline characteristics of 12,209 ARIC participants by serum potassium (mEq/L) at baseline.
| Characteristics | K < 4.0 | 4.0 ≤ K < 4.5 | 4.5 ≤ K < 5.0 | 5.0 ≤ K ≤ 5.5 | p-value* |
|---|---|---|---|---|---|
| n | 1619 | 4903 | 4178 | 1509 | |
| Age | 54.0 ± 5.77 | 53.7 ± 5.65 | 54.0 ± 5.76 | 54.0 ± 5.64 | 0.04 |
| African American race (%) | 45.3 | 25.8 | 14.2 | 8.2 | <0.01 |
| Female gender (%) | 70.0 | 57.5 | 50.8 | 49.0 | <0.01 |
| Family history of diabetes (%) | 24.0 | 22.7 | 22.5 | 21.3 | 0.35 |
| BMI (kg/m2) | 28.6 ± 5.90 | 27.4 ± 5.12 | 26.9 ± 4.70 | 26.1 ± 4.23 | <0.01 |
| Waist circumference (cm) | 97.9 ± 14.58 | 95.9 ± 13.25 | 95.5 ± 12.84 | 93.4 ± 12.42 | <0.01 |
| Hypertension present (%) | 63.6 | 29.2 | 22.6 | 19.7 | <0.01 |
| Systolic blood pressure (mmHg) | 124 ± 20 | 119 ± 18 | 119 ± 17 | 118 ± 16 | <0.01 |
| Serum K+ (mEq/l) | 3.68 ± 0.25 | 4.22 ± 0.14 | 4.67 ± 0.14 | 5.17 ± 0.16 | <0.01 |
| Serm Mg++ (mEq/l) | 1.58 ± 0.17 | 1.63 ± 0.15 | 1.66 ± 0.14 | 1.69 ± 0.14 | <0.01 |
| Serum Ca++ (mg/dl) | 9.71 ± 0.44 | 9.70 ± 0.41 | 9.81 ± 0.41 | 9.96 ± 0.40 | <0.01 |
| Serum Creatinine (mg/dl) | 1.06 ± 0.18 | 1.08 ± 0.18 | 1.10 ± 0.18 | 1.12 ± 0.18 | <0.01 |
| Fasting glucose (mg/dl) | 99.07 ± 10.27 | 97.81 ± 9.23 | 98.83 ± 8.93 | 99.84 ± 8.85 | <0.01 |
| Fasting insulin (pmol/l) | 95.27 ± 77.87 | 77.06 ± 56.76 | 74.70 ± 53.93 | 67.17 ± 42.48 | <0.01 |
| Leisure activity | 2.31 ± 0.58 | 2.38 ± 0.56 | 2.42 ± 0.56 | 2.47 ± 0.55 | <0.01 |
| Beta-blocker use (%) | 16.1 | 9.0 | 7.7 | 8 | <0.01 |
| Diuretic use (%) | 45.5 | 13.9 | 7.9 | 5.7 | <0.01 |
| Ace-inhibitor use (%) | 3.2 | 2.5 | 2.4 | 2.1 | 0.28 |
| Dietary potassium intake (mg/kcal) | 1.63 ± 0.43 | 1.65 ± 0.42 | 1.68 ± 0.41 | 1.70 ± 0.41 | <0.01 |
| Combine family annual income% | <0.01 | ||||
| >$50,000 | 15.6 | 24.3 | 28.1 | 34.3 | |
| $25,000-$49,999 | 31.4 | 37.0 | 38.6 | 39.1 | |
| $12,000-$24,999 | 25.5 | 20.8 | 19.2 | 15.0 | |
| <$5,000-$11,999 | 27.4 | 17.9 | 14.2 | 11.6 |
# values are mean ± standard deviation for continuous variables and % for categorical variables
from one-way ANOVA or chi-square test of overall difference
%1986 US dollars
During the first 9 years of follow-up, 1475 adults developed diabetes (according to the in-person visit-based definition). The crude incidence rate of diabetes was highest in adults with serum potassium <4.0 mEq/L, (24.6 per 1,000 person years); adults with higher potassium levels had progressively lower rates of diabetes (Table 2). The relative hazard of incident diabetes, adjusted for age, sex, race, and center was also highest for those with potassium of <4.0 mEq/L with a relative hazard (RH) (95% CI) of 2.05 (1.64-2.56) compared to those with a serum potassium levels of 5.0-5.5 mEq/L (Table 2).
Table 2.
Crude rates of incident diabetes and partially-adjusted relative hazards by serum potassium at baseline. Relative hazards are adjusted for age, sex, race, and center.
| Serum Potassium Levels (mEq/L) | ||||
|---|---|---|---|---|
| K < 4.0 | 4.0 ≤ K < 4.5 | 4.5 ≤ K < 5.0 | 5.0 ≤ K ≤ 5.5 | |
| Incident cases of diabetes (incident cases not on diuretics) | 284 (120) | 616 (485) | 456 (402) | 119 (111) |
| Person-years | 11567 | 37113 | 32073 | 11816 |
| Rate/1000 person-years | 24.55 | 16.60 | 14.22 | 11.82 |
| Age, sex, race, center adjusted RH | 2.05 | 1.52 | 1.37 | 1.00 |
| (95% CI) | (1.64-2.56) | (1.25-1.85) | (1.12-1.68) | (ref) |
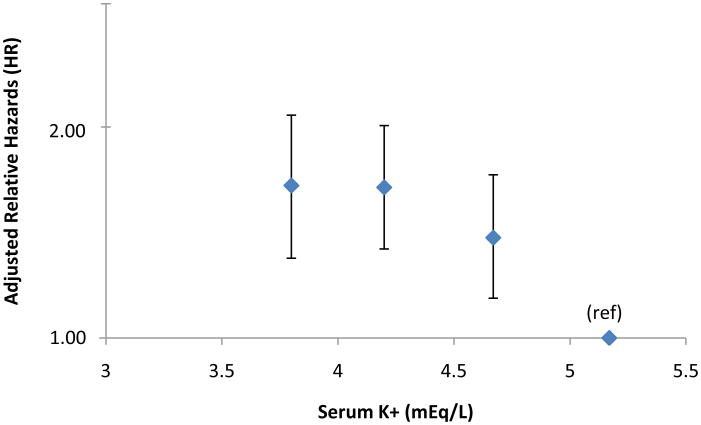
After adjustments for age, sex, race, center, BMI, waist circumference, serum magnesium, calcium, and creatinine levels, physical activity, parental history of diabetes, presence of hypertension, average systolic blood pressure of second and third measurements, fasting glucose and insulin levels, income, and use of beta-blockers, diuretics, and ACE-I, the Cox proportional hazard models revealed an increased risk of developing diabetes with lower potassium levels. When compared to those with a high-normal serum potassium (5.0-5.5 mEq/l), adults with serum potassium levels of < 4.0, 4.0-<4.5, and 4.5-<5.0 (mEq/L) had adjusted relative hazards (RH) (95% CI) of incident diabetes of 1.64 (1.29-2.08), 1.64 (1.34-2.01), and 1.39 (1.14-1.71) respectively, (p-trend <0.0001) (Figure 1). A similar inverse relationship was found when serum potassium was categorized differently according to quintiles or deciles, with all RHs being statistically significant. When serum potassium was used as a continuous variable, its coefficient revealed an inverse association with diabetes risk that was statistically significant (β= −0.27, p < 0.0001). We included estimated glomerular filtration rate (mL/min/1.73 m2), HOMA-IR, and alcohol consumption as continuous variables, and smoking status as a categorical covariate, all of which had no effect on the pattern of association.
Figure 1.
Adjusted relative hazards for incident diabetes by serum potassium at baseline. Relative hazards are adjusted for age, sex, race, center, BMI, waist circumference, serum magnesium, calcium, and creatinine levels, physical activity levels, parental history of diabetes, presence of hypertension, systolic blood pressure (average of 2nd and 3rd measurements), fasting glucose, fasting insulin, income, use of beta-blockers, use of diuretics, and use of ACE-I. Bars indicate 95% confidence interval. Results are plotted at the medians of the potassium categories (3.8, 4.2. 4.7, and 5.2 mEq/L).
In the stratified analyses by age, sex, BMI and household income, the inverse relationship between serum potassium and diabetes risk remained in fully adjusted models. The association was also similar among African-Americans and whites with no significant statistical interaction (p > 0.5). Adjusted relative hazards of incident diabetes for African Americans with a serum potassium of <4.0, 4.0-<4.5, and 4.5-<5.0 (mEq/L) were 2.42 (1.29-4.53), 2.14 (1.16-3.97), and 1.87 (1.00-3.49) respectively, compared to those with a serum potassium of 5.0-5.5 mEq/L; adjusted relative hazards of incident diabetes for whites for the same potassium groups were 1.46 (1.09-1.96), 1.50 (1.20-1.88), and 1.28 (1.03-1.59).
Sensitivity analyses
To confirm the robustness of the main findings, we conducted two types of supplementary analyses: 1) We used the interview-based definition of diabetes. 2) We conducted subgroup analyses based on use of medications.
When using the interview-based definition of diabetes during the main 9-year study period, 607 participants reported newly diagnosed diabetes, and there was a significant association between diagnosis of incident diabetes and serum potassium. Among these subjects, when compared to those with high-normal serum potassium (5.0-5.5 mEq/L), adults with serum potassium of < 4.0, 4.0-<4.5, and 4.5-<5.0, (mEq/L) had adjusted RHs (95% CI) of incident diabetes of 1.46 (0.99-2.16), 1.39 (0.99-1.95), and 1.47 (1.05-2.06), respectively (Table 3). Using our interview-based definition over the entire 17 year study period, 2552 adults reported a diagnosis of diabetes. The relationship between incident diabetes and baseline serum potassium during this longer follow-up period was still significant, with serum potassium levels less than 5.0 mEq/L being associated with a significantly higher risk of incident diabetes compared to subjects with a serum potassium of 5.0-5.5 mEq/L; however, as with the shorter follow-up period using the interview-based definition of diabetes, the graded association was no longer as evident (Table 3).
Table 3.
Summary of results from multivariate analyses assessing the relationship between risk of incident diabetes and serum potassium levels.
| Adjusted Relative Hazards (RH) for Incident Diabetes# | ||||
|---|---|---|---|---|
| Model (n total; cases of incident diabetes) | K < 4.0 | 4.0 ≤ K < 4.5 | 4.5 ≤ K < 5.0 | K 5.0-5.5 (ref)% |
| Main model (n=12,209; 1475) | 1.64 (1.29-2.08) | 1.64 (1.34-2.01) | 1.39 (1.14-1.71) | 1.00 |
| Subjects not on medications* (n=9,353; 930) | 1.57 (1.14-2.16) | 1.48 (1.17-1.88) | 1.38 (1.09-1.74) | 1.00 |
| Subjects not on diuretics (n=10,373; 1118) | 1.41 (1.06-1.88) | 1.51 (1.21-1.87) | 1.35 (1.09-1.67) | 1.00 |
| Subjects on diuretics (n=1835; 357) | 2.91 (1.41-6.00) | 2.85 (1.38-5.88) | 1.93 (0.92-4.08) | 1.00 |
| Subject self-report of diabetes, 9 years (n=12,209; 607) | 1.46 (0.99-2.16) | 1.39 (0.99-1.95) | 1.47 (1.05-2.06) | 1.00 |
| Subject self-report of diabetes, 17 years (n=12,209; 2552) | 1.24 (1.04-1.48) | 1.29 (1.11-1.49) | 1.31 (1.13-1.51) | 1.00 |
RH and OR adjusted for for age, sex, race, center, BMI, waist circumference, serum magnesium, calcium, and creatinine levels, physical activity, parental history of diabetes, presence of hypertension, systolic blood pressure, fasting glucose and insulin, income, use of beta-blockers, use of diuretics (unless indicated otherwise), and use of ACE-Is
subjects not on beta-blockers, ACE-inhibitors, diuretics, potassium or magnesium supplementation
% K= serum potassium in mEq/L
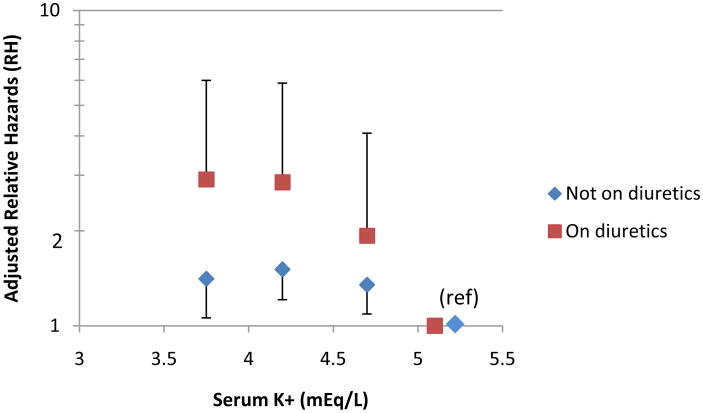
For subjects that were on diuretics (n= 1835) and those not on diuretics (n= 10,373), we found similar trends in both groups, with a higher risk of developing diabetes among those with lower potassium levels. Point estimates for the RHs were higher for each category of potassium for those on diuretics compared to those not on diuretics; however, confidence intervals for each category overlapped for the two groups of subjects (Figure 2, Table 3), with wide confidence intervals for the group on diuretics, likely due to the smaller sample size.
Figure 2.
Adjusted relative hazards of incident diabetes by potassium category for participants on diuretics and not on diuretics. Relative hazards are adjusted for age, sex, race, center, BMI, waist circumference, serum magnesium, calcium, and creatinine levels, physical activity level, parental history of diabetes, presence of hypertension, systolic blood pressure (average of 2nd and 3rd measurements), fasting glucose, fasting insulin, income, use of beta-blockers and use of ACE-I. Bars indicate 95% confidence interval. Results are plotted at the medians of the categories (3.8, 4.2. 4.7, and 5.2 mEq/L).
After excluding all subjects who were on diuretics, beta-blockers, ACE-I, potassium or magnesium supplements, there was a graded increase in RH of incident diabetes with lower serum potassium levels. When compared to those with high-normal serum potassium (5.0-5.5 mEq/L), adults with serum potassium of < 4.0, 4.0-<4.5, and 4.5-<5.0 (mEq/L) had adjusted RHs (95% CI) of incident diabetes of 1.57 (1.14-2.16), 1.48 (1.17-1.88), and 1.38 (1.09-1.74), respectively (Table 3).
Dietary analyses
Participants included in the dietary analyses had a mean dietary potassium intake of 2655 mg/day (recommended daily intake is 4700 mg/day) with a mean intake per kilocalorie of 1.66 mg/kcal. As expected, the correlation between serum potassium levels and dietary intake of potassium was modest (r= 0.06; 95% CI 0.042-0.077). We performed bivariate (unadjusted) analysis between dietary potassium intake and incident diabetes which revealed a significant graded increase in risk of incident diabetes with lower dietary potassium intake. Subjects in the lowest to higher quartiles of potassium intake had unadjusted RHs (95% CI) of incident diabetes of 1.37 (1.18-1.58), 1.19 (1.02-1.38), and 0.95 (0.81-1.11), respectively, compared to those in the highest quartile of potassium intake.
We conducted multivariate analyses of baseline dietary potassium intake and risk of incident diabetes during the 9 years of follow-up using Cox proportional hazard regression models. The covariates considered in these models were similar to the models with serum potassium as the main exposure and included the following potential confounders: age, sex, race, BMI, waist circumference, center, parental history of diabetes, dietary magnesium intake per total kilocalories consumed in a day, dietary calcium intake per total kilocalories consumed in a day, serum creatinine, hypertension, systolic blood pressure (average of 2nd and 3rd measurements), physical activity, income, and use of beta-blockers, diuretics, and ACE-I. In a no-interaction, multivariate model, there was no significant association between dietary potassium intake and diabetes risk. When compared to those in the highest potassium intake quartile (≥1.93mg/kcal), those with intakes of <1.37, 1.37-1.63, and 1.63-1.93 (mg/kcal) had RHs of incident diabetes of 1.03 (0.81-1.31), 1.05 (0.86-1.29), and 0.86 (0.72-1.03), respectively. When dietary potassium was used as a continuous variable or as a categorical variable alone, with a separate variable for total kilocalorie intake, there was still no significant association with risk of incident diabetes.
We did, however, observe a significant interaction between dietary potassium intake and income in the multivariable model (p=0.0089). Among those subjects in the lowest income group (<$12,000 annual family income in 1986 US dollars), there was a similar increase in risk of developing diabetes with lower dietary potassium intake. When compared to those in the highest potassium intake quartile (≥1.93mg/kcal), those with intakes of <1.37, 1.37-1.63, and 1.63-1.93 (mg/kcal) had RHs of incident diabetes of 1.81 (1.04-3.14), 1.70 (1.06-2.74), and 0.83 (0.52-1.31), respectively. A significant increase in risk of diabetes was not found among those subjects in higher income groups. In contrast, there was no significant interaction between dietary potassium intake and either age, sex, race, or BMI
Discussion
Our study suggests an inverse relationship between serum potassium levels and risk of incident diabetes in middle-aged adults. This relationship was independent of a wide array of potentially confounding factors, was stronger in thiazide users but present in their non-thiazide using counterparts, was still detectable more than 17 years later, and was robust in a variety of sensitivity analyses. In contrast, we did not find such a robust association between dietary potassium intake and incident diabetes after taking into account effect modification and adjusting for potential confounders.
Since the 1980's, several small-scale epidemiological studies of the effects of anti-hypertensive agents on risk of diabetes have been conducted, many of which suggested an increased risk of diabetes with the use of thiazide diuretics; however, many of these studies were not optimal due to sample size or study design (12). Since 2000, a few large-scale epidemiologic studies have been conducted, assessing the association of thiazide diuretic use and glucose metabolism and have found inconsistent relationships (12-14). Analysis of data from the Nurses' Health Studies I and II and Health Professionals Follow-up Study did find an increased risk of diabetes associated with diuretic use (14), while analysis of data from the ARIC Study and United Kingdom's General Practice Research Database did not find an independent association between thiazide diuretic use alone and increased risk of diabetes (12, 13). Analyses of data from clinical trials have focused on thiazide-induced hypokalemia. A review of data from 59 clinical trials, which had arms in which participants were on thiazide diuretics, found a significant inverse correlation between glucose and potassium levels and found that potassium supplementation was associated with smaller increases in glucose levels (1). A recent analysis of the SHEP trial found that low potassium was the primary mediator of the association between thiazide diuretics and increased risk of diabetes (5). None of the epidemiologic studies or clinical trials in our review of the literature has looked at the association of potassium levels, independent of diuretic use, and risk of diabetes, which was the aim of our study.
Strengths of this study include the population-based sampling method of ARIC, a biracial cohort, availability of blood measurements, extensive data on potential confounders, a large sample size that increased precision and permitted simultaneous statistical adjustment for multiple variables, and long duration of follow-up that offered the opportunity to study long-term risk., Also, the number of participants on medications at the baseline visit that could have affected serum potassium levels was relatively small, allowing for a fairly robust analysis of serum potassium levels unaffected by medications.
Nonetheless, several limitations of our study deserve mention. First, our main exposure, serum potassium, was based on a single measurement at the baseline visit and is subject to intra-individual variability. However, the short-term intra-individual variability of potassium has been assessed in ARIC and NHANES III. Both studies found serum potassium to have an intra-individual variability of approximately 5%, based on repeated measurements taken approximately 2 weeks apart, which is relatively low compared to other measures (15, 16). Accounting for this variability would likely strengthen the association found between serum potassium and risk of incident diabetes. Improper specimen handling would usually result in increased serum potassium (eg from hemolysis), which would also bias results toward the null. Hence, our findings are robust to this potential bias. One measure of fasting insulin, which we used in our model, is not a reliable measure of insulin levels or degree of insulin resistance. To help account for this unreliable measure, we included two measures of obesity, BMI and waist circumference, which could potentially better reflect degree of insulin resistance. The method of diabetes ascertainment differed in the in-person visit data and the telephone follow-up data. Based on other studies, both methods are valid for a diagnosis of diabetes (20) but may differ somewhat in their rate of case ascertainment.
An observational study cannot prove causality. It is possible that serum potassium could exert its effect on diabetes risk through other pathways and may not directly impact diabetes risk. However, earlier studies employing techniques including hyperglycemic clamps and experimentally-induced hypokalemia do suggest a direct and causal relationship with the induction of defects of insulin secretion by hypokalemia (2, 3, 17). While a direct relationship between serum potassium and glucose metabolism is possible as evidenced by these studies, other potential mediators of this relationship, such as aldosterone, should be considered. Measures of aldosterone and other hormones of the renin-angiotensin-aldosterone system, which directly affect serum potassium levels and could possibly affect glucose metabolism, were not available in this cohort.
Assessing a causal relationship between dietary potassium intake and diabetes is more difficult. First accurate measurement of dietary potassium intake is difficult by questionnaires. The reliability of dietary potassium is likely to be less than that of serum potassium, and measurement error could have affected the relationship seen between dietary measures as exposures and our outcome of interest. The reliability of dietary potassium has not been assessed in ARIC; however, estimates are reasonable and comparable to national estimates (18). Also, dietary potassium could be a marker of other substances in the diet which are contained in the same foods that are high in potassium and which could exert an effect on diabetes risk. One study looked at the relationship between risk of diabetes and dietary potassium in the Nurses' Health Study cohort. This study found an association between low potassium intake and increased risk of diabetes, but only controlled for two other dietary factors, dietary magnesium and dietary calcium, in the model (19). Other related factors could be mediators in this relationship. The interaction seen between dietary potassium and income also deserves further investigation, as other risk factors, including other dietary factors or lifestyle behaviors which could be associated with low income, could be mediating the higher risk of diabetes seen in this group of subjects.
The findings of this study deserve further investigation. The association between increased risk of diabetes and low serum potassium levels seen in this cohort should be assessed in other populations. Finally, clinical trials should be developed to assess if increasing serum potassium, either through medications, pharmacologic supplementation, or increased dietary intake, all relatively simple interventions, could indeed reduce the risk of incident diabetes.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022), and by National Institute of Diabetes, Digestive, and Kidney Diseases Grant (5R01-DK56918-03), Bethesda, MD. Dr. Chatterjee was supported by T32-HL007180. Dr. Selvin was supported by the NIH/NIDDK grants R21 DK080294 and K01 DK076595. Drs. Yeh and Brancati were supported by NIDDK Diabetes Research and Training Center (P60 DK079637). Dr. Brancati was supported by a grant from the National Institutes of Health, NIDDK, Bethesda, MD (K24 DK62222). The authors thank the staff and participants in the ARIC study for their important contributions. Dr. Chatterjee had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Findings presented at the American Diabetes Association's 69th Scientific Session, New Orleans, LA on June 8, 2009.
The authors have no financial disclosures.
References
- 1.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48(2):219–24. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 2.Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism. 1980;29(6):498–502. doi: 10.1016/0026-0495(80)90074-8. [DOI] [PubMed] [Google Scholar]
- 3.Helderman JH, Elahi D, Andersen DK, et al. Prevention of the glucose intolerance of thiazide diuretics by maintenance of body potassium. Diabetes. 1983 Feb;32(2):106–11. doi: 10.2337/diab.32.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Thornley-Brown D, Wang X, Wright JT, Jr, et al. Differing effects of antihypertensive drugs on the incidence of diabetes mellitus among patients with hypertensive kidney disease. Arch Intern Med. 2006;166(7):797–805. doi: 10.1001/archinte.166.7.797. [DOI] [PubMed] [Google Scholar]
- 5.Shafi T, Appel LJ, Miller ER, Klag MJ, Parekh RS. Changes in Serum Potassium Mediate Thiazide-Induced Diabetes. Hypertension. 2008;52:1022–1029. doi: 10.1161/HYPERTENSIONAHA.108.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 7.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 8.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999 Oct 11;159(18):2151–9. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 9.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–99. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 10.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253–9. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000 Mar 30;342(13):905–12. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 13.Burke TA, Sturkenboom MC, Ohman-Strickland PA, Wentworth CE, Rhoads GG. The effect of antihypertensive drugs and drug combinations on the incidence of new-onset type-2 diabetes mellitus. Pharmacoepidemiol Drug Saf. 2007;16(9):979–87. doi: 10.1002/pds.1444. [DOI] [PubMed] [Google Scholar]
- 14.Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29(5):1065–70. doi: 10.2337/diacare.2951065. [DOI] [PubMed] [Google Scholar]
- 15.Lacher DA, Hughes JP, Carroll MD. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clin Chem. 2005 Feb;51(2):450–2. doi: 10.1373/clinchem.2004.039354. [DOI] [PubMed] [Google Scholar]
- 16.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 17.Gorden P. Glucose intolerance with hypokalemia. Failure of short-term potassium depletion in normal subjects to reproduce the glucose and insulin abnormalities of clinical hypokalemia. Diabetes. 1973;22(7):544–551. doi: 10.2337/diab.22.7.544. [DOI] [PubMed] [Google Scholar]
- 18.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr J. 2009;8(1):14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992;55(5):1018–23. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. N Engl J Med. doi: 10.1056/NEJMoa0908359. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]