Abstract
Triolimus is a first-in-class, multi-drug loaded micelle containing paclitaxel, rapamycin and 17-AAG. In this study, we examine the anti-tumor mechanisms of action, efficacy and toxicity of Triolimus in vitro and in vivo. In vitro cytotoxicity testing of Triolimus was conducted using two aggressive adenocarcinomas including the lung cancer cell line, A549, and breast cancer cell line, MDA-MB-231. The three-drug combination of paclitaxel, rapamycin and 17-AAG displayed potent cytotoxic synergy in both A549 and MDA-MB-231 cell lines. Mechanistically, the drug combination inhibited both the Ras/Raf/MAPK and PI3K/Akt/mTOR pathways. Triolimus was advanced into tumor xenograft models for assessment of efficacy, toxicity and mechanisms of action. In vivo, a three-infusion schedule of Triolimus inhibited A549 and MDA-MB-231 tumor growth far more potently than paclitaxel-containing micelles and effected tumor cures in MDA-MB-231 tumor-bearing animals. Tumor growth delays resulted from a doubling in tumor cell apoptosis and a 50% reduction in tumor cell proliferation compared to paclitaxel-containing micelles. Enhanced anti-tumor efficacy was achieved without clinically significant increases in acute toxicity. Thus, Triolimus displays potent synergistic activity in vitro and anti-tumor activity in vivo with comparable toxicity to paclitaxel. These observations provide strong support for further development of Triolimus and an important proof of concept for safe, effective nanoparticle-based delivery of three complementary anti-cancer agents.
Keywords: Multi-targeting, Hsp90, mTOR, paclitaxel, micelles
Introduction
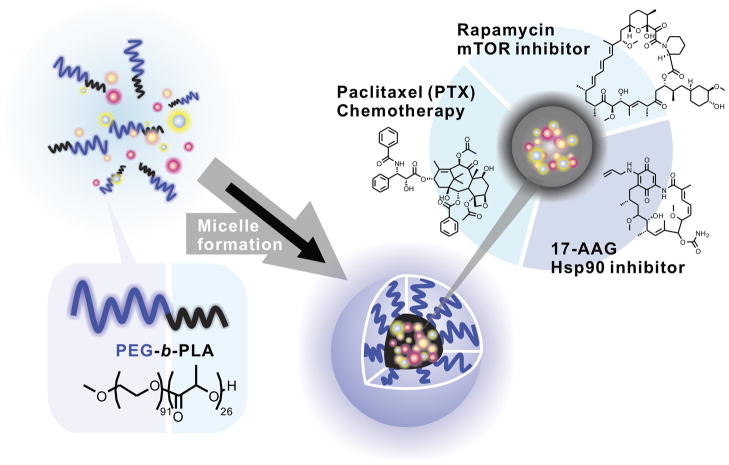
A growing appreciation of the complexity and heterogeneity of cancer cells has led to interest in combination therapies that simultaneously target multiple key pathways in carcinogenesis and tumor progression (1). Complementing this is a growing recognition that cancer cells possess a broad-ranging capacity to activate compensatory signaling pathways to overcome barriers presented by single agent therapy. To facilitate simultaneous, rational molecular multi-targeting, we employed a non-toxic, micellar nanocarrier capable of efficiently solubilizing multiple drugs at concentrations expected to have activity in vivo (2–4). For preclinical testing, a priori selection of drugs in multi-drug loaded micelles was based on known independent activity, clinical relevance and anticipated mechanisms of resistance. Paclitaxel is a cornerstone in the systemic management of an array of solid tumors including, notably, breast and lung cancer. Rapamycin has been reported to increase and prolong sensitivity to microtubule directed chemotherapeutic agents (5,6) and may exert independent activity in some settings (7). Finally, the prototypical Hsp90 inhibitor, 17-AAG, was selected for micelle inclusion due to its independent activity in several settings and documented capacity to target compensatory pathways activated by mTOR inhibition, including Akt activation (8–11). The resultant three-drug loaded micelle was designated Triolimus (Figure 1).
FIGURE 1.
Triolimus composition.
To assess the utility of Triolimus, we conducted formal combination index (i.e. synergy) analyses and mechanistic studies of these drugs, alone and in combination, in vitro as well as efficacy, toxicity and mechanistic studies in vivo.
Materials and Methods
Cell lines and pharmacologic agents
A549 and MDA-MB-231 cells were obtained from American Type Culture Collection (Manassas, VA) and passaged for fewer than 6 months after resuscitation. American Type Culture Collection cell line authentication includes short tandem repeat profiling, morphology monitoring, cytochrome C oxidase I testing and karyotyping. A549 cells were cultured in RPMI containing 5% heat inactivated fetal bovine serum and 1% penicillin/streptomycin. MDA-MB-231 cells were cultured in DMEM containing 5% fetal bovine serum and 1% penicillin/streptomycin. Paclitaxel, rapamycin and 17-AAG were obtained from LC Laboratories (Woburn, MA). Drug-loaded poly(ethyleneglycol)-block-poly(lactic-acid) (PEG-PLA) micelles were prepared and characterized as previously described (12,13). Briefly, PEG-PLA, paclitaxel, 17-AAG and rapamycin are dissolved in acetonitrile in a round-bottom flask. Acetonitrile is removed under reduced pressure and the resultant polymer film is rehydrated with double-distilled water. The aqueous solution is then centrifuged and filtered (0.45 μm). Prior to use, drug loading is quantified by reverse phase high-performance liquid chromatography. The drug ratio in Triolimus (paclitaxel:rapamycin:17-AAG, 2:1:2 by mass or 2:1:3 on a molar basis) was selected to permit mouse dosing of paclitaxel:rapamycin:17-AAG at 60 mg/kg:30 mg/kg:60 mg/kg as previously reported (12).
Immunoblotting and Immunohistochemistry
A549 and MDA-MB-231 cells were grown to approximately 70% confluence. Cells were then treated with DMSO vehicle control, paclitaxel (30 nM), rapamycin (30 nM), 17-AAG (30 nM), or combinations for 24 hours. The final concentration of DMSO was 0.1%. Cells were washed twice in cold PBS and protein isolated in cold NP-40 lysis buffer [50 mM HEPES (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 10% glycerol, 2.5 mM EGTA, 1 mM EDTA, 1 mM DTT, 100 mM PMSF, 200 mM Na3VO4, 2 M BGP, and 1 mg/ml of leupeptin and aprotinin]. Proteins were quantified using a standard Bradford absorbance assay. Proteins were separated on 4–20% gradient SDS-PAGE gels and transferred to PVDF membrane by electrophoretic transfer. Membranes were subsequently blocked in 5% non-fat milk in TBST buffer (1 mM Tris Base (pH 8.0), 150 mM NaCl, 0.5% Tween-20) or 5% BSA in TBST buffer. All primary antibodies for Western blot detection were from Cell Signaling Technology Inc. (Danvers, MA) unless otherwise stated. Primary antibodies for Western blot detection of Akt (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA), phospho-Akt (1:1000), ERK 1/2 (1:1000), phospho-ERK 1/2 (1:1000), eIF4E (1:1000), phospho-eIF4E (1:1000), 4E-BP1 (1:1000), phospho-4E-BP1 (1:1000), p70S6K (1:1000), and phospho-p70S6K (1:1000) were applied overnight at 4°C. Secondary antibody for detection was either goat anti-rabbit IgG-HRP (1:4000, Santa Cruz Biotechnology Inc., Santa Cruz, CA) or goat anti-mouse IgG-HRP (1:4000, Santa Cruz Biotechnology Inc., Santa Cruz, CA). Proteins were detected via enhanced chemiluminescence detection system (Amersham Biosciences).
For Ki-67 and cleaved caspase-3 immunohistochemistry, tissue was fixed for 24 hours in 10% neutral buffered formalin, dehydrated through graded ethyl alcohols, paraffin infiltrated and embedded. Tissue sections were cut at 5 μm and mounted on glass slides. Slides were deparaffinized in xylene and hydrated through graded ethyl alcohols to water. Slides were washed with PBS three times. Nonspecific binding was blocked with 10% goat serum in PBS for one hour and endogenous peroxidase was blocked with 0.3% hydrogen peroxide in PBS for 10 minutes. Endogenous biotin was blocked with 0.001% avidin in PBS for ten minutes and the avidin quenched with 0.001% biotin in PBS for ten minutes. The slides were then incubated with either anti-Ki-67 (US Biological, Swampscott, MA) at a 1:800 dilution in PBS with 1% goat serum or anti-cleaved caspase-3 (Asp175) (Cell Signaling Technology Inc., Danvers, MA) at a 1:800 dilution in PBS with 1% goat serum overnight at 4° C. After washing with PBS, the sections were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at 1:200 in PBS for one hour at room temperature. Slides were washed in PBS and then incubated 30 minutes at room temperature with Vectastain ABC Elite (Vector Laboratories, Burlingame, CA). Following PBS wash, slides were developed with DAB (Vector Laboratories, Burlingame, CA) and counterstained with Mayer’s hematoxylin. All reagents and chemicals were from Sigma, St. Louis, MO unless otherwise noted.
Proliferation assays
A549 and MDA-MB-231 cells were plated at 1500 cells per well in 96 well plates and allowed to adhere overnight. Medium was aspirated and replaced with fresh media containing DMSO control, paclitaxel, rapamycin, 17-AAG, or drug combinations at 0–10,000 nM. Final DMSO concentration was 0.1%. Cells were allowed to grow for 72 hours and cell proliferation was quantified using the WST-1 cell proliferation reagent (Roche Applied Science, Indianapolis, IN) according to manufacturer’s instructions. Dose-effect analyses were conducted using CompuSyn (ComboSyn, Inc., Paramus, NJ).
In vivo tumor control studies
Athymic nude mice (6–8 week old females) were obtained from Harlan Laboratories (Madison, WI). The care and treatment of experimental animals was in accordance with institutional guidelines. A549 cells (2 × 106) were implanted subcutaneously in the flank. MDA-MB-231 cells (2 × 106) were implanted in the mammary fat pad. For tumor growth delay studies, treatment was started when tumor volume reached approximately 200 mm3 for A549 tumors or when tumors were clearly palpable for MDA-MB-231 tumors. Following randomization, tumor-bearing animals were treated with micelles alone, paclitaxel-containing micelles (60 mg/kg) or Triolimus (17-AAG 60 mg/kg, paclitaxel 60 mg/kg, rapamycin 30 mg/kg) on days 0, 4 and 8 by tail vein injection. In the A549 model, an additional group was treated with isotonic saline. Tumor volume was determined by caliper measurement and calculated by the formula, volume = 0.5 (large diameter)(small diameter)2.
In vivo toxicity and mechanistic studies
A549 tumor bearing animals (200 mm3) were treated with micelles alone, paclitaxel-containing micelles (60 mg/kg) or Triolimus (17-AAG 60 mg/kg, paclitaxel 60 mg/kg, rapamycin 30 mg/kg) on days 0, 4 and 8 by tail vein injection. At day 11, animals were sacrificed and whole blood and serum were collected. Normal tissues (lung, liver, kidney and heart) and tumor were dissected and formalin-fixed. Complete blood counts and comprehensive metabolic panels were performed by the Clinical Pathology Laboratory of the University of Wisconsin School of Veterinary Medicine. Normal and tumor tissues were sectioned, H&E stained and examined by a pathologist blinded to treatment group. Tumor tissues were additionally stained for Ki-67 and cleaved caspase-3 as described above. Ki-67- and cleaved caspase-3-positive cells were counted by an observer blinded to treatment group. Total cell number was quantified using Image J (National Institutes of Health, USA).
Statistical analyses
Data are presented as means ± standard error. Multi-group comparisons were conducted by ANOVA. When appropriate, Tukey’s HSD test was applied to account for multiple comparisons. For two group comparisons, Student’s t-test was employed.
Results
Paclitaxel, rapamycin and 17-AAG display independent and synergistic cytotoxicity in vitro
Paclitaxel, rapamycin and 17-AAG exerted independent cytotoxic effects in vitro in A549 and MDA-MB-231 cells with IC50 values ranging from the mid-nanomolar range to the low-micromolar range (Table 1). MDA-MB-231 cells were more resistant to rapamycin and 17-AAG compared to A549 cells. In both cell lines, the three-drug combination displayed statistically significant, potent synergy whereas the affects of two-drug combinations were more varied. The two-drug combination of paclitaxel and 17-AAG displayed antagonism in A549 cells (CI > 1) but was modestly synergistic in MDA-MB-231 cells (CI < 1). Other two-drug combinations displayed synergistic cytotoxicity in both cell lines. The three-drug combination, either in an equimolar ratio or in the molar ratio in Triolimus (paclitaxel:rapamycin:17-AAG = 2:1:3), displayed combination indices < 1 throughout the fractional affect range indicating this combination exerts synergistic cytotoxicity over a broad range of cell kill. Triolimus displayed the greatest synergy compared to other tested two- and three-drug combinations.
Table 1.
Drug combination effects: combination index analysis of paclitaxel, rapamycin and 17-AAG (N = 4–5 per condition)
| IC50 (nM) | CI at Fa10 | CI at Fa25 | CI at Fa50 | CI at Fa75 | CI at Fa90 | Molar Ratio | |
|---|---|---|---|---|---|---|---|
| A549 | |||||||
| Paclitaxel | 100 ± 50 | - | - | - | - | - | - |
| Rapamycin | 50 ± 6 | - | - | - | - | - | - |
| 17-AAG | 380 ± 100 | - | - | - | - | - | - |
| Paclitaxel:Rapamycin | 23 ± 1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 1:1 |
| Paclitaxel:17-AAG | 260 ± 70 | 2.5 ± 0.8 | 2.2 ± 0.7 | 2.0 ± 0.6 | 1.8 ± 0.5 | 1.6 ± 0.4 | 1:1 |
| Rapamycin:17-AAG | 36 ± 2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 1:1 |
| Paclitaxel:Rapamycin:17-AAG | 29 ± 2 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 1:1:1 |
| Paclitaxel:Rapamycin:17-AAG | 27 ± 2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 2:1:3 |
| MDA-MB-231 | |||||||
| Paclitaxel | 120 ± 20 | - | - | - | - | - | - |
| Rapamycin | 440 ± 100 | - | - | - | - | - | - |
| 17-AAG | 4200 ± 1300 | - | - | - | - | - | - |
| Paclitaxel:Rapamycin | 100 ± 10 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 1:1 |
| Paclitaxel:17-AAG | 140 ± 10 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 1:1 |
| Rapamycin:17-AAG | 380 ± 40 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 1:1 |
| Paclitaxel:Rapamycin:17-AAG | 110 ± 10 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 1:1:1 |
| Paclitaxel:Rapamycin:17-AAG | 110 ± 10 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 2:1:3 |
IC50, half maximal inhibitory concentration (for drug combinations, this represents total drug concentration); CI, combination index; FaX, X% maximal fractional affect. Values represent mean ± standard error. CI = 1, < 1, and > 1 indicates additivity, synergy, and antagonism, respectively.
Mechanisms of synergistic toxicity
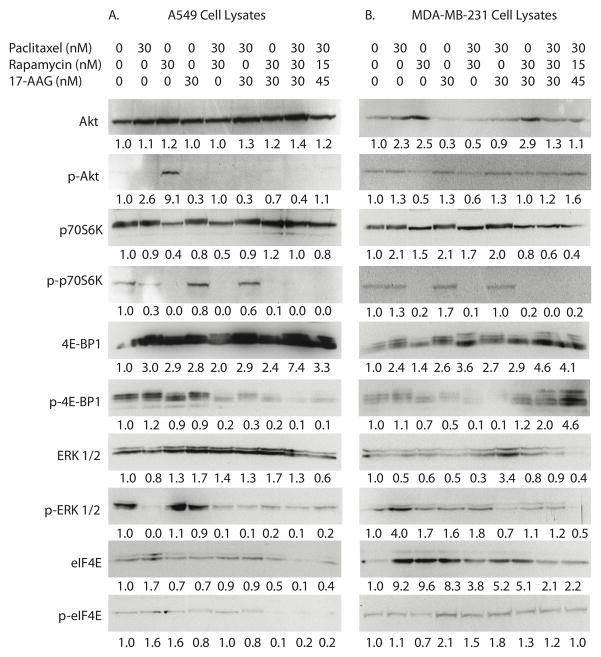
To identify potential mechanisms of synergistic cytotoxicity, A549 and MDA-MB-231 cells were treated with DMSO vehicle control (leftmost column), individual drugs or combinations. Expression and activation of key molecular targets in PI3K/Akt/mTOR and Ras/Raf/MAPK pathways were determined by Western blotting. In A549 cells, rapamycin monotherapy resulted in the expected inhibition of p70S6K phosphorylation (Figure 2A). Moreover, rapamycin monotherapy induced Akt activation. Inclusion of either paclitaxel or 17-AAG with rapamycin resulted in no change in mTOR inhibition as evidenced by persistent blockade of p70S6K phosphorylation. However, the addition of either paclitaxel or 17-AAG blocked compensatory Akt phosphorylation observed with rapamycin monotherapy. Paclitaxel, and all combination therapies, blocked basal ERK 1/2 activation whereas rapamycin and 17-AAG monotherapies did not. No Akt, p70S6K, 4E-BP1, ERK 1/2 or eIF4E activation was observed in cells treated with the three-drug combination. Responses to drug treatment differed in MDA-MB-231 cells (Figure 2B). Although rapamycin similarly inhibited p70S6K phosphorylation, compensatory Akt phosphorylation was not observed. Moreover, paclitaxel monotherapy appeared to modestly increase, rather than potently inhibit, ERK 1/2 phosphorylation. As in the case of A549 cells, MDA-MB-231 cells treated with the three-drug combination showed low levels of Akt, p70S6K, ERK 1/2 and eIF4E phosphorylation.
FIGURE 2. Expression and activation of PI3K/Akt/mTOR and Ras/Raf/MAPK pathway proteins in A549 (A) and MDA-MB-231 (B) cells in response to paclitaxel, rapamycin, 17-AAG and combinations.
Cells were exposed to the indicated concentrations of paclitaxel, rapamycin and/or 17-AAG for 24 hours. Total protein was collected and subjected to immunoblotting for the indicated proteins as described in Materials and Methods. Densitometric protein quantitation (normalized to untreated control cells) is shown.
Triolimus displays dramatic anti-tumor activity in vivo
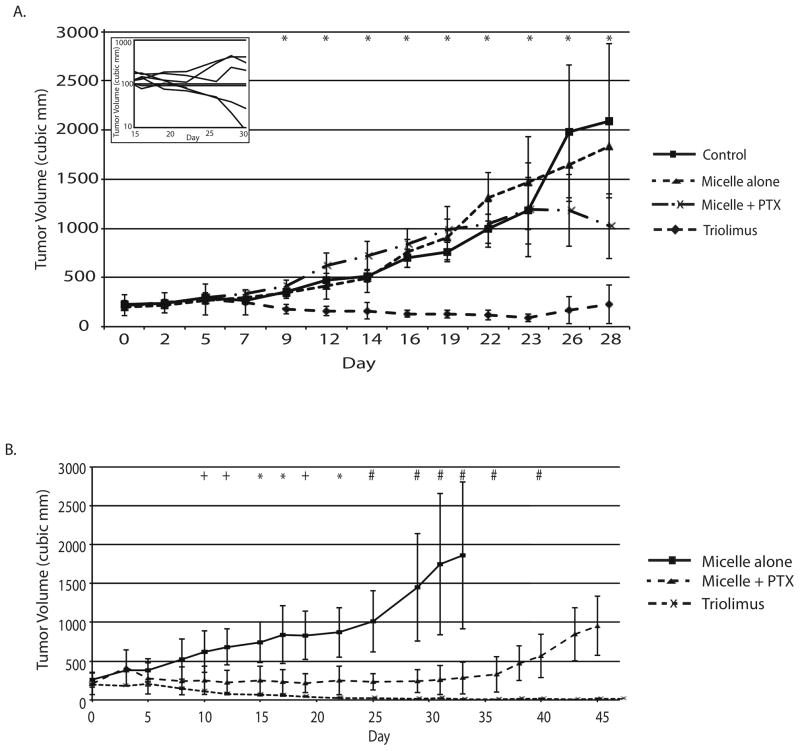
In A549 tumor bearing mice, statistically significant treatment effects were evident by day 9 and persisted throughout the course of the experiment (Figure 3A, ANOVA P < 0.05). Micelle alone treatment resulted in tumor growth indistinguishable from saline control. Paclitaxel-containing micelles had only modest effects on tumor growth with a statistically nonsignificant trend toward tumor growth delay evident late in the experimental course (Tukey’s HSD test, P > 0.05 at all timepoints). In contrast, Triolimus displayed potent and durable tumor control that differed significantly from saline control treatment from day 9 onward (Tukey’s HSD test, P < 0.05 at all timepoints).
FIGURE 3. Growth delay of A549 (A) and MDA-MB-231 (B) tumor xenografts by paclitaxel-containing micelles and Triolimus.
Mice bearing A549 flank (N ≥ 6 per group) or MDA-MB-231 mammary fat pad (N = 3 per group) tumors were treated with saline (A549 only), unloaded micelles, paclitaxel-containing micelles (60 mg/kg) or Triolimus (paclitaxel:rapamycin:17-AAG-loaded micelles (60:30:60 mg/kg)) by tail vein injection on days 0, 4 and 8. Tumor growth was determined as described in Materials and Methods. * P < 0.05, + P = 0.05 (ANOVA). Following day 23 MDA-MB-231 tumor volume measurements, a micelle alone treated animal required sacrifice due to large, symptomatic tumor. Thus, from day 25 on, paclitaxel-containing micelle group and Triolimus group were directly compared; # P < 0.05 (Student’s t-test). Inset displays individual A549 tumor volumes in Triolimus treated mice.
In MDA-MB-231 tumor bearing mice, statistically significant treatment effects were evident by day 15 (Figure 3B, ANOVA P < 0.05). Despite tumor delays with paclitaxel containing micelles, durable tumor control was not observed; all paclitaxel treated animals experienced persistent tumor regrowth by day 33. In contrast, Triolimus displayed much greater potency with durable tumor control. In fact, only one Triolimus treated animal eventually recurred with persistent tumor regrowth first evident at day 45. The remaining Triolimus treated animals were tumor-free over one year following treatment initiation.
Triolimus displays comparable toxicity to paclitaxel-containing micelles in vivo
Results of comprehensive metabolic and hematologic profiling following dosing with micelles alone, paclitaxel-containing micelles or Triolimus are shown in Table 2. Paclitaxel-containing micelles led to expected anemia with statistically significant reductions in hematocrit and hemoglobin of approximately 15%. Although not statistically significant, due to animal heterogeneity, reductions in white blood cell count were also observed. With the exception of mild hyperglycemia, Triolimus led to no obvious additional toxicity compared to paclitaxel-containing micelles. Interestingly, Triolimus effects on hemoglobin and hematocrit were less pronounced than paclitaxel-loaded micelles though this did not achieve statistical significance. Triolimus did induce mild hyperlipidemia compared to unloaded micelles with differences in total cholesterol reaching statistical significance.
Table 2.
Toxicity of Paclitaxel-containing Micelles and Triolimus (N = 6 per group).
| Micelle Alone (Mean ± SE) | Paclitaxel-containing Micelle (Mean ± SE) | Triolimus (Mean ± SE) | |
|---|---|---|---|
| Na (mM) | 144 ± 1 | 146 ± 1 | 148 ± 1 |
| K (mM) | 5.1 ± 0.5 | 4.1 ± 0.2 | 3.8 ± 0.2 |
| Cl (mM) | 112 ± 1 | 112 ± 1 | 116 ± 1 |
| CO2 (mM) | 22 ± 1 | 24 ± 1 | 22 ± 1 |
| Anion Gap (mM) | 15 ± 1 | 14 ± 1 | 14 ± 1 |
| Glucose (mg/dL) | 183 ± 18 | 202 ± 10 | 259 ± 7 # |
| BUN (mg/dL) | 22 ± 1 | 23 ± 1 | 18 ± 1 |
| Cr (mg/dL) | 0.2 ± 0.05 | 0.2 ± 0.02 | 0.2 ± 0.02 |
| Ca (mg/dL) | 9.1 ± 0.1 | 9.0 ± 0.1 | 9.2 ± 0.1 |
| Phos (mg/dL) | 6.1 ± 0.3 | 5.4 ± 0.2 | 5.9 ± 0.2 |
| Mg (mg/dL) | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| TProt (g/dL) | 4.7 ± 0.2 | 4.7 ± 0.1 | 4.7 ± 0.1 |
| Albumin (g/dL) | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 |
| AST (U/L) | 125 ± 25 | 66 ± 4 | 60 ± 4 |
| ALT (U/L) | 46 ± 8 | 32 ± 4 | 35 ± 3 |
| Alk Phos (U/L) | 68 ± 5 | 86 ± 9 | 99 ± 5 * |
| tBili (mg/dL) | ND | ND | ND |
| Total Chol (mg/dL) | 88 ± 5 | 107 ± 6 | 134 ± 7 * |
| Trig (mg/dL) | 82 ± 9 | 142 ± 30 | 140 ± 29 |
| HCT (%) | 42 ± 1 # | 36 ± 1 * | 39 ± 1 |
| Hgb (g/dL) | 13.8 ± 0.2 # | 11.5 ± 0.3* | 12.6 ± 0.2 |
| WBC (103/mm3) | 4.8 ± 1.3 | 1.8 ± 0.4 | 1.5 ± 0.4 |
| Platelets (103/mm3) | 1060 ± 186 | 1385 ± 183 | 1128 ± 86 |
Significantly different from micelle alone group (p < 0.0022).
Significantly different from paclitaxel-containing micelle group (p < 0.0022). Statistical significance of p < 0.0022 accounts for multiple comparison correction (Bonferroni correction for 23 comparisons). Uncorrected p values for all comparisons are available in Supplemental Table 1.
Renal function tests (BUN, Cr, electrolytes) revealed no acute renal toxicity with either paclitaxel-loaded micelle or Triolimus treatment. Similarly, liver function testing (AST, ALT, Alk Phos) revealed no differences between Triolimus and paclitaxel-containing micelles. Moreover, elevations in alkaline phosphatase seen with Triolimus compared to unloaded micelles were balanced by a trend toward lower transaminase levels (not significant). Unchanged serum CO2 levels and anion gap suggest neither paclitaxel-containing micelles nor Triolimus cause severe pulmonary toxicity.
Histologic assessment of normal organs (kidney, heart, liver and lungs) was accomplished in parallel. No treatment led to acute renal or cardiac toxicities evident on standard H&E evaluation (Supplemental Figure 1). In the micelle alone group, a single mouse demonstrated mild hepatic centrilobular chronic inflammation. Similarly, a single mouse in the Triolimus treated group demonstrated mild hepatic centrilobular chronic inflammation. No other signs of hepatic toxicity were evident in the remaining 16 animals. No drug-related pulmonary toxicity was evident. However, in five animals, small tumor deposits were identified in lungs. There was no obvious, treatment group-dependence to the presence of lung tumor deposits with lesions evident in 1, 2 and 2 of 6 animals in the micelle alone, paclitaxel-loaded micelle and Triolimus treated groups, respectively.
Triolimus-induced tumor growth delay is a consequence of both increased tumor cell apoptosis and reduced tumor cell proliferation
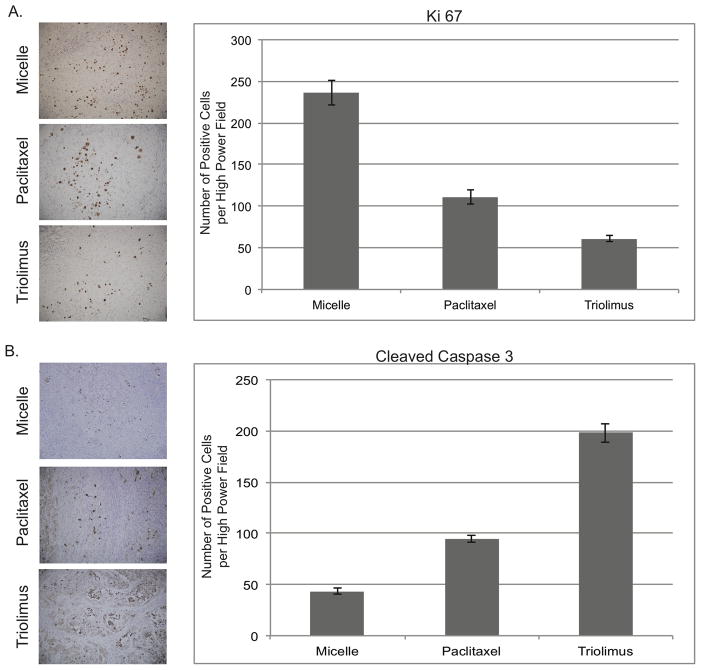
As shown in Figure 4A, Triolimus reduced tumor cell proliferation, assessed by Ki-67 staining, by nearly 80% compared to control treated animals. This compared to slightly more than a 50% reduction in proliferation induced by paclitaxel-containing micelles. Similar results were obtained when the percent Ki-67-positive cells was quantified (unloaded micelles 10.2 ± 0.8%; paclitaxel loaded micelles 5.0 ± 0.5%; Triolimus 2.3 ± 0.1% (ANOVA P < 0.0001, all pairwise comparisons P < 0.01 by Tukey’s HSD test)). In addition to the anti-proliferative effects, Triolimus more than quadrupled the number of apoptotic cells (Figure 4B) whereas paclitaxel-containing micelles approximately doubled the rate of apoptosis in tumor cells. Again, similar results were obtained when the percent cleaved caspase-3-positive cells was quantified (unloaded micelles 1.2 ± 0.1%; paclitaxel loaded micelles 3.1 ± 0.1%; Triolimus 6.1 ± 0.4% (ANOVA P < 0.0001, all pairwise comparisons P < 0.01 by Tukey’s HSD test)).
FIGURE 4. Impact of paclitaxel-containing micelles and Triolimus on tumor cell proliferation (A) and tumor cell apoptosis (B).
A549 tumor-bearing mice (N = 6 per group) were treated with unloaded micelles, paclitaxel-containing micelles (60 mg/kg) or Triolimus by tail vein injection on days 0, 4 and 8. Three days after final dosing, animals were sacrificed and tumors were dissected and fixed in formalin. Tumors were examined immunohistochemically for Ki-67- and cleaved caspase-3-positive cells. No more than 6 Ki-67-positive cells and 5 cleaved caspase-3-positive cells were identified per high power field in adjacent normal mouse tissue. Quantification and immunohistochemistry were performed as described in Materials and Methods. Differences were statistically significant (ANOVA P < 0.0001; Tukey’s HSD test P < 0.01 for all pairwise comparisons).
Discussion
This study examines the efficacy and safety of Triolimus, a novel systemic anti-neoplastic composed of micellized paclitaxel, rapamycin and 17-AAG, in tumor models. Using two widely employed, poorly differentiated, aggressive adenocarcinomas, A549 lung cancer and MDA-MB-231 breast cancer, Triolimus was subjected to evaluation in vitro and in vivo. Triolimus components exerted variable independent cytotoxic effects in both cell lines. As previously reported, paclitaxel displayed potent cytotoxic effects in both cell lines (14–16). Also consistent with prior reports, A549 cells proved relatively sensitive to both 17-AAG and mTOR inhibition whereas MDA-MB-231 cells proved relatively resistant (11,17,18). Combinations of paclitaxel, rapamycin and 17-AAG generally displayed synergistic cytotoxicity in both cell lines. Importantly, the three-drug combination, at the molar ratios of Triolimus, exerted the most potent synergistic cytotoxic effects in vitro in both A549 and MDA-MB-231 cells with combination indices less than 0.5.
Mechanisms of synergistic cytotoxicity were explored in both cell lines. In A549 cells, no single drug effectively blocked activation of both the PI3K/Akt/mTOR and the Ras/Raf/MAPK pathways. The mTOR target, p70S6K, was phosphorylated in A549 cells that were untreated or treated with paclitaxel or 17-AAG monotherapy. Similarly, ERK 1/2 was phosphorylated in untreated A549 cells as well as those treated with single agent rapamycin and 17-AAG. Drug combinations that prevented both p70S6K and ERK 1/2 phosphorylation proved synergistic. Of the drug combinations tested, only paclitaxel plus 17-AAG proved antagonistic in A549 cells and this regimen was the only combination tested that failed to block phosphorylation of p70S6K. Compensatory Akt phosphorylation was observed with rapamycin monotherapy as previously reported (19,20). Consistent with the rationale behind Triolimus design, as well as the synergistic cytotoxicity of 17-AAG and rapamycin, compensatory Akt phosphorylation driven by rapamycin was completely inhibited by the inclusion of 17-AAG.
Similar mechanisms of cytotoxic synergy in MDA-MB-231 cells are suggested. However, effects appear less straightforward. As with A549 cells, combinations that blocked both p70S6K and ERK 1/2 phosphorylation proved synergistic. However, the only drug combination that failed to inhibit activation of both of these key downstream targets, paclitaxel plus 17-AAG, also demonstrated mild cytotoxic synergy throughout the fractional effect range (CI = 0.6–0.7). Nonetheless, as in the case of A549 cells, this drug combination was the least synergistic of any combination tested. There are multiple potential explanations for the observed cell line differences including variations in basal activation of the PI3K/Akt/mTOR and the Ras/Raf/MAPK pathways, stark differences in cell sensitivity to monotherapies and alternative, unexplored, drug effects. Given the multitude of Hsp90 client proteins and the observation that the expression and activation of probed protein targets differed only modestly between three-drug treated and untreated MDA-MB-231 cells, it seems highly likely that unexplored drug effects are contributing to the synergistic activity observed.
Triolimus proved significantly more effective than paclitaxel-containing micelles in both the heterotopic A549 flank tumor model and the orthotopic MDA-MB-231 tumor model. In the latter, a single course of three Triolimus infusions resulted in tumor cures. Triolimus potency is attributable to marked increases in tumor cell apoptosis and concomitant reductions in tumor cell proliferation. These results are consistent with extensive preclinical data. Both 17-AAG and rapamycin have been shown to sensitize multiple cancer cell lines to paclitaxel-induced apoptosis (5, 21–24). Furthermore, all three agents have been implicated in cell cycle arrest and reductions in cell proliferation (25–27).
In vitro results suggest the striking activity of Triolimus in vivo may be a consequence of synergistic cytotoxicity. However, given the addition of both rapamycin (30 mg/kg) and 17-AAG (60 mg/kg) to paclitaxel (60 mg/kg), treatment intensification also likely contributes to the enhanced tumor growth delays observed with Triolimus. This treatment intensification is made possible through the use of the micellar delivery system. In the absence of PEG-PLA formulation, the maximum tolerated dose (MTD) of paclitaxel in mice is approximately 20 mg/kg in standard polyethoxylated castor oil (i.e. Cremophor EL, Taxol) (28). Triolimus permits simultaneous delivery of paclitaxel at three times this MTD as well as 17-AAG at approximately 75% of MTD and high doses of rapamycin (29–30). Each agent, when delivered parenterally, must be formulated with a solubilizer that can have independent toxicities. Paclitaxel is routinely dosed in polyethoxylated castor oil that has been associated with peripheral neurotoxicity and hypersensitivity reactions leading to potentially life-threatening events in up to 3% of patients (31). 17-AAG is commonly formulated in either polyethoxylated castor oil, with its attendant toxicities, or a dimethylsulfoxide/egg phospholipid (DMSO/EPL) vehicle. The latter has been implicated in significant gastrointestinal and other systemic toxicities (32,33). Additionally, preclinical mouse studies of paclitaxel in polyethoxlated castor oil and 17-AAG in DMSO/EPL demonstrated early deaths, attributable to additive vehicle toxicity, when the combination was delivered concurrently (34). Triolimus eliminates the need for multiple, unique drug vehicles, replacing them with non-toxic PEG-PLA. The use of PEG-PLA micelles in single drug delivery has shown considerable promise. Clinical trials of Genexol-PM, a PEG-PLA micelle formulation of paclitaxel in development by Samyang Research Corporation, have demonstrated superiority to conventional paclitaxel formulation through the obviation of premedication and safe dose-escalation (2,35). The use of PEG-PLA micelles for multi-drug delivery represents an attractive opportunity to leverage a novel drug delivery system for dose-escalated molecular multi-targeting.
A key clinical question when evaluating any dose-intensified regimen is whether improvements in efficacy are counterbalanced by increased toxicity thereby limiting the therapeutic ratio. Triolimus resulted in no animal deaths or significant weight loss in acute dosing studies (12). In acute toxicity studies in tumor bearing animals, Triolimus did not result in discernable histopathologic changes in kidney, heart, liver or lung. Similarly, comprehensive metabolic profiling demonstrated that Triolimus caused no clinically significant metabolic toxicity. Renal and liver function testing were essentially unremarkable compared to both paclitaxel-containing micelles and vehicle control. Alkaline phosphatase was modestly elevated with Triolimus treatment, however, total bilirubin remained undetectable, transaminases were unchanged (to lower) and hepatic synthetic function (albumin, total protein) was unmodified. Mild hyperlipidemia, including statistically significant hypercholesterolemia, and hyperglycemia were evident in Triolimus treated animals. As hyperlipidemia and hyperglycemia are dose-dependent side-effects of rapamycin and 17-AAG, respectively, these biochemical changes provide circumstantial evidence for the activity of these pharmacophores following Triolimus infusion and may provide useful markers for dosing strategies during clinical development (36,37).
Myelosuppression is a dose-limiting toxicity of taxanes and paclitaxel-containing micelles did result in hematologic toxicity including mild anemia and reductions in white blood cell counts. Triolimus did not enhance myelosuppression compared to paclitaxel-containing micelles. In fact, no anemia or thrombocytopenia was evident in Triolimus treated animals. Reductions in white blood cell counts were comparable in both paclitaxel and Triolimus treated groups but did not achieve statistical significance in part due to considerable variability in control animals. The absence of statistically significant neutropenia must be caveated by the recognition that host animals are immunosuppressed at baseline and ongoing studies are defining the hematologic toxicity of Triolimus in immunocompetent animals. More globally, potential disconnects between murine tolerability and human safety as well as extensive regulatory requirements necessitate further safety testing prior to advancing to human studies. Nonetheless, these data suggest Triolimus widens the therapeutic ratio compared to paclitaxel monotherapy.
Triolimus represents a novel systemic therapy that permits simultaneous, dose-escalated targeting of the microtubule apparatus, mTOR and Hsp90. Triolimus displays synergistic cytotoxicity in vitro, effectively inhibits the PI3K/Akt/mTOR and Ras/Raf/MAPK pathways, has impressive in vivo anti-tumor activity and is associated with modest toxicity. Results of these studies provide a strong foundation for further preclinical and clinical development of Triolimus and provide a proof of concept for the use of polymeric micelle nanocarriers in the rational design of combination cancer therapy.
Moreover, Triolimus reflects an emerging, and key, concept in combinatorial, cancer medicine. The accelerating pace of molecular target (and drug) identification, coupled with a growing understanding of molecular resistance mechanisms, continuously provides evidence to support novel drug combinations. In parallel, the development of a wide array of nanomaterials for clinical use offers new opportunities to deliver drug combinations (38–40). Insightful matching of pharmacophores with drug delivery vehicle will be essential in therapeutic design. For example, the PEG-PLA micellar construct of Triolimus is well-suited for efficient solubilization of the three synergistic, hydrophobic agents employed here (12,13,41). However, several rational drug combinations can be envisioned that do not share the physicochemical properties of paclitaxel, rapamycin and 17-AAG. In such cases, alternative drug delivery vehicles may prove superior to micelles. For example, Ahmed and colleagues endeavored to develop a therapeutic capable of simultaneously delivering the hydrophobic agent paclitaxel and the hydrophilic cytotoxin doxorubicin. In this case, biodegradable polymersomes were effectively exploited to solubilize paclitaxel in the thick polymersome hydrophobic membrane while encapsulating doxorubicin in the aqueous vesicular lumen (42,43). This illustrates the critical need for close collaboration of oncologists, biologists and pharmaceutical scientists in maximizing clinical progress (44).
Supplementary Material
Acknowledgments
GRANT SUPPORT:
Supported in part by NIH Grants 1UL1RR25011 (K. R. Kozak) and R21CA161537 (G. S. Kwon)
Footnotes
The authors have no conflicting financial interests
References
- 1.Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. N Engl J Med. 2011;364:985–7. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 2.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–16. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 3.Lim WT, Tan EH, Toh CK, Hee SW, Leong SS, Ang PC, et al. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol-PM) in patients with solid tumors. Ann Oncol. 2010;21:382–8. doi: 10.1093/annonc/mdp315. [DOI] [PubMed] [Google Scholar]
- 4.Xiao RZ, Zeng ZW, Zhou GL, Wang JJ, Li FZ, Wang AM. Recent advances in PEG-PLA block copolymer nanoparticles. Int J Nanomedicine. 2010;5:1057–65. doi: 10.2147/IJN.S14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10:7031–42. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 6.VanderWeele DJ, Zhou R, Rudin CM. Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol Cancer Ther. 2004;3:1605–13. [PubMed] [Google Scholar]
- 7.Wu P, Hu YZ. PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress. Curr Med Chem. 2010;17:4326–41. doi: 10.2174/092986710793361234. [DOI] [PubMed] [Google Scholar]
- 8.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis LK, Alsayed Y, Leleu X, Jia X, Singha UK, Anderson J, et al. Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res. 2006;12:6826–35. doi: 10.1158/1078-0432.CCR-06-1331. [DOI] [PubMed] [Google Scholar]
- 11.Roforth MM, Tan C. Combination of rapamycin and 17-allylamino-17-demethoxygeldanamycin abrogates Akt activation and potentiates mTOR blockade in breast cancer cells. Anticancer Drugs. 2008;19:681–8. doi: 10.1097/CAD.0b013e3283067681. [DOI] [PubMed] [Google Scholar]
- 12.Shin HC, Alani AW, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol Pharm. 2011;8:1257–65. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin HC, Alani AW, Rao DA, Rockich NC, Kwon GS. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J Control Release. 2009;140:294–300. doi: 10.1016/j.jconrel.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis A, Allerston CK, Jia H, Herzog B, Garza-Garcia A, Winfield N, et al. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J Med Chem. 2010;53:2215–26. doi: 10.1021/jm901755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS Pharm Sci Tech. 2008;9:486–93. doi: 10.1208/s12249-008-9063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Li H, Zuo M, Zhang Y, Liu H, Fang W, et al. Lx2-32c, a novel taxane and its antitumor activities in vitro and in vivo. Cancer Lett. 2008;268:89–97. doi: 10.1016/j.canlet.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005;65:7446–54. doi: 10.1158/0008-5472.CAN-04-4267. [DOI] [PubMed] [Google Scholar]
- 18.Ohara T, Takaoka M, Toyooka S, Tomono Y, Nishikawa T, Shirakawa Y, et al. Inhibition of mTOR by temsirolimus contributes to prolonged survival of mice with pleural dissemination of non-small-cell lung cancer cells. Cancer Sci. 2011;102:1344–9. doi: 10.1111/j.1349-7006.2011.01967.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 21.Faried LS, Faried A, Kanuma T, Nakazato T, Tamura T, Kuwano H, et al. Inhibition of the mammalian target of rapamycin (mTOR) by rapamycin increases chemosensitivity of CaSki cells to paclitaxel. Eur J Cancer. 2006;42:934–47. doi: 10.1016/j.ejca.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Munster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. See: E. A Sausville, Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters, Clin Cancer Res, 7: 2155–2158, 2001. Clin Cancer Res. 2001;7:2228–36. [PubMed] [Google Scholar]
- 23.Sain N, Krishnan B, Ormerod MG, De Rienzo A, Liu WM, Kaye SB, et al. Potentiation of paclitaxel activity by the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin in human ovarian carcinoma cell lines with high levels of activated AKT. Mol Cancer Ther. 2006;5:1197–208. doi: 10.1158/1535-7163.MCT-05-0445. [DOI] [PubMed] [Google Scholar]
- 24.Shafer A, Zhou C, Gehrig PA, Boggess JF, Bae-Jump VL. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int J Cancer. 2010;126:1144–54. doi: 10.1002/ijc.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–6. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- 26.Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91:1420–4. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hruban RH, Yardley JH, Donehower RC, Boitnott JK. Taxol toxicity. Epithelial necrosis in the gastrointestinal tract associated with polymerized microtubule accumulation and mitotic arrest. Cancer. 1989;63:1944–50. doi: 10.1002/1097-0142(19890515)63:10<1944::aid-cncr2820631013>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001;72:191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 29.Burger AM, Fiebig HH, Stinson SF, Sausville EA. 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. Anticancer Drugs. 2004;15:377–87. doi: 10.1097/00001813-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 31.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–85. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 32.Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–41. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 33.Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13:1775–82. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139–44. [PubMed] [Google Scholar]
- 35.Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18:2009–14. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 36.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–91. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 37.Nowakowski GS, McCollum AK, Ames MM, Mandrekar SJ, Reid JM, Adjei AA, et al. A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin Cancer Res. 2006;12:6087–93. doi: 10.1158/1078-0432.CCR-06-1015. [DOI] [PubMed] [Google Scholar]
- 38.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967–73. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 39.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363:2434–43. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 41.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27:2569–89. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–8. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. 2006;3:340–50. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 44.Author websites: Kozak Laboratory: http://www.humonc.wisc.edu/index.php/Kozak_Lab and Kwon Laboratory: http://apps.pharmacy.wisc.edu/sopdir/39
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.