Abstract
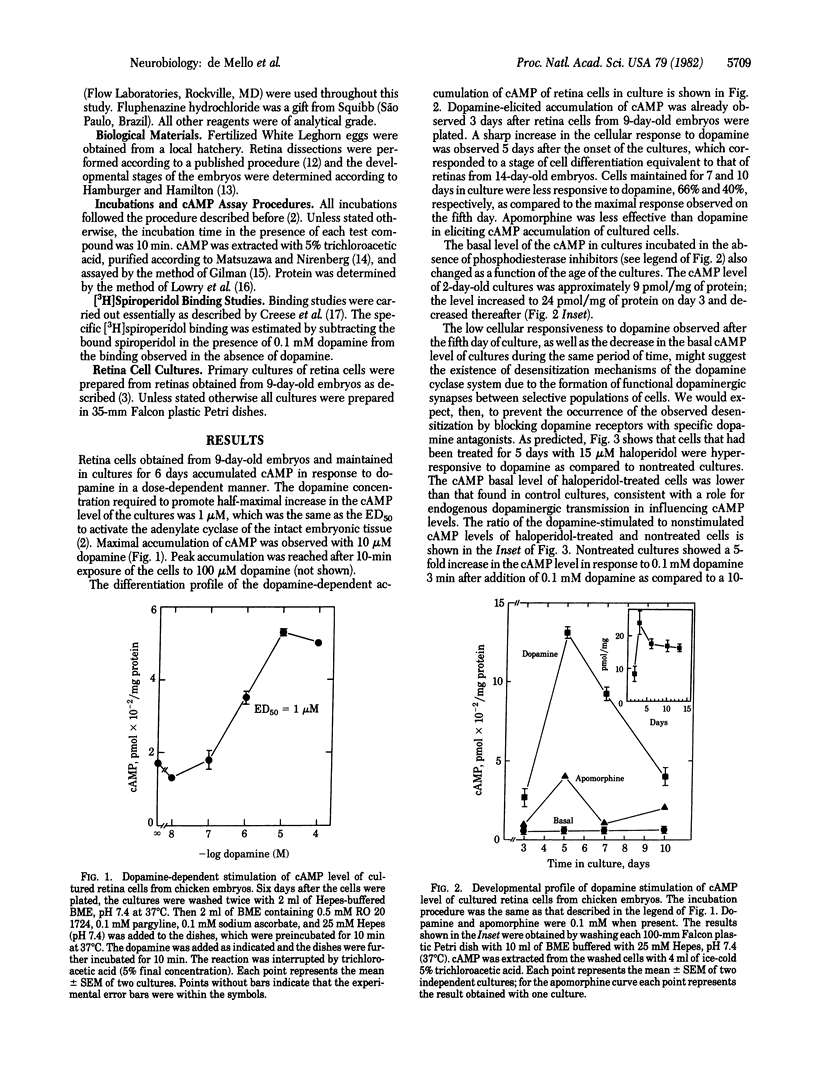
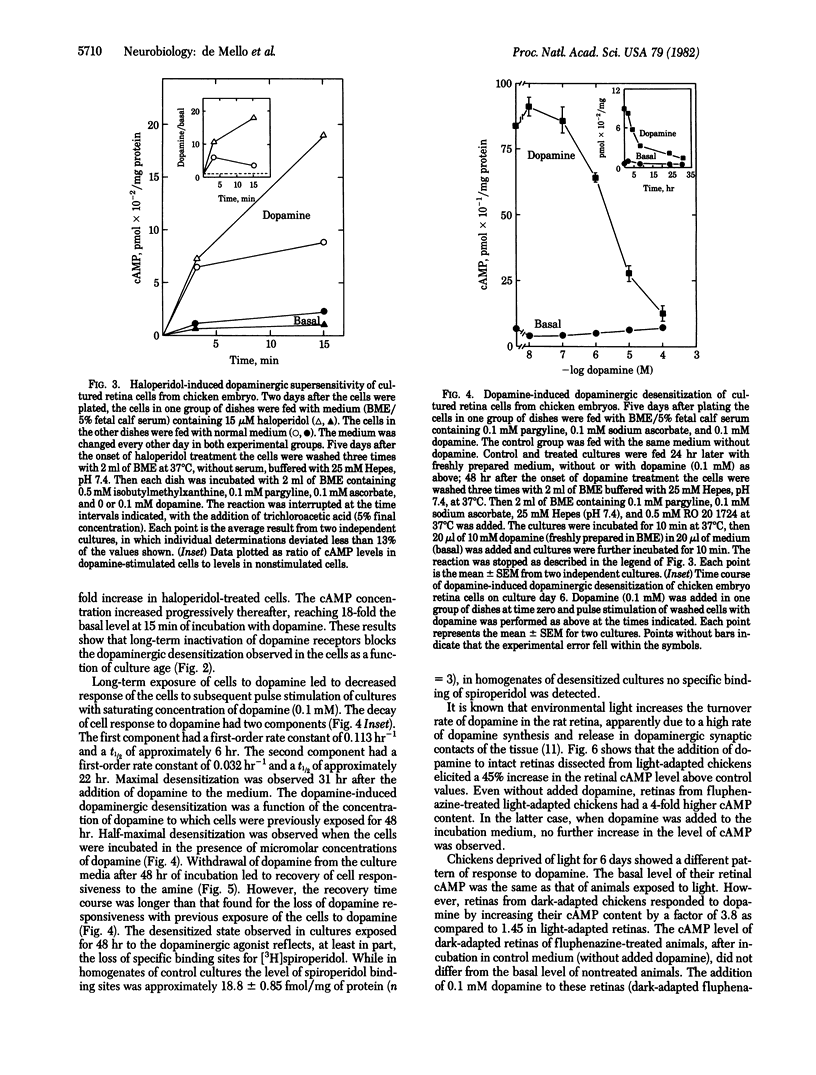
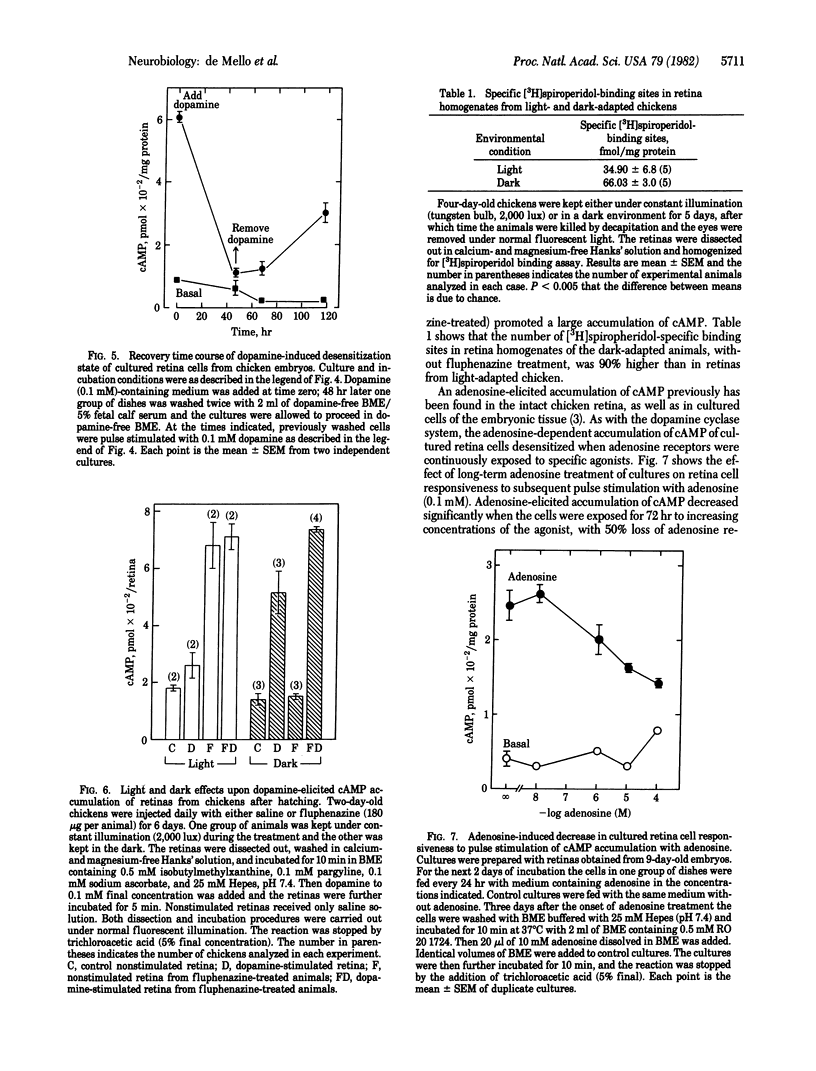
We have obtained evidence that receptor-stimulated adenylate cyclase activity [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] is regulated physiologically in both embryonic and mature neurons. In a series of experiments using cultured retina cells from chicken embryos, we found that dopamine-sensitive adenylate cyclase activity spontaneously desensitized as cultures differentiated. The cellular response to dopamine reached a maximum after 5 days in culture and then decreased to 40% during the next 5 days. This spontaneous desensitization appeared to be caused by functional dopaminergic transmission because it could be blocked by the dopamine antagonist haloperidol. The ability of added dopamine at 100 microM to cause near-complete desensitization is consistent with this conclusion. Pharmacologically induced desensitization required 31 hr for maximal effect and was half-maximal at 1-10 microM dopamine. Analogous desensitization of the adenosine-dependent adenylate cyclase system also was noted. When dopamine was removed from the medium of chronically treated cultures, cells resensitized to subsequent stimulation at a very slow rate. Resensitization likely depended on replacement of dopamine receptors because chronic dopamine treatment caused the disappearance of binding sites for the ligand [3H]spiroperidol. In a second series of experiments, using hatched animals, we found that similar regulation of dopamine receptor binding sites and activity could be elicited by manipulation of environmental light, a treatment thought to influence dopaminergic transmission. Retinas from animals in constant light had less specific [3H]spiroperidol binding (35 fmol/mg of protein) than did retinas from animals in constant darkness (66 fmol/mg of protein) and made less cAMP in response to added dopamine. Our results indicate that regulation of the dopamine receptor system begins early in development and continues to function in mature synapses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burt D. R., Creese I., Snyder S. H. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977 Apr 15;196(4287):326–328. doi: 10.1126/science.847477. [DOI] [PubMed] [Google Scholar]
- Creese I., Burt D. R., Snyder S. H. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977 Aug 5;197(4303):596–598. doi: 10.1126/science.877576. [DOI] [PubMed] [Google Scholar]
- Creese I., Sibley D. R. Receptor adaptations to centrally acting drugs. Annu Rev Pharmacol Toxicol. 1981;21:357–391. doi: 10.1146/annurev.pa.21.040181.002041. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- De Mello F. G. The ontogeny of dopamine-dependent increase of adenosine 3',5'-cyclic monophosphate in the chick retina. J Neurochem. 1978 Oct;31(4):1049–1053. doi: 10.1111/j.1471-4159.1978.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone P. M., Galli C. L., Garrison-Gund C. K., Neff N. H. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978 Nov 24;202(4370):901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuzawa H., Nirenberg M. Receptor-mediated shifts in cGMP and cAMP levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3472–3476. doi: 10.1073/pnas.72.9.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse S. Dopamine and the pharmacology of schizophrenia: the state of the evidence. J Psychiatr Res. 1974;11:107–113. doi: 10.1016/0022-3956(74)90081-8. [DOI] [PubMed] [Google Scholar]
- Matthysse S., Lipinski J. Biochemical aspects of schizophrenia. Annu Rev Med. 1975;26:551–565. doi: 10.1146/annurev.me.26.020175.003003. [DOI] [PubMed] [Google Scholar]
- Paes de Carvalho R., de Mello F. G. Adenosine-elicited accumulation of adenosine 3', 5'-cyclic monophosphate in the chick embryo retina. J Neurochem. 1982 Feb;38(2):493–500. doi: 10.1111/j.1471-4159.1982.tb08655.x. [DOI] [PubMed] [Google Scholar]
- Piddington R., Moscona A. A. Correspondence between glutamine synthetase activity and differentiation in the embryonic retina in situ and in cultrue. J Cell Biol. 1965 Oct;27(1):247–252. doi: 10.1083/jcb.27.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., De Mello F. G., Nirenberg M. Synapse turnover: the formation and termination of transient synapses. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4977–4981. doi: 10.1073/pnas.74.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Sporn J. R., Harden T. K., Wolfe B. B., Molinoff P. B. beta-Adrenergic receptor involvement in 6-hydroxydopamine-induced supersensitivity in rat cerebral cortex. Science. 1976 Nov 5;194(4265):624–626. doi: 10.1126/science.10626. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Daniels M. P., Nirenberg M. Muscarinic acetylcholine receptors of the developing retina. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5524–5528. doi: 10.1073/pnas.74.12.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Daniels M. P., Nirenberg M. Synapse and acetylcholine receptor synthesis by neurons dissociated from retina. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2370–2374. doi: 10.1073/pnas.73.7.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]


