Abstract
Assessment of antilipolytic insulin action is important in obesity research, but extensive isotopic tracer studies are not always feasible. We evaluated whether an index of antilipolytic insulin action could be derived from readily available insulin and glycerol concentrations obtained during clamps or oral glucose tolerance tests (OGTT). We evaluated data collected from 29 subjects who had undergone a 3-stage hyperinsulinemic-euglycemic clamp (4, 8, and 40 mU/m2/min) with infusion of [2H5]glycerol to calculate the glycerol rate of appearance (GLYRA). Exponential decay curves for GLYRA across insulin concentrations were generated for each individual and suppression of lipolysis was calculated as the insulin concentration needed to half-maximally suppress GLYRA (GLYRA EC50). Areas under the curve for glycerol (GLYAUC) and insulin (INSAUC) were calculated and their products (INSAUC×GLYAUC) were calculated as an index of insulin suppression of lipolysis. The clamp index was highly correlated with GLYRA EC50 (r = 0.862, p<0.001), as was an OGTT-derived index (r = 0.720, p<0.01). These findings suggest that the products of the insulin and glycerol AUC from either a clamp or an OGTT are good biomarkers of the antilipolytic action of insulin and are comparable to direct measurement by isotopic tracer methods.
Introduction
Insulin resistance is critical in the early development of type 2 diabetes. Thus, the measurement of insulin sensitivity has been extensively studied using a variety of techniques. Much of the focus on insulin sensitivity has been on glucoregulatory insulin action (i.e., primarily skeletal muscle insulin sensitivity), with less emphasis on antilipolytic insulin action (i.e., primarily adipose tissue insulin sensitivity).
Currently, the reference method for assessing glucoregulatory insulin action is the hyperinsulinemic-euglycemic clamp, which measures glucose disposal rate (GDR) during a high-dose (40 mU/m2/min) insulin infusion. This clamp technique can also be used to assess the antilipolytic action of insulin using lower-dose insulin infusions (e.g., 4 or 8 mU/m2/min) in conjunction with stable isotope glycerol tracers. The decrease in glycerol rate of appearance (GLYRA) from basal to steady-state low-dose insulin is an index of the suppression of lipolysis; multiple low-dose insulin steps are used to construct decay curves to assess the insulin concentration needed to half-maximally suppress GLYRA (EC50). However, such clamp methods are time-consuming, expensive, and simply not feasible in large populations. Thus, considerable attention has been given to finding indices of insulin action that can be derived from less costly and less complicated tests.
There are a number of good indices for assessing glucoregulatory insulin action using glucose and insulin concentrations obtained from fasted or oral glucose tolerance tests (OGTT)(1, 2). However, few studies have considered equivalent indices for assessing the antilipolytic action of insulin (3). Because the product of the insulin and glucose areas under the curve during an OGTT are a good index of peripheral glucoregulatory insulin action, we hypothesized that the product of insulin and glycerol areas may also be a good index of antilipolytic insulin action.
Methods
Subjects
We evaluated data from two different studies of healthy postmenopausal women (n=29) not on hormone therapy. Postmenopausal status was defined as cessation of menses for at least one year or hysterectomy with an FSH >30 mIU/mL. Women were excluded from the study if they had a history of hormone-sensitive cancer, fasted plasma glucose >126 mg/dL, uncontrolled hypertension (resting systolic blood pressure >150 mmHg or diastolic >90 mmHg), thyroid dysfunction (TSH <0.5 or >5.0 µU/L), hypertriglyceridemia (fasting triglycerides >400 mg/dL), or abnormal liver or renal function. All participants provided written informed consent to participate in the studies, which were approved by the Colorado Multiple Institutional Review Board.
Hyperinsulinemic, euglycemic clamp
Three-stage (4, 8 and 40 mU/m2/min) hyperinsulinemic-euglycemic clamps were administered according to the methods of DeFronzo et al (4). Clamps were performed on the Clinical Translational Research Center (CTRC) following a, 3-day standardized diet and a 12-hour fast. An IV catheter was placed in an antecubital vein for the infusion of insulin and 20% dextrose. A second catheter was placed retrograde to venous flow in the contralateral hand for blood sampling. The hand was kept in a warming box maintained at 60°C to produce arterialized blood samples (5). After the 90-minute basal period, insulin was infused at a priming dose for 10 minutes and then at a constant rate of 4 mU/m2/min for 80 minutes. For the second and third stages, the insulin infusion was increased to 8 and 40 mU/m2/min for each 90 minute stage. Additionally, a primed (1.5 µmol/kg), constant (~0.1 µmol/kg/min) infusion of [2H5]glycerol (Cambridge Isotope Laboratories, Inc., Andover, MA) was delivered throughout the 360 min to measure whole-body lipolysis. Plasma glucose was measured bedside every 5 minutes on an automated glucose analyzer (Analox Instruments, Lunenburg, MA). The dextrose infusion was adjusted to maintain plasma glucose at 90 mg/dL. Blood samples were collected at time 0 (fasting) and at 60, 75 and 90 minutes of the basal period and each insulin stage for determination of insulin, and glycerol (concentration and isotope enrichment). Steady-state plasma insulin (I) and whole body glucose disposal rate (M) were determined from the average insulin concentrations and steady-state glucose infusion rates, respectively, during the final 30 minutes of the 40 mU/m2/min dose insulin infusion. Whole body glucoregulatory insulin action was calculated as steady-state glucose disposal adjusted for insulin concentrations (M/I).
Plasma glycerol concentrations were measured by the CTRC Core laboratory. The analysis of [2H5]glycerol was done by the Colorado Clinical Nutrition Research Unit Mass Spectrometry Core Laboratory using a modification of the negative ion chemical ionization gas chromatography-mass spectrometry as previously described (6). The average rate of appearance of glycerol (GLYRA) over the last 30 minutes of each stage was calculated using the steady-state equation of Steele (7): GLYRA = F/ Ep; where F is the rate of infusion for [2H5]glycerol (0.10 µmol/kg/min), and E is the plateau plasma isotope enrichment.
Glucose tolerance test
A subset of the women (n=16) also had a 2-hour 75-g OGTT administered in the morning after an overnight fast. Blood samples were obtained before and 30, 60, 90, and 120 min after glucose ingestion for glucose, insulin, and glycerol determinations.
Data analysis
Exponential decay curves for GLYRA across the range of insulin concentrations were generated for each individual and suppression of lipolysis was calculated as the insulin concentration needed to half-maximally suppress GLYRA (GLYRA EC50). We were unable to generate exponential decay curves for 6 of the women due to missing enrichment values at one of the 3 insulin stages. Therefore, we also generated decay curves using glycerol concentrations ([GLY]) for all 29 women to calculate [GLY] EC50. Data are presented using both EC50 measures.
Total areas under the curve (AUC) for glycerol (GLYAUC) and insulin (INSAUC) during the clamps were calculated using the trapezoidal method. Likewise, total AUCs for glucose (GLUAUC), insulin (INSAUC), and glycerol (GLYAUC) during the OGTTs were calculated using the trapezoidal method.
GLYRA EC50 was the reference measure of insulin suppression of lipolysis. Steady-state glucose disposal adjusted for insulin concentrations (M/I) was the reference measure of insulin-mediated glucose uptake. The products of the clamp and OGTT insulin and glycerol areas (INSAUCxGLYAUC) were calculated as indices of insulin-mediated suppression of lipolysis. The product of the fasting insulin and glycerol concentrations (INS0xGLY0) was calculated as an index of basal antilipolytic insulin action. The products of the OGTT insulin and glucose areas (INSAUCxGLUAUC) were calculated as indices of insulin-stimulated glucose uptake. Pearson correlations were used to evaluate the strength of the relation between the reference measure and corresponding index. All statistical analyses were performed using PASW software (v18.0, SPSS, Inc., Chicago, IL).
Results
Study subjects were sedentary and overweight or mildly obese (mean±SD; BMI 30±5 kg/m2), but otherwise healthy, non-diabetic postmenopausal women (56±4 yr). Of the 29 women, 6 had elevated fasting glucose (101 – 118 mg/dL range). On average, the insulin concentration (EC50) needed to half-maximally suppress lipolysis (GLYRA) in these women was 11.6 µU/mL (Supplementary Figure 1, online). The 8 mU/m2/min dose of insulin resulted in near maximal suppression of lipolysis whereas the 4 mU/m2/min insulin infusion was, in most cases, sufficient to half-maximally suppress lipolysis.
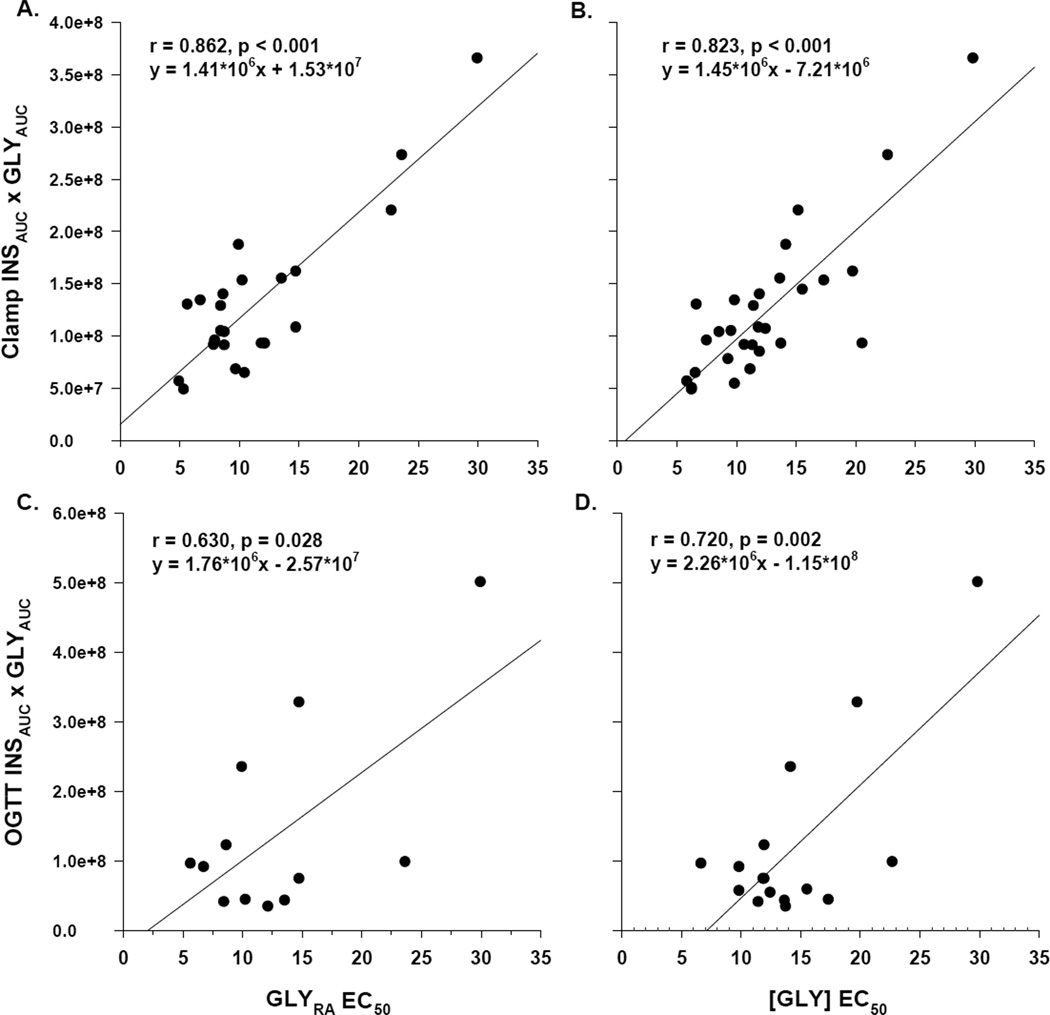
The clamp INSAUCxGLYAUC index correlated with the EC50 calculated from GLYRA (Figure 1, Panel A) and from [GLY] (Figure 1, Panel B); the strong correlations suggest good agreement between the INSAUCxGLYAUC index and the reference method. Likewise, the OGTT INSAUCxGLYAUC index (n=16) correlated with the EC50 calculated from GLYRA (Figure 1, Panel C) and from [GLY] (Figure 1, Panel D). The product of fasting insulin and glycerol concentrations (INS0xGLY0) obtained the morning of the clamp was also strongly correlated with antilipolytic insulin action (EC50 for [GLY]; r=0.868, p<0.001) and inversely correlated with glucoregulatory insulin action (M/I; r=−0.724, p<0.001). Although the correlations were not as strong as those observed with the clamp-derived index, there was good agreement between the OGTT-derived INSAUCxGLYAUC index and the reference method. Indeed, the correlation was similar to that observed between the OGTT-derived index of glucoregulatory insulin action (INSAUCxGLUAUC) and the reference clamp-derived M/I (r=−0.539, p<0.05).
Figure 1.
Correlation of indices versus reference measures of antilipolytic insulin action. The clamp-derived index (product of insulin and glycerol areas) was correlated with the directly measured EC50 for glycerol rate of appearance (panel A) and glycerol concentration (panel B). Likewise, in a subgroup of women, the OGTT-derived index was correlated with the directly measured EC50 for glycerol rate of appearance (panel C) and glycerol concentration (panel D).
Discussion
The findings suggest that the products of the insulin and glycerol AUC from either a clamp or an OGTT are good biomarkers of the antilipolytic action of insulin as measured via isotopic tracer methods. This has important implications for studies that lack the resources for analyzing isotopic tracers and performing extensive multi-stage clamps. For large studies that have the ability to perform glucose tolerance tests but not clamps, the measurement of glycerol and insulin provides a simple way to estimate antilipolytic insulin resistance from OGTT data. For studies that perform clamps, but do not have the mass spectrometry resources to analyze isotopic tracers, glycerol concentrations during a clamp may suffice. Moreover, the product of fasting insulin and fasting glycerol appeared to be an excellent surrogate index of insulin resistance, consistent with that previously reported for the product of fasting free fatty acid and insulin (8). Such indices of antilipolytic insulin action will complement those used to assess glucoregulatory insulin action, providing a more comprehensive measure of systemic insulin resistance.
The generalizability of these results are limited. We studied a small number of sedentary, overweight, relatively healthy, postmenopausal women. Thus, these indices of antilipolytic insulin action will need to be validated: 1) in larger, more diverse populations that have a wider range of insulin action (i.e., exercise-trained thru diabetes); and 2) prospectively across interventions known to improve insulin action (e.g., exercise training, diabetes medications). Nevertheless, the good correlations between the indices and the referent isotope methods suggest that they are good estimates of antilipolytic insulin action and potentially useful in larger studies with limited resources.
Supplementary Material
Acknowledgments
The authors wish to thank the staffs of the UCD Clinical Translational Research Center (CTRC) and Energy Balance Core of the Nutrition and Obesity Research Center (NORC) for their assistance in conducting this study. The authors would also like to thank the members of their research group for carrying out the day-to-day activities of the project and the study volunteers for their time and efforts. The following awards from the National Institutes of Health supported this research: R01 AG018198 and AG018857, K01 AG019630 (REV), F32 AG005899 (WSG), M01 RR000051 (CTRC), P30 DK048520 (NORC).
Footnotes
Conflict of Interest
The authors have no conflicts to disclose.
References
- 1.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30(1):89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral gluocse tolerance testing. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, DeFronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the antilipolytic effect of insulin. Acta Diabetol. 2008;45(3):147–150. doi: 10.1007/s00592-008-0033-z. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, MacDonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35(3):287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- 6.Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers. Anal Biochem. 1992;205:172–178. doi: 10.1016/0003-2697(92)90595-x. [DOI] [PubMed] [Google Scholar]
- 7.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, DeFronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the antilipolytic effect of insulin. Acta Diabetol. 2008;45(3):147–150. doi: 10.1007/s00592-008-0033-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.