Abstract
There is a lack of multi-center cost-identification studies for hematopoietic cell transplantation (HCT). We used a single longitudinal administrative claims database representing a national, commercially insured population to evaluate the feasibility of identifying HCT recipients and to establish a cohort of autologous and allogeneic HCT recipients to study inpatient and outpatient direct medical costs from transplant hospitalization through first 100 days post-transplantation. Using ICD-9 procedure and diagnosis codes, we identified 3,365 patients who had received their first transplant in the United States between 2007 and 2009 (autologous-1,678, allogeneic-1,320, graft source not specified-367). The median 100-day total costs for autologous HCT were $99,899 (interquartile range [IQR], $73,914–140,555) and for allogeneic HCT were $203,026 (IQR, $141,742–316,426). The majority of costs (>75%) occurred during the initial transplant hospitalization for both autologous and allogeneic HCT recipients. Costs were greater among pediatric (≤ 20 years) compared to adult (>20 years) recipients and this difference was more pronounced with allogeneic HCT. Using a claims database representing a national HCT population, we highlight the high costs associated with autologous and allogeneic HCT. Our study lays the foundation for using claims data for future research on economic aspects of HCT.
Keywords: Hematopoietic cell transplantation, Autologous, Allogeneic, Costs, Hospitalization
INTRODUCTION
Hematopoietic cell transplantation (HCT) is the preferred and potentially curative therapy for many patients with high-risk hematologic diseases. However, it is a highly specialized, resource intense and costly medical procedure. A recent report from the Agency for Healthcare Research and Quality highlighted that despite being a relatively uncommon procedure, HCT was among the top ten procedures with the highest increase in hospital costs from 2004 to 2007 in the United States (US); total national costs of HCT hospitalization increased from $694 million to $1.3 billion over this timeperiod.1
The costs of HCT in the US have been described previously in the literature.2–9 However, studies have been limited to single institution reports, have used different methodologies and time horizons to describe costs of transplantation, and have usually excluded costs of care outside the transplant center (e.g., home care, outpatient pharmacy services, and hospitalization in another center). There also exists substantial practice variation in HCT.10–12 Because of these limitations, the prevailing literature makes it difficult to compare costs of HCT among transplant centers and to estimate nationally representative costs for this procedure.
Administrative claims data are often used for studying economic aspects of disease and healthcare.13–15 These data are available for large and well defined populations, and depending on the database used, can provide detailed information on individual service utilization that can be aggregated by service type and episode. There are some limitations of using these data; they are not collected specifically for research, may not be generalizable and may be constrained by coverage and benefit restrictions. Most importantly, the perspective of costs included in a claims database has to be considered. Frequently, costs represent a payors perspective and include reimbursements made by insurers to individual hospitals and providers. The payments included can differ depending on the database used for analysis (e.g., private claims vs. government payors such as Medicare). Economic studies using administrative data in HCT are generally lacking.16
We conducted an exploratory study using Thomson Reuters MarketScan®, a single longitudinal database of administrative private insurance claims of patients distributed broadly in the US, to establish a cohort of autologous and allogeneic HCT recipients. Using this cohort, we describe direct medical costs over the first 100 days post-transplantation.
METHODS
Data Source
We used claims data from the Thomson Reuters MarketScan® commercial claims and encounters database to assemble a cohort of patients who had received an autologous or allogeneic HCT in the US. This database includes specific health services records for employees and their dependents in a selection of large employers, health plans and public and government organizations. The database captures the full continuum of care for insurance reimbursable services delivered across all settings including hospital stays, outpatient physician office visits, emergency department visits, home care services and outpatient prescription drug claims. Billing and encounter data are linked to detailed patient demographic and enrollment information across sites and types of providers over time and includes commercial heath data from approximately 100 payors. In 2007, this database contained health data for approximately 23 million people in the US. In comparison to other sources for claims data (e.g., Medicare that is mainly limited to patients older than 65 years and Medicaid that includes a relatively small proportion of HCT recipients), MarketScan® database has the advantage of representing a large cross-section of younger individuals with private health insurance. Patient data included in this analysis were deidentified and this study was considered exempt from approval by the Institutional Review Board of the University of Minnesota.
Patient Selection
Figure 1 shows the algorithm used to select patients for the study cohort. Patients were identified as HCT recipients if they had at least one inpatient claim with an International Classification of Diseases Ninth Edition (ICD-9) procedure code for HCT (see Table 1 for codes) between January 2007 and December 2009. Since we were interested in capturing costs over the first 100 days post-transplantation, we excluded patients whose 100 day period extended into the calendar year 2010 (i.e., HCT between 09/22/2009 and 12/31/2009). We identified 139 patients with a claim for a second HCT hospitalization that occurred more than 100 days from the first procedure during the study period of 2007–2009; these second transplant hospitalizations were excluded. We also excluded patients with two or more hospitalizations associated with an ICD-9 procedure code for HCT (>1 transplant procedure) within the 100 day period. We also excluded hospitalizations with claims of $0 and initial HCT hospitalizations longer than 100 days. Costs for the 27 HCT hospitalizations extending more than 100 days are reported separately in the results section. Our final cohort consisted of 3,365 hospitalizations for HCT, and since we considered the first transplant hospitalization only, the same number of patients.
Figure 1.
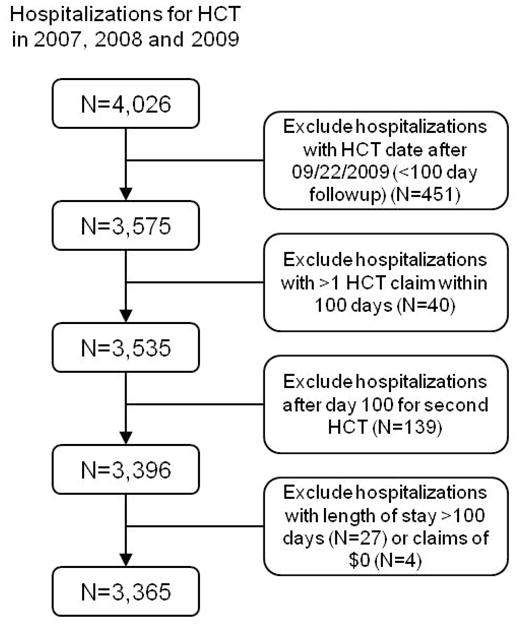
Patient selection criteria.
Table 1.
International Classification of Disease, Ninth Revision (ICD-9) procedure codes used to identify hematopoietic cell transplant (HCT) recipients and ICD-9 codes of diagnoses considered
| Procedure | Procedure code |
|---|---|
| Allogeneic hematopoietic cell transplant | 41.02, 41.03, 41.05, 41.06, 41.08 |
| Autologous hematopoietic cell transplant | 41.01, 41.04, 41.07, 41.09 |
| Hematopoietic cell transplant, graft source not specified | 41.00 |
| Disease | Diagnosis code |
| Acute myeloid leukemia | 205.0x, 205.3x, 206.0x, 207.0x, 207.2x |
| Acute lymphoblastic leukemia | 204.0x |
| Myelodysplastic syndrome | 238.72, 238.73, 238.75 |
| Lymphoma | 196.xx, 200.xx, 201.xx, 202.xx |
| Multiple myeloma and plasma cell neoplasms | 203.xx, 277.3x |
| Chronic lymphocytic leukemia | 204.1x |
| Aplastic anemia | 284.xx |
| Sarcomas | 171.xx |
Primary and secondary diagnosis codes on the HCT hospitalization claim were used to define the reason why the HCT was done. If the primary diagnosis code was one of the codes listed in Table 1, then that disease was defined as indication for HCT. If the primary diagnosis code was not one of the codes in Table 1, then all secondary diagnosis codes on the claim were assessed for presence of one of these codes. If one of these codes was present in any of the secondary positions, then that disease was defined as indication for HCT. Otherwise, the indication for HCT was defined as “other.”
Costs of Transplantation
The primary outcome measure was total medical costs of HCT from the date of HCT hospitalization through 100 days post-transplantation; total costs included costs for initial HCT hospitalization, any subsequent hospitalization and costs associated with outpatient visits and treatment. Cost estimates were based on paid amounts of adjudicated claims for inpatient and outpatient services, including insurance and health plan payments; however, patient co-payments and deductibles were not included. Also, costs did not include patient and caregiver non-medical costs (e.g., transportation, lodging, lost income) that are not captured by the MarketScan® claims database. Costs were not adjusted for inflation given the short timeframe considered in this analysis. The analysis presents costs from a payor perspective and reflects payments made by insurers.
Statistical Analysis
We designed the study to be descriptive only and present unadjusted expenditures. Summary statistics are reported as count and proportion or as median and range or interquartile range (IQR), as applicable. For selected diagnoses with an adequate sample size, we also present costs stratified by transplant type (autologous vs. allogeneic), patient age and by selected diagnoses. Costs are described for the inpatient and outpatient phase of treatment. The Chi-square test or analysis of variance was used to compare cohort subgroups, as appropriate.
RESULTS
Patient Demographics
Table 2 describes the demographic characteristics of our study cohort. Among the 3,365 patients included, 1,678 had received an autologous transplant, 1,320 had received an allogeneic transplant and the graft source was not specified for 367 patients. Multiple myeloma/plasma cell disorders (47%) and lymphoma (36%) were the most common diagnoses among patients receiving an autologous HCT while acute leukemia (46%) was the most common diagnosis among allogeneic HCT recipients. Our cohort represented centers from all geographic regions of the country.
Table 2.
Patient demographics by transplant type
| Variable | Transplant Type | ||
|---|---|---|---|
| Autologous HCT | Allogeneic HCT | Not Specified* | |
| Sample size | 1678 | 1320 | 367 |
| Year of transplant, N (%) | |||
| 2007 | 485 (28.9) | 400 (30.3) | 148 (40.3) |
| 2008 | 566 (33.7) | 414 (31.4) | 209 (56.9) |
| 2009† | 627 (37.4) | 506 (38.3) | 10 (2.7) |
| Age in years, median (range) | 55 (<1–91) | 50 (<1–77) | 50 (<1–85) |
| Age in years, N (%) | |||
| ≤20 | 132 (7.9) | 221 (16.7) | 49 (13.4) |
| 21–40 | 180 (10.7) | 231 (17.5) | 54 (14.7) |
| 41–60 | 892 (53.2) | 652 (49.4) | 189 (51.5) |
| > 60 | 474 (28.3) | 216 (16.4) | 75 (20.4) |
| Gender, N (%) | |||
| Male | 989 (58.9) | 743 (56.3) | 190 (51.8) |
| Female | 689 (41.1) | 577 (43.7) | 177 (48.2) |
| Diagnosis, N (%) | |||
| Acute myeloid leukemia | 39 (2.3) | 433 (32.8) | 43 (11.7) |
| Acute lymphoblastic leukemia | 6 (<1) | 179 (13.6) | 21 (5.7) |
| Myelodysplastic syndrome | 5 (<1) | 111 (8.4) | 3 (<1) |
| Lymphoma | 595 (35.5) | 187 (14.2) | 67 (18.3) |
| Multiple myeloma | 791 (47.1) | 47 (3.6) | 68 (18.5) |
| Chronic lymphocytic leukemia | 1 (<1) | 64 (4.9) | 8 (2.2) |
| Aplastic anemia | 4 (<1) | 33 (2.5) | 3 (<1) |
| Other | 237 (14.1) | 266 (20.2) | 154 (42.0) |
| Geographic region of transplant center, N (%)‡ | |||
| New England | 43 (2.6) | 49 (3.7) | 11 (3.0) |
| Middle Atlantic | 118 (7.0) | 85 (6.4) | 22 (6.0) |
| East North Central | 467 (27.8) | 349 (26.4) | 87 (23.7) |
| West North Central | 103 (6.1) | 92 (7.0) | 18 (4.9) |
| South Atlantic | 242 (14.4) | 166 (12.6) | 78 (21.3) |
| East South Central | 36 (2.2) | 22 (1.7) | 18 (4.9) |
| West South Central | 322 (19.2) | 295 (22.3) | 66 (18.0) |
| Mountain | 53 (3.2) | 37 (2.8) | 7 (1.9) |
| Pacific | 165 (9.8) | 125 (9.5) | 27 (7.4) |
| Unknown | 129 (7.7) | 100 (7.6) | 33 (9.0) |
Graft source could not be ascertained from claims data
Includes patients with at least 100 days followup (date of transplant prior to 09/22/2009)
States in the geographic regions are New England (ME, NH, VT, MA, RI, CT), Middle Atlantic (NY, PA, NJ), East North Central (WI, MI, IL IN, OH), West North Central (MO, ND, SD, NE, KS, MN, IA), South Atlantic (DE, MD, DC, VA, WV, NC, SC, GA, FL), East South Central (KY, TN, MS, AL), West South Central (OK, TX, AR, LA), Mountain (ID, MT, WY, NV, UT, CO, AZ, NM), and Pacific (AK, WA, OR, CA, HI)
We also compared the characteristics of patients identified through the MarketScan database with patients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) over the same time period (Supplemental Table 1). Overall, the demographic profile of patients from the two databases was similar. However, differences were noted in the recipient age distribution and diagnosis, and the geographic location of the transplant center.
Resource Utilization and Costs of Autologous HCT
Resource utilization and costs of autologous HCT are described in Table 3. Autologous HCT recipients had a median of 1 hospitalization (range, 1–8; including transplant hospitalization), were hospitalized for a median of 19 days (IQR, 15–23 days) and required a median of 12 clinic visits (IQR, 8–19 visits) from the date of transplant hospitalization through the first 100-days after transplantation. The median total costs incurred over this time period were $99,899 (IQR, $73,914–140,555) and the majority of these costs (79%) occurred during the initial transplant hospitalization (Figure 2).
Table 3.
Medical costs and resource utilization from date of transplant hospitalization to day 100 post-transplantation
| Cost category | Transplant Type | ||
|---|---|---|---|
| Autologous HCT | Allogeneic HCT | Not Specified* | |
| Sample size | 1678 | 1320 | 367 |
| Number of hospitalizations, median (range)† | 1 (1–8) | 1 (1–7) | 1 (1–6) |
| Number of hospitalizations, N (%) | |||
| 1 (HCT hospitalization) | 1275 (76.0) | 759 (57.5) | 254 (69.2) |
| 2 | 301 (17.9) | 370 (28.0) | 75 (20.4) |
| 3 | 69 (4.1) | 136 (10.3) | 26 (7.1) |
| ≥4 | 33 (2.0) | 55 (4.2) | 12 (3.3) |
| Total days hospitalized, median (IQR) | 19 (15–23) | 31 (23–45) | 20 (6–30) |
| Total days hospitalized, N (%) | |||
| ≤15 | 433 (25.8) | 125 (9.5) | 132 (36.0) |
| 16–30 | 1033 (61.6) | 523 (39.6) | 145 (39.5) |
| 31–60 | 180 (10.7) | 519 (39.3) | 76 (20.7) |
| ≥61 | 32 (1.9) | 153 (11.6) | 14 (3.8) |
| Number of outpatient clinic visits, median (range) | 12 (8–19) | 22 (13–32) | 13 (8–25) |
| Number of outpatient clinic visits, N (%) | |||
| ≤10 | 714 (42.6) | 267 (20.2) | 140 (38.2) |
| 11–20 | 599 (35.7) | 349 (26.4) | 110 (30.0) |
| 21–30 | 238 (14.2) | 344 (26.1) | 70 (19.1) |
| 31–40 | 95 (5.7) | 189 (14.3) | 26 (7.1) |
| ≥40 | 32 (1.9) | 171 (13.0) | 21 (5.7) |
| Total costs, median (IQR) | $99,899 (73,914–140,555) | $203,026 (141,742–316,426) | $106,782 (54,728–198,963) |
| Total outpatient costs, median (IQR) | $7,462 (3,079–16,038) | $20,767 (8,898–41,271) | $7,829 (1,771–22,756) |
| Total inpatient costs, median (IQR) | $88,429 (62,828–123,328) | $174,398 (116,996–269,129) | $90,000 (51,994–170,553) |
| Costs for HCT hospitalization, median (IQR) | $82,606 (59,165–110,881) | $151,899 (106,438–233,282) | $82,641 (46,377–145,326) |
| Costs for subsequent hospitalization, median (IQR)‡ | $0 (0-0) | $0 (0–22,585) | $0 (0–7,940) |
HCT – hematopoietic cell transplantation; IQR – interquartile range
Graft source could not be ascertained from claims data
Includes hospitalization for HCT
Shows costs for subsequent hospitalization for all patients in the cohort (subsequent hospitalization costs for patients who did not require hospitalization after the initial inpatient stay for HCT were considered to be $0); median costs for patients who received a subsequent hospitalization were as follows – autologous HCT (N=403) $17,425 (IQR, 9,414–46,953), allogeneic HCT (N=561) $30,561 (IQR, 13,484–89,805), not specified (N=113) $28,004 (IQR, 11,438–25,066)
Figure 2.
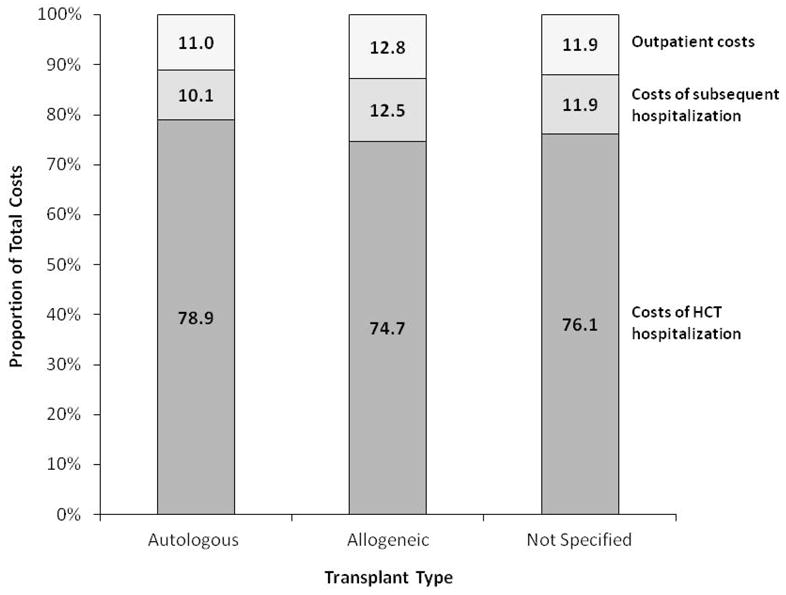
Relative contribution of costs of hematopoietic cell transplant hospitalization, costs of any subsequent hospitalization and outpatient costs to total costs of hematopoietic cell transplantation in the first 100 days after transplantation.
Autologous HCT in younger patients (≤20 years) was associated with higher costs and more hospitalized days compared to their older counterparts (Table 4). Among adult patients (>20 years), the median total costs for an autologous HCT for lymphoma were $102,458 and for myeloma were $90,702 (Table 5). The median total costs for autologous HCT for lymphoma in patients ≤ 20 years were $117,674.
Table 4.
Medical costs and resource utilization from date of transplant hospitalization to day 100 post-transplantation by age at transplantation
| Cost category | Age at transplant | |||
|---|---|---|---|---|
| ≤ 20 years | 21–40 years | 41–60 years | > 60 years | |
| Autologous HCT | ||||
| Sample size | 132 | 180 | 892 | 474 |
| Total days hospitalized, median (IQR) | 28 (22–39) | 20 (17–25) | 18 (15–22) | 18 (15–22) |
| Number of outpatient clinic visits, median (range) | 21 (12–30) | 11 (7–18) | 11 (7–18) | 12 (7–18) |
| Total costs, median (IQR) | $158,502 (115,542–255,544) | $106,929 (79,084–132,141) | $98,755 (75,650–132,141) | $87,374 (56,033–120,747) |
| Total outpatient costs, median (IQR) | $23,316 (8,420–44,712) | $7,623 (2,704–16,974) | $8,000 (3,966–15,504) | $5,348 (1,340–10,583) |
| Total inpatient costs, median (IQR) | $125,358 (96,917–212,197) | $94,454 (72,599–134,981) | $87,637 (63,990–118,303) | $79,001 (52,789–107,334) |
| Costs for HCT hospitalization, median (IQR) | $100,082 (66,290–139,767) | $89,509 (69,424–121,664) | $82,564 (62,013–108,942) | $74,997 (50,760–104,354) |
| Costs for subsequent hospitalization, median (IQR) | $2,086 (0–70,950) | $0 (0-0) | $0 (0-0) | $0 (0-0) |
| Allogeneic HCT | ||||
| Sample size | 221 | 231 | 652 | 216 |
| Total days hospitalized, median (IQR) | 42 (32–57) | 29 (23–43) | 28 (22–41) | 29 (21–43) |
| Number of outpatient clinic visits, median (range) | 21 (14–30) | 21 (10–31) | 22 (13–32) | 24 (13–32) |
| Total costs, median (IQR) | $303,709 (202,487–455,868) | $202,962 (138,545–306,313) | $192,228 (142,784–289,938) | $155,548 (111,727–267,893) |
| Total outpatient costs, median (IQR) | $27,751 (12,399–49,918) | $16,937 (6,820–36,019) | $20,949 (10,075–41,683) | $17,916 (4,829–35,921) |
| Total inpatient costs, median (IQR) | $258,790 (172,448–402,719) | $173,954 (120,865–273,036) | $164,309 (114,724–241,904) | $133,767 (93,265–233,221) |
| Costs for HCT hospitalization, median (IQR) | $224,180 (154,236–335,909) | $160,987 (110,613–244,312) | $145,457 (103,907–199,464) | $115,566 (81,277–186,135) |
| Costs for subsequent hospitalization, median (IQR) | $0 (0–31,869) | $0 (0–17,455) | $0 (0–22,346) | $0 (0–22,776) |
HCT – hematopoietic cell transplantation; IQR – interquartile range
Table 5.
Medical costs and resource utilization from date of transplant hospitalization to day 100 post-transplantation by age at transplantation for selected diagnoses
| Cost category | Age at transplant | |
|---|---|---|
| ≤ 20 years | > 20 years | |
| Autologous HCT for lymphoma | ||
| Sample size | 27 | 568 |
| Number of hospitalizations, median (range) | 1 (1–4) | 1 (1–5) |
| Total days hospitalized, median (IQR) | 22 (19–29) | 20 (18–24) |
| Number of outpatient clinic visits, median (range) | 13 (9–19) | 11 (7–18) |
| Total costs, median (IQR) | $117,674 (100,879–152,241) | $102,458 (79,053–140,529) |
| Total outpatient costs, median (IQR) | $11,016 (6,217–25,759) | $7,941 (3,109–17,449) |
| Total inpatient costs, median (IQR) | $101,945 (79,630–136,860) | $90,157 (69,863–125,482) |
| Costs for HCT hospitalization, median (IQR) | $90,727 (71,111–111,503) | $86,051 (66,861–115,439) |
| Costs for subsequent hospitalization, median (IQR) | $0 (0–13,942) | $0 (0-0) |
| Autologous HCT for multiple myeloma | ||
| Sample size | -- | 791 |
| Number of hospitalizations, median (range) | -- | 1 (1–5) |
| Total days hospitalized, median (IQR) | -- | 16 (14–20) |
| Number of outpatient clinic visits, median (range) | -- | 12 (8–18) |
| Total costs, median (IQR) | -- | $90,702 (67,213–119,894) |
| Total outpatient costs, median (IQR) | -- | $6,767 (2,840–13,264) |
| Total inpatient costs, median (IQR) | -- | $80,789 (56,809–107,292) |
| Costs for HCT hospitalization, median (IQR) | -- | $77,665 (54,833–102,072) |
| Costs for subsequent hospitalization, median (IQR) | -- | $0 (0-0) |
| Allogeneic HCT for AML, ALL and MDS | ||
| Sample size | 113 | 610 |
| Number of hospitalizations, median (range) | 2 (1–5) | 1 (1–5) |
| Total days hospitalized, median (IQR) | 43 (34–54) | 30 (24–42) |
| Number of outpatient clinic visits, median (range) | 20 (14–30) | 22 (13–32) |
| Total costs, median (IQR) | $302,822 (211,532–454,747) | $191,142 (137,752–286,097) |
| Total outpatient costs, median (IQR) | $31,463 (16,301–49,990) | $19,138 (8,769–39,125) |
| Total inpatient costs, median (IQR) | $254,485 (176,671–409,995) | $164,825 (116,576–250,468) |
| Costs for HCT hospitalization, median (IQR) | $200,000 (155,024–335,909) | $145,343 (107,978–206,978) |
| Costs for subsequent hospitalization, median (IQR) | $3,817 (0-0–34,162) | $0 (0–22,339) |
HCT – hematopoietic cell transplantation; IQR – interquartile range; AML – acute myeloid leukemia; ALL – acute lymphoblastic leukemia; MDS – myelodysplastic syndrome
Figure 3A shows the relative distribution of 100-day costs for autologous HCT by age, demonstrating higher costs for ≤ 20 year old recipients compared to patients >20 years of age. The total costs over the first 100 days for the majority of patients ≤ 20 years of age were between $100,001–200,000 (52%) compared to patients >20 years of age where the majority had total costs ≤ $100,000 (53%). Six percent of ≤ 20 year olds and 1% of patients >20 years had total costs of >$500,000.
Figure 3.
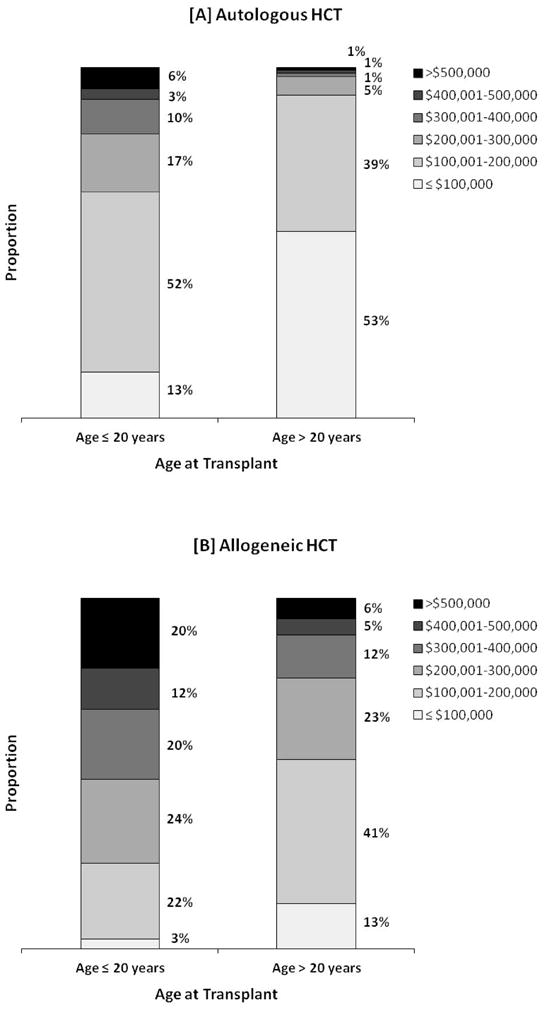
Distribution of total costs in the first 100 days after transplantation by age for recipients of [A] autologous HCT and [B] allogeneic HCT. Each stacked column represents proportion of patients with total costs within the specified range.
The MarketScan database does not contain information on patient survival. As a crude surrogate for 100-day mortality, we evaluated the rates of disenrollment with the assumption that it would be unlikely for patients receiving HCT to change their insurance plan within the first 100 days post-transplantation. Among autologous HCT recipients, 7.6% of patients ≤ 20 years, 6.1% 21–40 years, 5.9% 41–60 years and 8.0% >60 years of age disenrolled from their insurance plan. Their costs incurred were comparable to patients who maintained coverage for all 100 days; median total costs were $99,865 (IQR, $73,914–140,412; N=1566)) for patients enrolled for all 100 days and $100,840 (IQR, $73,459–147,400; N=112) for patients who were disenrolled prior to day 100 (P=0.51).
Resource Utilization and Costs of Allogeneic HCT
Allogeneic HCT recipients had a median of 1 hospitalization (range, 1–7), had a median of 31 hospitalized days (IQR, 23–45 days) and required a median of 22 clinic visits (IQR, 13-32 visits) during the study time period (Table 3). Their median total costs were $203,026 (IQR, $141,742–316,426). Similar to autologous HCT recipients, the majority of medical costs (75%) occurred during the initial transplant hospitalization (Figure 2).
Table 4 shows costs of allogeneic HCT by age at transplantation. Transplants were more expensive and associated with more hospitalized days in patients aged ≤ 20 years. Also, costs and resource utilization among allogeneic HCT recipients >60 years was no greater than that for adults who were ≤ 60 years of age. The median total costs of allogeneic HCT for acute leukemia and myelodysplastic syndromes among patients ≤ 20 years and >20 years were $302,822 and $191,142, respectively (Table 5).
Figure 3B also highlights that total costs for allogeneic HCT were greater among patients ≤ 20 years compared to patients >20 years of age. Total costs were >$500,000 for 20% and 6% of ≤ 20 year and >20 year old patients, respectively.
We also evaluated disenrollment rates for allogeneic HCT recipients to obtain a crude estimate of 100-day mortality. Proportion of patients disenrolled within 100-days by age were 5.9% among ≤ 20 year olds, 13.0% among 21–40 year olds, 12.9% among 41–60 year olds and 13.4% for patients >60 years of age. The total costs for patients who disenrolled and those who remained enrolled for all 100 days were comparable. The median total costs for the former (N=156) were $197,843 (IQR, $137,901–348,477) compared to $203,769 (IQR, $142,304–308,564) for patients who maintained enrollment for all 100 days (N=1164) (P=0.14).
Costs for Patients Hospitalized for >100 days
The initial transplant hospitalization lasted for >100 days in 27 patients (autologous HCT=3, allogeneic HCT=19, graft source not specified=5). Their median length of hospital stay was 112 days (range, 101–218 days) and the median total costs for their transplant hospitalization was $621,509 (IQR, $386,105–824,530).
DISCUSSION
Our study shows the feasibility of using a longitudinal administrative claims database representing a national, commercially insured population to identify HCT recipients and to study costs of transplantation. In comparison to previously reported single institution studies, our study draws on data representing a large number of transplant centers from throughout the country and highlights the high costs associated with autologous and allogeneic HCT over the first 100 days post-transplantation.
Within the first 100 days, hospitalization for the transplantation procedure was associated with the majority of total costs and the contribution of outpatient care and any subsequent hospitalizations to total expenses was relatively small. This is consistent with previous cost-identification studies of autologous and allogeneic HCT.2,3,5–7 As transplant centers, payors and policy makers consider ways to reduce costs of HCT, innovative methods for more economical delivery of care during this early post-transplant period would have the greatest impact.
We also show notable differences in costs through the first 100 days by age at transplantation in both autologous and allogeneic HCT recipients. In general, costs were greater among pediatric (≤ 20 years) compared to adult (>20 years) recipients and this difference was more pronounced with allogeneic HCT. Pediatric patients were also hospitalized for a longer time period compared to adults. For example, pediatric patients had 1.6 times higher median total costs and costs for outpatient care compared to adult recipients of allogeneic HCT for acute leukemia. The median total duration of hospitalization was 43 days versus 30 days, respectively. There is significant variation in transplant practices among physicians who take care of pediatric and adult HCT recipients.11 Our findings suggest the need for more research into the higher costs of HCT in children. Pediatric physician and center practices that may contribute to differences in costs need to be investigated further to identify opportunities where care could be delivered in a more cost-efficient manner without compromising patient outcomes.
Another finding of note from our analysis is that transplantation for older patients (>60 years) was not more expensive than that for adults ≤ 60 years of age. There exists a perception that older patients may be at higher risks for transplant related complications, which in turn may be associated with higher costs of transplantation. The relatively comparable costs for older and younger adults in our study may represent selection of healthier older patients for HCT and a greater proportion of older patients receiving reduced intensity transplants with their associated lower risk of complications within the first 100 days after HCT. Recent research has shown that adults ≥ 65 and <65 years have similar risks of overall and treatment related mortality after allogeneic HCT using reduced-intensity regimens.17 Although this needs more research, our preliminary findings are reassuring that HCT in older patients is not necessarily more expensive.
Our study shows that a large claims dataset from a national commercially insured population can be used to address several questions related to costs of HCT. Additional questions related to HCT costs that can addressed by this approach include costs related to HCT workup and graft acquisition, geographic variation in costs of HCT, costs associated with long-term care of HCT survivors and cost comparisons between various transplant and non-transplant treatments. However, some limitations of claims datasets need to be kept in mind. The costs represented in these data reflect costs from an insurer’s perspective and reflect the paid amounts of adjudicated claims to individual hospitals and providers. Claims data are not collected for research purposes and represent the coverage benefits of enrollees who receive a transplant. Cost estimates may not be generalizable. Costs reported are specific to the payors included in the database and reimbursement for transplantation through other payors (e.g., Medicare) may be different. Furthermore, some regions of the country and types of transplant centers may be differentially represented in a claims database. It is also difficult to describe costs by some important variables that can drive resource utilization and costs in HCT recipients (e.g., conditioning regimen intensity).
Notwithstanding these limitations, our study describes costs of HCT from a national claims database using a cohort of patients who received their transplants at multiple centers in the US and highlights the feasibility of using these data to study economic aspects of HCT. Our study provides a benchmark for costs for transplantation in the US and lays the foundation for further comprehensive research in this area.
Supplementary Material
Acknowledgments
Sources of Support:
Funding Support for this study was provided by the Cancer Outcomes and Survivorship Pilot Grant through the University of Minnesota Masonic Cancer Center.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Disclosure: None of the authors have any relevant financial conflicts of interest to disclose
Supplementary information is available at BMT’s website
References
- 1.Stranges E, Russo CA, Friedman B. HCUP Statistical Brief #82. Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2009. Procedures with the most rapidly increasing hospital costs, 2004–2007. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb82.pdf. [PubMed] [Google Scholar]
- 2.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Majhail NS, Mothukuri JM, Macmillan ML, et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr Blood Cancer. 2010;54:138–143. doi: 10.1002/pbc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Anasetti C, Kuntz KM, Patten J, Antin JH, Weeks JC. The costs and cost-effectiveness of unrelated donor bone marrow transplantation for chronic phase chronic myelogenous leukemia. Blood. 1998;92:4047–4052. [PubMed] [Google Scholar]
- 5.Lee SJ, Klar N, Weeks JC, Antin JH. Predicting costs of stem-cell transplantation. J Clin Oncol. 2000;18:64–71. doi: 10.1200/JCO.2000.18.1.64. [DOI] [PubMed] [Google Scholar]
- 6.Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14:197–207. doi: 10.1016/j.bbmt.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito AM, Zahrieh D, Cutler C, et al. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone Marrow Transplant. 2007;40:209–217. doi: 10.1038/sj.bmt.1705733. [DOI] [PubMed] [Google Scholar]
- 8.Bennett C, Waters T, Stinson T, et al. Valuing clinical strategies early in development: a cost analysis of allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;24:555–560. doi: 10.1038/sj.bmt.1701945. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo JD, Vogelsang GB, Krumm S, Frink B, Mock V, Bass EB. Outpatient-based bone marrow transplantation for hematologic malignancies: cost saving or cost shifting? J Clin Oncol. 1999;17:2811–2818. doi: 10.1200/JCO.1999.17.9.2811. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Astigarraga CC, Eapen M, et al. Variation in supportive care practices in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1231–1238. doi: 10.1016/j.bbmt.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26:2162–2170. doi: 10.1200/JCO.2007.15.0169. [DOI] [PubMed] [Google Scholar]
- 12.Majhail NS, Murphy EA, Omondi NA, et al. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant. 2011;17:956–961. doi: 10.1016/j.bbmt.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iezzoni LI. Assessing quality using administrative data. Ann Intern Med. 1997;127:666–674. doi: 10.7326/0003-4819-127-8_part_2-199710151-00048. [DOI] [PubMed] [Google Scholar]
- 14.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–55. doi: 10.1097/MLR.0b013e31819c95aa. [DOI] [PubMed] [Google Scholar]
- 15.Etzioni R, Riley GF, Ramsey SD, Brown M. Measuring costs: administrative claims data, clinical trials, and beyond. Med Care. 2002;40:III63–72. [PubMed] [Google Scholar]
- 16.Jones JA, Qazilbash MH, Shih YC, Cantor SB, Cooksley CD, Elting LS. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the Nationwide Inpatient Sample. Cancer. 2008;112:1096–1105. doi: 10.1002/cncr.23281. [DOI] [PubMed] [Google Scholar]
- 17.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


